Abstract
Lynch syndrome is the most common Mendelian disorder predisposing to hereditary colorectal cancer. Carriers of MSH6 mutations constitute less than 10% of total cases and present with a weaker clinical phenotype, including low levels of microsatellite instability (MSI-L) in colorectal tumors. The frequency of MSH6 mutation carriers among patients presenting with MSI-L colorectal cancer has yet to be determined, as has the appropriate genetic work-up in this context. We have reviewed here the clinicopathological characteristics, immunohistochemistry and genetic testing results for 71 patients at a single institution diagnosed with MSI-L colorectal cancers. Of 71 patients with MSI-L tumors, 21 underwent genetic testing for MSH6 mutations, three of them presented with loss of staining of MSH6 and only one carried a pathogenic germline MSH6 mutation in exon 4 (c.2677_2678delCT; p.Leu893Alafs*6). This latter patient had a significant family history and had a rectal primary that showed instability only in mononucleotide markers. In this cohort of MSI-L patients, we detected no notable clinicopathological and molecular characteristics that would help to distinguish a group most likely to harbor germline MSH6 mutations. Therefore, we conclude that the prevalence of MSH6 mutations among subjects with MSI-L tumors is very low. MSI analysis combined with immunohistochemistry of mismatch repair proteins adequately detects potential MSH6 carriers among MSI-L colorectal cancers.
Keywords: Microsatellite instability-low, Lynch syndrome, colorectal cancer, MSH6, mononucleotide markers
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States when both sexes are combined [1]. It has been estimated that 5 to 10% of CRC cases are secondary to a genetic condition, with Lynch syndrome (also termed hereditary nonpolyposis colorectal cancer) being the most common Mendelian disorder predisposing to CRC and accounting for 3 to 5% of all CRC [2, 3].
Lynch syndrome is secondary to the presence of germline mutations in the genes involved in the DNA mismatch repair (MMR) pathway: MLH1, MSH2, MSH6 and PMS2 [2, 4]. Mutations in MLH1 and MSH2 account for 90% of all identified mutations and MSH6 and PMS2 for 10% [2]. The molecular fingerprint of deficiency in the MMR system is the presence of microsatellite instability (MSI). MSI is detected in the majority of tumors arising in the context of Lynch syndrome by using either immunohistochemical (IHC) analysis of the MMR proteins or a standard PCR-based approach known as MSI analysis [5]. The phenotype of families with a diagnosis of Lynch syndrome includes increased life-time risks for the development of young-onset colorectal and endometrial cancers, as well as tumors of the ovaries, small bowel, urothelium, and other organs [2]. It has been observed that carriers of germline mutations in MSH6 have a distinct clinical phenotype with older age at diagnosis and lower life-time risk for colorectal and endometrial tumors compared to MLH1 and MSH2 mutation carriers [6–8]. In addition, it has been reported that tumors arising in the context of MSH6 mutations can present with intact expression of MMR proteins and may display low levels of microsatellite instability (MSI-L) or even microsatellite stability (MSS) [6–8].
At present, many academic institutions have established universal screening strategies for Lynch syndrome based on tumor studies with MSI and IHC and thus can systematically detect CRC cases with deficient MMR activity [9]. The implementation of such programs results in the subclassification of all CRC in either two (MMR-deficient and MMR-proficient) or three [MSI-high (MSI-H), MSI-L, and MSS] molecular subgroups [10]. Traditionally, tumors displaying MSI-L have been grouped along with those with MSS due to their similar clinical and pathological features and absence of a hereditary origin [10, 11]. However, it is currently unknown if patients presenting with tumors displaying MSI-L should be considered for genetic testing of Lynch syndrome based on previous studies associating MSH6 mutations with this tumor subtype [8, 12–15]. This is particularly critical in the context of patients with a CRC displaying MSI-L and either young onset at presentation or positive family history of Lynch syndrome–related tumors.
The aim of our current study was to assess the prevalence of MSH6 mutations in a cohort of patients diagnosed with colorectal tumors displaying MSI-L and to investigate the role of MSI analysis and IHC of the MMR proteins in identifying patients with Lynch syndrome among cases of CRC with MSI-L.
