Abstract
During 2001, 89 culture-confirmed cases of Yersinia pseudotuberculosis were reported in Finland; 55 (62%) were serotype O:1, and 34 (38%) were serotype O:3. Four major pulsed-field gel electrophoresis profiles were identified. A case-control study of 25 case patients and 71 healthy controls identified eating outside the home as a risk factor for infection.
Yersinia pseudotuberculosis infections occur primarily in the northern hemisphere (2, 7). The bacterium occurs in water and in the environment and has been found in various wild and domesticated animals (1, 3-6, 8, 10). However, the epidemiology of Y. pseudotuberculosis infections is unclear. In Finland, domestically produced iceberg lettuce was recently identified as a food vehicle in a nationwide outbreak (7).
Notification of a suspected food-borne outbreak among schoolchildren from municipality A in northern Finland was received by national authorities on 30 May 2001. Ten children were subsequently confirmed to have Y. pseudotuberculosis infection. A second cluster of six children with Y. pseudotuberculosis infection occurred in city B, in central Finland, in June 2001. No common exposures were identified by using hypothesis-generating telephone interviews with the parents of children in these two clusters. School holidays commenced further investigations. In total, 125 cases of Y. pseudotuberculosis infection were reported nationwide from 1 May to 31 July, and clinical laboratories were requested to submit isolates for further typing.
Y. pseudotuberculosis strains were verified, serotyped, and compared by pulsed-field gel electrophoresis (PFGE) with NotI and SpeI restriction enzymes as described earlier (7).
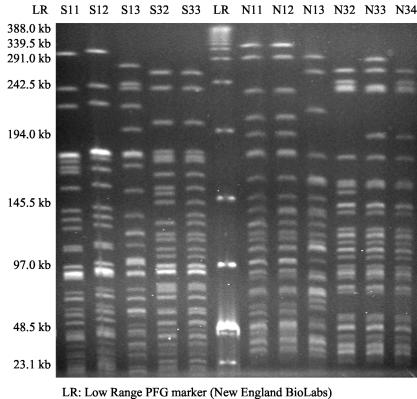
The banding patterns were named as follows. Serotype O:1 PFGE types were named, e.g., S11 (S, digested with SpeI; 1, serotype O:1; 1, type detected first), and serotype O:3 PFGE types were named, e.g., N32 (N, digested with NotI; 3, serotype O:3; 2, second detected type).
We conducted a case-control study. A case was defined as a resident of Finland aged 18 years or older with isolation of Y. pseudotuberculosis from stool or blood culture from 1 May to 31 July 2001. Five control subjects per case, randomly selected through the national population register, were matched for year of birth, gender, and postal code of residence. A self-administered questionnaire including informed consent was mailed to 39 case patients and 195 controls. Questions included those pertaining to symptoms and eating habits (specific diets, eating at home and outside home [work canteen or cafeteria, school canteen, restaurants or fast-food restaurants, or bars], and consumption of various types of fresh produce and pork). For case patients, questions referred to the 2 weeks before onset of symptoms. Controls were asked about the 2 weeks before questionnaire completion. Mantel-Haenszel matched odds were calculated, and the exact conditional maximum-likelihood method for fitting a logistic regression model to the data including 95% exact confidence intervals was used, using Epi-Info (version 6.04) and SAS (version 8.2) software.
Of the 87 fecal isolates and 2 blood isolates of Y. pseudotuberculosis submitted to the Laboratory of Enteric pathogens during March to August 2001, 55 (62%) were serotype O:1 and 34 (38%) were serotype O:3. Of the 34 strains obtained from case-control study subjects and available for further typing, serotype O:1 strains accounted for 25 (74%) isolates and serotype O:3 accounted for 9 (26%) isolates. Serologically grouped isolates could be further separated by PFGE (Fig. 1). Of all strains of Y. pseudotuberculosis isolated during March to August 2001, three PFGE clusters consisting of at least two isolates were identified among the 55 serotype O:1 strains. Four major profiles were identified among 34 serotype O:3 strains. In addition, three and five sporadic O:3 and O:1 profiles, respectively, were detected (Fig. 1). Overall, the four most common Y. pseudotuberculosis serotype O:1 and O:3 profiles (S11N11, S12N12, S32N32, and S33N33) accounted for 80% [(16 + 32 + 13 + 10)/89] of the isolates.
FIG. 1.
