Abstract
Two recently commercialized enzyme-linked immunosorbent assay kits, the SRSV(II)-AD (Denka Seiken Co. Ltd., Tokyo, Japan) and IDEIA NLV (DakoCytomation Ltd., Ely, United Kingdom) kits, that detect human norovirus (HuNV) antigens in stool samples were evaluated to assess whether they could be used instead of reverse transcription-PCR (RT-PCR) for routine diagnosis. The sensitivities and specificities of the two kits were tested with a panel of 103 stool samples containing HuNVs of 4 and 10 genetic subgroups within genogroups I and II (GI and GII), respectively, and 39 stool samples containing other enteric viruses. The Denka kit had a high sensitivity (>70% for 10 of the 14 subgroups) but a specificity of only 69%, and the Dako kit had a low sensitivity (<30% for 6 GII subgroups) but a high specificity of 100%. Statistical analysis suggests that HuNVs of four subgroups (subgroups GII/2, GII/5, GII/6, and GII/n) are likely to elude detection by the Dako kit. The two kits also demonstrated differences in reactivities. While the Dako kit discriminated between the GI and GII antigens of HuNVs, the Denka kit cross-reacted with samples containing all GI and GII subgroups of HuNVs. Moreover, the Denka kit also reacted with samples containing human sapovirus (HuSV). We demonstrate that the cross-reactivity of the Denka kit is not due to specific reactions with HuNV and HuSV antigens. These results indicate that neither the Denka kit nor the Dako kit has all the performance characteristics required to replace the RT-PCR methods used to detect HuNVs.
Human noroviruses (HuNVs), which are members of the genus Norovirus in the family Caliciviridae and which were previously known as Norwalk-like viruses (NLVs) or small round-structured viruses (SRSVs), are the leading cause of nonbacterial acute gastroenteritis outbreaks worldwide (7, 10, 15, 29). While HuNV infection causes many cases of illness and large economic losses (28, 30), outbreaks of HuNV infection are difficult to control because the virus is very contagious and is easily spread by multiple modes of transmission. A major obstacle in establishing the diagnosis of HuNV infection has been the absence of a sensitive and rapid diagnostic method suitable for use in public health laboratories and hospitals. Part of the difficulty in developing such a method has been the enormous genetic and antigenic diversity of this virus group. At present, most HuNVs have been classified genetically into two major groups, genogroups I and II (GI and GII, respectively), with GI comprising at least 7 subgroups and GII comprising at least 10 subgroups (4, 8, 9, 21, 40).
Historically, HuNVs were first detected by electron microscopy, but reverse transcription-PCR (RT-PCR) has now become the standard test for the diagnosis of HuNV infection. The RT-PCR assay is time-consuming, expensive, and not easy to perform. Because it is difficult to design broadly reactive primers, the ability to detect HuNVs by RT-PCR differs markedly between laboratories (39). Even in the same laboratories, the efficiency of amplification changes over time due to changes in the genetic groups of HuNVs circulating in the community (16, 32). To circumvent this problem of sequence mismatch, many laboratories use multiple sets of primers targeting different regions of the genome, at the cost of the simplicity of the procedures and the rapidity of results (7, 39, 40). The test can be further delayed because confirmation of the identities of the amplification products by either probe hybridization or sequencing is often required (3, 38). These difficulties with RT-PCR indicate that a much simpler and more rapid method needs to be developed to provide to researchers investigating HuNV outbreaks a more timely means of diagnosis.
An enzyme-linked immunosorbent assay (ELISA) would be an attractive supplement to RT-PCR methods for the screening of stool samples for HuNVs because of its simplicity and rapidity and because of the potential for widespread distribution on account of the standard technology used. To develop diagnostic ELISA methods, several research groups have prepared recombinant-expressed major capsid proteins (VP1), which spontaneously assemble into virus-like particles (VLPs), and polyclonal antibodies and monoclonal antibodies (MAbs) against the VLPs (5, 6, 11, 17, 18, 24-27, 31, 33). Comparison of the reactivities with VLPs of antibodies raised in animals by immunization of VLPs from different genetic groups has consistently suggested that GI and GII correspond to two distinct major antigenic types and that the genetic subgroups within GI and GII each correspond to distinct antigenic subtypes. While the rabbit anti-VLP polyclonal antibodies obtained in those studies were predominantly specific for the genetic subgroups of HuNVs used as the immunogen (11, 18, 24, 25), mouse MAbs recognizing epitopes common to either GI or GII, or both GI and GII, have also been reported (12, 14, 22, 42). These MAbs, which have the ability to react with a wide range of antigenically diverse HuNVs, could be useful for the development of a simple and rapid ELISA method for the detection of virus in stool samples.
Recently, two ELISA kits for the detection of HuNV antigens in stool samples have been commercialized: the SRSV(II)-AD kit, made by Denka Seiken Co. Ltd. (Tokyo, Japan), and the IDEIA NLV kit, made by DakoCytomation Ltd. (Ely, United Kingdom). These kits are distributed mainly in Japan and Europe, respectively, and neither kit is available on the U.S. market. Both kits use a sandwich-type format with MAbs to separately capture GI and GII antigens on the surfaces of two microwells (the GI and GII wells, respectively) and rabbit polyclonal antibodies conjugated to horseradish peroxidase to detect the captured GI and GII antigens.
The Denka kit uses two MAbs, MAbs NV3912 and NS14, and pools of the GI and GII antigen-directed polyclonal antibodies, which were prepared by immunizing rabbits with VLPs of 4 GI HuNV subgroups and 10 GII HuNV subgroups (37). MAb NV3912 reacts strongly only with VLPs of GI HuNVs (22). MAb NV14 reacts strongly with GII VLPs and weakly with GI VLPs. Neither MAb reacts with VLPs of Sapporo/82/JPN (22), the prototype strain of human sapovirus (HuSV), which is in the genus Sapovirus of the family Caliciviridae. The genetic subgroups of the 14 HuNVs used to immunize rabbits were not reported. The Dako kit is reported to use pools of MAbs specific for 4 and 10 genetic subgroups of GI and GII, respectively, but information on the properties of the capture and detector antibodies is not available (34).
