Abstract
Telomeres are repetitive sequence structures at the ends of linear chromosomes that consist of double-stranded DNA repeats followed by a short single-stranded DNA (ssDNA) protrusion. Telomeres need to be replicated each cell cycle and protected from DNA-processing enzymes, tasks that cells execute using specialized protein complexes such as telomerase (TERT), which aids in telomere maintenance and replication, and the shelterin complex, which protects chromosome ends. These complexes are also able to interact with a variety of other proteins, referred to as the telomere interactome, in order to fulfil their biological functions and control signaling cascades originating from telomeres. Given their essential role in genomic maintenance and cell cycle control, germline mutations in telomere-regulating proteins and their interacting partners have been found to underlie a variety of diseases and cancer-predisposition syndromes. These syndromes can be characterized by progressively shortening telomeres, in which carriers can present with organ failure due to stem cell senescence among other characteristics, or can also present with long or unprotected telomeres, providing an alternative route for cancer formation. This review, summarizes the critical roles that telomere-regulating proteins play in cell cycle control and cell fate and explores the current knowledge on different cancer-predisposing conditions that have been linked to germline defects in these proteins and their interacting partners.
Keywords: Telomeres, telomerase, shelterin, germline variation, cancer predisposition
Introduction
Telomeres are specialized structures at the ends of linear chromosomes that consist of arrays of repeated nucleotide sequences. Telomeres play an essential role in regulating genomic stability by allowing the cell to distinguish between chromosome ends and double-stand DNA breaks, a function that is controlled by adopting characteristic chromosomic structures and by complexes of telomere-binding proteins. Protein complexes also control telomere length, access of the DNA repair machinery, and telomere end-protection, which is also known as “capping” (1-3). In humans and other vertebrates, telomeres comprise 9-15 kilobases of double-stranded TTAGGG repeats, followed by a 50-300-nucleotide protrusion of G-rich ssDNA, known as the G-strand overhang (3-5).
Tight regulation of telomere length is essential for normal cell function. In the absence of telomere length maintenance mechanisms, the ends of chromosomes are shortened with each round of cell division due to the inability of the replication machinery to fully synthesize the 5′ end of the lagging strand (6), as well as the need to enzymatically generate the G-strand overhang (7-9). In order to counteract these effects, cells use a specialized enzyme complex called telomerase that is able to add TTAGGG repeats to the ends of chromosomes (10). In most somatic cells, telomerase is under stringent control and expressed only transiently and at low levels, the exception being cells that are undergoing rapid proliferation such as bone marrow precursors cells, as well as embryonic or adult stem cells (11-13). However, the level of telomerase expression in somatic tissues is insufficient to sustain indefinite cell proliferation, and so telomeres progressively erode with each cell division, ultimately resulting in cell senescence or death (reviewed in (14)).
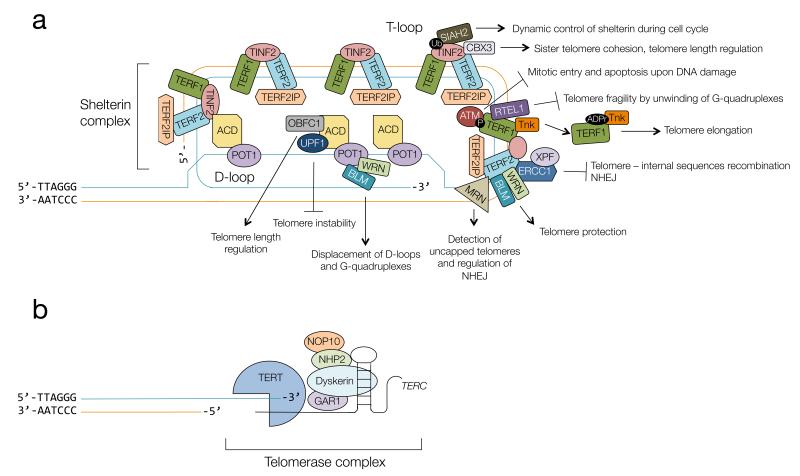
In addition to carefully regulating the length of telomeres, cells also need to distinguish telomere ends from DNA breaks that need to be repaired. Chromosome ends can adopt alternative structures that probably aid in this function. For example, the G-strand overhang is able to form G-quadruplexes, which are stacks of planar arrays of four G’s hydrogen-bonded by Hoogsteen base pairs (15, 16). This single-stranded overhang is also able to invade the double-stranded region of the telomere, forming what are referred to as a D- and T-loops and shielding the ssDNA from nucleases and DNA-processing enzymes (2, 17, 18) (Figure 1a). The shelterin complex, a large macromolecular structure composed of six proteins, also contributes to this function by binding to telomeres and inhibiting DNA repair, whilst also contributing to telomere length control by regulating access of telomerase to telomeres (2, 19) (Figure 1a). Alterations to telomere structure, or the loss of shelterin, results in defects in sister chromatid exchange, telomere fusions, polyploidization, telomere length dysregulation and other chromosomal abnormalities (2, 5, 20). The structures of telomerase and shelterin, as well as their roles in telomere protection and length regulation, are discussed in more detail in the next section.
Figure 1. Telomere-interacting proteins and their roles.
a) The shelterin complex, composed of six proteins (left), and several of its associated proteins are depicted with the biological functions they play in the cell (right). For simplicity, TERF1 and TERF2 homodimers are drawn as single boxes. The alternative T- and D-loop telomere structures are shown. Tnk: Tankyrase, Ub: ubiquitination, P: phosphorylation, ADPr: ADP ribosylation. b) The members of the telomerase complex are shown bound to the G-strand overhang with a simplified structure for TERC, which provides the template for repeat addition.
Telomerase and shelterin, with key roles in the regulation of telomere length and end-protection, have altered expression or are affected by somatic mutations in cancers (21-23), which confers upon malignant cells the ability to bypass senescence whilst promoting genomic instability. Many cancers display increased telomerase activity or have activated the alternative lengthening of telomeres (ALT) pathway leading to sustained telomere maintenance (reviewed in (24)). Further, ectopic expression of telomerase has been shown to elicit replicative immortality in certain cell types, a hallmark of cancer (25, 26). It has also been suggested that shelterin complex mutations might facilitate the acquisition of somatic aberrations, therefore driving cancer progression through accelerated tumor evolution (23, 27).
Here we discuss what is known about cancer predisposition syndromes driven by germline variants in telomere-regulating proteins.
Proteins involved in telomere maintenance
In this section, we briefly discuss the proteins and complexes involved in telomere maintenance.
Telomerase
This protein complex is formed by two core subunits: the catalytic telomerase reverse transcriptase (encoded by TERT) and the telomerase RNA component (TERC, also known as TR) (17, 28) (Figure 1b). Several additional accessory factors are necessary for telomerase assembly and activation, such as the auxiliary protein dyskerin (encoded by DKC1), the localization protein TCAB1 (encoded by WRAP53), EST1A (encoded by SMG6), which might aid in telomerase recruitment (29), and Pontin and Reptin (encoded by RUVBL1 and RUVBL2, respectively) (15).
The template for DNA synthesis is provided by TERC, a 451 nucleotide-long RNA subunit that can bind accessory proteins as well as TERT to ensure its own stability and telomerase biogenesis or localization (15, 30). The highly conserved TERT subunit provides the catalytic site for the addition of telomeric repeats to the end of chromosomes, and its amino acid sequence contains domains for binding TERC and for forming a closed ring-like structure that can bind the DNA-RNA duplex (15, 31). Dyskerin is an essential protein that associates with TERC and is required for its correct processing, as well as for telomerase activity and stability, and is found in a complex with both TERT and TERC when the catalytic enzyme is purified from cell lines (32, 33). Dyskerin can also bind the accessory proteins NOP10, NHP2 and GAR1 to form a subcomplex that is necessary for TERC accumulation in vivo (Reviewed in (12)) (Figure 1b). If proteins such as TCAB1 or EST1A are defective or abnormally expressed, then telomerase function is impaired due to mislocalization of TERC to the nucleoli or by telomere structural abnormalities (29, 34, 35).
In normal somatic cells, telomerase is assembled in Cajal bodies, which are specialized RNA-processing sub-organelles in the cell nucleus (36). It is then shipped to telomeres by TCAB1 and modified into an active conformation by Reptin and Pontin, by which point it is able to start adding nucleotides to the ends of telomeres (5, 37, 38). Telomerase is active during the S-phase of the cell cycle, and it is thought that it targets telomeres for elongation in a random fashion, despite some studies showing that it is recruited preferentially to the shortest telomeres (39-41). Regardless, telomerase expression is tightly regulated, as its unintended activity, or inactivity, can lead to tumor predisposition or stem cell exhaustion, respectively (42, 43).
Given the importance of the telomerase complex in telomere length maintenance, defects in any of its subunits or in proteins that assist its assembly and transport have been associated with premature aging, stem cell depletion, and predisposition to cancer and other syndromes (15, 44).
The shelterin complex
Despite being formed by only six proteins, shelterin displays a wide range of functions that include telomere length maintenance, protection from DNA repair mechanisms, and the regulation of signaling cascades from telomeres (5). It is composed of six core proteins: telomeric repeat-binding factors 1 and 2 (TERF1 and TERF2, also known as TRF1 and TRF2), TERF1-interacting protein 2 (TINF2, also known as TIN2), adrenocortical dysplasia protein homolog (ACD, also known as TPP1, TINT1, PTOP and PIP1), TERF2-interacting protein 1 (TERF2IP, also known as RAP1) and protection of telomeres 1 (POT1) (2) (Figure 1a). Although these proteins are fast-evolving, with the architecture of the complex being different in organisms such as ciliates and yeast when compared to mammals, the overall functionality of shelterin is highly conserved (45, 46).
TERF1 and TERF2 are double-stranded DNA-binding proteins that recognize telomeric repeats with high affinity upon homodimerization, while POT1, the most evolutionarily conserved member of shelterin, can specifically recognize telomeric ssDNA (2, 47-49). Therefore, the presence of several TTAGGG-binding domains in the complex gives shelterin its exquisite specificity for telomeric sequence. ACD binds to POT1, recruiting POT1 to telomeres and enhancing its recognition ability, whereas TERF2IP localizes to telomeres via its interaction with TERF2, and might have a role in the distribution of telomeres in post-mitotic nuclear assembly (2, 50-52). TINF2 is able to bind TERF1, TERF2 and the ACD/POT1 subcomplex, therefore bringing all the shelterin components together (15, 53) (Figure 1a).
