Abstract
Classical swine fever (CSF) is a highly contagious viral disease of pigs that has a tremendous socioeconomic impact. Vaccines are available for disease control. However, most industrialized countries are implementing stamping-out strategies to eliminate the disease and avoid trade restrictions. These restrictions can be avoided through the use of marker vaccines such as CP7_E2alf. Marker vaccines have to be accompanied by reliable and robust discriminatory assays. In this context, a multiplex microsphere immunoassay for serological differentiation of infected from vaccinated animals (DIVA) was developed to distinguish CSF virus (CSFV)-infected animals from CP7_E2alf-vaccinated animals. To this end, three viral proteins, namely, CSFV E2, CSFV Erns, and bovine viral diarrhea virus (BVDV) E2, were produced in insect cells using a baculovirus expression system; they were used as antigens in a microsphere immunoassay, which was further evaluated by testing a large panel of pig sera and compared to a well-characterized commercial CSFV E2 antibody enzyme-linked immunosorbent assays (ELISAs) and a test version of an improved CSFV Erns antibody ELISA. Under a cutoff median fluorescence intensity value of 5,522, the multiplex microsphere immunoassay had a sensitivity of 98.5% and a specificity of 98.9% for the detection of antibodies against CSFV E2. The microsphere immunoassay and the CSFV Erns ELISA gave the same results for 155 out of 187 samples (82.8%) for the presence of CSFV Erns antibodies. This novel multiplex immunoassay is a valuable tool for measuring and differentiating immune responses to vaccination and/or infection in animals.
INTRODUCTION
Classical swine fever (CSF) is a highly contagious and economically important viral disease of pigs and is notifiable to the World Organization for Animal Health (OIE). The causative agent, classical swine fever virus (CSFV), is a member of the Pestivirus genus within the Flaviviridae family. The other three members, namely, bovine viral diarrhea virus 1 (BVDV-1), bovine viral diarrhea virus 2 (BVDV-2), and border disease virus (BDV), can also infect domestic pigs and wild boar and cross-react with antibodies against CSFV, which might interfere with the serological diagnosis of CSF. The virus has a single-stranded positive-sense RNA genome of approximately 12.3 kb, which encodes a single polyprotein that is processed into four structural proteins, C, Erns, E1, and E2, and eight nonstructural proteins, Npro, p7, NS2, NS3, NS4A, NS4B, NS5A, and NS5B (1). Erns is heavily glycosylated and forms disulfide-linked homodimers (2, 3). Glycosylation and disulfide linkage are also important for E1 and E2. Glycoprotein E2 is involved in virus attachment and entry into target cells (4) and has been a target for the development of subunit vaccines (5–8). Both Erns and E2 provide protective immunity by inducing neutralizing antibodies in the host (9–11).
According to OIE animal health information (available in the World Animal Health Information Database), CSF has been confirmed in 21 countries since 2010. Within the European Union (EU), Council Directive 2001/89/EC has laid down community measures for the control of CSF within the EU, where the use of vaccines may be authorized only in emergencies. However, the use of conventional live attenuated vaccines in domestic pigs may result in restrictions in trading live pigs or pig products due to the fact that the vaccinated pigs cannot be serologically distinguished from those infected naturally with CSFV. In addition, the increasing number of wild boar in EU member states may pose a challenge to the control and eradication of the disease in wild boar populations. All of these factors emphasize the need for novel marker vaccines, which allow not only for differentiation of infected from vaccinated animals (DIVA) but also for oral vaccination of wild boar.
Several vaccines have been developed employing different approaches (12). Within the EU project “Epidemiology and control of classical swine fever (CSF) in wild boar and potential use of a newly developed live marker vaccine” (grant no. 501599), the marker vaccine candidate CP7_E2alf was initially tested. It is based on the backbone of the BVDV CP7 strain with the exchange of the E2 gene of CSFV strain Alfort/187 (6), which enables the differentiation of pigs infected with wild-type CSFV from those vaccinated by testing antibodies against CSFV Erns and E2. This chimeric vaccine candidate was further evaluated within the subsequent EU project “Improve tools and strategies for the prevention and control of classical swine fever” (grant no. 227003). This vaccine candidate provides early onset of protection against lethal challenge after intramuscular and oral immunization (13) and is safe for target and nontarget species (14). Efficacy has been shown in several trials (15–19), and licensing is under way. Besides efficacy and safety issues, marker vaccines have to be accompanied by reliable discriminatory assays (20). In the case of CP7_E2alf or similar vaccines, serological DIVA can be achieved by CSFV Erns and E2 enzyme-linked immunosorbent assays (ELISAs). While several fully validated CSFV E2 ELISAs are commercially available on the market from different suppliers, only the PrioCHECK CSFV Erns ELISA was found suitable for Erns-based marker serology. An improved version of this test was temporarily available for evaluation within the consortium, but further improvements are needed in terms of sensitivity, specificity, robustness, and reproducibility (15). Furthermore, testing the same sera with two different ELISA protocols would clearly increase hands-on time and labor intensity. In contrast, the xMAP technology (Luminex Corp., Austin, TX) allows the simultaneous detection of multiple targets, such as antibodies, in the same sample. The objective of this study was to develop a multiplex microsphere immunoassay for differentiating pigs infected with wild-type CSFV and those vaccinated with the marker vaccine candidate CP7_E2alf.
