Abstract
The D. melanogaster hematopoietic organ, called lymph gland, proliferates and differentiates throughout the larval period. The lymph gland of the late larva is comprised of a large primary lobe and several smaller secondary lobes. Differentiation into two types of hemocytes, plasmatocytes and crystal cells, is confined to the outer layer (cortical zone) of the primary lobe; the center of the primary lobe (medullary zone), as well as the secondary lobes, contain only proliferating prohemocytes. A small cluster of prohemocytes located at the posterior tip of the primary lobe serves as a signaling center (PSC) that inhibits precocious differentiation of the medullary zone. The larval lymph gland is stabilized by layers of extracellular matrix (basement membranes) that surround individual hemocytes, groups of hemocytes, as well as the lymph gland as a whole. In this paper we investigated the events shaping the lymph gland in the early pupa. The lymph gland dissociates and hemocytes disperse during the first 12h after puparium formation (APF), leaving behind empty husks of basement membrane. Prior to lymph gland dissociation, cells of the medullary zone differentiate, expressing the early differentiation marker Peroxidasin, as well as, in part, the late differentiation marker P1. Cells of the PSC spread out throughout the pupal lymph gland prior to their dispersal. Cells of the secondary lobes undergo a rapid phase of proliferation that lasts until 8h APF, followed by expression of Peroxidasin, and dispersal. These hemocytes do not express P1, indicating that they disperse prior to full maturation.
Keywords: Drosophila, blood, lymph gland, metamorphosis
Introduction
The hemolymph (“blood”) of D. melanogaster contains three types of mature hemocytes, the most prevalent of which is the plasmatocyte. The plasmatocyte carries out a phagocytic function, engulfing dead cells, debris and pathogens. The second most prevalent cell type is the crystal cell, which aids in the innate immune response as well as in wound healing by carrying out melanization. The third and most rare hemocyte type is the lamellocyte. The lamellocytes are large flat cells, which encapsulate and neutralize objects too large to be phagocytized (Evans et al., 2003; Crozatier and Meister, 2007; Krzemien et al., 2010). Lamellocytes are not seen in embryos or adults and are rarely found in larvae. However, upon immune challenge, via parasitization with a wasp egg, the number of lamellocytes increases substantially (Lanot et al 2001; Sorrentino et al 2002).
Blood formation in Drosophila takes place during two phases, the early embryo, and the larva. In the embryo, a subset of head mesodermal cells become specified as plasmatocytes and crystal cells (Tepass et al., 1994a; Lebestky et al., 2000; Alfonso and Jones, 2002); these cells play a role during embryonic development, and form the pool of differentiated, motile cells of the larval hemolymph. The second phase of blood formation takes place in the lymph gland, a hematopoietic organ that develops in close association with the cardio-vascular system (dorsal vessel) from the thoracic mesoderm of the embryo (Poulson, 1950; Rugendorff et al., 1994; Mandal et al., 2004). In the late embryo and early larva, the lymph gland forms a small, bilaterally paired cluster of undifferentiated blood progenitors “prohemocytes”. Prohemocytes proliferate rapidly during the larval period. In the late larva, the lymph gland comprises a pair of large primary lobes, which have developed from the small lymph gland primordium; to this, a series of secondary and tertiary lobes, located posterior to the primary lobes, have been added (Jung et al., 2005). Hemocyte maturation takes place in the primary lobes in a tightly regulated spatio-temporal pattern. The center of the primary lobes, called medullary zone, contains prohemocytes that remain in a slowly proliferating, undifferentiated state throughout the larval period. Cells of the medullary zone are considered “stem cell-like”, and are maintained in this state by the posterior signaling center (PSC) via a number of signaling pathways (Mandal et al., 2007; Krzemien et al., 2007). The medullary zone is surrounded by an outer cortical zone, in which cells differentiate, expressing a number of molecular and morphological markers of plasmatocytes and crystal cells (and, in cases of injury or infection, lamellocytes).
Under normal conditions, cells of the lymph gland remain together and do not disperse in the hemolymph. In that regard, Drosophila appears to present an exception, since in other insects, including other Dipterans, hemocytes of the lymph gland are constantly given off into the hemolymph (Akai and Sato, 1971; Hinks and Arnold, 1977; Nakahara et al., 2003). In Drosophila, only infection or injury (Rizki and Rizki, 1992; Sorrentino et al., 2002; Agaisse et al., 2003) triggers an increased proliferation and maturation in the lymph gland, and causes the release of hemocytes into circulation. In the absence of infection/injury, the lymph gland of Drosophila dissociates during the early pupal stage (Lanot et al., 2001). By 15h after puparium formation (APF), all lobes of the lymph gland have dissolved. Adult Drosophila have no compact blood forming organ. This may be an adaptation to the short life span of fruit flies; it is also possible that undifferentiated, pluripotent hemocyte progenitors remain active in circulation, or in the form of small clusters of cells which so far have eluded detection.
The process of lymph gland dissociation in the pupa has been analyzed electron microscopically (Lanot et al., 2001), but many questions have remained unanswered. For example, do cells of the lymph gland all differentiate before moving out into the hemolymph? Can one detect differences in the rate of maturation/dissociation between primary and secondary lobes? Do the zones of the primary lobe remain intact until the very end before lymph gland dissociation? What happens to the reticular extracellular matrix that surrounds individual hemocyte progenitors of the larval lymph gland? To address these questions, we have employed hemocyte specific markers to analyze the stages of lymph gland dissociation, in combination with transmission electron microscopy (TEM).
Materials and Methods
Antibodies
Antibodies used for Immunohistochemistry include: α-Mouse-Antennapedia (1:4) (Developmental Hybridoma Bank), α-rat BrdU (1:100) (Abcam), α-Mouse-GFP (1:400) (Sigma), α-Rabbit-GFP (1:2000) (Molecular Probes), α-Mouse-L1 (1:100) (Kurucz et al., 2003), α-Mouse P1 (1:100) (Kurucz et al., 2007), α-Mouse-Peroxidasin (1:500) (Nelson et al., 1994), α-Rabbit-ProPo (1:1000) (Muller et al., 1999). All secondary antibodies from Jackson Immunoresearch and used at 1:200 (FITC, CY3 and Biotin), 1:100 (CY5) and 1:500 (ALEXA).
