Abstract
Studies on candidate pandemic vaccines against avian influenza viruses have focused on H5N1, but viruses of other subtypes, such as A/H9N2, are also considered to have pandemic potential. We investigated the safety and immunogenicity of two immunizations with one of five different antigen doses (ranging from 3.75 to 45 μg of hemagglutinin antigen) of a nonadjuvanted whole-virus G9 lineage H9N2 influenza virus vaccine in healthy adults aged 18 to 49 years. The antibody responses were measured by hemagglutination inhibition (HI), microneutralization (MN), and single radial hemolysis (SRH) assays. To investigate a hypothesis that previous exposure to H2N2 viruses in subjects born in or before 1968 might prime for more robust antibody responses to H9N2 vaccination than that in subjects born after 1968, a post hoc age-stratified analysis of antibody responses was done. Both vaccinations in all dose groups were safe and well tolerated. No vaccine-related serious adverse events were reported, and the majority of the adverse reactions were rated as mild. The rates of injection site reactions were lower in the 3.75-μg- and 7.5-μg-dose groups than those in the higher-dose groups; the rates of systemic reactions were similar across all dose groups. The seroprotection rates among the different dose groups 21 days after the second immunization ranged from 52.8% to 88.9% as measured by HI assay, from 88.7% to 98.1% or 82.7% to 96.2% as measured by MN assay (MN titer cutoffs, 1:40 and 1:80, respectively), and from 94.2% to 100% as measured by SRH assay. Higher antibody responses were not induced in subjects born in or before 1968. These data indicate that a nonadjuvanted whole-virus H9N2 vaccine is well tolerated and immunogenic in healthy adults. (This study has been registered at ClinicalTrials.gov under registration no. NCT01320696.)
INTRODUCTION
A number of avian influenza virus subtypes have caused zoonotic infections in humans, including those of subtypes H5N1 (1), H9N2 (2), and, most recently, H7N9 (3) and H10N8 (4). Because the human population is largely immunologically naive to such viruses, there are concerns that a pandemic situation might occur if any of these viruses gains the capacity for efficient human-to-human transmission. The development of candidate pandemic vaccines to counter the threat of a pandemic resulting from avian influenza viruses is thus an important part of global pandemic preparedness programs (5, 6). To date, this effort has been concentrated largely on the development of candidate pandemic vaccines based on influenza viruses of the H5N1 subtype. A number of clinical studies have demonstrated whole-virus inactivated H5N1 vaccines to be immunogenic in adult, elderly, and pediatric populations without a requirement for adjuvantation (7–11). In the present study, we extend the clinical investigation of nonadjuvanted whole-virus avian influenza vaccines to include a vaccine against the H9N2 influenza virus subtype, which is enzootic in poultry across the Middle East and Asia and is considered to have pandemic potential (12, 13).
Three genetically and antigenically distinct H9N2 lineages, G1, G9, and Korean, have been reported (2), two of which (G1 and G9) have caused sporadic human infections. To date, human H9N2 infections have been associated with only mild disease (2, 14, 15), and there has been no documented transmission between humans. However, some seroepidemiological data suggest that there may be considerable underreporting of human H9N2 infections (16). Moreover, a substantial proportion of H9N2 viruses isolated in the Middle East and Asia contain an amino residue in the hemagglutinin (HA) receptor binding site (RBS) (13) that facilitates binding to α2,6-linked sialic acid receptors, such that the virus can replicate in human airway epithelial cells (17, 18). Several H9N2 isolates also contain amino residues in the HA RBS that facilitate transmission between mammals (13, 19). In addition, the cocirculation of H9N2 with other avian influenza viruses might result in the emergence of new virus strains with increased pathogenicities. Reassortment between H9N2, H5N1, and H7N3 viruses has been reported in the field (20–22), and increased pathogenicity and host range have been demonstrated experimentally for reassortants between H9N2 and H3N2 viruses (19, 23) and between H9N2 and the 2009 pandemic H1N1 virus (16, 24). Additionally, H9N2 viruses are reported to have contributed internal genes to H5N1, H7N9, and H10N8 viruses that have infected and killed humans (25, 26). The development of effective vaccines against H9N2 viruses, in addition to other avian influenza viruses with pandemic potential, might thus be an important aspect of pandemic preparedness.
We recently reported the nonclinical development of a Vero-derived whole-virus vaccine based on an H9N2 virus of the G9 lineage (27). In the present study (registered at ClinicalTrials.gov under registration no. NCT01320696), we undertook a double-blind randomized dose-finding phase I/II clinical trial to evaluate the safety and immunogenicity of this whole-virus H9N2 vaccine in healthy adults. To investigate a hypothesis that previous exposure to H2N2 viruses in subjects born in or before 1968 might prime for more robust antibody responses to H9N2 vaccination than that in subjects born after 1968 (28, 29), a post hoc age-stratified analysis of antibody responses was also done.
MATERIALS AND METHODS
Vaccine.
The nonadjuvanted inactivated whole-virus Vero cell culture-derived H9N2 vaccine was manufactured using a reverse genetics (RG)-modified strain A/chicken/Hong Kong/G9/97 (H9N2) virus (NIBRG-91) obtained from the National Institute for Biological Standards and Control (NIBSC) (United Kingdom). This vaccine was produced using a procedure identical to that used for the manufacture of licensed H5N1 and 2009 pandemic H1N1 (H1N1pdm09) whole-virus vaccines (30, 31). Briefly, the vaccine virus strain was grown in Vero cell culture and, after harvest, was double inactivated with formalin and UV irradiation and purified by continuous sucrose gradient centrifugation and ultra-/diafiltration steps prior to formulation. The HA content was determined by a single radial immunodiffusion assay.
Study design and procedures.
A phase I/II randomized double-blind study to assess the safety and immunogenicity of two immunizations with a whole-virus H9N2 influenza virus vaccine was undertaken in healthy adults aged 18 to 49 years at five study centers in the United States between 16 March and 20 October 2011, in accordance with the principles set forth in Title 21 of the U.S. Code of Federal Regulations, the International Committee on Harmonisation Guidelines for Good Clinical Practice, the Declaration of Helsinki, and local and national regulatory requirements. The study protocol was approved by the relevant ethics committees, prior institutional review board (IRB) approval was obtained from each institution that participated in the study, and written informed consent was provided by each study participant.
Approximately 275 subjects were randomized 1:1:1:1:1 (55 per arm) to five dose groups to receive two immunizations, 21 days apart, with a vaccine formulated to contain 3.75, 7.5, 15, 30, or 45 μg of HA antigen in a volume of 0.5 ml. Randomization was done centrally using an interactive voice response system, carried out in blocks using the random number generator algorithm of Wichmann and Hill (32), as modified by McLeod (33). The site staff and subjects remained blinded until the end of the study. Vaccination was administered by intramuscular injection into the deltoid muscle of the upper arm. Blood samples for the assessment of immune responses were drawn immediately before and 21 ± 2 days after each vaccination, as well as 180 ± 2 days after the first immunization.
The primary immunogenicity endpoints were the numbers of subjects with a hemagglutination inhibition (HI) antibody response to the vaccine strain associated with seroprotection and seroconversion 3 weeks after the second vaccination. Secondary immunogenicity endpoints included HI, microneutralization (MN), and single radial hemolysis (SRH) responses 3 weeks after each immunization and 6 months after the first immunization.
The subjects were provided with a subject diary to collect data on oral body temperature, solicited systemic and injection site reactions, and any other adverse events (AEs). The diary entries were evaluated and the data graded for severity and relatedness by the investigator. The fever and injection site reaction severity ratings were adapted from the FDA Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials (34). The primary safety endpoints were the frequency and severity of injection site and systemic reactions within 7 days after the first and second vaccinations. The secondary safety endpoints included the frequency and severity of AEs observed during the entire study period.
Laboratory methods.
The HI and MN assays were conducted by Focus Diagnostics, Inc. (Cypress, CA, USA), and the SRH assays were conducted by VisMederi srl (Siena, Italy). All assays were validated according to international standards (35, 36) to ensure that the performance characteristics of the methods met the requirements for use with the A/chicken/Hong Kong/G9/97 (H9N2) virus.
The HI assays were done according to standard methods using turkey erythrocytes. Briefly, serum samples were treated with a receptor-destroying enzyme, inactivated at 56°C, and serially diluted in 2-fold steps, starting at 1:10. Prior to the addition of the erythrocytes, the serum samples were preincubated at a ratio of 1:1 with H9N2 antigen (adjusted to an HA titer of about 3). Following the addition of the erythrocytes, the suspensions were carefully mixed and incubated at room temperature (RT) before determination of the HI titer. Serum samples with a negative signal in the first dilution were assigned a nominal HI titer of 1:5.
