Abstract
BACKGROUND
Idiopathic membranous nephropathy is an autoimmune disease. In approximately 70% of patients, it is associated with autoantibodies against the phospholipase A2 receptor 1 (PLA2R1). Antigenic targets in the remaining patients are unknown.
METHODS
Using Western blotting, we screened serum samples from patients with idiopathic membranous nephropathy, patients with other glomerular diseases, and healthy controls for antibodies against human native glomerular proteins. We partially purified a putative new antigen, identified this protein by means of mass spectrometry of digested peptides, and validated the results by analysis of recombinant protein expression, immunoprecipitation, and immunohistochemical analysis.
RESULTS
Serum samples from 6 of 44 patients in a European cohort and 9 of 110 patients in a Boston cohort with anti-PLA2R1–negative idiopathic membranous nephropathy recognized a glomerular protein that was 250 kD in size. None of the serum samples from the 74 patients with idiopathic membranous nephropathy who were sero-positive for anti-PLA2R1 antibodies, from the 76 patients with other glomerular diseases, and from the 44 healthy controls reacted against this antigen. Although this newly identified antigen is clearly different from PLA2R1, it shares some biochemical features, such as N-glycosylation, membranous location, and reactivity with serum only under nonreducing conditions. Mass spectrometry identified this antigen as thrombospondin type-1 domain-containing 7A (THSD7A). All reactive serum samples recognized recombinant THSD7A and immunoprecipitated THSD7A from glomerular lysates. Moreover, immunohistochemical analyses of biopsy samples from patients revealed localization of THSD7A to podocytes, and IgG eluted from one of these samples was specific for THSD7A.
CONCLUSIONS
In our cohort, 15 of 154 patients with idiopathic membranous nephropathy had circulating autoantibodies to THSD7A but not to PLA2R1, a finding that suggests a distinct subgroup of patients with this condition. (Funded by the French National Center for Scientific Research and others.)
Idiopathic membranous nephropathy is an autoimmune disease and a common cause of the nephrotic syndrome in adults.1 In 2009, the phospholipase A2 receptor 1 (PLA2R1), a protein that is expressed in glomerular podocytes, was discovered as the major antigen involved in the pathogenesis of adult idiopathic membranous nephropathy.2 As confirmed by a number of subsequent studies, about 70% of patients with idiopathic membranous nephropathy have circulating autoantibodies against PLA2R1.2-6 The remaining patients, approximately 30% of those with idiopathic membranous nephropathy, have no obvious secondary cause of the disease, and it is thought that other endogenous glomerular antigens may be involved in these cases.
In the current study, we analyzed serum samples from patients with idiopathic membranous nephropathy as well as samples from patients with other glomerular diseases and healthy controls, to identify circulating antibodies against glomerular antigens other than PLA2R1.
METHODS
PATIENTS
A diagnosis of membranous nephropathy was made by means of a renal biopsy. Serum samples were obtained from patients with membranous nephropathy and from patients with other glomerular diseases and healthy controls and were examined for anti-PLA2R1 antibodies with the use of an enzyme-linked immunosorbent assay (ELISA)7 and Western blot analysis.2 All study participants gave written informed consent, and the study was conducted in accordance with the provisions of the Declaration of Helsinki. A detailed description of the cohorts and the patient characteristics is provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
HUMAN RENAL TISSUE
Healthy portions of nephrectomized kidneys and kidney cortex from human donor kidneys that had been deemed unsuitable for transplantation were used to prepare a protein extract of glomeruli. Additional details can be found in the Methods section in the Supplementary Appendix.
WESTERN BLOT ANALYSIS
Gel electrophoresis and protein transfer to polyvinylidene difluoride membranes were performed according to standard protocols. For use as the primary antibody, the serum was diluted at a 1:100 ratio. For specific detection of THSD7A, we used a commercially available rabbit polyclonal antibody at a 1:1000 dilution (Atlas Antibodies).
IMMUNOPRECIPITATION
Human glomerular extracts were incubated overnight with serum samples from patients with membranous nephropathy, from patients with other glomerular diseases, and from healthy controls, and IgG4 affinity matrix (Life Technologies) was added. Immunoprecipitates were collected, electrophoresed, blotted, and examined for the presence of THSD7A protein with the use of anti-THSD7A antibodies (Atlas Antibodies) as described above.
MASS SPECTROMETRY
Gel regions corresponding to visible bands on Western blots were excised and subjected to in-gel tryptic digestion. Digested peptides were identified by mass spectrometry as described in the Supplementary Appendix.
HISTOLOGIC ANALYSIS AND AUTOANTIBODY ELUTION
Immunofluorescence and immunohistochemical analyses of THSD7A expression in healthy and diseased kidneys and autoantibody elution experiments were performed as described in the Supplementary Appendix.
