Abstract
BACKGROUND
In patients with severe hemophilia B, gene therapy that is mediated by a novel self-complementary adeno-associated virus serotype 8 (AAV8) vector has been shown to raise factor IX levels for periods of up to 16 months. We wanted to determine the durability of transgene expression, the vector dose–response relationship, and the level of persistent or late toxicity.
METHODS
We evaluated the stability of transgene expression and long-term safety in 10 patients with severe hemophilia B: 6 patients who had been enrolled in an initial phase 1 dose-escalation trial, with 2 patients each receiving a low, intermediate, or high dose, and 4 additional patients who received the high dose (2×1012 vector genomes per kilogram of body weight). The patients subsequently underwent extensive clinical and laboratory monitoring.
RESULTS
A single intravenous infusion of vector in all 10 patients with severe hemophilia B resulted in a dose-dependent increase in circulating factor IX to a level that was 1 to 6% of the normal value over a median period of 3.2 years, with observation ongoing. In the high-dose group, a consistent increase in the factor IX level to a mean (±SD) of 5.1±1.7% was observed in all 6 patients, which resulted in a reduction of more than 90% in both bleeding episodes and the use of prophylactic factor IX concentrate. A transient increase in the mean alanine aminotransferase level to 86 IU per liter (range, 36 to 202) occurred between week 7 and week 10 in 4 of the 6 patients in the high-dose group but resolved over a median of 5 days (range, 2 to 35) after prednisolone treatment.
CONCLUSIONS
In 10 patients with severe hemophilia B, the infusion of a single dose of AAV8 vector resulted in long-term therapeutic factor IX expression associated with clinical improvement. With a follow-up period of up to 3 years, no late toxic effects from the therapy were reported. (Funded by the National Heart, Lung, and Blood Institute and others; ClinicalTrials.gov number, NCT00979238.)
Hemophilia B, an X-linked recessive bleeding disorder, results from a defect in the gene encoding coagulation factor IX, a serine protease that is critical for blood clotting. Patients with functional plasma levels of factor IX that are less than 1% of the normal value (1 IU per deciliter) have a severe phenotype characterized by frequent spontaneous bleeding episodes that result in chronic, debilitating arthropathy and occasionally death.1 Current treatment to prevent these bleeding episodes entails lifelong intravenous injections of factor IX every 2 or 3 days. Although this treatment is effective in preventing spontaneous bleeding episodes, it is not curative and is invasive and inconvenient. In addition, the prophylactic administration of clotting factor concentrate, on average, costs approximately $250,000 per year, which is not affordable for most patients with hemophilia, resulting in a reduction in life expectancy for those with a severe bleeding phenotype.2 Novel clotting formulations with longer half-lives represent a major advance but still require lifelong intravenous administration at a high financial cost.3
In contrast, somatic gene therapy for hemophilia B offers the potential for cure through persistent, endogenous production of factor IX after the transfer of a normal copy of the factor IX gene. Hemophilia B is an ideal target for gene therapy, especially since a small increase in plasma factor IX levels above 1% of physiologic levels substantially ameliorates the severe bleeding phenotype. In a variety of animal models,4–6 vectors that are based on adeno-associated virus (AAV), a nonpathogenic parvovirus, have shown the greatest promise for gene therapy in patients with hemophilia B, largely because of the ability of these vectors to mediate long-term expression of factor IX at therapeutic levels after a single infusion.4–6 However, in two early trials of hemophilia B gene therapy that used either intramuscular or liver-targeted delivery of AAV factor IX vectors based on AAV serotype 2, investigators did not achieve stable expression of factor IX in the plasma of patients with severe hemophilia B.7,8 In addition, liver toxicity was observed in patients in the liver-targeted study, an adverse event that may have been due to the activation of capsid-specific T cells after the infusion of the high vector dose.8
On the basis of these results, we developed a variation of this approach that involves a single intravenous infusion of a novel serotype 8 pseudo-typed, self-complementary AAV (AAV8) vector expressing a codon-optimized factor IX transgene (scAAV2/8-LP1-hFIXco) and tested it in a phase 1 clinical trial in six patients with severe hemophilia B.9–12 Factor IX expression at 1 to 6% of the normal value was established in all six patients. Asymptomatic, transient elevations in serum liver enzymes, possibly as a result of a cellular immune response to the AAV8 capsid, were observed in the two patients who received the high dose of the vector. This complication had not been observed in animal models.12
Here, we report on the durability of expression of the therapeutic transgene and long-term clinical outcomes of AAV-mediated gene transfer for the treatment of hemophilia B. In addition, we describe the consistency of the dose–response relationship in an expanded high-dose cohort, as well as the incidence and kinetics of elevated liver enzymes. We also report on late toxic events to date in this small subset of patients.
METHODS
STUDY PATIENTS
A total of 10 men with severe hemophilia B each received a single dose of the scAAV2/8-LP1-hFIXco vector, administered through a peripheral vein, according to the trial protocol.9 The eligibility criteria and the plan for study investigations are outlined in Tables S1 and S2, respectively, in the Supplementary Appendix (available with the full text of this article at NEJM.org). The absence of detectable antibodies against AAV8 capsid protein was a critical enrollment criterion.9 (See the Supplementary Appendix for details.)
