Abstract
The objective of this study was to determine a chemopreventive activity of Korean red ginseng extract (KRG) in diethylnitrosamine (DEN) induced hepatocarcinogenesis in rats. After acclimatization for a week, Sprague-Dawley rats were randomized into five groups (n = 15) and fed either KRG (0.5, 1 or 2%) or control diets for 10 weeks. After two weeks of starting of experimental diets, the rats were initiated hepatocarcinogenesis by injection of DEN and were then subjected to two-thirds partial hepatectomy at five-week for developing the medium-term bioassay system. Both 0.5 and 1% KRG diets suppressed the area (55 and 60%; p= 0.0251 and 0.0144) and number (39 and 59%; p= 0.0433 and 0.0012) of glutathione S-transferase placental form (GST-P) positive foci when compared to the DEN-control group. The production of thiobarbituric acid reactive substances (TBARS) was significantly reduced in 0.5 and 1% KRG-treated rats. The supplementation of 1% KRG diet significantly elevated the levels of total glutathione (tGSH) and glutathione-related enzymes including cytosolic glutathione S-transferase (GST) and glutathione peroxidase (GPx) activities. It was also observed in cDNA microarray that the gene expressions (Cyp2c6, Cyp2e1, Cyp3a9, and Mgst1) involved in the xenobiotics metabolism via cytochrome P450 signaling pathway were down-regulated in the 1% KRG diet-treated group when compared to the DEN-control. The chemopreventive effects of KRG could be affected by 1) the decrease of lipid peroxidation, 2) the increase of tGSH content and GSH-dependent enzyme activities, and 3) the decrease of the gene expression profile involved in cytochrome P450 signaling pathway. These results suggest that KRG may prove to be a therapeutic agent against hepatocarcinogenesis.
Keywords: Korean red ginseng, rat, glutathione S-transferase placental form positive foci, hepatocarcinogenesis, antioxidant.
Introduction
Liver cancer is the second cause of cancer death in men and the sixth in women. The main risk factor for liver cancer is the elevated prevalence of chronic hepatitis B virus infection 1. It has been shown that a diet rich in dietary antioxidants and phytochemicals may decrease the risk of hepatocellular carcinoma, a primary malignant cancer of the liver. Hence, identifying the promising chemopreventive agents in diets and their underlying molecular mechanisms have been considered to be the best strategy to protect against hepatocarcinogenesis 2.
Panax ginseng C.A. Meyer was traditionally used as a medicinal plant in Asian countries, and it has now gained worldwide popularity 3. Ginseng is identified to contain ginsenosides, phenolic compounds, polysaccharides, and polyacetylenes, which are known to have a chemopreventive effect through antioxidant, apoptotic, and anti-cell proliferation in various cancers 4-8. Red ginseng is heated panax ginseng produced by steaming followed by drying, and contains higher amount of ginsenosides and polyphenolics than white ginseng 9, 10. The heat processing converts ginsenosides into other types of ginsenosides, including ginsenoside Rh2 and Rg3, and produces the antioxidant agents and phenolic compounds such as maltol 11, 12. Since Korea red ginseng (KRG) has unique anti-carcinogenic compounds, it has been suggested that KRG has more potent chemopreventive activity than fresh and white ginseng 13. In fact, it has been demonstrated that KRG extract has a chemopreventive effect on the hepatotoxins-induced liver cancer in rats 13. Also, KRG, specifically ginsenosides Rg3, Rg5 and Rh2, has been shown to increase apoptosis in human hepatocellular carcinoma cells 14. Ginsenoside Rh2 exhibited the apoptotic properties through caspase-3 activation in SK-HEP-1 cell lines 15. Moreover, ginsenoside Rg3 has been shown to induce apoptosis in human hepatocellular carcinoma cells and to inhibit liver cancer growth in vivo via alterations of Bcl-2 family proteins 16, 17. It was reported that compound K [20-O-β-(D-glucopyranosyl)-20(S)-protopanaxadiol], which is an intestinal metabolite of the protopanaxadiol-type ginsenoside, suppressed cell proliferation and induced apoptosis in hepatocellular cancer cell via a Bid-mediated pathway 18.
Although the beneficial effects of KRG are well documented, the administration of high dose of KRG might be detrimental through their toxicity. Several studies have reported that overdose and long-term usage of ginseng are associated with side effects such as hypertension, nausea, diarrhea, insomnia, and headache, known as ginseng abuse syndrome 19, 20. Ginsenoside Rh2, which is one of active ginsenosides of KRG, is known to have anticancer activities, while it showed cytotoxic effects to human hepatocyte cells 21. These evidences suggest that comparative studies between various concentrations of red ginseng for chemoprevention of hepatocarcinogenesis need to be performed, and that the proper usage of ginseng on liver cancer has to be established.
The objective of this study is to determine the potential chemopreventive effects of various concentrations of KRG extract on hepatocarcinogenesis in rats. We hypothesized that the proper amount of KRG extract may prevent hepatocarcinogenesis through modulation of the liver oxidative environment, but that the chemopreventive effects may differ based on the concentrations. Subsequently, the underlying mechanisms were investigated to determine whether these different concentrations of KRG extract minimize oxidative damage via the modulation of the cellular redox environment on rat hepatocarcinogenesis.
