Abstract
Interleukin-18 (IL-18) is an important cytokine involved in innate and acquired immunity. In this study, we cloned the full-length chicken IL-18 (ChIL-18) gene from specific-pathogen-free (SPF) chicken embryo spleen cells and provided evidence that the ChIL-18 gene in a recombinant plasmid was successfully expressed in chicken DT40 cells. ChIL-18 significantly enhanced gamma interferon (IFN-γ) mRNA expression in chicken splenocytes, which increased IFN-γ-induced nitric oxide (NO) synthesis by macrophages. The potential genetic adjuvant activity of the ChIL-18 plasmid was examined in chickens by coinjecting ChIL-18 plasmid and inactivated Newcastle disease virus (NDV) vaccine. ChIL-18 markedly elevated serum hemagglutination inhibition (HI) titers and anti-hemagglutinin-neuraminidase (anti-HN)-specific antibody levels, induced the secretion of both Th1- (IFN-γ) and Th2- (interleukin-4) type cytokines, promoted the proliferation of T and B lymphocytes, and increased the populations of CD3+ T cells and their subsets, CD3+ CD4+ and CD3+ CD8+ T cells. Furthermore, a virus challenge revealed that ChIL-18 contributed to protection against Newcastle disease virus challenge. Taken together, our data indicate that the coadministration of ChIL-18 plasmid and NDV vaccine induces a strong immune response at both the humoral and cellular levels and that ChIL-18 is a novel immunoadjuvant suitable for NDV vaccination.
INTRODUCTION
Newcastle disease (ND) is a serious avian disease that causes substantial economic loss and remains a major threat to the poultry industry (1, 2). Outbreaks of ND among poultry occur worldwide, and the pathogenic form of the virus is a disease listed in the World Organization for Animal Health (OIE) Terrestrial Animal Health Code and must be reported to the OIE (3), which results in severe trade limitations (4, 5). Currently, vaccination is the major tool for controlling infection by Newcastle disease virus (NDV). The NDV vaccine strains LaSota, B1, Mukteswar, and V4 are used widely in China. However, virulent NDV strains are still frequently isolated in vaccinated birds, indicating that NDV remains an ongoing threat to commercial flocks of birds (6). Therefore, it is necessary to develop more efficacious vaccines to prevent NDV infection.
Many techniques have been developed to increase the immunogenicity of vaccines. Among these, cytokines are effective immunomodulators in animal models or in clinical testing (7–10). Among the large number of cytokines, interleukin-18 (IL-18) is a strong stimulator of T helper type 1 (Th1) responses and activates natural killer (NK) cells, stimulates the synthesis of other immunoactive cytokines from Th1 cells, monocytes, and NK cells, and synergizes with IL-12 in the maturation of Th1 cells and the suppression of IgE synthesis by B cells (11–13). Thus, IL-18 functions as an adjuvant (14, 15). However, depending on the cytokine environment, IL-18 may also promote Th2-type responses (16, 17) and antibody formation (18).
The isolation and characterization of chicken IL-18 (ChIL-18) were reported in 2000 by Schneider et al. (19), and when expressed in Escherichia coli, ChIL-18 induced gamma interferon (IFN-γ) production. Gobel et al. (20) reported that ChIL-18 has many biological effects, including the activation and proliferation of Th cells and the induction of CD4+ T cells to secrete IFN-γ. Hung et al. (21) confirmed that chicken IL-18 recombinant proteins exert in vivo biological functions through stimulating humoral and cell-mediated immunities in order to enhance antigen-specific immunity and vaccine efficacy. Although many studies have shown that recombinant ChIL-18 boosts the immune responses to avian virus vaccines (22–24), few studies have investigated the modulatory effect of using a eukaryotic expression plasmid carrying ChIL-18 as a molecular genetic adjuvant to enhance these vaccines. In this study, we cloned the full-length ChIL-18 gene from specific-pathogen-free (SPF) chicken embryo spleen cells and report on a eukaryotic expression plasmid carrying ChIL-18 as a genetic adjuvant, coadministered with an inactivated NDV vaccine, which induced strong immune responses in chickens at both the humoral and cellular levels.
MATERIALS AND METHODS
Chicken embryos, animals, and vaccine.
Specific-pathogen-free (SPF) Roman chickens and chicken embryos were obtained from the Beijing Experimental Animal Research Center. Chinese standard virulent NDV (strain F48E9, 105.0 50% lethal dose [LD50]/ml) grown in the allantoic cavity of SPF chicken embryos was obtained from the China Institute of Veterinary Drug Control and used as the challenge virus. Four hemagglutination (HA) units of NDV antigen (strain LaSota) were provided by the China Animal Health and Epidemiology Center. NDV LaSota (107.0 50% egg infective dose [EID50]/ml; obtained from Yi Kang Co., Ltd., Liaoning, China) was inoculated into the allantoic cavities of 9-day-old SPF chicken embryos; the embryos that died within 24 h were discarded, and the allantoic fluids were harvested from the infected embryos at 48 h postinfection and inactivated by treatment with 0.2% formalin. The inactivated virus was emulsified with mineral oil to make an oil-formulated inactivated NDV vaccine (LaSota). One dose of this vaccine contained the equivalent of 108.0 EID50 before inactivation by formalin. Procedures and testing associated with the inactivated vaccine were performed according to the OIE Terrestrial Manual 2012 (3).
Plasmids, cells, and reagents.
pMD18-T and E. coli strain JM109 were purchased from the TaKaRa Corporation (Dalian, China), and pcDNA3.1/HisB was obtained from the Invitrogen Corporation (NY, USA). Chicken DT40 cells were purchased from the Jennio Bio Corporation (Guangzhou, China) and maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C with 5% CO2. Concanavalin A (ConA), phorbol-12-myristate-13-acetate (PMA), and 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTT) were purchased from the Jiu Shi Corporation (Zhengzhou, China) and RPMI 1640 culture medium from the Sino-American Corporation (USA).
Construction of a eukaryotic expression plasmid carrying ChIL-18.
The allantoic cavity of each 11-day-old SPF chicken embryo was inoculated with 40 μl of NDV LaSota (107.0 EID50/ml). The embryos that died within 24 h were discarded. The spleens were collected from the infected embryos at 48 h postinfection. Total RNA was extracted from the embryo spleens with TRIzol reagent (Invitrogen, USA). cDNA was synthesized using Moloney murine leukemia virus (MMLV) reverse transcriptase, according to the Invitrogen manual. Reverse transcription-PCR (RT-PCR) primers were designed based on the full-length ChIL-18 gene. The sense primer sequence was 5′-CGG AAT TCA TGA GCT GTG AAG AGA TCG CA-3′, and the antisense sequence was 5′-CAC TCG AGT CAT AGG TTG TGC CTT TCA TTA TG-3′. The PCR consisted of 35 cycles of denaturation at 94°C for 30 s, annealing at 52°C for 40 s, and extension at 72°C for 1 min, with a final extension at 72°C for 10 min. Full-length ChIL-18 amplified PCR products were cloned into the pMD18-T plasmid, named pMD18-T-ChIL-18. Next, pMD18-T-ChIL-18 was digested by EcoRI and XhoI, and the 0.6-kb digested ChIL-18 gene was purified by agarose electrophoresis and mixed with the 5.5-kb pcDNA3.1/HisB vector in a 4:1 ratio; the mixture was incubated overnight at 16°C in the presence of T4 DNA ligase. Next, the ligated product was transformed into competent E. coli cells. Recombinant plasmids were identified by EcoRI and XhoI digestion. The resulting constructed recombinant plasmid was designated pcDNA3.1/His-ChIL-18.
Expression analysis of the recombinant ChIL-18 plasmid in chicken DT40 cells.