The importance of the shelterin complex is evidenced by the fact that null mutations in the majority of its components result in embryonic lethality in mice (54-57). One of the most important functions of shelterin is to protect chromosome ends from DNA repair nucleases, which it achieves by inhibiting six DNA damage signaling pathways: ATM- and ATR-signaling, classical non-homologous end joining (NHEJ), alternative NHEJ, homologous recombination, and resection (20). Shelterin also has an important role in regulating telomere structure and length. TERF1 and TERF2 have DNA remodeling activities, being able to bend DNA and contributing to T-loop formation, whereas POT1 is able to regulate telomere length by contributing to the nucleolytic processing of the telomeric 5′ end and control telomerase access to the end of chromosomes (2, 47, 58, 59). The absence of functional shelterin therefore leads to polyploidization, fragile telomeres and sister chromatid exchanges, among other chromosomal aberrations (20).
The telomere interactome
The telomere interactome consists of the telomerase and the shelterin complexes along with an extended network of interacting partners, which aid in regulating diverse signaling cascades originating from chromosome ends (60). In this section, we discuss the different molecular processes that have been linked to the telomere interactome, as well as other non-telomeric functions for telomeric proteins relevant for cancer formation.
DNA repair signaling pathways and cell cycle control
Components of the shelterin complex, as mentioned above, are known to interact with DNA repair signaling pathways to control cell fate when telomeres are damaged. TERF1 co-immunoprecipitates with ATM, and is phosphorylated by it in vivo upon DNA damage, which prevents the cell from entering mitosis or apoptosis, and therefore results in a reduction in radiation sensitivity (61). It can also interact with RTEL1, a protein that aids in telomere elongation, to suppress telomere fragility possibly by unwinding G-quadruplexes (62-64). TERF1 can also bind EB1 (encoded by MAPRE1) and tankyrase (encoded by TNKS1), conferring it a role in microtubule polymerization and telomere elongation in a cell cycle-dependent manner (65-67) (Figure 1a).
TERF2 can recruit the ERCC1/XPF (encoded by ERCC4) heterodimer to the telomeric complex, which helps protect against telomere recombination with interstitial telomere-related sequences, and at the same time prevent NHEJ by blocking its access to the G-strand overhang (68). It is also able to recruit the MRE11A-RAD50-NBN (MRN) complex to telomeres, where it acts to detect the presence of uncapped telomeres (69, 70), and can also bind the Werner (WRN) and Bloom (BLM) helicases to stimulate their activity (71, 72). Other identified TERF2 binding partners are Apollo (encoded by DCLRE1B), Ku70 (encoded by XRCC6), PARP1/2 (Reviewed in (73)), and the chromatin regulator UBR5 (74) (Figure 1a).
Other members of the shelterin complex also interact with proteins involved in cell cycle regulation and genomic stability. For example, TINF2 can interact with the E3 ubiquitin ligase SIAH2, which regulates its stability allowing for dynamic control of the shelterin complex during the cell cycle (75). It can also bind CBX3 to maintain sister telomere cohesion and regulate telomere length (76). TERF2IP can interact with the nuclear envelope protein SUN1, which has a critical role in cell cycle progression by tethering telomeres to the nuclear membrane after mitosis (52), and with Ku80 (encoded by XRCC5) (77), a protein required for double-strand DNA repair. POT1 can physically interact with the WRN and BLM helicases, stimulating their activity and allowing efficient displacement of D-loops and G-quadruplexes (78, 79). ACD can physically bind the helicase and RNA surveillance protein UPF1 to prevent telomere instability and prevent activation of the DNA damage response (80), and can also interact with OBFC1 to regulate telomere length (81) (Figure 1a).
Other non-telomeric functions of telomeric proteins
TERF2IP has been found to have additional roles outside telomere maintenance. It has been shown that it can associate with the inhibitor of the NF-κB kinase (IKK) complex, thus positively regulating the NF-κB signaling pathway (82). Additionally, it can bind to extratelomeric chromosomal regions and possibly interact with other factors, thereby participating in subtelomeric gene silencing, metabolism control and interstitial genomic stability (83).
The catalytic subunit of telomerase also has other ‘non-canonical’ functions. TERT can act as a transcriptional modulator of the Wnt-β-catenin signaling pathway by associating with SMARCA4, a member of the SWI/SNF chromatin-remodeling complex, and can also physically occupy Wnt-dependent promoters (84). Also, in addition to using TERC as its RNA subunit, TERT is also able to bind the RNA component of mitochondrial RNA processing endoribonuclease (RMRP). The TERT-RMRP complex is then able to process RMRP into small interfering RNAs (siRNAs) to modulate its own levels, and it remains possible that TERT-RMRP is able to regulate the expression of other genes via the generation of siRNAs (85).
The association of telomeric proteins with these diverse factors provides a glimpse of the immensely complex interaction network that surrounds the telomere maintenance machinery, and its inherent connection to cell cycle regulation and DNA repair. The fact that some of the abovementioned proteins have ‘non-canonical’ roles outside of their role in safeguarding telomere length and end-protection has significantly extended our understanding of other biological processes, such as the regulation of important developmental pathways, mitochondrial function and cancer.
Telomere syndromes: variants in telomere-regulating genes and cancer predisposition
The contribution of acquired alterations in telomere-regulating genes and their interacting partners to cancer development is well documented. For example, telomerase has been found activated in hepatocellular carcinoma (86), melanoma (87), glioma and other cancers with low self-renewal rates (88), as well as other tissues (89), as it helps cells acquire replicative immortality. Moreover, the shelterin complex member POT1 has been reported to be somatically inactivated in chronic lymphocytic leukemia (23), where it causes telomere de-protection and length extension. DNA repair genes, such as XRCC5, XRCC6 and PARP1 are also frequent targets of somatic alteration in B cell lymphomas (90), and the NF-κB signaling pathway is frequently activated in breast cancer (91) and lymphomas (92), where it contributes to cell survival. It remains possible, given the important roles of these DNA repair and NF-κB pathway proteins in telomere maintenance (93-96), that patients that have somatically-acquired mutations in them display telomeric abnormalities. However, germline mutations in these genes also underlie an important proportion of hereditary cancer predisposition syndromes (Supplementary Tables 1-3). In this section we discuss the different disorders attributed to malfunction of components of the telomere interactome, including hereditary telomere dysfunction.
Telomere shortening syndromes
Progressive shortening of telomeres is seen in patients carrying germline mutations inactivating telomerase or telomere maintenance mechanisms, as their cells are unable to counteract telomere attrition after each cell division (41). In these individuals, telomeres might quickly reach their critical minimum size in highly proliferating tissues such as the bone marrow, and organ failure might ensue as stem cells senesce. However, in spite of this seemingly cancer-protecting effect, these individuals are also cancer-prone. The mechanism by which this happens is largely unexplained, although the fusion of chromosome ends, possibly by NHEJ, is potentially involved, as it has been been observed in mutation carriers and in Terc−/− mice (97).
Although telomere-shortening syndromes are precipitated via a common mechanism, their clinical presentations can be quite variable. This is, in part, due to a phenomenon known as genetic anticipation (41). Telomere length is a heritable trait, and because telomere shortening also occurs in germ cells, offspring of mutation carriers inherit chromosomes with shorter telomeres, and thus some patients will present earlier with a more severe phenotype than others (98). This is thought to be the reason behind the observation that, within a pedigree, the disease can evolve from generation to generation, becoming increasingly more severe. It might also be the case that offspring that inherit a wild-type copy of the disease-causing gene sometimes present with mild symptoms, as they will have inherited shorter telomeres. However, although this observation has been made in mice, it remains unclear whether this applies to humans (41, 42). It is worth mentioning that progressive telomere shortening is one of only two known molecular mechanism for genetic anticipation, the other one being the trinucleotide repeat expansion that underlies conditions such as Huntington’s disease and myotonic dystrophy (41).
Germline mutations in several of the telomere maintenance genes cause dyskeratosis congenita (DC), a rare multisystem disorder characterized by cutaneous abnormalities such as aberrant skin pigmentation, mucosal leukoplakia and nail dystrophy, in addition to bone marrow failure and cancer predisposition (99). About 10% of DC patients develop cancer, usually in the third decade of life, much younger than in the general population (100). Carcinomas of the bronchus, colon, larynx, pancreas, esophagus, skin and tongue, as well as leukemias and lymphomas are common in these patients (99). It has been estimated that DC patients have a 11-fold increase in cancer incidence compared to the general population, and that their risk, and cancer susceptibility spectrum, are similar to those of Fanconi anemia (FA) patients (100).
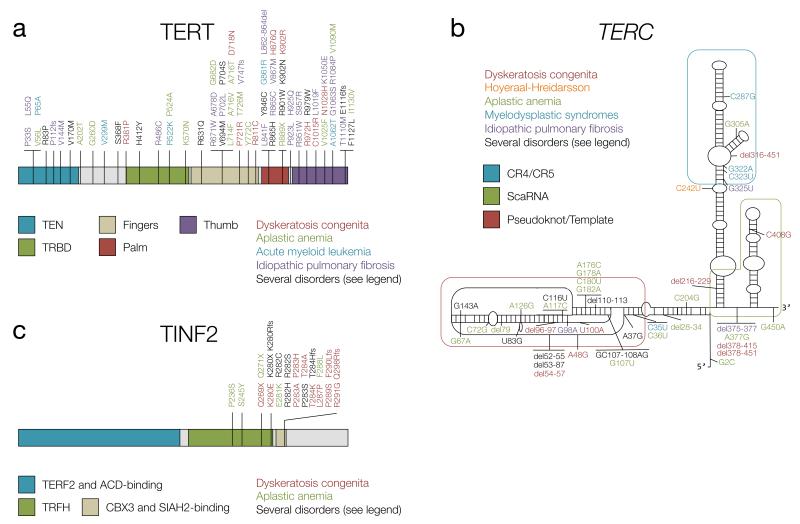
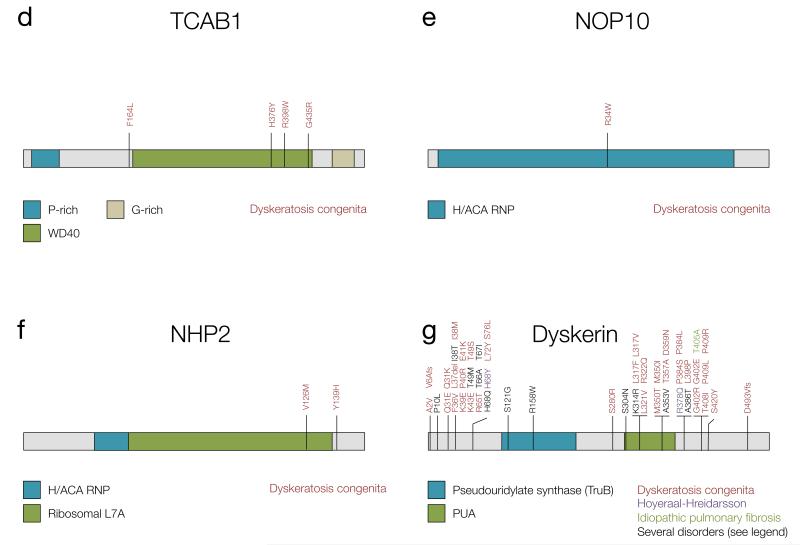
As DC is caused by mutations in several genes, it presents with different modes of inheritance. The autosomal dominant form, referred to as AD-DC, can be caused by mutations in TERT, TERC and TINF2, and it has been shown that mutations in these genes can act as either haploinsufficient or dominant negative alleles (reviewed in (101)) (Figure 2a-c). Variants in TINF2 have been shown to affect the CBX3/SIAH2 binding motif, affecting sister telomere cohesion (76). The autosomal recessive form (AR-DC) can be caused by variants in TCAB1, as well as in the genes coding for the telomerase accessory proteins NOP10 and NHP2, and are predicted to cause telomerase loss of function (41) (Figure 2d-f). The X-linked form of DC, caused by mutations in dyskerin (Figure 2g), is the most common and most severe, with more than 40% of patients developing bone marrow failure by the age of 10 years (102).