RESULTS
SCREENING OF SERUM SAMPLES WITH A HUMAN GLOMERULAR PROTEIN EXTRACT
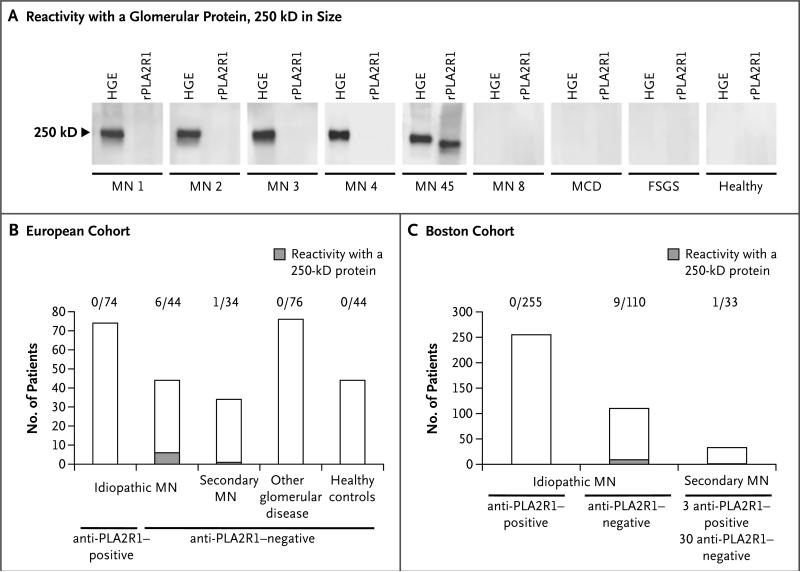
We screened serum samples from patients with membranous nephropathy and relevant controls against total proteins in human glomerular extracts. Serum reactivity was initially investigated by Western blotting under nonreducing conditions in serum from 65 patients with membranous nephropathy (of whom 30 were negative for anti-PLA2R1 antibodies), 76 patients with other proteinuric or autoimmune kidney diseases, and 44 healthy controls. All samples that were known to be positive for anti-PLA2R1 antibodies, as tested previously by ELISA, reacted with native and recombinant PLA2R1 by means of Western blotting (MN 45 in Fig. 1A). Samples from 4 patients with idiopathic membranous nephropathy, all of whom were seronegative for anti-PLA2R1 antibodies, recognized a glomerular protein approximately 250 kD in size (MN 1 to MN 4 in Fig. 1A). Samples from patients with other glomerular diseases or from healthy controls did not show similar reactivity (Fig. 1A, and Fig. S1 in the Supplementary Appendix). Screening was extended to 152 patients with membranous nephropathy (118 with idiopathic and 34 with secondary membranous nephropathy) (Table S1 in the Supplementary Appendix). A total of 7 serum samples, all of which were negative for anti-PLA2R1 antibodies, showed reactivity in the same 250-kD region (Fig. 1B).
Figure 1. Detection of Autoantibodies against an Unknown Antigen in Patients with Membranous Nephropathy.
Panel A shows reactivity of serum samples with human glomerular extracts (HGE). Four of the initially tested samples from patients with membranous nephropathy (MN) reacted with a glomerular protein approximately 250 kD in size (MN 1 through MN 4) but not with the recombinant phospholipase A2 receptor (rPLA2R1). Panels B and C show overall reactivity in the European and Boston cohorts, respectively. FSGS denotes focal segmental glomerulo-sclerosis, and MCD minimal-change disease. The number above each column indicates the fraction of samples positive for a 250-kD protein.
In an independent cohort at Boston University comprising 365 patients with idiopathic membranous nephropathy and 33 with secondary membranous nephropathy (Table S2 in the Supplementary Appendix), 10 additional patients who were seronegative for anti-PLA2R1 antibodies, as determined by Western blot analysis and ELISA, were identified; serum samples from these 10 patients showed similar reactivity to a 250-kD protein present in human glomeruli (Fig. 1C, and Table S3 in the Supplementary Appendix).
The screening thus identified a putative auto-antigen of 250 kD that was present in normal human glomeruli. This protein was found to be N-glycosylated to a lesser extent than PLA2R1 and was expressed almost exclusively in cell membranes, suggesting that it was an as-yet unknown antigen that was different from PLA2R1 (Fig. S2 in the Supplementary Appendix). We also screened the serum from patients with idiopathic membranous nephropathy against the three paralogs of PLA2R1 (MRC1, MRC2, and LY75),8 but we did not observe any reactivity (Fig. S3 and S4 in the Supplementary Appendix).
IDENTIFICATION OF THE ANTIGEN AS THROMBOSPONDIN TYPE-1 DOMAIN-CONTAINING 7A
We used two independent strategies to identify the antigen. In the first, we performed gel electrophoresis and Western blot analysis of glomerular proteins, either untreated or treated with N-glycopeptidase F alone or with both N-glycopeptidase F and neuraminidase; these analyses revealed three different bands — at 250 kD, at 225 kD, and at 200 kD (Fig. S2B in the Supplementary Appendix). In the second strategy, glomerular proteins were partially proteolyzed with trypsin, with and without prior treatment with N-glycopeptidase F. All gel regions corresponding to the Western blot signals were excised and analyzed by mass spectrometry, and a list of candidate protein antigens was generated (Table S4 in the Supplementary Appendix). Candidate proteins were ranked according to the expected characteristics of the newly identified antigen with respect to molecular mass, N-glycosylation, membrane location, and expression in human kidney or glomeruli. We used commercially available antibodies and transiently transfected human embryonic kidney (HEK) 293 cells to specifically test for candidate antigens (data not shown) and finally identified thrombospondin type-1 domain-containing 7A (THSD7A) as the protein of interest.
