Abstract
Investigations on the functional niche of organisms have primarily focused on differences among species and tended to neglect the potential effects of intraspecific variability despite the fact that its potential ecological and evolutionary importance is now widely recognized. In this study, we measured the distribution of functional traits in an entire population of largemouth bass (Micropterus salmoides) to quantify the magnitude of intraspecific variability in functional traits and niche (size, position, and overlap) between age classes. Stable isotope analyses (δ13C and δ15N) were also used to determine the association between individual trophic ecology and intraspecific functional trait variability. We observed that functional traits were highly variable within the population (mean coefficient variation: 15.62% ± 1.78% SE) and predominantly different between age classes. In addition, functional and trophic niche overlap between age classes was extremely low. Differences in functional niche between age classes were associated with strong changes in trophic niche occurring during ontogeny while, within age classes, differences among individuals were likely driven by trophic specialization. Each age class filled only a small portion of the total functional niche of the population and age classes occupied distinct portions in the functional space, indicating the existence of ontogenetic specialists with different functional roles within the population. The high amplitude of intraspecific variability in functional traits and differences in functional niche position among individuals reported here supports the recent claims for an individual-based approach in functional ecology.
Keywords: Functional traits, niche, ontogeny, overlap, stable isotope analyses
Introduction
Biodiversity studies have primarily focused on the role of species richness (i.e., the number of species; Tilman 1997), although biodiversity has a multitude of facets (Gaston 1996; Purvis and Hector 2000) and the ecological characteristics and roles of species are not equal (Tilman 1997; Lavorel and Garnier 2002; Bolnick et al. 2011). During the last decade, there has been an increasing body of literature calling for the use of functional approach to understand and quantify biological diversity, notably in the general context of human-induced perturbations (Mouillot et al. 2013). Functional diversity approaches are based on the functional traits of species, that is, any biological attributes that impact fitness through effects on growth, reproduction, or survival of organisms (Violle et al. 2007), and it has been applied to address many ecological questions (Petchey and Gaston 2002; Mouillot et al. 2004; Mason et al. 2005). For animal species, these functional traits are typically obtained based on morphological measurements to estimate vital functions (e.g., locomotion and food acquisition in fish, Dumay et al. 2004; Mason et al. 2008; Villéger et al. 2010; Albouy et al. 2011 and foraging movements in birds, Ricklefs 2012).
In the meantime, population ecologists have reported the existence of intraspecific variability in morphological traits in animal taxa driven, for instance, by resource polymorphism, trophic specialization, ontogeny, or sexual dimorphism (Smith and Skulason 1996; Hjelm et al. 2000, 2001; Svanbäck and Eklöv 2002; Bolnick et al. 2003). However, despite the existence of such variations, functional ecologists have primarily focused on the differences in functional traits among species without accounting for the potential effects of intraspecific (i.e., between and within populations) variations in animal populations (Wilson et al. 1999; Ackerly and Cornwell 2007; Violle et al. 2012). This stands on the assumption that intraspecific variation was negligible compared to interspecific variation when studying functional ecology at the community level (McGill et al. 2006; Jung et al. 2010; Albert et al. 2011). Accordingly, low levels of intraspecific variation have been reported in the literature (Garnier et al. 2001; Dumay et al. 2004; Buckley et al. 2010). However, because intraspecific variations in functional traits can affect ecological interactions (Bolnick et al. 2011) and ecosystem functioning (Harmon et al. 2009; Rudolf and Rasmussen 2013a), it has been claimed that functional ecology should become more individual based than species based (McGill et al. 2006; Petchey and Gaston 2006; Cianciaruso et al. 2009; Violle et al. 2012).
This is especially true if intraspecific variation in functional traits is naturally present in wild populations. Indeed, within a population, the functional characteristics of individuals can change as individuals usually undergo morphological shifts during ontogeny (Miller and Rudolf 2011), and this intraspecific variations are ecologically relevant because they are often associated with ontogenetic shift in habitat and trophic niches (Ingram and Shurin 2009). Functional traits can also differ within life stages as individuals might exploit different ecological niches (e.g., resource polymorphism, Skulason and Smith 1995; Cucherousset et al. 2011). Depending upon their intensity, these two mechanisms can subsequently translate into different scenarios of niche overlap among life stages, ranging from no niche overlap between juveniles and adults to a high niche overlap whereby one life stage could be totally nested within the space of the other (Hammerschlag-Peyer et al. 2011). Previous studies have revealed that invertebrate consumers without metamorphosis could display an average of 40% niche overlap between life stages (Woodward and Hildrew 2002). It has suggested that this low ontogenetic niche overlap could decrease the stability of ecological networks (Rudolf and Lafferty 2011). However, there are still no empirical studies that have quantified the degree of variability and overlap in functional traits and the associated variation in trophic niche within a top predator species.
In this study, we quantified the distribution of functional traits describing food acquisition and locomotion (Villéger et al. 2010; Albouy et al. 2011) within a whole population of top predator fish to determine (1) the magnitude of intraspecific changes in functional traits during ontogeny; (2) functional niche position, size, and overlap between age classes; and (3) the association between functional and trophic (stable isotope) variability at the individual level.