Three research groups have evaluated these kits independently (34, 37, 41). The Denka kit had a sensitivity, specificity and agreement (accuracy) of 68, 76, and 69%, respectively, on the basis of the RT-PCR results for 1,502 stool samples collected from patients involved in outbreaks of gastroenteritis in Japan (37) and a sensitivity, specificity and agreement of 86, 67, and 80%, respectively, on the basis of the RT-PCR results for 20 stool samples (41). The Dako kit had a sensitivity, specificity, and agreement of 56, 98, and 81%, respectively, on the basis of the results of RT-PCR for 479 stool samples from patients involved in more than 150 outbreaks in the United Kingdom (34). These values suggest that the kits perform well, with the Denka kit having a greater sensitivity and the Dako kit having a greater specificity, and have attracted the interest of many researchers working with HuNV outbreaks. However, two observations described in the papers attracted our attention. First, the Dako kit did not detect three viruses in 1 of 12 genetic subgroups tested (subgroup GII/2), but information on the composition of the genetic subgroups in the test panel was not presented. The papers evaluating the Denka kit provided no information on the efficiency of the kit for the detection of individual genetic subgroups. Because the genetic subgroups of HuNVs circulating in communities can vary enormously (16, 32), information on the efficiencies of the kits for the detection of individual genetic groups is crucial for the application of the kits to field studies. Second, while the Dako kit discriminated between the GI and the GII antigens, the GI and GII wells of the Denka kit appeared to cross-react with both antigens. A prerequisite for such cross-reactivity is the use of both capture and detector antibodies with the capability to recognize epitopes common to GI and GII HuNVs. However, the rabbit anti-VLP antibodies produced in many laboratories were generally specific for genetic subgroups (11, 18, 24, 25), and the GI wells of the Denka kit were coated with MAb NV3912, reportedly specific only for VLPs of GI antigens. The authors of the papers evaluating the Denka kit did not provide information on how frequently this cross-reactivity occurred and did not discuss those results.
The aim of the present study was to independently evaluate and compare the Denka and Dako kits and to determine their sensitivities and specificities for the detection of HuNVs of the most common genetic subgroups recently identified in the United States (7). The study specifically addressed the questions of whether particular genetic subgroups eluded detection by the kits and whether the cross-reactivity of the Denka kit represented reactions with HuNV antigens. The answers to these questions should help scientists in public health laboratories and hospitals who might be interested in using these kits in investigations of outbreaks of HuNV infections.
MATERIALS AND METHODS
Panel of stool samples.
The kits were evaluated with a panel of 104 HuNV-positive reference stool samples that had yielded strong signals by RT-PCR when their amplified products were stained with ethidium bromide and 33 negative reference stool samples containing rotavirus, astrovirus, and enterovirus, as detected by ELISA, RT-PCR, or virus isolation (Table 1). In addition, one norovirus sample of GIV/1 (7, 20, 40) was included. Of these, 98 positive and 23 negative reference samples were used to evaluate both kits. The 104 positive reference samples were collected from 35 outbreaks of acute gastroenteritis that occurred in the United States between June 1999 and June 2002. The 33 negative reference samples were collected from children less than 5 years of age with diarrhea.
TABLE 1.
Detection of human viruses in stool samples with two ELISA kitsa
| Virus and genetic group | No. of outbreaks | No. of samples positive/no. tested (%)
|
|
|---|---|---|---|
| Denka kit | Dako kit | ||
| HuNV | |||
| GI/1 | 2 | 8/8 (100) | 5/8 (63) |
| GI/2 | 3 | 5/8 (63) | 4/8 (50) |
| GI/3 | 3 | 8/8 (100) | 3/6 (50) |
| GI/4 | 1 | 7/8 (88) | 7/8 (88) |
| Sum | 9 | 28/32 (88) | 19/30 (63) |
| GII/1 | 2 | 6/6 (100) | 5/6 (83) |
| GII/1b | 2 | 6/6 (100) | 2/6 (33) |
| GII/2 | 2 | 5/6 (83) | 0/6 (0) |
| GII/3 | 2 | 4/8 (50) | 1/8 (13) |
| GII/4 | 2 | 5/7 (71) | 3/4 (75) |
| GII/5 | 3 | 3/8 (38) | 0/8 (0) |
| GII/6 | 4 | 5/8 (63) | 1/8 (13) |
| GII/7 | 2 | 7/8 (88) | 4/8 (50) |
| GII/j | 3 | 8/8 (100) | 2/7 (29) |
| GII/n | 3 | 5/6 (83) | 1/6 (17) |
| Sum | 25 | 54/71 (76) | 19/67 (28) |
| GIV/1 | 1 | 1/1 (100) | 1/1 (100) |
| Total | 35 | 83/104 (80) | 39/98 (40) |
| Non-HuNVs | |||
| HuSV | 1 | 4/6 (67) | NA |
| RV | 6/24 (25) | 0/23 (0) | |
| ASV | 2/9 (22) | NA | |
| EnV | NA | 0/9 (0) | |
| Total | 36 | 12/39 (31) | 0/32 (0) |
Abbreviations: RV, rotavirus; ASV, astrovirus; EnV, enterovirus; NA, samples not available.
The Denka kit was also tested with six HuSV-positive stool samples from patients involved in an outbreak that had yielded strong signals by RT-PCR. These six samples were included in the negative reference panel. The HuNVs in the reference panel were detected by RT-PCR with either the region B-specific (2) or the region A-specific (3) primer set, and the HuSVs were detected with primer set p289-p290 alone (19).
Genetic group.
The genetic groups of the HuNV isolates used in this study were determined on the basis of a genetic classification scheme described previously (4), with modification (7). Briefly, a unique sequence of the amplification product either in region B (a 172-base region located between nucleotides 265 and 94 from the 3′ end of open reading frame [ORF] ORF1 in the equivalent location of the Bristol virus genome) or in region C (a 277-base region located between nucleotides 312 and 588 from the 5′end of ORF2), or in both regions, was assigned to a specified genetic group if the sequence had less than a 15% difference in nucleotide identity with the sequences of one of the following reference strains (GenBank accession numbers are given in parentheses): GI/1, Norwalk/8FIIa/1968 (M87661); GI/2, Southampton/1991/UK (L07418); GI/3, Desert Shield 395/1990/SA (U04469); GI/4, Ciba 407/1987/JP (AB022679); GII/1, Hawaii/1971/US (U07611); GII/1b, Wortley/1990/UK (AJ277618); GII/2, Melksham/1989/UK (X81879); GII/3, Toronto 24/1991/CA (U02030); GII/4, Bristol/1993/UK (X76716); GII/5, Hillingdon/1994/UK (AJ277607); GII/6, Seacroft/1990/UK (AJ277620); GII/7, Leeds/1990/UK (AJ277608); and GIV/1, Alphatron/1998/NL (AF195847). The designations GII/j and GII/n refer to genetic subgroups suggested to be distinct from the groups indicated above.