The recombinant pcDNA3.1/His-ChIL-18 plasmid in different doses (1.0, 2.5, and 5.0 μg) was transfected into DT40 cells with Lipofectamine 2000 reagent (Invitrogen, USA), according to the manufacturer's instructions. In order to detect the expression of the His-tagged ChIL-18 and endogenous ChIL-18 proteins, Western blotting was performed as previously described (25). Briefly, after 48 h of transfection, the cells were washed and collected from plates in phosphate-buffered saline (PBS) solution, resuspended with 2× SDS sample buffer, and boiled for 5 min. The proteins were then resolved in an 8 to 12% SDS-PAGE gel and transferred to a nitrocellulose membrane. Next, the membrane was blocked with 5% skim milk powder (Jiu Shi Corporation, China) overnight at 4°C. After three washes, the membrane was incubated with mouse anti-His tag monoclonal antibody (1:2,000 dilution; Tiangen, China), rabbit anti-ChIL-18 polyclonal antibody (1:1,500 dilution; Cusabio Biotech, USA), and mouse anti-β-actin monoclonal antibody (1:5,000 dilution; Abcam, United Kingdom) at 37°C for 1 h. After three washes, the membrane was incubated with horseradish peroxidase (HRP)-labeled goat anti-mouse IgG antibody or HRP-labeled goat anti-rabbit IgG antibody (1:500 dilution; Cusabio Biotech) at 37°C for 1 h, followed by enhanced chemiluminescence (ECL) detection (Tiangen).
The His-tagged ChIL-18 protein expressed in DT40 cells was purified on a nickel-chelating agarose affinity column (Amersham Biosciences, USA), according to the manufacturer's instructions. Briefly, DT40 cells were transfected using 10 μg of recombinant pcDNA3.1/His-ChIL-18 plasmid, the cells were collected and incubated with lysis buffer (100 mM KCl, 5 mM MgCl2, 10 mM HEPES, 1 mM dithiothreitol [DTT], and 0.5% NP-40), and the supernatant of the cell lysates was applied to an Ni-affinity chromatography column preequilibrated with buffer A. The proteins were eluted in a gradient of 0.0 to 0.5 M imidazole in buffer A. The eluted proteins were analyzed by SDS-PAGE. The His-tagged ChIL-18 was pooled and dialyzed overnight against a solution containing 50 mM potassium phosphate, 50 mM NaCl, and 50% glycerol (pH 8.0). The protein concentrations of the samples were measured using the Bradford assay and bovine serum albumin as a standard.
Bioassays of ChIL-18 expressed in eukaryotic cells.
A sensitive bioassay for chicken interleukin-18 based on the inducible release of IFN-γ was performed as described previously (21, 26). Briefly, splenocytes were separated from 21-day-old SPF chickens, seeded at a density of 1 × 107 cells/ml in a 24-well plate, treated with 50 μl of different concentrations (25, 50, 100, and 200 ng/ml) of purified His-tagged ChIL-18 protein expressed in DT40 cells or nonstimulated splenocytes as a negative control, and incubated in triplicate per individual animal at 41°C for 24 h. The IFN-γ mRNA expression of pretreated splenocytes was determined using real-time RT-PCR. Total RNAs were isolated using the TRIzol reagent. cDNA was synthesized using MMLV reverse transcriptase, according to the Invitrogen manual. Quantitative PCR (qPCR) was performed using 2× qPCR SYBR green mix (ABgene, Epsom, United Kingdom) with 5 μM primers. The reactions were run on a realplex (Eppendorf, Germany) using a two-step cycling program: 95°C for 15 min, followed by 35 cycles of 95°C for 15 s, 58°C for 30 s, and 70°C for 30 s. A melting curve (55 to 95°C) was generated at the end of each run to verify the specificity. The primers for chicken actin were forward primer, 5′-GAT TTC GAG CAG GAG ATG GCC ACA G-3′, and reverse primer, 5′-GAT CCA CAT CTG CTG GAA GGT GGA C-3′. The primers for chicken IFN-γ were forward primer, 5′-GAC ATC TCC CAG AAG CTA TCT GAG C-3′, and reverse primer, 5′-GAG CAC AGG AGG TCA TAA GAT GC-3′. Meanwhile, the splenocyte supernatants were harvested from the cells treated with the purified His-tagged ChIL-18 protein and then transferred for culturing chicken macrophages to determine the synthesis of IFN-γ-induced nitric oxide (NO). The chicken macrophages (2 × 105/100 μl/well) isolated from 21-day-old SPF chickens were incubated with the splenocyte supernatants, as described above, in a 96-well plate at 37°C and 5% CO2 for 24 h. The NO levels in the culture medium of macrophages were quantified using the Griess assay kit (Promega), according to the manufacturer's instructions.
Immunization of chickens.
All animal experiments were approved by the Henan University of Science and Technology Animal Care and Use Committee. A set of 200 21-day-old SPF Roman chickens was randomly divided into five groups (n = 40 per group) and intramuscularly immunized two times on days 0 and 14 with a mixture of 100 μl of oil-formulated inactivated NDV vaccine (108.0 EID50/0.1 ml LaSota) and (group 5) 100 μl of phosphate-buffered saline (PBS), (group 4) 250 μg of pcDNA3.1/His-ChIL-18, or (group 3) 250 μg pcDNA3.1/HisB. Group 1 was immunized with PBS; for group 2, 250 μg of pcDNA3.1/His-ChIL-18 was used as a control (Table 1).
TABLE 1.
Animal groups and experimental design
| Group | Immunogen | Vaccination on days 0 and 14a |
|---|---|---|
| 1 | PBS | 100 μl PBS |
| 2 | ChIL-18 plasmid | 250 μg pcDNA3.1/His-ChIL-18 |
| 3 | Vaccine plus control plasmid | 108.0 EID50 plus 250 μg pcDNA3.1/HisB |
| 4 | Vaccine plus ChIL-18 plasmid | 108.0 EID50 plus 250 μg pcDNA3.1/His-ChIL-18 |
| 5 | Inactivated NDV vaccineb | 108.0 EID50 plus 100 μl PBS |
All chickens were challenged on day 28.
Inactivated NDV vaccine (strain LaSota, 108.0 EID50/vaccine), prepared with oil/water as an adjuvant.
Chickens (n = 10 per group) were euthanized to collect thymus, bursa of Fabricius (BF), and whole-blood samples at 7 and 21 days after the first immunization. Serum was separated from whole blood for the determination of hemagglutination inhibition (HI) and IgG titers and cytokine assays. Peripheral blood lymphocytes were separated from 10 chickens per group at 28 days after the first vaccination for a flow cytometric assay. Two weeks after the second immunization, each of the remaining 20 chickens was intramuscularly challenged with strong virulent NDV (F48E9) at a dose of 104 LD50 in 1 ml. The chickens were observed daily for clinical signs and mortality within 10 days postchallenge, and the results were recorded. Cloacal swabs were collected from the dead and infected birds, and NDV was isolated from the cloacal swabs and detected by RT-PCR. Briefly, the cloacal swabs were placed in PBS solution (pH 7.0) containing penicillin (10,000 units/ml), and the suspensions were incubated for 2 h, centrifuged at 1,000 × g for 10 min at 25°C, and inoculated in 0.2 ml into the allantoic cavity of 11-day-old SPF chicken embryos. The allantoic fluids were collected form the inoculated embryos 48 h after inoculation to detect the NDV hemagglutinin-neuraminidase (HN) gene RNA. The RT-PCR protocol was performed as described above. The RT-PCR primers were designed based on the NDV HN gene. The sense primer sequence was 5′-GAA TCC ATG GAC CGC GTG GTT AAC-3′ and the antisense sequence was 5′-CTC GAG CGT CTT CCC AAC CAT CCT-3′. The details of the animal experiment time points are shown in Fig. 1.
FIG 1.