Materials and Methods
Model species and sampling
Largemouth bass (Micropterus salmoides), a top predator freshwater fish species that has been introduced in more than 50 countries and reported to display ecological impacts (Cucherousset and Olden 2011), was selected as a model species. Largemouth bass undergo strong trophic niche shift during ontogeny as the species diet change from zooplankton to macroinvertebrates and prey fish (Post 2003). The sampled population was located in southwest France in a 1500 m2 private pond where angling was prohibited. This pond has been stocked approximately 15 years before sampling with largemouth bass and roach (Rutilus rutilus), a native cyprinid prey fish. Seine netting was used in July 2010 to collect largemouth bass and the pond was fully drained in October 2010 for maintenance purposes, ensuring that all individuals of the population were captured (ntotal = 105). Collected specimens were euthanized using an overdose of anesthetic and preserved at −18°C.
Data acquisition
In the laboratory, a set of 19 measurements describing the morphological characteristics of individuals was performed on each specimen directly using a scale and a digital caliper or through picture analyses (ImageJ). This set included mass (M), standard body length (Bl), body depth (Bd), caudal peduncle minimal depth (CPd), maximal caudal fin depth (CFd), caudal fin surface (CFs), eye diameter (Ed), distance between the center of the eye to the bottom of the head (Eh), total gut length (Gl), maximal gill raker length (GRl), head depth along the vertical axis of the eye (Hd), distance from the top of the mouth to the bottom of the head along the head depth axis (Mo), distance between the insertion of the pectoral fin to the bottom of the body (PFi), body depth at the level of the pectoral fin insertion (PFb), pectoral fin length (PFl), pectoral fin surface (PFs), body width (Bw), mouth depth (Md), and mouth width (Mw; Villéger et al. 2010; Albouy et al. 2011). Scales were collected in the antero-medial region of each individual for age determination (Britton et al. 2010), and individuals were subsequently grouped into three age classes: age-0, age-1, and ≥age-2. Finally, a sample of white dorsal muscle was collected on each specimen, oven dried (60°C for 48 h) and analyzed for stable isotope values (δ13C and δ15N) at the Cornell Isotope Laboratory (COIL, Ithaca, NY).
Statistical analyses
We selected 16 complementary functional traits (Table 1) to reflect ecological functions of interest (i.e., multifaceted strategies associated locomotion and food acquisition) and which can be easily quantified on a large number of individuals (Dumay et al. 2004). Following these criteria and on the basis of published literature (Sibbing and Nagelkerke 2000; Mouillot et al. 2007; Schleuter et al. 2012; Reecht et al. 2013), functional traits were quantified using the aforementioned measurements. Functional traits described food acquisition (i.e., oral gape surface, oral gape shape, oral gape position, eye diameter, gill raker length, gut length), locomotion (i.e., eye position, body section shape, body section area, pectoral fin position, pectoral fin shape, caudal peduncle throttling, caudal fin shape, fins area ratio, fins area) or both (mass) in fish (Villéger et al. 2010; Albouy et al. 2011; Mouillot et al. 2013; details in Table 1). For instance, oral gape shape is associated to prey shape and capture. Specifically, individuals with lower oral gape shape tend to feed on benthic prey while individuals with higher oral gape shape tend to filter water for feeding (Karpouzi and Stergiou 2003). Pectoral fin position represents fish maneuverability and its position in the water column (Bellwood and Wainwright 2001; Bellwood et al. 2002; Wainwright et al. 2002; see details in Table 1 for other functional traits). Except mass, these functional traits are unitless ratio that are a priori independent of individual body size (Winemiller 1991; Villéger et al. 2010) to ensure that changes measured across age classes were not solely driven by changes in individual size.
Table 1.
List of the 16 functional traits associated with food acquisition and locomotion (adapted from Villéger et al. 2010). The letter in brackets indicates the function associated with each trait (F, food acquisition and L, locomotion). Coefficients of variation (CV) measured in the population
| Functional traits | Measure | Ecological meaning | CV, % |
|---|---|---|---|
| Mass (F/L) | log (M + 1) | Volume, muscle mass | 24.71 |
| Oral gape surface (F) | 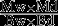 |
Maximum prey size or ability to filter water | 18.61 |
| Oral gape shape (F) | 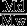 |
Prey shape and food acquisition | 6.50 |
| Oral gape position (F) |  |
Position of prey in the water | 16.03 |
| Eye diameter (F) | 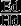 |
Prey detection | 14.80 |
| Gill raker length (F) |  |
Filtration capacity or gill protection | 31.39 |
| Gut length (F) | 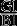 |
Digestibility of food | 10.16 |
| Eye position (L) | 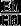 |
Position in the water column | 11.51 |
| Body section shape (L) | 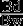 |
Position in the water column and hydrodynamism | 5.03 |
| Body section area (L) | 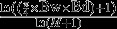 |
Mass distribution along the body and hydrodynamism | 22.05 |
| Pectoral fin position (L) | 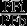 |
Maneuverability and position in the water column | 6.73 |
| Pectoral fin shape (L) | 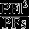 |
Propulsion and/or maneuverability | 19.64 |
| Caudal peduncle throttling (L) | 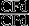 |
Swimming endurance | 12.77 |
| Caudal fin shape (L) | 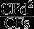 |
Endurance, acceleration, and/or maneuverability | 18.14 |
| Fins area ratio (L) | 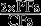 |
Swimming type (pectoral or caudal fin propulsion) | 19.63 |
| Fins area (L) | 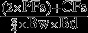 |
Endurance, acceleration, and/or maneuverability | 12.23 |
M, mass; Bl, standard body length; Bd, body depth; CPd, caudal peduncle minimal depth; CFd, maximal caudal fin depth; CFs, caudal fin surface; Ed, eye diameter; Eh, distance between the centre of the eye to the bottom of the head; Gl, total gut length; GRl, maximal gill raker length; Hd, head depth along the vertical axis of the eye; Mo, distance from the top of the mouth to the bottom of the head along the head depth axis; PFi, distance between the insertion of the pectoral fin to the bottom of the body; PFb, body depth at the level of the pectoral fin insertion; PFl, pectoral fin length; PFs, pectoral fin surface; Bw, body width; Md, mouth depth; Mw: mouth width.