Panel of VLPs.
A panel of VLPs was prepared from seven HuNV-containing samples (the designations in parentheses indicate the abbreviations for the recombinant-expressed protein name; GenBank accession numbers): Desert Shield 395/1990/SA (rDSV), Hawaii/1971/US (rHV), and Chesterfield/434/1997/US (rCFV; AY054300) in HuNV/GII/2; Toronto 24/1991/CA (rTV) and Burwash Landing/331/1995/US (rBLV; AF414425) in HuNV/GII/4; White River/290/1994/US (rWRV; AF414423) in HuNV/GII/5; and Florida/269/1993/US (rFV; AF414407) in HuNV/GII/6. The VLPs from Parkville/1994/US (rPV; U73124) in HuSV/GI/2 were also included in the panel. The expression and purification of these VLPs were performed by previously described methods (6). rCFV, rBLV, rWRV, rFV, and rPV were generated in the Respiratory and Enteric Viruses Branch at the Centers for Disease Control and Prevention (Atlanta, Ga.). The cloned cDNAs of rDSV, rHV, and rTV inserted in either plasmid or baculovirus expression vectors were generated in the Laboratory of Infectious Disease at the National Institute of Allergy and Infectious Disease, National Institutes of Health (Bethesda, Md.), and were kindly provided by Kim Green. The protein concentrations of the purified VLPs were determined with a Micro BCA protein assay reagent kit (Pierce Biotechnology Inc., Rockford, Ill.) with bovine serum albumin used as the standard. The purities of the VLPs were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by staining with Coomassie blue.
ELISA.
The stool samples were individually weighed to determine the accuracies of the concentrations, and the coded suspensions were randomly distributed in the GI and GII wells coated with anti-GI and anti-GII MAbs, respectively, provided with the kits. The assay was repeated twice, and the procedures described in the instructions were strictly followed.
Briefly, for the Denka kit, a 10% stool suspension made in 2.5% veal infusion broth was mixed with an equal volume of sample extraction buffer provided with the kit. The reactions for the capture and detection of HuNV antigens were performed separately in 100 μl for 1 h at room temperature. After the wells were washed, 3,3′,5,5′-tetramethylbenzidine and hydrogen peroxide were added and a colorimetric reaction was conducted for 30 min at room temperature. The optical densities (ODs) at 450 and 630 nm were measured with a MRX Revelation spectrophotometer (Dynex Technologies, Inc., Chantilly, Va.), and the average OD value for two blanks obtained with buffer in place of the 10% stool suspension was subtracted from the OD values for the test samples and the controls. Samples with OD values greater than the average (mean) OD values for the two positive controls were regarded as positive, as indicated in the kit's instructions.
For the Dako kit, 10% stool suspensions were prepared in the buffer provided with the kit. The capture and detection reactions were performed simultaneously in a volume of 200 μl for 2 h at room temperature; 100 μl of the 10% stool suspension and 100 μl of the horseradish peroxidase-conjugated anti-GI and anti-GII polyclonal antibodies were successively added to the GI and GII wells, respectively. The procedures for the colorimetric reaction were the same as those used with the Denka kit. Samples with OD values greater than the average OD values for the two negative controls plus 0.1 were regarded as positive.
Purification of HuNVs from stool samples.
Two GI- and GII-containing stool samples were selected and purified by isopycnic CsCl density gradient centrifugation and subsequent dialysis against TN buffer (0.01 M Tris hydrochloride [pH 7.5], 0.15 M NaCl) with Float-A-Lyzer dialysis membranes with a molecular mass cutoff of 25,000 Da (Spectrum Laboratories Inc., Rancho Dominguez, Calif.), as described previously (13). Before purification, each of these samples had high OD values in the two wells: one sample, HuNV/GI/3, had ODs of 2.959 and 1.100 in the GI and GII wells, respectively, and the other, HuNV/GII/7, had ODs of 1.679 and 2.919 in the GI and GII wells, respectively.
Analyses of the data.
The sensitivity, specificity, and agreement (Table 2) were calculated as described previously (23): sensitivity was the percentage of positive reference samples that were positive by ELISA; specificity was the percentage of negative reference samples that were negative by ELISA; and agreement was the percentage of the results that were fully concordant, with the positive reference samples being positive by ELISA and the negative reference samples being negative by ELISA.
TABLE 2.
Sensitivities, specificities, and rates of agreement of the Denka and Dako kits
| Kit | Well | No. of reference samples
|
Overall properties of the two kits
|
|||
|---|---|---|---|---|---|---|
| HuNVa | Non-HuNVb | Sensitivity (%) | Specificity (%) | Agreement (%) | ||
| Denka | GI | 32 | 39 | 81 | 72 | 76 |
| GII | 71 | 39 | 69 | 85 | 75 | |
| Both | 103 | 39 | 80 | 69 | 77 | |
| Dako | GI | 30 | 32 | 60 | 100 | 81 |
| GII | 67 | 32 | 28 | 100 | 52 | |
| Both | 97 | 32 | 39 | 100 | 54 | |
Stool samples with HuNV used as positive reference samples.
Stool samples with other viruses (HuSV, rotavirus, astrovirus, and enterovirus) used as negative reference samples.
Analyses of OD values were performed by statistical methods (1) with the SAS programming software package, version 9 (35). To improve the normality of the distribution, the OD values were logarithmically transformed before the analyses. After logarithmic transformation, we tested the normality of the distribution of the OD values by the Shapiro-Wilk W test, and the results were plotted with a box-and-whisker plot. A two-sample paired t test with the OD values obtained from the same samples was used to assess whether the mean OD values between the GI and GII wells or between the two kits differed between the sample populations. The Bonferroni modified t test was conducted with the OD values from the different samples to estimate differences in the means of the OD values for populations between the positive and negative reference samples. In these analyses, a null hypothesis was set to no difference, and a probability to accept this hypothesis was indicated by P values.