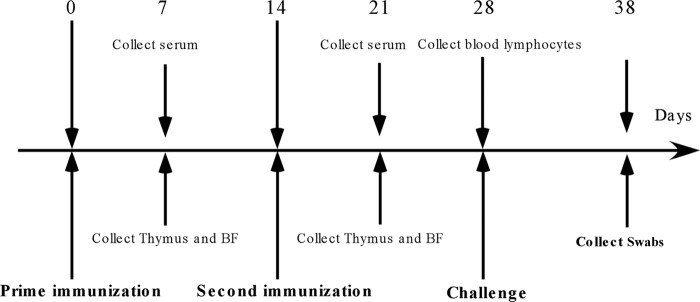
Experimental scheme of immunization, sample collection, and challenge. Numbers represent the days with respect to vaccination.
Hemagglutination inhibition assay.
Serum samples were collected for the HI test, as described by the OIE (3). Briefly, serum was inactivated by incubation for 30 min at 56°C, transferred in duplicate to 96-well round-bottom plates, and serially diluted 2-fold in PBS. Next, 4 units of NDV (LaSota) antigen was added to each well in a volume of 50 μl. The content of each well was gently mixed with a multichannel pipette, and the plates were incubated for 60 min at room temperature. Finally, 50 μl of a 0.5% SPF chicken erythrocyte suspension in PBS was added to each well, and hemagglutination was allowed to proceed for 1 h at room temperature. The HI titer was defined as the highest serum dilution capable of preventing hemagglutination.
Detection of antigen-specific antibodies.
Specific anti-hemagglutinin-neuraminidase (HN) IgG from chicken serum samples was analyzed by enzyme-linked immunosorbent assay (ELISA). The ELISA plates were coated with 10 μg/ml recombinant NDV-HN protein (expressed in Pichia pastoris and kept in our lab). The plates were blocked with 1% bovine serum albumin (BSA) for 2 h at 37°C. Sera that were serially diluted at 1-log intervals (1:10 to 1:105) were then incubated on plates for 2 h at 37°C. Next, the plates were incubated for 1 h at 37°C in 50 μl of a 1:1,000 dilution of horseradish peroxidase (HRP)-conjugated goat anti-chicken IgG (GenScript Co., Ltd., China). Finally, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added, the reaction was stopped by adding 2 N H2SO4, and the absorbance at 450 nm (A450) was read. Each serum sample was assayed in triplicate. The results were plotted as the optical density (OD) versus dilution (log scale). The titers at half-maximal optical density were calculated using the logarithm of the titer values (27).
Th1-type and Th2-type cytokine assays.
Levels of Th1-type cytokines (IFN-γ) and Th2-type cytokines (IL-4) in serum samples from chickens were determined using commercial chicken cytokine gamma interferon (IFN-γ) and interleukin-4 (IL-4) ELISA kits (Cusabio Biotech, USA). The procedures were performed according to the manufacturer's instructions. Briefly, the reagents, serum samples, and standards were prepared as per the instructions. A blank well without any solution was set, and 50 μl of standard and serum sample was added per well. Next, 50 μl of HRP conjugate (1×) was added to each well and incubated for 30 min at 37°C. This was then aspirated and washed 5 times with wash buffer (200 μl). Ninety microliters of TMB substrate was added to each well and incubated for 20 min at 37°C, protected from light. Fifty microliters of stop solution was added to each well, and the absorbance at 450 nm (A450) was read.
Lymphocyte proliferation response and immunophenotyping assay.
The lymphocyte proliferation response was determined as previously described (28, 29). Briefly, thymus and bursa of Fabricius (BF) samples were collected from immunized chickens at 7 and 21 days after the first immunization. The thymus and BF samples were processed to make single-cell suspensions. The thymus lymphocyte cell suspension (1 × 107 cells/ml) was seeded in a 96-well plate and treated with 50 μl of ConA (40 μg/ml) at 40°C and 5% CO2 for 48 h, and then the plate was incubated with 10 μl of 5 mg/ml MTT for 3 h. Finally, 100 μl of 10% (wt/vol) SDS in 0.01 M HCl was added to the plate and allowed to incubate for 2 h. A spectrophotometric measurement was taken at 570 nm. For the BF lymphocyte proliferation response, BF lymphocyte cell suspensions (1 × 107 cells/ml) were incubated with 50 μl of PMA (1 μg/ml) in a 96-well plate at 40°C and 5% CO2 for 24 h. Finally, 100 μl of 10% (wt/vol) SDS in 0.01 M HCl was added and allowed to incubate for a further 2 h before a spectrophotometric measurement was taken at 570 nm.
The flow cytometric assay with the peripheral blood lymphocytes was performed as previously described (30). Peripheral blood lymphocytes (2 × 106 cells/ml) formed a complex with monoclonal antibody phycoerythrin (PE)-labeled anti-chicken CD3+ cells and then were incubated with PE-labeled anti-chicken CD4+ cells and fluorescein isothiocyanate (FITC)-conjugated anti-chicken CD8+ cells (SouthernBiotech) for 1 h at 4°C. PE- and FITC-conjugated isotype antibodies were used as controls. The cells were analyzed by fluorescence-activated cell sorting (BD Biosciences, USA).
Statistical analysis.
Statistical analyses were performed using unpaired t tests or one-way analysis of variance (ANOVA) F-statistics, followed by use of the GraphPad Prism 5 software. The data are presented as the mean ± standard deviation (SD). Tukey's multiple comparison tests were used to assess differences among the five experimental groups, with differences considered significant at a P value of <0.05 or <0.01.
RESULTS
Construction and expression of a full-length ChIL-18 expression plasmid.
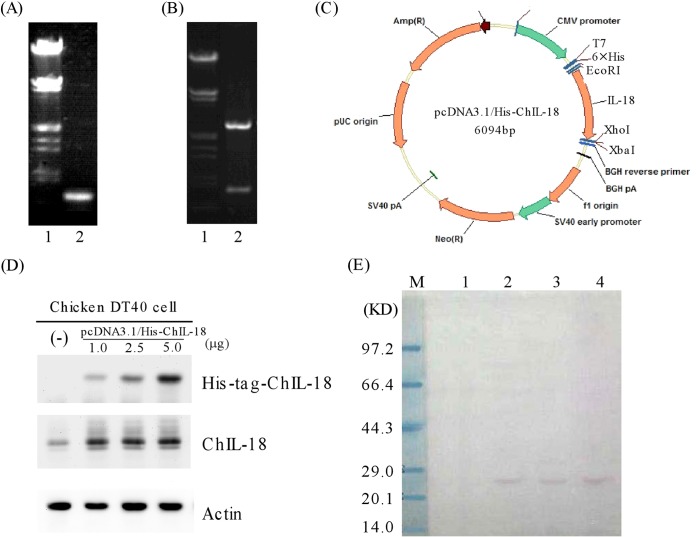
The full-length ChIL-18 PCR product produced a 0.6-kb fragment (Fig. 2A). Sequencing analysis confirmed that the full-length ChIL-18 gene contained 594 nucleotides that encoded a 198-amino-acid protein. The gene sequence was identical to the published ChIL-18 sequence (20), except at one base position, while the predicted amino acid sequences were identical. Compared with the previously published mature ChIL-18 gene sequence (19), the sequence of the mature gene (bp 88 to 594, 169 amino acids) differed at position 557 (C to T), which also generated an amino acid mutation (Ser to Phe). Next, the full-length ChIL-18 gene (594 bp) was digested by EcoRI and XhoI and ligated into the pcDNA3.1/HisB plasmid. The length of the recombinant plasmid was about 6.1 kb, which resolved into two fragments of 5.5 kb and 0.6 kb when digested with EcoRI and XhoI, respectively, as expected (Fig. 2B). The resulting recombinant plasmid was designated pcDNA3.1/His-ChIL-18 (Fig. 2C).
FIG 2.