The enzyme-linked immunosorbent assay (ELISA)-based MN assay is based on the assay originally developed for serological evaluation of H5N1 vaccines (37) and subsequently widely used for the evaluation of pandemic H1N1 vaccines (38–41). In brief, duplicates of heat-inactivated serum samples were serially diluted with phosphate-buffered saline (PBS) in 2-fold steps, starting at a 1:5 dilution. Fifty microliters of each dilution was transferred to a microtiter plate, mixed 1:1 with H9N2 virus (adjusted to 100× the 50% tissue culture infective dose [TCID50]/50 μl), and incubated for 1 h at RT. After the addition of 100 μl/well MDCK cell suspension (5 × 105/ml), the plates were incubated for 18 to 22 h at 37°C, fixed with a methanol-acetone mixture, and incubated with a primary antibody (anti-influenza A nucleoprotein mouse monoclonal antibody) and then with a horseradish peroxidase-conjugated rabbit anti-mouse IgG secondary antibody. Following substrate (o-phenylenediamine [OPD]) addition and incubation, stop solution was added, and the absorbance (optical density [OD]) of the wells at 450/620 nm was read. The neutralization titer of a sample was determined as the highest serum dilution with an OD lower than or equal to the 50% neutralization point of the plate, calculated from the OD values of the virus control wells (medium plus test dilution of virus plus MDCK cells) and of the negative cell control wells (medium plus MDCK cells). The titer of each sample was defined as the geometric mean of the titers from the two individual determinations. Serum samples with a negative signal in the first dilution were assigned a nominal MN titer of 1:5. The SRH assay was done essentially as previously described (42). SRH areas of ≤4 mm2 were considered negative.
Statistical analyses.
Assuming a dropout rate of around 9%, 50 subjects per dose group would be evaluable for immunogenicity. The 50 evaluable subjects ensured a width of the exact 95% confidence interval (CI) for the percentage of subjects who achieved seroprotection and seroconversion based on the Clopper-Pearson method of <29% in each dose group. With 275 vaccinated subjects, there would be a 93% chance to observe an AE with an underlying incidence rate of 1:100.
Point estimates of the response rates and exact 95% CIs were computed for the primary immunogenicity endpoints. In addition, a logistic regression model that included dose levels, baseline antibody titers, age, and gender as independent factors was fitted. Point estimates of the geometric means and two-sided 95% CIs were calculated for all continuous secondary immunogenicity endpoints for each vaccine dose within an analysis of covariance (ANCOVA) framework. The model includes dose, age, and gender as fixed effects and baseline values as covariates. The antibody titers were log-transformed prior to the analysis. The least-squares means and two-sided 95% CIs were computed for each dose group, and the differences between the means were estimated and back transformed by exponentiation. This analysis was carried out separately for antibody responses measured by HI, MN, and SRH 3 weeks after the first and second vaccination and 6 months after the first vaccination. The correlation between the seroprotective HI titer and SRH area cutoffs (1:40 and 25 mm2, respectively) and different MN titer cutoff values was analyzed according to Cohen's kappa coefficient (43). Point estimates and exact two-sided 95% CIs were calculated for all safety endpoints. All analyses were carried out utilizing the statistical software package SAS version 9.1.3 (SAS Institute, Inc., Cary, NC, USA). A post hoc analysis of antibody responses, comprising descriptive statistics of the response rates and continuous immunogenicity endpoints in subjects born either in or before 1968 or after 1968, was carried out.
RESULTS
Study population.
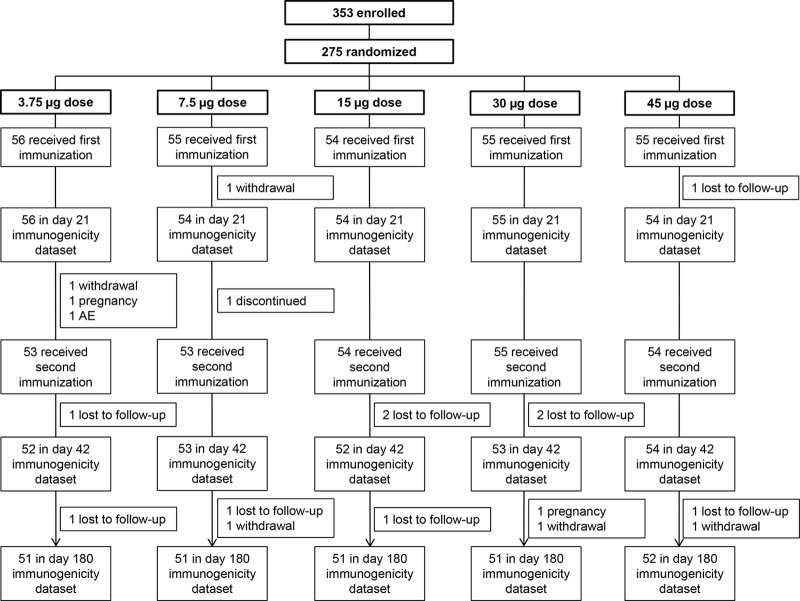
The trial profile is shown in Fig. 1. A total of 353 subjects were enrolled in the study and screened, of whom 275 were eligible, randomized to receive the first immunization, and included in the safety data set. Two hundred seventy-three subjects were included in the immunogenicity analyses 3 weeks after the first immunization. A total of 269 subjects received the second vaccination, and 264 and 256 subjects were included in the immunogenicity analyses 3 weeks after the second immunization and 6 months after the first immunization, respectively. The dose groups were balanced with respect to all baseline demographic characteristics (Table 1).
FIG 1.
Flow chart of the study participants through the trial.
TABLE 1.
Demographic and clinical characteristics of the participants at baseline
| Patient characteristic | Data by dose groupa |
||||
|---|---|---|---|---|---|
| 3.75 μg (n = 56) | 7.5 μg (n = 55) | 15 μg (n = 54) | 30 μg (n = 55) | 45 μg (n = 55) | |
| Male sex (no. [%]) | 23 (41.1) | 28 (50.9) | 23 (42.6) | 24 (43.6) | 26 (47.3) |
| Age (mean ± SD) (yr) | 32.5 ± 8.6 | 32.6 ± 8.7 | 33.8 ± 8.1 | 35.8 ± 9.3 | 34.1 ± 9.0 |
| Born (no. [%]): | |||||
| In or before 1968 | 12 (21.4) | 9 (16.4) | 10 (18.5) | 17 (30.9) | 15 (27.3) |
| After 1968 | 44 (78.6) | 46 (83.6) | 44 (81.5) | 38 (69.1) | 40 (72.7) |
| Wt (mean ± SD) (kg) | 78.6 ± 15.4 | 79.5 ± 16.9 | 80.7 ± 15.4 | 80.3 ± 18.6 | 83.3 ± 17.1 |
| Ht (mean ± SD) (cm) | 171.2 ± 7.4 | 172.2 ± 10.1 | 171.5 ± 10.2 | 170.9 ± 9.6 | 172.3 ± 8.9 |
| Race (no. [%]) | |||||
| White | 45 (80.4) | 45 (81.8) | 41 (75.9) | 44 (80.0) | 42 (76.4) |
| Black or African-American | 9 (16.1) | 7 (12.7) | 11 (20.4) | 9 (16.4) | 11 (20.0) |
| Asian | 1 (1.8) | 2 (3.6) | 2 (3.7) | 2 (3.6) | 1 (1.8) |
| Other | 1 (1.8) | 1 (1.8) | 0 (0.0) | 0 (0.0) | 1 (1.8) |
| Ethnicity (no. [%]) | |||||
| Hispanic or Latino | 4 (7.1) | 5 (9.1) | 2 (3.7) | 4 (7.3) | 4 (7.3) |
| Not Hispanic or Latino | 52 (92.9) | 50 (90.9) | 52 (96.3) | 51 (92.7) | 51 (92.7) |
n, total number of participants per group.
Safety.
Both vaccinations were safe and well tolerated in all dose groups. The reactions were transient and predominantly mild. No vaccine-related serious AEs were reported. Injection site and systemic reactions were reported less frequently after the second immunization than after the first. The injection site and systemic reactions after the first vaccination are shown in Table 2. The rates of injection site reactions within 7 days of the first and second vaccinations ranged from 16.1% to 41.8% and from 13.2% to 37.0%, respectively, among the different dose groups. The majority of the reported injection site reactions were injection site pain and tenderness, with very few reported cases of other injection site reactions. The rates of injection site reactions were lower at the lower dose levels of 3.75 and 7.5 μg (ranges, 16.1% to 18.2% and 13.2% to 15.1% after the first and second immunizations, respectively) than those at the 15-, 30-, and 45-μg dose levels (ranges, 32.7% to 41.8% and 21.8% to 37.0% after the first and second immunizations, respectively).
TABLE 2.