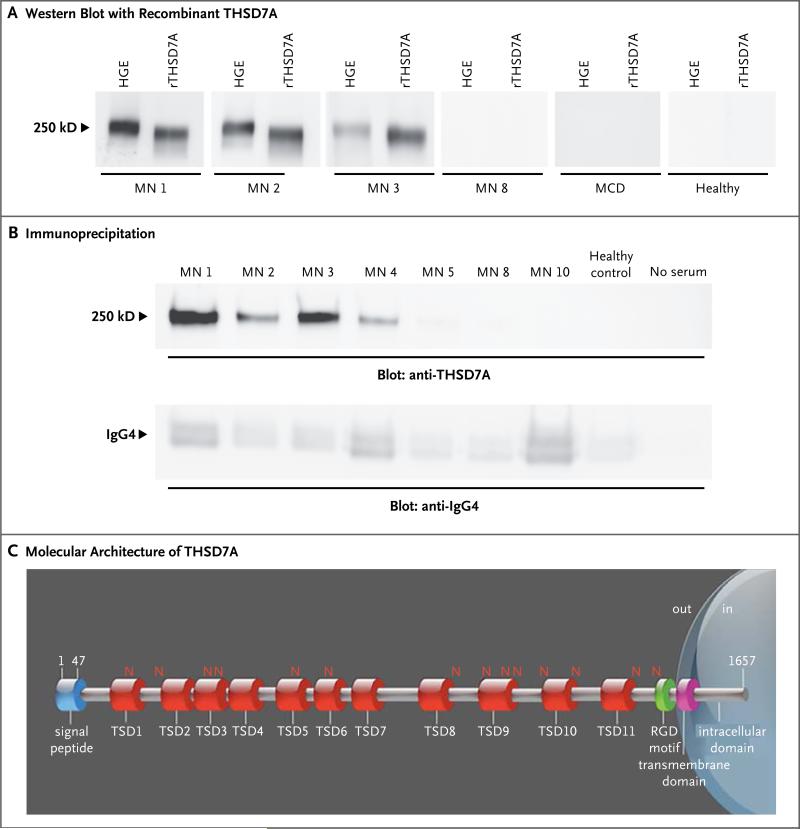
All 17 serum samples previously shown to be reactive with the 250-kD protein (7 samples from the European cohort and 10 from the Boston cohort) — but none of the samples that were positive for anti-PLA2R1 antibodies or the control samples — also recognized recombinant THSD7A (Fig. 2A). Native and recombinant THSD7A proteins showed the same mass shift after deglycosylation, yet the recombinant protein migrated to a slightly lower position, suggesting a minor difference in post-translational modification (Fig. 2A, and Fig. S5 in the Supplementary Appendix). All reactive serum samples, but not control samples, were able to immunoprecipitate THSD7A from glomerular lysates (Fig. 2B). THSD7A is a 250-kD type I transmembrane protein with a large extracellular region, a single transmembrane domain, and a short intracellular tail. The extracellular part contains 11 thrombospondin type-1 repeats and 1 arginine–glycine–aspartic acid (RGD) motif (Fig. 2C).9
Figure 2. Identification of the Target Antigen.
Panel A shows reactivity of serum samples with human glomerular extracts (HGE) and recombinant thrombospondin type-1 domain-containing 7A (rTHSD7A). Only samples from patients with membranous nephropathy (MN) that previously recognized the 250-kD protein present in HGE also recognized rTHSD7A (MN 1, MN 2, and MN 3). Panel B shows results of immunoprecipitation experiments. All serum samples from patients with membranous nephropathy that previously recognized rTHSD7A precipitated the antigen from HGE. No immunoprecipitation occurred with serum from healthy controls or with no serum (i.e., with water substituted for serum in the experiment). IgG4 was efficiently immunoprecipitated in all conditions (lower panel). Panel C shows the molecular architecture of THSD7A, with a large extracellular region comprising 11 thrombospondin type-1 domains (TSDs), 14 glycosylation sites (N), and 1 predicted arginine–glycine–aspartic acid (RGD) motif. The scheme was built according to the UniProt accession number Q9UPZ6 for THSD7A and ScanProsite tool from ExPASy.
