Abstract
The objective of a systemically administered cancer gene therapy is to achieve gene expression that is isolated to the tumor tissue. Unfortunately, viral systems have strong affinity for the liver, and delivery from non-viral cationic systems often results in high expression in the lungs. Non-specific delivery to these organs must be overcome if tumors are to be aggressively treated with genes such as IL-12 which activates a tumor immune response, and TNF-alpha which can induce tumor cell apoptosis. Techniques which have led to specific expression in tumor tissue include receptor targeting through ligand conjugation, utilization of tumor specific promoters and viral mutation in order to take advantage of proteins overexpressed in tumor cells. This review analyzes these techniques applied to liposomal, PEI, dendrimer, stem cell and viral gene delivery systems in order to determine the techniques that are most effective in achieving tumor specific gene expression after systemic administration.
Keywords: Gene Therapy, Systemic, Viral, Non-Viral, Dendrimer, Delivery System
Introduction
The vast majority of reported viral and non-viral gene delivery systems do not preferentially target tumor tissue. Viral systems including vaccinia virus, adenovirus, and lentivirus deliver their payload primarily to the liver, while non-viral systems such as cationic liposomes and polymers principally deliver to lung tissue [1-4]. Researchers take advantage of the innate targeting of these systems, using viral and non-viral vectors to target hepatocellular carcinomas and non-small cell lung carcinomas respectively [5-8]. However, when targeting other cancers or metastases not resident in the liver and lung, accumulation in these organs hinders effective cancer therapy.
Methods for locally administering gene delivery systems include intracranial, intratumoral and intravenous injection as well as inhalation. Localized delivery techniques have been shown to be effective in treating primary tumors [9]. Intratumoral delivery of viral gene delivery systems targets the tumor tissue and while there is some distribution of the virus outside of the tumor, the toxicity and immunogenicity are tolerable. Inhalation of nanoparticles results in the payload selectively reaching the lungs, while intracranial tumor injection is effective for circumventing the blood brain barrier [10, 11]. Although some of these localized methods of administration may be sufficient for primary tumor targeting, undetected metastases will need to be targeted with an intravenous injection of a delivery system that can home to tumor cells systemically. As primary tumors can often be removed surgically, metastases should be the priority when developing a delivery system for treating cancer. A tumor-specific, systemically-delivered vector would be able to capitalize on the growing number of genes that have been shown to hinder the growth of metastatic cancer cells.
Many of the genes that could be used to aggressively promote cancer cell apoptosis or stimulate an immune response to a tumor will result in unacceptable toxicity if delivered primarily to the lungs and liver [12, 13]. Two cytokines that have been shown to deter metastasis and effectively treat already established metastases are IL-12 and IL-2 [14-16]. When delivered to the microenvironment of a metastatic tumor, IL-12 can polarize T helper cells towards a TH1 phenotype, which produces IFN-gamma and activates a cytotoxic T-cell response. IL-2 has been shown to illicit a response from natural killer and cytotoxic T-cells. While both of these cytokines can prevent metastatic tumor growth when delivered locally, systemic delivery that is non-specific has significant toxicity [12, 17]. It is largely because of this dose-dependent toxicity that clinical trials using systemic delivery of IL-12 have had limited success.
Other attractive genes for delivery encode ligands that target death receptors leading to apoptosis, including tumor necrosis factor-alpha (TNF-alpha), CD95/FAS and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [13, 18, 19]. The appeal of a suicide protein being delivered to tumor cells is undeniable, but as with immune-stimulating cytokines, the non-specific systemic delivery of a suicide gene is not well tolerated [20]. Considering recent concerns of hepatotoxicity, even the relatively non-toxic TRAIL must be dosed conservatively as it moves into the clinic [21]. Herpes simplex virus thymidine kinase (HSV/tk) is another gene commonly chosen for delivery [22]. Accumulation of thymidine kinase within a target cell is toxic upon administration of a pyrimidine or purine analog drug, converting the drug to its active form in the cytoplasm [23]. As with the other genes mentioned above, non-specific delivery of HSV/tk will result in death of bystander cells. Whether specificity is incurred by targeting of tumor tissue, selective internalization into tumor cells or use of tumor-specific promoters, selective gene expression must be achieved if we are to aggressively target tumor metastases with genes that promote cell death.
This review focuses on viral and non-viral systems that have been reported to achieve specific gene expression in tumor tissue. Unless stated otherwise, the delivery system was administered via tail vein injection. The type of delivery system is introduced in each section with a description of its natural targets, followed by an analysis of studies that have manipulated the system so that gene expression is greater in the tumor than in other major organs. In addition, we discuss gene delivery using stem cells which have been reported to naturally migrate to tumor tissue.
Non-Viral Systems
Lipoplex
Cationic lipoplexes are composed of a cationic lipid and a neutral lipid or cholesterol [2, 24]. The negatively-charged DNA is compacted and forms a complex with the positively-charged lipid, resulting in the formation of a lipoplex. Lipoplexes interact with the cell membrane and internalize into the cell through endocytosis. It is thought that the lipid components, once internalized, lead to destabilization of the lipid complex, fusion with the endosomal membrane, and cytoplasmic delivery of the DNA. DNA can reach the nucleus when the nuclear membrane breaks down during cell division, and thus rapidly-dividing cells are generally more easily transfected. When compared with viral delivery systems, the non-viral cationic lipoplex is considered less immunogenic, and able to hold a larger payload. The drawbacks include toxicity of cationic lipids and relatively inefficient and non-specific delivery [25].
Cationic lipoplexes deliver genes primarily to the lungs and secondarily to the liver [26]. Luciferase expression is observed in the liver and lungs with relatively low expression in flank tumors of a mouse model. Coating of the cationic lipoplex with polyethylene glycol (PEG) can be employed to increase circulation time with the hope of enhancing delivery to the leaky capillary bed of tumor tissue [1, 27]. However, several groups have reported that increased levels of PEG do not improve distribution to the tumor tissue, and delivery is predominantly to the lungs and liver [1, 28].
To increase the tumor specificity of systemically-delivered cationic lipoplexes, one group used both PEG shielding and integrin targeting [27, 29, 30]. These cationic lipoplexes have a short triethylene glycol coating and a peptide targeted to integrins, which are overexpressed in many neuroblastoma cell lines. The PEG and integrin-targeting peptides are connected to the liposome through linkages that are cleavable by endosomal furin, cathepsinB, or esterases. After binding and internalization into tumor cells, the peptides and PEG are cleaved from the liposome, allowing for destabilization and delivery of the nucleic acid. This technique has resulted in highly specific tumor targeting upon systemic administration. Tissue analysis showed that accumulation of luciferase DNA was at least two-fold greater in the tumor tissue when compared to the lung, liver and spleen, but more importantly, expression of the luciferase gene was 130-fold greater in the tumor tissue than in the other organs. When this system was used to deliver IL-2/IL-12 cytokines to subcutaneous neuroblastoma tumors in a mouse model, 1/3 of the tumors were eradicated and 2/3 of the tumors had markedly decreased growth. It is important to mention that the expression data took into account the entire tumor and organ. When measuring expression, data is usually presented as per organ, per mg protein, or per mg tissue. Since the liver certainly weighs much more than the tumor in any realistic clinical scenario, presenting data as per organ is often the least impressive and possibly the most relevant reporting method. However, data reported as “per organ” can be misleading if tumors are allowed to achieve grotesque proportions in animal models. Therefore, it is preferable to include both measurements to allow comparison with published studies.