Two separate lots of scAAV2/8-LP1-hFIXco vector were manufactured by Children’s GMP in Memphis, Tennessee, and were characterized, as described previously.9–11,13 The vector titer was determined by means of a recently described gelbased assay.14 In the initial study group, six patients were sequentially enrolled between March 2010 and January 2011 to receive one of three vector doses—a low dose (2×1011 vector genomes [vg] per kilogram of body weight), an intermediate dose (6×1011 vg per kilogram), or a high dose (2×1012 vg per kilogram) — with two patients in each dose cohort. The early results for these patients have been reported previously.9 The next four patients were enrolled between February 2012 and December 2012; each received the high dose. All the patients provided written informed consent.
SAFETY AND EFFICACY ASSESSMENTS
After vector infusion, we monitored plasma factor IX activity, vector shedding, and humoral and cellular immune responses against capsid and factor IX, in addition to performing routine laboratory studies.8,15 We used a standard, one-stage assay of the activated partial-thromboplastin time to assess factor IX coagulation activity and an immunoassay to measure antigen levels. (Details about these assays are provided in the Supplementary Appendix.) Using validated records of patients, we calculated the annual number of bleeding episodes and the amount of factor IX concentrate that was administered as prophylactic therapy.
STUDY OUTCOMES
The primary outcome of the study was the safety of vector infusion at the three different doses. We assessed efficacy (a secondary outcome) by analyzing three measurements: factor IX activity (as measured at least 10 days after the last dose of factor IX concentrate), the annual number of bleeding episodes, and the annual amount of factor IX concentrate that was administered.
STATISTICAL ANALYSIS
We used one-sample Wilcoxon signed-rank tests to determine whether there was a significant difference in the distributions of the annual number of bleeding episodes and the use of factor IX concentrate 1 year before gene transfer, as compared with 1 year after gene transfer. The analyses were restricted to data from these time periods to ensure that data would be complete for all patients, since the majority of patients who were treated in the high-dose cohort had been followed for just over 1 year. However, we report a summary of the annual number of bleeding episodes and factor IX use over a longer follow-up period (up to 3 years) for the six patients in the initial dose-escalation cohort.
RESULTS
PATIENTS
Of the 10 patients with severe hemophilia B who were enrolled in the study, all had negative test results for human immunodeficiency virus (Table 1). All the patients except Patient 7 had evidence of serologic immunity to hepatitis B surface antigen after vaccination. All the patients except Patients 1, 4, and 6 had evidence of previous infection with hepatitis C virus (HCV), on the basis of testing that was positive for HCV antibodies but negative for HCV RNA.
Table 1.
Characteristics of the Patients at Baseline and after Gene Transfer, According to Vector Dose.*
| Variable | Low Dose | Intermediate Dose | High Dose | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | |
| At baseline | ||||||||||
| Mean age — yr | 31 | 64 | 43 | 29 | 32 | 27 | 22 | 38 | 44 | 33 |
| Factor IX mutation | 31280 G→A E387K |
2-bp del frameshift |
30097 G→T W215C |