CHARACTERIZATION OF ANTI-THSD7A AUTOANTIBODIES
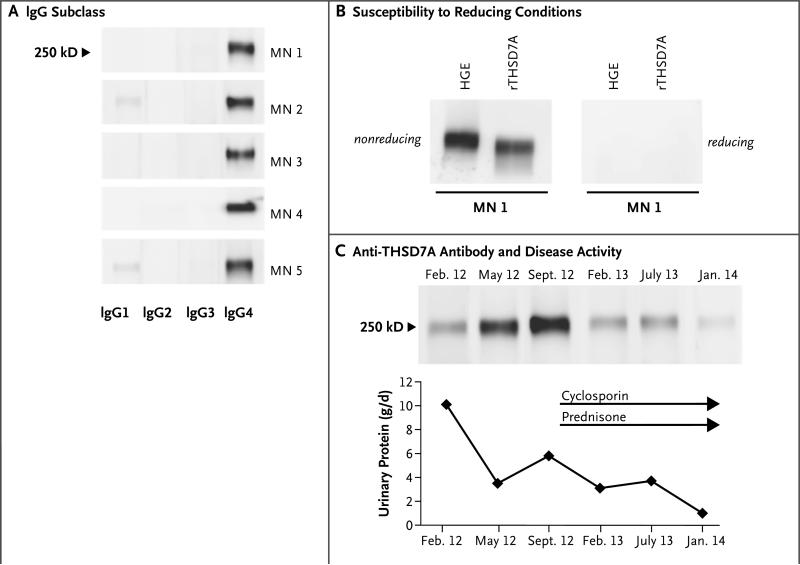
To determine the IgG subtypes of anti-THSD7A autoantibodies, we used secondary antibodies against IgG1 to IgG4. IgG4 was the predominant IgG subclass of anti-THSD7A, although other IgG subtypes were weakly present in most serum samples (Fig. 3A). This finding was in accordance with enhanced staining for IgG4 in all available biopsy samples (data not shown). Moreover, reactivity of all the positive serum samples to both the 250-kD glomerular protein and recombinant THSD7A was lost when gels were run under reducing conditions (Fig. 3B). Thus, the subtype of most anti-THSD7A autoantibodies, like that of most anti-PLA2R1 autoantibodies, is IgG4, and the anti-THSD7A autoantibodies recognize one or more conformation-dependent epitopes in both native and recombinant THSD7A.
Figure 3. Characterization of Anti-THSD7A Autoantibodies.
Panel A shows that anti-THSD7A autoantibodies are predominantly of the IgG4 subclass. Other IgG subtypes were also present in most patients. Panel B shows that serum reactivity against THSD7A is lost in the presence of a reducing agent, a finding that suggests that the autoantibody recognizes a conformation-dependent epitope. Panel C shows results of Western blotting of equal volumes of glomerular proteins with serial serum samples from one patient with idiopathic membranous nephropathy. After an initial lessening of proteinuria, severe edema and severe proteinuria developed in the patient in September 2012. Immunosuppressive treatment with cyclosporine and prednisone induced a partial remission in January 2014. This resolution of disease activity is reflected by a decrease of the Western blot signal after initiation of immunosuppressive treatment.
ANTI-THSD7A AUTOANTIBODIES AND DISEASE ACTIVITY
Serial serum samples from three patients who were positive for anti-THSD7A antibodies were available for analysis of antibody levels by semi-quantitative Western blot analysis. In one patient, protein levels in the urine were very high at the time of diagnosis; they were decreased with pharmacologic inhibition of the renin–angiotensin system 3 months later but were still in the nephrotic range (Fig. 3C). The protein levels increased 4 months later, and the proteinuria was accompanied by a marked increase in antibody signal. Owing to the development of severe edema, immunosuppressive therapy was started with cyclosporine and low-dose prednisone, which resulted in a decrease in the anti-THSD7A antibody level and in the level of protein in the urine to the subnephrotic range. A second patient had severe proteinuria and was treated with corticotropin analogues, resulting in a decline in the anti-THSD7A antibody level and in the level of protein in the urine (data not shown). In contrast, in a third patient who did not receive immunosuppressive therapy owing to minor clinical problems, the antibody level remained consistently high with sustained proteinuria in the nephrotic range (data not shown). Taken together, these results suggest an association between the antibody level and clinical disease activity.