Intraspecific differences in functional traits were quantified using a multiple-trait approach. A synthetic multidimensional functional space was built by computing a principal components analysis (PCA) on the functional traits measured on all individuals (after scaling each trait to a mean of 0 and a standard deviation of 1; Villéger et al. 2008). The four-first principal components (eigenvalues > 1) were then used as synthetic axes (Villéger et al. 2008). Differences in niche position between age classes were tested using PERMANOVA on the first four axes. To quantify the effect of ontogeny on niche size, we calculated the functional niche size of each age class as the amount of space filled in the multiple dimensional functional space from the PCA axes (hull area, Villéger et al. 2008). Then the levels of functional niche overlap between three age classes were calculated following Villéger et al. (2013). As the number of individuals varied between age classes, we also computed bootstrapped functional niche size and overlap values (n = 10,000) based on the minimum number of individuals within the three age classes. In addition, we tested whether intraspecific variability affects the estimates of niche size obtained with a restricted number of individuals. We thus computed functional niche size on 10,000 random subsets of 15 individuals from the entire population. This sample size is similar to the number of individuals considered per species in study on functional diversity in fish communities (e.g., Mason et al. 2008; Villéger et al. 2010; Albouy et al. 2011; Mouchet et al. 2012). These bootstrapped functional niche size values were subsequently compared to the observed functional niche size of the entire population.
We also tested whether stable isotope values (δ13C and δ15N) and trophic niche position differ between age classes using PERMANOVA and Kruskal–Wallis tests. Stable isotope niche size and niche overlap were quantified using the convex hull area (TA) in δ13C-δ15N bi-plot space (Layman et al. 2007) for each age class. Although convex hull area could be affected by the number of individuals analyzed, it represents in this study the entire trophic niche in the population as all individuals were sampled. In addition, the core of the stable isotope niche was also quantified using standard ellipse area corrected for small sample sizes (SEAc; Jackson et al. 2011, 2012). Comparisons of stable isotope niche size between age classes were performed using Bayesian estimates of standard ellipse areas (SEAB; Jackson et al. 2011). Finally, correlations between each of the four PCA axes and each stable isotope value (δ13C and δ15N) were tested using Pearson correlations with Bonferroni corrections for multiple tests. All statistical analyses were conducted in R (R Development Core Team 2011).
Results
Total length ranged from 62 to 139 mm, from 150 to 211 mm, and from 236 to 323 mm for age-0 (n = 33), age-1 (n = 64) and ≥age-2 (n = 8), respectively, and total length did not overlap between age classes. High intraspecific variations were observed for each of the 16 functional traits (mean coefficient variation: 15.62% ± 1.78% SE; Table 1). The four-first PCA axes explained 70.6% of the total inertia (PC1 = 28.9%, PC2 = 24.1%, PC3 = 10.3%, PC4 = 7.3%, respectively; Table 2). Specifically, PC1 was mainly driven by mass and functional traits related to locomotion; as PC1 values increased, individuals were more elongated and maneuverable (rounded pectoral fin shape) with a higher endurance (thicker caudal peduncles). PC2 was principally associated with functional traits related to food acquisition; as PC2 values increased, individuals displayed mouth in a more ventral position and laterally flattened with larger eyes and closer to the mouth.
Table 2.