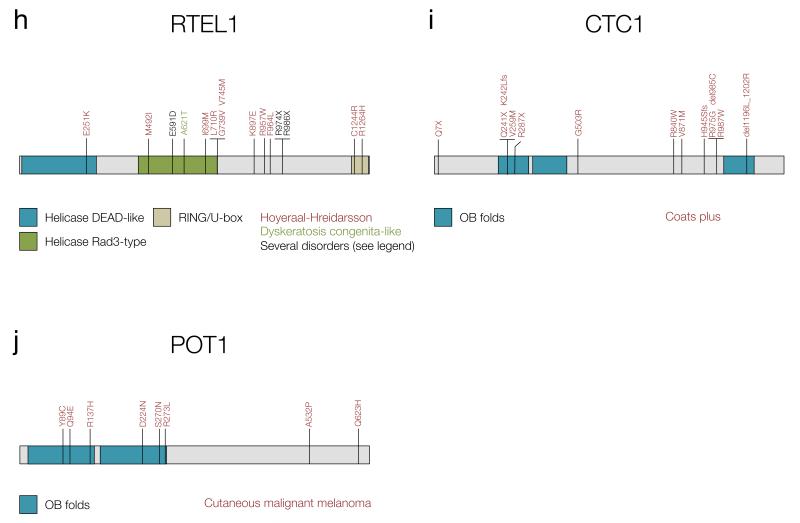
Figure 2. Germline mutations in telomere-interacting proteins found in familial cancer-predisposition syndromes.
The mutations depicted in panels a-c have been compiled from references (101) and (103). Protein structure has been taken from Ensembl release 75 (165) unless specified otherwise. a) Mutations in TERT. R83P: AA and MDS, V170M: AA and IPF, S368F: AA and IPF, H412Y: AA and AML, R631Q: DC and IPF, V694M: AA and IPF, P704S: DC, AA and IPF, Y846C: AA and DC, R865H: AA and IPF, R901W: DC and HHS, K902N: DC and AA, R979W: AA and DC, E116fs: AA and IPF, F1127L: DC and HHS. b) Mutations in TERC. The structure is taken from reference (103). A37G: DC and IPF, del52-55: DC and MDS, del 53-87: AA and IPF, U83G: AA and MDS, GC107-108AG: DC and AA, del 110-113: AA and MDS, C116U: AA and MDS, G143A: DC and AA. c) Mutations in TINF2. Structure taken from references (2) and (75). K280X: DC, HHS and Revesz syndrome, K280Rfs: DC and Revesz syndrome, R282S: DC and Revesz syndrome, R282C: DC and AA, R282H: DC, HHS and Revesz syndrome, P283S: DC and HHS, T284Hfs: DC and AA. d) Mutations in TCAB1. Protein structure taken from (166). Mutations taken from reference (34). e) Mutations in NOP10 were taken from reference (103). f) Mutations in NHP2 were taken from reference (103). g) Mutations in dyskerin, encoded by DKC1, were taken from references (103) and (127). P10L: DC and HHS, I38T: DC and HHS, T49M: DC and HHS, T66A: DC and HHS, T67I: DC and HHS, H68Q: DC and HHS, S121G: DC and HHS, R158W: DC and HHS, S304N: DC and HHS, K314R: DC and HHS, A353V: DC and HHS, A386T: DC and HHS. h) Mutations in RTEL1. Mutations have been taken from references (62), (104), (167) and (168). E591D: DC and HHS, R974X: DC and HHS, R986X: DC and HHS. i) Mutations in CTC1. Structure taken from reference (169) and mutations from (107). j) Mutations in POT1. Structure and mutations taken from references (141, 142).
In addition to classic DC, other telomere shortening syndromes have been described in the literature. Hoyeraal-Hreidarsson syndrome (HHS) is recognized as a clinically severe form of DC presenting with developmental delay, microcephaly and immunodeficiency, in addition to the characteristics mentioned above. It is caused by rare variants in dyskerin (reviewed in (101)), TERT and TINF2 (collected in (103)), recessive mutations in RTEL1 (104), and has also been associated with a rare variant in TERC (105) (Figure 2a-c,g,h). Like individuals with DC, HHS patients also seem to have increased chromosomal instability, although they usually die in their first decade of life due to progressive bone marrow failure (104). Other conditions with overlapping phenotypes are Revesz and Coats plus syndromes, in which patients present with exudative retinopathy, intracranial calcifications, and neurological and bone marrow defects (41). In these disorders, germline mutations have been found in telomeric proteins such as TINF2 (106) and telomere maintenance component 1 (CTC1), which lead to extensive telomere de-protection (107) (Figure 2c,i). Although their cells may show markers of genomic instability such as spontaneous DNA damage, anaphase bridges and sister telomere losses (104, 108), cancer is not commonly seen in patients with these more severe syndromes, as they succumb to other complications at a young age.
Aplastic anemia (AA) is a bone marrow disorder, characterized by pancytopenia (low counts of red blood cells, white blood cells and platelets). It can be acquired upon exposure to radiation or toxic chemicals. An inherited form has been associated with mutations in the shelterin complex members TERF1 (an intronic variant) and TINF2, and in the telomerase components TERT and TERC (101, 109, 110) (Figure 2a-c). Mutations in these genes are found as heterozygous variants, and are thought to result in haploinsufficiency (101). Patients with AA resulting from mutations in the abovementioned genes have shorter telomeres than controls (111) and may develop cancer, especially leukemias and lymphomas (112). There are a number of related conditions, such as FA, paroxysmal nocturnal hemoglobinuria (PNH) and myelodysplastic syndromes (MDS). All of these may be characterized by blood and bone marrow abnormalities as well as significantly shorter telomeres (113-115), and can be associated with germline mutations in proteins part of the telomere interactome such as XPF in the case of FA (116) and in TERC or its promoter in the case of MDS and PNH (101, 117) (Figure 2b). FA patients often progress towards MDS or acute myeloid leukemia (AML), which has also been associated with rare germline mutations in TERT (113, 118). MDS are sometimes considered a form of cancer, as they are clonal diseases arising from a single cell, and indeed, about 30% of MDS patients develop AML (101). Recently, an unexpected high incidence of diverse cancers, including pancreatic, gastric, prostatic and lymphoma, has been seen in a small cohort of PNH patients (119). This observation suggests that this condition might predispose to the development of neoplasias, although more studies with larger patient cohorts are necessary in order to establish the link definitively.
Germline mutations in other genes part of the telomere interactome can also cause a different spectrum of short telomere syndromes. The Nijmegen breakage syndrome (NBS) is characterized by hypersensitivity to ionizing radiation, immunodeficiency and a strong predisposition to malignancy. It is caused by recessive mutations in the NBN gene, rendering a defective protein that causes impaired ATM phosphorylation, a delayed cell cycle arrest and an accumulation of somatic mutations (78). In accordance with the role of the MRN complex at telomeres, cells isolated from NBS patients display accelerated telomere attrition and an increased number of telomere fusions. Another closely related condition is ataxia telangiectasia (A-T), which is defined by cerebellar degeneration in addition to all NBS characteristics mentioned above (120). A-T is caused by deleterious germline variants in ATM, leading to a defective response to DNA damage and impaired cell cycle control. A-T cells have accelerated telomere shortening, genomic instability and altered telomere-nuclear matrix interactions (78). NBS and A-T patients are strongly predisposed to cancer, and they develop predominantly lymphomas and B- and T-cell leukemias, although solid tumors have also been observed (120). Rare variants in other members of the MRN complex also give rise to cancer-predisposing syndromes, for example, deleterious mutations in MRE11 cause a similar disorder to A-T (121), and some germline variants in NBN and RAD50 have been found associated with childhood acute lymphoblastic leukemia (122, 123).
The Bloom (BS) and Werner (WS) syndromes are caused by autosomal recessive mutations inactivating two interacting partners of TERF2 and POT1, the DNA helicases BLM and WRN, respectively. BS can present with male infertility, skin pigmentation aberrations and a predisposition to malignancy (78). WS is characterized by premature aging, short stature, endocrine disorders and an elevated frequency of cancer (124). WS patients present with an excess of cancers, especially thyroid carcinomas, osteosarcomas, melanomas, meningiomas and leukemias, and cancer is one of the main causes of death in these patients (124). BS patients have an elevated incidence of lymphomas, leukemias, gastrointestinal tract neoplasias, genital and urinary tract tumors and cutaneous neoplasias, and often present with multiple malignancies (125). Cells lacking functional WRN display catastrophic telomere loss and an increased frequency of chromosomal fusions, and cells from BS patients have increased chromosomal breakage and sister-chromatid exchanges (78).
There are other telomere-shortening syndromes that manifest later in life. Idiopathic pulmonary fibrosis (IPF) is a chronic and progressive lung disease characterized by usual interstitial pneumonia that manifests in middle-aged to older patients (126). It has been estimated that 8-15% of familial cases carry haploinsufficient mutations in TERT or TERC, and because IPF affects as many as 63 people per 100,000 in the United States alone, it is the most common manifestation of telomerase gene mutation carriers (41, 101, 126) (Figure 2a,b). In one family, it has also been associated with a mutation in dyskerin (127) (Figure 2g). IPF cases have a higher incidence of lung cancer according to several studies, and it has been found to be one of the common causes of death in these patients (for a review see reference (126)). Individuals that carry germline mutations in telomerase components may also present for the first time with adult-onset liver cirrhosis (41, 128), which often progresses to hepatocellular carcinoma.