Materials and Methods
Animals
After obtaining Institutional Animal Care and Use Committee (IACUC) approval, male Sprague-Dawley rats (Four week-old) were supplied from the Animal Care Facility (Seoul National University, Seoul, Korea), and were then acclimatized for a week. Rats were randomized into five groups and fed either Korean red ginseng extract (KRG; n = 15/group; 0.5, 1, or 2%) diets or control diet for 10 weeks. Animals were kept in polycarbonate cages under standard conditions (room temperature 23 ± 2ºC, relative humidity 55 ± 5 %, 12-hr light/dark cycle), given food and water ad libitum, recorded daily and weighed weekly. After two weeks of starting of experimental diets, all of the rats except a control group were initiated hepatocarcinogenesis by the injection of DEN (200 mg/kg body weight; Sigma Chemical Co., St. Louis, MO, USA) dissolved in saline. In addition, they were subjected to two-thirds of partial hepatectomy (PH) after 3 weeks according to a modified medium-term bioassay protocol 22. The control group was treated with saline and a sham operation. This study was terminated at ten weeks, and the liver sections were fixed in 10% neutral buffered formalin. Two aliquots (0.1 and 5g, respectively) of each liver were quickly removed and kept at -80ºC for total RNA extraction and glutathione content determination.
Diets
KRG extract was received from Cheong-Kwan-Jang (Seoul, Korea) in high value of commercial concentrated pure extract prepared from Korean red ginseng root (6-year-old Panax ginseng C.A Meyer). The moisture of KRG extract was approximately 40%, the amount of crude ginsenoside was 70 mg/g, and the total concentration of ginsenosides was 20 mg/g. The composition of the basal diet is given in Table 1. The KRG extract was substituted for part of cornstarch in the experimental KRG diets. The prepared dietary foods were stored at -20ºC.
Table 1.
Composition of control and experimental diets (g / 100g)
| Components | Control | 0.5% KRG | 1% KRG | 2% KRG |
|---|---|---|---|---|
| KRG extract | - | 0.5 | 1 | 2 |
| Corn starch | 55.2 | 54.7 | 54.2 | 53.2 |
| Casein | 20.00 | 20.00 | 20.00 | 20.00 |
| Corn Oil | 15.00 | 15.00 | 15.00 | 15.00 |
| α-Cellulose | 5.00 | 5.00 | 5.00 | 5.00 |
| Mineral mixa | 3.5 | 3.5 | 3.5 | 3.5 |
| Vitamin mixa | 1.00 | 1.00 | 1.00 | 1.00 |
| DL-Methionine | 0.30 | 0.30 | 0.30 | 0.30 |
a Mineral and vitamin mixtures for AIN-76 diet.
Sample preparation
Five gram of liver was homogenized in Tris-HCl buffered solution (pH 7.4) and centrifuged at 10,000×g for 20 min, followed by re-centrifugation of the supernatant at 100,000×g for 60 min. The supernatant was considered as the cytosol, and the pellet as the microsome. The microsomal pellet was re-suspended in 20% glycerol buffered solution. The entire fractionation procedure was conducted at 4ºC 23.
Immunohistochemical staining for glutathione S-transferase placental form positive foci (GST-P+ foci)
GST-P+ foci, DEN-initiated lesions, is thought to be a biomarker of hepatocellular carcinoma 24. At autopsy, 2-3 mm of liver sections were fixed in ice-cold acetone for immunohistochemistry of GST-P+ foci using an anti-mouse GST-P antibody (Medical Biological Laboratories Co, Nagoya, Japan). The avidin-biotin-peroxidase complex method (Vectastain ABC kit, Vector Lab. Inc., Burlingame, CA) was used to visualize GST-P+ foci that reflect putative preneoplastic lesions. The areas and numbers of the GST-P+ foci (> 0.2 mm in diameter) in liver sections were measured using an image analyzer with a microscope (Quantinet 520, Cambridge Instruments, Cambridge, UK) 23.
Determinations of lipid peroxidation
Hepatic lipid peroxidation (thiobarbituric acid reactive substances, TBARS) was determined by the reaction between TBA and malondialdehyde formed from peroxidation of lipids 25. In short, 200μL of 0.375% thiobarbituric acid-15% trichloroacetic acid-0.25 N HCl were added to 100 μL of rat liver microsomal suspension, and the mixture was incubated in a boiling water bath for 15 min. After that, it was centrifuged at 1,000×g for 10 min. Malondialdehyde in the supernatant was measured at 532 nm. Protein was quantified using a modified Lowry method 26, with bovine serum albumin as a standard.
Determinations of total glutathione (tGSH) contents and GSH-dependent enzymes
tGSH content (both reduced and oxidized glutathione; GSH and GSSG) was measured by the 5,5'-dithiobis-2-nitrobenzoic acid (DTNB) and glutathione reductase (GR) recycling procedure. The existing GSSG can be converted to GSH by adding GR and NADPH. After that, tGSH can be determined by measuring the reaction of GSH with DTNB 27. The tGSH content was expressed as moles of reduced GSH equivalents per liver (g). Glutathione S-transferase (GST) activities in the hepatic cytosolic fraction were determined through monitoring the conjugation of GSH with 1-chloro-2,4-dinitrobenzen (CDNB) at 340 nm by using a dual beam spectrophotometer (Beckman DU650, Beckman Coulter Inc, Miami, FL) 28. The glutathione peroxidase (GPx) was measured indirectly by a coupled reaction with GR. The GSSG catalyzed by GPx is recycled to GSH by GR and NADPH. Hepatic cytosolic fraction was added in the reaction mixture contained 50 mM Tris-HCl buffer (pH 7.6), 0.1 mM EDTA, 0.25 mM GSH, 1 unit/ml GR, and 0.12 mM NADPH. The reaction was initiated by adding the cumene hydroperoxide, and the oxidation of NADPH to NADP+ was monitored at 340 nm 29. For measuring the cytosolic GR activity, hepatic cytosolic fraction was added in the reaction mixture containing a 0.2 mM potassium phosphate buffer (pH 7.0), 2mM EDTA, 20 mM GSSG, and 2mM NADPH. The oxidation of NADPH to NADP+ was monitored at 340 nm 30. The levels of GPx and GR were defined as the amount of enzyme causing the oxidation of 1 nmol of NADPH (extinction coefficient, 6.22mM-1cm-1) per minute and per mg protein.
cDNA microarray analysis
cDNA microarray analysis was used to examine differential gene expression between 0% and 1% KRG extract group. Total RNA was isolated from 6 tissues randomly selected in each group using the Trizol extraction reagent (Invitrogen, Carlsbad, CA). Digital Genomics Inc. (Seoul, Korea) performed duplicate examinations on GenePlorerTMtwin chipTM-Rat 5K containing 4,863 gene probes with mixed total RNA. Global median, intensity/location-dependent normalization was performed to analyze all data. Genes were considered differentially expressed when the log2 ratio was more than 1 or less than -1. To identify the pathway altered by the 1% KRG extract, differentially expressed gene data underwent further analyses using KEGG pathway. A DAVID (the database for annotation, visualization and integrated discovery) bioinformatics resources (http://david.abcc.ncifcrf.gov/) was used to perform a functional analysis for those genes 31.