MATERIALS AND METHODS
Serum samples.
A total of 365 serum samples were analyzed in this study (Table 1). These included 33 samples from a German reference panel for ELISA batch release, 8 of which were from pigs infected with non-CSFV pestiviruses (BVDV/BDV), and 186 samples collected from different CP7_E2alf (no C-strain) vaccination/challenge studies at the Friedrich-Loeffler Institut (FLI), Greifswald-Insel Riems, Germany. All the German samples had been tested for CSFV E2 antibodies and some (187 samples) for CSFV Erns antibodies (15, 18). Sixty-six CSFV E2-positive samples were derived from experimental infections of pigs with different CSFV genotypes at the EU and OIE Reference Laboratory for CSF (TiHo), Hannover, Germany, and 80 CSFV E2-negative samples were from the National Veterinary Institute (SVA), Uppsala, Sweden. CSFV E2 antibodies were tested using an IDEXX HerdCheck CSFV Ab ELISA (IDEXX Laboratories, Shiphol-Rijk, The Netherlands), and CSFV Erns antibodies were tested using a PrioCHECK Erns ELISA (Prionics Lelystad BV, Lelystad, The Netherlands).
TABLE 1.
Serum samples with CSFV E2 and Erns ELISA results
| Providera | Source | No. of samples | E2 ELISAb | Erns ELISAc |
|---|---|---|---|---|
| FLI | Vaccination/challenge | 186 | 186 (175/11) | 75 (111/64) |
| German serum batch, CSFV | 25 | 25 (24/1) | 12 (12/0) | |
| German serum batch, BVDV/BDVd | 8 | 8 (0/8) | Not done | |
| TiHo | CSF reference sera | 66 | 66 (66/0) | Not done |
| SVA | Swedish swine sera | 80 | 80 (0/80) | Not done |
| Total (all providers) | 365 | 365 (265/100) | 187 (123/64) |
FLI, Friedrich-Loeffler Institut, Greifswald-Insel Riems, Germany; TiHo, the EU and OIE Reference Laboratory for CSF, Hannover, Germany; SVA, National Veterinary Institute, Uppsala, Sweden.
Values represent the number of samples tested by the CSFV E2 ELISA (number of positive samples/number of negative samples).
Values represent the number of samples tested by the CSFV Erns ELISA (number of positive samples/number of negative samples).
These sera were used for Luminex detection of CSFV E2 antibody only; they were not included in any group for the purpose of the Luminex DIVA.
PCR amplification and cloning.
Two microliters of plasmids containing CSFV E2, CSFV Erns, or BVDV Erns was used for amplification of the target gene regions in a 25-μl reaction volume, including 12.5 μl of water, 2.5 μl of 10× buffer, 2 μl of 2.5 mM deoxynucleoside triphosphate (dNTP) mix, 2.5 μl of 10 μM each primer, and 1.25 U PfuUltra DNA polymerase (Agilent Technologies, Inc., Santa Clara, CA). The primers are listed in Table 2. The fragment was excised from the gel, purified using the PCR cleanup system (Promega Co., Madison, WI), and cloned into pFast Bac/HBM TOPO vector (Invitrogen, Carlsbad, CA). The positive plasmids, designated pFastBac/HBM-CSFVErns, pFastBac/HBM-BVDVErns, and pFastBac/HBM-CSFVE2, were verified by DNA sequencing.
TABLE 2.
Primers and nucleotide sequences
| Primer | Nucleotide sequence |
|---|---|
| CSFV-Erns-F | GAAAATATAACTCAATGGAACCTG |
| CSFV-Erns-R | GGCATAGGCACCAAACCAG |
| BVDV-Erns-F | GAGAACATAACGCAATGGAACT |
| BVDV-Erns-R | TGCATATGCCCCAAACCA |
| CSFV-E2-F | CAGCTAGCCTGCAAGGAAGAT |
| CSFV-E2-R (truncated) | TTCTGCGAAGTAATCTGAGTGGC |
Expression of CSFV E2, CSFV Erns, and BVDV Erns using a baculovirus expression system.