Pearson correlation coefficients between the four principal components analysis axes and the 16 functional traits. Significant P-values are in bold
| Functional traits | PC1 (28.9%) | PC2 (24.1%) | PC3 (10.3%) | PC4 (7.3%) |
|---|---|---|---|---|
| Mass | 0.86 | 0.32 | −0.12 | 0.04 |
| Oral gape surface | 0.30 | −0.61 | 0.08 | −0.21 |
| Oral gape shape | 0.06 | 0.20 | −0.60 | −0.19 |
| Oral gape position | 0.35 | −0.74 | 0.20 | 0.10 |
| Eye diameter | −0.59 | 0.65 | −0.05 | −0.17 |
| Gill raker length | 0.08 | 0.73 | −0.34 | −0.04 |
| Gut length | 0.38 | 0.53 | 0.09 | −0.19 |
| Eye position | 0.41 | −0.74 | 0.15 | 0.02 |
| Body section shape | 0.12 | 0.08 | 0.11 | −0.85 |
| Body section area | −0.79 | −0.45 | 0.16 | −0.07 |
| Pectoral fin position | −0.10 | −0.51 | 0.01 | −0.52 |
| Pectoral fin shape | −0.79 | 0.18 | 0.37 | 0.04 |
| Caudal peduncle throttling | 0.65 | 0.44 | 0.53 | 0.00 |
| Caudal fin shape | 0.66 | 0.41 | 0.50 | 0.04 |
| Fins area ratio | 0.52 | −0.41 | −0.62 | 0.08 |
| Fins area | 0.78 | −0.04 | −0.01 | −0.09 |
The position of individuals in the functional space differed significantly among the three age classes (PERMANOVA P < 0.001, Fig. 1A and B). Observed functional niche size (hull area) decreased with age classes and was relatively low compared to the niche size of the entire population (Fig. 1A and B). While there were more age-1 (n = 64) than age-0 (n = 33) individuals in the population, they had a smaller observed functional niche size than age-0 individuals. The smallest observed functional niche size was displayed by ≥age-2 individuals (Table 3 and Fig. 1). Bootstrap tests revealed that, when considering only eight individuals, the functional niche size of age-0 and age-1 were not significantly different from the functional niche size of adults (Table 3). However, when considering 33 individuals, the functional niche size of age-1 was significantly lower than that of age-0 (Table 3). There was no functional niche overlap between ≥age-2 and age-0 classes and between ≥age-2 and age-1 classes. The functional niche overlap between age-0 and age-1 classes was 0.52%. Bootstrap tests considering only eight individuals in each age class also revealed a very low overlap between age-0 and age-1 classes (mean = 0.003% ± 0.0008% SE). When considering a random subsample of 15 individuals (i.e., 14.3% of the entire population), functional niche size estimate corresponded on average to only 7.9% (SE: ±2.5%) of the total functional niche size. This indicated that accounting for a restricted number of individuals in such a heterogeneous population disproportionately affects estimates of functional diversity in the population.
Figure 1.
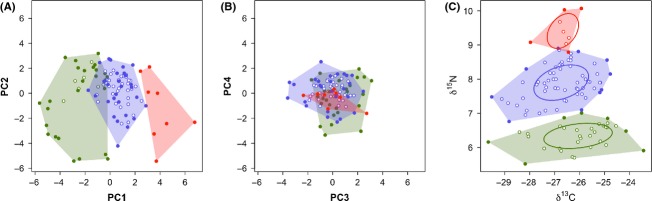
Distribution of the three age classes (green: age-0, blue: age-1, red: ≥age-2) in the functional and trophic spaces. (A) PC1 and PC2 of the functional space, (B) PC3 and PC4 of the functional space, and (C) stable isotope values (δ13C and δ15N). Colored polygons represent the niche size (convex hull area) of each age class, and filled points are vertices of the convex hull computed in four dimensions. Colored ellipses represent the standard ellipse area (SEAc) calculated for each age class based on stable isotope values.
Table 3.
Number of individuals in each age class, observed and bootstrapped functional niche size values considering only eight or 33 individuals (95% confidence interval) and trophic niche size (convex hull: TA; standard ellipse area: SEAc; Bayesian estimates of the standard ellipse area: SEAB) of the three age classes (age-0, age-1, ≥age-2)
| Functional niche | Trophic niche | ||||||
|---|---|---|---|---|---|---|---|
| n | Observed | Bootstrappedn = 8 | Bootstrappedn = 33 | TA | SEAc | SEAB | |
| Age-0 | 33 | 243.69 | 1.45–47.81 | – | 5.41 | 1.53 | 1.82 |
| Age-1 | 64 | 96.60 | 0.77–9.13 | 31.57–68.56 | 6.39 | 1.68 | 1.77 |
| ≥Age-2 | 8 | 5.94 | – | – | 1.34 | 0.95 | 1.69 |
Trophic niche position significantly differed between the three age classes (PERMANOVA, P < 0.001, Fig. 1C). Specifically, δ15N values (mean: 6.34 ‰ (±0.06 SE), 7.90 ‰ (±0.06 SE) and 9.41 ‰ (±0.17 SE) for age-0, age-1 and ≥age-2, respectively) significantly differed between age classes (Kruskal–Wallis test, P < 0.01, Fig. 1C), suggesting an increased trophic position during ontogeny. The origin of the carbon consumed by largemouth bass slightly but significantly changed during ontogeny as δ13C values (mean: −26.06 ‰ (±0.24 SE), −26.74 ‰ (±0.14 SE) and −26.67 ‰ (±0.21 SE) for age-0, age-1, and ≥age-2, respectively) differed significantly between age classes (Kruskal–Wallis test, P = 0.01, Fig. 1C). Interestingly, within each age class, the range of δ13C values was high, but the trophic niche size of each age classes was relatively low compared to the entire population. Age-0 individuals (TA = 5.41 and SEAc = 1.53) filled slightly less trophic space than age-1 individuals (TA = 6.39 and SEAc = 1.68) and these two age classes filled more trophic space than ≥age-2 (TA = 1.34 and SEAc = 0.95; Table 3; Fig. 1C). Although these differences in trophic niche size were not significant between age classes (age-0: SEAB = 1.82, age-1: SEAB = 1.77, ≥age-2: SEAB = 1.69, P > 0.05), each age class could also be considered to occupy distinct trophic niche as there was no or only little trophic niche overlap between age classes (age-0 vs. age-1: 0 and 0%; age-0 vs. ≥age-2: 0 and 0%; age-1 vs. ≥age-2: 0.28 and 0% for TA and SEAc, respectively).