Statistical analyses
All results were shown as the means ± SE (n = 15) for each group. All data were analyzed using one-way ANOVA followed by Duncan's post-hoc test using SPSS (version 11.5, SPSS). Correlations between variables were calculated using a Pearson correlation.
Results
Body weight and liver weight of rats
Animals of all groups were kept under close observation for intake of diet and fluid, rate of weight gain, and general health. There was no significant difference in final body weight, liver weight, or relative liver weight (percentage liver weight per body weight) among the groups (Table 2).
Table 2.
Final body weight, food intake, liver weight, and relative liver weight
| Group | Final body weight (g) | Food intake (g/d) | Liver weight (g) | Relative liver weight (%)a |
|---|---|---|---|---|
| Control | 389.16 ± 9.24ns | 14.91 ± 0.59ns | 9.93 ± 0.32 ns | 2.55 ± 0.02 ns |
| DEN-Con | 368.81 ± 12.26 | 14.44 ± 0.87 | 8.74 ± 0.45 | 2.36 ± 0.06 |
| KRG 0.5 | 376.54 ± 15.47 | 14.56 ± 0.92 | 8.79 ± 0.45 | 2.33 ± 0.03 |
| KRG 1 | 378.74 ± 9.62 | 14.55 ± 0.89 | 8.76 ± 0.45 | 2.31 ± 0.07 |
| KRG 2 | 373.07 ± 11.46 | 14.31 ± 0.92 | 9.15 ± 0.41 | 2.45 ± 0.06 |
aRelative liver weight (%) = [Liver weight (g) / Body weight (g) * 100].
All values are means ± SE (n = 15 per group), and “ns” means “not significantly different” among groups.
KRG extract suppressed the formation of preneoplastic foci
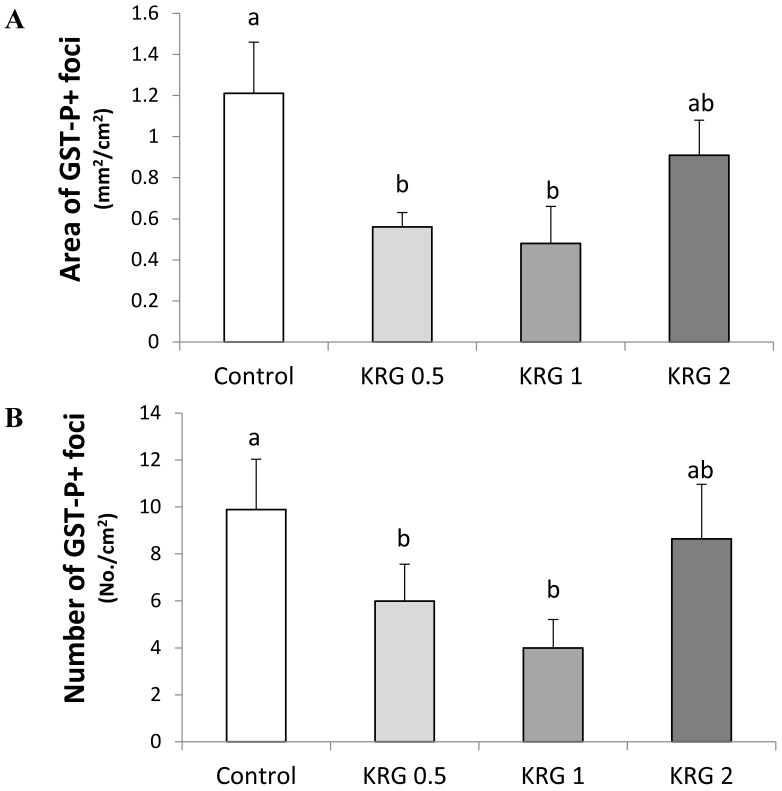
GST-P+ foci were developed in all groups treated with DEN. The area (mm2/cm2) and number (No./cm2) of the GST-P+ foci (mean diameter >0.2 mm) in the DEN-control were 1.21 and 9.93, respectively. The values were significantly lower to 0.55 and 6.04 (55% and 39% reduction; p=0.0251 and 0.0433, respectively) in the KRG 0.5% group and 0.49 and 4.03 (60% and 59% reduction; p=0.0144 and 0.0012, respectively) in the KRG 1% group, respectively when compared to the DEN-control (Fig. 1A and B). By contrast, the frequency of GST-P+ foci was not significantly different between the KRG 2%-supplemented group and the DEN-control (Fig. 1A and B).
Figure 1.
Effects of Korea red ginseng extract on the area and number of placental glutathione S-transferase (GST-P) positive foci in DEN-induced and PH-promoted hepatic carcinogenesis in rat. (A) The area of GST-P+ foci. (B) The number of GST-P+ foci. Values are means ± SE (n = 15) from image reading of stained GST-P+ foci. Means with different letters are significantly different, p < 0.05, whereas means with similar letters are not different from each other.