Wang et al. were able to achieve good tumor specificity using a cationic lipid system consisting of DOTAP and cholesterol [31]. Messenger RNA for luciferase is condensed with protamine which then associates with the cationic liposome. The resulting complex is PEGylated, and anisamide is attached so that the particles will target cancer cells overexpressing the sigma receptor. This system results in at least 10-fold greater expression per mg of tumor tissue compared to liver tissue. When the suicide gene thymidine kinase from Herpes simplex virus is delivered systemically using this system, the tumors show a marked decrease in growth [31]. To assess if the delivery system was causing toxicity, serum concentrations of liver enzymes, alanine transaminase and aspartate transaminase were assayed along with blood urea nitrogen levels which can reveal kidney damage. After repeated treatment with the liposomes, all of these toxicity indicators fell within their normal range, suggesting that this therapy could be tolerable at clinical dosages.
To target prostate cancer, Ikegami et al. used a monoclonal antibody that binds to prostate-specific membrane antigen (PSMA) [32]. This membrane protein is present in prostate cancers and is not expressed in normal tissue. The antibody is coupled to polylysine, which is then used to condense DNA. The targeted polylysine is mixed with cationic liposomes and the resulting complex is used to deliver luciferase and HSV/tk to prostate tumors. With this system, luciferase expression was at least 17-fold higher per mg prostate tumor than in lung, liver and kidneys. When the HSV/tk gene was delivered to treat tumors, tumor mass was reduced by approximately 50% [32].
Ligand targeting of lipoplexes seems to increase gene expression in the tumor, but besides the examples cited above, there are limited papers showing specificity for the tumor over lungs or liver tissue. Another strategy used to incur specificity takes advantage of promoters specific to the tumor tissue. Although the lipoplexes may still deliver DNA to the lungs and liver, gene expression only occurs in the tumor tissue where the promoter is active. Li et al. searched the literature for genes often overexpressed in pancreatic cancer and found that mRNA of cholecystokinin type A receptor (CCKAR) had a promoter that allowed gene expression in pancreatic adenocarcinomas but not in normal pancreatic tissue. Incorporating this promoter into a luciferase plasmid which was then delivered with a DOTAP/Chol lipoplex, this group achieved gene expression in the tumor that was 20-fold greater per mg protein than in the lung [33]. In contrast, using a standard CMV promoter, luciferase expression was slightly greater in the lung than in the tumor.
All of the lipoplex examples described above use liposomes formulated with cationic lipids. Heyes et al. achieved tumor-specific luciferase expression using anionic liposomes which are thought to have less interaction with the lungs than cationic liposomes [34]. By first condensing DNA with either polylysine or polyethylenimine (PEI) and then interacting this with their neutral, anionic and PEG-conjugated lipids, the group was able to form a liposome that encapsulated a single copy of luciferase plasmid. While cationic liposomes distribute to the lungs, these anionic liposomes primarily accumulated in the liver and spleen with 4-fold lower accumulation in the tumor. Although many of the particles distributed to other organs, the expression level per gram tumor tissue was over 100-fold higher than in the liver, spleen or lungs. Without ligand or antibody targeting, these liposomes produced tumor-specific gene expression, relying only on the leaky vasculature of the tumor. Although DNA is much harder to encapsulate in anionic liposomes, it may be worth the effort if they can successfully circumvent the excessive lung delivery from cationic lipids.
Polyethylenimines (PEIs)
PEIs are branched or linear cationic polymers that have the ability to condense DNA, internalize into cells and facilitate endosomal escape [35, 36]. PEI is attractive as a carrier because of its ability to rupture cellular endosomes through the “proton sponge” mechanism [36]. It is thought that the amine groups on the PEI act as a buffer, binding up protons and causing the accumulation of Cl- during endosome acidification. This in turn leads to osmotic swelling and rupture of the endosome. Considering this mechanism of action, it should not be surprising that PEIs have been shown to have considerable non-specific cytotoxicity. Although the toxicity decreases for smaller or linear PEIs, transfection efficiency also suffers. Research focused on developing biodegradable PEIs that are less toxic has shown some success [37]. When delivered in vivo, PEI tends to accumulate in the lung, similar to cationic liposomes [36].
Using a highly branched low molecular weight PEI, Li et al. were able to achieve reasonable tumor specificity by attaching a targeting peptide specific for fibroblast growth factor [35]. The particles were positively-charged with a diameter of 300 nm. Without the targeting peptide, luciferase activity per mg protein was 2-fold greater in the lungs than the tumor. After attachment of the peptide, luciferase signal was 2-fold greater in the tumor than in the lungs and 4-fold greater than in the liver.
Kircheis et al. had success in getting tumor-specific delivery, also through the use of small molecular weight PEIs conjugated to transferrin [37-40]. These researchers report that they were able to shield non-specific charge interactions by increasing the density of transferrin conjugated to PEI. The reduction in surface charge lowered luciferase expression in normal tissue and resulted in tumor luciferase expression that was 100-fold greater than in the lung on a per organ basis. Attaching PEG to the system improved delivery to the tumor tissue by 10-fold but decreased specificity slightly. When DNA encoding TNF-alpha was targeted to mouse tumors using both the PEGylated and non-PEGylated transferrin receptor-targeted PEI systems, tumors showed necrosis and considerably slower growth. Substituting EGF for transferrin, this system was also specific against EGFR-overexpressing tumors [40]. The group contributes their success to two factors: reduced surface charge and large particles. While most researchers formulate particles in the 100-300 nm range, these PEI particles were in the 800-1200 nm range which may allow extravasation into the leaky tumor vasculature while limiting delivery to normal tissue. A drawback is that this large particle size could possibly restrict delivery to tumors that have a tighter vasculature, but the specificity is compelling.
Dendrimers
Since their creation in the late 1970s, dendrimers have been used for applications ranging from gene and drug delivery to nano-engineering and diagnostic sensors [41]. Dendrimers consist of a core molecule with functional branches that start from the core and branch outwards. When formed by divergent methods, each layer is added by stepwise synthesis. The generation number of a dendrimer refers to the number of synthesis steps or layers of branches. Because their synthesis is tightly controlled, dendrimers have well-defined size and chemical properties. There are many dendrimers, but the commercially available cationic dendrimers used for gene delivery are polyamidoamine (PAMAM) and polypropylenimine (PPI) dendrimers. PAMAM dendrimers consist of an ethylenediamine or ammonia core, and are built by iterative addition of methylacrylate followed by ethylenediamine which adds an amide group. PPI dendrimers are built upon a butylenediamine core with polypropylenimine branches. Endosome escape is thought to be mediated by the proton sponge mechanism mentioned above, similar to PEI. While it is generally accepted that PAMAM dendrimers accumulate in the lungs, there is recent evidence that PPI dendrimers have innate tumor specificity when delivered systemically [42]. Furthermore, those PPI dendrimers that accumulate in the tumor tissue can induce tumor-specific gene expression.