31290 G→A A309T |
20518 C→T R180W |
−52 del C | 3-bp del frameshift |
1277 C→T T426I |
698 C→A A233D |
385 G→T G129X |
| Cross-reacting material status† | + | − | + | + | + | − | − | + | + | − |
| Factor IX prophylaxis | Twice weekly |
Twice weekly |
Twice weekly |
Once weekly |
Twice weekly |
Three times weekly |
Once weekly |
On demand |
On demand |
On demand |
| Infection markers | ||||||||||
| Hepatitis B surface antigen/ antibody | −/+ | −/+ | −/+ | −/+ | −/+ | −/+ | −/− | −/+ | −/+ | −/+ |
| HIV antibody | − | − | − | − | − | − | − | − | − | − |
| Hepatitis C antibody | − | + | + | − | + | − | + | + | + | + |
| Hepatitis C RNA | − | − | − | − | − | − | − | − | − | − |
| Anti-AAV8 IgG antibody titers — relative units | 1 | 12 | 37 | 1 | 5 | 8 | 1 | 6 | 6 | 5 |
| Anti-AAV2 IgG antibody titers — relative units | 5 | 20 | 77 | 12 | 10 | 22 | 22 | 39 | 16 | 9 |
| After gene transfer | ||||||||||
| Steady-state factor IX activity — % of normal value‡ | 2.17±0.83 | 1.4±0.3 | 2.86±1.23 | 2.17±0.72 | 3.56±1.65 | 7.21±2.92 | 5.00±0.63 | 6.67±1.50 | 5.23±1.54 | 2.89±1.62 |
| Steady-state factor IX antigen levels in patients with negative results for cross-reacting material — % of normal value‡ | NA | 1.1±0.4 | NA | NA | NA | 5.5±0.5 | 4.13±0.7 | NA | NA | 2.3±0.9 |
| Elevation in ALT (wk after infusion) | No | No | No | No | Yes (wk 8) |
Yes (wk 9) |
Yes (wk 7) |
No | No | Yes (wk 9) |
| Presence of factor IX neutralizing antibodies | No | No | No | No | No | No | No | No | No | No |
| Follow-up period (mo) | 48 | 44 | 42 | 41 | 40 | 39 | 24 | 22 | 20 | 16 |
| Annual use of factor IX concentrate | ||||||||||
| Before gene transfer — IU/kg | 3608§ | 4284 § | 2486 § | 1515 § | 2678 § | 2509 § | 5719 ¶ | 3130 § | 1367 ¶ | 1714¶ |
| After gene transfer — IU/kg (% reduction) | 683 (81)‖ | 3000 (30)‖ | 1835 (26)‖ | 143 (91)‖ | 2090 (22)‖ | 0 (100)‖ | 89 (98)¶ | 95 (97) ¶ | 50 (96) ¶ | 241 (86)¶ |
| Annual bleeding episodes | ||||||||||
| Before gene transfer — no. | 3§ | 13§ | 12§ | 12§ | 15‖ | 3‖ | 22¶ | 20¶ | 36¶ | 29¶ |
| After gene transfer — no. (% reduction) | 1 (63)‖ | 7(46)‖ | 19 (158)‖ | 2 (87)‖ | 10 (34)‖ | 0 (100)‖ | 1 (95)¶ | 1 (95)¶ | 1 (98)¶ | 2 (93)¶ |
Plus–minus values are means ±SD. A plus sign indicates positive results, and a minus sign negative results. AAV denotes adeno-associated virus, ALT alanine aminotransferase, del deletion, HIV human immunodeficiency virus, and NA not applicable.
Patients who have positive results for cross-reacting material have near-normal levels (at least 30%) of dysfunctional protein in their plasma. Factor IX antigen is not detectable in patients who have negative results for cross-reacting material.
Steady-state factor IX activity and antigen levels were defined as the mean of all levels measured from 4 months after gene transfer through the most recent follow-up assessment, with the latter measurement performed at least 10 days after the most recent administration of factor IX concentrate.
This value is the mean for 2 years.
This value is the mean for 1 year.
This value is the mean for 3 years.
The systemic infusion of scAAV2/8-LP1-hFIXco was not associated with clinically significant changes in pulse, respiration rate, blood pressure, or temperature either during or after vector infusion (Fig. S1 in the Supplementary Appendix), despite the presence of the vector genome in saliva, urine, semen, stool, or plasma for up to 6 weeks after gene transfer (Fig. S2 in the Supplementary Appendix). None of the patients reported any new symptoms during or after vector administration, including symptoms that are typically associated with viral infection (e.g., fever, sweating, muscle ache, abdominal pain, and diarrhea).