Injection site and systemic reactions after first immunization
| Reaction type and severity | % (95% CI) with reaction by dose groupa |
||||
|---|---|---|---|---|---|
| 3.75 μg (n = 56) | 7.5 μg (n = 55) | 15 μg (n = 54) | 30 μg (n = 55) | 45 μg (n = 55) | |
| Injection site | 16.1 (7.6–28.3) | 18.2 (9.1–30.9) | 35.2 (22.7–49.4) | 32.7 (20.7–46.7) | 41.8 (28.7–55.9) |
| Severity | |||||
| Mild | 16.1 | 18.2 | 29.6 | 30.9 | 36.4 |
| Moderate | 0.0 | 0.0 | 5.6 | 0.0 | 5.5 |
| Severe | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Unknown | 0.0 | 0.0 | 0.0 | 1.8 | 0.0 |
| Type | |||||
| Pain | 5.4 (1.1–14.9) | 14.5 (6.5–26.7) | 13.0 (5.4–24.9) | 18.2 (9.1–30.9) | 27.3 (16.1–41.0) |
| Tenderness | 16.1 (7.6–28.3) | 14.5 (6.5–26.7) | 25.9 (15.0–39.7) | 30.9 (19.1–44.8) | 34.5 (22.2–48.6) |
| Redness | 0.0 (0.0–6.4) | 0.0 (0.0–6.5) | 0.0 (0.0–6.6) | 0.0 (0.0–6.5) | 1.8 (0.0–9.7) |
| Swelling | 0.0 (0.0–6.4) | 0.0 (0.0–6.5) | 0.0 (0.0–6.6) | 0.0 (0.0–6.5) | 0.0 (0.0–6.5) |
| Induration | 0.0 (0.0–6.4) | 0.0 (0.0–6.5) | 0.0 (0.0–6.6) | 0.0 (0.0–6.5) | 3.6 (0.4–12.5) |
| Bruising | 0.0 (0.0–6.4) | 1.8 (0.0–9.7) | 1.9 (0.0–9.9) | 0.0 (0.0–6.5) | 1.8 (0.0–9.7) |
| Systemic | 12.5 (5.2–24.1) | 25.5 (14.7–39.0) | 25.9 (15.0–39.7) | 20.0 (10.4–33.0) | 23.6 (13.2–37.0) |
| Severity | |||||
| Mild | 10.7 | 21.8 | 22.2 | 18.2 | 20.0 |
| Moderate | 0.0 | 1.8 | 3.7 | 1.8 | 3.6 |
| Severe | 1.8 | 0.0 | 0.0 | 0.0 | 0.0 |
| Unknown | 0.0 | 1.8 | 0.0 | 0.0 | 0.0 |
| Type | |||||
| Feverb | 0.0 (0.0–6.4) | 0.0 (0.0–6.5) | 0.0 (0.0–6.6) | 0.0 (0.0–6.5) | 0.0 (0.0–6.5) |
| Malaise | 1.8 (0.0–9.6) | 3.6 (0.4–12.5) | 3.7 (0.5–12.7) | 1.8 (0.0–9.7) | 3.6 (0.4–12.5) |
| Shivering | 0.0 (0.0–6.4) | 3.6 (0.4–12.5) | 0.0 (0.0–6.6) | 0.0 (0.0–6.5) | 3.6 (0.4–12.5) |
| Fatigue | 5.4 (1.1–14.9) | 16.4 (7.8–28.8) | 7.4 (2.1–17.9) | 3.6 (0.4–12.5) | 3.6 (0.4–12.5) |
| Headache | 3.6 (0.4–12.3) | 16.4 (7.8–28.8) | 18.5 (9.3–31.4) | 7.3 (2.0–17.6) | 10.9 (4.1–22.2) |
| Sweating | 0.0 (0.0–6.4) | 1.8 (0.0–9.7) | 0.0 (0.0–6.6) | 0.0 (0.0–6.5) | 1.8 (0.0–9.7) |
| Muscle pain | 1.8 (0.0–9.6) | 9.1 (3.0–20.0) | 1.9 (0.0–9.9) | 5.5 (1.1–15.1) | 9.1 (3.0–20.0) |
| Joint pain | 0.0 (0.0–6.4) | 0.0 (0.0–6.5) | 0.0 (0.0–6.6) | 3.6 (0.4–12.5) | 1.8 (0.0–9.7) |
Data are for reactions occurring within 21 days of vaccination, unless otherwise indicated. n, total number of participants per group.
Reaction occurred within 7 days.
The rates of systemic reactions were comparable across the different dose groups, ranging from 12.5% to 25.9% after the first vaccination and from 5.6% to 13.0% after the second vaccination. Headache and fatigue were the most commonly reported systemic reactions, with rates of ≤18.5% and ≤7.3% after the first and second vaccination, respectively, for headache, and rates of ≤16.4% and ≤5.7%, respectively, for fatigue. No subjects developed fever after the first vaccination; one subject in the 45-μg-dose group reported moderate fever (38.4°C) after the second vaccination.
Hemagglutination inhibition, virus-neutralizing, and single radial hemolysis antibody responses.
The antibody responses induced by the whole-virus H9N2 vaccine, as assessed by HI, MN, and SRH assays, are shown in Tables 3, 4, and 5, respectively. Two immunizations with the whole-virus H9N2 vaccine induced substantial serum antibody responses in all dose groups. The seroprotection rates determined by the HI assay 3 weeks after the second vaccination ranged from 52.8% in the 7.5-μg-dose group to 88.9% in the 45-μg-dose group (Table 3). The HI seroconversion rates after the second immunization ranged from 50.9% in the 7.5-μg-dose group to 88.9% in the 45-μg-dose group. The HI geometric mean titers (GMTs) ranged from 5.3 to 6.1 at baseline and from 32.5 to 104.3 after the second immunization. The geometric mean fold rise (GMFR) of the HI antibody responses compared to baseline after the second vaccination ranged from 5.7 in the 3.75-μg-dose group to 19.8 in the 45-μg-dose group. The ANCOVA of the HI antibody GMTs induced after the second immunization showed a statistically significant effect of vaccine dose and baseline antibody titer (P < 0.001) on the HI antibody responses, as well as age (P = 0.014) and gender (P = 0.012). A pairwise comparison of the antibody GMTs across the dose groups indicated differences in the vaccine-induced HI antibody titers after the second immunization between all doses except between the 3.75- and 7.5-μg-dose groups and the 15- and 30-μg-dose groups. The HI antibody levels determined 6 months after the first immunization were lower than those at 3 weeks after the second immunization; however, they were substantially higher than those at baseline, with seroprotection rates ranging from 19.6% in the 3.75-μg-dose group to 59.6% in the 45-μg-dose group.
TABLE 3.
Hemagglutinin inhibition antibody responses to H9N2 viruses 3 weeks after each immunization and 6 months after first immunization
| Antibody response | Data by dose group |
||||
|---|---|---|---|---|---|
| 3.75 μg | 7.5 μg | 15 μg | 30 μg | 45 μg | |
| Geometric mean titer (mean [95% CI]) | |||||
| Baseline | 5.6 (4.9–6.5) | 6.1 (5.4–6.8) | 5.8 (5.2–6.5) | 5.7 (5.0–6.4) | 5.3 (5.0–5.5) |
| Day 21 | 18.2 (13.5–24.6) | 27.5 (18.3–41.1) | 45.6 (31.6–65.9) | 59.1 (39.4–88.7) | 81.7 (58.3–114.5) |
| Day 42 | 32.5 (24.6–42.9) | 40.2 (27.8–57.9) | 62.7 (44.4–88.5) | 71.7 (51.0–100.9) | 104.3 (77.5–140.3) |
| Day 180 | 14.6 (11.7–18.2) | 22.3 (15.3–32.4) | 24.2 (17.9–32.7) | 33.1 (25.3–43.2) | 41.5 (32.0–53.7) |
| Seroprotection (no./total no. [%; 95% CI])a | |||||
| Baseline | 1/56 (1.8; 0.0–9.6) | 1/54 (1.9; 0.0–9.9) | 1/54 (1.9; 0.0–9.9) | 1/55 (1.8; 0.0–9.7) | 0/54 (0.0; 0.0–6.6) |
| Day 21 | 17/56 (30.4; 18.8–44.1) | 22/54 (40.7; 27.6–55.0) | 33/54 (61.1; 46.9–74.1) | 35/55 (63.6; 49.6–76.2) | 40/54 (74.1; 60.3–85.0) |
| Day 42 | 28/52 (53.8; 39.5–67.8) | 28/53 (52.8; 38.6–66.7) | 39/52 (75.0; 61.1–86.0) | 42/53 (79.2; 65.9–89.2) | 48/54 (88.9; 77.4–95.8) |
| Day 180 | 10/51 (19.6; 9.8–33.1) | 17/51 (33.3; 20.8–47.9) | 22/51 (43.1; 29.3–57.8) | 28/51 (54.9; 40.3–68.9) | 31/52 (59.6; 45.1–73.0) |
| Seroconversion (no./total no. [%; 95% CI])b | |||||
| Day 21 | 16/56 (28.6; 17.3–42.2) | 21/54 (38.9; 25.9–53.1) | 32/54 (59.3; 45.0–72.4) | 35/55 (63.6; 49.6–76.2) | 40/54 (74.1; 60.3–85.0) |
| Day 42 | 27/52 (51.9; 37.6–66.0) | 27/53 (50.9; 36.8–64.9) | 38/52 (73.1; 59.0–84.4) | 41/53 (77.4; 63.8–87.7) | 48/54 (88.9; 77.4–95.8) |
| Day 180 | 9/51 (17.6; 8.4–30.9) | 17/51 (33.3; 20.8–47.9) | 21/51 (41.2; 27.6–55.8) | 28/51 (54.9; 40.3–68.9) | 31/52 (59.6; 45.1–73.0) |
| GMFR (mean [95% CI])c | |||||
| Day 21 | 3.2 (2.4–4.3) | 4.5 (3.1–6.6) | 7.8 (5.5–11.2) | 10.4 (7.0–15.6) | 15.5 (11.1–21.7) |
| Day 42 | 5.7 (4.3–7.5) | 6.6 (4.8–9.1) | 10.7 (7.6–15.0) | 12.7 (9.1–17.8) | 19.8 (14.8–26.6) |
| Day 180 | 2.7 (2.2–3.3) | 3.9 (2.8–5.4) | 4.3 (3.2–5.9) | 6.1 (4.7–7.9) | 8.1 (6.3–10.3) |
Hemagglutinin inhibition (HI) titer, ≥1:40.