KRG extract suppressed the lipid peroxidation
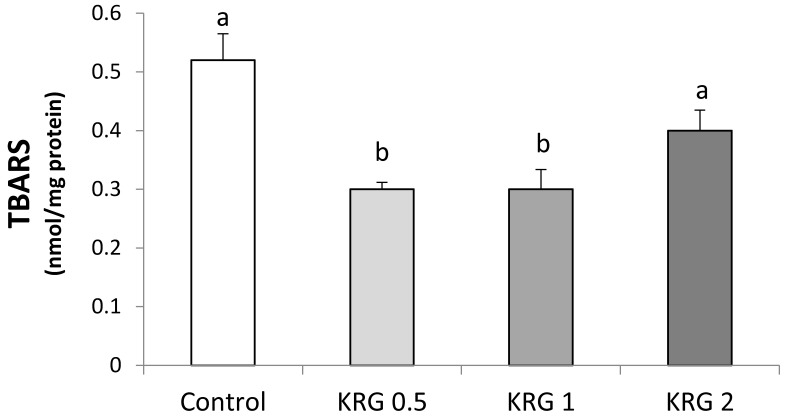
The animals supplemented with KRG (0.5 or 1 %) showed a lower level of lipid peroxidation compared to the DEN-control (Fig. 2). TBARS was significantly elevated after the DEN treatment followed by PH in a positive correlation with the area of GST-P+ foci (r = 0.99, p = 0.011).
Figure 2.
Effects of Korea red ginseng extract on Thiobarbituric acid (TBARS) in DEN-induced and PH-promoted hepatic carcinogenesis in rat. Values are means ± SE (n = 15). Means with different letters are significantly different, p < 0.05, whereas means with similar letters are not different from each other.
KRG 1% extract induced the levels of total glutathione and glutathione-dependent enzymes
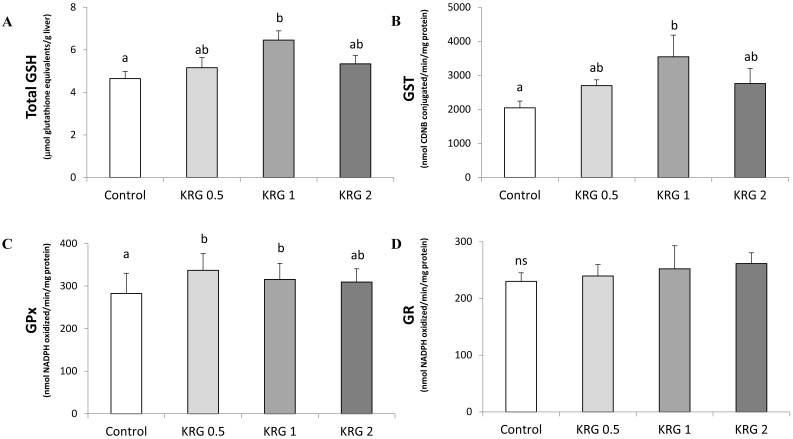
Hepatic tGSH content of the 1% KRG-supplemented rats was significantly increased when compared to the DEN-control (Fig. 3A). In addition, the cytosolic GST activity of the 1% KRG supplemented rats was also significantly increased when compared to the DEN-control (Fig. 3B). The activity of GPx was significantly elevated by the 0.5% and 1% KRG, when compared to the DEN-control (Fig. 3C). However, there was no significant difference in the activity of GR between groups (Fig. 3D).
Figure 3.
Effects of Korea red ginseng extract on glutathione level and glutathione-dependent enzyme activities in DEN-induced and PH-promoted hepatic carcinogenesis in rat. (A) tGSH Content. (B) GST, (C) GPx, and (D) GR activities. Values are means ± SE (n = 15). Means with different letters are significantly different, p < 0.05, whereas means with similar letters are not different from each other.
KEGG pathways based on differentially expressed genes altered by the 1% KRG diet
Since the KRG 1% extract group has the most protective effects (based on Fig. 1), it was further studied to identify genes using the cDNA microarray. Of 4,863 gene probes on cDNA arrays, it was identified that 19 genes were up-regulated, and 114 genes were down-regulated in the 1% KRG extract diet. When functional analysis of differentially expressed genes was performed using the DAVID web-based program, 5 KEGG pathways were significantly enriched in the 1% KRG extract diet (Table 3). Importantly, the most relevant GO terms was metabolism of xenobiotics via cytochrome P450 (Cyp P450, p = 0.0144), in which four genes (Cyp2c6, Cyp2e1, Cyp3a9, Mgst1) were significantly down-regulated.
Table 3.
KEGG analysis with significant enrichment of genes differentially expressed by 1% Korean red ginseng intake on hepatocarcinogenesis
| GO terms | p-value | Up-regulated | Down-regulated |
|---|---|---|---|
| rno00830:Retinol metabolism | 0.0138 | - | Cyp2c6, Cyp3a9, Cyp4a3, Rdh10 |
| rno00980:Metabolism of xenobiotics by cytochrome P450 | 0.0144 | - | Cyp2c6, Cyp2e1, Cyp3a9, Mgst1 |
| rno03320:PPAR signaling pathway | 0.0226 | - | Acadm, Acsl1, Cyp4a3, Slc27a2 |
| rno00982:Drug metabolism | 0.0234 | - | Cyp2c6, Cyp2e1, Cyp3a9, Mgst1 |
| rno00591:Linoleic acid metabolism | 0.0322 | - | Cyp2c6, Cyp2e1, Cyp3a9 |
Discussion
In this study, various concentrations of KRG extract were utilized to investigate whether 1) KRG extract may play an important role in modulating redox status, and 2) the optimum intake of KRG may suppress hepatocarcinogenesis in carcinogen-treated rats. It was hypothesized that KRG extract may prevent hepatocarcinogenesis through modulation of the liver redox environment and oxidative stress, but that the chemopreventive effects may differ based on the concentration.