FACTOR IX ACTIVITY
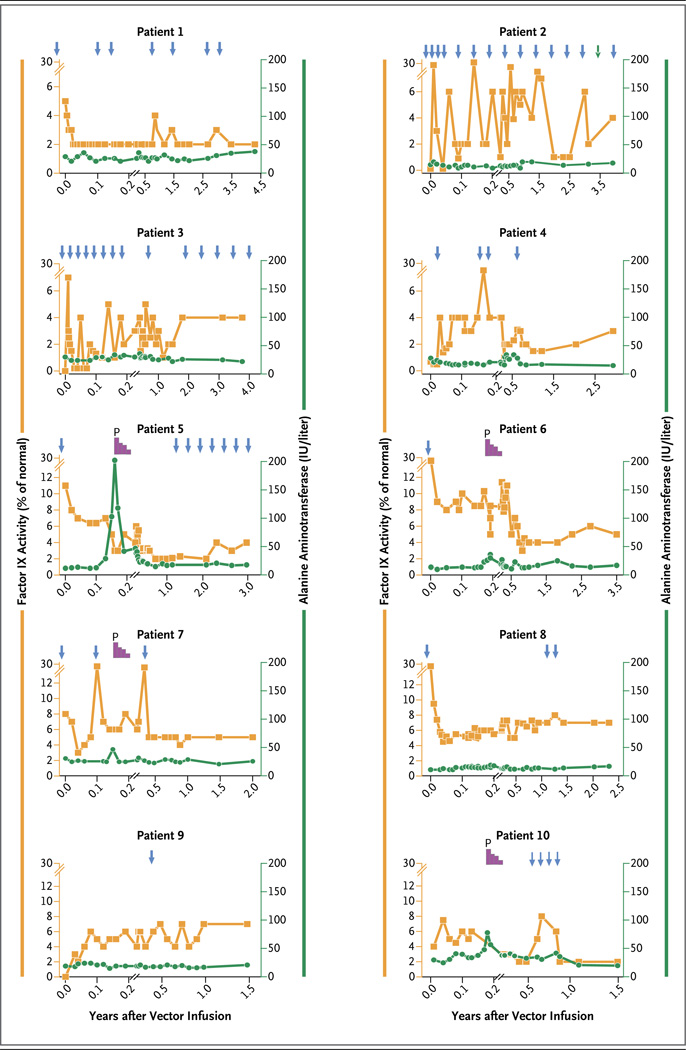
Steady-state transgenic factor IX activity of 1 to 6% of the normal value was reached within 4 months after vector infusion and remained stable for a median of 3.2 years (range, 1.5 to 4.3) (Fig. 1). The increase in transgenic factor IX activity appeared to be vector dose-dependent, with a mean (±SD) level of factor IX coagulation activity that was approximately 1.8±0.7% of the normal value in the low-dose cohort and that increased to 2.5±0.9% in the intermediate-dose cohort. At least 1 year after receiving the vector infusion, the six patients in the high-dose group had a doubling in the steady-state plasma level of factor IX (5.1±1.7%) (Table 1). The ratio of factor IX antigen to factor IX coagulation activity was 1:1 in four patients (Patients 2, 6, 7, and 10) who had a factor IX gene mutation that resulted in no production of endogenous factor IX, thus permitting accurate assessment of the level of transgenic factor IX antigen (Table 1). This finding suggested that endogenously expressed factor IX was biologically active. Of the seven patients who were receiving factor IX concentrate prophylactically at the time of vector infusion, four (Patients 1, 4, 6, and 7) were able to discontinue therapy without having spontaneous bleeding. In contrast, Patient 2 (in the low-dose group) and Patient 3 (in the intermediate-dose group) were not able to stop prophylaxis after vector infusion because of severe preexisting hemophilic arthropathy. When these patients were assessed at a minimum of 10 days after the administration of factor IX concentrate, factor IX levels were consistently at 1% in Patient 2 approximately 2 years after gene transfer and ranged from 1 to 3% in Patient 3. Collectively, these data suggest that there was endogenous synthesis of factor IX in these two patients after gene transfer. In Patient 5, the first patient enrolled in the high-dose group, prophylaxis with factor IX concentrate was reinitiated because of recurrent episodes of bleeding in his hands and knees approximately 1 year after gene transfer. However, his factor IX level without prophylaxis was 2% at 3 years after gene transfer.
Figure 1. Summary of Factor IX Activity and Liver Function after Vector Infusion in 10 Patients with Hemophilia B.
The mean (±SD) levels of factor IX coagulation activity were determined at the indicated time points with the use of a one-stage clotting assay after the infusion of a low dose of vector (in Patients 1 and 2), an intermediate dose (in Patients 3 and 4), or a high dose (in Patients 5 through 10), as shown on the left y axis. The arrows at the top of the graphs show the time points for treatment with factor IX concentrate. The decline in plasma factor IX activity that was observed in some patients (i.e., Patients 1, 5, 6, 7, and 8) in the initial phase after gene transfer probably represents a washout of the factor IX concentrate from the circulation. Levels of serum alanine aminotransferase (ALT) over time are shown on the right y axis. The duration of treatment with prednisolone (P), starting at a dose of 60 mg per day that was followed by a gradual tapering of the dose, is shown in purple at the top of the graphs for Patients 5, 6, 7, and 10. The left y axis has been adjusted for the patients in the high-dose group to reflect higher factor IX levels in these patients. The x axis has been adjusted to show with greater detail the kinetics of elevations in ALT levels in these patients.