≥4-fold titer increase compared to baseline.
Geometric mean fold rise (GMFR) compared to baseline.
TABLE 4.
Neutralizing antibody responses to H9N2 viruses 3 weeks after each immunization and 6 months after first immunization
| Antibody response | Data by dose group |
||||
|---|---|---|---|---|---|
| 3.75 μg | 7.5 μg | 15 μg | 30 μg | 45 μg | |
| Geometric mean titer (mean [95% CI]) | |||||
| Baseline | 7.2 (5.7–9.1) | 9.0 (6.9–11.7) | 7.5 (6.0–9.4) | 8.8 (6.3–12.1) | 6.2 (5.2–7.4) |
| Day 21 | 57.3 (37.5–87.5) | 129.5 (81.1–206.6) | 142.5 (91.3–222.5) | 233.5 (159.1–342.7) | 272.6 (185.0–401.6) |
| Day 42 | 164.3 (118.8–227.4) | 230.8 (151.3–351.9) | 255.1 (181.0–359.6) | 339.4 (247.7–465.1) | 416.3 (300.2–577.4) |
| Day 180 | 37.4 (25.9–53.9) | 67.0 (42.3–106.2) | 66.1 (46.8–93.5) | 101.5 (72.3–142.4) | 117.0 (82.5–165.8) |
| Seroprotection (MN titer ≥ 1:40) (no./total no. [%; 95% CI]) | |||||
| Baseline | 1/56 (1.8; 0.0–9.6) | 8/54 (14.8; 6.6–27.1) | 4/54 (7.4; 2.1–17.9) | 5/55 (9.1; 3.0–20.0) | 3/54 (5.6; 1.2–15.4) |
| Day 21 | 33/56 (58.9; 45.0–70.1) | 41/54 (75.9; 62.4–86.5) | 45/54 (83.3; 70.7–92.1) | 51/55 (92.7; 82.4–98.0) | 50/54 (92.6; 82.1–97.9) |
| Day 42 | 50/52 (96.2; 86.8–99.5) | 47/53 (88.7; 77.0–95.7) | 50/52 (96.2; 86.8–99.5) | 52/53 (98.1; 89.9–100.0) | 53/54 (98.1; 90.1–100.0) |
| Day 180 | 26/51 (51.0; 36.6–65.2) | 32/51 (62.7; 48.1–75.9) | 37/51 (72.5; 58.3–84.1) | 43/51 (84.3; 71.4–93.0) | 42/52 (80.8; 67.5–90.42) |
| Seroprotection (MN titer ≥ 1:80) (no./total no. [%; 95% CI]) | |||||
| Baseline | 1/56 (1.8; 0.0–9.6) | 4/54 (7.4; 2.1–17.9) | 2/54 (3.7; 0.5–12.7) | 5/55 (9.1; 3.0–20.0) | 1/54 (1.9; 0.0–9.9) |
| Day 21 | 24/56 (42.9; 29.7–56.8) | 34/54 (63.0; 48.7–75.7) | 39/54 (72.2; 58.4–83.5) | 43/55 (78.2; 65.0–88.2) | 48/54 (88.9; 77.4–95.8) |
| Day 42 | 43/52 (82.7; 69.7–91.8) | 44/53 (83.0; 70.2–91.9) | 45/52 (86.5; 74.2–94.4) | 51/53 (96.2; 87.0–99.5) | 51/54 (94.4; 84.6–98.8) |
| Day 180 | 13/51 (25.5; 14.3–39.6) | 24/51 (47.1; 32.9–61.5) | 24/51 (47.1; 32.9–61.5) | 33/51 (64.7; 50.1–77.6) | 34/52 (65.4; 50.9–78.0) |
| Seroconversion (no./total no. [%; 95% CI])a | |||||
| Day 21 | 40/56 (71.4; 57.8–82.7) | 44/54 (81.5; 68.6–90.7) | 47/54 (87.0; 75.1–94.6) | 52/55 (94.5; 84.9–98.9) | 53/54 (98.1; 90.1–100.0) |
| Day 42 | 49/52 (94.2; 84.1–98.8) | 49/53 (92.5; 81.8–97.9) | 49/52 (94.2; 84.1–98.8) | 51/53 (96.2; 87.0–99.5) | 53/54 (98.1; 90.1–100.0) |
| Day 180 | 34/51 (66.7; 52.1–79.2) | 41/51 (80.4; 66.9–90.2) | 44/51 (86.3; 73.7–94.3) | 47/51 (92.2; 81.1–97.8) | 52/52 (100.0; 93.2–100.0) |
| GMFR (mean [95% CI])b | |||||
| Day 21 | 8.0 (5.5–11.4) | 14.4 (9.6–21.8) | 18.9 (12.5–28.6) | 26.7 (18.1–39.2) | 44.1 (30.4–63.9) |
| Day 42 | 22.2 (16.3–30.1) | 25.5 (17.4–37.2) | 34.0 (23.7–48.7) | 38.9 (27.2–55.7) | 67.4 (48.4–93.8) |
| Day 180 | 5.4 (4.0–7.4) | 9.1 (6.1–13.5) | 10.3 (7.5–14.0) | 13.3 (9.8–18.0) | 19.9 (14.6–27.1) |
≥4-fold titer increase compared to baseline.
Geometric mean fold rise (GMFR) compared to baseline.
TABLE 5.
Single radial hemolysis antibody responses to H9N2 viruses 3 weeks after each immunization and 6 months after first immunization
| Antibody response | Data by dose group |
||||
|---|---|---|---|---|---|
| 3.75 μg | 7.5 μg | 15 μg | 30 μg | 45 μg | |
| Geometric mean titer (mean [95% CI]) | |||||
| Baseline | 13.7 (10.2–18.5) | 12.9 (9.6–17.4) | 16.5 (12.2–22.3) | 14.9 (11.2–19.8) | 12.8 (9.6–17.1) |
| Day 21 | 37.0 (29.2–46.9) | 50.0 (43.7–57.2) | 52.3 (45.4–60.3) | 60.1 (53.7–67.2) | 65.7 (59.7–72.4) |
| Day 42 | 51.5 (44.5–59.6) | 56.6 (51.7–61.9) | 60.7 (55.3–66.6) | 63.4 (58.4–68.8) | 70.9 (66.3–75.7) |
| Day 180 | 48.9 (41.7–57.3) | 56.6 (51.1–62.5) | 57.4 (52.7–62.6) | 62.7 (59.1–66.6) | 66.2 (61.5–71.2) |
| Seroprotection (no./total no. [%; 95% CI])a | |||||
| Baseline | 22/56 (39.3; 26.5–53.2) | 20/54 (37.0; 24.3–51.3) | 26/54 (48.1; 34.3–62.2) | 17/55 (30.9; 19.1–44.8) | 18/54 (33.3; 21.1–47.5) |
| Day 21 | 45/56 (80.4; 67.6–89.8) | 48/54 (88.9; 77.4–95.8) | 49/54 (90.7; 79.7–96.9) | 53/55 (96.4; 87.5–99.6) | 53/54 (98.1; 90.1–100.0) |
| Day 42 | 49/52 (94.2; 84.1–98.8) | 52/53 (98.1; 89.9–100.0) | 51/52 (98.1; 89.7–100.0) | 52/53 (98.1; 89.9–100.0) | 54/54 (100.0; 93.4–100.0) |
| Day 180 | 46/51 (90.2; 78.6–96.7) | 49/51 (96.1; 86.5–99.5) | 50/51 (98.0; 89.6–100.0) | 51/51 (100.0; 93.0–100.0) | 51/52 (98.1; 89.7–100.0) |
| Seroconversion (no./total no. [%; 95% CI])b | |||||
| Day 21 | 30/56 (53.6; 39.7–67.0) | 39/54 (72.2; 58.4–83.5) | 35/54 (64.8; 50.6–77.3) | 42/55 (76.4; 63.0–86.8) | 42/54 (77.8; 64.4–88.0) |
| Day 42 | 35/52 (67.3; 52.9–79.7) | 42/53 (79.2; 65.9–89.2) | 38/52 (73.1; 59.0–84.4) | 43/53 (81.1; 68.0–90.6) | 44/54 (81.5; 68.6–90.7) |
| Day 180 | 30/51 (58.8; 44.2–72.4) | 43/51 (84.3; 71.4–93.0) | 36/51 (70.6; 56.2–82.5) | 43/51 (84.3; 71.4–93.0) | 40/52 (76.9; 63.2–87.5) |
| GMFR (mean [95% CI])c | |||||
| Day 21 | 2.7 (2.1–3.5) | 3.9 (2.9–5.2) | 3.2 (2.4–4.1) | 4.0 (3.1–5.3) | 5.1 (3.8–7.0) |
| Day 42 | 3.7 (2.8–4.8) | 4.5 (3.3–6.1) | 3.7 (2.8–5.0) | 4.4 (3.4–5.8) | 5.5 (4.1–7.5) |
| Day 180 | 3.4 (2.6–4.5) | 4.5 (3.4–6.0) | 3.7 (2.8–4.9) | 4.5 (3.4–5.9) | 5.1 (3.8–7.0) |
Single radial hemolysis (SRH) area of ≥25 mm2.