Construction and expression of a full-length ChIL-18 expression plasmid. (A) Lane 1, marker λDNA/EcoRI+HindIII; lane 2, PCR products of the full-length ChIL-18 gene. (B) Lane 1, marker λDNA/EcoRI+HindIII; lane 2, identification of pcDNA3.1/His-ChIL-18 recombinant plasmid digested with EcoRI and XhoI. (C) Structure of the pcDHA3.1/His-ChIL-18 recombinant plasmid. (D) Western blots were prepared with extracts from DT40 cells with untransfected (−) or transfected recombinant pcDNA3.1/His-ChIL-18 plasmid with different doses (1.0, 2.5, and 5.0 μg) for 48 h. The blots were then probed with antibodies against His tag, ChIL-18, and actin. (E) Western blot analysis of purified His-tagged ChIL-18 protein expressed in DT40 cells. Lane M, protein size marker; lane 1, empty expression plasmid pcDNA3.1/His, negative control; lanes 2 to 4, purified His-tagged ChIL-18 protein collection fractions by Ni-affinity chromatography.
To test if the recombinant pcDNA3.1/His-ChIL-18 plasmid worked, the expression analysis of the plasmid was performed in chicken DT40 cells. The recombinant plasmid was transfected into DT40 cells. The recombinant plasmid resulted in the expression of the ChIL-18 fusion protein with the His tag. In order to avoid the influence of the chicken DT40 cell endogenous IL-18 protein on the analysis, anti-His tag monoclonal antibody was used for the Western blotting analysis. The level of actin was measured as a loading control. We found that the ChIL-18 protein was specifically recognized by the anti-His tag monoclonal antibody (Fig. 2D), whereas no band was detected from the cells transfected with pcDNA3.1/His control plasmid. Furthermore, the recombinant plasmid was expressed in DT40 cells in a dose-dependent manner (Fig. 2D, compare lane 2 with lanes 3 and 4). His-tagged ChIL-18 protein was purified from transfected DT40 cells by Ni-affinity chromatography. The purified ChIL-18 protein has a major band with an Mr of approximately 25,000 and a specific probe by the anti-His tag monoclonal antibody (Fig. 2E). Together, the results provided evidence that the ChIL-18 gene in a recombinant plasmid was successfully expressed in chicken DT40 cells.
Bioassays of ChIL-18 expressed in eukaryotic cells.
To further evaluate bioassays of ChIL-18 expressed in eukaryotic cells, a sensitive bioassay for chicken interleukin-18 based on the inducible release of IFN-γ was performed. We found that the IFN-γ mRNA levels in splenocytes were significantly enhanced by stimulation with purified His-tagged ChIL-18 protein expressed in eukaryotic DT40 cells (Fig. 3A). Moreover, significantly elevated IFN-γ-induced NO production by macrophages was observed in those cultured with purified His-tagged ChIL-18 protein pretreated splenocyte supernatants (Fig. 3B). Therefore, this result indicates that the ChIL-18 protein expressed in eukaryotic DT40 cells significantly enhanced IFN-γ mRNA expression in chicken splenocytes and IFN-γ-induced NO production by macrophages.
FIG 3.
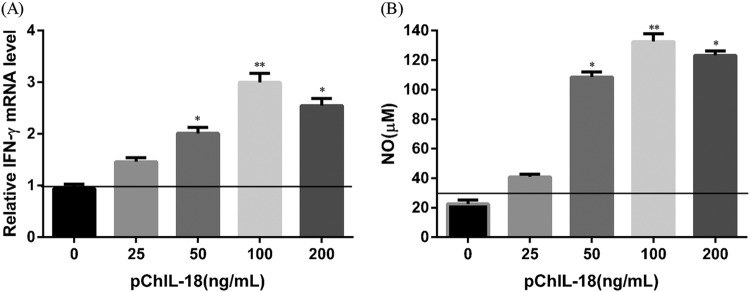
Bioassays of ChIL-18 expressed in eukaryotic cells. (A) Relative IFN-γ transcript level in splenocytes stimulated with recombinant chicken IL-18. Splenocytes (1 × 107 cells/ml) isolated from SPF chickens were cultured with 50 μl of different concentrations (25, 50, 100, and 200 ng/ml) of purified His-tagged ChIL-18 protein expressed in DT40 cells, or nonstimulated splenocytes as a negative control. Quantitative RT-PCR was performed with the total RNAs purified from the stimulated splenocytes. The level of IFN-γ transcripts was normalized to the actin transcript level. The experiment was performed in triplicate. (B) IFN-γ-induced nitric oxide (NO) synthesis by macrophages. Chicken macrophages (2 × 105/100 μl/well) were isolated from SPF chickens and cultured with the medium collected from splenocyte-culturing supernatants pretreated with purified His-tagged ChIL-18 protein expressed in DT40 cells at 37°C and 5% CO2 for 24 h in triplicate. The error bars indicate the SD. *, P < 0.05, and **, P < 0.01, compared with nonstimulated splenocytes or macrophages.
ChIL-18 stimulates significant antigen-specific immune responses.
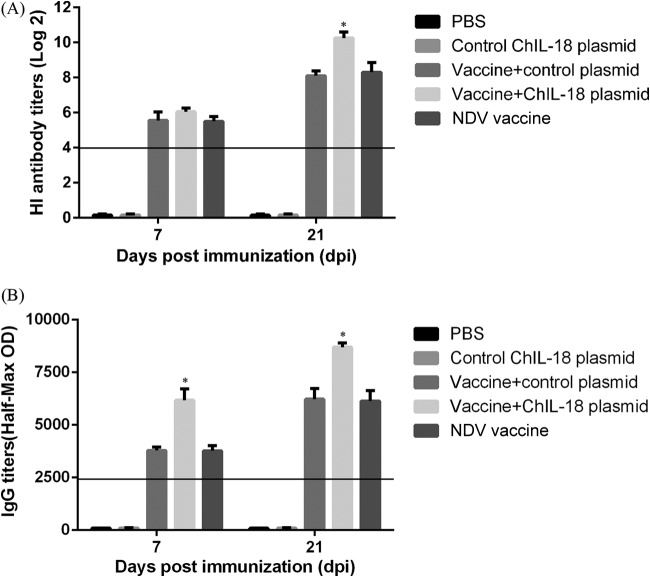
To determine the antigen-specific immune responses to immunization, serum samples from immunized chickens were taken on days 7 and 21 after the first immunization and tested for HI and anti-HN antibody titers. The HI antibody titers were significantly induced in the three groups of chickens immunized with the vaccine after the first immunization. The HI antibody titers of chickens immunized with the vaccine combined with ChIL-18 plasmid did not increase significantly (P > 0.05) compared to those in the chickens immunized with the vaccine only at day 7. However, at day 21, the HI antibody titers of the chickens immunized with the vaccine combined with ChIL-18 plasmid were significantly higher than those in the other groups (P < 0.05) (Fig. 4A). Anti-HN IgG antibodies were consistently observed in the three NDV vaccine-immunized groups on day 7 after the first immunization, and the antibody levels increased following the second vaccination. IL-18 enhanced the IgG antibody secretion levels, and the effect was greater than that induced by the NDV vaccine only (Fig. 4B). These data suggest that ChIL-18 stimulates significant antigen-specific immune responses.
FIG 4.
Effect of ChIL-18 to NDV vaccination on antigen-specific HI titers and anti-HN IgG antibodies. Chicken serum samples were collected on days 7 and 21 postimmunization, and the serum HI titers (A) and IgG titers (B) were analyzed by HI assay and ELISA, respectively. The data presented are the means ± SD of the results from 10 separate experiments. *, P < 0.05 compared with chickens immunized with the inactivated NDV vaccine.
ChIL-18 increases the production of both Th1- and Th2-type cytokines.