FACTOR IX PROPHYLAXIS AND BLEEDING EPISODES
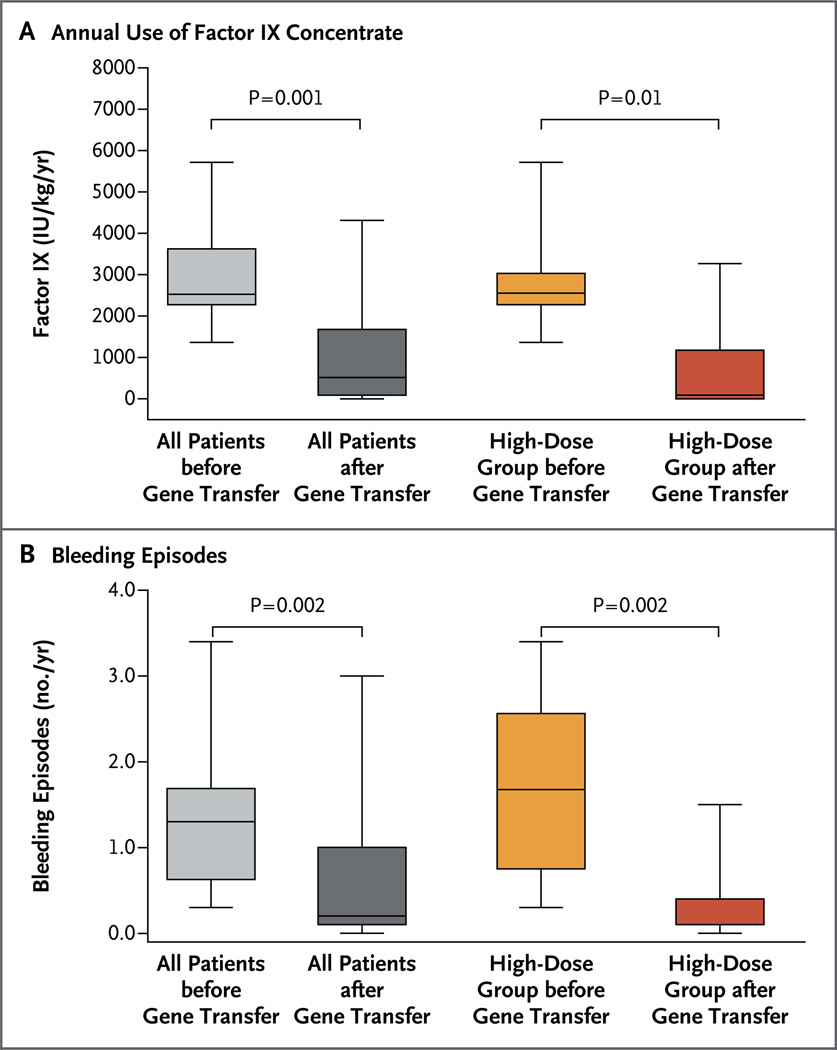
The annual amount of factor IX concentrate that was administered to all 10 patients dropped from a median of 2613 IU per kilogram (interquartile range, 1671 to 4513) in the year before vector infusion to 206 IU per kilogram (interquartile range, 79 to 948) in the year after vector infusion (P = 0.002), a relative reduction of approximately 92% (Fig. 2A). In the high-dose group, the amount of factor IX concentrate that was used dropped from a median of 2613 IU per kilogram (interquartile range, 1627 to 3487) to 92 IU per kilogram (interquartile range, 38 to 395), a relative reduction of 96% (P = 0.03). Despite this decline in factor use, the annual number of bleeding episodes for the entire cohort of 10 patients decreased from a median of 15.5 bleeding episodes (interquartile range, 10.3 to 19.3) 1 year before vector infusion to 1.5 episodes (interquartile range, 1.0 to 4.0) in the year after vector infusion, a relative reduction of 90% (P = 0.009) (Fig. 2B). When only patients in the high-dose group were considered, the annual number of bleeding episodes decreased from a median of 16.5 episodes (interquartile range, 12.5 to 27.0) to 1.0 episode (interquartile range, 0.8 to 2.5), a relative reduction of 94% (P = 0.03).
Figure 2. Effect of Gene Transfer on the Administration of Factor IX Concentrate and the Number of Bleeding Episodes before and after Gene Transfer.
Shown are box plots comparing the year before gene transfer and the year after gene transfer with respect to the mean amount of factor IX concentrate that was administered (Panel A) and the number of bleeding episodes (Panel B) in the 10 study patients. The top and bottom of each box represent the interquartile range, the horizontal line within the box represents the median (with the absence of a horizontal line indicating a median >10 times the 99th percentile), and the I bars represent the minimum and maximum values.
ADVERSE EVENTS
A total of 128 adverse events were reported in the 10 patients after vector infusion, with 110 of these events (86%) judged to be unrelated to the study agent (Table 2). The majority of adverse events (125 events [98%]) were mild in severity (grade 1), according to the National Cancer Institute Common Terminology Criteria for Adverse Events (see the Results section in the Supplementary Appendix). The most common study-related adverse event was an asymptomatic elevation in the alanine aminotransferase (ALT) level, which occurred approximately 7 to 10 weeks after vector infusion in 4 of the 6 patients in the high-dose group (Table 3). Each of these patients received a tapering dose of prednisolone, starting at 60 mg per day, which resulted in resolution of the elevated ALT level over a median period of 5 days (range, 2 to 35). Subsequently, factor IX levels were reduced by 50 to 70%, as compared with values before the onset of the elevated ALT level, especially among patients for whom more than 2 days had elapsed from the increase in ALT to the initiation of treatment with prednisolone. No patient had a recurrent episode of an elevated ALT level.
Table 2.