Material and methods
Patients and samples
A total of 71 patients with a diagnosis of CRC displaying MSI-L were included in this study. This cohort of patients was obtained from two prospective institutional databases at The University of Texas MD Anderson Cancer Center (UTMDACC) in the period from 2003 to 2012: 1) genetic counseling database, which includes all patients undergoing genetic counseling; and 2) CRC database, which collects clinical, pathologic, and molecular data of patients with CRC undergoing surgical resection. These two databases contained a total 933 registered cases. Only cases displaying MSI-L by MSI analysis that had results for IHC of MMR proteins were included in the current study. Both the MSI analysis and the IHC were requested by providers per standard of care and in the context of an institutional initiative to have all surgically resected colorectal tumors undergo universal screening for Lynch syndrome through tumor studies, initiated in 2010. Clinical, laboratory, and pathology information was retrieved from the electronic medical record. Information regarding the family history was collected by certified genetic counselors at the time of the initial assessment. In those cases not referred for genetic counseling, information on the family history was retrieved directly from the medical record. Personal and family history was assessed for the risk of carrying a germline mutation using the PREMM1,2,6 risk prediction model [16]. Pedigrees made as part of the genetic counseling visit or using the information collected in the chart were assessed for fulfilment of Amsterdam I/II and Bethesda guidelines [17, 18]. This study was approved by the Institutional Review Board of UTMDACC.
MSI analysis and immunohistochemical examination
For MSI analysis, DNA was extracted from microdissected formalin-fixed, paraffin-embedded tumor and normal tissue sections. The MSI status was ascertained using a panel with seven microsatellite markers (BAT25, BAT26, BAT40, D2S123, D5S346, D17S250, and TGFβRII) and was performed at the Molecular Diagnostic Laboratory in UTMDACC. A microsatellite marker was considered positive when an allelic shift was present in the tumor compared with normal tissue. A case was considered MSI-H when three of the loci (≥30%) tested were positive, MSI-L when one or two (<30%) were positive, and MSS when all loci tested were negative. We included in this study only CRC cases displaying MSI-L [5]. To assess the phenotypic differences of MSI-L tumors presenting with mononucleotide compared with dinucleotide allelic changes, we classified patients in two categories: 1) a mononucleotide group, which included patients with tumors with changes in at least one mononucleotide allele in the MSI analysis; and 2) a dinucleotide group, which included patients with changes in dinucleotide alleles only. Immunohistochemical staining was performed on 5-µm sections of formalin-fixed, paraffin-embedded tumor blocks using a panel of four mouse monoclonal antibodies against the mismatch repair proteins MLH1 (550838, clone G168-15, BD Pharmingen, San Diego, CA), MSH2 (NA27, clone FE11, Calbiochem, EMD Millipore, Billerica, MA), MSH6 (610919, clone 44, BD Pharmingen), and PMS2 (556415, clone A16-4, BD Pharmingen). Loss of expression in the tumor cells was considered solely when there was normal nuclear staining in adjacent non-neoplastic cells, which served as internal controls. The sections stained with these antibodies were reviewed by two expert pathologists (R.R.B. and M.W.T.) to confirm the absence of MSH6 staining.
DNA sequencing
To assess for the presence of germline mutations and large deletions and duplications in the MSH6 gene, DNA extracted from blood was tested by full genomic sequencing of the 10 exons of the gene and by gene dosage analysis [multiplex ligation-dependent probe amplification (MLPA)]. These analyses were performed in a CLIA-certified laboratory (City of Hope Molecular Diagnostic Laboratory, Duarte, CA, or Mayo Medical Laboratories, Rochester, MN). Prior to the ordering of genetic testing, patients received appropriate genetic counseling by certified genetic counselors, who were also in charge of disclosing the results to the patients in person or by phone. In addition, germline DNA sequencing on a research basis was performed on an ABI 3730XL sequencer using Big Dye Terminator cycle chemistry at the Sequencing and Microarray Facility Core of UTMDACC for two patients: one patient who was found to harbor a germline MSH6 mutation by clinical testing and with the research analysis performed to confirm the result, and one patient who fulfilled Amsterdam I criteria and was not offered genetic testing at the time of the initial genetic evaluation. PCR conditions and primers used for those cases tested on a research basis are available upon request.