GLOMERULAR THSD7A EXPRESSION IN PATIENTS WITH MEMBRANOUS NEPHROPATHY AND CONTROLS
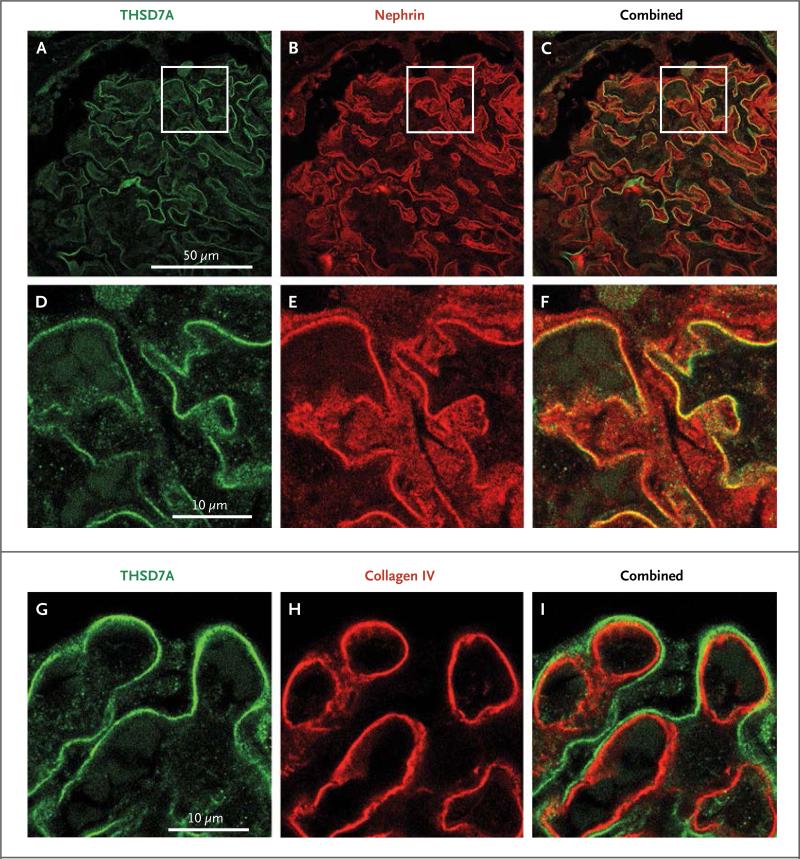
Immunofluorescence staining of renal-biopsy samples from healthy controls with anti–THSD7A-specific antibodies showed prominent linear glomerular expression of THSD7A (Fig. 4A and 4D). There was no staining when the primary antibody was omitted (data not shown). The staining pattern for nephrin, a transmembrane protein located at the intercellular slit diaphragm of podocyte foot processes, was very similar to that of THSD7A (Fig. 4B and 4E). Moreover, both molecules showed strong colocalization, suggesting that THSD7A is located on or within close proximity of podocyte foot processes (Fig. 4C and 4F, and Fig. S6 in the Supplementary Appendix). On the other hand, THSD7A did not colocalize with markers of the glomerular basement membrane, such as collagen type IV (Fig. 4I, and Fig. S6 in the Supplementary Appendix) and fibronectin (Fig. S7 in the Supplementary Appendix), or with CD34, a marker of endothelial cells (Fig. S8 in the Supplementary Appendix). The abluminal position of THSD7A relative to collagen type IV and fibronectin suggests that THSD7A is expressed on podocyte foot processes rather than on the glomerular basement membrane.
Figure 4. Expression of THSD7A in Normal Human Glomeruli.
Immunofluorescence experiments were performed on renal-biopsy samples from healthy controls who underwent nephrectomy. Panel A shows staining for THSD7A (green) which is found along the glomerular capillary wall in a linear aspect. Panel B shows staining for nephrin (red), a podocyte membrane protein. Panel C shows strong colocalization of THSD7A and nephrin, suggesting that THSD7A is expressed on glomerular podocytes. Panels D, E, and F are enlarged images of the boxed areas in Panels A, B, and C, respectively. Panels G, H, and I show staining for THSD7A, collagen type IV, and both, respectively. THSD7A is localized in an abluminal position relative to collagen type IV, which suggests that it is expressed on glomerular podocytes rather than on the glomerular basement membrane itself.
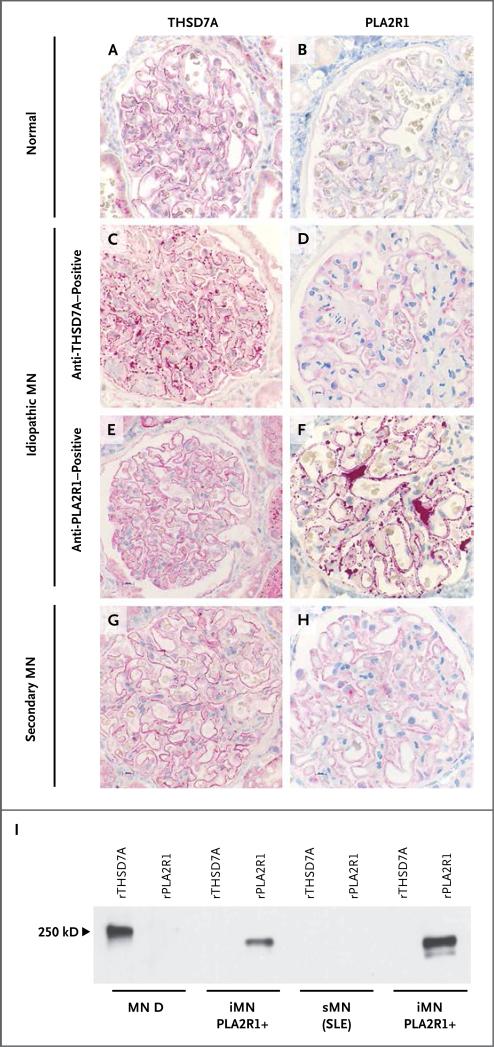
Immunohistochemical staining of THSD7A further revealed a linear positivity in the biopsy samples from healthy controls (Fig. 5A); PLA2R1 is less well expressed than is THSD7A (Fig. 5B). In all 5 available biopsy samples from patients with membranous nephropathy who were positive for anti-THSD7A antibodies, immunohisto-chemical analysis revealed markedly enhanced granular staining for THSD7A (Fig. 5C); PLA2R1 staining was normal in these patients (Fig. 5D). In contrast, all patients with detectable anti-PLA2R1 serum antibodies had normal staining for THSD7A along the capillary walls (Fig. 5E) but enhanced granular staining for PLA2R1 (Fig. 5F), as described previously.10-12 Three of these patients had scant and unspecific positivity for THSD7A within proteinaceous material in the capsular space of a few glomeruli (data not shown). All 50 biopsy samples from patients with membranous nephropathy and no detectable autoantibodies had normal staining for both THSD7A and PLA2R1 (Fig. 5G and 5H).