Summary of Adverse Events.*
| Event | No. of Events | Grade | Relation to Study Agent |
|---|---|---|---|
| Constitutional | |||
| Lethargy | 1 | 1 | Possible |
| Skin | |||
| Acne | 1 | 1 | Unlikely |
| Infected hair follicle | 1 | 1 | Unlikely |
| Infection | |||
| Upper respiratory tract infection | 5 | 1 | Unlikely |
| Staphylococcus septic arthritis | 1 | 4 | Unlikely |
| Respiratory | |||
| Cough | 1 | 1 | Unlikely |
| Hay fever | 2 | 1 | Unlikely |
| Sore throat | 1 | 1 | Unlikely |
| Cardiovascular | |||
| Supraventricular tachycardia | 1 | 4 | Unlikely |
| High blood pressure | 1 | 1 | Unlikely |
| Gastrointestinal | |||
| Nausea | 1 | 1 | Unlikely |
| Heartburn | 1 | 1 | Unlikely |
| Mouth ulcer | 1 | 1 | Unlikely |
| Stomach cramps | 1 | 1 | Unlikely |
| Diarrhea | 1 | 1 | Unlikely |
| Bleeding hemorrhoid | 1 | 1 | Unlikely |
| Hepatobiliary | |||
| Hyperbilirubinemia (Gilbert’s syndrome) | 1 | 1 | Unlikely |
| Increase in alanine aminotransferase† | 1 | 3 | Related |
| Increase in alanine aminotransferase† | 1 | 2 | Related |
| Increase in alanine aminotransferase† | 4 | 1 | Related |
| Genitourinary | |||
| Nocturia | 1 | 1 | Unlikely |
| Increase in serum creatinine | 3 | 1 | Unlikely |
| Hematuria | 3 | 1 | Unlikely |
| General hematologic | |||
| Leukocytosis | 7 | 1 | Unlikely |
| Anemia | 11 | 1 | Possible |
| Thrombocytosis | 6 | 1 | Unlikely |
| Neutropenia | 10 | 2 | Unlikely |
| Thrombocytopenia | 10 | 1 | Unlikely |
| Bleeding in joint or soft tissue | |||
| Finger | 3 | 1 | Unlikely |
| Wrist | 2 | 1 | Unlikely |
| Elbow | 7 | 1 | Unlikely |
| Shoulder | 7 | 1 | Unlikely |
| Hip | 2 | 1 | Unlikely |
| Knee | 11 | 1 | Unlikely |
| Ankle | 7 | 1 | Unlikely |
| Soft tissue (once in thigh and once in calf) | 2 | 1 | Unlikely |
| Musculoskeletal | |||
| Back pain | 1 | 1 | Unlikely |
| Joint pain | 4 | 1 | Unlikely |
| Toe fracture | 1 | 1 | Unlikely |
| Metabolic | |||
| Hyperkalemia | 2 | 1 | Unlikely |
The grade of the adverse event was determined according to the modified, symptom-specific National Cancer Institute Common Terminology Criteria for Adverse Events. The trial steering committee determined the likelihood of whether the adverse event was related to the study agent.
Increases in alanine aminotransferase levels are listed separately for three patients because of different grades of toxicity.
Table 3.
Peak ALT Levels in the High-Dose Cohort.*
| Patient No. |
Average ALT Level at Baseline† |
Peak ALT Level |
Time from Baseline to Peak ALT Level |
Time from First Increase in ALT Level to Initiation of Prednisolone |
Time from Initiation of Prednisolone to Normalization of ALT Level |
Total Duration of Prednisolone Use |
Relative Reduction in Factor IX Level after an Elevated ALT Level‡ |
|---|---|---|---|---|---|---|---|
| IU/liter | day (wk) | days | wk | % | |||
| 5 | 13 | 202 | 58 (8) | 7 | 35 | 12 | 70 |
| 6 | 13 | 36 | 69 (9) | 5 | 2 | 8 | 54 |
| 7 | 29 | 43 | 55 (7) | 2 | 2 | 8 | 0 |
| 8 | 13 | 17 | 47 (6) | NA | NA | NA | NA |
| 9 | 17 | 20 | 63 (9) | NA | NA | NA | NA |
| 10 | 32 | 64 | 66 (9) | 7 | 7 | 8 | 50 |
The upper limit of the normal range for alanine aminotransferase (ALT) was 45 IU per liter. NA denotes not applicable.
The baseline ALT level was measured during the period from 6 weeks before gene transfer to 6 weeks after gene transfer.
Shown is the relative reduction as compared with the factor IX level 4 to 6 weeks after gene transfer.
Although elevated ALT levels were observed after vector infusion only in the high-dose group, an increase in the number of AAV8 capsid−reactive T cells was detectable in peripheral blood within 3 months after the infusion of the intermediate dose and high dose of vector, as reported previously.9 Long-term immune monitoring of some of these patients also showed the presence of capsid-reactive T cells in peripheral blood at late time points (>1 year after infusion) on enzyme-linked immunosorbent spot (ELISPOT) assay, but this finding was not associated with an elevation in liver enzyme levels (Fig. S3A in the Supplementary Appendix). The same ELISPOT assay showed T-cell reactivity in three of the four additional patients in the high-dose group, including Patient 8, in whom there was no increase in the ALT level at any time after gene transfer (Fig. S3B in the Supplementary Appendix). Subtle differences in capsid-specific T-cell subsets were observed in various patients, a finding suggesting that more proinflammatory cytokines were produced in the patient with an elevated ALT level (Patient 10) than in the patient without such an elevation (Patient 8) (Fig. S4 in the Supplementary Appendix).
No increase in levels of neutralizing antibodies against factor IX was observed in any of the 10 study patients (data not shown). In contrast, anti-AAV8 IgG antibody titers increased over time, from a baseline mean value of 11±6 relative units per milliliter to a peak mean value of 387±175 relative units per milliliter at a median of 13 weeks (range, 3 to 27) (Fig. S5 in the Supplementary Appendix). These antibody titers remained at high levels for at least 12 months after vector infusion. In comparison, there were only small increases in anti-AAV IgG antibody titers against most of the other serotypes of AAV that were tested after vector infusion (Table S3 and Fig. S4 in the Supplementary Appendix). These findings suggest that there was minimal cross-reactivity between anti-AAV8 antibodies and epitopes on the capsid of some of the other serotypes.