Fly Lines
Protein trap line, ZCL1973X (Kelso et al., 2004), which labels Trol expression with GFP. G147, a GFP trap line associated with a microtubule protein (Morin et al., 2001).
Pupal Fixation
Pupae were pinned dorsal side up and their pupal case removed. Pupal lymph gland was carefully exposed before a fixative (1:3 of 37% Formaldehyde: 1X PBS) solution was applied to the sample for 20 minutes. Pupae were then washed 3 times in 0.1% PBT for 15 minutes each. Immunohistochemistry was then carried out.
Immunohistochemistry
Samples were washed with 0.1% PBT three times for 15 minutes each at room temperature while rotating. Samples were then blocked for 30 minutes in a solution of 10% NGS made in 0.1% PBT. Samples then placed in 1° antibody solution made in 10% NGS and left to rotate overnight at 4°C. Samples were then washed and blocked as described above and placed in a 2° antibody conjugated to a fluorescent marker. Samples were left in 2° antibody to rotate overnight at 4°C. Samples were then washed as described above and mounted in vectashield containing a 1:500 concentration of Toto-3-iodide (invitrogen).
BrdU incubation, fixation and staining
Dissected pupal lymph glands were placed in a solution of 75 μg/ml of BrdU. Lymph glands were allowed to incubate in BrdU solution for 40–60 minutes on ice. Tissue was fixed in a 1:3 solution of 37% Formaldehyde: 1X PBS for 20–30 minutes. Samples were washed 4 times for 10 minutes each in 0.3% PBST, and blocked in 10% NGS for 30 minutes. Samples were incubated in primary antibody overnight at 4°C on rotator. Samples were washed four times, for 10 minutes each, in 0.3% PBST. After blocking for 30 minutes in 10% NGS samples were incubated in 2° antibody overnight at 4°C. After washing in 0.3% PBST twice for 10 minutes, tissue was fixed for a second time in a 1:3 solution of 37% Formaldehyde and 1X PBS for 15 minutes, followed by washing in 0.3% PBST three times for 10 minutes each. DNA was denatured for 30 minutes by adding 2N HCl. After three washes in 0.3% PBST,10 minutes each, samples were blocked for 30 minutes. Samples were incubated in anti-BrdU antibody overnight at 4°C. Samples were washed with 0.3% PBST four times for 10 minutes each, blocked for 30 minutes in 10% NGS, and incubated in 2° antibody overnight at 4°C. This was followed by washing samples with 0.3% PBST 4 times for 10 minutes. Samples were mounted in Vectashield.
EM Fixation
Fixative solution contained 0.2 mL EM Phosphate Buffer A, 0.8 mL EM Phosphate Buffer B, 0.4 mL 16% Paraformaldehyde, 0.125 mL 50% Glutaraldehyde and 0.475 mL DI water; kept on ice. Dissected tissue was fixed for 24 hours at 4°C, followed by washing 5 times for 10 minutes in phosphate buffer (1.0 mL EM Phosphate Buffer A, 4.0 mL EM Phosphate Buffer B, 5.0 mL DI water). Postfixation in 1% osmium tetroxide made in phosphate buffer (0.1M pH 7.3) (0.5 ml DI water, 0.5 ml 4% osmium tetroxide and 1 ml phosphate buffer 0.2 M pH 7.3) at 4°C, in dark, for 60 minutes. Washing 4 times for 10 minutes in DI water. Tissue was dehydrated through acetone series at 4°C (50%, 70% and 96% acetone for 10 minutes each; 100% acetone 3 times for 10 minutes each). For embedding tissue was placed in 1:3 Epon:acetone mixture for 2 hours at room temperature, while rotating. Placed in 1:1 Epon:acetone mixture for 3 hours at room temperature while rotating. Placed in 3:1 Epon:acetone mixture overnight at room temperature. Placed in Epon overnight. Blocks were polymerized for 16 hours at 60°C in flexible plastic mold.
Confocal
BioRad Radiance 2000 confocal microscope with LaserSharp 2000 acquisition software; 40x oil immersion lens.
Results
Lymph gland hemocytes express differentiation markers prior to dissociation
In order to determine whether all the cells of the lymph gland differentiated before their release and dispersal and at what time point this occurred, we analyzed animals at white prepupal stage and 2, 4, 8 and 10 hour after puparium formation (APF), using the ZCL1973X (Trol-GFP protein trap) line that expresses GFP in the lymph gland (Fig. 1A–K). As a differentiation marker for plamatocytes, we labeled dissected lymph glands with anti-Peroxidasin (Pxn; early differentiation marker) and P1 (late differentiation marker); in addition, Propo, a marker for differentiated crystal cells, was used (Jung et al., 2005).