As discussed above, mutations in the same genes can give rise to a broad range of phenotypes. For example, rare variants in telomerase components can cause DC, AA or IPF, and mutations in TINF2 can result in DC, AA or Revesz syndrome (Figure 2a-c, Supplementary Table 1). How this happens is largely unexplained, though the phenomenon of genetic anticipation, which includes factors such as inherited short telomeres, may play an important role. It is noteworthy however, that these syndromes have largely overlapping characteristics that will often present concurrently in the same individual; for example, IPF patients with telomerase mutations are at an increased risk of developing bone marrow failure, and AA patients are conversely more prone to developing IPF (41, 129). Nonetheless, it remains to be shown if factors such as the positions where the mutations lie within TERC or the telomeric proteins contribute to the spectrum of telomere shortening syndromes.
Germline mutations can underlie long telomeres and cancer predisposition
Long telomeres might also be a risk factor for cancer development. For example, it has been observed that both breast cancer cases and women at high genetic risk for developing the disease have longer telomeres than controls, with telomere length displaying a positive correlation with risk (130, 131). In fact, BRCA1 and BRCA2 mutation carriers have recently been found to have longer telomeres than controls (132), and longer telomeres were also associated with a worse prognosis in a subset of breast cancer patients with advanced disease (131). Other cancers in which longer telomeres have been associated with increased risk are non-Hodgkin lymphoma (133), melanoma (134) and lung cancer in both smokers (135) and non-smokers (136). Interestingly, longer telomeres have also been associated with risk of colorectal cancer, although only in young individuals, as risk is associated with shorter telomere length in older individuals (137).
The complex relationship between telomere length and cancer risk might seem paradoxical at first, as both long and short telomeres are associated with an increased cancer incidence. However, there are other factors likely to influence this risk. For example, longer telomeres in younger individuals might be predictive of a malfunction in the telomere maintenance machinery (137), which might lead to an activation of telomerase and therefore a longer cell lifespan, allowing a higher accumulation of somatic mutations. Conversely, as discussed in the previous section, chromosomes with short telomeres are prone to genomic instability and chromosomal rearrangements, and because telomeres shorten with age, older individuals might be at an increased risk to develop malignancy-associated chromosomal lesions. The fact that telomere lengths outside an optimal range results in cancer predisposition suggests that there is a delicate balance to be struck for tissue homeostasis.
The genomic variation underlying telomere length has been investigated in an unbiased manner by genome-wide association studies (GWAS). Variants in the TERC, TERT, RTEL1, OBFC1 and CTC1 loci have been found to influence mean telomere length, in addition to several other genomic regions (138, 139), observations replicated in numerous studies. In some cases, such as for CTC1 and TERC, it has been suggested by in vitro experiments or analysis of patterns of genome-wide expression data, that the variants associated with longer telomeres are also correlated with changes in gene expression (138-140), and that this in turn may increase telomerase expression. However, to our knowledge, no specific germline genetic changes had been associated with an increased telomere length in humans until recently.
Recently, two studies identified rare, germline variants in POT1 predisposing to the development of familial melanoma (141, 142) (Figure 2j). Carrier individuals in these cohorts had significantly longer and more fragile telomeres than controls, and in some cases developed not only melanoma but also cancer in other tissues. Some of the variants identified abolish the binding of POT1 to ssDNA, and thus it is possible that carriers are predisposed to malignancy via telomere uncapping and a more permissive extension of chromosome ends. However, the biological mechanism underlying the strong cancer predisposition observed in carriers requires further investigation.
Other cancer-predisposition syndromes arising from mutations in the telomere interactome
There are other cancer-predisposition conditions caused by mutations in telomere-interacting components that do not have a clear effect on telomere length (or in which telomere length has not been measured thus far). For example, a germline mutation in the promoter of TERT was recently discovered to co-segregate with melanoma in a 14-case German pedigree (143). This rare variant creates an E-twenty six/ternary complex factor (Ets/TCF)-binding motif, increasing telomerase expression as shown by luciferase reporter assays. At present it is unclear if this effect is translated to TERT protein levels or if it has any effect on telomere length, but similar TERT promoter mutations have been associated with poor outcome parameters such as increased Breslow thickness and tumor ulceration (144). Following these reports, several other studies have found other somatically-acquired mutations in the promoter of TERT that also increase TERT expression. However, in at least one of these studies, these mutations were associated with shorter telomeres, contrary to what would be expected given the role of TERT in telomere length maintenance (145). This seemingly counterintuitive result could be due to these mutations arising late in the tumor evolutionary history, helping cells survive in the late stages when extensive chromosomal instability has taken place (145). Therefore, the presence of activating TERT promoter mutations and increased telomerase expression in a tumor is not predictive of longer telomere length. It would be expected that individuals harbouring TERT activating germline mutations have increased telomerase expression levels and augmented telomere length. However, it remains to be determined whether the additional ‘non canonical’ roles of TERT affect the relationship between telomerase expression levels and telomere length.
Defects in one of the roles of TERT outside telomere maintenance, affecting the TERT-RMRP complex, have been found to underlie cartilage-hair hypoplasia (CHH) (146). CHH patients present with short stature, hair anomaly, immunodeficiency and predisposition to malignancy. The causal mutations, found in both the promoter and the transcribed region of RMRP, are predicted to decrease RMRP levels and modify ribosomal processing leading to altered cytokine signaling and cell cycle progression (146). CHH patients have a tendency to develop cancer, principally lymphoma, although basal cell carcinoma has also been observed (147, 148).
As previously discussed, TERT can also associate with SMARCA4, a member of the SWI/SNF chromatin-remodelling complex, and regulate Wnt-dependent promoters (84). Recently, germline deleterious mutations in the SMARCA4 gene were found in all available affected members of four families prone to ovarian small cell carcinoma, and loss of heterozygosity or second somatic SMARCA4 mutations were observed in the tumors from these patients (149). Interestingly, TERT promoter mutations associated with increased TERT mRNA expression and longer telomere length have also been observed in clear cell ovary carcinomas (150). These TERT mutations are mutually exclusive with loss of expression of ARID1A, another member of the SWI/SNF complex that includes SMARCA4, and the most common alteration seen in ovarian cancer (150). These data might suggest that mutations affecting the SWI/SNF complex members and TERT mRNA levels act on the same biological pathway, although this hypothesis requires further investigation.
Germline mutations affecting other proteins that interact with members of the shelterin complex and that increase cancer risk have been described. For example, a GWAS and a subsequent independent replication found a SNP in SIAH2, encoding an interacting partner of TINF2, to be associated with ER-positive breast cancer in Japanese and Chinese cohorts (151, 152). Rare germline variants in the TERF2IP-interacting protein Ku80 and the TERF2-interacting partner PARP1 have also been found in diffuse large B-cell lymphomas, in which DNA repair genes have been found to be selectively inactivated possibly leading to increased survival in cells that have suffered extensive DNA damage (90). Ku70 polymorphisms have also been reported to be associated with gastric cancer (153), and PARP1 polymorphisms with melanoma (154).
Moreover, efforts are currently on going with the aim of identifying novel telomere-interacting proteins, which then might provide valuable biological insight into the mechanisms of cancer development. For example, a recently described chromatin purification method identified proteins such as BRIP1, NRIP1 and HMBOX1 bound to telomeres maintained by the ALT pathway (155). HMBOX1 has also recently been found to bind both telomeres and telomerase, contributing to telomere elongation (156). All these proteins have been linked to cancer predisposition or differentiation (157-159), and thus their newly discovered association with telomeres might provide mechanistic clues to their role in cancer. Another recent RNA pull-down experiment identified 115 proteins that bind specifically to telomeric repeat-containing RNA (TERRA), the result of telomere transcription (160). Among these proteins are HMGA1, HMGB1, HNRNPA1 and HNRNPM, all of which have previously been implicated in cancer development (161-164).
Summary and concluding remarks
Cells need a way to tell apart the ends of linear chromosomes from accidental DNA breakage, as failing to get this distinction right can predispose an organism to the development of cancer due to the unnecessary “repair” of chromosome ends. Telomeres serve this purpose by adopting alternative structures such as G-quadruplexes and D- and T-loops, which help shield the ends of chromosomes from nucleases and DNA-processing enzymes. Dedicated complexes, such as telomerase and shelterin, aid in telomere protection and maintenance functions and also regulate signaling cascades that originate from telomeres by intervening in a number of biological pathways. These pathways intrinsically link telomere status with cell cycle control, and contribute to determine cell fate when DNA damage takes place.
In cancer, somatic mutations in the telomere proteins and their interaction partners occur frequently, as these allow the bypass of senescence or death checkpoints and the subsequent accumulation of potentially advantageous mutations. In humans, rare germline variants that impair the function of telomere proteins have been found to underlie a wide spectrum of diseases and cancer-predisposition syndromes. The majority of the conditions described in the literature have progressive shortening of telomeres as a common characteristic, but they can present with distinct phenotypes, different ages of onset and varying severity. This heterogeneity in clinical presentation can be attributed to the phenomenon of genetic anticipation, and possibly other causes such as individual genetic make-up and lifestyle choices. Despite the fact that cells from individuals with impaired telomerase activity show signs of early entry into senescence and can therefore lead to organ failure, these cells are also prone to genomic rearrangements, such as telomeric fusions and sister chromatid exchanges, and thus predispose their host to neoplasia.
Long telomeres are also a risk factor for cancer development; for example, it has been observed that in some cases, breast cancer, lymphoma and melanoma patients have longer telomeres than controls. The intricate relationship between telomere length and cancer predisposition, which includes cell replicative ability and protection from chromosomal instability, explains the need for tight, cell cycle-dependent regulation of telomere proteins. Progress has been made over recent years to elucidate the functions of these proteins, and important roles have been found in DNA repair pathway inhibition, cell fate decision upon DNA damage, facilitation of DNA replication and even non-telomeric roles such as regulation of gene silencing and metabolism control.
These discoveries have allowed us to appreciate the myriad of biological pathways that influence telomeric functions and the effects of particular inherited and acquired mutations in telomere genes. Current research, exploring novel genes that participate in telomere-regulating functions, the mechanisms by which the telomeric proteins contribute to biological cell cycle progression and their influence on telomere dysregulation should extend our understanding of the biology of cancer predisposition and hopefully, in the future, aid in clinical decision-making and patient management.
Supplementary Material
Acknowledgments
Financial support:
C.D.R.-E., M.d.C.V.H. and D.J.A. were supported by Cancer Research UK and the Wellcome Trust (WT098051). C.D.R.-E. was also supported by the Consejo Nacional de Ciencia y Tecnología of Mexico. N.K.H. was supported by a fellowship from the National Health and Medical Research Council of Australia (NHMRC).
Footnotes
Conflicts of interest:
The authors declare no conflicts of interest.