Statistical Analysis
Descriptive statistics were summarized for frequency distributions of categorical variables and mean distributions of continuous variables. Wilcoxon rank-sum test was performed on continuous variables and Chi-square or Fisher exact tests were performed on categorical variables to compare the differences of frequency or mean distribution for clinical, demographic or marker variables between categories of outcome variables. P-values less than 0.05 were considered statistically significant. All tests were two-sided. Data was processed and analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Results
Patients
Seventy-one patients diagnosed with CRC were included in this study after being identified as having a tumor displaying MSI-L by MSI analysis. The main characteristics of the study population are presented in Table 1. Twenty-two subjects had the primary tumor located in the right colon, 19 in the left colon, and 30 in the rectum. Twenty-three patients received neoadjuvant treatment. Thirty-two patients were diagnosed with advanced disease. Twenty-four tumors presented with pathologic features associated with MSI such as low grade and mucinous differentiation. Regarding family history, 29 patients received an overall risk score >5% for carrying a germline mutation in one of the MMR genes using the PREMM1,2,6 model, which has been identified as a cost-effective strategy for screening for Lynch syndrome in the general population [19]. Thirteen patients had a first-degree relative with CRC, and two patients fulfilled Amsterdam I criteria.
Table 1.
Clinicopathologic features of the 71 patients with microsatellite instability-low (MSI-L) tumors
| Characteristic | No. of patients (%) |
|---|---|
| Age at diagnosis, years (range) | 53.25 (29–80) |
| Female | 33 (46.5) |
| Race/ethnicity | |
| White | 50 (70.4) |
| Black | 10 (14.1) |
| Hispanic | 9 (12.7) |
| Other | 2 (2.8) |
| Colorectal cancer site | |
| Right colon | 22 (31.0) |
| Left colon | 19 (26.8) |
| Rectum | 30 (42.3) |
| Synchronous CRC | 3 (4.2) |
| Tumor stage | |
| Tis/I | 8 (11.3) |
| II | 12 (16.9) |
| III | 19 (26.8) |
| IV | 32 (45.1) |
| Neoadjuvant treatment | 23 (32.4) |
| Tumor histology | |
| Poorly differentiated | 16 (22.5) |
| Mucinous | 8 (11.3) |
| Family history | |
| Revised Bethesda | 37 (52.1) |
| Amsterdam I | 2 (2.8) |
| FDR with CRC | 13 (18.3) |
| PREMM1,2,6 score >5% | 29 (40.8) |
| Genetic counseling | 55 (77.5) |
| Germline testing | 21 (29.6) |
CRC, colorectal cancer; FDR, first-degree relative.
Genetic counseling
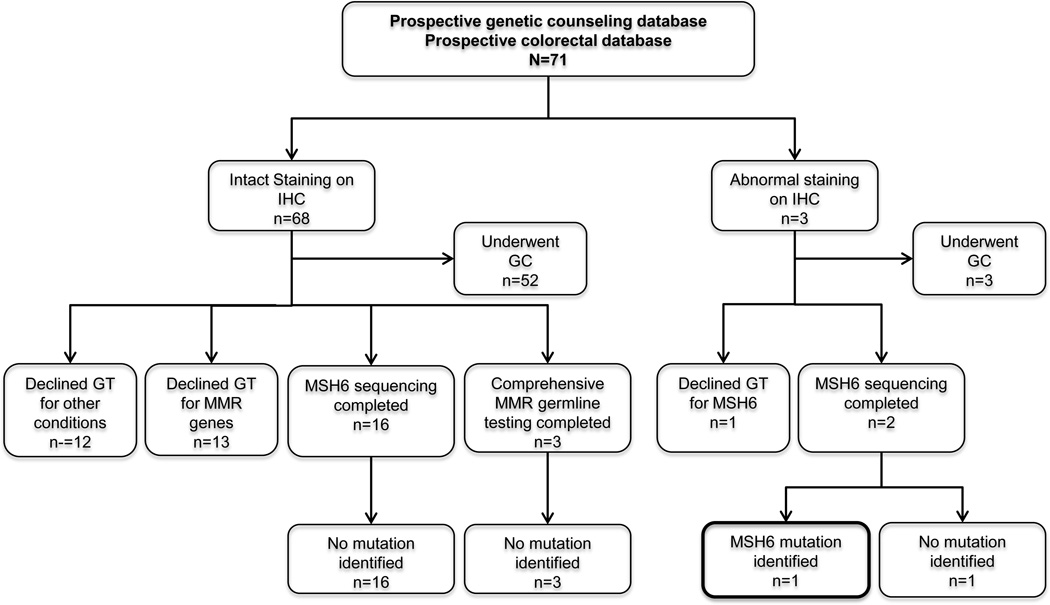
Fifty-eight patients (82%) were referred for genetic counseling to the Familial High-Risk Gastrointestinal Cancer Program in UTMDACC by their care providers based on clinical criteria that included young age of onset, significant family history of CRC or other tumors, results of tumor studies consistent with a suspicion of Lynch Syndrome or MSIL results. A total of 21 patients elected to proceed with germline testing of MMR genes to be evaluated for Lynch syndrome based on genetic counseling recommendations (Figure 1). The characteristics of those patients who had genetic testing and those who did not are summarized in Table 2; there were no statistically significant differences between the two groups in their clincopathological characteristics with the exception of the number of patients with a PREMM1,2,6 score >5%. Within the group of 21 patients who had genetic testing, 18 underwent MSH6 germline testing and three underwent comprehensive testing of MLH1, MSH2, and MSH6 genes.