DISCUSSION
In this study involving 10 men with severe hemophilia B, the systemic infusion of AAV8 vector containing a codon-optimized factor IX transgene resulted in long-term endogenous factor IX expression at therapeutic levels. A single intravenous infusion of scAAV2/8-LP1-hFIXco resulted in an increase in plasma factor IX activity from baseline values that ranged from less than 1% of the normal value to steady-state levels of 1 to 6% of the normal value in all 10 patients. Among patients receiving a low or intermediate dose of vector, the increase in factor IX activity was modest (to 1 to 3% of the normal value). In contrast, remarkably consistent factor IX expression (mean, 5.1±1.7% of the normal value) was observed in the 6 patients receiving the high dose of vector. AAV8-mediated factor IX expression remained relatively stable over a period of up to 4 years, in keeping with the stability of transgene expression in animal models, including nonhuman primates.12 Moreover, long-term follow-up suggests that episodes of elevated ALT levels have not recurred and that there have been no new late toxic events.
Of the seven patients who were receiving prophylaxis with factor IX concentrate before gene transfer, four (Patients 1, 4, 6, and 7) were able to stop regular factor IX replacement therapy, and most of the others were able to increase the interval between prophylactic infusions. The overall reduction in the amount of factor IX concentrate that was administered over the duration of the study was more than 3 million units, resulting in a financial savings of more than $2.5 million based on 2014 prices. The ultimate savings cannot currently be calculated, since the cost of a single dose of vector has not been determined and questions remain about whether the dose might ultimately need to be repeated. Despite the reduction in the use of factor IX concentrate, the average annual number of bleeding episodes was consistently lower after gene transfer, most notably in patients in the high-dose cohort, for whom the mean steady-state factor IX expression was around 5% of the normal value. This reduction in the number of annual bleeding episodes occurred despite higher overall physical activity levels reported by all patients, including participation in sports activities that had previously been associated with bleeding. This improvement in the bleeding phenotype among patients in the high-dose cohort is consistent with the natural bleeding tendency in patients with mild hemophilia who have plasma factor IX levels of 5 to 40%. Such patients have either no or very few spontaneous bleeding episodes but are at risk for excessive hemorrhage after trauma or surgery.16
As expected, long-lasting AAV8 capsid-specific humoral immunity developed in all the patients who underwent gene transfer. Although the increase in anti-AAV8 IgG levels did not have direct clinical consequences as far as we can tell, its persistence at high titers would preclude subsequent gene transfer with vector of the same serotype if transgene expression fell below therapeutic levels. However, preclinical studies suggest that it may be possible to achieve successful transduction in patients with preexisting anti-AAV8 antibodies after the infusion of AAV vectors pseudotyped with alternative serotypes, such as AAV5, that have low cross-reactivity with anti-AAV8 antibodies.11
The major vector-related adverse event was a dose-dependent, asymptomatic increase in the serum ALT level associated with a decline in factor IX levels, suggesting a loss of transduced hepatocytes (Table 3). Expansion of the high-dose cohort showed that this adverse event was common, occurring in four of the six patients. The increase in serum ALT levels occurred consistently at 7 to 10 weeks after gene transfer, thus defining the critical period of monitoring and pharmacologic intervention. The precise pathophysiological basis for the hepatocellular toxicity, which has also been observed with other serotypes and genomic configurations, remains unclear.8 Apart from the dose of vector, there were no identifiable clinical differences between the patients who had an increase in the ALT level and those who did not have an increase. In any case, prednisolone was effective in limiting the hepatocellular toxicity as well as preserving the expression of transgenic factor IX, especially when such treatment was initiated early. Although data from patients undergoing liver transplantation suggest that the administration of prednisolone at the doses used in this study may be safe even in patients with active HCV infection, since any increase in HCV viremia would be transient, future studies should be focused on improving the quality and potency of AAV vectors so that the risk of liver toxicity can be eliminated.17
In conclusion, we found that a single infusion of AAV8 vector resulted in durable factor IX expression and long-lasting amelioration of bleeding episodes in patients with severe hemophilia B. The findings with respect to the safety of this approach are encouraging, with the main vector-related adverse event being an elevated serum ALT level, an effect that appears to be readily attenuated by a short, tapering course of prednisolone.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the views of the National Health Service, the National Institute for Health Research, or the Department of Health in the United Kingdom.
Supported by a grant from the National Heart, Lung, and Blood Institute (HL094396), the United Kingdom Medical Research Council, the Katharine Dormandy Trust, the United Kingdom Department of Health, NHS Blood and Transplant, a grant from the Biomedical Research Centre of the National Institute for Health Research (NIHR), Department of Health, to the University College London Hospitals NHS Foundation Trust and University College London, the Royal Free Hospital Charity, an NIHR Program Grant (RP-PG-0310-1001), the Assisi Foundation of Memphis, the American Lebanese Syrian Associated Charities, and the Howard Hughes Medical Institute.