SRH area of ≥25 mm2 after immunization in case of negative baseline sample (≤4 mm2) or ≥50% increase in SRH area if baseline sample is >4 mm2.
Geometric mean fold rise (GMFR) compared to baseline.
The antibody responses determined by the MN (Table 4) and SRH (Table 5) assays confirmed the immunogenicity of the whole-virus H9N2 vaccine. Both assays were more sensitive than the HI assay for detecting antibodies induced by the H9N2 vaccine, demonstrating higher rates of seroprotection and seroconversion after vaccination. As there is no standard neutralization assay for influenza viruses and, as such, no internationally accepted titer cutoff for seroprotection as for the HI assay, we calculated seroprotection based on two different cutoff titers, i.e., 1:40 and 1:80. After the second vaccination, the seroprotection rates determined by the MN assay ranged from 88.7% to 98.1% (MN titer cutoff, 1:40) and 82.7% to 96.2% (MN titer cutoff, 1:80), among the different dose groups (Table 4), with seroconversion rates ranging from 94.2% to 98.1%. The seroprotection rates determined by the SRH assay after the second immunization ranged from 94.2% to 100%, and the seroconversion rates ranged from 67.3% to 81.5% (Table 5).
The baseline GMTs determined by the MN assay (range, 6.2 to 9.0) were similar to those determined by the HI assay; the baseline SRH GMTs (range, 12.8 to 16.5) were higher than those determined by the HI and MN assays. The GMTs after the second immunization as determined by the MN and SRH assays ranged from 164.3 to 416.3 and from 51.5 to 70.9, respectively. The GMFRs after the second immunization determined by the MN and SRH assays were 22.2 to 67.4 and 3.7 to 5.5, respectively. The ANCOVA of the MN and SRH antibody GMTs induced after the second immunization showed a statistically significant effect of vaccine dose (P < 0.001) and baseline antibody titer (P < 0.001) but not of gender (P = 0.212). Age had a significant effect on MN titers (P = 0.004) but not on SRH titers (P = 0.201). Consistent with the higher sensitivities of the MN and SRH assays, the dose responses determined by these assays were less marked than that measured by the HI assay. A pairwise comparison of the MN and SRH antibody GMTs across the dose groups after the second immunization showed no statistically significant differences in the vaccine-induced MN antibody titers between the 3.75- and 7.5-μg, 7.5- and 15-μg, 15- and 30-μg, and 30- and 45-μg-dose groups, and there were no statistically significant differences in the vaccine-induced SRH antibody titers between the 3.75- and 7.5-μg, 7.5- and 15-μg, 7.5- and 30-μg, and 15- and 30-μg-dose groups. The ranges of the seroprotection rates persisting 6 months after the first immunization as determined by the MN assay were 51.0% to 84.3% using the MN titer cutoff of 1:40 and 25.5% to 65.4% using the MN titer cutoff of 1:80; the range as determined by the SRH assay was 90.2% to 100.0%.
An analysis of the MN and HI responses according to Cohen's kappa coefficient (43) demonstrated the best agreement between the HI titer cutoff of 1:40 and the MN 1:80 cutoff (overall kappa, 0.725; 95% CI, 0.688 to 0.762). The best kappa coefficient agreement between the SRH area seroprotection cutoff of 25 mm2 and an MN titer cutoff occurred at an MN titer of 1:20 (overall kappa, 0.539; 95% CI, 0.492 to 0.585).
Post hoc analyses of the effect of age and baseline antibody titers on hemagglutination inhibition, virus-neutralizing, and single radial hemolysis antibody responses.
Some previous studies of H9N2 vaccines based on a G1 lineage virus had reported that the HI and MN antibody responses induced in individuals born in or before 1968 were higher than those in individuals born after 1968, possibly as a result of immunological priming by previous exposure to H2N2 viruses, which circulated widely between 1957 and 1968 but not afterwards (28, 29). In contrast, a more recent study did not find similar age-related differences in the antibody responses to an H9N2 vaccine based on a G9 lineage virus (44), which is the lineage of the virus from which the vaccine in the present study was derived.
To investigate this phenomenon in the present study, a post hoc stratification of the participants was done according to their birth either in or before 1968 or after 1968. Descriptive statistics of the response rates and continuous immunogenicity endpoints were calculated. Due to the inclusion of multiple dose groups of a relatively small size in this study, the dose groups were pooled for the purpose of this post hoc analysis, as was also done for previous investigations into age group-specific antibody responses to H9N2 vaccines (29). Figure 2 shows these data for the HI, MN, and SRH antibody responses at baseline, 21 days after the first and second vaccinations, and 6 months after the first vaccination. For all three serological assays, the antibody responses at all time points are similar in the two age groups. There were no statistically significant differences in the antibody responses for any of the assays at any of the time points, except for a statistically significant difference in the SRH antibody response observed at baseline, for which higher titers were measured in the older age group.
FIG 2.
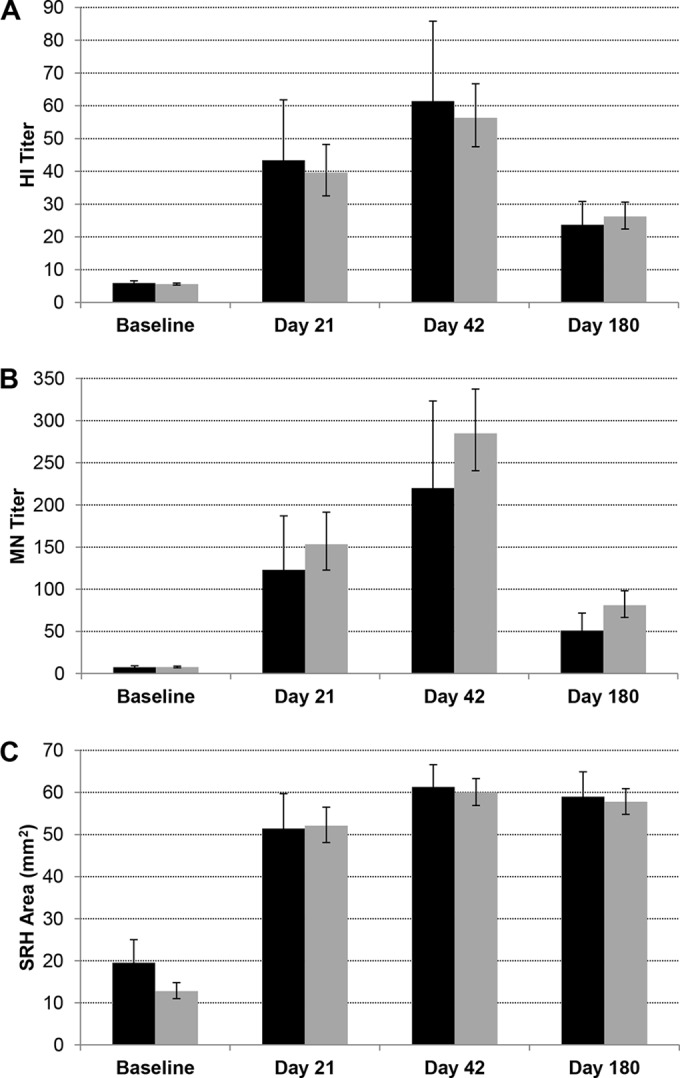
Effect of age group on antibody responses to H9N2 vaccine. Antibody responses at baseline, 21 days after the first and second vaccinations, and 180 days after the first vaccination, as determined by an HI assay (A), MN assay (B), and SRH assay (C) in participants born in or before 1968 (black bars) and after 1968 (gray bars). The data are the geometric mean titers (GMTs) or geometric mean SRH area, and 95% CIs, pooled for all dose groups.