Figure 1.
Diagram of patients with CRC displaying MSI-L included in this study. IHC, immunohistochemistry; GC, genetic counseling; GT, genetic testing; MMR, mismatch repair.
Table 2.
Clinicopathologic characteristics of patients by status of germline testing
| Characteristics | Germline testing N = 21, (%) |
No germline testing N = 50, (%) |
P-value |
|---|---|---|---|
| Age at diagnosis, years (range) | 52.3 (30–69) | 53.7 (29–80) | NS |
| Female | 11 (52.4) | 22 (44) | |
| Race/ethnicity | NS | ||
| White | 14 (66.7) | 36 (72) | |
| Black | 2 (9.5) | 8 (16) | |
| Hispanic | 4 (19.0) | 5 (10) | |
| Other | 1 (4.8) | 1 (2) | |
| Colorectal cancer site | NS | ||
| Right colon | 8 (38.1) | 14 (28) | |
| Left colon | 5 (23.8) | 14 (28) | |
| Rectum | 8 (38.1) | 22 (44) | |
| Tumor stage | NS | ||
| Tis/I | 3 (14.3) | 5 (10) | |
| II | 4 (19.0) | 8 (16) | |
| III | 7 (33.3) | 12 (24) | |
| IV | 7 (33.3) | 25 (50) | |
| Any MSI-H histology | 8 (38.1) | 16 (32) | NS |
| Family history | |||
| Revised Bethesda | 12 (57.1) | 25 (50) | 0.067 |
| Amsterdam I/II | 2 (9.5) | 0 | |
| FDR with CRC | 5 (23.8) | 8 (16) | NS |
| PREMM1,2,6 score total | 7.1 (1.8–20.7) | 5 (1.5–21.6) | 0.075 |
| PREMM1,2,6 score >5% | 13 (61.9) | 16 (32) | 0.019 |
| Number displaying mononucleotide alleles shifted | 8 (38.1) | 12 (24) | NS |
| Genetic counseling | 21 (100) | 34 (68) | 0.0018 |
Borderline and significant P-values are displayed. CRC, colorectal cancer; FDR, first-degree relative; NS, not significant.
Tumor studies and genetic testing results
For MSI analysis, a panel of seven microsatellites was used at our institution in all of the 71 patients included in this report. Sixty tumors (85%) had results for the complete panel of seven markers, nine had valid results for six, and two had only five markers tested. The variation in the total number of markers tested in our patient population was secondary to technical failures. A total of seven tumors had two allelic markers shifted and the rest showed instability in only one marker.
Research studies have demonstrated that MSH6 is involved in the recognition of single-base mispairs and small insertion/deletion loops and that human tumor cell lines with MSH6 mutations exhibit primarily changes in mononucleotide and not dinucleotide repeats [20, 21]. Therefore, we were interested in comparing phenotypic characteristics in MSI-L patients presenting with alterations in mononucleotide repeats and in those with changes restricted to dinucleotides. Fifty-one (72%) tumors had microsatellite instability restricted to dinucleotide markers only. The marker most commonly altered was D2S123, followed by D17S250 and TGFβRII (Table 4). Mononucleotide alleles were shifted in 20 tumors (28%), with BAT40 being the marker most commonly shifted (13 tumors), followed by BAT25 (7 cases). MSI-L tumors with mononucleotide changes were more frequently located in the left side of the colon, which is consistent with previous reports [13], although this difference did not reach the level of statistical significance (45% versus 20%, P = 0.09). No other clinicopathological characteristics or family history differed significantly between the two groups, apart from the fact that the only MSH6 mutation carrier identified in this cohort presented with microsatellite instability in mononucleotide repeats only.