δ13C values were significantly and positively correlated with PC2 (Pearson correlation, Bonferroni correction, r = 0.31, P < 0.006, Fig. 2). δ15N values increased significantly with PC1 (Pearson correlation, Bonferroni correction, r = 0.72, P < 0.006; Fig. 2). PC3 and PC4 were not significantly correlated to δ13C (Pearson correlation, Bonferroni correction, r = 0.06, P = 0.560 and r = 0.01, P = 0.894, respectively) and to δ15N values (Pearson correlation, Bonferroni correction, r = −0.24, P = 0.014 and r = 0.16, P = 0.109, respectively).
Figure 2.
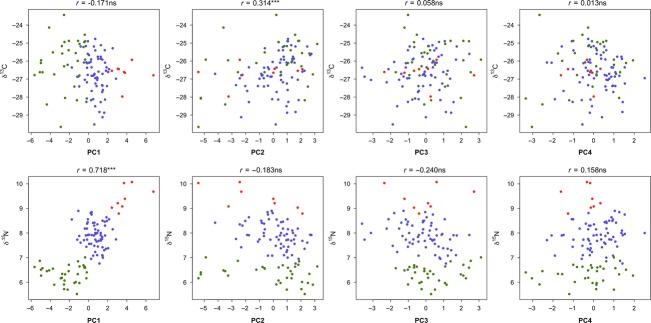
Pearson correlations between individual values on the four principal components analysis axes and stable isotope values (δ13C and δ15N, trophic niche; green = age-0; blue = age-1; red = ≥age-2). ns: not significant; ***: P < 0.006 (Bonferroni correction).
Discussion
The present study demonstrated that the variation in the functional attributes of individuals within a top predator population was high, principally driven by ontogenetic shifts (differences among age classes) coupled to interindividual variability (differences within age classes). Overall, the level of overlap in functional niche among the three age classes was null or extremely low. Specifically, the patterns of ontogenetic niche shift demonstrated that age-0 individuals overlapped only slightly with age-1 individuals that displayed a totally distinct niche than ≥age-2 individuals. Additionally, we found that age-0 and age-1 individuals significantly differed in terms of functional niche size, that is, the amount of space occupied in the functional space. All of these observations indicated that these three age classes should be considered as distinct functional entities when investigating the functional properties of populations or the functional diversity of communities as this source of variation could disproportionally affect the estimates of functional diversity. Furthermore, variations of 16 functional traits within the population were associated with significant changes in stable isotope values. A vast majority of animal species may modify their trophic resource use during ontogeny (Werner and Hall 1988) and this source of intraspecific variations can affect ecosystem functioning (Rudolf and Rasmussen 2013a). In the present study, despite the absence of differences in trophic niche size, there was no or extremely low overlap between the three age classes. Overall, these results demonstrated that largemouth bass should be considered as an ontogenetic ecological specialist, which could potentially reduce the level of stability in ecological networks such as food webs (Rudolf and Lafferty 2011).
Particularly, there was a strong association between ontogenetic trophic niche shift and several functional traits. The significant positive relationship between δ15N values and PC1 indicated that in addition to increased mass, most functional traits related to locomotion varied significantly. This might indicate change in locomotion attributes associated with foraging behavior and the mobility of prey encountered during ontogeny as largemouth bass diet shits from consuming zooplankton, macroinvertebrates to fish (Post 2003). During ontogeny, individuals displayed deeper body, thicker caudal peduncles, and more rounded pectoral fins and become more elongated and maneuverable with a higher endurance (Bellwood and Wainwright 2001; Wainwright et al. 2002) to mainly forage on prey fish in the pelagic area (Blake 2004), leading to higher δ15N values. Such morphological changes during ontogeny are relatively common in predatory fish (Amundsen et al. 2003; Johansson et al. 2006; Zimmerman et al. 2009). Interestingly, age-0 and age-1 individuals displayed a relatively wide range of δ13C values which indicated that, within the same age class and with similar δ15N values, individuals consumed prey with different origins such as aquatic and terrestrial invertebrates (Cucherousset et al. 2007). These results therefore suggest the potential existence of trophic specialization associated with differences in functional traits within life stages (Wilson et al. 1996; Svanbäck and Eklöv 2002). Moreover, the positive relationship between δ13C values and functional traits related to food acquisition suggested that individuals with higher PC2 values (i.e., larger eyes which were closer to the head, ventral position, and laterally flattened mouth) preying upon invertebrates or insects in the littoral zone (Winemiller 1991; Karpouzi and Stergiou 2003; Pouilly et al. 2003), leading to higher δ13C values.
Several studies have demonstrated that intraspecific variation in functional traits could be negligible compared to interspecific variation (McGill et al. 2006; Jung et al. 2010; Albert et al. 2011). At the opposite, the magnitude of intraspecific variation in functional traits observed in the present study suggests that, irrespective of its drivers, it should be considered (Albert et al. 2011, 2012; De Bello et al. 2011; Rudolf and Rasmussen 2013a). Therefore, we argue that intraspecific variability in predatory species should be explicitly accounted for when studying functional diversity of communities as distinct ecological entities can actually be discriminated within a population (Violle et al. 2012). Furthermore, as these ontogenetic differences can affect community structure and ecosystem functioning (Rudolf and Rasmussen 2013b), intraspecific variability in functional traits is likely to be important in ecosystem ecology. Using mean functional trait values across all age classes to estimate the diversity of communities is not appropriate as functional traits variation is dynamical and related to changes of population demographic structure (Rudolf and Rasmussen 2013b; Rudolf et al. 2014). All of these findings reinforce the need of quantifying intraspecific functional variability and the general idea that a shift from “species level” to “individual level” may enhance the ability of ecologists to understand and predict ecological patterns and processes (Bolnick et al. 2011; Violle et al. 2012).