References
- 1.Blackburn EH, Gall JG. A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. Journal of molecular biology. 1978 Mar 25;120(1):33–53. doi: 10.1016/0022-2836(78)90294-2. [DOI] [PubMed] [Google Scholar]
- 2.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes & development. 2005 Sep 15;19(18):2100–10. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 3.van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998 Feb 6;92(3):401–13. doi: 10.1016/s0092-8674(00)80932-0. [DOI] [PubMed] [Google Scholar]
- 4.Farr C, Fantes J, Goodfellow P, Cooke H. Functional reintroduction of human telomeres into mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 1991 Aug 15;88(16):7006–10. doi: 10.1073/pnas.88.16.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Sullivan RJ, Karlseder J. Telomeres: protecting chromosomes against genome instability. Nature reviews Molecular cell biology. 2010 Mar;11(3):171–81. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonetti D, Martina M, Falcettoni M, Longhese MP. Telomere-end processing: mechanisms and regulation. Chromosoma. 2013 Oct 12; doi: 10.1007/s00412-013-0440-y. [DOI] [PubMed] [Google Scholar]
- 7.Makarov VL, Hirose Y, Langmore JP. Long G tails at both ends of human chromosomes suggest a C strand degradation mechanism for telomere shortening. Cell. 1997 Mar 7;88(5):657–66. doi: 10.1016/s0092-8674(00)81908-x. [DOI] [PubMed] [Google Scholar]
- 8.Wright WE, Tesmer VM, Huffman KE, Levene SD, Shay JW. Normal human chromosomes have long G-rich telomeric overhangs at one end. Genes & development. 1997 Nov 1;11(21):2801–9. doi: 10.1101/gad.11.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob NK, Kirk KE, Price CM. Generation of telomeric G strand overhangs involves both G and C strand cleavage. Molecular cell. 2003 Apr;11(4):1021–32. doi: 10.1016/s1097-2765(03)00131-x. [DOI] [PubMed] [Google Scholar]
- 10.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–13. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 11.Wright WE, Piatyszek MA, Rainey WE, Byrd W, Shay JW. Telomerase activity in human germline and embryonic tissues and cells. Developmental genetics. 1996;18(2):173–9. doi: 10.1002/(SICI)1520-6408(1996)18:2<173::AID-DVG10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002 Jan 21;21(4):564–79. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- 13.Chiu CP, Dragowska W, Kim NW, Vaziri H, Yui J, Thomas TE, et al. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem cells. 1996 Mar;14(2):239–48. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- 14.Stewart SA, Weinberg RA. Telomerase and human tumorigenesis. Seminars in cancer biology. 2000 Dec;10(6):399–406. doi: 10.1006/scbi.2000.0339. [DOI] [PubMed] [Google Scholar]
- 15.Nandakumar J, Cech TR. Finding the end: recruitment of telomerase to telomeres. Nature reviews Molecular cell biology. 2013 Feb;14(2):69–82. doi: 10.1038/nrm3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ray S, Bandaria JN, Qureshi MH, Yildiz A, Balci H. G-quadruplex formation in telomeres enhances POT1/TPP1 protection against RPA binding. Proceedings of the National Academy of Sciences of the United States of America. 2014 Feb 25;111(8):2990–5. doi: 10.1073/pnas.1321436111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez P, Blasco MA. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nature reviews Cancer. 2011 Mar;11(3):161–76. doi: 10.1038/nrc3025. [DOI] [PubMed] [Google Scholar]
- 18.Doksani Y, Wu JY, de Lange T, Zhuang X. Super-resolution fluorescence imaging of telomeres reveals TRF2-dependent T-loop formation. Cell. 2013 Oct 10;155(2):345–56. doi: 10.1016/j.cell.2013.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelleher C, Kurth I, Lingner J. Human protection of telomeres 1 (POT1) is a negative regulator of telomerase activity in vitro. Molecular and cellular biology. 2005 Jan;25(2):808–18. doi: 10.1128/MCB.25.2.808-818.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sfeir A, de Lange T. Removal of shelterin reveals the telomere end-protection problem. Science. 2012 May 4;336(6081):593–7. doi: 10.1126/science.1218498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nature reviews Genetics. 2005 Aug;6(8):611–22. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 22.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. European journal of cancer. 1997 Apr;33(5):787–91. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 23.Ramsay AJ, Quesada V, Foronda M, Conde L, Martinez-Trillos A, Villamor N, et al. POT1 mutations cause telomere dysfunction in chronic lymphocytic leukemia. Nature genetics. 2013 May;45(5):526–30. doi: 10.1038/ng.2584. [DOI] [PubMed] [Google Scholar]
- 24.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nature reviews Genetics. 2010 May;11(5):319–30. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 26.Bodnar AG, Ouellette M, Frolkis M, Holt SE, Chiu CP, Morin GB, et al. Extension of lifespan by introduction of telomerase into normal human cells. Science. 1998 Jan 16;279(5349):349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 27.Donate LE, Blasco MA. Telomeres in cancer and ageing. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011 Jan 12;366(1561):76–84. doi: 10.1098/rstb.2010.0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blackburn EH. Switching and signaling at the telomere. Cell. 2001 Sep 21;106(6):661–73. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- 29.Redon S, Reichenbach P, Lingner J. Protein RNA and protein protein interactions mediate association of human EST1A/SMG6 with telomerase. Nucleic acids research. 2007;35(20):7011–22. doi: 10.1093/nar/gkm724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egan ED, Collins K. Biogenesis of telomerase ribonucleoproteins. Rna. 2012 Oct;18(10):1747–59. doi: 10.1261/rna.034629.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gillis AJ, Schuller AP, Skordalakes E. Structure of the Tribolium castaneum telomerase catalytic subunit TERT. Nature. 2008 Oct 2;455(7213):633–7. doi: 10.1038/nature07283. [DOI] [PubMed] [Google Scholar]
- 32.Kannan K, Nelson AD, Shippen DE. Dyskerin is a component of the Arabidopsis telomerase RNP required for telomere maintenance. Molecular and cellular biology. 2008 Apr;28(7):2332–41. doi: 10.1128/MCB.01490-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen SB, Graham ME, Lovrecz GO, Bache N, Robinson PJ, Reddel RR. Protein composition of catalytically active human telomerase from immortal cells. Science. 2007 Mar 30;315(5820):1850–3. doi: 10.1126/science.1138596. [DOI] [PubMed] [Google Scholar]
- 34.Zhong F, Savage SA, Shkreli M, Giri N, Jessop L, Myers T, et al. Disruption of telomerase trafficking by TCAB1 mutation causes dyskeratosis congenita. Genes & development. 2011 Jan 1;25(1):11–6. doi: 10.1101/gad.2006411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang ML, Tong XJ, Fu XH, Zhou BO, Wang J, Liao XH, et al. Yeast telomerase subunit Est1p has guanine quadruplex-promoting activity that is required for telomere elongation. Nature structural & molecular biology. 2010 Feb;17(2):202–9. doi: 10.1038/nsmb.1760. [DOI] [PubMed] [Google Scholar]
- 36.Cristofari G, Adolf E, Reichenbach P, Sikora K, Terns RM, Terns MP, et al. Human telomerase RNA accumulation in Cajal bodies facilitates telomerase recruitment to telomeres and telomere elongation. Molecular cell. 2007 Sep 21;27(6):882–9. doi: 10.1016/j.molcel.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 37.Venteicher AS, Abreu EB, Meng Z, McCann KE, Terns RM, Veenstra TD, et al. A human telomerase holoenzyme protein required for Cajal body localization and telomere synthesis. Science. 2009 Jan 30;323(5914):644–8. doi: 10.1126/science.1165357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venteicher AS, Meng Z, Mason PJ, Veenstra TD, Artandi SE. Identification of ATPases pontin and reptin as telomerase components essential for holoenzyme assembly. Cell. 2008 Mar 21;132(6):945–57. doi: 10.1016/j.cell.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Y, Sfeir AJ, Zou Y, Buseman CM, Chow TT, Shay JW, et al. Telomere extension occurs at most chromosome ends and is uncoupled from fill-in in human cancer cells. Cell. 2009 Aug 7;138(3):463–75. doi: 10.1016/j.cell.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemann MT, Strong MA, Hao LY, Greider CW. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001 Oct 5;107(1):67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 41.Armanios M, Blackburn EH. The telomere syndromes. Nature reviews Genetics. 2012 Oct;13(10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hao LY, Armanios M, Strong MA, Karim B, Feldser DM, Huso D, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005 Dec 16;123(6):1121–31. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez-Suarez E, Samper E, Ramirez A, Flores JM, Martin-Caballero J, Jorcano JL, et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. The EMBO journal. 2001 Jun 1;20(11):2619–30. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011 Jan 6;469(7328):102–6. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin H, Liu D, Wan M, Safari A, Kim H, Sun W, et al. TPP1 is a homologue of ciliate TEBPbeta and interacts with POT1 to recruit telomerase. Nature. 2007 Feb 1;445(7127):559–62. doi: 10.1038/nature05469. [DOI] [PubMed] [Google Scholar]
- 46.Linger BR, Price CM. Conservation of telomere protein complexes: shuffling through evolution. Critical reviews in biochemistry and molecular biology. 2009 Nov-Dec;44(6):434–46. doi: 10.3109/10409230903307329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bianchi A, Smith S, Chong L, Elias P, de Lange T. TRF1 is a dimer and bends telomeric DNA. The EMBO journal. 1997 Apr 1;16(7):1785–94. doi: 10.1093/emboj/16.7.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Court R, Chapman L, Fairall L, Rhodes D. How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high-resolution crystal structures. EMBO reports. 2005 Jan;6(1):39–45. doi: 10.1038/sj.embor.7400314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Loayza D, Parsons H, Donigian J, Hoke K, de Lange T. DNA binding features of human POT1: a nonamer 5′-TAGGGTTAG-3′ minimal binding site, sequence specificity, and internal binding to multimeric sites. The Journal of biological chemistry. 2004 Mar 26;279(13):13241–8. doi: 10.1074/jbc.M312309200. [DOI] [PubMed] [Google Scholar]