The PFGE patterns of the most common genotypes of Y. pseudotuberculosis isolated during 2001 in Finland. The lanes show the DNA of serotype O:1 strains, S11, S12, and S13 (digested with SpeI) and N11 and N12 (digested with NotI); and the DNA of serotype O:3 strains, S32 and S33 (digested with SpeI) and N32 and N33 (digested with NotI). Of the main O:1 PFGE patterns, 32 strains represented pattern S12N12, 16 strains represented pattern S11N11, and 2 strains represented pattern S13N13. Of the main serotype O:3 PFGE patterns, 13 strains were of S32N32, 10 strains were of S33N33, 5 strains were of S33N32, and 3 strains were of S32N34.
Municipality A and city B strains consisted of clusters S33N33 and S33N32, respectively. The only common factor between the children in both clusters was the school. Children might have become ill from contaminated food served at the schools. These PFGE profiles were not observed elsewhere, indicating the possibility of a common source.
Overall, the 89 cases were scattered around the country. Thirty-nine (44%) culture-confirmed cases were eligible for the case-control study. Fifty (56%) cases were excluded because they were too young (48 cases) or reported late (2 cases). Twenty-nine (74%) case patients returned the questionnaire. Three further case patients were excluded because no controls were available, and one was excluded because of travel away from home, leaving 25 cases for analysis. Of controls, 124 (64%) returned the questionnaire. Fifty-three controls were excluded due to gastrointestinal symptoms (12 controls) or lack of corresponding case patient (41 controls), leaving 71 controls.
Among case patients, the median age was 48 years (range, 18 to 87 years), and 13 (52%) were men. The most common clinical symptoms were abdominal cramps (92%), fever (83%), and articular or back pain (54%). Only about half (52%) of the patients had diarrhea. Symptom duration was >14 days for 61% of the case patients (median, 16 days).
Eating iceberg lettuce was associated with Y. pseudotuberculosis infection in the matched analysis (matched odds ratio [MOR], 5.7; 95% confidence interval [CI], 1.6 to 47.7) (Table 1). The case patients were also more likely than the controls to have eaten outside their home (MOR, 11.2; 95% CI, 2.6 to 390.4) (Table 1). After adjusting for variables that were significantly associated with illness in the initial univariate analysis or were considered potential confounders (iceberg lettuce, Chinese cabbage, carrots, and pot salad), case patients were 10.3 times more likely than controls to have eaten outside their home (95% CI, 1.2 to infinity; P = 0.03). Other variables were no longer significant. Practices in commercial and institutional kitchens might favor the enrichment of Y. pseudotuberculosis compared with home kitchen practices, regardless of vehicle. However, as identification of cases associated with a restaurant or canteen usually prompts search for additional cases, the possibility of detection bias should also be considered. Fresh produce was the suspected source of a Y. pseudotuberculosis outbreak among Finnish schoolchildren in 1984 (9).
TABLE 1.
Exposures to selected food items and eating preferences among case patients with culture-confirmed Y. pseudotuberculosis infection and controls matched by age, gender, and area of residence
| Exposure | No. of case patients/ no. total (%) | No. of controls/ no. total (%) | Matched odds ratio (95% CI) | P value |
|---|---|---|---|---|
| Foodstuff | ||||
| Iceberg lettuce | 15/19 (79) | 25/62 (40) | 5.7 (1.6-47.7) | 0.01 |
| Chinese cabbage | 10/19 (53) | 18/63 (29) | 2.6 (0.8-9.0) | 0.17 |
| Carrots | 19/20 (95) | 46/66 (70) | 6.5 (0.9-130.8) | 0.13 |
| Pot salad | 18/22 (82) | 39/65 (60) | 2.3 (0.9-10.3) | 0.14 |
| Leaf salad | 10/17 (59) | 29/61 (48) | 1.3 (0.4-4.3) | 0.88 |
| Cauliflower | 8/19 (42) | 27/65 (42) | 1.1 (0.3-3.7) | 0.83 |
| White cabbage | 6/17 (35) | 15/64 (23) | 1.2 (0.3-4.0) | 0.99 |
| Ready-to-eat-salads | 4/20 (20) | 21/62 (34) | 0.6 (0.1-1.9) | 0.46 |
| Pork | 20/22 (91) | 55/67 (82) | 1.7 (0.4-11.8) | 0.75 |
| Eating preferences | ||||
| Ate outside home | 24/25 (96) | 44/69 (64) | 11.2 (2.6-390.4) | 0.003 |
| Ate fresh produce in workplace restaurant or canteen | 11/24 (46) | 10/53 (19) | 3.3 (1.1-13.6) | 0.06 |
| Ate fresh produce at home | 17/23 (74) | 52/65 (80) | 0.6 (0.2-2.7) | 0.9 |
| Ate fresh produce in fast-food restaurant? | 10/23 (44) | 18/51 (35) | 1.5 (0.5-5.1) | 0.64 |
| Ate fresh produce in restaurant | 12/23 (52) | 14/49 (29) | 2.5 (0.8-6.5) | 0.15 |
| Vegetarian diet | 1/25 (4) | 5/68 (7) | 0.7 (0.03-4.7) | 0.95 |
Few countries conduct surveillance for Y. pseudotuberculosis, limiting understanding of its epidemiology. A low threshold of reporting for suspected food-borne outbreaks nationally, combined with continuous laboratory surveillance, enabled rapid outbreak detection. Investigation was strengthened by ongoing submission of Y. pseudotuberculosis isolates for further characterization. Since eating outside the home was strongly associated with the illness, hygienic measures in the food chain and institutional kitchens handling fresh produce need to be evaluated. Characterization of the growth of Y. pseudotuberculosis in various environmental conditions and in fresh produce is also required.