We next examined the levels of Th1 (IFN-γ) and Th2 (IL-4) cytokines from chickens after coimmunization with the NDV vaccine and ChIL-18 plasmid. IFN-γ secretion was significantly increased after vaccination with ChIL-18 at day 7, and the highest level of IFN-γ secretion was observed in the immunization group with the NDV vaccine and ChIL-18 plasmid at day 21. The levels of IFN-γ secretion in the ChIL-18 control group were significantly higher those than in the vaccine-only group (P < 0.01) (Fig. 5A). Although the levels of IL-4 secretion in the ChIL-18 control groups did not differ significantly from those in the vaccine-only group (P > 0.05), the IL-4 secretion levels in the vaccine-plus-ChIL-18 plasmid group were significantly higher than those in the other groups (P < 0.05) (Fig. 5B). These results indicate that ChIL-18 improved the secretion of both the Th1 and Th2 cytokines.
FIG 5.
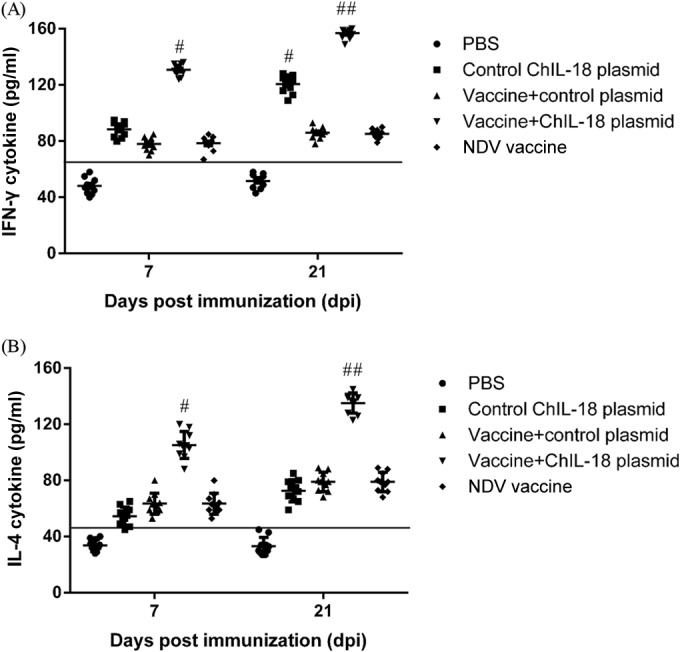
Effect of ChIL-18 to NDV vaccination on Th1/Th2 cytokine production in chicken serum samples. The chickens were immunized two times, and chicken serum samples were collected on days 7 and 21 postimmunization. Cytokine release was measured using commercial chicken cytokine gamma interferon (IFN-γ) and interleukin 4 (IL-4) ELISA kits. The data presented are the means ± SD of the results from 10 separate experiments. #, P < 0.05, and ##, P < 0.01, compared to chickens immunized with the inactivated NDV vaccine.
ChIL-18 promotes T- and B-lymphocyte proliferation responses.
To further determine the effect of ChIL-18 on cellular and humoral immunity, T- and B-lymphocyte proliferation experiments were performed. The T-lymphocyte proliferation responses of chickens immunized with the vaccine plus ChIL-18 were enhanced at 7 days and 21 days after the first immunization compared with those in chickens immunized with the inactivated NDV vaccine (Fig. 6A). The B-lymphocyte proliferation responses were similar to the T-lymphocyte responses (Fig. 6B). Interestingly, the chickens immunized with the ChIL-18 plasmid only also produced significantly higher B-lymphocyte proliferation responses than did the PBS group. Although the proliferation of B lymphocytes of chickens immunized with the ChIL-18 plasmid only was not significantly increased compared with that in the chickens immunized with the vaccine only, the responses of the chickens immunized with the vaccine plus ChIL-18 were significantly higher than those for the inactivated NDV vaccine groups (P < 0.01). These data indicate that the presence of the ChIL-18 plasmid promoted T- and B-lymphocyte-proliferative responses.
FIG 6.
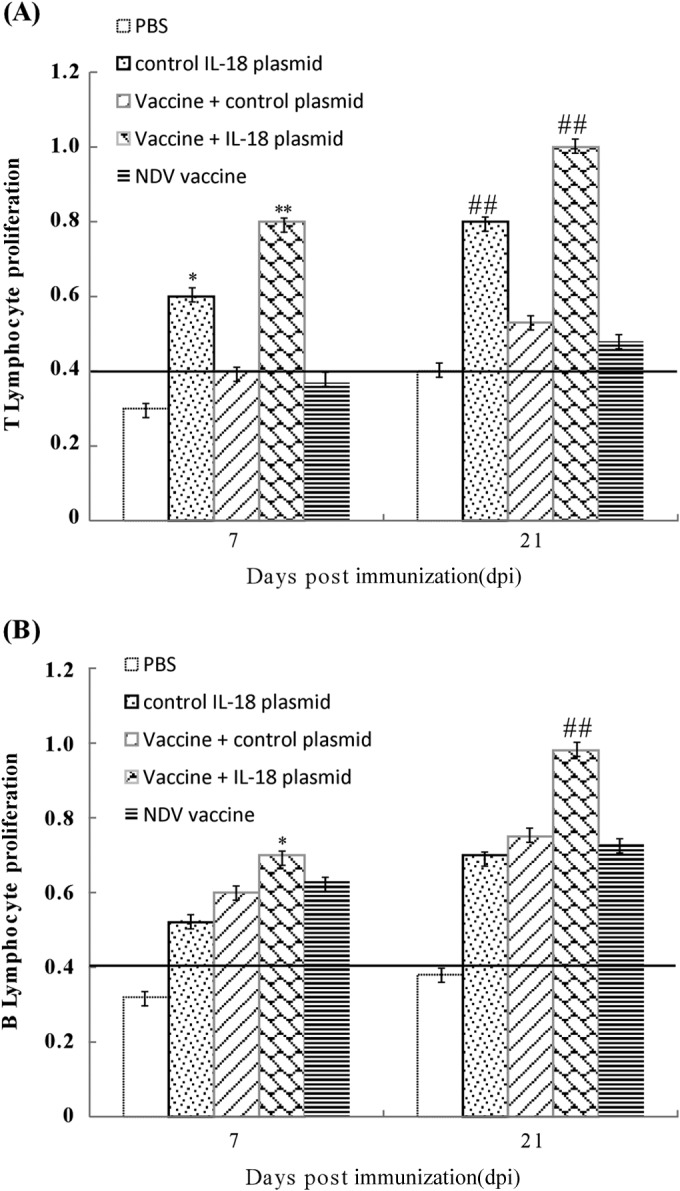
ChIL-18 significantly stimulates chicken peripheral T- and B-lymphocyte proliferation. Chickens were immunized two times, and chicken thymus and bursa of Fabricius samples were collected on days 7 and 21 postimmunization. The T- and B-lymphocyte-proliferative responses were evaluated by MTT assay. The data are the means ± SD of the results from 10 separate experiments. *, P < 0.05, and **, P < 0.01 (day 7); #, P < 0.05, and ##, P < 0.01 (boost), compared to chickens immunized with the inactivated NDV vaccine.
ChIL-18 stimulates CD4+ and CD8+ T cells.
At 28 days after the first immunization, chicken peripheral blood lymphocytes were separated, and the percentages of T-cell subsets were observed by flow cytometry. The percentages of total CD3+ T cells and their subsets (CD4+ T cells [CD3+ CD4+] and CD8+ T cells [CD3+ CD8+]) in the peripheral blood lymphocyte populations were significantly increased in chickens immunized with the vaccine plus ChIL-18 compared with those in the chickens immunized with the vaccine only (Table 2). These data suggest that ChIL-18 stimulated both CD4+ and CD8+ T cells.
TABLE 2.