We thank all the study patients for their interest and participation in this study; Paul Lloyd-Evans, Keith Smith, Rose Sheridan, Michelle Quaye, Julia Wilkinson, Jennifer L. Larkin, and Victor Santana for their help with trial-related activities, including vector importation and qualification, data entry, and regulatory affairs; and Professor Johnny Mahlangu for his assistance in monitoring one of the study patients.
APPENDIX
The authors’ names and academic degrees are as follows: Amit C. Nathwani, M.B., Ch.B., Ph.D., Ulrike M. Reiss, M.D., Edward G.D. Tuddenham, M.B., B.S., Cecilia Rosales, Ph.D., Pratima Chowdary, M.B., B.S., Jenny McIntosh, Ph.D., Marco Della Peruta, Ph.D., Elsa Lheriteau, Ph.D., Nishal Patel, F.R.C.P., F.R.C.Path., Deepak Raj, M.B., B.S., Ph.D., Anne Riddell, B.Sc., Jun Pie, B.S.N., Savita Rangarajan, M.B., B.S., David Bevan, M.B., B.S., Michael Recht, M.D., Yu-Min Shen, M.D., Kathleen G. Halka, M.D., Etiena Basner-Tschakarjan, M.D., Ph.D., Federico Mingozzi, Ph.D., Katherine A. High, M.D., James Allay, Ph.D., Mark A. Kay, M.D., Catherine Y.C. Ng, M.S., Junfang Zhou, M.D., Maria Cancio, M.D., Christopher L. Morton, B.S., John T. Gray, Ph.D., Deokumar Srivastava, Ph.D., Arthur W. Nienhuis, M.D., and Andrew M. Davidoff, M.D.
The authors’ affiliations are as follows: the Katharine Dormandy Haemophilia Centre and Thrombosis Unit, Royal Free NHS Trust (A.C.N., E.G.D.T., P.C., J.M., A.R., J.P.), the Department of Haematology, University College London Cancer Institute (A.C.N., E.G.D.T., C.R., J.M., M.D.P., E.L., N.P., D.R.), and St. Thomas’ Hospital (S.R., D.B.), London, NHS Blood and Transplant, Watford (A.C.N., C.R., D.R.), and Basingstoke and North Hampshire Foundation Trust, Basingstoke (S.R.) — all in the United Kingdom; the Departments of Hematology (U.M.R., J.T.G., A.W.N.), Surgery (C.Y.C.N., J.Z., M.C., C.L.M., A.M.D.), and Biostatistics (D.S.), St. Jude Children’s Research Hospital, and Poplar Healthcare (J.A.), Memphis, TN; the Hemophilia Center, Oregon Health and Science University, Portland (M.R.); the Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas (Y.-M.S.), and Scott and White Healthcare, Temple Clinic, Temple (K.G.H.) — both in Texas; the Center for Cellular and Molecular Therapeutics, Children’s Hospital of Philadelphia, Philadelphia (E.B.-T., F.M., K.A.H.); and the Departments of Pediatrics and Genetics, Stanford University School of Medicine, Palo Alto, CA (M.A.K.).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Nathwani AC, Tuddenham EG. Epidemiology of coagulation disorders. Baillieres Clin Haematol. 1992;5:383–439. doi: 10.1016/s0950-3536(11)80025-9. [DOI] [PubMed] [Google Scholar]
- 2.Ponder KP, Srivastava A. Walk a mile in the moccasins of people with haemophilia. Haemophilia. 2008;14:618–620. doi: 10.1111/j.1365-2516.2008.01660.x. [DOI] [PubMed] [Google Scholar]
- 3.Pipe SW. The hope and reality of long-acting hemophilia products. Am J Hematol. 2012;87(Suppl 1):S33–S39. doi: 10.1002/ajh.23146. [DOI] [PubMed] [Google Scholar]
- 4.Herzog RW, Hagstrom JN, Kung SH, et al. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94:5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 6.Snyder RO, Miao CH, Patijn GA, et al. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997;16:270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- 7.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 8.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 9.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector–mediated gene transfer in hemophilia B. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathwani AC, Gray JT, Ng CY, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nathwani AC, Gray JT, McIntosh J, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathwani AC, Rosales C, McIntosh J, et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther. 2011;19:876–885. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allay JA, Sleep S, Long S, et al. Good manufacturing practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum Gene Ther. 2011;22:595–604. doi: 10.1089/hum.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fagone P, Wright JF, Nathwani AC, Nienhuis AW, Davidoff AM, Gray JT. Systemic errors in quantitative PCR titration of self-complementary AAV vectors and improved alternative methods. Hum Gene Ther Methods. 2011;23:1–7. doi: 10.1089/hgtb.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 16.den Uijl IE, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17:41–44. doi: 10.1111/j.1365-2516.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 17.Takada Y, Kaido T, Asonuma K, et al. Randomized, multicenter trial comparing tacrolimus plus mycophenolate mofetil to tacrolimus plus steroids in hepatitis C virus-positive recipients of living donor liver transplantation. Liver Transpl. 2013;19:896–906. doi: 10.1002/lt.23679. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.