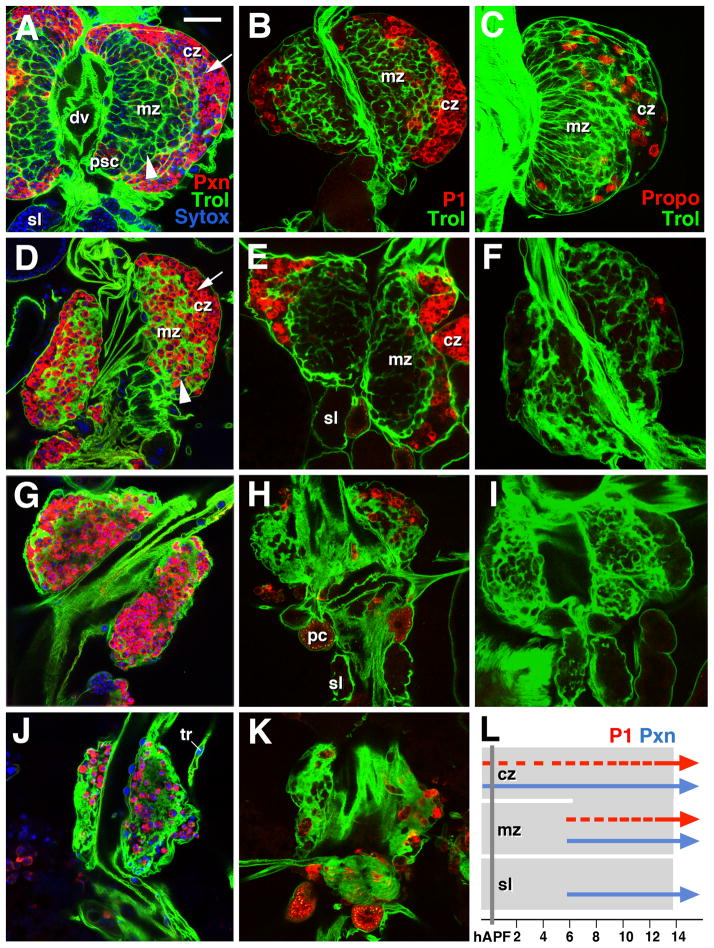
Figure 1. Expression of hemocyte differentiation markers in the pupal lymph gland.
Panels show Z-projections of confocal sections of central part of primary lymph gland lobe. Expression of GFP driven by Trol-Gal4 in extracellular matrix outlines the lymph gland (green). Panels of left column (A, D, G, J) show labeling with early plasmatocyte marker anti-Peroxidasin (Pxn, red). Second column (B, E, H, K) represents labeling with late differentiation marker P1 (red); in right column (C, F, I), crystal cell marker Propo was used (red). Upper row (A, B, C) represents late third instar to white prepupa (0–2h APF), second row (D–F) 2–4h pupa, third row (G–I) 8h pupa, and fourth row (J, K) 10h pupa. Panel L provides a timeline (in h after puparium formation) of the expression pattern of P1 (red) and Peroxidasin (blue) in different domains of pupal lymph gland. The top portion of the figure represents the cortical zone. Below this is seen the medullary zone and finally the secondary lobes. White arrowheads in A and D indicate medullary zone (mz) which, until about 6hAPF, can be clearly distinguished from cortical zone (cz; white arrows in A and D).
Other abbreviations: dv dorsal vessel; sl secondary lobe; pc pericardial cell; tr trachea.
Bar: 20μm (A–K)
In late larvae, expression of Pxn is restricted to the cortical zone (Fig. 1A). Around 4h APF, we observe sporadic Pxn signal in the medullary zone (Fig. 1D), and by 8h, most, if not all cells of the pupal lymph gland are Pxn-positive (Fig. 1G). At the same time, the structural distinction between a medullary and cortical zone breaks down. Thus, in late larvae, the medullary zone is distinguished from the cortical zone by a denser Trol-positive ECM reticulum, as well as a higher packing density of hemocytes (Fig. 1A). By 8h APF, hemocytes are packed at equal density throughout the lymph gland (Fig. 1G).
The expression of P1 is temporally offset relative to Pxn. By 4h APF, when many central (medullary) cells are already Pxn-positive, P1 is still restricted to the cortical zone (Fig. 1E). However, by 8h and 10h APF, P1-positive cells are clustered throughout the lymph gland (Fig. 1H, K respectively).
Crystal cells, labeled by Propo, form a small population of hemocytes that are evenly scattered throughout the cortical zone of late larvae (Fig. 1C). In the pupal lymph gland, crystal cells are almost entirely absent; we only saw small groups of 1–3 crystal cells in the periphery of lymph glands of pupae up to 2h APF (Fig. 1F). Our findings indicate that hemocytes differentiate before they move out of the lymph gland; crystal cells appear to be the first cells to leave.
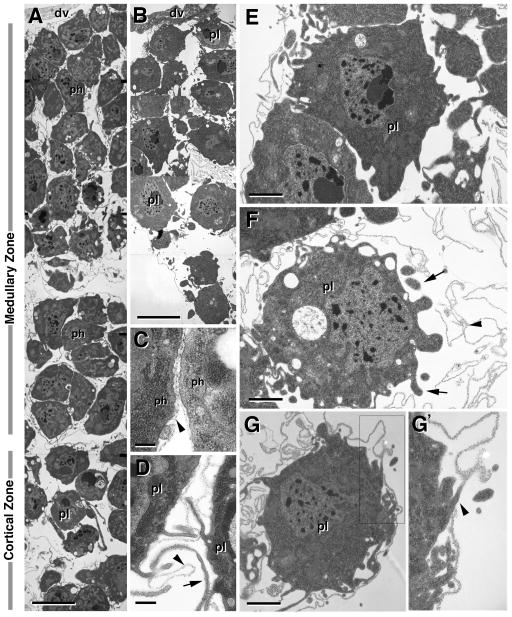
The expression of differentiation markers presented above is paralleled by morphological signs of differentiation, as documented by TEM. Undifferentiated blood progenitors of the larval medullary zone are characterized by a higher nucleus-cytoplasm ratio than hemocytes in the cortical zone (Fig. 2A). They also lack the filiform processes and membrane ruffles typical for mature hemocytes. In the 4h pupa, some cells with the characteristics of undifferentiated blood progenitors still persist (Fig. 2B, E), but mature plasmatocytes predominate throughout the lymph gland (Fig. 2F).
Figure 2. Ultrastructure of the lymph gland of the third instar larva and 4h pupa.