Russ et al. and Chisholm et al., have used generation 2 and 3 PPI dendrimers and found that gene expression is prevalent in the tumor tissues with up to100-fold lower expression in all other organs including the liver, spleen, kidney, heart and lung on a per organ basis [42, 43]. Another group found that generation 3 PPI delivery resulted in slightly greater delivery to the liver than to the tumor tissue, with little expression in lung and other tissues on a per organ basis [41, 44]. To improve specificity, they conjugated transferrin to the dendrimers, resulting in tumor tissue expression that was 4-fold greater than in liver tissue. When this system was used to deliver TNF-alpha with a tumor-specific promoter, the treatment led to complete regression of 90% of tumors in mice [45]. This impressive result used the innate targeting of PPI dendrimers combined with ligand targeting and a tumor-specific promoter. The synergy of these tumor-specific techniques allowed the researchers to aggressively treat with a potent therapeutic gene, leading to tumor regression that is rarely seen in treatments.
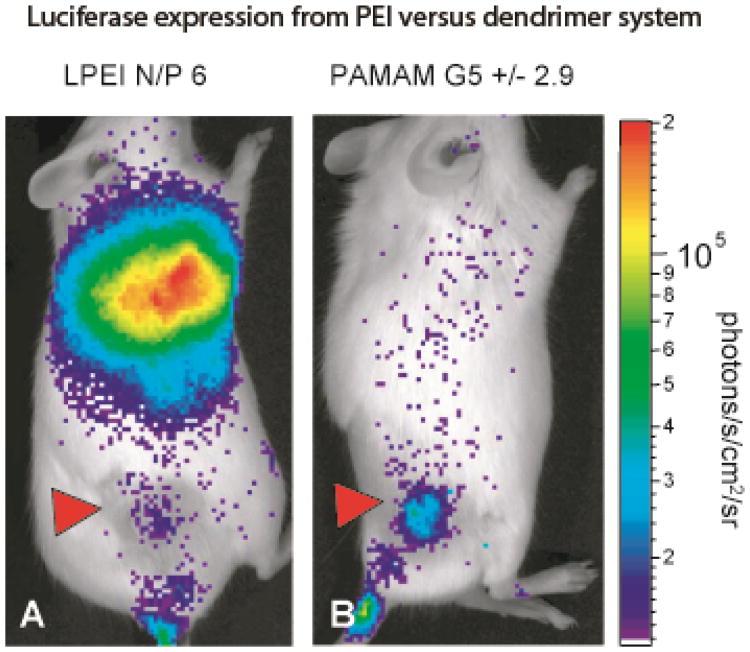
An analysis of PPI dendrimer biodistribution showed that there was little particle accumulation in the lung and, in all three of these studies, the dendrimer size was between 100 nm and 300 nm [42]. PPI dendrimers are perhaps the only system that appears to show an inherent homing to tumor tissue with little delivery to normal tissue throughout the body. This homing mechanism may involve precise control of particle size and/or recruitment of specific serum proteins that promote preferential uptake by tumors. Although PAMAM dendrimers do not have a tumor-specific biodistribution like PPI dendimers, Navarro et al. demonstrated that the gene expression could still be specific to the tumor [46]. Both a control PEI system and PAMAM denderimers were shown to gather primarily in the lung tissue, but while PEI resulted in a strong luciferase signal from the lungs, PAMAM luciferase expression was isolated to the tumor (Figure 1). These initial studies achieving tumor-specific gene expression certainly warrant the need for further investigation into dendrimers for gene delivery.
Fig 1.
Luciferase expression is shown after delivery of Luciferase plasmid from either PEI or PAMAM dendrimers. Although both systems accumulate in the lungs, PEI results in luciferase expression primarily in the lung while PAMAM dendrimers results in tumor-specific luciferase expression. (G. Navarro et al., journal of controlled release, 146 (2010) 99-105.)
Stem Cells
Mesenchymal stem cells (MSCs) are nonhemapoetic stem cells that originate in the bone marrow and in umbilical cord tissue. They are highly proliferative, easily isolated and can be genetically modified in culture to express genes that potentially could produce therapeutic effects. The excitement around MSCs as a gene delivery tool comes from the fact that they have been observed to accumulate in tumor tissue in a manner similar to how they migrate to any wound environment [47, 48]. The hope is that MSC's can be isolated, cultured, transfected with a therapeutic gene and administered systemically, whereupon they would migrate selectively to the tumor tissue and produce a therapeutic protein that would eliminate the tumor. Cell-based therapies such as these have great appeal, since there is no transfer of DNA into the patients' cells and, the protein will be expressed as long as the stem cells remain viable. Some sources show stem cells producing protein for up to 21 days, whereas other therapies seem to induce expression on the scale of 3 days [49].
Studies using stem cells have shown specific delivery to gliomas, lung tumors, melanomas and breast tumors, delivering genes such as IL-12 and IFN-beta [50, 51]. While the specificity of these therapies is impressive, it has been suggested that tumor targeting could be an artifact of the tumor system [52]. In these studies, the mice bear a tumor from a human cancer cell line and the stem cells being used are derived from humans [48, 50, 51]. It is possible that the human derived MSCs are attracted to chemokines or surface markers from the human tumor tissue and not from the normal mouse tissue. Indeed, when other groups have performed studies using tumor cell lines derived from mouse tumors and MSCs isolated from mice, they find that there is considerable accumulation and expression from MSCs in the lungs, liver and spleen [53-55]. Not surprisingly, the specificity using mouse derived MSCs and tumors is not as impressive as found with human-derived cells in a mouse model. However, even though delivery from studies utilizing mouse MSCs is not perfectly specific, systemic toxicity during treatment seems low and these MSC therapies have effectively treated tumors in mice. Lung tumors treated with stem cells bearing the gene for CX3CL1, an immunostimulatory cytokine, showed a marked decrease in metastasis and an overall increase in survival time of the mouse [53]. Human-derived Ewing Sarcoma tumors that were treated with murine-derived MSCs made to express IL-12 showed a significant reduction in size compared to untreated tumors [54].