Figure 5. Expression Level of THSD7A and PLA2R1 in Patients with Idiopathic and Secondary Membranous Nephropathy and in a Healthy Control.
Immunohistochemical staining for THSD7A and PLA2R1 was performed in renal-biopsy specimens from healthy controls and from patients with membranous nephropathy. Panels A and B show normal staining for THSD7A and PLA2R1, respectively, in a control biopsy specimen. Panel C shows markedly enhanced staining for THSD7A in a patient with serum anti-THSD7A antibodies, whereas PLA2R1 staining, as shown in Panel D, is normal. Conversely, the biopsy specimen from a patient with serum anti-PLA2R1 antibodies shows that staining for THSD7A was not increased, as shown in Panel E, but staining for PLA2R1 was increased, as shown in Panel F. The biopsy specimen from a patient with secondary membranous nephropathy (sMN) due to systemic lupus erythematosus (SLE) and no detectable serum antibodies against THSD7A or PLA2R1 has normal staining for both antigens, as seen in Panels G and H. Panel I shows the reactivity of IgG eluted from patient biopsy samples with recombinant THSD7A and PLA2R1. Only the IgG eluted from the biopsy sample from a patient with idiopathic membranous nephropathy (iMN) and detectable serum anti-THSD7A antibodies recognized THSD7A (MN D; see also Table S3 in the Supplementary Appendix). The eluates from two biopsy samples from patients with idiopathic membranous nephropathy and serum anti-PLA2R1 autoantibodies recognized PLA2R1 but not THSD7A. IgG eluted from one patient with lupus membranous nephropathy and no detectable serum antibodies against THSD7A or PLA2R1 recognized neither antigen.
The PLA2R1 antigen is known to colocalize with IgG4 in subepithelial deposits in patients with PLA2R1-associated idopathic membranous nephropathy.2 Similarly, we found colocalization of IgG4 and THSD7A in biopsy samples from THSD7A-seropositive patients (Fig. S9 in the Supplementary Appendix). Moreover, we found local activation of complement, as shown by strong granular staining for C5b-9 (Fig. S10 in the Supplementary Appendix).
Despite the apparent expression of the target antigen on the basal surface of the podocyte, we also looked for a circulating soluble form of the antigen, as well as THSD7A-containing circulating immune complexes that might be deposited in a similar subepithelial position. We were not able to identify either a circulating soluble form of THSD7A or THSD7A–anti-THSD7A immune complexes (Fig. S11 in the Supplementary Appendix).
IgG eluted from the frozen remnant biopsy core from one patient with anti-THSD7A antibodies specifically recognized recombinant THSD7A on a Western blot, whereas IgG eluted from two PLA2R1-associated cases did not recognize THSD7A but specifically recognized PLA2R1 (Fig. 5I). IgG eluted from a biopsy sample from a patient with class V lupus nephritis recognized neither antigen.
CHARACTERISTICS OF PATIENTS POSITIVE FOR ANTI-THSD7A OR ANTI-PLA2R1 AUTOANTIBODIES
Given the apparent mutual exclusivity of anti-THSD7A and anti-PLA2R1 autoantibodies, we compared the clinical characteristics of the patients with each type of autoantibody. We could not find any significant difference between the two groups with respect to age; levels of urinary protein, serum albumin, and serum creatinine; or status with respect to the receipt of immunosuppressive therapy in the first 6 months after diagnosis (Table S5 and Fig. S12 in the Supplementary Appendix). However, there were significantly more women in the group that was positive for anti-THSD7A autoantibodies (65% vs. 27%), a finding that might also represent a sampling error owing to the small number of patients.
DISCUSSION
In the current study, we identified THSD7A as a second autoantigen involved in adult idiopathic membranous nephropathy. Approximately 2.5 to 5% of the patients with idiopathic membranous nephropathy whom we evaluated had autoantibodies against THSD7A, which corresponds to 8 to 14% of the patients who are seronegative for anti-PLA2R1 antibodies. The percentage of patients with anti-THSD7A autoantibodies was higher in the European cohort, which comprised patients who had received no treatment for membranous nephropathy and from whom serum samples were obtained close to the time of the biopsy, than in the Boston cohort, which was more cross-sectional in nature and may have included patients who were already in immunologic remission.