The plasmids were transformed into MAX efficiency DH10Bac-competent Escherichia coli cells (Invitrogen, Carlsbad, CA) to generate recombinant bacmids, which were further verified based on the phenotypic characteristics and analytical PCR according to the manufacturer's instructions. One microgram of bacmid DNA was transfected into Spodoptera frugiperda (sf9) cells using 6 μl of Cellfectin II reagent (Invitrogen). The recombinant viruses were harvested after 96 h, and the virus titers were determined by a BacPAK baculovirus rapid titer kit (Clontech Laboratories, Mountain View, CA) as per the manufacturer's instructions. The protein expression was optimized by infecting the cells at a density of 2 × 106 with multiplicity of infection (MOI) values of 1 and 2. Twenty-four hours postinfection, 0.5% fetal bovine serum (FBS) was added, and the cells were collected at 48, 72, and 96 h postinfection and centrifuged at 800 × g for 5 min. Pellets were washed with 1 ml phosphate-buffered saline (PBS) and, if not used immediately, stored at −20°C. To analyze the proteins, 75 μl of lysis buffer (200 mM Tris, 20% glycerol, 5 mM EDTA, 4% SDS, 50 mM dithiothreitol, 6 units of Benzonase) was added to the cell pellet and incubated at 4°C for 30 min. The pellet and supernatant were analyzed by SDS-PAGE and Western blot analysis using an anti-His monoclonal antibody (Sigma, St. Louis, MO) and sera positive for CSFV E2, CSFV Erns, or BVDV Erns.
Affinity purification of recombinant proteins.
One milliliter of cell lysis buffer (25 mM HEPES [pH 7.4], 100 mM NaCl, 1% Triton X-100, 1× protease inhibitor) was added to 1.2 × 107 cells and incubated on ice for 45 min. The cells were sheared by brief sonication, and soluble proteins were recovered in the supernatant following ultracentrifugation at 18,000 × g for 30 min at 4°C. A HisTrap chelating HP column (GE Healthcare, Uppsala, Sweden) was equilibrated with 10 column volumes of buffer A (50 mM HEPES [pH 7.4], 500 mM NaCl, 20 mM imidazole). After loading the sample, the column was washed with 10 volumes of buffer B (50 mM HEPES [pH 7.4], 400 mM NaCl, 30 mM imidazole). The proteins were eluted from the column with 100, 300, or 500 mM imidazole. The elution fractions were concentrated with a 10-kDa molecular mass cutoff Amicon Ultra-4 centrifugal filter device (Millipore Ireland B.V., County Cork, Ireland).
Microsphere immunoassay.
Coupling of the recombinant proteins (CSFV E2, CSFV Erns, BVDV Erns) to carboxylated microspheres (Luminex Corp., Austin, TX) was mediated by 1-ethyl-3-3-dimethylaminopropyl carbodiimide (Pierce, Rockford, IL), as previously described (21). The optimal amount of protein, dilution of serum, and blocking solutions were determined experimentally. The same amounts of secondary antibody and conjugate were used as described previously (21). The coupled microspheres were resuspended in 500 μl of PBS-TBN (PBS, 0.1% bovine serum albumin [BSA], 0.02% Tween 20, 0.05% azide [pH 7.4]) and stored at 4°C in darkness. The coupling reaction was confirmed by testing positive sera.
The immunoassay was performed in 96-well plates. The coupled microspheres were resuspended in 4-fold-diluted PBS with 1% BSA, and 50 μl of microspheres was transferred to each well of the plate. Fifty microliters of 4-fold-diluted PBS with 1% BSA and 50 μl of sera were added to the respective wells and incubated at 37°C for 30 min on a plate shaker at 300 rpm. The microspheres were washed twice with PBS and separated by a microsphere magnet in a HydroFlex microplate washer (Tecan Group Ltd., Männedorf, Switzerland). Thereafter, 50 μl of 2-μg/ml biotinylated anti-swine IgG (Jackson ImmunoResearch, West Grove, PA) in the 4-fold-diluted PBS with 1% BSA was added to each well and incubated for another 30 min at 300 rpm. Following the same washing step as before, 50 μl of 10-μg/ml streptavidin-R-phycoerythrin conjugate (ProZyme, Inc., Hayward, CA) in PBS with 1% BSA was added to each well and incubated at 37°C for 30 min on a plate shaker at 300 rpm. After two washes, the microspheres were resuspended in 100 μl of the 4-fold-diluted PBS with 1% BSA and analyzed by a Luminex 200 analyzer. Median fluorescence intensity (MFI) values were calculated based on the measurement of 100 beads per sample. Receiver operating characteristic (ROC) analysis of the microsphere immunoassay and ELISA were performed using MedCalc software (MedCalc Software, Mariakerke, Belgium) to determine the cutoff value of the microsphere immunoassay.
RESULTS
Expression and purification of recombinant proteins.
Three viral proteins were produced in sf9 cells using a baculovirus expression system. The recombinant proteins contained a 6×His tag at the C terminus for affinity purification. The expression of the recombinant proteins was driven by a baculovirus-specific strong promoter of Autographa californica multiple nuclear polyhedrosis virus (AcMNPV). Two CSFV proteins, E2 and Erns, were found to have high yields when sf9 cells were infected with an MOI of 2, and proteins were harvested at 96 h posttransfection. Following lysis of the cells, the two proteins were readily detected by Western blot analysis using an anti-His monoclonal antibody (Fig. 1). While E2 protein was present both in soluble format and in association with cell membranes in the pellet after centrifugation, the Erns protein was mostly expressed in insoluble form or associated with cell membranes. The molecular mass of the E2 protein was about 55 kDa and that of the Erns protein was 35 kDa, indicating that neither of the two proteins was fully glycosylated. The two proteins reacted well with positive serum in a Western blot analysis, indicating that they retained their antigenicity. The two proteins were purified using nickel (Ni2+) affinity chromatography, and the purified proteins were verified by Western blot analysis. Similar to CSFV Erns expression, the BVDV Erns protein was found mainly in insoluble form when cells were infected with an MOI of 1 and harvested at 72 h posttransfection. The molecular mass of the BVDV Erns protein was less than that of CSFV Erns, indicating that it was not fully glycosylated.