Viral Systems
Viral gene delivery attempts to hijack the gene delivery system of viruses in order to achieve selective expression of a therapeutic protein in tumor cells. Although some viruses including reovirus, autonomous parvoviruses and newcastle disease virus have been reported to be tumor specific, there are limited data showing organ analysis of gene expression after systemic administration [56]. Adenovirus is one of the best studied viral gene delivery systems because of its simple and efficient binding and entry mechanism; however, its natural affinity for liver cells has hindered specific delivery in vivo [57]. Lentivirus, herpes virus, and vaccinia virus are not inherently tumor targeting but are capable of being modified in order to achieve tumor specificity. Modifications can be made to the viral membrane proteins either genetically or through direct protein insertion in an attempt to target receptors and motifs overexpressed in the tumor. Another technique is to genetically modify the viral particles so that genes will only be expressed in tumor cells that have the proper protein signature [58]. These methods have been employed in a number of viral systems with varying levels of success in avoiding non-specific organ delivery.
Vaccinia Virus
Vaccinia virus has a strong eukaryotic promoter and unlike most viruses, has genes encoding for transcriptional proteins, allowing for very high gene expression that is not dependent on the host cell undergoing replication [58]. Since live vaccinia virus has been used extensively for small pox vaccination, its safety is well documented. To take advantage of this virus for tumor targeting gene delivery, Puhlmann et al. have mutated the vaccinia genome so that it no longer makes functional thymidine kinase, a protein essential for DNA synthesis and viral replication [58]. Thymidine kinase is found in tumor cells and when this modified vaccinia virus encoding luciferase DNA is injected systemically, tumor expression of luciferase per mg protein is at least 250-fold higher than in any of the normal tissues. This group thoroughly characterized their vaccinia gene delivery system and in at least 4 different studies, found great specificity for the tumor tissue [18, 58-60]. In addition, they replaced luciferase with genes that encode for various therapeutic genes and successfully treated tumors in mice and rabbits. In one study, the delivered DNA coding for cytosine deaminase, which converts non-toxic 5-fluorocytosine into a toxic form, allowed for selective killing of liver metastases from a colorectal tumor cell line. The mice treated with this vaccinia virus lived significantly longer and were cured in 14% of the cases [60]. In another study, the thymidine kinase-negative vaccinia viral particles were loaded with DNA encoding endothelial cell monocyte activating polypeptide II, which sensitizes cells to TNF-alpha [18]. Using this system, TNF-alpha resistant melanoma cells were specifically targeted upon subsequent systemic treatment with TNF-alpha, leading to tumor regression. The specificity of this system has also been established in rabbits; however, the immune response to the viral particles leads to rapid clearance [59]. Although the vaccinia virus is cleared quickly, with this level of specificity it may be possible to treat aggressively and elicit a therapeutic response.
Herpes Virus
Herpes simplex virus preferentially infects cancer cells over normal cells and has therefore been investigated for tumor gene delivery [22]. However, most systems using Herpes rely on intratumoral injection to avoid the non-specific delivery that occurs upon systemic administration. One group has increased specificity by designing a mutant Herpes virus that has both copies of the gamma-delta 34.5 gene deleted [61]. Deletion of this gene results in a herpes virus that can only replicate in mitogen-activated protein kinase kinase (MEK) expressing cells. MEK is a survival gene that allows cells to resist apoptosis, and is overexpressed in many cancers. Administration of this modified virus via intraperitoneal injection showed specific luciferase expression in tumor xenografts in nude mice. Observation of the whole mouse with a luciferase imaging device, showed no detectable delivery to other organs. Following intravenous injection of the viral particles, they found significant reduction in tumor growth simply from the virus infecting tumor cells. The viral system has no therapeutic effect on cancer cells that lack MEK and they report that systemic delivery is just as effective as intratumoral injection. This system demonstrates that ligand and antibody targeting may not be necessary if gene expression in the organs can be adequately limited using cellular proteins that are specifically expressed at high levels in cancer cells. Although this system delivers DNA to other organs, expression of the DNA is restricted to the tumor.
Lentivirus
The retroviral lentivirus has the advantage of transfecting quiescent cells and can lead to long-term stable gene expression due to integration of its DNA into the cell genome. Lentiviruses are inherently not tumor specific, and when ligands and targeting molecules are attached to the viral envelope, transfection efficiency is reduced [62]. To overcome this hurdle, Morizono et al. used a lentivirus with a modified chimeric Sindbis virus envelope [57]. The advantage of this chimeric viral system is that when antibodies were bound to the virus, they resulted in specific binding in vitro and, importantly, allowed for efficient gene delivery. Unfortunately, this system showed high transfection of liver and spleen cells in vivo, but by mutating the E2 envelope protein, this natural tropism could be greatly reduced [57]. Incorporating a P-glycoprotein antibody in the viral envelope allowed for targeting of melanoma cells expressing P-glycoprotein. Mice with melanoma metastases in the lungs showed high expression of the luciferase marker gene while mice that had not been injected with cancer cells had no detectable gene expression in the lungs and very limited expression in the liver and spleen. These data were obtained by ex-vivo imaging of tumors and organs with a luciferase imaging device. Expression of the luciferase gene was dependent on incorporation of the antibody for P-glycoprotein. The extensive manipulations of this system demonstrate the difficulties of producing a viral system that results in tumor-specific gene expression. Genetic modification and incorporation of antibodies can be complicated but, as shown with the specificity of this system, the effort can produce a potentially powerful vector.
SiRNA Delivery Systems
The discovery of siRNA in 1998 unveiled the ability of small double stranded DNA to induce post-transcriptional gene silencing [63]. When the small RNAs are delivered to the cytoplasm of target cells, they get taken up by the RNA-Induced Silencing Complex (RISC) [64]. The RNA strands are separated and used to bind and guide cleavage of mRNAs in a sequence homology-dependent manner. The potential of siRNA for cancer therapy was quickly realized and delivery with the standard viral and non-viral techniques has been thoroughly characterized. Genes that are often targeted by siRNA treatment are those overexpressed in tumors, including cell cycle proteins involved with G2/M transition, vasculature growth, cell migration, adaptation to hypoxia, cell survival and cell growth [64]. One advantage of using siRNA which downregulates the overexpressed proteins is that non-specific delivery is often less toxic than the delivery of plasmid DNAs that encode genes such as IL-12 and TNF-alpha. However, to limit the toxicity that does exist and to increase knockdown at the tumor site, many groups have added ligands to delivery systems to increase tumor specificity [65].
Unlike delivery of plasmid, it is difficult to accurately measure knockdown of genes systemically. Techniques for measuring knockdown include measuring the reduction of GFP and luciferase in transfected tumor cell lines as well as comparing RT-PCR levels of the target gene before and after delivery in the tumor tissue [66-68]. Biodistribution studies most often involve observation of fluorescently tagged siRNA to determine if localization is restricted to tumor tissue [68, 69]. While this accurately measures biodistribution of the siRNA it does not directly measure gene silencing. Since there is not a common or practical method for comparing knockdown of genes in the various tissues after systemic delivery of siRNA we have not conducted a thorough investigation of systemically administered siRNA delivery systems.