KRG is a traditional medicine to treat a variety of disorders, including cancers 32. The DEN model for this study is a pre-clinical model of hepatocellular cancer that exhibits many phenotypic characteristics relevant to the liver cancer 22. We observed that the chemopreventive effects of KRG extract on rat hepatocarcinogenesis initiated by DEN and promoted by PH range between 0.5-1% 33. These ranges significantly reduced the area and number of GST-P positive foci when compared to the control. However, the KRG 2% diet had no suppressive effect on DEN-induced hepatocarcinogenesis in terms of the area and number of GST-P foci. We previously reported that white panax ginseng has a chemopreventive effect on hepatocarcinogenesis in 2% concentration 34. The average value of ginsenosides in four-year-old white panax ginseng was 1.348% 35, whereas the concentration of ginsenosides in six-year-old KRG was 2%. In addition, red ginseng has more active deglycosylated derivatives including ginsenosides Rg3 than white ginseng by the heat processing 11. Therefore, the different therapeutic ranges for these white and red ginsengs can be explained by higher amount of active ginsenosides in KRG.
High-dose KRG, however, might reduce its chemopreventive effects through induction of its toxicity. It was previously reported that high intakes of well-known chemopreventive compounds, such as indole-3-carbinol, caffeic acid, and ferulic acid were associated with increased risks of cancers 36-39. A previous study showed similar outcomes that high-dose intake of chemopreventive compounds lost its chemopreventive efficacy. The effects of pfaffia paniculata root (Brazilian ginseng) on hepatocarcinogenesis were evaluated at 0.5, 2, and 10% in mice, and a 2% dose was shown to be the most effective on suppressing tumor incidence than 0.5 or 10% dose. Especially, the 10% dose had no suppressive effect on DEN-induced hepatocarcinogenesis in female mice 40. One potential mechanism is that high dose intake of phytochemicals as xenobiotics may induce the activities of hepatic Cyp P450 and reduce phase II enzymes, which enhance oxidative stress response and hepatocarcinogenesis 38.
In the dosage calculation of this study, the amounts of KRG intake in 0.5 and 1% KRG group were 165.5 and 331 mg/kg/day in rats, and these groups have chemopreventive effects on carcinogen-treated rats. The results were similar to the previous study in which red ginseng extract suppressed skin tumor in rats in a dose-dependent manner at 50-400 mg/kg 41. Moreover, it has been reported that there was no toxic effect in rats fed on ginseng extract at dose levels of 105-210 mg/kg/day for 25 weeks 42. It has been shown that human equivalent intake of 0.5-1% KRG is 26.84-53.68 mg/kg/day for human 43, which equals 2-4 g intake of KRG for an individual of 75 kg body weight. It has been shown that KRG at dose of 3-6 g/day for eight weeks improved the antioxidant enzymes and oxidative stress markers in healthy human 44. By contrast, there was no chemopreventive effect of the 662 mg/kg/day intake of KRG (2%) on carcinogen-treated rats in this study. However, there were no significant difference in the body weight and relative weight of liver (Table 2). In addition, it has been suggested that the No Observed Adverse Effect Level (NOAEL) of KRG was 2 g/kg/day in rats fed the KRG extract for 4 weeks 45. Based on our results, even though there are no toxic effects, we suggest that more than the 8 g/day intake of KRG may not improve the redox status of glutathione in human.
Oxidative stress represents an imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense system 46. High ROS production has been shown to lead to DNA damage, mutations of tumor suppressors, gene instability, and carcinogenesis, and damage other molecules including the fatty acid side chains of lipids in the membranes of the cell 47. Direct measurement of ROS has not established well due to their instability, so ROS generation is usually indirectly assayed by detecting specific biomarkers, such as lipid peroxidation (TBARS analysis) 48. It has been shown previously that KRG has a potent antioxidant activity 49, and red ginseng oil has a protective effect on liver damages by inducing the antioxidant enzymes activity and by inhibiting lipid peroxidation in vitro and in vivo 50. In addition, KRG extract has been shown to be a chemopreventive effect via improvement of antioxidant capacity in hepatotoxin-treated rats 51. Similar to these previous reports, we demonstrated for the first time that 0.5% and 1% KRG suppress the level of TBARS, a lipid peroxidation biomarker compared to control. It suggests that KRG has an antioxidant property, which may contribute to inhibition of lipid peroxidation, and suppress hepatocarcinogenesis.
Liver detoxification process metabolizes carcinogens by Phase I (i.e. Cytochrome P450), Phase II enzymes (i.e. GST), and antioxidant enzymes including GR and GPx 52, 53. In phase II of detoxification, glutathione (GSH and GSSG) and its related enzymes such as GST, GPx, and GR play an important antioxidant role, preventing damage caused by ROS. Elevations of these enzymes may suppress the process of liver disease development such as liver cancer 54. The combination of carcinogen injection and PH are vulnerable with an oxidative damage that may lead to lipid peroxidation and hepatocarcinogenesis 55. In our study, the induction of tGSH level by the 1% KRG extract may be associated with the reduction of oxidative stress. We also observed that the 1% KRG extract increased cytosolic GST and GPx activities when compared to the control, suggesting that the increase of cytosolic GST activity would improve the cellular detoxifying potential. Moreover, the increase in the activities of GPx in the KRG fed rats would help to speed up the redox cycling. It suggests that KRG-regulated both cytosolic GST and GPx activities may relate to the cell protection against oxidative damage by catalyzing the elimination of peroxide.