RESULTS
The assays were repeated twice, with good reproducibility, and we obtained essentially the same results each time (data not shown). The analyses described below were based on the results obtained by one of the two assays. The statistical analyses were conducted with log OD values, which showed normal distributions and which had similar standard deviations within the GI and GII groups.
Overall properties of the two kits. (i) Sensitivity.
The two kits showed marked differences in sensitivities for the detection of GI and GII antigens. While the Denka kit detected HuNVs of all 14 genetic subgroups and the sensitivities for the detection of 10 of these subgroups were >70% (with a sensitivity of 100% for the detection of 5 subgroups), the Dako kit detected HuNVs of only 12 subgroups and the sensitivities for the detection of 5 GII subgroups were <30% (Table 1). The overall sensitivities, specificities, and levels of agreement were 80, 69, and 77%, respectively, for the Denka kit and 39, 100, and 54%, respectively, for the Dako kit (Table 2).
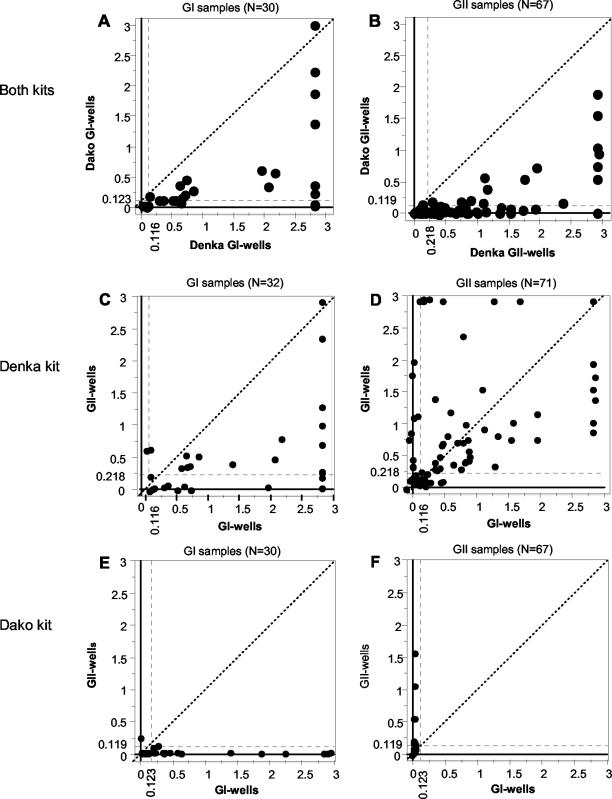
This difference in sensitivities between the two kits was clearly visualized by OD values of 30 for GI-containing samples and 67 for GII-containing samples determined with the GI and GII wells, respectively (Fig. 1A and B, respectively). All positive OD values determined with the Denka kit were higher than those determined with the Dako kit, except for those for three GI-containing samples. The observed difference in OD values was confirmed by the results of the two-sample paired t test, which showed significant differences between the two kits for both GI-containing (P < 0.0001) and GII-containing (P < 0.0001) samples.
FIG. 1.
Comparison of OD values between Denka and Dako kits (A and B) and the GI and GII wells of the Denka kit (C and D) and the Dako kit (E and F), respectively. Points represent OD values in the GI and GII wells obtained from the same sample. The left (A, C, and E) and right (B, D, and F) groups of graphs provide OD values for samples with GI HuNVs and GII HuNVs, respectively. Dashed lines on horizontal and vertical axes indicate cutoff OD values. The dashed diagonal lines correspond to the locations where the OD values in the two wells are the same.
Of note, one sample with GIV/1 was positive with both of the kits; it was positive in both wells of the Denka kit (ODs, 0.162 and 2.495 in the GI and GII wells, respectively) but only in the GII well of the Dako kit (OD, 1.274) (Table 1).
(ii) Cross-reactivity.
The two kits demonstrated marked differences in cross-reactivities (Table 2). The Denka kit detected 59% of the GII antigens in the GI wells and 63% of the GI antigens in the GII wells. The Dako kit detected no GII antigens in the GI wells and only 7% of the GI antigens in the GII wells.
This difference in cross-reactivity was further demonstrated by the distributions of the OD values on x-y plots (Fig. 1C to F). While many OD values determined with the Denka kit were distributed in a large area outside of the two cutoff values (Fig. 1C and D), all but two of the OD values determined with the Dako kit were distributed inside of the cutoff values (Fig. 1E and F). Furthermore, for the Denka kit, samples containing five GII subgroups (subgroups GII/1, GII/1b, GII/2, GII/3, and GII/4) had mean OD values that were higher in the GI wells than those in the homologous GII wells.
Consistent with this observation, the results of the two-sample paired t test did not indicate a significant difference in the OD values for the 72 GII-containing samples between the GI and GII wells of the Denka kit (P = 0.684), while the OD values for the 32 GI-containing samples differed significantly between the GI and GII wells (P < 0.0001).
(iii) Specificity.
The Dako kit gave more specific results than the Denka kit. None of the negative reference samples were positive in tests with the Dako kit, but 12 (31%) of 39 were positive in tests with the Denka kit (Table 1 and Table 2).
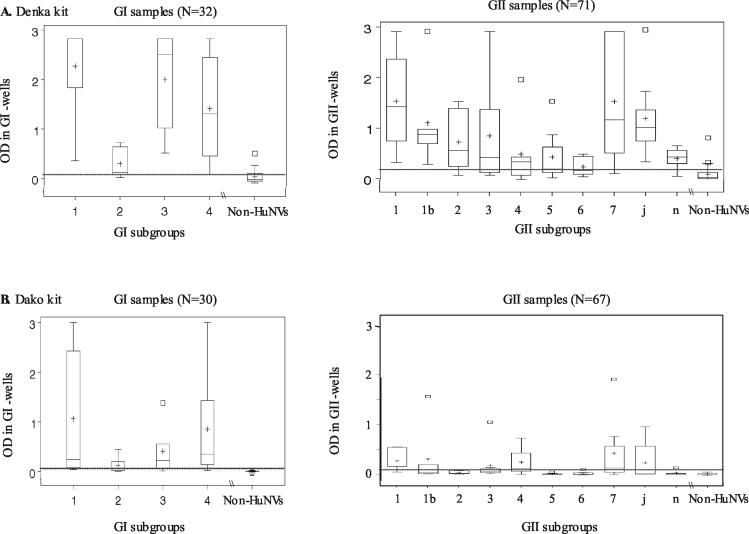
Eight of the 12 samples with false-positive results, 6 of which contained rotavirus and 2 of which contained astrovirus, were confirmed to be negative for HuNVs by RT-PCR with primers specific for region B. The OD values for these eight samples with false-positive results ranged from 0.118 to 0.505 (mean OD, 0.215) in the GI wells and from 0.039 to 0.807 (mean OD, 0.227) in the GII wells, and the values for three of these samples were outliers of the estimated normal distribution (Fig. 2).