FIG 1.
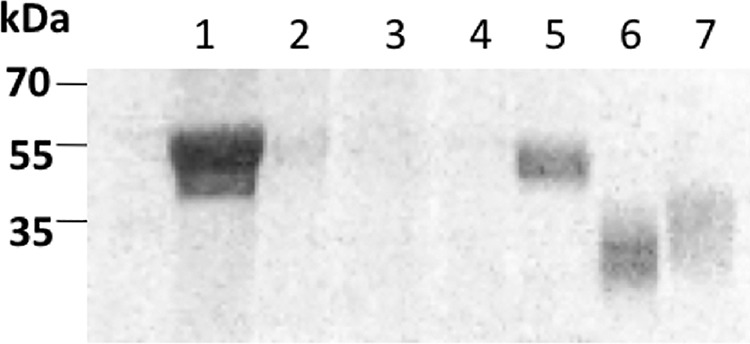
Western blot detection of three viral glycoproteins produced in sf9 cells. Lanes 1 to 5 show purification of the recombinant CSFV E2 protein (lane 1, cell lysate; lane 2, supernatant; lane 3, flow-through fraction; lane 4, wash; lane 5, elution fraction). Two Erns proteins of BVDV (lane 6) and CSFV (lane 7) were also purified in the similar way. Anti-His monoclonal antibody was used in the analysis.
Optimization of the microsphere immunoassay.
To set up an indirect multiplex immunoassay, each set of microspheres was coupled with one of the viral antigens, namely, CSFV E2, CSFV Erns, or BVDV Erns. The amount of each antigen was determined by comparing the MFI values of a range of antigens coupled to 1.2 million microspheres. It was found that the optimal amount was 20 μg for CSFV E2, 120 μg for CSFV Erns, and 20 μg for BVDV Erns, which gave higher MFI values for the positive sera and lower MFI values for negative sera (see Fig. S1 in the supplemental material).
To investigate the possible effects of salt concentration on the specific binding between antibodies and antigens, five different concentrations (0, 100, 200, 300, and 400 mM) of NaCl solution were added to the CSFV Erns immunoassay, which had showed initially some degree of cross-reactivity with antibodies against BVDV Erns. With the increasing salt concentrations, MFI values for the specific binding of CSFV Erns antigen to CSFV-positive sera decreased by 28% and nonspecific binding to the CP7_E2alf-positive serum increased by 38% (see Fig. S2 in the supplemental material). This suggests that the lower the salt concentration was, the higher the specific binding between CSFV Erns antigen and antibodies became. To further reduce the salt concentration, 4-fold-diluted PBS was used in the multiplex immunoassay. Finally, a 500-fold dilution of serum was found to be optimal for testing all three antigens.
Evaluation of the multiplex immunoassay for the detection of CSFV E2 and Erns antibodies.
To evaluate the sensitivity and specificity for E2 antibody detection, a total of 365 samples were tested by the multiplex immunoassay, and the results were compared to those of the ELISAs. Based on the ROC analysis, a cutoff MFI value of >5,522 was chosen for the evaluation of the diagnostic sensitivity and specificity for E2 antibody detection. Out of the 365 samples, 360 samples were correctly diagnosed, resulting in a sensitivity of 98.5% and a specificity of 98.9%. The 95% confidence intervals (CI) were 96.3 to 99.6% for the sensitivity and 94 to 100% for the specificity. The area under the ROC curve (AUC) was 0.999 (P < 0.0001). The 8 BVDV/BDV serum samples gave negative results by the immunoassay. Four false-negative serum samples, which had positive ELISA results, were taken from vaccinated pigs at 1 and 3 months postvaccination and from vaccinated/challenged animals (one wild boar and one domestic pig) at 4 days postinfection (d.p.i.) (15). The MFI values for the four samples ranged from 4,498 to 5,367. In addition, one sample, which was taken from a challenged pig at 10 d.p.i., tested positive by the immunoassay but negative by the commercial CSFV E2 ELISA kit.