To understand the genetic metabolic adaptation, we performed cDNA microarray and identified 133 differentially expressed genes comparing between the 1% KRG diet and the DEN-control group. When the differentially expressed genes underwent KEGG pathway analysis with the DAVID web-based program, we observed that 5 KEGG pathways such as Retinol metabolism and/or Metabolism of xenobiotics by Cyp P450 and PPAR signaling pathway were over-represented. The gene expressions of Cyp2c6, Cyp2e1, Cyp3a9 and Mgst1 in Metabolism of xenobiotics via Cyp P450 were down-regulated in the 1% KRG-treated rats when compared to the control. It has been shown that the Cyp P450 involved in the phase I xenobiotic metabolism is a key enzyme in cancer formation and cancer treatment 56. It has also been suggested that red ginseng has an inhibitory effect on the Cyp P450 activities in rat liver 56, and ginsenoside, such as Rg3, suppressed the Cyp P450 enzymes activity 57. It has been shown that CYP2E1 acts as a lipid peroxidation inducer 58. Also, it was reported that the intake of garlic powder decreased the preneoplastic foci formation and contributed to chemoprevention against the rat hepatocarcinogenesis through the suppression of CYP2E1 58. Based on these previous reports and our data, we suggest that KRG may have a beneficial effect on the inhibition of lipid peroxidation via modulation of Cyp P450 enzymes during hepatocarcinogenesis.
In conclusion, the 0.5~1% dose of Korean red ginseng has a chemopreventive effect on chemically-induced rat hepatocarcinogenesis by suppression of oxidative stress and modulation of redox-enzymes. Additionally, more than the 2% dose of KRG may lose the chemopreventive efficacy due to the fact that it cannot improve carcinogen detoxifying enzyme. Ginseng is a popular chemopreventive compound; however the lack of the dose ranging trials may result in overdose. We report for the first time to determine the chemopreventive effects of KRG at the certain concentration range. Since we did not look at all the possible signaling pathways that are considered targets of KRG, further in vitro and in vivo tests are needed to elucidate the role of high-dose ginseng on rat hepatocarcinogenesis. Thus, investigating the detailed molecular mechanisms of the anti-oxidative effects of KRG is essential to further understand the chemopreventive effects of KRG. Despite these limitations, our results have shown that KRG has a chemopreventive effect via the modulation of the cellular redox environment to minimize oxidative damage, and we suggested that KRG could be a potential therapeutic agent against hepatocarcinogenesis.
Acknowledgments
We are very thankful for Dr.Sanghui Kweon, Dr. Younkyoung Suh, Dr. Mi-Joung Kim, and Yoonjung Kim, Department of Food and Nutrition, Seoul National University, Seoul, South Korea for their support with the animal study.
Abbreviations
- Cyp P450
cytochrome P450
- CDNB
1-chloro-2,4-dinitrobenzen
- DAVID
database for annotation, visualization and integrated discovery
- DEN
diethylnitrosamine
- DTNB
5,5'-dithiobis-2-nitrobenzoic acid
- GPx
glutathione peroxidase
- GR
glutathione reductase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GST
glutathione S-transferase
- GST-P+ foci
glutathione S-transferase placental form positive foci
- KRG
Korea red ginseng
- NOAEL
no observed adverse effect level
- PH
partial hepatectomy
- ROS
reactive oxygen species
- TBARS
thiobarbituric acid reactive substances
- tGSH
total glutathione.
References
- 1.Jemal A, Bray F, Center MM. et al. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61:69–90. doi: 10.3322/caac.20107. doi:10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Mann CD, Neal CP, Garcea G. et al. Phytochemicals as potential chemopreventive and chemotherapeutic agents in hepatocarcinogenesis. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation. 2009;18:13–25. doi: 10.1097/CEJ.0b013e3282f0c090. doi:10.1097/CEJ.0b013e3282f0c090. [DOI] [PubMed] [Google Scholar]
- 3.Yun TK. Experimental and epidemiological evidence on non-organ specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutation research. 2003;523-524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 4.Kim HS, Lee EH, Ko SR. et al. Effects of ginsenosides Rg3 and Rh2 on the proliferation of prostate cancer cells. Archives of pharmacal research. 2004;27:429–35. doi: 10.1007/BF02980085. [DOI] [PubMed] [Google Scholar]
- 5.Park J, Lee KY, Oh YJ. et al. Activation of caspase-3 protease via a Bcl-2-insensitive pathway during the process of ginsenoside Rh2-induced apoptosis. Cancer letters. 1997;121:73–81. doi: 10.1016/s0304-3835(97)00333-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee L-S, Cho C-W, Hong H-D. et al. Hypolipidemic and antioxidant properties of phenolic compound-rich extracts from white ginseng (Panax ginseng) in cholesterol-fed rabbits. Molecules. 2013;18:12548–60. doi: 10.3390/molecules181012548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng H, Li S, Fan Y. et al. Comparative studies of the antiproliferative effects of ginseng polysaccharides on HT-29 human colon cancer cells. Medical oncology. 2011;28:175–81. doi: 10.1007/s12032-010-9449-8. [DOI] [PubMed] [Google Scholar]
- 8.Yang MC, Seo DS, Choi SU. et al. Polyacetylenes from the roots of cultivated-wild ginseng and their cytotoxicity in vitro. Archives of pharmacal research. 2008;31:154–9. doi: 10.1007/s12272-001-1134-1. [DOI] [PubMed] [Google Scholar]
- 9.Chung I-M, Kim J-W, Seguin P. et al. Ginsenosides and phenolics in fresh and processed Korean ginseng (Panax ginseng CA Meyer): Effects of cultivation location, year, and storage period. Food chemistry. 2012;130:73–83. [Google Scholar]