Acknowledgments
We thank Sarah O'Brien for critical evaluation of the manuscript. We thank Jukka Ollgren and Pekka Holmström for help with statistical analysis and Tarja Heiskanen and Liisa Immonen for serotyping of the isolates. We thank all national and local authorities, clinical laboratories, and food control authorities that participated in the outbreak investigations.
REFERENCES
- 1.Fukushima, H., M. Gomyoda, K. Shiozawa, S. Kaneko, and M. Tsubokura. 1988. Yersinia pseudotuberculosis infection contracted through water contaminated by a wild animal. J. Clin. Microbiol. 26:584-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukushima, H., Y. Matsuda, R. Seki, M. Tsubokura, N. Takeda, F. N. Shubin, I. K. Paik, and X. B. Zheng. 2001. Geographical heterogeneity between Far Eastern and Western countries in prevalence of the virulence plasmid, the superantigen Yersinia pseudotuberculosis-derived mitogen, and the high-pathogenicity island among Yersinia pseudotuberculosis strains. J. Clin. Microbiol. 39:3541-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashidani, H., N. Kanzaki, Y. Kaneko, A. T. Okatani, T. Taniguchi, K. Kaneko, and M. Ogawa. 2002. Occurrence of yersiniosis and listeriosis in wild boars in Japan. J. Wildl. Dis. 38:202-205. [DOI] [PubMed] [Google Scholar]
- 4.Inoue, M., H. Nakashima, T. Ishida, M. Tsubokura, and R. Sakazaki. 1988. Isolation of Yersinia pseudotuberculosis from water. Zentbl. Bakteriol. Mikrobiol. Hyg. B 186:338-343. [PubMed] [Google Scholar]
- 5.Nikolova, S., Y. Tzvetkov, H. Najdenski, and A. Vesselinova. 2001. Isolation of pathogenic yersiniae from wild animals in Bulgaria. J. Vet. Med. B Infect. Dis. Vet. Public Health 48:203-209. [DOI] [PubMed] [Google Scholar]
- 6.Niskanen, T., M. Fredriksson-Ahomaa, and H. Korkeala. 2002. Yersinia pseudotuberculosis with limited genetic diversity is a common finding in tonsils of fattening pigs. J. Food Prot. 65:540-545. [DOI] [PubMed] [Google Scholar]
- 7.Nuorti, J. P., T. Niskanen, S. Hallanvuo, J. Mikkola, E. Kela, M. Hatakka, M. Fredriksson-Ahomaa, O. Lyytikäinen, A. Siitonen, H. Korkeala, and P. Ruutu. 2004. A widespread outbreak of Yersinia pseudotuberculosis O:3 infection from iceberg lettuce in Finland. J. Infect. Dis. 189:766-774. [DOI] [PubMed] [Google Scholar]
- 8.Pocock, M. J., J. B. Searle, W. B. Betts, and P. C. White. 2001. Patterns of infection by Salmonella and Yersinia spp. in commensal house mouse (Mus musculus domesticus) populations. J Appl. Microbiol. 90:755-760. [DOI] [PubMed] [Google Scholar]
- 9.Tertti, R., K. Granfors, O. P. Lehtonen, J. Mertsola, A. L. Makela, I. Valimaki, P. Hanninen, and A. Toivanen. 1984. An outbreak of Yersinia pseudotuberculosis infection. J. Infect. Dis. 149:245-250. [DOI] [PubMed] [Google Scholar]
- 10.Toma, S. 1986. Human and nonhuman infections caused by Yersinia pseudotuberculosis in Canada from 1962 to 1985. J. Clin. Microbiol. 24:465-466. [DOI] [PMC free article] [PubMed] [Google Scholar]