Comment on endosomal escape mechanism
We wish to point out that all of these synthetic delivery systems include some mechanism of endosomal escape (e.g., proton sponge) in order to avoid lysosomal degradation and facilitate cytoplasmic delivery. While entrapment in the endosomal/lysosomal pathway ultimately prevents therapeutic gene expression, it should be recognized that the rupture of intracellular organelles is not well-tolerated by cells, and may contribute to the significant toxicities observed with these systems. For example, the formation of inflammasomes in response to lysosomal rupture is well-documented, and cationic components used in many delivery systems have been shown to trigger this pathway [70]. The inflammation that results from rupturing endosomes and lysosomes might prove to be beneficial for eliciting an immune response against tumors, especially if endosomal rupture can be limited to tumor cells. Although pathogens utilize endosomal rupture to access the cytoplasm, these organisms also possess molecules that inhibit inflammasome activation, thereby preventing activation of an immune response at the site of infection [71]. It follows that the current dependence on endosomolytic components while omitting other molecules (e.g., caspase inhibitors) that prevent inflammasome formation and cytokine production will inevitably trigger immune responses that may affect therapy.
Concluding Remarks
Developing a systemic gene delivery system that is tumor specific involves choosing a basic delivery method and then overcoming the natural tropism of that system in order to achieve tumor specificity. Once tumor specificity is realized, a therapeutic gene must be chosen that will maximize treatment potential. Studying the systems that have been successful gives an indication of which delivery strategy and techniques have the best chance of resulting in effective cancer treatment.
Gene delivery from viral particles and cationic polymers to the liver and lungs results in non-specificity that is almost impossible to overcome. Given the incredible amount of resources that have gone into the two fields of liposomal and viral gene therapy, it is surprising that so few systems exist that can be delivered systemically and result in tumor-specific gene expression. Although gene delivery by polypropylenimine dendrimers is far less studied, three groups have already demonstrated tumor-specific delivery from the particles. Given the limited research that has gone into PPI dendrimers and the level of success that has been achieved by these groups, dendrimers show potential to be a potent gene delivery tool.
Once a system has been chosen, modifications must be made to improve the specificity of gene expression in tumor tissue. In this review, we described 15 systems that showed specificity upon systemic administration. Although there may be other examples of tumor-specific systems that we have not discussed here, this cross-section of papers should give an indication of the techniques that are successful in achieving specificity. Of the systems that were reviewed, 8 used ligand targeting, 5 used innate or passive accumulation in tumor tissue (by stem cells, dendrimers and anionic liposomes), 2 used a tumor-specific promoter and 2 used viral mutations that made gene expression specific to tumor cells. The two systems that used viral mutations incurred specificity to thymidine- and MEK-expressing cells, both effective techniques for overcoming the innate targeting to the liver. Requiring a tumor-specific cellular protein for viral replication and gene expression is an elegant way of obtaining specificity. Similarly, for non-viral systems, the incorporation of tumor-specific promoters can be used to exploit cancer-specific cytoplasmic proteins for improving gene expression specificity. However, since tumor cells are derived from normal cells, there are few unique promoters that result in gene expression in tumor tissue and not normal tissue.
The bulk of the modifications that allowed for specific delivery to cancer cells involved attaching targeting ligands to the delivery system. Whether conjugated to PEG or directly to the delivery particle, antibodies and ligands targeting EGF, p-glycoprotein and transferrin have shown to be effective for targeting tumor tissue. Using cell proteins present in the nucleus or cytoplasm of tumor cells to incur specificity is elegant, but for now, the most effective and common method for increasing selectivity is to target proteins overexpressed on the tumor cell surface. Ultimately, the most impressive specificity will come from combining multiple techniques, as demonstrated by Koppu et al. [45]. Their dendrimer based therapy utilized the tumor-specific accumulation of PPI dendrimers combined with ligand targeting and a tumor-specific promoter to deliver TNF-alpha. Treatment led to complete regression in 90% of the tumors; the most successful systemic gene therapy covered in this review.
After realizing tumor specificity, the most commonly chosen therapeutic genes are IL-12 which can activate a T-cell response or alternatively TNF-alpha which induces apoptosis. Utilizing the natural killing ability of the immune system or hijacking the suicide pathways that lead to cell death is a sophisticated way to target cancer if sufficient specificity can be achieved. Given the limited access of systemically-administered chemotherapies to poorly vascularized regions of the tumor and the ability of cancer to become resistant to chemotherapy, activation of the immune system may be a more potent method for eliminating metastatic cancer. An IL-12 gene therapy does not need to reach every cell in the tumor in order to be effective. If enough cells are producing the immunostimulatory cytokine, T-cell presence within the tumor could reach levels necessary for treating the entire tumor.
The advantages of gene therapy are clear. Compared to chemotherapy, gene delivery is a more subtle treatment, turning cancer cells into factories that produce proteins leading to their death and that of surrounding tumor cells. Chemotherapeutic agents and proteins administered systemically are removed quickly from circulation and tumor cells that receive chemotherapies begin clearing the drugs as soon as they are delivered. In contrast, gene therapies can produce multiple days of continuous protein production, resulting in a persistent therapeutic effect. Furthermore, if those genes are expressed specifically by the tumor tissue, there will be limited damage to normal cells as protein production and concentration will be maximal surrounding the cancer cells. With an increased ability to achieve tumor specific gene expression, gene therapy will hopefully provide an effective alternative to conventional chemotherapy and a tool for aggressively treating cancer.