- 10.Yun TK, Lee YS, Lee YH. et al. Anticarcinogenic effect of Panax ginseng C.A. Meyer and identification of active compounds. Journal of Korean medical science. 2001;16(Suppl):S6–18. doi: 10.3346/jkms.2001.16.S.S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nam K. The comparative understanding between red ginseng and white ginsengs, processed ginsengs (Panax ginseng C.A. Meyer) J Ginseng Res. 2005;29:1–18. [Google Scholar]
- 12.Kim GN, Lee JS, Song JH. et al. Heat processing decreases Amadori products and increases total phenolic content and antioxidant activity of Korean red ginseng. Journal of medicinal food. 2010;13:1478–84. doi: 10.1089/jmf.2010.1076. doi:10.1089/jmf.2010.1076. [DOI] [PubMed] [Google Scholar]
- 13.Wu XG, Zhu DH, Li X. Anticarcinogenic effect of red ginseng on the development of liver cancer induced by diethylnitrosamine in rats. Journal of Korean medical science. 2001;16(Suppl):S61–5. doi: 10.3346/jkms.2001.16.S.S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park HM, Kim SJ, Kim JS. et al. Reactive oxygen species mediated ginsenoside Rg3- and Rh2-induced apoptosis in hepatoma cells through mitochondrial signaling pathways. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2012;50:2736–41. doi: 10.1016/j.fct.2012.05.027. doi:10.1016/j.fct.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 15.Oh JI, Chun KH, Joo SH. et al. Caspase-3-dependent protein kinase C delta activity is required for the progression of Ginsenoside-Rh2-induced apoptosis in SK-HEP-1 cells. Cancer letters. 2005;230:228–38. doi: 10.1016/j.canlet.2004.12.043. doi:10.1016/j.canlet.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Liu L, Yu Y. et al. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Molecular medicine reports. 2012;5:1295–8. doi: 10.3892/mmr.2012.808. doi:10.3892/mmr.2012.808. [DOI] [PubMed] [Google Scholar]
- 17.Jiang JW, Chen XM, Chen XH. et al. Ginsenoside Rg3 inhibit hepatocellular carcinoma growth via intrinsic apoptotic pathway. World journal of gastroenterology: WJG. 2011;17:3605–13. doi: 10.3748/wjg.v17.i31.3605. doi:10.3748/wjg.v17.i31.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song G, Guo S, Wang W. et al. Intestinal metabolite compound K of ginseng saponin potently attenuates metastatic growth of hepatocellular carcinoma by augmenting apoptosis via a Bid-mediated mitochondrial pathway. Journal of agricultural and food chemistry. 2010;58:12753–60. doi: 10.1021/jf103814f. doi:10.1021/jf103814f. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RK. Ginseng abuse syndrome. Problems with the panacea. JAMA: the journal of the American Medical Association. 1979;241:1614–5. [PubMed] [Google Scholar]
- 20.Seely D, Dugoua JJ, Perri D. et al. Safety and efficacy of panax ginseng during pregnancy and lactation. The Canadian journal of clinical pharmacology = Journal canadien de pharmacologie clinique. 2008;15:e87–94. [PubMed] [Google Scholar]
- 21.Wei G-Q, Zheng Y-N, Li W. et al. Structural modification of ginsenoside Rh2 by fatty acid esterification and its detoxification property in antitumor. Bioorganic & medicinal chemistry letters. 2012;22:1082–5. doi: 10.1016/j.bmcl.2011.11.104. [DOI] [PubMed] [Google Scholar]
- 22.Ito N, Tsuda H, Tatematsu M. et al. Enhancing effect of various hepatocarcinogens on induction of preneoplastic glutathione S-transferase placental form positive foci in rats--an approach for a new medium-term bioassay system. Carcinogenesis. 1988;9:387–94. doi: 10.1093/carcin/9.3.387. [DOI] [PubMed] [Google Scholar]
- 23.Lee HJ, Lee SA, Choi H. Dietary administration of inositol and/or inositol-6-phosphate prevents chemically-induced rat hepatocarcinogenesis. Asian Pacific journal of cancer prevention: APJCP. 2005;6:41–7. [PubMed] [Google Scholar]
- 24.Sato K. Glutathione S-transferases and hepatocarcinogenesis. Japanese journal of cancer research: Gann. 1988;79:556–72. doi: 10.1111/j.1349-7006.1988.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods in enzymology. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 26.Lowry OH, Rosebrough NJ, Farr AL. et al. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951;193:265–75. [PubMed] [Google Scholar]
- 27.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods in enzymology. 1985;113:548–55. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 28.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. The Journal of biological chemistry. 1974;249:7130–9. [PubMed] [Google Scholar]
- 29.Tappel AL. Glutathione peroxidase and hydroperoxides. Methods in enzymology. 1978;52:506–13. doi: 10.1016/s0076-6879(78)52055-7. [DOI] [PubMed] [Google Scholar]
- 30.Carlberg I, Mannervik B. Glutathione reductase. Methods in enzymology. 1985;113:484–90. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 31.Cho Y, Kim H, Turner ND. et al. A chemoprotective fish oil- and pectin-containing diet temporally alters gene expression profiles in exfoliated rat colonocytes throughout oncogenesis. The Journal of nutrition. 2011;141:1029–35. doi: 10.3945/jn.110.134973. doi:10.3945/jn.110.134973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yun TK, Choi SY. Non-organ specific cancer prevention of ginseng: a prospective study in Korea. International journal of epidemiology. 1998;27:359–64. doi: 10.1093/ije/27.3.359. [DOI] [PubMed] [Google Scholar]
- 33.Lee H-J, Hong M-K, Kim H, Chemopreventive effect of Korean red ginseng extract in rat hepatocarcinogenesis. AACR Meeting Abstracts. 2005. p928. [DOI] [PMC free article] [PubMed]
- 34.Kim H, Lee H-J, Kim DJ. et al. Panax ginseng exerts antiproliferative effects on rat hepatocarcinogenesis. Nutrition Research. 2013;33:753–60. doi: 10.1016/j.nutres.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Soldati F, Sticher O. HPLC separation and quantitative determination of ginsenosides from Panax ginseng, Panax quinquefolium and from ginseng drug preparations. Planta medica. 1980;39:348–57. doi: 10.1055/s-2008-1074929. [DOI] [PubMed] [Google Scholar]