Acknowledgments
We are grateful to S. Blanchet and N. Charpin for their help during fieldwork, to S. Boutes for access to the field site, and to M. Alp for editing the English style. We thank J. R. Britton for help in scale reading. ZT was supported by Chinese Scholarship Council (CSC) and JC by an “ERG Marie Curie” grant (PERG08-GA-2010-276969) in the laboratory EDB, part of the Laboratoire d'Excellence (LABEX) entitled TULIP ANR-10-LABX-41 ; ANR-11-IDEX-0002-02.
Conflict of Interest
None declared.
References
- Ackerly DD, Cornwell WK. A trait-based approach to community assembly: partitioning of species trait values into within- and among-community components. Ecol. Lett. 2007;10:135–145. doi: 10.1111/j.1461-0248.2006.01006.x. [DOI] [PubMed] [Google Scholar]
- Albert CH, Grassein F, Schurr FM, Vieilledent G, Violle C. When and how should intraspecific variability be considered in trait-based plant ecology? Perspect. Plant Ecol. Evol. Syst. 2011;13:217–225. [Google Scholar]
- Albert CH, De Bello F, Boulangeat I, Pellet G, Lavorel S, Thuiller W. On the importance of intraspecific variability for the quantification of functional diversity. Oikos. 2012;121:116–126. [Google Scholar]
- Albouy C, Guilhaumon F, Villéger S, Mouchet M, Mercier L, Culioli J, et al. Predicting trophic guild and diet overlap from functional traits: statistics, opportunities and limitations for marine ecology. Mar. Ecol. Prog. Ser. 2011;436:17–28. [Google Scholar]
- Amundsen P-A, Bøhn T, Popova O, Staldvik F, Reshetnikov Y, Kashulin N, et al. Ontogenetic niche shifts and resource partitioning in a subarctic piscivore fish guild. Hydrobiologia. 2003;497:109–119. [Google Scholar]
- Bellwood D, Wainwright P. Locomotion in labrid fishes: implications for habitat use and cross-shelf biogeography on the Great Barrier Reef. Coral Reefs. 2001;20:139–150. [Google Scholar]
- Bellwood DR, Wainwright PC, Fulton CJ, Hoey A. Assembly rules and functional groups at global biogeographical scales. Funct. Ecol. 2002;16:557–562. [Google Scholar]
- Blake R, W Fish functional design and swimming performance. J. Fish Biol. 2004;65:1193–1222. [Google Scholar]
- Bolnick DI, Svanbäck R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, et al. The ecology of individuals: incidence and implications of individual specialization. Am. Nat. 2003;161:1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, et al. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011;26:183–192. doi: 10.1016/j.tree.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JR, Harper DM, Oyugi DO. Is the fast growth of an equatorial Micropterus salmoides population explained by high water temperature? Ecol. Freshw. Fish. 2010;19:228–238. [Google Scholar]
- Buckley YM, Ramula S, Blomberg SP, Burns JH, Crone EE, Ehrlén J, et al. Causes and consequences of variation in plant population growth rate: a synthesis of matrix population models in a phylogenetic context. Ecol. Lett. 2010;13:1182–1197. doi: 10.1111/j.1461-0248.2010.01506.x. [DOI] [PubMed] [Google Scholar]
- Cianciaruso MV, Batalha MA, Gaston KJ, Petchey OL. Including intraspecific variability in functional diversity. Ecology. 2009;90:81–89. doi: 10.1890/07-1864.1. [DOI] [PubMed] [Google Scholar]
- Cucherousset J, Olden JD. Ecological impacts of nonnative freshwater fishes. Fisheries. 2011;36:215–230. [Google Scholar]
- Cucherousset J, Aymes JC, Santoul F, Céréghino R. Stable isotope evidence of trophic interactions between introduced brook trout Salvelinus fontinalis and native brown trout Salmo trutta in a mountain stream of south-west France. J. Fish Biol. 2007;71:210–223. [Google Scholar]
- Cucherousset J, Acou A, Blanchet S, Britton JR, Beaumont WRC, Gozlan RE. Fitness consequences of individual specialisation in resource use and trophic morphology in European eels. Oecologia. 2011;167:75–84. doi: 10.1007/s00442-011-1974-4. [DOI] [PubMed] [Google Scholar]
- De Bello F, Lavorel S, Albert CH, Thuiller W, Grigulis K, Dolezal J, et al. Quantifying the relevance of intraspecific trait variability for functional diversity. Methods Ecol. Evol. 2011;2:163–174. [Google Scholar]
- Dumay O, Tari PS, Tomasini JA, Mouillot D. Functional groups of lagoon fish species in Languedoc Roussillon, southern France. J. Fish Biol. 2004;64:970–983. [Google Scholar]
- Garnier E, Laurent G, Bellmann A, Debain S, Berthelier P, Ducout B, et al. Consistency of species ranking based on functional leaf traits. New Phytol. 2001;152:69–83. doi: 10.1046/j.0028-646x.2001.00239.x. [DOI] [PubMed] [Google Scholar]
- Gaston KJ. Biodiversity: a biology of numbers and difference. Cambridge, MA: Blackwell Science; 1996. [Google Scholar]