A, B: Low magnification electron micrographs of primary lymph gland lobe of third instar larva (A) and 4h pupa (B). Medial at top; note wall of dorsal vessel (dv) visible in both panels. At larval stage, cells in the medial two thirds of the lymph gland form part of the medullary zone, as indicated to the left of panel A. These cells are more densely packed and exhibit the ultrastructural features of prohemocytes (ph). In the periphery of the larval lymph gland (cortical zone) cells are spaced further apart and are mostly differentiated plasmatocytes (pl). In the early pupa (B), the lymph gland has significantly decreased in diameter, and a clear distinction between medullary zone and cortical zone is no longer visible. Most cells represent differentiated plasmatocytes. C, D: Basement membranes deposited in the cleft between two adjacent prohemocytes (arrowhead in C) and plasmatocytes (arrowhead in D; arrow points at extension of plasmatocyte membrane). E–G: Plasmatocytes in 4h pupal lymph gland. Many plasmatocytes have already moved out of the lymph gland, leaving behind the basement membranes that originally surrounded them (arrowhead; arrow points at extensions of plasmatocyte membrane). Plasmatocyte shown at bottom of G appears to be in the process of leaving, as indicated by the wide gap between the cell and the basement membrane (double arrowhead).
Bars: 5μm (A, B); 1μm (E, F, G); 0.1μm (C, D)
An Antennapedia-positive PSC persists in the early pupa
The PSC of the late larva forms a bilateral cluster of cells located at the posterior margin of the primary lobes (Fig. 3A). Antennapedia (Antp)-positive cells at numbers comparable to those typical for the larval PSC persisted until 10h APF, immediately before dissociation of the lymph gland. After that, Antp expression is no longer associated with the dorsal vessel, indicating that PSC cells, along with other hemocytes, disperse into the hemolymph. Interestingly, in 8h, 10h and 12h pupae, Antp-positive cells appeared more widely distributed over the small remainder of the lymph gland (Fig. 3B–D), rather then forming a tight cluster as in the larva. This could indicate that hemocytes in the hours immediately before dispersal mix more freely than at the larval stage.
Figure 3. Fate of the Posterior Signaling Center (PSC) in the dissociating pupal lymph gland.
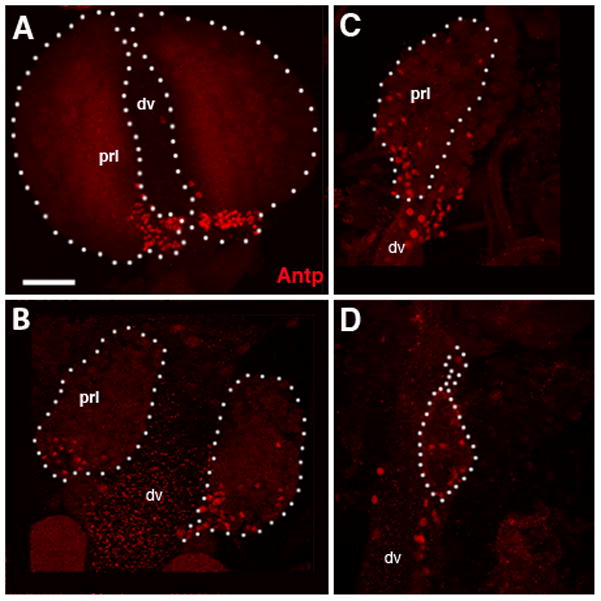
Expression of PSC marker Antennapedia (Antp). Panels show confocal sections of late larval (A), 8h pupal (B),10 hour pupal (C) and 12 hour pupal (D) lymph gland lobes labeled with anti-Antennapedia (Antp, red). Lymph gland lobes are demarcated with a dashed outline. In pupa, the Antp-positive cells appear to migrate away from the Posterior Signaling Center (PSC) and spread out throughout the lymph gland.
Other abbreviations: prl primary lobe; sl secondary lobe; dv dorsal vessel.
Bar: 20μm (A–D)
The secondary lobes undergo a growth spurt prior to lymph gland dissociation
The secondary and higher order lobes of the larva do not generally express any of the differentiation markers found in the cortical zone of the primary lobes as most of the cells are undifferentiated (Jung et al., 2005). This prompted the question whether hemocytes of the secondary lobes (which dissociate at the same time as the primary lobes) disperse as undifferentiated blood progenitors, or go through an accelerated phase of maturation prior to dispersal. Using the markers Pxn and P1 we observed that secondary lobe hemocytes do initiate differentiation during a short time interval: from 4h APF onward, most, if not all, cells of the secondary lobes are Pxn-positive (Fig. 4A–C). However, expression of the differentiation marker P1 is never initiated (Fig. 4F), indicating that dispersal of the secondary lobe cells takes place before they have terminally differentiated. It should be noted that, as in the larva, expression of the crystal cell marker Propo is never observed in the secondary lobes.
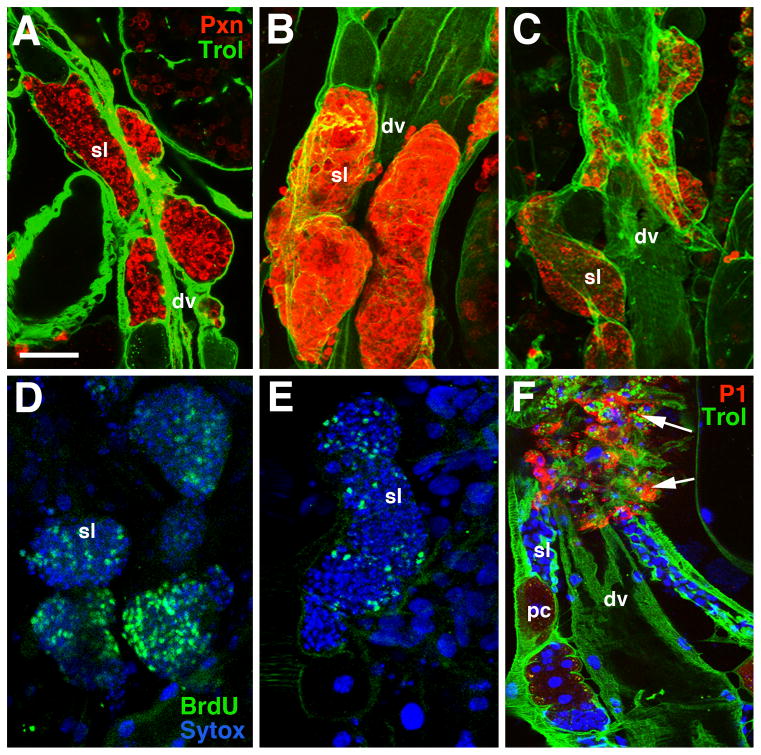
Figure 4. Pupal development of the secondary lymph gland lobes.