- 36.Conney AH. Enzyme induction and dietary chemicals as approaches to cancer chemoprevention: the Seventh DeWitt S. Goodman Lecture. Cancer research. 2003;63:7005–31. [PubMed] [Google Scholar]
- 37.Tanaka T, Kojima T, Kawamori T. et al. Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogenesis. 1993;14:1321–5. doi: 10.1093/carcin/14.7.1321. [DOI] [PubMed] [Google Scholar]
- 38.Hodek P, Křížková J, Burdová K. et al. Chemopreventive compounds—view from the other side. Chemico-biological interactions. 2009;180:1–9. doi: 10.1016/j.cbi.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Martin KR, Appel CL. Polyphenols as dietary supplements: a double-edged sword. Nutrition and Dietary Supplements. 2010;2:1–12. [Google Scholar]
- 40.da Silva TC, Paula da Silva A, Akisue G. et al. Inhibitory effects of Pfaffia paniculata (Brazilian ginseng) on preneoplastic and neoplastic lesions in a mouse hepatocarcinogenesis model. Cancer letters. 2005;226:107–13. doi: 10.1016/j.canlet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Xiaoguang C, Hongyan L, Xiaohong L. et al. Cancer chemopreventive and therapeutic activities of red ginseng. Journal of ethnopharmacology. 1998;60:71–8. doi: 10.1016/s0378-8741(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 42.Popov IM, Goldwag WJ. A review of the properties and clinical effects of ginseng. Am J Chin Med (Gard City N Y) 1973;1:263–70. doi: 10.1142/s0192415x73000280. [DOI] [PubMed] [Google Scholar]
- 43.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2008;22:659–61. doi: 10.1096/fj.07-9574LSF. doi:10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY, Park JY, Kang HJ. et al. Beneficial effects of Korean red ginseng on lymphocyte DNA damage, antioxidant enzyme activity, and LDL oxidation in healthy participants: a randomized, double-blind, placebo-controlled trial. Nutrition journal. 2012;11:47.. doi: 10.1186/1475-2891-11-47. doi:10.1186/1475-2891-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SJ, Lim KH, Noh JH. et al. Subacute oral toxicity study of korean red ginseng extract in sprague-dawley rats. Toxicol Res. 2013;29:285–92. doi: 10.5487/TR.2013.29.4.285. doi:10.5487/TR.2013.29.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valko M, Rhodes CJ, Moncol J. et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. doi:10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbeck's archives of surgery / Deutsche Gesellschaft fur Chirurgie. 2006;391:499–510. doi: 10.1007/s00423-006-0073-1. doi:10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 48.Samoylenko A, Hossain JA, Mennerich D. et al. Nutritional countermeasures targeting reactive oxygen species in cancer: From mechanisms to biomarkers and clinical evidence. Antioxidants & redox signaling. 2013;19:2157–96. doi: 10.1089/ars.2012.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huu Tung N, Uto T, Morinaga O. et al. Pharmacological effects of ginseng on liver functions and diseases: a minireview. Evidence-based complementary and alternative medicine: eCAM. 2012;2012:173297.. doi: 10.1155/2012/173297. doi:10.1155/2012/173297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bak MJ, Jun M, Jeong WS. Antioxidant and Hepatoprotective Effects of the Red Ginseng Essential Oil in H(2)O(2)-Treated HepG2 Cells and CCl(4)-Treated Mice. International journal of molecular sciences. 2012;13:2314–30. doi: 10.3390/ijms13022314. doi:10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abdel-Wahhab MA, Hassan NS, El-Kady AA. et al. Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2010;48:733–42. doi: 10.1016/j.fct.2009.12.006. doi:10.1016/j.fct.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Manson MM, Ball H, Barrett MC. et al. Mechanism of action of dietary chemoprotective agents in rat liver: induction of phase I and II drug metabolizing enzymes and aflatoxin B1 metabolism. Carcinogenesis. 1997;18:1729–38. doi: 10.1093/carcin/18.9.1729. [DOI] [PubMed] [Google Scholar]
- 53.Vásquez-Garzón VR, Arellanes-Robledo J, Garcia-Roman R. et al. Inhibition of reactive oxygen species and pre-neoplastic lesions by quercetin through an antioxidant defense mechanism. Free radical research. 2008;43:128–37. doi: 10.1080/10715760802626535. [DOI] [PubMed] [Google Scholar]
- 54.Czeczot H, Scibior D, Skrzycki M. et al. Glutathione and GSH-dependent enzymes in patients with liver cirrhosis and hepatocellular carcinoma. Acta biochimica Polonica. 2006;53:237–42. [PubMed] [Google Scholar]
- 55.Sanchez-Perez Y, Carrasco-Legleu C, Garcia-Cuellar C. et al. Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer letters. 2005;217:25–32. doi: 10.1016/j.canlet.2004.07.019. doi:10.1016/j.canlet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Jeong TC, Kim HJ, Park JI. et al. Protective effects of red ginseng saponins against carbon tetrachloride-induced hepatotoxicity in Sprague Dawley rats. Planta medica. 1997;63:136–40. doi: 10.1055/s-2006-957630. doi:10.1055/s-2006-957630. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Zhang JW, Li W. et al. Ginsenoside metabolites, rather than naturally occurring ginsenosides, lead to inhibition of human cytochrome P450 enzymes. Toxicol Sci. 2006;91:356–64. doi: 10.1093/toxsci/kfj164. doi:10.1093/toxsci/kfj164. [DOI] [PubMed] [Google Scholar]
- 58.Park KA, Kweon S, Choi H. Anticarcinogenic effect and modification of cytochrome P450 2E1 by dietary garlic powder in diethylnitrosamine-initiated rat hepatocarcinogenesis. Journal of biochemistry and molecular biology. 2002;35:615–22. doi: 10.5483/bmbrep.2002.35.6.615. [DOI] [PubMed] [Google Scholar]