We also found anti-THSD7A antibodies in one woman with membranous nephropathy and systemic lupus erythematosus and in one older man with membranous nephropathy and prostate cancer.13 However, the renal-biopsy samples from these patients showed no features typical of lupus nephritis or secondary membranous nephropathy; in addition, their anti-THSD7A serum antibodies were mainly of the IgG4 subclass, and the renal-biopsy samples showed enhanced staining for IgG4, a finding that is similar to that in all other anti-THSD7A–positive patients and that is a feature of idiopathic rather than secondary membranous nephropathy.10,11 It is thus possible that these patients have two independent diseases, idiopathic membranous nephropathy and lupus or cancer. Furthermore, we could not find any reactivity against THSD7A among healthy controls and among patients with other protein-uric or renal autoimmune diseases. However, a number of patients were seronegative for both antigens. Anti-PLA2R1 autoantibodies are known to disappear before the full clinical resolution of disease activity,6,14 and our finding that anti-THSD7A antibody levels also fell with a decrease in disease activity suggests that the same may be true for THSD7A-associated membranous nephropathy. Staining of the biopsy specimen for the presence of the antigen (THSD7A or PLA2R1) may identify a small additional percentage of patients whose serum was sampled after immunologic remission had occurred.12,15 Taken together, THSD7A- and PLA2R1-associated membranous nephropathy may now account for approximately 75 to 85% of cases, which further helps to distinguish idiopathic from secondary membranous nephropathy. The remaining 15 to 25% of cases may involve as-yet unidentified autoantigens that are distinct from THSD7A and PLA2R1 or may have been wrongly classified as idiopathic owing to an undetected secondary cause of the disease.
THSD7A was initially characterized as an endothelial protein that is expressed in the placental vasculature.9 We have shown by immunohistologic analysis that THSD7A is concentrated at the basal aspect of the podocyte, and we did not find any expression in glomerular endothelial cells. This finding is consistent with recent data showing that THSD7A is more highly expressed in podocytes than in glomerular endothelial and mesangial cells.16 On the basis of these experimental data, our current immunohistologic findings, and previous data from patients with other forms of human membranous nephropathy,2,17 we believe that the likely mechanism of THSD7A-associated membranous nephropathy involves in situ immune complex formation with podocyte-associated THSD7A. Our inability to detect circulating soluble THSD7A or THSD7A– anti-THSD7A immune complexes further supports this view. However, we cannot completely rule out the presence of circulating soluble THSD7A or THSD7A–anti-THSD7A immune complexes because of a possible lack of sensitivity of our assays.
THSD7A and PLA2R1 have quite similar structural and biochemical properties. Both proteins are expressed on podocyte membranes and have high molecular masses and a large extra-cellular region consisting of multiple and repeated disulfide-bonded and N-glycosylated domains. In addition, autoantibodies to both THSD7A and PLA2R1 are predominantly of the IgG4 subclass and recognize their target antigen exclusively under nonreducing conditions, suggesting the presence of one or more conformational epitopes in each protein. Furthermore, renal-biopsy samples from patients who were positive for anti-THSD7A antibodies had increased staining for the antigen within immune deposits, a phenomenon also seen in patients who are positive for anti-PLA2R1 antibodies,10-12 and IgG eluted from a biopsy sample from a patient with anti-THSD7A serum autoantibodies recognized recombinant THSD7A but not PLA2R1.
The fact that patients seem to mount an autoimmune response against either THSD7A or PLA2R1, but not both, is of particular interest. A priori, the probability that all patients who were positive for anti-THSD7A autoantibodies would be negative for anti-PLA2R1 autoantibodies owing to chance alone was less than 0.5% among the patients in our European cohort, for whom all samples were assayed against both recombinant molecules. The reasons for this mutual exclusivity of the two antigens remain only speculative at this point. It is known from other autoimmune diseases that a similar histologic and clinical phenotype can arise from disparate antigen–antibody interactions. For example, most patients with myasthenia gravis have auto-antibodies to the acetylcholine receptor, whereas some have IgG4 antibodies to muscle-specific kinase, yet all the patients have the same clinical presentation.18 Whether genetic susceptibility factors such as HLA phenotype, as described for PLA2R1-associated membranous nephropathy,19 also apply to patients with THSD7A-associated membranous nephropathy can be determined only in studies of much larger cohorts. In fact, it is the observed mutual exclusivity of these two antigens that lends credence to our proposal that THSD7A- and PLA2R1-associated membranous nephropathy represent two separate molecular entities and that the respective antigens are primary targets in this disease. It seems unlikely that these antibodies are epiphenomena resulting from podocyte injury and exposure of cryptic antigens, since one would have expected antibodies to both antigens to be found in several cases. At this stage, however, we do not know why antibodies to PLA2R1 develop in some persons with idiopathic membranous nephropathy, antibodies to THSD7A in others, and antibodies to as-yet unidentified antigens in still others.
In conclusion, we have identified a group of patients with idiopathic membranous nephropathy whose circulating autoantibodies are specific for THSD7A and not PLA2R1, suggesting that patients who are positive for anti-THSD7A autoantibodies represent a distinct subgroup with this disease. This finding not only furthers our understanding of the pathophysiological basis of membranous nephropathy but also allows for the potential identification and monitoring of patients who are positive for anti-THSD7A autoantibodies, by both serologic testing and histologic staining for the antigen.