Acknowledgments
We would like to thank Dr. Kristine Mann and Dr. Richard Kullberg for their critical reading of this manuscript. We are grateful for support from the UC Denver Breast Cancer Spore, the Susan G. Komen Foundation, KG111128 and NIH/NIGMS, RO1GM093287-01A1
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gjetting T, Arildsen NS, Christensen CL, Poulsen TT, Roth JA, Handlos VN, Poulsen HS. In vitro and in vivo effects of polyethylene glycol (PEG)-modified lipid in DOTAP/cholesterol-mediated gene transfection. International journal of nanomedicine. 2010;5:371–383. doi: 10.2147/ijn.s10462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tros de Ilarduya C, Sun Y, Duzgunes N. Gene delivery by lipoplexes and polyplexes. European journal of pharmaceutical sciences: official journal of the European Federation for Pharmaceutical Sciences. 2010;40:159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Wang S, Shan B, Sang M, Liu S, Wang G. Advances in viral-vector systemic cytokine gene therapy against cancer. Vaccine. 2010;28:3883–3887. doi: 10.1016/j.vaccine.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Young LS, Searle PF, Onion D, Mautner V. Viral gene therapy strategies: from basic science to clinical application. The Journal of pathology. 2006;208:299–318. doi: 10.1002/path.1896. [DOI] [PubMed] [Google Scholar]
- 5.Tong AW. Small RNAs and non-small cell lung cancer. Current molecular medicine. 2006;6:339–349. doi: 10.2174/156652406776894554. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Siddiq A, Emdad L, Rajasekaran D, Gredler R, Shen XN, Santhekadur PK, Srivastava J, Robertson CL, Dmitriev I, Kashentseva EA, Curiel DT, Fisher PB, Sarkar D. Insulin-like Growth Factor-binding Protein-7 (IGFBP7): A Promising Gene Therapeutic for Hepatocellular Carcinoma (HCC) Molecular therapy: the journal of the American Society of Gene Therapy. 2013 doi: 10.1038/mt.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Zeng X, Lin Y, Yin C, Zhang X, Ning BF, Zhang Q, Zhang JP, Qiu L, Qin XR, Chen YX, Xie WF. Recombinant adenovirus carrying the hepatocyte nuclear factor-1alpha gene inhibits hepatocellular carcinoma xenograft growth in mice. Hepatology. 2011;54:2036–2047. doi: 10.1002/hep.24647. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, Zhang HW, Zhang ZR. Growth inhibition of the pulmonary metastatic tumors by systemic delivery of the p27 kip1 gene using lyophilized lipid-polycation-DNA complexes. The journal of gene medicine. 2009;11:535–544. doi: 10.1002/jgm.1322. [DOI] [PubMed] [Google Scholar]
- 9.Zhao L, Wu J, Zhou H, Yuan A, Zhang X, Xu F, Hu Y. Local gene delivery for cancer therapy. Current gene therapy. 2011;11:423–432. doi: 10.2174/156652311797415854. [DOI] [PubMed] [Google Scholar]
- 10.Shin JY, Lim HT, Minai-Tehrani A, Noh MS, Kim JE, Kim JH, Jiang HL, Arote R, Kim DY, Chae C, Lee KH, Kim MS, Cho MH. Aerosol delivery of beclin1 enhanced the anti-tumor effect of radiation in the lungs of K-rasLA1 mice. Journal of radiation research. 2012;53:506–515. doi: 10.1093/jrr/rrs005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Lang F, Xie X, Prabhu S, Xu J, Sampath D, Aldape K, Fuller G, Puduvalli VK. Efficacy of adenovirally expressed soluble TRAIL in human glioma organotypic slice culture and glioma xenografts. Cell death & disease. 2011;2:e121. doi: 10.1038/cddis.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wojas-Turek J, Pajtasz-Piasecka E, Rossowska J, Piasecki E, Dus D. Antitumor effect of murine dendritic and tumor cells transduced with IL-2 gene, Folia histochemica et cytobiologica/Polish Academy of Sciences. Polish Histochemical and Cytochemical Society. 2012;50:414–419. doi: 10.5603/19750. [DOI] [PubMed] [Google Scholar]
- 13.Zhang HY, Man JH, Liang B, Zhou T, Wang CH, Li T, Li HY, Li WH, Jin BF, Zhang PJ, Zhao J, Pan X, He K, Gong WL, Zhang XM, Li AL. Tumor-targeted delivery of biologically active TRAIL protein. Cancer gene therapy. 2010;17:334–343. doi: 10.1038/cgt.2009.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallaj-Nezhadi S, Lotfipour F, Dass C. Nanoparticle-mediated interleukin-12 cancer gene therapy, Journal of pharmacy & pharmaceutical sciences: a publication of the Canadian Society for Pharmaceutical Sciences. Societe canadienne des sciences pharmaceutiques. 2010;13:472–485. doi: 10.18433/j3630v. [DOI] [PubMed] [Google Scholar]
- 15.Kanaoka E, Takahashi K, Yoshikawa T, Jizomoto H, Nishihara Y, Uchida N, Maekawa R, Hirano K. A significant enhancement of therapeutic effect against hepatic metastases of M5076 in mice by a liposomal interleukin-2 (mixture) Journal of controlled release: official journal of the Controlled Release Society. 2002;82:183–187. doi: 10.1016/s0168-3659(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 16.Dow S, Elmslie R, Kurzman I, MacEwen G, Pericle F, Liggitt D. Phase I study of liposome-DNA complexes encoding the interleukin-2 gene in dogs with osteosarcoma lung metastases. Human gene therapy. 2005;16:937–946. doi: 10.1089/hum.2005.16.937. [DOI] [PubMed] [Google Scholar]
- 17.Salem ML, Gillanders WE, Kadima AN, El-Naggar S, Rubinstein MP, Demcheva M, Vournakis JN, Cole DJ. Review: novel nonviral delivery approaches for interleukin-12 protein and gene systems: curbing toxicity and enhancing adjuvant activity. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2006;26:593–608. doi: 10.1089/jir.2006.26.593. [DOI] [PubMed] [Google Scholar]
- 18.Gnant MF, Berger AC, Huang J, Puhlmann M, Wu PC, Merino MJ, Bartlett DL, Alexander HR, Jr, Libutti SK. Sensitization of tumor necrosis factor alpha-resistant human melanoma by tumor-specific in vivo transfer of the gene encoding endothelial monocyte-activating polypeptide II using recombinant vaccinia virus. Cancer research. 1999;59:4668–4674. [PubMed] [Google Scholar]
- 19.Wajant H. CD95L/FasL and TRAIL in tumour surveillance and cancer therapy. Cancer treatment and research. 2006;130:141–165. doi: 10.1007/0-387-26283-0_7. [DOI] [PubMed] [Google Scholar]
- 20.Yerbes R, Palacios C, Lopez-Rivas A. The therapeutic potential of TRAIL receptor signalling in cancer cells. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2011;13:839–847. doi: 10.1007/s12094-011-0744-4. [DOI] [PubMed] [Google Scholar]
- 21.Volkmann X, Fischer U, Bahr MJ, Ott M, Lehner F, Macfarlane M, Cohen GM, Manns MP, Schulze-Osthoff K, Bantel H. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46:1498–1508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 22.Kolodkin-Gal D, Edden Y, Hartshtark Z, Ilan L, Khalaileh A, Pikarsky AJ, Pikarsky E, Rabkin SD, Panet A, Zamir G. Herpes simplex virus delivery to orthotopic rectal carcinoma results in an efficient and selective antitumor effect. Gene Ther. 2009;16:905–915. doi: 10.1038/gt.2009.44. [DOI] [PubMed] [Google Scholar]