- Hammerschlag-Peyer CM, Yeager LA, Araújo MS, Layman CA. A hypothesis-testing framework for studies investigating ontogenetic niche shifts using stable isotope ratios (ed S Thrush) PLoS ONE. 2011;6:e27104. doi: 10.1371/journal.pone.0027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon LJ, Matthews B, Des Roches S, Chase JM, Shurin JB, Schluter D. Evolutionary diversification in stickleback affects ecosystem functioning. Nature. 2009;458:1167–1170. doi: 10.1038/nature07974. [DOI] [PubMed] [Google Scholar]
- Hjelm J, Persson L, Christensen B. Growth, morphological variation and ontogenetic niche shifts in perch (Perca fluviatilis) in relation to resource availability. Oecologia. 2000;122:190–199. doi: 10.1007/PL00008846. [DOI] [PubMed] [Google Scholar]
- Hjelm J, Svanback R, Bystrom P, Persson L, Wahlstrom E. Diet-dependent body morphology and ontogenetic reaction norms in Eurasian perch. Oikos. 2001;95:311–323. [Google Scholar]
- Ingram T, Shurin JB. Trait-based assembly and phylogenetic structure in northeast Pacific rockfish assemblages. Ecology. 2009;90:2444–2453. doi: 10.1890/08-1841.1. [DOI] [PubMed] [Google Scholar]
- Jackson AL, Inger R, Parnell AC, Bearhop S. Comparing isotopic niche widths among and within communities: SIBER – Stable Isotope Bayesian Ellipses in R: bayesian isotopic niche metrics. J. Anim. Ecol. 2011;80:595–602. doi: 10.1111/j.1365-2656.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- Jackson MC, Donohue I, Jackson AL, Britton JR, Harper DM, Grey J. Population-level metrics of trophic structure based on stable isotopes and their application to invasion ecology (ed S Thrush) PLoS ONE. 2012;7:e31757. doi: 10.1371/journal.pone.0031757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson F, Rådman P, Andersson J. The relationship between ontogeny, morphology, and diet in the Chinese hook snout carp (Opsariichthys bidens. Ichthyol. Res. 2006;53:63–69. [Google Scholar]
- Jung V, Violle C, Mondy C, Hoffmann L, Muller S. Intraspecific variability and trait-based community assembly. J. Ecol. 2010;98:1134–1140. [Google Scholar]
- Karpouzi VS, Stergiou KI. The relationships between mouth size and shape and body length for 18 species of marine fishes and their trophic implications. J. Fish Biol. 2003;62:1353–1365. [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 2002;16:545–556. [Google Scholar]
- Layman CA, Arrington DA, Montaña CG, Post DM. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology. 2007;88:42–48. doi: 10.1890/0012-9658(2007)88[42:csirpf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mason NWH, Mouillot D, Lee WG, Wilson JB. Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos. 2005;111:112–118. [Google Scholar]
- Mason NWH, Lanoiselée C, Mouillot D, Wilson JB, Argillier C. Does niche overlap control relative abundance in French lacustrine fish communities? A new method incorporating functional traits. J. Anim. Ecol. 2008;77:661–669. doi: 10.1111/j.1365-2656.2008.01379.x. [DOI] [PubMed] [Google Scholar]
- McGill B, Enquist B, Weiher E, Westoby M. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Miller TEX, Rudolf VHW. Thinking inside the box: community-level consequences of stage-structured populations. Trends Ecol. Evol. 2011;26:457–466. doi: 10.1016/j.tree.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Mouchet MA, Bouvier C, Bouvier T, Troussellier M, Escalas A, Mouillot D. Genetic difference but functional similarity among fish gut bacterial communities through molecular and biochemical fingerprints. FEMS Microbiol. Ecol. 2012;79:568–580. doi: 10.1111/j.1574-6941.2011.01241.x. [DOI] [PubMed] [Google Scholar]
- Mouillot D, Mason WHN, Dumay O, Wilson JB. Functional regularity: a neglected aspect of functional diversity. Oecologia. 2004;142:353–359. doi: 10.1007/s00442-004-1744-7. [DOI] [PubMed] [Google Scholar]
- Mouillot D, Dumay O, Tomasini JA. Limiting similarity, niche filtering and functional diversity in coastal lagoon fish communities. Estuar. Coast. Shelf Sci. 2007;71:443–456. [Google Scholar]
- Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 2013;28:167–177. doi: 10.1016/j.tree.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Petchey OL, Gaston KJ. Functional diversity (FD), species richness and community composition. Ecol. Lett. 2002;5:402–411. [Google Scholar]
- Petchey OL, Gaston KJ. Functional diversity: back to basics and looking forward. Ecol. Lett. 2006;9:741–758. doi: 10.1111/j.1461-0248.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- Post DM. Individual variation in the timing of ontogenetic niche shifts in largemouth bass. Ecology. 2003;84:1298–1310. [Google Scholar]
- Pouilly M, Lino F, Bretenoux J-G, Rosales C. Dietary-morphological relationships in a fish assemblage of the Bolivian Amazonian floodplain. J. Fish Biol. 2003;62:1137–1158. [Google Scholar]