Flow cytometric analysis of CD3+ T cells and CD3+ CD4+ and CD3+ CD8+ T-cell subsets from peripheral blood lymphocytes of immunized chickensa
| Treatment | % of peripheral blood lymphocytes in T cells with: |
||
|---|---|---|---|
| CD3+ | CD3+ CD4+ CD8− | CD3+ CD8+ CD4− | |
| PBS | 32.1 ± 2.12 | 14.5 ± 1.09 | 8.5 ± 1.56 |
| ChIL-18 plasmid | 49.3 ± 1.38b | 21.7 ± 1.85b | 17.2 ± 2.16b |
| Vaccine plus control plasmid | 41.5 ± 1.52 | 14.9 ± 1.75 | 13.2 ± 1.65 |
| Vaccine plus ChIL-18 plasmid | 64.5 ± 2.17c | 31.5 ± 2.23c | 24.6 ± 1.62c |
| NDV vaccine | 40.3 ± 1.39 | 15.2 ± 2.05 | 12.3 ± 2.21 |
Chickens (n = 10) were euthanized on day 28 after the first immunization, and peripheral blood lymphocytes were collected for immunophenotyping. The data presented are means ± SD.
P < 0.05 compared with chickens immunized with the inactivated NDV vaccine.
P < 0.01 compared with chickens immunized with the inactivated NDV vaccine.
ChIL-18 significantly promotes immune protection against NDV challenge.
To evaluate whether ChIL-18 promotes immune protection against NDV infection, 2 weeks after the second vaccination, each of the remaining 20 chickens was intramuscularly challenged with strong virulent NDV (F48E9) at a dose of 104 LD50. The chickens were observed daily for clinical signs and mortality within 10 days postchallenge, and the results were recorded. In order to determine NDV infection, cloacal swabs were collected from dead and infected birds, and NDV was isolated from the cloacal swabs and detected by RT-PCR. Based on positive cloacal swab virus isolation test results, dead birds and birds with clinical signs were determined to be infected birds. We found that all chickens immunized with PBS or ChIL-18 control plasmid were infected with the neuropathological clinical syndromes of depression, paralysis, standing instability, cough and asthma, and neck twist, and all died within 7 days postchallenge. Five chickens immunized with the NDV vaccine or NDV vaccine plus control plasmid were infected within 10 days, and five of these died, whereas the chickens immunized with the vaccine plus ChIL-18 plasmid did not show any clinical symptoms and did not die after 10 days postchallenge (Table 3). All cloacal swabs collected from dead birds and birds with clinical signs based on virus isolation test results had a positive rate of 100% (50/50). These data indicate that ChIL-18 significantly promoted immune protection against NDV challenge.
TABLE 3.
Protective efficacy against a virulent NDV in vaccinated chickensa
| Treatment | Morbidity and mortality results |
||
|---|---|---|---|
| No. infected (n = 20)b | No. that died (n = 20) | Protection rate (%)c | |
| PBS | 20 | 20 | 0 |
| Control IL-18 plasmid | 20 | 20 | 0 |
| Vaccine plus control plasmid | 5 | 5 | 75 |
| Vaccine plus IL-18 plasmid | 0 | 0 | 100d |
| NDV vaccine | 5 | 5 | 75 |
Two weeks after the second vaccination, each of the remaining 20 chickens was intramuscularly challenged with strong virulent NDV (strain F48E9). The chickens were recorded daily for clinical signs and mortality over the next 10 days.
Number of chickens infected as defined by clinical signs.
Percentage of protection rate at 10 days postchallenge, calculated as the number of uninfected chickens/total number of chickens. All cloacal swabs collected from dead birds and birds with clinical signs based on virus isolation test results had a positive rate of 50/50 (100%).
P < 0.05 compared to chickens immunized with the NDV vaccine.
DISCUSSION
In this study, we cloned the full-length ChIL-18 gene from SPF chicken embryo spleen cells. A sequence analysis demonstrated that the full-length gene is 594 bp in length and encodes a 198-amino-acid protein, which is identical to the sequence published by Schneider et al. (19). The homologies between the nucleotide and amino acid sequences were 99.8% and 100%, respectively. There was only one difference between the gene fragment sequence encoding the mature protein and the gene sequence of white Laihang chickens published by Wang et al. (identification rate, 99.8%) (31). The mutation of C to T was present at nucleotide (nt) 557, and this change in nucleotide sequence caused a mutant amino acid sequence (Ser to Phe; identification rate, 99.4%). These data indicate that the sequences of ChIL-18 cDNA from different chicken species were highly homologous.
Many studies have shown that ChIL-18 is expressed in non-chicken-derived cells (21, 32). However, there are no documents showing whether ChIL-18 plasmid expresses IL-18 in chicken cells. To address this, the expression analysis of the recombinant pcDNA3.1/His-ChIL-18 plasmid was performed in chicken DT40 cells. The DT40 chicken B-cell line is derived from an avian leukosis virus (ALV)-induced bursal lymphoma (33). Moreover, it is arrested at a bursal stem cell stage of differentiation, suggesting it may be best characterized as being a bursal pre-B-cell line (34). We found that the ChIL-18 protein was specifically recognized by the anti-His tag monoclonal antibody in a dose-dependent manner. The purified ChIL-18 protein has a major band with an Mr of approximately 25,000 and a specific probe by the anti-His Tag monoclonal antibody. Therefore, this paper provides evidence that the ChIL-18 gene in a recombinant plasmid was successfully expressed in chicken DT40 cells. To further evaluate the bioassays of the full-length ChIL-18 gene expressed in eukaryotic cells, a sensitive in vitro bioassay for ChIL-18 based on the inducible release of IFN-γ was performed. We found that the ChIL-18 protein expressed in eukaryotic DT40 cells significantly enhanced IFN-γ mRNA expression in chicken splenocytes, which increased IFN-γ-induced nitric oxide (NO) synthesis by macrophages. These results show that the amino acid mutation of Ser to Phe in the ChIL-18 protein does not modify the activity of IL-18.
Cytokines play an important role in the development of the immune system and in the response to infection (35). IL-18 is a strong IFN-γ-inducing factor that enhances immune responses when used as an adjuvant. The concept of using IL-18 to boost immune responses after vaccination has been investigated for chicken vaccines (23, 24, 32, 36–38). Hung et al. (21) described that the full-length and mature chicken IL-18 purified recombinant protein effectively enhanced cell-mediated and humoral immunity. However, the short half-lives of recombinant cytokines and the side effects caused by repetitive administration are major disadvantages for their use (39). Previous reports showed that the direct injection of cytokine genes into muscles might modulate immune responses (18, 40, 41). Here, we report a eukaryotic expression plasmid carrying ChIL-18 in order to investigate its immunogenic potential as a genetic adjuvant for inducing immune responses in chickens. Upon NDV vaccination, the ChIL-18 plasmid stimulated significant antigen-specific immune responses. The level of HI antibodies present at 7 days following the coinoculation of ChIL-18 eukaryotic expression plasmid and NDV vaccine was higher than that induced by inoculation with the vaccine only, and this enhancement was still detectable up to 21 days postinoculation. Moreover, ChIL-18 promoted T- and B-lymphocyte proliferation responses, which were higher than those in the group inoculated with vaccine only on days 7 and 21 after the first vaccination. These data indicate that ChIL-18 induces a strong immune response at both the humoral and the cellular level.