FIG. 2.
Box-and-whisker plots of homologous OD values, showing the 95% central ranges derived from an estimated normal distribution of OD values obtained with the Denka kit (A) and the Dako kit (B) before logarithmic transformation. The left and right panels indicate OD values for samples with GI and GII HuNVs, respectively. The bottom, middle, and top lines of each box correspond to the 25, 50, and 75% cumulative frequencies of the observed values, respectively. The upper and lower whiskers extending from the box correspond to 2.5 and 97.5% cumulative frequencies, respectively (either the whisker or the box line, or both, overlap each other for some subgroups). The plus signs and squares mark the mean OD value and the relative outliers, respectively. The dashed line indicates cutoff OD value. Non-HuNVs, negative reference samples.
For the remaining four samples from an HuSV outbreak with false-positive results, sequencing of the RT-PCR products from two of the four samples indicated different sequences: one closely related to Parkville/1994/US in HuSV/GI/2 and another closely related to London/1992/UK in HuSv/GII/1 (36). Three of the four samples, including the sample with HuSV of GI/2, were positive in both the GI and the GII wells, and their OD values ranged from 0.154 to 2.938 (mean OD, 0.931) in the GI wells and from 0.140 to 1.964 (mean OD, 0.828) in the GII wells. The sample with HuSV of GII/1 tested negative in both wells.
These results suggest that increasing cutoff values would not increase the specificity of the Denka kit. Unfortunately, the samples with HuSVs were not tested with the Dako kit because sufficient supplies of these samples were not available.
Detection of specific subgroups with the kits.
To assess whether particular subgroups elude detection by the kits, we analyzed the OD values obtained for the HuNVs of individual subgroups (Fig. 2). The two kits differed markedly in their ability to detect the 4 GI subgroups and 10 GII subgroups. With the Denka kit, the median values were above the cutoff values for all but 1 of the 14 subgroups (subgroups GII/6) and the 25% lower-border values were below the cutoff point for 4 subgroups (subgroups GII/3, GII/4, GII/5, and GII/6) (Fig. 2A). By contrast, for the Dako kit, the median values were below the cutoff values for 8 subgroups (subgroups GI/2, GII/1b, GII/2, GII/3, GII/5, GII/6, GII/j, and GII/n) and the 25% lower-border values were below the cutoff values for 10 subgroups (subgroups GII/4 and GII/7 along with the 8 subgroups indicated above) (Fig. 2B).
The results of the analysis by the Bonferroni modified t test indicated that the OD values determined with the Denka kit differed significantly from the negative reference values for all 14 subgroups. However, the values obtained with the Dako kit differed significantly from the negative reference values for only 10 subgroups, and the values for the remaining 4 subgroups did not show the difference (for GII/2, P = 0.064; for GII/5, P = 0.787; for GII/6, P = 0.820; and for GII/n, P = 0.427). These results indicate that these four GII subgroups are likely to elude detection with the Dako kit.
Determination of source of cross-reactive property of the Denka kit.
To understand the basis for the cross-reactivity observed with the Denka kit, we examined the ability of the kit to discriminate the GI and GII antigens of the VLPs versus those of native virus in stool samples.
(i) Test for a common epitope(s) on VLPs.
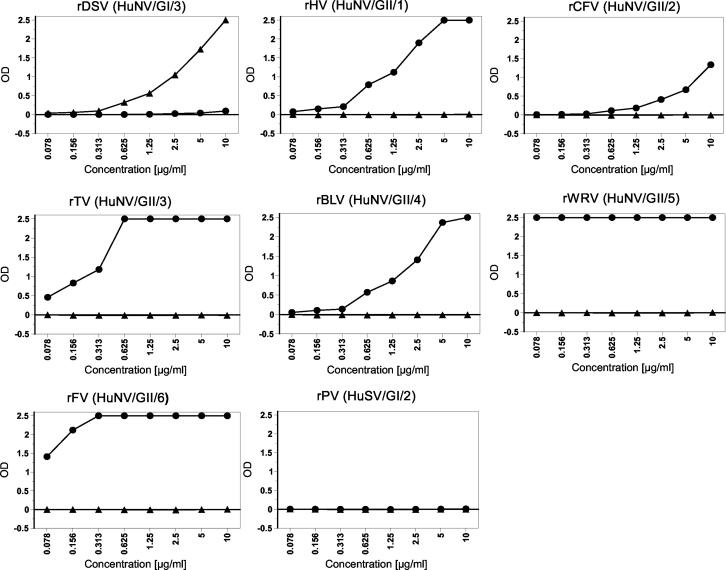
To determine whether the GI and GII wells of the Denka kit reacted with a common epitope(s), we tested the kit with a panel of VLPs prepared from seven HuNV subgroups and one HuSV subgroup. The Denka kit detected all VLPs from HuNVs in the GI or GII wells with the homologous genogroup of the VLPs but did not detect the VLPs at any concentrations in the wells with the heterologous genogroup (Fig. 3). In addition, the kit did not detect the HuSV antigen of rPV (Parkville/1994/US). These results ruled out the possibility that the cross-reactivity of the Denka kit represents a reaction with a common epitope(s) shared by VLPs and native virus in stool samples.
FIG. 3.
Results of evaluation of the Denka kit with a panel of VLPs (see the text for details). ▴, OD values in GI wells; •, OD values in GII wells.
The kit showed the lowest detection threshold (<78 ng/ml) for rWRV (GII/5) and rFV (GII/6) and the highest detection threshold (∼313 ng/ml) for rCFV (GII/2), but this difference did not correlate with the observed differences in the sensitivities of detection of the different HuNV subgroups in stool samples (Table 1).
(ii) Test for a common epitope(s) on native virus.