CSFV Erns antibody detection was evaluated with 187 samples that had been tested by a test version of the PrioCHECK CSFV Erns ELISA, which was the only kit available then but needed further optimization and thorough validation. The ELISA results were used as arbitrary references for preliminary comparison with the Luminex assay. Under the cutoff value of >4,070.75, as suggested by ROC analysis, 155 out of the 187 samples showed the same results by the microsphere immunoassay and by ELISA. The samples with different results are listed in Table S1 in the supplemental material. While 15 samples were found Erns antibody negative by the immunoassay but positive by ELISA, 17 samples were regarded Erns antibody positive by the immunoassay but negative by ELISA. It has to be noted that 13 out of the 15 immunoassay-negative samples were taken within 10 days after infection/vaccination, when the Erns antibodies were likely present in a very low titer, and that 14 out of the 17 immunoassay-positive samples were taken at least 14 days after vaccination/infection when specific IgG usually develops upon infection/vaccination. Given the unsatisfactory performance of the CSFV Erns ELISA, the immunoassay seemed to have improved sensitivity and specificity for CSFV Erns antibody detection compared to those of the ELISA. All 80 serum samples from a naive pig population in Sweden tested negative by the immunoassay, with a median MFI value of 269. Five out of the 66 CSF reference serum samples were scored Erns antibody negative by the immunoassay, with MFI values ranging from 537 to 2,018. Because the PrioCHECK CSFV Erns ELISA was a prototype version and not a fully validated kit, specificity and sensitivity values were not calculated, as they would not have accurately reflected the full capability of the assay.
Detection of BVDV Erns antibodies upon vaccination.
The multiplex microsphere immunoassay detected antibodies against BVDV Erns in the vaccinated pig population. Thirty-four pigs were vaccinated with the CP7_E2alf marker vaccine candidate and sampled 3 weeks postvaccination and up to 6 months postvaccination (15, 18). The samples were tested by the multiplex immunoassay for the detection of BVDV Erns antibodies. The MFI values ranged from 608 to 11,190, with a median value of 1,403. The 95% confidence interval for the median MFI was 1,096 to 2,152. When the 80 negative Swedish serum samples were tested, the MFI values for BVDV Erns antibody detection were between 27 and 6,720, with a median value of 762. The 95% confidence interval for the median was 664 to 912. As the MFI values from vaccinated pigs were slightly higher than those from naive pigs, a very low-level antibody response to BVDV Erns antigen in pigs upon vaccination with CP7_E2alf was suggested. The antibody response was consistently observed regardless of the sampling time points (1, 3, and 6 months postvaccination) or the vaccination route (oral or intramuscular). The low-level and slow development of BVDV Erns antibodies was in sharp contrast to the rapid development of CSFV E2 antibodies to high titers.
DIVA potential of the multiplex microsphere immunoassay.
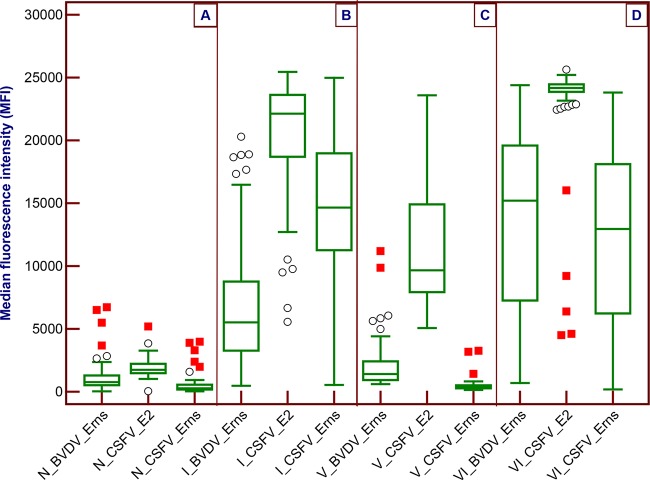
To evaluate the potential to differentiate wild-type CSFV infection from marker vaccination, the 356 samples tested were grouped into one of four populations: naive (Swedish swine sera; n = 80); wild-type CSFV infected (n = 96), including CSF reference sera, German CSFV batch and part of vaccination/challenge; marker vaccinated (n = 34); and vaccinated and subsequently infected (n = 146). The marker-vaccinated and the vaccinated-and-subsequently-infected groups were from the vaccination/challenge. The results are presented as a box-and-whisker plot in Fig. 2, and the median MFI values and 95% confidence intervals are listed in Table 3.
FIG 2.
DIVA potential of the multiplex microsphere immunoassay for the simultaneous detection of three antibodies in naive (A), wild-type CSFV-infected (B), CP7_E2alf-vaccinated (C), and vaccinated/infected (D) populations. The box-and-whisker plot shows the median fluorescence intensity (MFI) values from the multiplex immunoassay, with median values shown in the line within the boxes. The boundaries of the boxes indicate the 25th and 75th percentiles, and error bars above and below the boxes indicate the 10th and 90th percentiles. Outside and outlier values are represented by circles and filled squares, respectively.
TABLE 3.
Antibody detection results in the four pig populations
| Antibody | Median MFI value (95% confidence interval) in: |
|||
|---|---|---|---|---|
| Naive pigs | Infected pigs | Vaccinated pigs | Vaccinated/challenged pigs | |
| CSFV E2 | 1,742 (1,577–1,913) | 22,128a (20,781–22,793) | 9,664 (8,688–12,402) | 24,170 (24,071–24,273) |
| CSFV Erns | 269 (224–381) | 14,651 (13,713–16,015) | 366 (294–435) | 12,949 (9,983–15,043) |
| BVDV Erns | 762 (664–912) | 5,514 (4,160–6,238) | 1,403 (1,096–2,152) | 15,207 (13,419–16,716) |
Numbers in bold type are theoretically positive.