- 50.Hockemeyer D, Palm W, Else T, Daniels JP, Takai KK, Ye JZ, et al. Telomere protection by mammalian Pot1 requires interaction with Tpp1. Nature structural & molecular biology. 2007 Aug;14(8):754–61. doi: 10.1038/nsmb1270. [DOI] [PubMed] [Google Scholar]
- 51.Wang F, Podell ER, Zaug AJ, Yang Y, Baciu P, Cech TR, et al. The POT1-TPP1 telomere complex is a telomerase processivity factor. Nature. 2007 Feb 1;445(7127):506–10. doi: 10.1038/nature05454. [DOI] [PubMed] [Google Scholar]
- 52.Crabbe L, Cesare AJ, Kasuboski JM, Fitzpatrick JA, Karlseder J. Human telomeres are tethered to the nuclear envelope during postmitotic nuclear assembly. Cell reports. 2012 Dec 27;2(6):1521–9. doi: 10.1016/j.celrep.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ye JZ, Donigian JR, van Overbeek M, Loayza D, Luo Y, Krutchinsky AN, et al. TIN2 binds TRF1 and TRF2 simultaneously and stabilizes the TRF2 complex on telomeres. The Journal of biological chemistry. 2004 Nov 5;279(45):47264–71. doi: 10.1074/jbc.M409047200. [DOI] [PubMed] [Google Scholar]
- 54.Chiang YJ, Kim SH, Tessarollo L, Campisi J, Hodes RJ. Telomere-associated protein TIN2 is essential for early embryonic development through a telomerase-independent pathway. Molecular and cellular biology. 2004 Aug;24(15):6631–4. doi: 10.1128/MCB.24.15.6631-6634.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hockemeyer D, Daniels JP, Takai H, de Lange T. Recent expansion of the telomeric complex in rodents: Two distinct POT1 proteins protect mouse telomeres. Cell. 2006 Jul 14;126(1):63–77. doi: 10.1016/j.cell.2006.04.044. [DOI] [PubMed] [Google Scholar]
- 56.Karlseder J, Kachatrian L, Takai H, Mercer K, Hingorani S, Jacks T, et al. Targeted deletion reveals an essential function for the telomere length regulator Trf1. Molecular and cellular biology. 2003 Sep;23(18):6533–41. doi: 10.1128/MCB.23.18.6533-6541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Celli GB, de Lange T. DNA processing is not required for ATM-mediated telomere damage response after TRF2 deletion. Nature cell biology. 2005 Jul;7(7):712–8. doi: 10.1038/ncb1275. [DOI] [PubMed] [Google Scholar]
- 58.Stansel RM, de Lange T, Griffith JD. T-loop assembly in vitro involves binding of TRF2 near the 3′ telomeric overhang. The EMBO journal. 2001 Oct 1;20(19):5532–40. doi: 10.1093/emboj/20.19.5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hockemeyer D, Sfeir AJ, Shay JW, Wright WE, de Lange T. POT1 protects telomeres from a transient DNA damage response and determines how human chromosomes end. The EMBO journal. 2005 Jul 20;24(14):2667–78. doi: 10.1038/sj.emboj.7600733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Songyang Z, Liu D. Inside the mammalian telomere interactome: regulation and regulatory activities of telomeres. Critical reviews in eukaryotic gene expression. 2006;16(2):103–18. doi: 10.1615/critreveukargeneexpr.v16.i2.10. [DOI] [PubMed] [Google Scholar]
- 61.Kishi S, Zhou XZ, Ziv Y, Khoo C, Hill DE, Shiloh Y, et al. Telomeric protein Pin2/TRF1 as an important ATM target in response to double strand DNA breaks. The Journal of biological chemistry. 2001 Aug 3;276(31):29282–91. doi: 10.1074/jbc.M011534200. [DOI] [PubMed] [Google Scholar]
- 62.Deng Z, Glousker G, Molczan A, Fox AJ, Lamm N, Dheekollu J, et al. Inherited mutations in the helicase RTEL1 cause telomere dysfunction and Hoyeraal-Hreidarsson syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2013 Sep 3;110(36):E3408–16. doi: 10.1073/pnas.1300600110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vannier JB, Pavicic-Kaltenbrunner V, Petalcorin MI, Ding H, Boulton SJ. RTEL1 dismantles T loops and counteracts telomeric G4-DNA to maintain telomere integrity. Cell. 2012 May 11;149(4):795–806. doi: 10.1016/j.cell.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 64.Sfeir A, Kosiyatrakul ST, Hockemeyer D, MacRae SL, Karlseder J, Schildkraut CL, et al. Mammalian telomeres resemble fragile sites and require TRF1 for efficient replication. Cell. 2009 Jul 10;138(1):90–103. doi: 10.1016/j.cell.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakamura M, Zhou XZ, Kishi S, Lu KP. Involvement of the telomeric protein Pin2/TRF1 in the regulation of the mitotic spindle. FEBS letters. 2002 Mar 13;514(2-3):193–8. doi: 10.1016/s0014-5793(02)02363-3. [DOI] [PubMed] [Google Scholar]
- 66.Smith S, Giriat I, Schmitt A, de Lange T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science. 1998 Nov 20;282(5393):1484–7. doi: 10.1126/science.282.5393.1484. [DOI] [PubMed] [Google Scholar]
- 67.Smith S, de Lange T. Tankyrase promotes telomere elongation in human cells. Current biology : CB. 2000 Oct 19;10(20):1299–302. doi: 10.1016/s0960-9822(00)00752-1. [DOI] [PubMed] [Google Scholar]
- 68.Zhu XD, Niedernhofer L, Kuster B, Mann M, Hoeijmakers JH, de Lange T. ERCC1/XPF removes the 3′ overhang from uncapped telomeres and represses formation of telomeric DNA-containing double minute chromosomes. Molecular cell. 2003 Dec;12(6):1489–98. doi: 10.1016/s1097-2765(03)00478-7. [DOI] [PubMed] [Google Scholar]
- 69.Zhu XD, Kuster B, Mann M, Petrini JH, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nature genetics. 2000 Jul;25(3):347–52. doi: 10.1038/77139. [DOI] [PubMed] [Google Scholar]
- 70.Lamarche BJ, Orazio NI, Weitzman MD. The MRN complex in double-strand break repair and telomere maintenance. FEBS letters. 2010 Sep 10;584(17):3682–95. doi: 10.1016/j.febslet.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Opresko PL, von Kobbe C, Laine JP, Harrigan J, Hickson ID, Bohr VA. Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. The Journal of biological chemistry. 2002 Oct 25;277(43):41110–9. doi: 10.1074/jbc.M205396200. [DOI] [PubMed] [Google Scholar]
- 72.Barefield C, Karlseder J. The BLM helicase contributes to telomere maintenance through processing of late-replicating intermediate structures. Nucleic acids research. 2012 Aug;40(15):7358–67. doi: 10.1093/nar/gks407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xin H, Liu D, Songyang Z. The telosome/shelterin complex and its functions. Genome biology. 2008;9(9):232. doi: 10.1186/gb-2008-9-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okamoto K, Bartocci C, Ouzounov I, Diedrich JK, Yates JR, 3rd, Denchi EL. A two-step mechanism for TRF2-mediated chromosome-end protection. Nature. 2013 Feb 28;494(7438):502–5. doi: 10.1038/nature11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhanot M, Smith S. TIN2 stability is regulated by the E3 ligase Siah2. Molecular and cellular biology. 2012 Jan;32(2):376–84. doi: 10.1128/MCB.06227-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Canudas S, Houghtaling BR, Bhanot M, Sasa G, Savage SA, Bertuch AA, et al. A role for heterochromatin protein 1gamma at human telomeres. Genes & development. 2011 Sep 1;25(17):1807–19. doi: 10.1101/gad.17325211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Connor MS, Safari A, Liu D, Qin J, Songyang Z. The human Rap1 protein complex and modulation of telomere length. The Journal of biological chemistry. 2004 Jul 2;279(27):28585–91. doi: 10.1074/jbc.M312913200. [DOI] [PubMed] [Google Scholar]
- 78.Kong CM, Lee XW, Wang X. Telomere shortening in human diseases. The FEBS journal. 2013 Jul;280(14):3180–93. doi: 10.1111/febs.12326. [DOI] [PubMed] [Google Scholar]
- 79.Opresko PL, Mason PA, Podell ER, Lei M, Hickson ID, Cech TR, et al. POT1 stimulates RecQ helicases WRN and BLM to unwind telomeric DNA substrates. The Journal of biological chemistry. 2005 Sep 16;280(37):32069–80. doi: 10.1074/jbc.M505211200. [DOI] [PubMed] [Google Scholar]
- 80.Chawla R, Redon S, Raftopoulou C, Wischnewski H, Gagos S, Azzalin CM. Human UPF1 interacts with TPP1 and telomerase and sustains telomere leading-strand replication. The EMBO journal. 2011 Oct 5;30(19):4047–58. doi: 10.1038/emboj.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wan M, Qin J, Songyang Z, Liu D. OB fold-containing protein 1 (OBFC1), a human homolog of yeast Stn1, associates with TPP1 and is implicated in telomere length regulation. The Journal of biological chemistry. 2009 Sep 25;284(39):26725–31. doi: 10.1074/jbc.M109.021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Teo H, Ghosh S, Luesch H, Ghosh A, Wong ET, Malik N, et al. Telomere-independent Rap1 is an IKK adaptor and regulates NF-kappaB-dependent gene expression. Nature cell biology. 2010 Aug;12(8):758–67. doi: 10.1038/ncb2080. [DOI] [PubMed] [Google Scholar]
- 83.Martinez P, Thanasoula M, Carlos AR, Gomez-Lopez G, Tejera AM, Schoeftner S, et al. Mammalian Rap1 controls telomere function and gene expression through binding to telomeric and extratelomeric sites. Nature cell biology. 2010 Aug;12(8):768–80. doi: 10.1038/ncb2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009 Jul 2;460(7251):66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maida Y, Yasukawa M, Furuuchi M, Lassmann T, Possemato R, Okamoto N, et al. An RNA-dependent RNA polymerase formed by TERT and the RMRP RNA. Nature. 2009 Sep 10;461(7261):230–5. doi: 10.1038/nature08283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac-Sage P, Laurent C, et al. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nature communications. 2013;4:2218. doi: 10.1038/ncomms3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013 Feb 22;339(6122):957–9. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Jr., et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proceedings of the National Academy of Sciences of the United States of America. 2013 Apr 9;110(15):6021–6. doi: 10.1073/pnas.1303607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vinagre J, Almeida A, Populo H, Batista R, Lyra J, Pinto V, et al. Frequency of TERT promoter mutations in human cancers. Nature communications. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 90.de Miranda NF, Peng R, Georgiou K, Wu C, Falk Sorqvist E, Berglund M, et al. DNA repair genes are selectively mutated in diffuse large B cell lymphomas. The Journal of experimental medicine. 2013 Aug 26;210(9):1729–42. doi: 10.1084/jem.20122842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiao X, Wood LD, Lindman M, Jones S, Buckhaults P, Polyak K, et al. Somatic mutations in the Notch, NF-KB, PIK3CA, and Hedgehog pathways in human breast cancers. Genes, chromosomes & cancer. 2012 May;51(5):480–9. doi: 10.1002/gcc.21935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gilmore TD, Kalaitzidis D, Liang MC, Starczynowski DT. The c-Rel transcription factor and B-cell proliferation: a deal with the devil. Oncogene. 2004 Mar 25;23(13):2275–86. doi: 10.1038/sj.onc.1207410. [DOI] [PubMed] [Google Scholar]