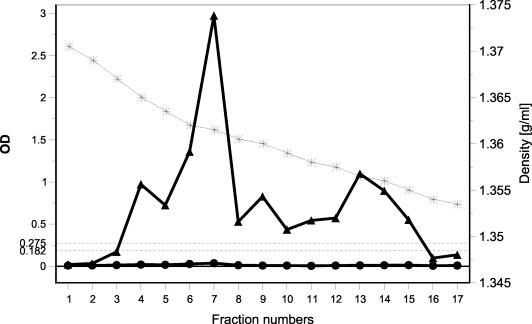
To determine whether the GI and GII wells of the Denka kit reacted with a common epitope(s) on native virus in stool samples, we purified a GI/3 antigen from stool samples by CsCl density gradient centrifugation and subsequent dialysis. The cross-reactivity of the GI antigen in the GII wells was completely eliminated by the purification. The gradient fractions corresponding to the intact virions (fraction 7, with a buoyant density of 1.36 g/ml) and the disrupted virions (fractions 12 to 15, with buoyant densities of ∼1.357 to 1.353) showed high OD values only in the GI wells (Fig. 4). Similarly, purification and dialysis conducted with another sample containing HuNV subgroup GII/7 completely eliminated the cross-reactivity in the GI wells (data not shown).
FIG. 4.
Reactivity of HuNV GI/3 antigen with the Denka kit after purification from stool samples by isopycnic CsCl density gradient centrifugation followed by dialysis. *, CsCl density; ▴, OD values in GI wells; •, OD vales in GII wells. Dashed lines indicate the cutoff values in GI wells (OD, 0.182) and GII wells (OD, 0.275).
These results rule out the possibility that the cross-reactivity represented the reaction with a common epitope(s) present on native virus and indicate that it was due to a substance(s) present in the stool samples.
In an attempt to identify this substance(s), we selected an additional six stool samples and added CsCl (final concentration, 1.36 g/ml) to the samples during sample preparation and dialyzed them against TN buffer before the ELISA. These treatments of the samples substantially eliminated the cross-reactivities for three of the six samples tested (data not shown).
DISCUSSION
Because the RT-PCR assay used to diagnose HuNV infections at present is time-consuming and difficult to perform, we evaluated two recently commercialized ELISA kits to determine whether they could be used instead of the RT-PCR assay in public health laboratories. Earlier reports of studies that evaluated these kits suggested that they performed well but also questioned whether particular genetic subgroups eluded detection and whether the cross-reactivity of the Denka kit represented a reaction with HuNV antigens. It was important to answer these questions before the distribution of these kits to state public health laboratories to apply them to the investigation of HuNV outbreaks is considered. The panel of stool samples used in this study differed in composition from those used in the preceding studies. First, the HuNV-positive reference samples were composed of only those stool samples that gave a strong signal by RT-PCR products with ethidium bromide staining. Second, the stool samples were selected so that the most common genetic groups previously identified (7) were almost evenly distributed in the panel.
For the Dako kit, the sensitivity and agreement determined in this study were substantially lower than those determined previously (34). We found that 8 of the 14 subgroups tested (subgroups GI/2, GII/1b, GII/2, GII/4, GII/5, GII/6, GII/j, and GII/n) had OD values markedly lower than those for the positive reference samples, and the sensitivities for six of these samples were <30%. Importantly, the OD values for 4 subgroups (subgroups GII/2, GII/5, GII/6, and GII/n) did not differ significantly from those for the negative reference samples, suggesting that these GII subgroups are not likely to be detected by the Dako kit. This finding suggests that inclusion of the samples with HuNVs in these four subgroups caused the major differences in sensitivity and agreement between the present study and previous studies. Of note, we used only stool samples that were strongly positive for HuNVs by RT-PCR. The results could be much worse in field studies, in which stool samples have various concentrations of HuNV antigens. Since the eight subgroups mentioned above have been responsible for many outbreaks in the past several years in the United States and many other countries (7, 39), we conclude that the present version of the Dako kit is too insensitive for routine diagnostic use and would yield many false-negative results.
We also found that the cross-reactivities of the GI and GII wells of the Denka kit occurred with all GI and GII subgroups of HuNVs tested. Importantly, the OD values for the samples containing GII were not statistically significantly different between the GI and GII wells, and both the GI and GII wells gave positive OD values for three of seven stool samples collected from one HuSV outbreak. These results could be obtained from a specific reaction with HuNV and HuSV antigens only when the reaction occurred with an epitope(s) common to the GI and GII antigens of HuNV and HuSV antigens, a possibility that was not predicted from previous reports. Such a common epitope(s) either could be shared by VLPs and native virus in stool samples or could be maintained by the native virus alone. Both of these possibilities were ruled out by the results of the test with the panel of VLPs and those from virus purification. When the virus in the stool samples was purified, the cross-reactivity disappeared. We do not know what substance(s) is responsible for this cross-reactivity, but our results suggest that it has a molecular mass <25,000 Da and can be detached from the antigen by either exposure to a high concentration of CsCl or dialysis against TN buffer, or a combination of both treatments. Taken together, we conclude that the present version of the Denka kit has too many nonspecific reactions for routine diagnostic use and would yield many false-positive results. The kit might be used to screen large numbers of stool samples for HuNVs, for which RT-PCR methods are difficult to apply, but the results for positive samples must be confirmed by RT-PCR.
While each of the two commercial kits evaluated in this study has a problem with sensitivity or specificity, the early prototype ELISAs for detection of antigens of specific HuNV subgroups (e.g., subgroups GI/1, GI/4, and GII/4) had previously reported (11, 14, 25) high sensitivities of 83 to 100% when stool samples with HuNVs whose sequences were known were used and did not have problems because of the cross-reactive substance(s) present in stool samples. In one of those studies (11), 1% normal rabbit serum was added to the detector antibody solution to reduce background reactivity. Our study indicates that further improvements to the kits are required. For the Denka kit, nonspecific reactions of the reagents in the assay with a substance(s) in stool samples occur frequently, and specificity could be increased by either improving fecal sample processing to remove or reduce the cross-reactive substance(s) or modifying the composition of the detector antibody solution, as reported previously (11), or both. For the Dako kit, some common subgroups are not well detected, and the sensitivity could be improved by including additional antibodies specific for these groups. With these improvements, the next generations of the kits might provide simple and rapid diagnostic tests needed for the timely diagnosis of HuNV infections in investigations of outbreaks.