DISCUSSION
A nonadjuvanted Vero cell culture-derived whole-virus H9N2 influenza vaccine is safe and well-tolerated in healthy adults aged 18 to 49 years. No vaccine-related serious AEs were reported, and the majority of the adverse reactions were rated as mild, consistent with the safety profile of the Vero-derived whole-virus H5N1 and H1N1 vaccines previously reported in clinical trials in healthy adults (7, 11, 38). The rates of injection site reactions were lower at the lower dose levels of 3.75 and 7.5 μg than those at the 15-, 30-, and 45-μg-dose levels. The rates of systemic reactions were comparable across all dose groups. Only one case of fever was reported, which was in a participant who received the highest vaccine dose. The rates of injection site and systemic reactions were similar to those reported for other nonadjuvanted H9N2 vaccines in this age group (28, 29, 44) but lower than those reported in a study of H9N2 vaccines containing a novel oil-in-water adjuvant (45).
With respect to immunogenicity, substantial HI, MN, and SRH antibody responses were induced by the nonadjuvanted whole-virus H9N2 vaccine. There was a statistically significant dose effect on the antibody responses measured by all assays, but this was less marked for the MN and SRH assays, which were more sensitive for detecting H9N2 antibodies than was the HI assay. The seroprotection rates among the different dose groups after the second immunization ranged from 52.8% to 88.9% as measured by the HI assay, from 88.7% to 98.1% or 82.7% to 96.2% as measured by the MN assay (for MN titer cutoffs of 1:40 and 1:80, respectively), and from 94.2% to 100% as measured by the SRH assay. The HI and MN data compare favorably with those reported for other nonadjuvanted whole-virus, virosomal, subunit, and alum-adjuvanted whole-virus H9N2 vaccines (28, 29, 44, 45), particularly with respect to the MN antibody GMTs, which were substantially higher for the Vero-derived whole-virus H9N2 vaccine in the present study than the MN GMTs reported in these previous studies. Similar rates of MN antibody responses were reported for a subunit vaccine adjuvanted with the novel oil-in-water adjuvant MF59 (45); however, substantially higher HI antibody titers were induced by the MF59-adjuvanted vaccine than by nonadjuvanted vaccines used in the present and previous studies. These data are consistent with studies of H5N1 vaccines which also reported that MF59 adjuvanted vaccines induce very high HI titers (46–48). SRH antibody responses to H9N2 vaccines were not reported in previous studies of H9N2 vaccines (28, 29, 44, 45), and as such, no comparison can be made with respect to SRH antibody responses induced in the present and previous studies.
In the present study, the antibody responses detected by the HI assay were lower than those detected by the MN and SRH assays. The lower sensitivity of the HI assay than that of the MN assay for detecting H9 antibodies has also been reported in previous studies (28, 29), as well as for other avian influenza viruses (37). These observations suggest that the HI assay might not be optimal for detecting H9 antibodies, similar to the situation with antibodies against H5N1 viruses, for which high rates of seroprotection based on the HI assay are difficult to achieve without the use of novel oil-in-water adjuvants.
The current immunogenicity criteria for pandemic influenza virus vaccines are based on those developed for seasonal influenza virus vaccines, where an HI titer of 1:40 or an SRH area of 25 mm2 are the serological criteria used by regulatory authorities as markers for vaccine-induced seroprotection. However, it is not known how these correlates relate to protection from pandemic influenza virus vaccines. In the European Union (EU), nonadjuvanted whole-virus H5N1 vaccines have been licensed based on antibody responses detected by MN and SRH assays (49, 50). For the MN assay, no standardized assay and no internationally accepted cutoff for seroprotection exist. In the present study, an analysis of the MN and HI responses according to Cohen's kappa coefficient (43) demonstrated the best agreement between the HI titer cutoff of 1:40 and the MN cutoff of 1:80 (overall kappa, 0.725; 95% CI, 0.688 to 0.762). However, the best kappa coefficient agreement between the SRH area seroprotection cutoff of 25 mm2 and an MN titer cutoff occurs at an MN titer of 1:20 (overall kappa, 0.539; 95% CI, 0.492 to 0.585), emphasizing the uncertainty surrounding the choice of the cutoff titer for the MN assay. The higher MN titer corresponding best to the HI titer cutoff of 1:40, compared to the lower MN titer which correlates best with the 25 mm2 SRH area cutoff, might be due to the relatively low sensitivity of the HI assay compared to that of the SRH assay.
Based on the findings of the present study, a 15-μg dose of the whole-virus H9N2 vaccine would be required to meet all three EU immunogenicity criteria based on the HI assay data. In contrast, a much lower dose of only 3.75 μg would comfortably meet all immunogenicity criteria based on the SRH assay data. There are no widely accepted criteria for licensure based on MN assays; however, if the same immunogenicity criteria are applied to the MN assay results, irrespective of whether a seroprotection cutoff titer of 1:20, 1:40, or 1:80 is used, these criteria are also all comfortably met for the 3.75 μg dose. These data would indicate that it is necessary to review the appropriateness of the HI cutoff of 1:40 as the sole criterion for licensure of pandemic influenza virus vaccines, particularly in view of the importance of antigen sparing in a pandemic situation.
Some previous studies of H9N2 vaccines based on a G1 lineage virus, which were undertaken in the United Kingdom, reported that the HI and MN antibody responses induced in individuals born in or before 1968 were higher than those born after that year (28, 29). These studies suggested that older individuals were immunologically primed to the H9N2 vaccine, possibly by previous exposure to H2N2 viruses (28, 29). In contrast, a more recent study of an H9N2 virus based on a G9 lineage virus and performed in the United States did not find such age-related differences in the antibody responses (44). In the present study of a G9 lineage vaccine, undertaken in the United States, there was also no difference in the HI and MN antibody responses in subjects born after 1968 and those in subjects born in or before 1968, in agreement with the previous study of a G9 lineage H9N2 vaccine undertaken in the United States. There was a significantly higher SRH antibody response in the older age group at baseline than in the younger age group. However, this difference was not significant at any other time point. The reason for the difference in the SRH antibody titers between the two age groups at baseline is not clear. SRH data were not reported for previous studies of H9N2 vaccines (28, 29, 44, 45). Taken together, the present and previous data indicate that the effects of antigenic priming by potential previous exposure to H2N2 viruses might be different for H9N2 vaccines of the G1 and G9 lineages or that there might be differences in the United Kingdom and U.S. demographics with respect to previous exposure of the population to H2N2 viruses.
Although for licensing purposes, serum HI, SRH, or MN antibody titers are accepted as being indicative of vaccine efficacy, other vaccine-induced immune responses may also contribute to the amelioration of disease severity and/or the prevention of virus shedding. Neuraminidase (NA)-inhibiting (NAi) antibodies have the potential to prevent virus release, which in turn can reduce the severity of influenza disease (16), and NA-specific antibodies have been demonstrated to correlate with protection in animals and humans (51, 52). The immunological response to NA might be of particular importance in the event of an H9N2 pandemic, in which the majority of the population would be naive to the H9 HA protein but may have preexisting immunity to N2 NA as a result of earlier exposure to seasonal H2N2 or H3N2 viruses. In this respect, a limitation of the present study is that we did not investigate the induction of NAi antibody responses. However, in nonclinical studies, the whole-virus H9N2 vaccine induced high levels of functional antibodies capable of inhibiting the function of the H9N2 neuraminidase (27). High-titer NAi antibodies have also been demonstrated in clinical studies of a whole-virus H5N1 vaccine (10, 53), suggesting that the effective induction of NAi antibodies is a consistent feature of whole-virus influenza virus vaccines. Taken together, the clinical and preclinical data generated in studies of the nonadjuvanted whole-virus H9N2 vaccine suggest that it would be a safe, well-tolerated, and effective intervention in the event of a pandemic caused by an H9N2 influenza virus. These data also extend the clinical data set generated for other nonadjuvanted candidate pandemic influenza virus vaccines based on H5N1 and H1N1 viruses (7–11, 38, 40, 54, 55) and support the general safety and immunogenicity profiles of such vaccines for prepandemic and pandemic vaccination.
ACKNOWLEDGMENTS
We thank Theresa Petrohoy, Julie McLaren, Karima Benamara, Miranda Chapman, Smita Barua, Kieran Saul, Christine Wu, and Nina Peschel of the Baxter clinical study team and Cheryl Main from the DVC team for their role in this study.
This work was supported by federal (U.S. government) funds from the Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under contract HHS0100200600013C to the DynPort Vaccine Company LLC (DVC), a CSC company.
The content of this publication does not necessarily reflect the views or policies of the U.S. Department of Health and Human Services, and the mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. government.
G.A., B.G.-I., M.V.W.v.d.V., S.F., M.K., D.P., O.K., and P.N.B. report being employed by Baxter. G.A., B.G.-I., M.V.W.v.d.V., S.F., M.K., O.K., and P.N.B. report having an equity interest in Baxter, and O.K. and P.N.B. report holding patents on influenza vaccines derived from Vero cell cultures. M.K.H. and W.E.-A. report being employed by the DynPort Vaccine Company, and M.K.H. reports having an equity investment in CSC, the parent company of DVC.