The naive population was negative for CSFV E2 and Erns antibodies and had slightly higher MFI values for BVDV Erns antibody. In the infected group, CSFV E2 antibodies were positive in all samples with high MFI values, whereas CSFV Erns antibodies were positive in 89 out of 96 samples. The negative samples included three reference samples against the live attenuated Riems strain, which induced a late or low Erns antibody response upon vaccination. Cross-reactivity of the BVDV Erns antigen with CSFV Erns antibodies was observed. In the vaccinated population, except for two samples, the MFI values for the CSFV E2 antibodies were high above the cutoff values, while the CSFV Erns antibodies remained negative. The MFI values for the BVDV Erns antibodies were similar to those found in the naive population. Finally, in the more complicated vaccination/challenge group, while the CSFV Erns MFI values were similar to those in the infected group, the CSFV E2 MFI values were the highest among all groups. Two samples were E2 antibody negative, and three positive outliers had relatively low MFI values (6,300 to 16,000). The two negative sera were sampled at 4 days p.i. However, when sampled at 7 days p.i., both became strongly positive, with MFI values of >23,000. The three outliers with low MFI values were also sampled at 4 days p.i. and reached high MFI values within 3 days.
DISCUSSION
Marker vaccines, such as CP7_E2alf, have to be accompanied by reliable discriminatory assays. However, currently available approaches have shown drawbacks in either sensitivity/specificity or large-scale application (e.g., the detection of the CSFV Erns antibodies by ELISA is problematic, which is likely due to the cross-reactivity with antibodies against BVDV or BDV). Within the framework of the EU project “CSFV_goDIVA,” two CSFV Erns-based ELISAs were developed using viral antigens produced in E. coli and evaluated for the detection and differentiation of infected from vaccinated animals and for further discrimination of pestivirus antibodies (22). The current study took a different approach; the three viral proteins were produced in insect cells using a baculovirus expression system (aiming for a better posttranslational modification of the proteins) and used as antigens in a microsphere immunoassay. Unlike the ELISA, in the Luminex assay, antigens are coupled to suspension microspheres, which allows a high degree of multiplexing capacity by mixing sets of microspheres coupled with different antigens. For example, a 30-plex Luminex panel is commercially available for the detection and quantification of human cytokines, chemokines, and growth factors in serum, plasma, and tissue culture supernatant in less than 1 day (Life Technologies), which would be difficult to achieve by ELISA. Conversely, it takes more effort and time, and it is always a great challenge to develop and validate such a multiplex microsphere immunoassay.
It has been reported that CSFV E2 produced using a baculovirus system results in higher sensitivities and specificities when evaluated in different ELISA formats (23). In this study, none of the viral proteins was fully glycosylated, which may be a reason for the lower sensitivity of Erns antibody detection. While BVDV Erns had slightly higher background binding and cross-reacted with CSFV Erns antibodies, the CSFV E2 and Erns antigens reacted specifically to the respective antibodies. In addition, the immunoassay has improved CSFV Erns antibody detection (compared to that of the ELISA), which is likely due to better binding between the antibody and antigen. Nevertheless, the multiplex immunoassay can be readily used as an alternative method for the diagnosis of CSF.
Following vaccination with CP7_E2alf, the BVDV Erns antibody response was surprisingly weak. With the exchange of the CSFV E2 gene, the tissue tropism of the BVDV-backboned chimeric vaccine candidate has been changed and the virus has a very limited replication in pigs (6), which is barely detectable by a highly sensitive real-time reverse transcriptase PCR (RT-PCR) (15). Compared to the potent E2 protein, the BVDV Erns protein induced a weak immune response in pigs vaccinated with the marker vaccine candidate CP7_E2alf. In most cases, the BVDV Erns MFI values from the vaccinated pigs were similar to those found in naive pigs, while the E2 MFI values were much higher than the values obtained from naive pigs. It has been demonstrated that the BVDV Erns can induce a rapid immune response in calves within 2 weeks of postexperimental infection with BVDV-1 or atypical bovine pestivirus, and the MFI values increased up to 20,000 (cutoff value, 2,800) (24). The chimeric nature of the marker vaccine candidate CP7_E2alf may be the reason for the observed difference of the BVDV Erns protein in inducing immune responses in pigs vaccinated with CP7_E2alf and in calves infected with wild-type BVDV.
The focus of this study was on the DIVA potential of the multiplex microsphere immunoassay, which is an essential component of the marker vaccine concept and DIVA diagnostics. In the naive pig population, the antibodies against all three viral proteins were regarded negative. In the infected group, where the CSFV E2 and Erns antibodies should theoretically be positive, all 96 serum samples tested positive for the E2 antibody, and 89 out of 96 samples were positive for the CSFV Erns antibody by the multiplex immunoassay. The seven Erns antibody-negative samples were from four pigs infected with the Riems strain and from three pigs that had a low neutralization peroxidase-linked assay (NPLA) titer of 60 using the CSFV Alfort strain. Cross-reactivity of the BVDV Erns antigen with CSFV Erns antibodies was observed in this group, which was also reported recently (17, 25). In contrast, the CSFV Erns antibodies remained negative in the vaccinated group, and E2 antibodies were all positive except two samples that had NPLA titers of 20 and 160. The distinct feature of CSFV Erns antibody detection in the two groups confirmed the DIVA potential of the multiplex microsphere immunoassay.