- 93.Samper E, Goytisolo FA, Slijepcevic P, van Buul PP, Blasco MA. Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the Gstrand overhang. EMBO reports. 2000 Sep;1(3):244–52. doi: 10.1093/embo-reports/kvd051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Celli GB, Denchi EL, de Lange T. Ku70 stimulates fusion of dysfunctional telomeres yet protects chromosome ends from homologous recombination. Nature cell biology. 2006 Aug;8(8):885–90. doi: 10.1038/ncb1444. [DOI] [PubMed] [Google Scholar]
- 95.Beneke S, Cohausz O, Malanga M, Boukamp P, Althaus F, Burkle A. Rapid regulation of telomere length is mediated by poly(ADP-ribose) polymerase-1. Nucleic acids research. 2008 Nov;36(19):6309–17. doi: 10.1093/nar/gkn615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Akiyama M, Yamada O, Hideshima T, Yanagisawa T, Yokoi K, Fujisawa K, et al. TNFalpha induces rapid activation and nuclear translocation of telomerase in human lymphocytes. Biochemical and biophysical research communications. 2004 Apr 2;316(2):528–32. doi: 10.1016/j.bbrc.2004.02.080. [DOI] [PubMed] [Google Scholar]
- 97.Vulliamy T, Marrone A, Goldman F, Dearlove A, Bessler M, Mason PJ, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001 Sep 27;413(6854):432–5. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 98.Goldman F, Bouarich R, Kulkarni S, Freeman S, Du HY, Harrington L, et al. The effect of TERC haploinsufficiency on the inheritance of telomere length. Proceedings of the National Academy of Sciences of the United States of America. 2005 Nov 22;102(47):17119–24. doi: 10.1073/pnas.0505318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de la Fuente J, Dokal I. Dyskeratosis congenita: advances in the understanding of the telomerase defect and the role of stem cell transplantation. Pediatric transplantation. 2007 Sep;11(6):584–94. doi: 10.1111/j.1399-3046.2007.00721.x. [DOI] [PubMed] [Google Scholar]
- 100.Alter BP, Giri N, Savage SA, Rosenberg PS. Cancer in dyskeratosis congenita. Blood. 2009 Jun 25;113(26):6549–57. doi: 10.1182/blood-2008-12-192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carroll KA, Ly H. Telomere dysfunction in human diseases: the long and short of it! International journal of clinical and experimental pathology. 2009;2(6):528–43. [PMC free article] [PubMed] [Google Scholar]
- 102.Knight SW, Heiss NS, Vulliamy TJ, Greschner S, Stavrides G, Pai GS, et al. X-linked dyskeratosis congenita is predominantly caused by missense mutations in the DKC1 gene. American journal of human genetics. 1999 Jul;65(1):50–8. doi: 10.1086/302446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Podlevsky JD, Bley CJ, Omana RV, Qi X, Chen JJ. The telomerase database. Nucleic acids research. 2008 Jan;36(Database issue):D339–43. doi: 10.1093/nar/gkm700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Le Guen T, Jullien L, Touzot F, Schertzer M, Gaillard L, Perderiset M, et al. Human RTEL1 deficiency causes Hoyeraal-Hreidarsson syndrome with short telomeres and genome instability. Human molecular genetics. 2013 Aug 15;22(16):3239–49. doi: 10.1093/hmg/ddt178. [DOI] [PubMed] [Google Scholar]
- 105.Vulliamy TJ, Kirwan MJ, Beswick R, Hossain U, Baqai C, Ratcliffe A, et al. Differences in disease severity but similar telomere lengths in genetic subgroups of patients with telomerase and shelterin mutations. PloS one. 2011;6(9):e24383. doi: 10.1371/journal.pone.0024383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Savage SA, Giri N, Baerlocher GM, Orr N, Lansdorp PM, Alter BP. TINF2, a component of the shelterin telomere protection complex, is mutated in dyskeratosis congenita. American journal of human genetics. 2008 Feb;82(2):501–9. doi: 10.1016/j.ajhg.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anderson BH, Kasher PR, Mayer J, Szynkiewicz M, Jenkinson EM, Bhaskar SS, et al. Mutations in CTC1, encoding conserved telomere maintenance component 1, cause Coats plus. Nature genetics. 2012 Mar;44(3):338–42. doi: 10.1038/ng.1084. [DOI] [PubMed] [Google Scholar]
- 108.Gu P, Chang S. Functional characterization of human CTC1 mutations reveals novel mechanisms responsible for the pathogenesis of the telomere disease Coats plus. Aging cell. 2013 Dec;12(6):1100–9. doi: 10.1111/acel.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Savage SA, Calado RT, Xin ZT, Ly H, Young NS, Chanock SJ. Genetic variation in telomeric repeat binding factors 1 and 2 in aplastic anemia. Experimental hematology. 2006 May;34(5):664–71. doi: 10.1016/j.exphem.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 110.Walne AJ, Vulliamy T, Beswick R, Kirwan M, Dokal I. TINF2 mutations result in very short telomeres: analysis of a large cohort of patients with dyskeratosis congenita and related bone marrow failure syndromes. Blood. 2008 Nov 1;112(9):3594–600. doi: 10.1182/blood-2008-05-153445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ball SE, Gibson FM, Rizzo S, Tooze JA, Marsh JC, Gordon-Smith EC. Progressive telomere shortening in aplastic anemia. Blood. 1998 May;15(10):3582–92. [PubMed] [Google Scholar]
- 112.Tzankov A, Medinger M. Aplastic anemia: possible associations with lymphoproliferative neoplasms. International journal of laboratory hematology. 2014 Jun;36(3):382–7. doi: 10.1111/ijlh.12224. [DOI] [PubMed] [Google Scholar]
- 113.Leteurtre F, Li X, Guardiola P, Le Roux G, Sergere JC, Richard P, et al. Accelerated telomere shortening and telomerase activation in Fanconi’s anaemia. British journal of haematology. 1999 Jun;105(4):883–93. doi: 10.1046/j.1365-2141.1999.01445.x. [DOI] [PubMed] [Google Scholar]
- 114.Lange K, Holm L, Vang Nielsen K, Hahn A, Hofmann W, Kreipe H, et al. Telomere shortening and chromosomal instability in myelodysplastic syndromes. Genes, chromosomes & cancer. 2010 Mar;49(3):260–9. doi: 10.1002/gcc.20737. [DOI] [PubMed] [Google Scholar]
- 115.Karadimitris A, Araten DJ, Luzzatto L, Notaro R. Severe telomere shortening in patients with paroxysmal nocturnal hemoglobinuria affects both GPI- and GPI+ hematopoiesis. Blood. 2003 Jul 15;102(2):514–6. doi: 10.1182/blood-2003-01-0128. [DOI] [PubMed] [Google Scholar]
- 116.Bogliolo M, Schuster B, Stoepker C, Derkunt B, Su Y, Raams A, et al. Mutations in ERCC4, encoding the DNA-repair endonuclease XPF, cause Fanconi anemia. American journal of human genetics. 2013 May 2;92(5):800–6. doi: 10.1016/j.ajhg.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keith WN, Vulliamy T, Zhao J, Ar C, Erzik C, Bilsland A, et al. A mutation in a functional Sp1 binding site of the telomerase RNA gene (hTERC) promoter in a patient with Paroxysmal Nocturnal Haemoglobinuria. BMC blood disorders. 2004 Jun 22;4(1):3. doi: 10.1186/1471-2326-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Calado RT, Regal JA, Hills M, Yewdell WT, Dalmazzo LF, Zago MA, et al. Constitutional hypomorphic telomerase mutations in patients with acute myeloid leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2009 Jan 27;106(4):1187–92. doi: 10.1073/pnas.0807057106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ojeda E, Fores R, Cabero M, Morillo D, Bautista G, Navarro B, et al. Paroxysmal Nocturnal Hemoglobinuria and Cancer: High Incidence Of Cancer In a Large Series Of PNH Patients In a Single Center. Blood. 2013 2013 Nov 15;122(21):4871. [Google Scholar]
- 120.Shiloh Y. Ataxia-telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annual review of genetics. 1997;31:635–62. doi: 10.1146/annurev.genet.31.1.635. [DOI] [PubMed] [Google Scholar]
- 121.Stewart GS, Maser RS, Stankovic T, Bressan DA, Kaplan MI, Jaspers NG, et al. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxiatelangiectasia- like disorder. Cell. 1999 Dec 10;99(6):577–87. doi: 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- 122.Mosor M, Ziolkowska I, Pernak-Schwarz M, Januszkiewicz-Lewandowska D, Nowak J. Association of the heterozygous germline I171V mutation of the NBS1 gene with childhood acute lymphoblastic leukemia. Leukemia. 2006 Aug;20(8):1454–6. doi: 10.1038/sj.leu.2404285. [DOI] [PubMed] [Google Scholar]
- 123.Mosor M, Ziolkowska-Suchanek I, Nowicka K, Dzikiewicz-Krawczyk A, Januszkiewicz-Lewandowska D, Nowak J. Germline variants in MRE11/RAD50/NBN complex genes in childhood leukemia. BMC cancer. 2013;13:457. doi: 10.1186/1471-2407-13-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goto M, Miller RW, Ishikawa Y, Sugano H. Excess of rare cancers in Werner syndrome (adult progeria) Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1996 Apr;5(4):239–46. [PubMed] [Google Scholar]
- 125.Arora H, Chacon AH, Choudhary S, McLeod MP, Meshkov L, Nouri K, et al. Bloom syndrome. International journal of dermatology. 2014 Mar 6; doi: 10.1111/ijd.12408. [DOI] [PubMed] [Google Scholar]
- 126.Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clinical epidemiology. 2013;5:483–92. doi: 10.2147/CLEP.S54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kropski JA, Mitchell DB, Markin C, Polosukhin VV, Choi LA, Johnson JE, et al. A novel dyskerin (DKC1) mutation is associated with Familial Interstitial Pneumonia. Chest. 2014 Feb 6; doi: 10.1378/chest.13-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hartmann D, Srivastava U, Thaler M, Kleinhans KN, N’Kontchou G, Scheffold A, et al. Telomerase gene mutations are associated with cirrhosis formation. Hepatology. 2011 May;53(5):1608–17. doi: 10.1002/hep.24217. [DOI] [PubMed] [Google Scholar]
- 129.Parry EM, Alder JK, Qi X, Chen JJ, Armanios M. Syndrome complex of bone marrow failure and pulmonary fibrosis predicts germline defects in telomerase. Blood. 2011 May 26;117(21):5607–11. doi: 10.1182/blood-2010-11-322149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gramatges MM, Telli ML, Balise R, Ford JM. Longer relative telomere length in blood from women with sporadic and familial breast cancer compared with healthy controls. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010 Feb;19(2):605–13. doi: 10.1158/1055-9965.EPI-09-0896. [DOI] [PubMed] [Google Scholar]