REFERENCES
- 1.Gambotto A, Barratt-Boyes SM, de Jong MD, Neumann G, Kawaoka Y. 2008. Human infection with highly pathogenic H5N1 influenza virus. Lancet 371:1464–1475. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- 2.Peiris M, Yuen KY, Leung CW, Chan KH, Ip PL, Lai RW, Orr WK, Shortridge KF. 1999. Human infection with influenza H9N2. Lancet 354:916–917. doi: 10.1016/S0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 3.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. 2013. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med 368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Yuan H, Gao R, Zhang J, Wang D, Xiong Y, Fan G, Yang F, Li X, Zhou J, Zou S, Yang L, Chen T, Dong L, Bo H, Zhao X, Zhang Y, Lan Y, Bai T, Dong J, Li Q, Wang S, Zhang Y, Li H, Gong T, Shi Y, Ni X, Li J, Zhou J, Fan J, Wu J, Zhou X, Hu M, Wan J, Yang W, Li D, Wu G, Feng Z, Gao GF, Wang Y, Jin Q, Liu M, Shu Y. 2014. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet 383:714–721. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. 2012. Pandemic influenza preparedness and response: a WHO guidance document. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2009/9789241547680_eng.pdf?ua=1. [PubMed] [Google Scholar]
- 6.World Health Organization. 2012. Responding to the avian influenza pandemic threat: recommended strategic actions. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/influenza/WHO_CDS_CSR_GIP_05_8-EN.pdf?ua=1. [Google Scholar]
- 7.Ehrlich HJ, Müller M, Oh HM, Tambyah PA, Joukhadar C, Montomoli E, Fisher D, Berezuk G, Fritsch S, Löw-Baselli A, Vartian N, Bobrovsky R, Pavlova BG, Pöllabauer EM, Kistner O, Barrett PN, Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team . 2008. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 358:2573–2584. doi: 10.1056/NEJMoa073121. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich HJ, Müller M, Fritsch S, Zeitlinger M, Berezuk G, Löw-Baselli A, van der Velden MV, Pöllabauer EM, Maritsch F, Pavlova BG, Tambyah PA, Oh HM, Montomoli E, Kistner O, Noel Barrett P. 2009. A cell culture (Vero)-derived H5N1 whole-virus vaccine induces cross-reactive memory responses. J Infect Dis 200:1113–1118. doi: 10.1086/605608. [DOI] [PubMed] [Google Scholar]
- 9.Tambyah PA, Wilder-Smith A, Pavlova BG, Barrett PN, Oh HM, Hui DS, Yuen KY, Fritsch S, Aichinger G, Loew-Baselli A, van der Velden M, Maritsch F, Kistner O, Ehrlich HJ. 2012. Safety and immunogenicity of two different doses of a Vero cell-derived, whole virus clade 2 H5N1 (A/Indonesia/05/2005) influenza vaccine. Vaccine 30:329–335. doi: 10.1016/j.vaccine.2011.10.088. [DOI] [PubMed] [Google Scholar]
- 10.van der Velden MV, Fritz R, Pöllabauer EM, Portsmouth D, Howard MK, Kreil TR, Dvorak T, Fritsch S, Vesikari T, Diez-Domingo J, Richmond P, Lee BW, Kistner O, Ehrlich HJ, Barrett PN, Aichinger G. 2014. Safety and immunogenicity of a Vero cell culture-derived whole-virus influenza A(H5N1) vaccine in a pediatric population. J Infect Dis 209:12–23. doi: 10.1093/infdis/jit498. [DOI] [PubMed] [Google Scholar]
- 11.van der Velden MV, Aichinger G, Pöllabauer EM, Löw-Baselli A, Fritsch S, Benamara K, Kistner O, Müller M, Zeitlinger M, Kollaritsch H, Vesikari T, Ehrlich HJ, Barrett PN. 2012. Cell culture (Vero cell) derived whole-virus non-adjuvanted H5N1 influenza vaccine induces long-lasting cross-reactive memory immune response: homologous or heterologous booster response following two dose or single dose priming. Vaccine 30:6127–6135. doi: 10.1016/j.vaccine.2012.07.077. [DOI] [PubMed] [Google Scholar]
- 12.Dong G, Luo J, Zhang H, Wang C, Duan M, Deliberto TJ, Nolte DL, Ji G, He H. 2011. Phylogenetic diversity and genotypical complexity of H9N2 influenza A viruses revealed by genomic sequence analysis. PLoS One 6:e17212. doi: 10.1371/journal.pone.0017212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusaro A, Monne I, Salviato A, Valastro V, Schivo A, Amarin NM, Gonzalez C, Ismail MM, Al-Ankari AR, Al-Blowi MH, Khan OA, Maken Ali AS, Hedayati A, Garcia Garcia J, Ziay GM, Shoushtari A, Al Qahtani KN, Capua I, Holmes EC, Cattoli G. 2011. Phylogeography and evolutionary history of reassortant H9N2 viruses with potential human health implications. J Virol 85:8413–8421. doi: 10.1128/JVI.00219-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butt KM, Smith GJ, Chen H, Zhang LJ, Leung YH, Xu KM, Lim W, Webster RG, Yuen KY, Peiris JS, Guan Y. 2005. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J Clin Microbiol 43:5760–5767. doi: 10.1128/JCM.43.11.5760-5767.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin YP, Shaw M, Gregory V, Cameron K, Lim W, Klimov A, Subbarao K, Guan Y, Krauss S, Shortridge K, Webster R, Cox N, Hay A. 2000. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc Natl Acad Sci U S A 97:9654–9658. doi: 10.1073/pnas.160270697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Qin K, Wang J, Pu J, Tang Q, Hu Y, Bi Y, Zhao X, Yang H, Shu Y, Liu J. 2011. High genetic compatibility and increased pathogenicity of reassortants derived from avian H9N2 and pandemic H1N1/2009 influenza viruses. Proc Natl Acad Sci U S A 108:4164–4169. doi: 10.1073/pnas.1019109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matrosovich MN, Krauss S, Webster RG. 2001. H9N2 influenza A viruses from poultry in Asia have human virus-like receptor specificity. Virology 281:156–162. doi: 10.1006/viro.2000.0799. [DOI] [PubMed] [Google Scholar]
- 18.Wan H, Perez DR. 2007. Amino acid 226 in the hemagglutinin of H9N2 influenza viruses determines cell tropism and replication in human airway epithelial cells. J Virol 81:5181–5191. doi: 10.1128/JVI.02827-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sorrell EM, Wan H, Araya Y, Song H, Perez DR. 2009. Minimal molecular constraints for respiratory droplet transmission of an avian-human H9N2 influenza A virus. Proc Natl Acad Sci U S A 106:7565–7570. doi: 10.1073/pnas.0900877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guan Y, Shortridge KF, Krauss S, Chin PS, Dyrting KC, Ellis TM, Webster RG, Peiris M. 2000. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J Virol 74:9372–9380. doi: 10.1128/JVI.74.20.9372-9380.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal M, Yaqub T, Reddy K, McCauley JW. 2009. Novel genotypes of H9N2 influenza A viruses isolated from poultry in Pakistan containing NS genes similar to highly pathogenic H7N3 and H5N1 viruses. PLoS One 4:e5788. doi: 10.1371/journal.pone.0005788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P, Tang Y, Liu X, Liu W, Zhang X, Liu H, Peng D, Gao S, Wu Y, Zhang L, Lu S, Liu X. 2009. A novel genotype H9N2 influenza virus possessing human H5N1 internal genomes has been circulating in poultry in eastern China since 1998. J Virol 83:8428–8438. doi: 10.1128/JVI.00659-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wan H, Sorrell EM, Song H, Hossain MJ, Ramirez-Nieto G, Monne I, Stevens J, Cattoli G, Capua I, Chen LM, Donis RO, Busch J, Paulson JC, Brockwell C, Webby R, Blanco J, Al-Natour MQ, Perez DR. 2008. Replication and transmission of H9N2 influenza viruses in ferrets: evaluation of pandemic potential. PLoS One 3:e2923. doi: 10.1371/journal.pone.0002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimble JB, Sorrell E, Shao H, Martin PL, Perez DR. 2011. Compatibility of H9N2 avian influenza surface genes and 2009 pandemic H1N1 internal genes for transmission in the ferret model. Proc Natl Acad Sci U S A 108:12084–12088. doi: 10.1073/pnas.1108058108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.García-Sastre A, Schmolke M. 2014. Avian influenza A H10N8–a virus on the verge? Lancet 383:676–677. doi: 10.1016/S0140-6736(14)60163-X. [DOI] [PubMed] [Google Scholar]
- 26.Liu D, Shi W, Gao GF. 2014. Poultry carrying H9N2 act as incubators for novel human avian influenza viruses. Lancet 383:869. doi: 10.1016/S0140-6736(14)60386-X. [DOI] [PubMed] [Google Scholar]