Acknowledgments
We gratefully acknowledge Susan M. Adams and Stephan S. Monroe in the Respiratory and Enteric Viruses Branch for help with preparation of the test panels. We thank Craig Borkowf, Rosane Nisenbaum, and Aaron Curns for help with statistical analysis and Claudia C. Chesley for editorial assistance.
REFERENCES
- 1.Altman D. G. 1991. Practical statistics for medical research. Chapman & Hall, London, United Kingdom.
- 2.Anderson, A., A. G. Heryford, J. P. Sarisky, C. Higgins, S. S. Monroe, R. S. Beard, C. M. Newport, J. L. Cashdollar, G. S. Fout, D. E. Robbins, S. A. Seys, K. J. Musgrave, C. Medus, J. Vinje, J. S. Bresee, H. M. Mainzer, and R. I. Glass. 2003. A waterborne outbreak of Norwalk-like virus among snowmobilers—Wyoming, 2001. J. Infect. Dis. 187:303-306. [DOI] [PubMed] [Google Scholar]
- 3.Ando, T., S. S. Monroe, J. R. Gentsch, Q. Jun, D. C. Lewis, and R. I. Glass. 1995. Detection and differentiation of antigenically distinct small round-structured viruses (Norwalk-like viruses) by reverse transcription-PCR and Southern hybridization. J. Clin. Microbiol. 33:64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ando, T., J. S. Noel, and R. L. Fankhauser. 2000. Genetic classification of “Norwalk-like viruses.” J. Infect. Dis. 181(Suppl. 2):S336-S348. [DOI] [PubMed] [Google Scholar]
- 5.Baric, R. S., B. Yount, L. Lindesmith, P. R. Harrington, S. R. Greene, F.-C. Tseng, N. Davis, R. E. Johnston, D. G. Klapper, and C. L. Moe. 2002. Expression and self-assembly of Norwalk virus capsid protein from Venezuelan equine encephalitis virus replicons. J. Virol. 76:3023-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belliot, G., J. S. Noel, J.-F. Li, Y. Seto, C. D. Humphrey, T. Ando, R. I. Glass, and S. S. Monroe. 2001. Characterization of capsid genes, expression in the baculovirus system, of three new genetically distinct strains of “Norwalk-like viruses.” J. Clin. Microbiol. 39:4288-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fankhauser, R. L., S. S. Monroe, J. S. Noel, C. D. Humphrey, J. S. Bresee, U. D. Parashar, T. Ando, and R. I. Glass. 2002. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 186:1-7. [DOI] [PubMed] [Google Scholar]
- 8.Green, J., J. Vinje, C. I. Gallimore, M. Koopmans, A. Hale, and D. W. G. Brown. 2000. Capsid protein diversity among Norwalk-like viruses. Virus Genes 20:227-236. [DOI] [PubMed] [Google Scholar]
- 9.Green, K. Y., R. M. Chanock, and A. Z. Kapikian. 2001. Human caliciviruses, p. 841-874. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott, Williams & Wilkins, Baltimore, Md.
- 10.Greening, G. E., M. Mirams, and T. Berke. 2001. Molecular epidemiology of “Norwalk-like viruses” associated with gastroenteritis outbreaks in New Zealand. J. Med. Virol. 64:58-66. [DOI] [PubMed] [Google Scholar]
- 11.Hale, A. D., S. E. Crawford, M. Ciarlet, J. Green, C. Gallimore, D. W. G. Brown, X. Jiang, and M. K. Estes. 1999. Expression and self-assembly of Grimsby virus: antigenic distinction from Norwalk and Mexico viruses. Clin. Diagn. Lab. Immunol. 6:142-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy, M. E., T. N. Tanaka, N. Kitamoto, L. J. White, J. M. Ball, X. Jiang, and M. K. Estes. 1996. Antigenic mapping of the recombinant Norwalk virus capsid protein using monoclonal antibodies. Virology 217:252-261.8599210 [Google Scholar]
- 13.Hayashi, Y., T. Ando, E. Utagawa, S. Sekine, S. Okada, K. Yabuuchi, T. Miki, and M. Ohashi. 1989. Western blot (immunoblot) assay of small, round-structured virus associated with an acute gastroenteritis outbreak in Tokyo. J. Clin. Microbiol. 27:1728-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herrmann, J. E., N. R. Blacklow, S. M. Matsui, T. L. Lewis, M. K. Estes, J. M. Ball, and J. P. Brinker. 1995. Monoclonal antibodies for detection of Norwalk virus antigen in stools. J. Clin. Microbiol. 33:2511-2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inouye, K., K. Yamashita, S. Yamadera, M. Yoshikawa, N. Kato, and N. Okabe. 2000. Surveillance of viral gastroenteritis in Japan: pediatric cases and outbreak incidents. J. Infect. Dis. 181(Suppl. 2):S270-S274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iritani, N., Y. Seto, K. Haruki, M. Kimura, M. Ayata, and H. Ogura. 2000. Major change in the predominant type of “Norwalk-like viruses” in outbreaks of acute nonbacterial gastroenteritis in Osaka City, Japan, between April 1996 and March 1999. J. Clin. Microbiol. 38:2649-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang, X., M. Wang, D. Y. Graham, and M. K. Estes. 1992. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang, X., D. Cubitt, J. Hu, X. Dai, J. Treanor, D. O. Matson, and L. K. Pickering. 1995. Development of an ELISA to detect MX virus, a human calicivirus in the Snow Mountain agent genogroup. J. Gen. Virol. 76:2739-2747. [DOI] [PubMed] [Google Scholar]
- 19.Jiang, X. P. W. Huang, W. M. Zhong, T. Farkas, D. W. Cubitt, and D. O. Matson. 1999. Design and evaluation of a primer pair that detects both Norwlk- and Sapporo-like caliciviruses by RT-PCR. J. Virol. Methods 83:145-154. [DOI] [PubMed] [Google Scholar]
- 20.Karst, S. M., C. E. Wobus, M. Lay, J. Davidson, and H. W. Virgin IV. 2003. STAT1-dependent innate immunity to Norwalk-like virus. Science 299:1575-1578. [DOI] [PubMed] [Google Scholar]
- 21.Katayama, K., H. Shirato-Horikoshi, S. Kojima, T. Kageyama, T. Oka, F. B. Hoshino, S. Fukushi, M. Shinohara, K. Uchida, Y. Suzuki, T. Gojobori, and N. Takeda. 2002. Phylogenetic analysis of the complete genome of 18 Norwalk-like viruses. Virology 299:225-239. [DOI] [PubMed] [Google Scholar]
- 22.Kitamoro, N., T. Tanaka, K. Natori, N. Takeda, S. Nakata, X. Jiang, and M. K. Estes. 2002. Cross-reactivity among several recombinant calicivirus-like particles (VLPs) with monoclonal antibodies obtained from mice immunized orally with one type of VLP. J. Clin. Microbiol. 40:2459-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelsey, J. L., A. S. Whittemore, A. S. Evans, and W. D. Thompson. 1996. Methods in observational epidemiology, p. 341-363. Monograph in epidemiology and biostatistics, vol. 26. Oxford University Press, New York, N.Y.