- 23.Rath P, Shi H, Maruniak JA, Litofsky NS, Maria BL, Kirk MD. Stem cells as vectors to deliver HSV/tk gene therapy for malignant gliomas. Current stem cell research & therapy. 2009;4:44–49. doi: 10.2174/157488809787169138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan Y, Zhang S, Wang B, Yang B, Zhi D. The biological routes of gene delivery mediated by lipid-based non-viral vectors. Expert opinion on drug delivery. 2009;6:1351–1361. doi: 10.1517/17425240903287153. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Upponi JR, Torchilin VP. Design of multifunctional non-viral gene vectors to overcome physiological barriers: dilemmas and strategies. International journal of pharmaceutics. 2012;427:3–20. doi: 10.1016/j.ijpharm.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Anchordoquy T. Drug delivery trends in clinical trials and translational medicine: challenges and opportunities in the delivery of nucleic acid-based therapeutics. Journal of pharmaceutical sciences. 2011;100:38–52. doi: 10.1002/jps.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tagalakis AD, Grosse SM, Meng QH, Mustapa MF, Kwok A, Salehi SE, Tabor AB, Hailes HC, Hart SL. Integrin-targeted nanocomplexes for tumour specific delivery and therapy by systemic administration. Biomaterials. 2011;32:1370–1376. doi: 10.1016/j.biomaterials.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Bradshaw-Pierce EL, Delille A, Gustafson DL, Anchordoquy TJ. In vivo comparative study of lipid/DNA complexes with different in vitro serum stability: effects on biodistribution and tumor accumulation. Journal of pharmaceutical sciences. 2008;97:237–250. doi: 10.1002/jps.21076. [DOI] [PubMed] [Google Scholar]
- 29.Mustapa MF, Grosse SM, Kudsiova L, Elbs M, Raiber EA, Wong JB, Brain AP, Armer HE, Warley A, Keppler M, Ng T, Lawrence MJ, Hart SL, Hailes HC, Tabor AB. Stabilized integrin-targeting ternary LPD (lipopolyplex) vectors for gene delivery designed to disassemble within the target cell. Bioconjugate chemistry. 2009;20:518–532. doi: 10.1021/bc800450r. [DOI] [PubMed] [Google Scholar]
- 30.Grosse SM, Tagalakis AD, Mustapa MF, Elbs M, Meng QH, Mohammadi A, Tabor AB, Hailes HC, Hart SL. Tumor-specific gene transfer with receptor-mediated nanocomplexes modified by polyethylene glycol shielding and endosomally cleavable lipid and peptide linkers. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24:2301–2313. doi: 10.1096/fj.09-144220. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Su HH, Yang Y, Hu Y, Zhang L, Blancafort P, Huang L. Systemic delivery of modified mRNA encoding herpes simplex virus 1 thymidine kinase for targeted cancer gene therapy. Molecular therapy: the journal of the American Society of Gene Therapy. 2013;21:358–367. doi: 10.1038/mt.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikegami S, Yamakami K, Ono T, Sato M, Suzuki S, Yoshimura I, Asano T, Hayakawa M, Tadakuma T. Targeting gene therapy for prostate cancer cells by liposomes complexed with anti-prostate-specific membrane antigen monoclonal antibody. Human gene therapy. 2006;17:997–1005. doi: 10.1089/hum.2006.17.997. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Ding Q, Li Y, Miller SA, Abbruzzese JL, Hung MC. Suppression of pancreatic tumor progression by systemic delivery of a pancreatic-cancer-specific promoter driven Bik mutant. Cancer letters. 2006;236:58–63. doi: 10.1016/j.canlet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Heyes J, Palmer L, Chan K, Giesbrecht C, Jeffs L, MacLachlan I. Lipid encapsulation enables the effective systemic delivery of polyplex plasmid DNA. Molecular Therapy. 2007;15:713–720. doi: 10.1038/sj.mt.6300101. [DOI] [PubMed] [Google Scholar]
- 35.Li D, Ping Y, Xu F, Yu H, Pan H, Huang H, Wang Q, Tang G, Li J. Construction of a star-shaped copolymer as a vector for FGF receptor-mediated gene delivery in vitro and in vivo. Biomacromolecules. 2010;11:2221–2229. doi: 10.1021/bm100141y. [DOI] [PubMed] [Google Scholar]
- 36.Patnaik S, Gupta KC. Novel polyethylenimine-derived nanoparticles for in vivo gene delivery. Expert opinion on drug delivery. 2013;10:215–228. doi: 10.1517/17425247.2013.744964. [DOI] [PubMed] [Google Scholar]
- 37.Kircheis R, Wightman L, Schreiber A, Robitza B, Rossler V, Kursa M, Wagner E. Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application. Gene Ther. 2001;8:28–40. doi: 10.1038/sj.gt.3301351. [DOI] [PubMed] [Google Scholar]
- 38.Ogris M, Walker G, Blessing T, Kircheis R, Wolschek M, Wagner E. Tumor-targeted gene therapy: strategies for the preparation of ligand-polyethylene glycol-polyethylenimine/DNA complexes. Journal of Controlled Release. 2003;91:173–181. doi: 10.1016/s0168-3659(03)00230-x. [DOI] [PubMed] [Google Scholar]
- 39.Kursa M, Walker GF, Roessler V, Ogris M, Roedl W, Kircheis R, Wagner E. Novel shielded transferrin-polyethylene glycol-polyethylenimine/DNA complexes for systemic tumor-targeted gene transfer. Bioconjugate chemistry. 2003;14:222–231. doi: 10.1021/bc0256087. [DOI] [PubMed] [Google Scholar]
- 40.Wolschek MF, Thallinger C, Kursa M, Rossler V, Allen M, Lichtenberger C, Kircheis R, Lucas T, Willheim M, Reinisch W, Gangl A, Wagner E, Jansen B. Specific systemic nonviral gene delivery to human hepatocellular carcinoma xenografts in SCID mice. Hepatology. 2002;36:1106–1114. doi: 10.1053/jhep.2002.36372. [DOI] [PubMed] [Google Scholar]
- 41.Dufes C, Uchegbu IF, Schatzlein AG. Dendrimers in gene delivery. Adv Drug Deliver Rev. 2005;57:2177–2202. doi: 10.1016/j.addr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Chisholm EJ, Vassaux G, Martin-Duque P, Chevre R, Lambert O, Pitard B, Merron A, Weeks M, Burnet J, Peerlinck I, Dai MS, Alusi G, Mather SJ, Bolton K, Uchegbu IF, Schatzlein AG, Baril P. Cancer-specific transgene expression mediated by systemic injection of nanoparticles. Cancer research. 2009;69:2655–2662. doi: 10.1158/0008-5472.CAN-08-2657. [DOI] [PubMed] [Google Scholar]
- 43.Russ V, Gunther M, Halama A, Ogris M, Wagner E. Oligoethylenimine-grafted polypropylenimine dendrimers as degradable and biocompatible synthetic vectors for gene delivery. Journal of controlled release: official journal of the Controlled Release Society. 2008;132:131–140. doi: 10.1016/j.jconrel.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 44.Dufes C, Keith WN, Bilsland A, Proutski I, Uchegbu IF, Schatzlein AG. Synthetic anticancer gene medicine exploits intrinsic antitumor activity of cationic vector to cure established tumors. Cancer research. 2005;65:8079–8084. doi: 10.1158/0008-5472.CAN-04-4402. [DOI] [PubMed] [Google Scholar]