A–C: confocal sections of secondary lobes (sl) of 4h (A), 8h (B) and 10h pupa (C), labeled with ZCL1973X (Trol; green) and anti-Peroxidasin (Pxn, red). D, E: Labeling of proliferating cells in secondary lobes of 4h (D) and 8h (E) lymph gland with anti-BrdU (green), following 45min incubation of dissected lymph glands in BrdU-containing solution. F: Labeling of 10h secondary lobe with anti-P1 (red). P1-positive cells (arrows) are dispersed hemocytes; cells within the secondary lobes (sl) are P1-negative.
Other abbreviations: dv dorsal vessel; pc pericardial cell
Bar: 20μm (A–D)
We noted that the secondary lobes transiently increase in size between the 4 and 8 hour pupal time points, before diminishing by 10 hours (Fig. 4A–C). To assess whether cell division was in fact taking place, 4 and 8 hour pupal lymph glands were incubated with BrdU. For both time points, high numbers of Brdu-positive cells were seen in the secondary lobes (Fig. 4D–E respectively); by contrast, no BrdU incorporation takes place in the primary lobes during this time interval (data not shown).
The reticular ECM during hemocyte dispersal
During the larval period, blood progenitors surround themselves with a thin lamella of extracellular matrix. It contains collagen and laminin (Jung et al., 2005; Krzemien et al., 2010; Grigorian et al., 2011), as well as perlecan (in Drosophila: Trol; Fig. 1A). Electron microscopically, the ECM lamella is approximately 30–50 nm thick and has a granulated appearance (Fig. 2C). Characteristically, ECM lamellae do not adhere tightly to the cell surface, but leave an open space of variable diameter (Fig. 2D). Trol driven GFP visualizes impressively the ECM of the larval lymph gland. In the medullary zone, each cell, or small groups of cells, are surrounded by ECM lamellae; in the cortical zone, many lamellae have disappeared, and larger clusters of (differentiated) hemocytes occupy a common “chamber” bounded by ECM (Fig. 1A–C).
As the lymph gland dissociates in the pupa and hemocytes disperse in the hemolymph, they leave behind the ECM lamellae. As shown in Figs. 5A and B, the lymph gland of a 10h pupa consists of empty shells of folded ECM lamellae, almost devoid of hemocytes. What this implies is that hemocytes, rather than digesting the ECM, push through gaps or only digest small, localized areas in the ECM lamellae as they move out of the lymph gland. A figure suggesting a hemocyte in the process of “escaping” is shown in panel G of Fig. 2. We were not able to determine for how much longer, after 12h APF, the lymph gland associated ECM remains. The finding that ECM stays behind after the cells that produced it have left is unexpected, since in other tissues that have been studied, cell migration or dispersal is preceded by the disappearance (digestion) of the ECM. For example, in case of the wing, a thick, multilayered membrane underlies the epithelium in the 6h pupa (Srivastava et al., 2007). During eversion of the wing, which is accompanied by large scale cell movement, this ECM membrane has all but disappeared. The lymph gland associated ECM may have special properties that render it particularly stable, so that it can persist in the absence of cells; another difference between lymph gland and wing metamorphosis that may be relevant is that hemocytes merely have to break through the surrounding ECM lamella, after which they become freely motile cells, whereas the wing epithelium moves as an intact layer, and remnants of ECM (if they existed) would impede this movement.
Figure 5. Ultrastructure of the 10h pupal lymph gland.
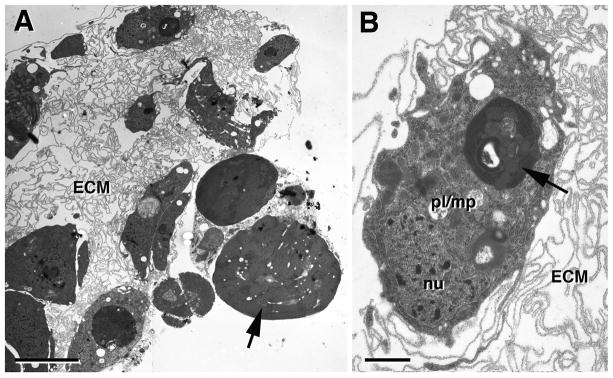
A: Low power electron micrograph showing remnant of lymph gland in the 10h pupa. Few cells, typically phagocytotically active plasmatocytes (pl), are left. Aside from these cells, the lymph gland forms a “husk” of folded basement membranes (ECM) that at earlier stages contained hemocytes. B: Phagocytotically active plasmatocyte (macrophage; pl/mp), containing lysosomes with electron dense cellular debris (arrow).
Bars: 5μm (A); 1μm (B)
Discussion
The time course of production of hemocytes and their release into circulation shows considerable differences between insect groups. In most cases studied (e.g., Akai and Sato, 1971; Nakahara et al., 2003), bursts of hemocyte proliferation and release are coupled to the molting cycle of the larva. On top of this cyclic pattern, the ratio of differentiated versus undifferentiated cells generally increases with larval age. With the onset of metamorphosis, compact hematopoietic organs which have been described for many insects (Han et al., 1998; Crossley, 1964; Monpeyssin and Beaulaton, 1978; Hoffmann et al., 1979) typically disappear after a final burst of hemocyte release. In other words, adults of most insect species, lack compact hematopoietic organs. The latter is true also for Drosophila, but in terms of earlier production and release of hemocytes during the larval stage, one notes that hemocyte proliferation does not appear to be coupled as strictly to the molting cycle and release does not occur at all prior to metamorphosis in Drosophila. Only in cases of infection or wounding, is the production of hemocytes stimulated, and their release into circulation seen. The same applies to a number of mutations [e.g., Hopscotch (Hop), encoding a Drosophila Janus Kinase) in which hemocyte proliferation and/or differentiation is increased (Harrison et al., 1995; Minakhina and Steward, 2006). The absence of hemocyte release during larval stages was experimentally confirmed by analyzing labeled clones of hemocyte progenitors induced in embryos (Holz et al., 2003). These clones came to occupy the lymph glands of larvae, but did not spread into circulation until immediately before pupation.