- Purvis A, Hector A. Getting the measure of biodiversity. Nature. 2000;405:212–219. doi: 10.1038/35012221. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. Available via http://www.R-project.org/ [Google Scholar]
- Reecht Y, Rochet M-J, Trenkel VM, Jennings S, Pinnegar JK. Use of morphological characteristics to define functional groups of predatory fishes in the Celtic Sea: functional groups and ecomorphology. J. Fish Biol. 2013;83:355–377. doi: 10.1111/jfb.12177. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE. Species richness and morphological diversity of passerine birds. Proc. Natl Acad. Sci. 2012;109:14482–14487. doi: 10.1073/pnas.1212079109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf VHW, Lafferty KD. Stage structure alters how complexity affects stability of ecological networks: stage structure and network stability. Ecol. Lett. 2011;14:75–79. doi: 10.1111/j.1461-0248.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- Rudolf VHW, Rasmussen NL. Ontogenetic functional diversity: size structure of a keystone predator drives functioning of a complex ecosystem. Ecology. 2013a;94:1046–1056. doi: 10.1890/12-0378.1. [DOI] [PubMed] [Google Scholar]
- Rudolf VHW, Rasmussen NL. Population structure determines functional differences among species and ecosystem processes. Nat. Commun. 2013b;4:2318. doi: 10.1038/ncomms3318. [DOI] [PubMed] [Google Scholar]
- Rudolf VHW, Rasmussen NL, Dibble CJ, Van Allen BG. Resolving the roles of body size and species identity in driving functional diversity. Proc. Biol. Sci. 2014;281:20133203. doi: 10.1098/rspb.2013.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleuter D, Daufresne M, Veslot J, Mason NWH, Lanoiselée C, Brosse S, et al. Geographic isolation and climate govern the functional diversity of native fish communities in European drainage basins: functional diversity of European fish communities. Glob. Ecol. Biogeogr. 2012;21:1083–1095. [Google Scholar]
- Sibbing F, Nagelkerke LJ. Resource partitioning by Lake Tana barbs predicted from fish morphometrics and prey characteristics. Rev. Fish Biol. Fisheries. 2000;10:393–437. [Google Scholar]
- Skulason S, Smith TB. Resource polymorphisms in vertebrates. Trends Ecol. Evol. 1995;10:366–370. doi: 10.1016/s0169-5347(00)89135-1. [DOI] [PubMed] [Google Scholar]
- Smith TB, Skulason S. Evolutionary Significance of Resource Polymorphisms in Fishes, Amphibians, and Birds. Annu. Rev. Ecol. Syst. 1996;27:111–133. [Google Scholar]
- Svanbäck R, Eklöv P. Effects of habitat and food resources on morphology and ontogenetic growth trajectories in perch. Oecologia. 2002;131:61–70. doi: 10.1007/s00442-001-0861-9. [DOI] [PubMed] [Google Scholar]
- Tilman D. The influence of functional diversity and composition on ecosystem processes. Science. 1997;277:1300–1302. [Google Scholar]
- Villéger S, Mason NWH, Mouillot D. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology. 2008;89:2290–2301. doi: 10.1890/07-1206.1. [DOI] [PubMed] [Google Scholar]
- Villéger S, Miranda JR, Hernández DF, Mouillot D. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 2010;20:1512–1522. doi: 10.1890/09-1310.1. [DOI] [PubMed] [Google Scholar]
- Villéger S, Grenouillet G, Brosse S. Decomposing functional β-diversity reveals that low functional β-diversity is driven by low functional turnover in European fish assemblages: decomposing functional β-diversity. Glob. Ecol. Biogeogr. 2013;22:671–681. [Google Scholar]
- Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, et al. Let the concept of trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, et al. The return of the variance: intraspecific variability in community ecology. Trends Ecol. Evol. 2012;27:244–252. doi: 10.1016/j.tree.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Wainwright P, Bellwood D, Westneat M. Ecomorphology of locomotion in Labrid fishes. Environ. Biol. Fishes. 2002;65:47–62. [Google Scholar]
- Werner EE, Hall DJ. Ontogenetic habitat shifts in bluegill: the foraging rate-predation risk trade-off. Ecology. 1988;69:1352–1366. [Google Scholar]
- Wilson DS, Muzzall PM, Ehlinger TJ. Parasites, morphology, and habitat use in a Bluegill sunfish (Lepomis macrochirus) Population. Copeia. 1996;1996:348–354. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytol. 1999;143:155–162. [Google Scholar]
- Winemiller KO. Ecomorphological diversification in lowland freshwater fish assemblages from five biotic regions. Ecol. Monogr. 1991;61:343. [Google Scholar]
- Woodward G, Hildrew AG. Body-size determinants of niche overlap and intraguild predation within a complex food web. J. Anim. Ecol. 2002;71:1063–1074. [Google Scholar]
- Zimmerman MS, Schmidt SN, Krueger CC, Van der Zanden MJ, Eshenroder RL. Ontogenetic niche shifts and resource partitioning of lake trout morphotypes. Can. J. Fish. Aquat. Sci. 2009;66:1007–1018. [Google Scholar]