This study also assessed the distributions and ratios of CD4+ (CD3+ CD4+) and CD8+ (CD3+ CD8+) T-cell subsets. We found that ChIL-18 increased CD3+ T-cell populations, particularly CD4+ and CD8+ T cells. CD4+ T cells can be subdivided into Th1 and Th2 cells. Th1 cells mainly produce IFN-γ, which is critical to cell-mediated immunity, whereas Th2 cells mainly produce IL-4, which enhances antibody production and improves humoral immunity. IFN-γ secreted by Th1 cells can inhibit Th2 cell activity, whereas IL-4 secreted by Th2 cells can block the proliferation of Th1 cells (42, 43). However, upon exiting the thymus, naive T cells can secrete both Th1 and Th2 cytokines; these are termed Th0 cells, and their cytokine patterns become fixed as either Th1 or Th2 (44). IL-18 generally induces Th1 responses that enhance the production of cytokines such as IFN-γ (20). Here, we also examined the levels of Th1 (IFN-γ) and Th2 (IL-4) cytokines in chickens following coimmunization with NDV vaccine and ChIL-18 plasmid. Interestingly, besides its effect on IFN-γ production, the fact that ChIL-18 also promoted the production of IL-4 suggests a variation in Th2 response; this study suggests that ChIL-18 may induce a mixed Th1/Th2 response resembling a Th0 response. Consistent with this, recent studies demonstrated that IL-18 also promotes Th2-type responses and increases dendritic cell numbers in mouse lymph nodes (45). Furthermore, Szeto, Gillespie, and Mathieson (46) demonstrated that IL-18 potentially induced IgG1, IgE, and Th2 cytokine (IL-4, IL-5, and IL-10) production in murine experimental models. The detailed mechanisms by which ChIL-18 enhances innate immunity are not well understood, suggesting the need for future studies to better understand the adjuvant activity of ChIL-18.
To further evaluate the influence of ChIL-18 plasmid as an adjuvant for the immune protection provided by NDV vaccine against NDV infection, chickens were intramuscularly challenged with strong virulent NDV (F48E9) on day 28 postimmunization. At 10 days postchallenge, the protective rate of the group receiving NDV only was 75%, whereas the protective rate of chickens immunized with the vaccine plus ChIL-18 plasmid (100%) was significantly increased compared to that for chickens immunized with the vaccine only (P < 0.05). Thus, ChIL-18 significantly promotes immune protection against NDV challenge.
In summary, this study indicates that the coadministration of ChIL-18 plasmid and NDV vaccine induces a strong immune response at both the humoral and cellular levels and suggests that ChIL-18 is a novel immunoadjuvant suitable for use in the NDV vaccine.
ACKNOWLEDGMENTS
This work was supported by grants 31101792 and 31201928 from the National Natural Science Foundation of China and grant 2012GGJS-077 from the Foundation for University Key Teacher by Higher Education of Henan Province.
We thank Edanz Editing Ltd. for editing the manuscript.
We declare no conflicts of interest.
REFERENCES
- 1.Awan MA, Otte MJ, James AD. 1994. The epidemiology of Newcastle disease in rural poultry: a review. Avian Pathol 23:405–423. doi: 10.1080/03079459408419012. [DOI] [PubMed] [Google Scholar]
- 2.Mayo MA. 2002. A summary of taxonomic changes recently approved by ICTV. Arch Virol 147:1655–1663. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- 3.OIE. 2012. Manual of diagnostic tests and vaccines for terrestrial animals. Office International des Epizooties, Paris, France: http://www.oie.int/international-standard-setting/terrestrial-manual/. [Google Scholar]
- 4.Alexander DJ, Aldous EW, Fuller CM. 2012. The long view: a selective review of 40 years of Newcastle disease research. Avian Pathol 41:329–335. doi: 10.1080/03079457.2012.697991. [DOI] [PubMed] [Google Scholar]
- 5.Cattoli G, Susta L, Terregino C, Brown C. 2011. Newcastle disease: a review of field recognition and current methods of laboratory detection. J Vet Diagn Invest 23:637–656. doi: 10.1177/1040638711407887. [DOI] [PubMed] [Google Scholar]
- 6.Liang R, Cao DJ, Li JQ, Chen J, Guo X, Zhuang FF, Duan MX. 2002. Newcastle disease outbreaks in western China were caused by the genotypes VIIa and VIII. Vet Microbiol 87:193–203. doi: 10.1016/S0378-1135(02)00050-0. [DOI] [PubMed] [Google Scholar]
- 7.Hughes HP, Babiuk LA. 1992. The adjuvant potential of cytokines. Biotechnol Ther 3:101–117. [PubMed] [Google Scholar]
- 8.Matsui M, Moriya O, Belladonna ML, Kamiya S, Lemonnier FA, Yoshimoto T, Akatsuka T. 2004. Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice. J Virol 78:9093–9104. doi: 10.1128/JVI.78.17.9093-9104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noll A, Autenrieth IB. 1996. Immunity against Yersinia enterocolitica by vaccination with Yersinia HSP60 immunostimulating complexes or Yersinia HSP60 plus interleukin-12. Infect Immun 64:2955–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tovey MG, Lallemand C. 2010. Adjuvant activity of cytokines. Methods Mol Biol 626:287–309. doi: 10.1007/978-1-60761-585-9_19. [DOI] [PubMed] [Google Scholar]
- 11.Fukao T, Matsuda S, Koyasu S. 2000. Synergistic effects of IL-4 and IL-18 on IL-12-dependent IFN-gamma production by dendritic cells. J Immunol 164:64–71. doi: 10.4049/jimmunol.164.1.64. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto T, Okamura H, Tagawa YI, Iwakura Y, Nakanishi K. 1997. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-γ production from activated B cells. Proc Natl Acad Sci U S A 94:3948–3953. doi: 10.1073/pnas.94.8.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimoto T, Takeda K, Tanaka T, Ohkusu K, Kashiwamura S, Okamura H, Akira S, Nakanishi K. 1998. IL-12 up-regulates IL-18 receptor expression on T cells, Th1 cells, and B cells: synergism with IL-18 for IFN-gamma production. J Immunol 161:3400–3407. [PubMed] [Google Scholar]
- 14.Eberl M, Beck E, Coulson PS, Okamura H, Wilson RA, Mountford AP. 2000. IL-18 potentiates the adjuvant properties of IL-12 in the induction of a strong Th1 type immune response against a recombinant antigen. Vaccine 18:2002–2008. doi: 10.1016/S0264-410X(99)00532-0. [DOI] [PubMed] [Google Scholar]
- 15.Hansen G, Yeung VP, Berry G, Umetsu DT, DeKruyff RH. 2000. Vaccination with heat-killed Listeria as adjuvant reverses established allergen-induced airway hyperreactivity and inflammation: role of CD8+ T cells and IL-18. J Immunol 164:223–230. doi: 10.4049/jimmunol.164.1.223. [DOI] [PubMed] [Google Scholar]
- 16.Smeltz RB, Chen J, Hu-Li J, Shevach EM. 2001. Regulation of interleukin (IL)-18 receptor alpha chain expression on CD4(+) T cells during T helper (Th)1/Th2 differentiation. Critical downregulatory role of IL-4. J Exp Med 194:143–153. doi: 10.1084/jem.194.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leite-De-Moraes MC, Hameg A, Pacilio M, Koezuka Y, Taniguchi M, Van Kaer L, Schneider E, Dy M, Herbelin A. 2001. IL-18 enhances IL-4 production by ligand-activated NKT lymphocytes: a pro-Th2 effect of IL-18 exerted through NKT cells. J Immunol 166:945–951. doi: 10.4049/jimmunol.166.2.945. [DOI] [PubMed] [Google Scholar]