- 131.Svenson U, Nordfjall K, Stegmayr B, Manjer J, Nilsson P, Tavelin B, et al. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer research. 2008 May 15;68(10):3618–23. doi: 10.1158/0008-5472.CAN-07-6497. [DOI] [PubMed] [Google Scholar]
- 132.Pooley KA, McGuffog L, Barrowdale D, Frost D, Ellis SD, Fineberg E, et al. Lymphocyte telomere length is long in BRCA1 and BRCA2 mutation carriers regardless of cancer-affected status. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014 Jun;23(6):1018–24. doi: 10.1158/1055-9965.EPI-13-0635-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lan Q, Cawthon R, Shen M, Weinstein SJ, Virtamo J, Lim U, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of non-Hodgkin lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009 Dec 1;15(23):7429–33. doi: 10.1158/1078-0432.CCR-09-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Anic GM, Sondak VK, Messina JL, Fenske NA, Zager JS, Cherpelis BS, et al. Telomere length and risk of melanoma, squamous cell carcinoma, and basal cell carcinoma. Cancer epidemiology. 2013 Aug;37(4):434–9. doi: 10.1016/j.canep.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shen M, Cawthon R, Rothman N, Weinstein SJ, Virtamo J, Hosgood HD, 3rd, et al. A prospective study of telomere length measured by monochrome multiplex quantitative PCR and risk of lung cancer. Lung cancer. 2011 Aug;73(2):133–7. doi: 10.1016/j.lungcan.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lan Q, Cawthon R, Gao Y, Hu W, Hosgood HD, 3rd, Barone-Adesi F, et al. Longer telomere length in peripheral white blood cells is associated with risk of lung cancer and the rs2736100 (CLPTM1L-TERT) polymorphism in a prospective cohort study among women in China. PloS one. 2013;8(3):e59230. doi: 10.1371/journal.pone.0059230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Boardman LA, Litzelman K, Seo S, Johnson RA, Vanderboom RJ, Kimmel GW, et al. The association of telomere length with colorectal cancer differs by the age of cancer onset. Clinical and translational gastroenterology. 2014;5:e52. doi: 10.1038/ctg.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nature genetics. 2013 Apr;45(4):422–7. 7e1–2. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mangino M, Hwang SJ, Spector TD, Hunt SC, Kimura M, Fitzpatrick AL, et al. Genomewide meta-analysis points to CTC1 and ZNF676 as genes regulating telomere homeostasis in humans. Human molecular genetics. 2012 Dec 15;21(24):5385–94. doi: 10.1093/hmg/dds382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jones AM, Beggs AD, Carvajal-Carmona L, Farrington S, Tenesa A, Walker M, et al. TERC polymorphisms are associated both with susceptibility to colorectal cancer and with longer telomeres. Gut. 2012 Feb;61(2):248–54. doi: 10.1136/gut.2011.239772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, et al. POT1 loss-of-function variants predispose to familial melanoma. Nature genetics. 2014 May;46(5):478–81. doi: 10.1038/ng.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shi J, Yang XR, Ballew B, Rotunno M, Calista D, Fargnoli MC, et al. Rare missense variants in POT1 predispose to familial cutaneous malignant melanoma. Nature genetics. 2014 May;46(5):482–6. doi: 10.1038/ng.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013 Feb 22;339(6122):959–61. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 144.Heidenreich B, Nagore E, Rachakonda PS, Garcia-Casado Z, Requena C, Traves V, et al. Telomerase reverse transcriptase promoter mutations in primary cutaneous melanoma. Nature communications. 2014;5:3401. doi: 10.1038/ncomms4401. [DOI] [PubMed] [Google Scholar]
- 145.Liu T, Wang N, Cao J, Sofiadis A, Dinets A, Zedenius J, et al. The age- and shorter telomere-dependent TERT promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene. 2013 Oct 21; doi: 10.1038/onc.2013.446. [DOI] [PubMed] [Google Scholar]
- 146.Hermanns P, Bertuch AA, Bertin TK, Dawson B, Schmitt ME, Shaw C, et al. Consequences of mutations in the non-coding RMRP RNA in cartilage-hair hypoplasia. Human molecular genetics. 2005 Dec 1;14(23):3723–40. doi: 10.1093/hmg/ddi403. [DOI] [PubMed] [Google Scholar]
- 147.Martin AN, Li Y. RNase MRP RNA and human genetic diseases. Cell research. 2007 Mar;17(3):219–26. doi: 10.1038/sj.cr.7310120. [DOI] [PubMed] [Google Scholar]
- 148.Castori M, Morrone A, Kanitakis J, Grammatico P. Genetic skin diseases predisposing to basal cell carcinoma. European journal of dermatology : EJD. 2012 May-Jun;22(3):299–309. doi: 10.1684/ejd.2011.1633. [DOI] [PubMed] [Google Scholar]
- 149.Witkowski L, Carrot-Zhang J, Albrecht S, Fahiminiya S, Hamel N, Tomiak E, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nature genetics. 2014 May;46(5):438–43. doi: 10.1038/ng.2931. [DOI] [PubMed] [Google Scholar]
- 150.Wu RC, Ayhan A, Maeda D, Kim KR, Clarke BA, Shaw P, et al. Frequent somatic mutations of the telomerase reverse transcriptase promoter in ovarian clear cell carcinoma but not in other major types of gynaecological malignancy. The Journal of pathology. 2014 Mar;232(4):473–81. doi: 10.1002/path.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elgazzar S, Zembutsu H, Takahashi A, Kubo M, Aki F, Hirata K, et al. A genome-wide association study identifies a genetic variant in the SIAH2 locus associated with hormonal receptor-positive breast cancer in Japanese. Journal of human genetics. 2012 Dec;57(12):766–71. doi: 10.1038/jhg.2012.108. [DOI] [PubMed] [Google Scholar]
- 152.Zhang B, Li Y, Zheng X, Zuo X, Zhou F, Liang B, et al. A common variant in the SIAH2 locus is associated with estrogen receptor-positive breast cancer in the Chinese Han population. PloS one. 2013;8(11):e79365. doi: 10.1371/journal.pone.0079365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang MD, Wang HC, Chang WS, Tsai CW, Bau DT. Genetic polymorphisms of DNA double strand break gene Ku70 and gastric cancer in Taiwan. BMC cancer. 2011;11:174. doi: 10.1186/1471-2407-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Macgregor S, Montgomery GW, Liu JZ, Zhao ZZ, Henders AK, Stark M, et al. Genomewide association study identifies a new melanoma susceptibility locus at 1q21.3. Nature genetics. 2011 Nov;43(11):1114–8. doi: 10.1038/ng.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dejardin J, Kingston RE. Purification of proteins associated with specific genomic Loci. Cell. 2009 Jan 9;136(1):175–86. doi: 10.1016/j.cell.2008.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kappei D, Butter F, Benda C, Scheibe M, Draskovic I, Stevense M, et al. HOT1 is a mammalian direct telomere repeat-binding protein contributing to telomerase recruitment. The EMBO journal. 2013 Jun 12;32(12):1681–701. doi: 10.1038/emboj.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rafnar T, Gudbjartsson DF, Sulem P, Jonasdottir A, Sigurdsson A, Jonasdottir A, et al. Mutations in BRIP1 confer high risk of ovarian cancer. Nature genetics. 2011 Nov;43(11):1104–7. doi: 10.1038/ng.955. [DOI] [PubMed] [Google Scholar]
- 158.Dai J, Zhang C, Tian Z, Zhang J. Expression profile of HMBOX1, a novel transcription factor, in human cancers using highly specific monoclonal antibodies. Experimental and therapeutic medicine. 2011 May;2(3):487–90. doi: 10.3892/etm.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heim KC, White KA, Deng D, Tomlinson CR, Moore JH, Freemantle SJ, et al. Selective repression of retinoic acid target genes by RIP140 during induced tumor cell differentiation of pluripotent human embryonal carcinoma cells. Molecular cancer. 2007;6:57. doi: 10.1186/1476-4598-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Scheibe M, Arnoult N, Kappei D, Buchholz F, Decottignies A, Butter F, et al. Quantitative interaction screen of telomeric repeat-containing RNA reveals novel TERRA regulators. Genome research. 2013 Dec;23(12):2149–57. doi: 10.1101/gr.151878.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shah SN, Cope L, Poh W, Belton A, Roy S, Talbot CC, Jr., et al. HMGA1: a master regulator of tumor progression in triple-negative breast cancer cells. PloS one. 2013;8(5):e63419. doi: 10.1371/journal.pone.0063419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tang D, Kang R, Zeh HJ, 3rd, Lotze MT. High-mobility group box 1 and cancer. Biochimica et biophysica acta. 2010 Jan-Feb;1799(1-2):131–40. doi: 10.1016/j.bbagrm.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhou ZJ, Dai Z, Zhou SL, Fu XT, Zhao YM, Shi YH, et al. Overexpression of HnRNP A1 promotes tumor invasion through regulating CD44v6 and indicates poor prognosis for hepatocellular carcinoma. International journal of cancer Journal international du cancer. 2013 Mar 1;132(5):1080–9. doi: 10.1002/ijc.27742. [DOI] [PubMed] [Google Scholar]
- 164.Xu Y, Gao XD, Lee JH, Huang H, Tan H, Ahn J, et al. Cell type-restricted activity of hnRNPM promotes breast cancer metastasis via regulating alternative splicing. Genes & development. 2014 Jun 1;28(11):1191–203. doi: 10.1101/gad.241968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Flicek P, Amode MR, Barrell D, Beal K, Billis K, Brent S, et al. Ensembl 2014. Nucleic acids research. 2014 Jan;42(Database issue):D749–55. doi: 10.1093/nar/gkt1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mahmoudi S, Henriksson S, Weibrecht I, Smith S, Soderberg O, Stromblad S, et al. WRAP53 is essential for Cajal body formation and for targeting the survival of motor neuron complex to Cajal bodies. PLoS biology. 2010;8(11):e1000521. doi: 10.1371/journal.pbio.1000521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ballew BJ, Yeager M, Jacobs K, Giri N, Boland J, Burdett L, et al. Germline mutations of regulator of telomere elongation helicase 1, RTEL1, in Dyskeratosis congenita. Human genetics. 2013 Apr;132(4):473–80. doi: 10.1007/s00439-013-1265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Walne AJ, Vulliamy T, Kirwan M, Plagnol V, Dokal I. Constitutional mutations in RTEL1 cause severe dyskeratosis congenita. American journal of human genetics. 2013 Mar 7;92(3):448–53. doi: 10.1016/j.ajhg.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Polvi A, Linnankivi T, Kivela T, Herva R, Keating JP, Makitie O, et al. Mutations in CTC1, encoding the CTS telomere maintenance complex component 1, cause cerebroretinal microangiopathy with calcifications and cysts. American journal of human genetics. 2012 Mar 9;90(3):540–9. doi: 10.1016/j.ajhg.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.