- 27.Wodal W, Falkner FG, Kerschbaum A, Gaiswinkler C, Fritz R, Kiermayr S, Portsmouth D, Savidis-Dacho H, Coulibaly S, Piskernik C, Hohenadl C, Howard MK, Kistner O, Barrett PN, Kreil TR. 2012. A cell culture-derived whole-virus H9N2 vaccine induces high titer antibodies against hemagglutinin and neuraminidase and protects mice from severe lung pathology and weight loss after challenge with a highly virulent H9N2 isolate. Vaccine 30:4625–4631. doi: 10.1016/j.vaccine.2012.04.102. [DOI] [PubMed] [Google Scholar]
- 28.Nicholson KG, Thompson CI, Klap JM, Wood JM, Batham S, Newman RW, Mischler R, Zambon MC, Stephenson I. 2009. Safety and immunogenicity of whole-virus, alum-adjuvanted whole-virus, virosomal, and whole-virus intradermal influenza A/H9N2 vaccine formulations. Vaccine 28:171–178. doi: 10.1016/j.vaccine.2009.09.103. [DOI] [PubMed] [Google Scholar]
- 29.Stephenson I, Nicholson KG, Glück R, Mischler R, Newman RW, Palache AM, Verlander NQ, Warburton F, Wood JM, Zambon MC. 2003. Safety and antigenicity of whole virus and subunit influenza A/Hong Kong/1073/99 (H9N2) vaccine in healthy adults: phase I randomised trial. Lancet 362:1959–1966. doi: 10.1016/S0140-6736(03)15014-3. [DOI] [PubMed] [Google Scholar]
- 30.Kistner O, Howard MK, Spruth M, Wodal W, Brühl P, Gerencer M, Crowe BA, Savidis-Dacho H, Livey I, Reiter M, Mayerhofer I, Tauer C, Grillberger L, Mundt W, Falkner FG, Barrett PN. 2007. Cell culture (Vero) derived whole virus (H5N1) vaccine based on wild-type virus strain induces cross-protective immune responses. Vaccine 25:6028–6036. doi: 10.1016/j.vaccine.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kistner O, Crowe BA, Wodal W, Kerschbaum A, Savidis-Dacho H, Sabarth N, Falkner FG, Mayerhofer I, Mundt W, Reiter M, Grillberger L, Tauer C, Graninger M, Sachslehner A, Schwendinger M, Brühl P, Kreil TR, Ehrlich HJ, Barrett PN. 2010. A whole virus pandemic influenza H1N1 vaccine is highly immunogenic and protective in active immunization and passive protection mouse models. PLoS One 5:e9349. doi: 10.1371/journal.pone.0009349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wichmann BA, Hill ID. 1982. Algorithm 183. An efficient and portable pseudo-random number generator. Appl Stat 31:188–190. [Google Scholar]
- 33.McLeod A. 1985. Remark AS R58. A remark on algorithm AS 183. An efficient and portable pseudo-random number generator. Appl Stat 121:811–822. [Google Scholar]
- 34.U.S. FDA. 2011. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm091977.pdf. [Google Scholar]
- 35.U.S. FDA. 2001. Guidance for industry: bioanalytical method validation U.S. Department of Health and Human Services, Center for Drug Evaluation and Research, Center for Veterinary Medicine, U.S. FDA, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf. [Google Scholar]
- 36.ICH. 2005. ICH harmonised tripartite guideline: validation of analytical procedures: text and methodology. Q2(R1). International Conference of Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, Geneva, Switzerland http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf. [Google Scholar]
- 37.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, Lim W, Fukuda K, Cox NJ, Katz JM. 1999. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol 37:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ehrlich HJ, Müller M, Kollaritsch H, Pinl F, Schmitt B, Zeitlinger M, Loew-Baselli A, Kreil TR, Kistner O, Portsmouth D, Fritsch S, Maritsch F, Aichinger G, Pavlova BG, Barrett PN. 2012. Pre-vaccination immunity and immune responses to a cell culture-derived whole-virus H1N1 vaccine are similar to a seasonal influenza vaccine. Vaccine 30:4543–4551. doi: 10.1016/j.vaccine.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, Basser RL. 2009. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med 361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 40.Loew-Baselli A, Pavlova BG, Fritsch S, Poellabauer EM, Draxler W, Kistner O, Behre U, Angermayr R, Neugebauer J, Kirsten K, Förster-Waldl E, Koellges R, Ehrlich HJ, Barrett PN. 2012. A non-adjuvanted whole-virus H1N1 pandemic vaccine is well tolerated and highly immunogenic in children and adolescents and induces substantial immunological memory. Vaccine 30:5956–5966. doi: 10.1016/j.vaccine.2012.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, Nissen M, Marshall H, Booy R, Heron L, Hartel G, Lai M, Basser R, Gittleson C, Greenberg M. 2010. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA 303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 42.Schild GC, Pereira MS, Chakraverty P. 1975. Single-radial-hemolysis: a new method for the assay of antibody to influenza haemagglutinin. Applications for diagnosis and seroepidemiologic surveillance of influenza. Bull World Health Organ 52:43–50. [PMC free article] [PubMed] [Google Scholar]
- 43.Agresti A, Coull BA. 1996. Order-restricted tests for stratified comparisons of binomial proportions. Biometrics 52:1103–1111. doi: 10.2307/2533072. [DOI] [PubMed] [Google Scholar]
- 44.Atmar RL, Keitel WA, Quarles JM, Cate TR, Patel SM, Nino D, Wells J, Arden N, Guo K, Hill H, Couch RB. 2011. Evaluation of age-related differences in the immunogenicity of a G9 H9N2 influenza vaccine. Vaccine 29:8066–8072. doi: 10.1016/j.vaccine.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, Pompey J, Cate TR, Couch RB. 2006. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis 43:1135–1142. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- 46.Vesikari T, Karvonen A, Tilman S, Borkowski A, Montomoli E, Banzhoff A, Clemens R. 2010. Immunogenicity and safety of MF59-adjuvanted H5N1 influenza vaccine from infancy to adolescence. Pediatrics 126:e762–e770. doi: 10.1542/peds.2009-2628. [DOI] [PubMed] [Google Scholar]
- 47.Vesikari T, Forstén A, Herbinger KH, Della Cioppa G, Beygo J, Borkowski A, Groth N, Bennati M, von Sonnenburg F. 2012. Safety and immunogenicity of an MF59-adjuvanted A/H5N1 pre-pandemic influenza vaccine in adults and the elderly. Vaccine 30:1388–1396. doi: 10.1016/j.vaccine.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Vesikari T, Forstén A, Borkowski A, Gaitatzis N, Banzhoff A, Clemens R. 2012. Homologous and heterologous antibody responses to a one-year booster dose of an MF59 adjuvanted A/H5N1 pre-pandemic influenza vaccine in pediatric subjects. Hum Vaccin Immunother 8:921–928. doi: 10.4161/hv.20248. [DOI] [PubMed] [Google Scholar]
- 49.EMA. 2012. Pandemic influenza vaccine H5N1 Baxter AG: pandemic influenza vaccine (H1N1) (whole virion, inactivated, prepared in cell culture). European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001200/human_med_001215.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124. [Google Scholar]
- 50.EMA. 2012. EPAR summary for the public: Vepacel influenza vaccine (whole virion, inactivated). European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/002089/WC500124226.pdf. [Google Scholar]
- 51.Couch RB, Atmar RL, Franco LM, Quarles JM, Wells J, Arden N, Niño D, Belmont JW. 2013. Antibody correlates and predictors of immunity to naturally occurring influenza in humans and the importance of antibody to the neuraminidase. J Infect Dis 207:974–981. doi: 10.1093/infdis/jis935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rockman S, Brown LE, Barr IG, Gilbertson B, Lowther S, Kachurin A, Kachurina O, Klippel J, Bodle J, Pearse M, Middleton D. 2013. Neuraminidase-inhibiting antibody is a correlate of cross-protetion against lethal H5N1 influenza virus in ferrets immunized with seasonal influenza vaccine. J Virol 87:3053–3061. doi: 10.1128/JVI.02434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fritz R, Sabarth N, Kiermayr S, Hohenadl C, Howard MK, Ilk R, Kistner O, Ehrlich HJ, Barrett PN, Kreil TR. 2012. A Vero cell-derived whole-virus H5N1 vaccine effectively induces neuraminidase-inhibiting antibodies. J Infect Dis 205:28–34. doi: 10.1093/infdis/jir711. [DOI] [PubMed] [Google Scholar]
- 54.Barrett PN, Portsmouth D, Ehrlich HJ. 2010. Developing cell culture-derived pandemic vaccines. Curr Opin Mol Ther 12:21–30. [PubMed] [Google Scholar]
- 55.Barrett PN, Portsmouth D, Ehrlich HJ. 2013. Vero cell culture-derived pandemic influenza vaccines: preclinical and clinical development. Expert Rev Vaccines 12:395–413. doi: 10.1586/erv.13.21. [DOI] [PubMed] [Google Scholar]