- 24.Kobayashi, S., K. Sakae, K. Natori, N. Takeda, T. Miyamura, and Y. Suzuki. 2000. Serotype-specific antigen ELISA for detection of Chiba virus in stool. J. Med. Virol. 62:233-238. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi, S., K. Sakae, Y. Suzuki, K. Shinozaki, M. Okada, H. Ishiko, K. Kawata, K. Suzuki, K. Natori, T. Miyamura, and N. Takeda. 2000. Molecular cloning, expression, and antigenisity of Seto virus belonging to genotype I Norwalk-like viruses. J. Clin. Microbiol. 38:3492-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leite, J. P. G., T. Ando, J. S. Noel, B. Jiang, C. D. Humphrey, J. F. Lew, K. Y. Green, R. I. Glass, and S. S. Monroe. 1996. Characterization of Toronto virus capsid protein expressed in baculovirus. Arch. Virol. 141:865-875. [DOI] [PubMed] [Google Scholar]
- 27.Lew, J. F., A. Z. Kapikian, X. Jiang, M. K. Estes, and K. Y. Green. 1994. Molecular characterization and expression of the capsid protein of a Norwalk-like virus recovered from a Desert Shield troop with gastroenteritis. Virology 200:319-325. [DOI] [PubMed] [Google Scholar]
- 28.Lindqvist, R., Y. Andersson, J. Lindback, M. Wegscheider, Y. Eriksson, L. Tidestrom, A. Lagerqvist-Widh, K.-O. Hedlund, S. Lofdahl, L. Svensson, and A. Norinder. 2001. A one-year study of foodborne illness in the municipality of Uppsala, Sweden. Emerg. Infect. Dis. 7:588-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopman, B. A., G. K. Adak, M. H. Reacher, and D. W. G. Brown. 2003. Two epidemiologic patterns of norovirus outbreaks: surveillance in England and Wales, 1992-2000. Emerg. Infect. Dis. 9:71-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicollier-Jamot, B., V. Pico, P. Pothier, and E. Kohli. 2003. Molecular cloning, expression, self-assembly, antigenicity, and seroepidemiology of a genotype II norovirus isolated in France. J. Clin. Microbiol. 41:3901-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noel, J. S., R. L. Fankhauser, T. Ando, S. S. Monroe, and R. I. Glass. 1999. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J. Infect. Dis. 179:1334-1344. [DOI] [PubMed] [Google Scholar]
- 33.Pletneva, M. A., S. V. Sosnovtsev, and K. Green. 2001. The genome of Hawaii virus and its relationship with other members of Caliciviridae. Virus Genes 23:5-16. [DOI] [PubMed] [Google Scholar]
- 34.Richards, A. F., B. Lopman, A. Gunn, A. Curry, D. Ellis, H. Cotterill, S. Ratcliffe, M. Jenkins, H. Appleton, C. I. Gallimore, J. J. Gary, and D. W. G. Brown. 2003. Evaluation of a commercial ELISA for detecting Norwalk-like virus antigen in faeces. J. Clin. Virol. 26:109-115. [DOI] [PubMed] [Google Scholar]
- 35.SAS Institute, Inc. 1999. SAS OnlineDoc. SAS Institute, Inc. Cary, N.C.
- 36.Schuffenecker, I., T. Ando, D. Thouvenot, B. Lina, and M. Aymard. 2001. Genetic classification of “Sapporo-like viruses.” Arch. Virol. 146:2115-2132. [DOI] [PubMed] [Google Scholar]
- 37.Uchino, K., Y. Iwagami, and T. Tanaka. 2002. Newly developed Norwalk-like viruses detection ELISA by combination with monoclonal antibodies and hyperimmune rabbit sera. Nippon Rinsho 60:1188-1193. (In Japanese.) [PubMed] [Google Scholar]
- 38.Vinje, J., and M. P. G. Koopmans. 2000. Simultaneous detection and genotyping of “Norwalk-like viruses” by oligonucleotide array in a reverse line blot hybridization format. J. Clin. Microbiol. 38:2595-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinje, J., H. Vennema, L. Maunula, C.-H. von Bonsdorff, M. Hoehne, E. Schreier, A. Richards, J. Green, D. Brown, S. S. Beard, S. S. Monroe, E. de Bruin, L. Svensson, and M. P. G. Koopmans. 2003. International collaborative study to compare reverse transcriptase PCR assay for detection and genotyping of noroviruses. J. Clin. Microbiol. 41:1423-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinje, J., R. A. Hamidjaja, and D. Sobsey. 2004. Development and application of a capsid VP1 (region D) based reverse transcription PCR assay for genotyping of genogroup I and II noroviruses. J. Virol. Methods 116:109-117. [DOI] [PubMed] [Google Scholar]
- 41.Yamagami, T., H. Asakawa, and Y. Oishi. 2002. Evaluation of Norwalk virus detection kit by enzyme immunoassay. Rinsho Biseibutsu Jinsoku Shindan Kenkyukai Shi 13:15-18. (In Japanese.) [PubMed] [Google Scholar]
- 42.Yoda, T., Y. Suzuki, Y. Terano, K. Yamazaki, N. Sakon, T. Kuzuguchi, H. Oda, and T. Tsukamoto. 2003. Precise characterization of norovirus (Norwalk-like virus)-specific monoclonal antibodies with broad reactivity. J. Clin. Microbiol. 41:2367-2371. [DOI] [PMC free article] [PubMed] [Google Scholar]