Supplementary Material
Acknowledgments
Supported by grants from the French National Center for Scientific Research, the French National Research Agency (ANR-11-LABX-0028-01), Fondation pour la Recherche Médicale (FRM ING20140129210), Centre Hospitalier Universitaire de Nice and Direction Générale de l’Offre de Soins of the French Ministry of Health (PHRC 2011-A01302-39 and NCT01897961, to Drs. Lambeau and Seitz-Polski), and Deutsche Forschungsgemeinschaft (STA 193/9-1 and STA193/9-2, to Dr. Stahl); by a Gerok fellowship granted by the Deutsche Forschungsgemeinschaft to the University of Hamburg (KFO 228, to Dr. Tomas); and by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK097053, to Dr. Beck; and R01 DK090029, to Dr. Salant).
We thank Ursula Kneissler for performing immunohisto-chemical analyses and confocal microscopy; Christine Payré for help in the initial biochemical characterization of THSD7A-positive patients; Eugen Kinzler for collecting the patient data; Dr. Andreas Gross and personnel at Asklepios Klinik Barmbek for collaborating in the collection of kidney tissue; Dr. Jens Gerth and personnel at Heinrich-Braun-Klinikum Zwickau for collaborating in the collection of serum samples; Sjoerd Timmermans and Jan Willem Cohen Tervaert (for the Limburg Renal Registry); and Anna-Lena Berg, Gerald Appel, Jai Radhakrishnan, Pietro Canetta, and Tanuja Mishra for providing serum samples.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Deegens JK, Wetzels JF. Membranous nephropathy in the older adult: epidemiology, diagnosis and management. Drugs Aging. 2007;24:717–32. doi: 10.2165/00002512-200724090-00002. [DOI] [PubMed] [Google Scholar]
- 2.Beck LH, Jr, Bonegio RG, Lambeau G, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoxha E, Harendza S, Zahner G, et al. An immunofluorescence test for phospholipase-A₂-receptor antibodies and its clinical usefulness in patients with mem branous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–32. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 4.Hofstra JM, Beck LH, Jr, Beck DM, Wetzels JF, Salant DJ. Anti-phospholipase A2 receptor antibodies correlate with clinical status in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2011;6:1286–91. doi: 10.2215/CJN.07210810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hofstra JM, Debiec H, Short CD, et al. Antiphospholipase A2 receptor antibody titer and subclass in idiopathic membranous nephropathy. J Am Soc Nephrol. 2012;23:1735–43. doi: 10.1681/ASN.2012030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–66. doi: 10.1681/ASN.2013040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dähnrich C, Komorowski L, Probst C, et al. Development of a standardized ELISA for the determination of autoantibodies against human M-type phospholipase A2 receptor in primary membranous nephropathy. Clin Chim Acta. 2013;421:213–8. doi: 10.1016/j.cca.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 8.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002;1572:364–86. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang CH, Su PT, Du XY, et al. Thrombospondin type I domain containing 7A (THSD7A) mediates endothelial cell migration and tube formation. J Cell Physiol. 2010;222:685–94. doi: 10.1002/jcp.21990. [DOI] [PubMed] [Google Scholar]
- 10.Hoxha E, Kneißler U, Stege G, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 11.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–15. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 12.Debiec H, Ronco P. PLA2R autoanti-bodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–90. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 13.Beck LH., Jr Membranous nephropathy and malignancy. Semin Nephrol. 2010;30:635–44. doi: 10.1016/j.semnephrol.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Seitz-Polski B, Payré C, Ambrosetti D, et al. Prediction of membranous nephropathy recurrence after transplantation by monitoring of anti-PLA2R1 (M-type phospholipase A2 receptor) autoantibodies: a case series of 15 patients. Nephrol Dial Transplant. 2014 Jul 25; doi: 10.1093/ndt/gfu252. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 15.Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2013;28:1839–44. doi: 10.1093/ndt/gfs439. [DOI] [PubMed] [Google Scholar]
- 16.Ju W, Greene CS, Eichinger F, et al. Defining cell-type specificity at the transcriptional level in human disease. Genome Res. 2013;23:1862–73. doi: 10.1101/gr.155697.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debiec H, Guigonis V, Mougenot B, et al. Antenatal membranous glomerulonephritis due to anti-neutral endopeptidase antibodies. N Engl J Med. 2002;346:2053–60. doi: 10.1056/NEJMoa012895. [DOI] [PubMed] [Google Scholar]
- 18.Hoch W, McConville J, Helms S, Newsom-Davis J, Melms A, Vincent A. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med. 2001;7:365–8. doi: 10.1038/85520. [DOI] [PubMed] [Google Scholar]
- 19.Stanescu HC, Arcos-Burgos M, Medlar A, et al. Risk HLA-DQA1 and PLA2R1 alleles in idiopathic membranous nephropathy. N Engl J Med. 2011;364:616–26. doi: 10.1056/NEJMoa1009742. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.