In the current study we investigated the differentiative processes occurring in the Drosophila lymph gland prior to its dissociation. We found that one of the differentiation markers, Pxn, appears to be expressed in all hemocytes before they dissociate. Thus, both medullary zone of the primary lobe and the secondary lobes, domains which only contain undifferentiated prohemocytes in the larva, show labeling with anti-Pxn in the early pupa. Pxn, an ECM protein, is synthesized by hemocytes among other cell types (Nelson et al., 1994), and is considered an early differentiation marker (Jung et al., 2005). A later differentiation marker, P1, appears only in a fraction of cells of the cortical zone in the larvae. In the 4h pupa, P1 is still not expressed in the primary lobe medullary zone; only immediately prior to complete dissociation around 12 h did most of the (remaining) cells of the primary lobe express P1. Cells of the secondary lobes, on the other hand, remained negative to the very end, demonstrating that these cells dissociated as incompletely differentiated hemocytes.
As described previously by Lanot et al. (2001), crystal cells were quickly lost after the onset of metamorphosis. We found the persistence of small numbers of crystal cells until 2 hours following the onset of pupation; by 6 hours crystal cells appeared to be completely absent from the lymph gland. Furthermore, as shown previously (Lanot et al., 2001), lamellocytes, the third type of Drosophila hemocytes, are never found in the pupal lymph gland. Lamellocytes are only rarely seen under normal conditions in either larva or adult (Lanot et al., 2001); they are induced by infection or wounding (Markus et al., 2005; Rizki and Rizki, 1992).
Past studies had demonstrated the presence of mitotic cells (1–2%) in all lymph gland lobes of mid-third and wandering larva (Lanot et al., 2001). For the pupa, we observed a high level of cell division in the secondary lobes at 4h APF and slightly less at 8h APF; the primary lobes were virtually devoid of any proliferation until dissociation of the lymph gland occurred around 12h APF. This finding supports the view that secondary lobes (which also appear later in development) are always at an earlier state of differentiation than primary lobes: cells remain mitotically active to the very end of lymph gland life, and concomitantly do not complete differentiation, as evidenced by the lack of P1 expression (see above).
How does the release of blood cells from the insect lymph gland compare to the corresponding process in vertebrates? Can one detect similarities that extend to the mechanisms controlling blood cell release across phylum boundaries? The blood forming tissue in most vertebrates is the bone marrow. The bone marrow is formed by blood vessels, capillaries and wide, endothelium bound venous sinuses. The spaces in between this vascular tissue are filled with stromal cells, blood stem and progenitor cells, and blood cells at successive stages maturation (Lichtman, 1981; Petrides and Dittmann, 1990). Fully differentiated, postmitotic blood cells form a pool (“postmitotic pool”) of cells that is released in a controlled manner into the sinuses; here, blood cells can stay for extended period of time before moving out into circulation.
The process of releasing blood cells from the stroma into the sinuses (marrow egress) involves dynamic changes in both the blood cells themselves, as well as the cellular elements that form the physical boundary between bone marrow stroma and the blood. This boundary is formed by three layers: the endothelium lining the sinuses, specialized stromal cells called “adventitial cells” (Weiss, 1980; Lichtman, 1981; Petrides and Dittmann, 1990; Inoue and Osmond, 2001), and a basement membrane secreted by both cell types and deposited in the space between them. Blood cells and their progenitors themselves are not individually surrounded by a basement membrane, which constitutes a marked difference to the condition found in the insect hematopoietic tissues. When crossing from the stroma into the lumen of the sinuses, blood cells move in between clefts of adventitial cells, and create pores in the basement membrane and the endothelial cells. This multistep process is orchestrated by multiple signaling interactions that are incompletely understood. A variety of signals derived from both the bone marrow stroma, as well as from peripheral blood cells and endothelial cells act on receptors on the blood cells-to-be-released, and cause changes in their adhesiveness, motility, and secretion of extracellular proteins required to pave the way from stroma into the lumen of sinuses (Lapidot and Petit, 2002; Velders and Fibbe, 2005; Christopher and Link, 2007). A well known signal that inhibits blood cell egress from the marrow is stromal derived factor 1 (SDF1); opposing signals, such as granulocyte colony-stimulating factor (G-CSF) act by degrading SDF1 and thereby allowing blood cells to pass into circulation. G-CSF and other signals also increase the release of metalloproteases from egressing blood cells, which may be important to locally remove the basement membranes that these blood cells have to traverse.
Localized digestion of basement membranes, possibly combined with an increased motility appear to be the main processes that play a role in release of blood cells from the lymph gland of Drosophila. The underlying molecular mechanisms and its control by signaling pathways is currently not known. Extracellular matrix proteins assembled as basement membranes play a fundamental role in morphogenesis and organogenesis (Ekblom et al., 1985; Doihara et al., 2009; Mitsunaga-Nagatsubo et al., 2009; Tsang et al., 2010). Hemocytes are known to produce and secrete proteins used for stabilizing basement membranes around other tissues. For example, the highly conserved ECM protein SPARC is secreted by hemocytes and acts to stabilize collagen IV in the basement membranes surrounding muscles and other tissues (Martinek et al., 2008; Koehler et al., 2009). However, no hemocyte derived protein has been described yet that would degrade extracellular matrix, including the basement membranes that captivate hemocytes within the lymph gland. It is possible that an increase in motility could be sufficient for hemocytes to tear and break through the encapsulating basement membranes. Thus, during normal development, a signal triggered by the ecdyson peak that promotes metamorphosis could induce both the proliferative/differentiative events that happen rapidly in the few hours between pupation and lymph gland dissociation, as well as an increased motility of hemocytes. A similar signal could act as part of the immune response that is able to trigger premature lymph gland dissociation in larvae infected with various parasites (Lanot et al., 2001; Sorrentino et al., 2002; Chiu and Govind, 2002).