It is also possible that the vaccinated pigs or wild boar are infected with wild-type viruses in the field. As the BVDV Erns protein present in the marker vaccine induced a very weak immune response and cross-reacted with the CSFV Erns antibody, the detection of BVDV Erns antibody was unreliable for distinguishing the infected group from the vaccination/challenge group. However, E2 antibody detection in the vaccination/challenge group had a characteristic phenomenon (high median MFI values with short ranges), which was also observed in another study where ELISA was used to detect E2 antibodies (18). As this characteristic profile of E2 antibody detection was observed in neither the infected group nor the vaccinated group, it can be a strong indication of the vaccination and subsequent infection status. Importantly, the detection of CSFV Erns antibody in a population indicates wild-type infection that will surely require more thorough investigations.
In conclusion, the recombinant CSFV E2, CSFV Erns, and BVDV CP7 Erns proteins were produced using a baculovirus expression system and used for developing a multiplex microsphere immunoassay. The assay detected and differentiated antibodies raised against wild-type CSFV infection and vaccination with CP7_E2alf. It also revealed an unexpectedly slow development of BVDV Erns antibody in the vaccinated pigs. This microsphere immunoassay provides a platform to multiplex additional antigens for the simultaneous detection and differentiation of different porcine pathogens.
ACKNOWLEDGMENTS
We thank Katrin Pannhorst and Denise Meyer (EU and OIE Reference Laboratory for CSF, Hannover, Germany), Åse Uttenthal (Technical University of Denmark, National Veterinary Institute, Lindholm, Denmark), Marie-Frédérique Le Potier (ANSES, Ploufragan, France), and Eva Liljekvist (National Veterinary Institute, Uppsala, Sweden) for providing the serum samples.
This work was supported by EU projects CSFV_goDIVA and RAPIDIA-FIELD, the Award of Excellence (Excellensbidrag) to S. Belák, and by the Royal Swedish Academy of Agriculture and Forestry (KSLA) to L. Liu.
L.L., S. Belák, and H.X. contributed to the study design; R.H., B.V., and H.X. performed the experiments; R.H., L.L., and H.X. analyzed the data; R.H. drafted the manuscript; and all the authors revised the manuscript.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00271-14.
REFERENCES
- 1.Lindenbach BD, Thiel H-J, Rice CM. 2007. Flaviviridae: the viruses and their replication, p 1101–1152. In Knipe DM, Howley PM (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 2.Langedijk JP, van Veelen PA, Schaaper WM, de Ru AH, Meloen RH, Hulst MM. 2002. A structural model of Pestivirus E(rns) based on disulfide bond connectivity and homology modeling reveals an extremely rare vicinal disulfide. J Virol 76:10383–10392. doi: 10.1128/JVI.76.20.10383-10392.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiel H-J, Stark R, Weiland E, Rümenapf T, Meyers G. 1991. Hog cholera virus: molecular composition of virions from a pestivirus. J Virol 65:4705–4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulst MM, Moormann RJ. 1997. Inhibition of pestivirus infection in cell culture by envelope proteins E(rns) and E2 of classical swine fever virus: E(rns) and E2 interact with different receptors. J Gen Virol 78:2779–2787. [DOI] [PubMed] [Google Scholar]
- 5.Moormann RJ, Bouma A, Kramps JA, Terpstra C, De Smit HJ. 2000. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet Microbiol 73:209–219. doi: 10.1016/S0378-1135(00)00146-2. [DOI] [PubMed] [Google Scholar]
- 6.Reimann I, Depner K, Trapp S, Beer M. 2004. An avirulent chimeric Pestivirus with altered cell tropism protects pigs against lethal infection with classical swine fever virus. Virology 322:143–157. doi: 10.1016/j.virol.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 7.Koenig P, Lange E, Reimann I, Beer M. 2007. CP7_E2alf: a safe and efficient marker vaccine strain for oral immunisation of wild boar against classical swine fever virus (CSFV). Vaccine 25:3391–3399. doi: 10.1016/j.vaccine.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Li HY, Tian DY, Han QY, Zhang X, Li N, Qiu HJ. 2011. A novel alphavirus replicon-vectored vaccine delivered by adenovirus induces sterile immunity against classical swine fever. Vaccine 29:8364–8372. doi: 10.1016/j.vaccine.2011.08.085. [DOI] [PubMed] [Google Scholar]