- 18.Kim JJ, Nottingham LK, Tsai A, Lee DJ, Maguire HC, Oh J, Dentchev T, Manson KH, Wyand MS, Agadjanyan MG, Ugen KE, Weiner DB. 1999. Antigen-specific humoral and cellular immune responses can be modulated in rhesus macaques through the use of IFN-gamma, IL-12, or IL-18 gene adjuvants. J Med Primatol 28:214–223. doi: 10.1111/j.1600-0684.1999.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 19.Schneider K, Puehler F, Baeuerle D, Elvers S, Staeheli P, Kaspers B, Weining KC. 2000. cDNA cloning of biologically active chicken interleukin-18. J Interferon Cytokine Res 20:879–883. doi: 10.1089/10799900050163244. [DOI] [PubMed] [Google Scholar]
- 20.Gobel TW, Schneider K, Schaerer B, Mejri I, Puehler F, Weigend S, Staeheli P, Kaspers B. 2003. IL-18 stimulates the proliferation and IFN-gamma release of CD4+ T cells in the chicken: conservation of a Th1-like system in a nonmammalian species. J Immunol 171:1809–1815. doi: 10.4049/jimmunol.171.4.1809. [DOI] [PubMed] [Google Scholar]
- 21.Hung LH, Li HP, Lien YY, Wu ML, Chaung HC. 2010. Adjuvant effects of chicken interleukin-18 in avian Newcastle disease vaccine. Vaccine 28:1148–1155. doi: 10.1016/j.vaccine.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 22.Degen WG, van Zuilekom HI, Scholtes NC, van Daal N, Schijns VE. 2005. Potentiation of humoral immune responses to vaccine antigens by recombinant chicken IL-18 (rChIL-18). Vaccine 23:4212–4218. doi: 10.1016/j.vaccine.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Chen HY, Yang MF, Cui BA, Cui P, Sheng M, Chen G, Wang SJ, Geng JW. 2010. Construction and immunogenicity of a recombinant fowlpox vaccine coexpressing S1 glycoprotein of infectious bronchitis virus and chicken IL-18. Vaccine 28:8112–8119. doi: 10.1016/j.vaccine.2010.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su BS, Shen PC, Hung LH, Huang JP, Yin HS, Lee LH. 2011. Potentiation of cell-mediated immune responses against recombinant HN protein of Newcastle disease virus by recombinant chicken IL-18. Vet Immunol Immunopathol 141:283–292. doi: 10.1016/j.vetimm.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 25.Shi W, Liu Q, Zhang J, Sun J, Jiang X, Geng J, Wang F, Xiao Y, Li H, Zhao X. 2014. Co-expression of EtMic2 protein and chicken interleukin-18 for DNA vaccine against chicken coccidiosis. Res Vet Sci 97:64–70. doi: 10.1016/j.rvsc.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Puehler F, Göbel T, Breyer U, Ohnemus A, Staeheli P, Kaspers B. 2003. A sensitive bioassay for chicken interleukin-18 based on the inducible release of preformed interferon-gamma. J Immunol Methods 274:229–232. doi: 10.1016/S0022-1759(02)00515-X. [DOI] [PubMed] [Google Scholar]
- 27.van Houten NE, Zwick MB, Menendez A, Scott JK. 2006. Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide. Vaccine 24:4188–4200. doi: 10.1016/j.vaccine.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Li JR, Huang YW, Liang XY, Meng SS. 2001. Enhanced immunogenicity of plasmid encoding polyprotein gene of infectious bursal disease virus by co-administration of chicken interleukin 2 (IL-2). Sheng Wu Gong Cheng Xue Bao 17:652–657. (In Chinese.) doi: 10.3321/j.issn:1000-3061.2001.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Sasai K, Yoshimura K, Lillehoj HS, Withanage GS, Fukata T, Baba E, Arakawa A. 1997. Analysis of splenic and thymic lymphocyte subpopulations in chickens infected with Salmonella Enteritidis. Vet Immunol Immunopathol 59:359–367. doi: 10.1016/S0165-2427(97)00082-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Yue S, Hu J, Huang Y, Chen J, Wang T, Fan Z, Zhao H. 2010. Expression and bioactivity assay of mature chicken interleukin-18 protein mutant using Pichia pastoris expression system. Wei Sheng Wu Xue Bao 50:1258–1263. (In Chinese.) [PubMed] [Google Scholar]
- 32.Lim KL, Jazayeri SD, Yeap SK, Alitheen NBM, Bejo MH, Ideris A, Omar AR. 2012. Co-administration of avian influenza virus H5 plasmid DNA with chicken IL-15 and IL-18 enhanced chickens immune responses. BMC Vet Res 8:132. doi: 10.1186/1746-6148-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winding P, Berchtold MW. 2001. The chicken B cell line DT40: a novel tool for gene disruption experiments. J Immunol Methods 249:1–16. doi: 10.1016/S0022-1759(00)00333-1. [DOI] [PubMed] [Google Scholar]
- 34.Arakawa H, Buerstedde JM. 2004. Immunoglobulin gene conversion: insights from bursal B cells and the DT40 cell line. Dev Dyn 229:458–464. doi: 10.1002/dvdy.10495. [DOI] [PubMed] [Google Scholar]
- 35.Schijns VE. 2000. Immunological concepts of vaccine adjuvant activity. Curr Opin Immunol 12:456–463. doi: 10.1016/S0952-7915(00)00120-5. [DOI] [PubMed] [Google Scholar]
- 36.Lim KL, Jazayeri SD, Yeap SK, Mohamed Alitheen NB, Bejo MH, Ideris A, Omar AR. 2013. Antibody and T cell responses induced in chickens immunized with avian influenza virus N1 and NP DNA vaccine with chicken IL-15 and IL-18. Res Vet Sci 95:1224–1234. doi: 10.1016/j.rvsc.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Chen HY, Cui P, Cui BA, Li HP, Jiao XQ, Zheng LL, Cheng G, Chao AJ. 2011. Immune responses of chickens inoculated with a recombinant fowlpox vaccine coexpressing glycoprotein B of infectious laryngotracheitis virus and chicken IL-18. FEMS Immunol Med Microbiol 63:289–295. doi: 10.1111/j.1574-695X.2011.00850.x. [DOI] [PubMed] [Google Scholar]
- 38.Marshall DJ, Rudnick KA, McCarthy SG, Mateo LR, Harris MC, McCauley C, Snyder LA. 2006. Interleukin-18 enhances Th1 immunity and tumor protection of a DNA vaccine. Vaccine 24:244–253. doi: 10.1016/j.vaccine.2005.07.087. [DOI] [PubMed] [Google Scholar]
- 39.Barouch DH, Letvin NL, Seder RA. 2004. The role of cytokine DNAs as vaccine adjuvants for optimizing cellular immune responses. Immunol Rev 202:266–274. doi: 10.1111/j.0105-2896.2004.00200.x. [DOI] [PubMed] [Google Scholar]
- 40.Barouch DH, Truitt DM, Letvin NL. 2004. Expression kinetics of the interleukin-2/immunoglobulin (IL-2/Ig) plasmid cytokine adjuvant. Vaccine 22:3092–3097. doi: 10.1016/j.vaccine.2004.01.065. [DOI] [PubMed] [Google Scholar]
- 41.Yoon HA, Aleyas AG, George JA, Park SO, Han YW, Kang SH, Cho JG, Eo SK. 2006. Differential segregation of protective immunity by encoded antigen in DNA vaccine against pseudorabies virus. Immunol Cell Biol 84:502–511. doi: 10.1111/j.1440-1711.2006.01463.x. [DOI] [PubMed] [Google Scholar]
- 42.Ferrara JL. 2000. Cytokines and the regulation of tolerance. J Clin Invest 105:1043–1044. doi: 10.1172/JCI9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roncarolo MG, Levings MK. 2000. The role of different subsets of T regulatory cells in controlling autoimmunity. Curr Opin Immunol 12:676–683. doi: 10.1016/S0952-7915(00)00162-X. [DOI] [PubMed] [Google Scholar]
- 44.Howe RC, Wondimu A, Demissee A, Frommel D. 1995. Functional heterogeneity among CD4+ T-cell clones from blood and skin lesions of leprosy patients. Identification of T-cell clones distinct from Th0, Th1 and Th2. Immunology 84:585–594. [PMC free article] [PubMed] [Google Scholar]
- 45.Pollock KG, Conacher M, Wei XQ, Alexander J, Brewer JM. 2003. Interleukin-18 plays a role in both the alum-induced T helper 2 response and the T helper 1 response induced by alum-adsorbed interleukin-12. Immunology 108:137–143. doi: 10.1046/j.1365-2567.2003.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szeto C, Gillespie KM, Mathieson PW. 2000. Levamisole induces interleukin-18 and shifts type 1/type 2 cytokine balance. Immunology 100:217–224. doi: 10.1046/j.1365-2567.2000.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]