A signaling pathway known to be involved in hemocyte motility in the embryo is the PVR (PDGF-VEGF-Related) pathway. Hemocytes derived from the head mesoderm spread out along stereotyped pathways (Tepass et al., 1994a) throughout the embryo. One should note that this phase of hemocyte dispersal, which is formally comparable to the dissociation of the lymph gland considered here, takes place in the absence of basement membranes around hemocytes. Thus, the population of head mesoderm cells that adopts the fate of prohemocytes in the stage 11 embryo does so before basement membranes have formed (Tepass and Hartenstein, 1994b). Prohemocytes initially form a loose mass of cells in the head of the embryo, lacking a capsule similar to the one that surrounds the prohemocytes of the larval lymph gland. Subsequently, in response to PVR signals presented by various sources, prohemocytes migrate away from the head mesoderm and, at the same time, differentiate into plasmatocytes with phagocytotic activity (Cho et al., 2002; Bruckner et al., 2004). Lack of PVR, aside from causing an increase of cell death, also has an effect on hemocyte spreading. PVR signaling is also required for directed migration of larval hemocytes towards wounded tissue (Wood et al., 2006). A role of this pathway, or any other mechanism controlling motility, in hemocyte dispersal during early metamorphosis remains to be seen.
Acknowledgments
This research was supported by the Ruth L. Kirschstein National Research Service Award GM007185 and the HFSP Grant RGP0015/2008-C to VH. We would also like to thank I. Ando, U. Banerjee, L. Cooley, J. Fessler, L. Fessler, F. Kafatos, the Bloomington Stock Center, and the Developmental Studies Hybridoma Bank for fly strains and antibodies. We would also like to thank Marianne Cilluffo and the Brain Research Institute Electron Microscopy Core at UCLA for help in processing our TEM samples.
References
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell. 2003;5:441–50. doi: 10.1016/s1534-5807(03)00244-2. [DOI] [PubMed] [Google Scholar]
- Akai H, Sato S. An ultrastructural study of the haemopoietic organs of the silkworm, Bombyx mori. Journal of Insect Physiology. 1971;17:1665–1676. [Google Scholar]
- Alfonso TB, Jones BW. gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev Biol. 2002;248:369–83. doi: 10.1006/dbio.2002.0740. [DOI] [PubMed] [Google Scholar]
- Bruckner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Chiu H, Govind S. Natural infection of D. melanogaster by virulent parasitic wasps induces apoptotic depletion of hematopoietic precursors. Cell Death Differ. 2002;9:1379–81. doi: 10.1038/sj.cdd.4401134. [DOI] [PubMed] [Google Scholar]
- Cho NK, Keyes L, Johnson E, Heller J, Ryner L, Karim F, Krasnow MA. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell. 2002;108:865–76. doi: 10.1016/s0092-8674(02)00676-1. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Crossley AC. Experimental analysis of the origins and physiology of haemocytes in the blue blow fly Calliphora erythrocephala (Meig) J Exp Zool. 1964;157:375–97. doi: 10.1002/jez.1401570309. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–26. doi: 10.1111/j.1462-5822.2007.00930.x. [DOI] [PubMed] [Google Scholar]
- Doihara T, Miguchi Y, Miyawaki K, Shimokawa T, Hamada F, Kobayashi N, Matsuda S. Spatiotemporal distribution patterns of oligosaccharides during early embryogenesis in the starfish Patiria pectinifera. Dev Genes Evol. 2009;219:199–206. doi: 10.1007/s00427-009-0280-1. [DOI] [PubMed] [Google Scholar]
- Ekblom P, Sariola H, Thesleff I. Basement membrane and organogenesis. Prog Clin Biol Res. 1985;171:109–21. [PubMed] [Google Scholar]
- Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–90. doi: 10.1016/s1534-5807(03)00335-6. [DOI] [PubMed] [Google Scholar]
- Han SS, Lee MH, Kim WK, Wago H, Yoe SM. Hemocytic Differentiation in Hemopoietic Organ of Bombyx mori Larvae. Zoolog Sci. 1998;15:371–9. doi: 10.2108/zsj.15.371. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14:2857–65. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks CF, Arnold JW. Haemopoiesis in Lepidoptera. II. The role of the haemopoietic organs. Can J Zool. 1977;55:1740–1755. [Google Scholar]
- Hoffmann JA, Zachary D, Hoffmann D, Brehelin M. Postembryonic development and differentiation: hemopoietic tissues and their functions in some insects. In: Gupta AP, editor. Insect Hemocytes. Cambridge University Press; London: 1979. [Google Scholar]
- Holz A, Bossinger B, Strasser T, Janning W, Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–62. doi: 10.1242/dev.00702. [DOI] [PubMed] [Google Scholar]
- Inoue S, Osmond DG. Basement membrane of mouse bone marrow sinusoids shows distinctive structure and proteoglycan composition: a high resolution ultrastructural study. Anat Rec. 2001;264:294–304. doi: 10.1002/ar.1166. [DOI] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–33. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kelso RJ, Buszczak M, Quinones AT, Castiblanco C, Mazzalupo S, Cooley L. Flytrap, a database documenting a GFP protein-trap insertion screen in Drosophila melanogaster. Nucleic Acids Res. 2004;32:D418–20. doi: 10.1093/nar/gkh014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler A, Desser S, Chang B, MacDonald J, Tepass U, Ringuette M. Molecular evolution of SPARC: absence of the acidic module and expression in the endoderm of the starlet sea anemone, Nematostella vectensis. Dev Genes Evol. 2009;219:509–21. doi: 10.1007/s00427-009-0313-9. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Crozatier M, Vincent A. Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int J Dev Biol. 2010;54:1117–25. doi: 10.1387/ijdb.093053jk. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–8. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Markus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, Zettervall CJ, Hultmark D, Ando I. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007;17:649–54. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Zettervall CJ, Sinka R, Vilmos P, Pivarcsi A, Ekengren S, Hegedus Z, Ando I, Hultmark D. Hemese, a hemocyte-specific transmembrane protein, affects the cellular immune response in Drosophila. Proc Natl Acad Sci U S A. 2003;100:2622–7. doi: 10.1073/pnas.0436940100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol. 2001;230:243–57. doi: 10.1006/dbio.2000.0123. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–81. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–9. doi: 10.1126/science.288.5463.146. [DOI] [PubMed] [Google Scholar]
- Lichtman MA. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp Hematol. 1981;9:391–410. [PubMed] [Google Scholar]
- Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–23. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–4. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus R, Kurucz E, Rus F, Ando I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett. 2005;101:108–11. doi: 10.1016/j.imlet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Martinek N, Shahab J, Saathoff M, Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci. 2008;121:1671–80. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- Minakhina S, Steward R. Melanotic mutants in Drosophila: pathways and phenotypes. Genetics. 2006;174:253–63. doi: 10.1534/genetics.106.061978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsunaga-Nakatsubo K, Akimoto Y, Kawakami H, Akasaka K. Sea urchin arylsulfatase, an extracellular matrix component, is involved in gastrulation during embryogenesis. Dev Genes Evol. 2009;219:281–8. doi: 10.1007/s00427-009-0289-5. [DOI] [PubMed] [Google Scholar]
- Monpeyssin M, Beaulaton J. Hemocytopoiesis in the oak silkworm Antheraea pernyi and some other lepidoptera. I. Ultrastructural study of normal processes. J Ultrastruct Res. 1978;64:35–45. doi: 10.1016/s0022-5320(78)90005-9. [DOI] [PubMed] [Google Scholar]
- Morin X, Daneman R, Zavortink M, Chia W. A protein trap strategy to detect GFP-tagged proteins expressed from their endogenous loci in Drosophila. Proc Natl Acad Sci U S A. 2001;98:15050–5. doi: 10.1073/pnas.261408198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HM, Dimopoulos G, Blass C, Kafatos FC. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem. 1999;274:11727–35. doi: 10.1074/jbc.274.17.11727. [DOI] [PubMed] [Google Scholar]
- Nakahara Y, Kanamori Y, Kiuchi M, Kamimura M. In vitro studies of hematopoiesis in the silkworm: cell proliferation in and hemocyte discharge from the hematopoietic organ. J Insect Physiol. 2003;49:907–16. doi: 10.1016/s0022-1910(03)00149-5. [DOI] [PubMed] [Google Scholar]
- Nelson RE, Fessler LI, Takagi Y, Blumberg B, Keene DR, Olson PF, Parker CG, Fessler JH. Peroxidasin: a novel enzyme-matrix protein of Drosophila development. EMBO J. 1994;13:3438–47. doi: 10.1002/j.1460-2075.1994.tb06649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides PE, Dittmann KH. How do normal and leukemic white blood cells egress from the bone marrow? Morphological facts and biochemical riddles. Blut. 1990;61:3–13. doi: 10.1007/BF01739426. [DOI] [PubMed] [Google Scholar]
- Poulson D. Histogenesis, organogenesis, and differentiation in the embryo of Drosophila melanogaster. In: Demerec M, editor. Biology of Drosophila. Wiley; New York: 1950. pp. 168–274. [Google Scholar]
- Rizki TM, Rizki RM. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol. 1992;16(2–3):103–10. doi: 10.1016/0145-305x(92)90011-z. [DOI] [PubMed] [Google Scholar]
- Rugendorff A, Younossi-Hartenstein A, Hartenstein V. Embryonic origin and differentiation of the Drosophila heart. Roux’s Arch Dev Biol. 1994;203:266–280. doi: 10.1007/BF00360522. [DOI] [PubMed] [Google Scholar]
- Sorrentino RP, Carton Y, Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol. 2002;243:65–80. doi: 10.1006/dbio.2001.0542. [DOI] [PubMed] [Google Scholar]
- Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc Natl Acad Sci U S A. 2007;104:2721–6. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U, Fessler LI, Aziz A, Hartenstein V. Embryonic origin of hemocytes and their relationship to cell death in Drosophila. Development. 1994a;120:1829–37. doi: 10.1242/dev.120.7.1829. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994b;161:563–96. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Tsang KY, Cheung MC, Chan D, Cheah KS. The developmental roles of the extracellular matrix: beyond structure to regulation. Cell Tissue Res. 2010;339:93–110. doi: 10.1007/s00441-009-0893-8. [DOI] [PubMed] [Google Scholar]
- Velders GA, Fibbe WE. Involvement of proteases in cytokine-induced hematopoietic stem cell mobilization. Ann N Y Acad Sci. 2005;1044:60–9. doi: 10.1196/annals.1349.008. [DOI] [PubMed] [Google Scholar]
- Weiss L. The haemopoietic microenvironment of bone marrow: an ultrastructural study of the interactions of blood cells, stroma and blood vessels. Ciba Found Symp. 1980;71:3–19. doi: 10.1002/9780470720547.ch2. [DOI] [PubMed] [Google Scholar]
- Wood W, Faria C, Jacinto A. Distinct mechanisms regulate hemocyte chemotaxis during development and wound healing in Drosophila melanogaster. J Cell Biol. 2006;173:405–16. doi: 10.1083/jcb.200508161. [DOI] [PMC free article] [PubMed] [Google Scholar]