- 45.Koppu S, Oh YJ, Edrada-Ebel R, Blatchford DR, Tetley L, Tate RJ, Dufes C. Tumor regression after systemic administration of a novel tumor-targeted gene delivery system carrying a therapeutic plasmid DNA. Journal of Controlled Release. 2010;143:215–221. doi: 10.1016/j.jconrel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 46.Navarro G, Maiwald G, Haase R, Rogach AL, Wagner E, de Ilarduya CT, Ogris M. Low generation PAMAM dendrimer and CpG free plasmids allow targeted and extended transgene expression in tumors after systemic delivery. Journal of controlled release: official journal of the Controlled Release Society. 2010;146:99–105. doi: 10.1016/j.jconrel.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 47.Cihova M, Altanerova V, Altaner C. Stem cell based cancer gene therapy. Mol Pharm. 2011;8:1480–1487. doi: 10.1021/mp200151a. [DOI] [PubMed] [Google Scholar]
- 48.Uchibori R, Tsukahara T, Mizuguchi H, Saga Y, Urabe M, Mizukami H, Kume A, Ozawa K. NF-kappaB activity regulates mesenchymal stem cell accumulation at tumor sites. Cancer research. 2013;73:364–372. doi: 10.1158/0008-5472.CAN-12-0088. [DOI] [PubMed] [Google Scholar]
- 49.Uchibori R, Okada T, Ito T, Urabe M, Mizukami H, Kume A, Ozawa K. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. The journal of gene medicine. 2009;11:373–381. doi: 10.1002/jgm.1313. [DOI] [PubMed] [Google Scholar]
- 50.Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer research. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 51.Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. Journal of the National Cancer Institute. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 52.Wolf D, Rumpold H, Koeck R, Gunsilius E. Re: Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. Journal of the National Cancer Institute. 2005;97:540–541. doi: 10.1093/jnci/dji088. author reply 541-542. [DOI] [PubMed] [Google Scholar]
- 53.Xin H, Kanehira M, Mizuguchi H, Hayakawa T, Kikuchi T, Nukiwa T, Saijo Y. Targeted delivery of CX3CL1 to multiple lung tumors by mesenchymal stem cells. Stem Cells. 2007;25:1618–1626. doi: 10.1634/stemcells.2006-0461. [DOI] [PubMed] [Google Scholar]
- 54.Duan X, Guan H, Cao Y, Kleinerman ES. Murine bone marrow-derived mesenchymal stem cells as vehicles for interleukin-12 gene delivery into Ewing sarcoma tumors. Cancer. 2009;115:13–22. doi: 10.1002/cncr.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xiang J, Tang J, Song C, Yang Z, Hirst DG, Zheng QJ, Li G. Mesenchymal stem cells as a gene therapy carrier for treatment of fibrosarcoma. Cytotherapy. 2009;11:516–526. doi: 10.1080/14653240902960429. [DOI] [PubMed] [Google Scholar]
- 56.Kirn D, Niculescu-Duvaz I, Hallden G, Springer CJ. The emerging fields of suicide gene therapy and virotherapy. Trends in molecular medicine. 2002;8:S68–73. doi: 10.1016/s1471-4914(02)02318-3. [DOI] [PubMed] [Google Scholar]
- 57.Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, Wu L, Chen IS. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nature medicine. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 58.Puhlmann M, Brown CK, Gnant M, Huang J, Libutti SK, Alexander HR, Bartlett DL. Vaccinia as a vector for tumor-directed gene therapy: biodistribution of a thymidine kinase-deleted mutant. Cancer gene therapy. 2000;7:66–73. doi: 10.1038/sj.cgt.7700075. [DOI] [PubMed] [Google Scholar]
- 59.Gnant MF, Noll LA, Irvine KR, Puhlmann M, Terrill RE, Alexander HR, Jr, Bartlett DL. Tumor-specific gene delivery using recombinant vaccinia virus in a rabbit model of liver metastases. Journal of the National Cancer Institute. 1999;91:1744–1750. doi: 10.1093/jnci/91.20.1744. [DOI] [PubMed] [Google Scholar]
- 60.Gnant MF, Puhlmann M, Bartlett DL, Alexander HR., Jr Regional versus systemic delivery of recombinant vaccinia virus as suicide gene therapy for murine liver metastases. Annals of surgery. 1999;230:352–360. doi: 10.1097/00000658-199909000-00008. discussion 360-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veerapong J, Bickenbach KA, Shao MY, Smith KD, Posner MC, Roizman B, Weichselbaum RR. Systemic delivery of (gamma1)34.5-deleted herpes simplex virus-1 selectively targets and treats distant human xenograft tumors that express high MEK activity. Cancer research. 2007;67:8301–8306. doi: 10.1158/0008-5472.CAN-07-1499. [DOI] [PubMed] [Google Scholar]
- 62.Han X, Kasahara N, Kan YW. Ligand-directed retroviral targeting of human breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:9747–9751. doi: 10.1073/pnas.92.21.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 64.Resnier P, Montier T, Mathieu V, Benoit JP, Passirani C. A review of the current status of siRNA nanomedicines in the treatment of cancer. Biomaterials. 2013;34:6429–6443. doi: 10.1016/j.biomaterials.2013.04.060. [DOI] [PubMed] [Google Scholar]
- 65.Bora RS, Gupta D, Mukkur TK, Saini KS. RNA interference therapeutics for cancer: challenges and opportunities (review) Molecular medicine reports. 2012;6:9–15. doi: 10.3892/mmr.2012.871. [DOI] [PubMed] [Google Scholar]
- 66.Gao J, Yu Y, Zhang Y, Song J, Chen H, Li W, Qian W, Deng L, Kou G, Chen J, Guo Y. EGFR-specific PEGylated immunoliposomes for active siRNA delivery in hepatocellular carcinoma. Biomaterials. 2012;33:270–282. doi: 10.1016/j.biomaterials.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 67.Gomes-da-Silva LC, Santos AO, Bimbo LM, Moura V, Ramalho JS, Pedroso de Lima MC, Simoes S, Moreira JN. Toward a siRNA-containing nanoparticle targeted to breast cancer cells and the tumor microenvironment. International journal of pharmaceutics. 2012;434:9–19. doi: 10.1016/j.ijpharm.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 68.Xiang B, Dong DW, Shi NQ, Gao W, Yang ZZ, Cui Y, Cao DY, Qi XR. PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials. 2013;34:6976–6991. doi: 10.1016/j.biomaterials.2013.05.055. [DOI] [PubMed] [Google Scholar]
- 69.Shu Y, Haque F, Shu D, Li W, Zhu Z, Kotb M, Lyubchenko Y, Guo P. Fabrication of 14 different RNA nanoparticles for specific tumor targeting without accumulation in normal organs. Rna. 2013;19:767–777. doi: 10.1261/rna.037002.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lonez C, Vandenbranden M, Ruysschaert JM. Cationic lipids activate intracellular signaling pathways. Adv Drug Deliv Rev. 2012;64:1749–1758. doi: 10.1016/j.addr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. Journal of immunology. 2011;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]