- 9.van Zijl M, Wensvoort G, de Kluyver E, Hulst M, van der Gulden H, Gielkens A, Berns A, Moormann R. 1991. Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J Virol 65:2761–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rümenapf T, Stark R, Meyers G, Thiel H-J. 1991. Structural proteins of hog cholera virus expressed by vaccinia virus: further characterization and induction of protective immunity. J Virol 65:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.König M, Lengsfeld T, Pauly T, Stark R, Thiel H-J. 1995. Classical swine fever virus: independent induction of protective immunity by two structural glycoproteins. J Virol 69:6479–6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beer M, Reimann I, Hoffmann B, Depner K. 2007. Novel marker vaccines against classical swine fever. Vaccine 25:5665–5670. doi: 10.1016/j.vaccine.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Leifer I, Lange E, Reimann I, Blome S, Juanola S, Duran JP, Beer M. 2009. Modified live marker vaccine candidate CP7_E2alf provides early onset of protection against lethal challenge infection with classical swine fever virus after both intramuscular and oral immunization. Vaccine 27:6522–6529. doi: 10.1016/j.vaccine.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 14.König P, Blome S, Gabriel C, Reimann I, Beer M. 2011. Innocuousness and safety of classical swine fever marker vaccine candidate CP7_E2alf in nontarget and target species. Vaccine 30:5–8. doi: 10.1016/j.vaccine.2011.10.064. [DOI] [PubMed] [Google Scholar]
- 15.Blome S, Aebischer A, Lange E, Hofmann M, Leifer I, Loeffen W, Koenen F, Beer M. 2012. Comparative evaluation of live marker vaccine candidates “CP7_E2alf” and “flc11” along with C-strain “Riems” after oral vaccination. Vet Microbiol 158:42–59. doi: 10.1016/j.vetmic.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Blome S, Gabriel C, Schmeiser S, Meyer D, Meindl-Bohmer A, Koenen F, Beer M. 2014. Efficacy of marker vaccine candidate CP7_E2alf against challenge with classical swine fever virus isolates of different genotypes. Vet Microbiol 169:8–17. doi: 10.1016/j.vetmic.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Eblé PL, Geurts Y, Quak S, Moonen-Leusen HW, Blome S, Hofmann MA, Koenen F, Beer M, Loeffen WL. 2013. Efficacy of chimeric Pestivirus vaccine candidates against classical swine fever: protection and DIVA characteristics. Vet Microbiol 162:437–446. doi: 10.1016/j.vetmic.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel C, Blome S, Urniza A, Juanola S, Koenen F, Beer M. 2012. Towards licensing of CP7_E2alf as marker vaccine against classical swine fever-duration of immunity. Vaccine 30:2928–2936. doi: 10.1016/j.vaccine.2012.02.065. [DOI] [PubMed] [Google Scholar]
- 19.Tignon M, Kulcsar G, Haegeman A, Barna T, Fabian K, Levai R, Van der Stede Y, Farsang A, Vrancken R, Belak K, Koenen F. 2010. Classical swine fever: comparison of oronasal immunisation with CP7E2alf marker and C-strain vaccines in domestic pigs. Vet Microbiol 142:59–68. doi: 10.1016/j.vetmic.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 20.European Commission. 2003. Diagnostic techniques and vaccines for foot-and-mouth disease, classical swine fever, avian influenza and some other important OIE list A diseases. Report of the Scientific Committee on Animal Health and Animal Welfare (Adopted 24–25th April 2003), p 1–150. Health and Consumer Protection Directorate-General, European Commission, Brussels, Belgium. [Google Scholar]
- 21.Vijayaraghavan B, Xia H, Harimoorthy R, Liu L, Belá S. 2012. Evaluation of envelope glycoprotein E(rns) of an atypical bovine pestivirus as antigen in a microsphere immunoassay for the detection of antibodies against bovine viral diarrhea virus 1 and atypical bovine Pestivirus. J Virol Methods 185:193–198. doi: 10.1016/j.jviromet.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aebischer A, Müller M, Hofmann MA. 2013. Two newly developed E(rns)-based ELISAs allow the differentiation of classical swine fever virus-infected from marker-vaccinated animals and the discrimination of pestivirus antibodies. Vet Microbiol 161:274–285. doi: 10.1016/j.vetmic.2012.07.046. [DOI] [PubMed] [Google Scholar]
- 23.Moser C, Ruggli N, Tratschin JD, Hofmann MA. 1996. Detection of antibodies against classical swine fever virus in swine sera by indirect ELISA using recombinant envelope glycoprotein E2. Vet Microbiol 51:41–53. doi: 10.1016/0378-1135(96)00019-3. [DOI] [PubMed] [Google Scholar]
- 24.Larska M, Polak MP, Liu L, Alenius S, Uttenthal A. 2013. Comparison of the performance of five different immunoassays to detect specific antibodies against emerging atypical bovine pestivirus. J Virol Methods 187:103–109. doi: 10.1016/j.jviromet.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder S, von Rosen T, Blome S, Loeffen W, Haegeman A, Koenen F, Uttenthal A. 2012. Evaluation of classical swine fever virus antibody detection assays with an emphasis on the differentiation of infected from vaccinated animals. Rev Sci Tech 31:997–1010. [DOI] [PubMed] [Google Scholar]