Table 4.
Clinicopathologic characteristics of patients classified by the presence of microsatellite instability in mononucleotide alleles versus in dinucleotide alleles only
| Characteristic | Mononucleotide N = 20, (%) |
Dinucleotide N = 51, (%) |
|---|---|---|
| Age at diagnosis, years (range) | 50.5 (29–67) | 54.4 (30–80) |
| Female | 10 (50) | 23 (45.1) |
| Race/ethnicity | ||
| White | 16 (80) | 34 (66.7) |
| Black | 1 (5) | 9 (17.6) |
| Hispanic | 3 (15) | 6 (11.8) |
| Other | 0 | 2 (3.9) |
| Colorectal cancer site | ||
| Right colon | 4 (20) | 18 (35.3) |
| Left colon | 9 (45) | 10 (19.6) |
| Rectum | 7 (35) | 23 (45.1) |
| Synchronous CRC | 1 (5) | 2 (3.9) |
| Tumor stage | ||
| Tis/I | 2 (10) | 6 (11.8) |
| II | 3 (15) | 9 (17.6) |
| III | 5 (25) | 14 (27.5) |
| IV | 10 (50) | 22 (43.1) |
| Any MSI-high histology | 8 (40) | 16 (31.4) |
| Family history | ||
| Revised Bethesda | 14 (70) | 23 (45.1) |
| Amsterdam I | 0 | 2 (3.9) |
| FDR with CRC | 6 (30) | 7 (13.7) |
| PREMM126 total | 7 (1.5–21.6) | 5 (1.5–13.7) |
| PREMM126 score >5% | 9 (45) | 20 (39.2) |
| MSI makers displaying instability | ||
| BAT25 | 7 (35) | 0 |
| BAT26 | 3 (15) | 0 |
| BAT40 | 13 (65) | 0 |
| D2S123 | 3 (15) | 25 (49.0) |
| D5S346 | 0 | 6 (11.8) |
| D17S250 | 0 | 12 (23.5) |
| TGFβRII | 0 | 12 (23.5) |
| Patients who had genetic counseling | 17 (85) | 38 (74.5) |
| Patients who underwent genetic testing | 8 (40) | 13 (25.5) |
| Genetic testing positive | 1 (5) | 0 |
CRC, colorectal cancer; FDR, first-degree relative.
Sixty-eight of the 71 tumors displaying MSI-L had intact staining of all four MMR proteins by IHC. Three tumors showed abnormal staining: two presented with loss of MSH6 only and one with loss of MSH2 and MSH6. The main clinical and pathological characteristics of these three subjects are detailed and compared in Table 3. The two patients with isolated loss of MSH6 underwent comprehensive germline testing of MSH6 (NM_000179.2), and one of them was found to be heterozygous for a small deletion located in exon 4 (c.2677_2678delCT; p.Leu893Alafs*6) resulting in a frameshift mutation at codon 893, thus leading to a truncation of the protein with major disruption in its structure. The PREMM1,2,6 score of this individual was as high as 20.66%. The patient was diagnosed at age 52 with a tumor located in the rectum and presented with MSI only in mononucleotide alleles (BAT25 and BAT26). In addition, he had a personal history of small bowel carcinoma and 3 years after the diagnosis of the rectal tumor developed early-stage prostate carcinoma. The family history was significant: his mother and maternal grandmother both had ovarian carcinoma with serous histology in their 60s, and one maternal aunt had breast cancer in her 30s. The paternal history was relevant only for the father, who had a diagnosis of prostate cancer in his late 80s. The other patient did not harbor mutations in MSH6. The patient who presented with loss of staining in both MSH2 and MSH6 was offered genetic testing in the context of his clinical care and also as part of a research protocol and declined on both occasions.
Table 3.
Characteristics of the three patients presenting with loss of staining in MSH6
| Clinical characteristic | Patient #1 | Patient #2 | Patient #3 |
|---|---|---|---|
| Age at diagnosis, years | 52 | 67 | 44 |
| Sex | Male | Male | Male |
| Tumor site | Rectum | Rectum | Rectum |
| IHC performed in the original biopsy | No | Yes | Yes |
| IHC results | Loss of MSH6 | Loss of MSH2/MSH 6 | Loss of MSH6 |
| Number of MS alleles shifted in MSI analysis | 2/7 | 1/7 | 2/7 |
| BAT25 | BAT40 | BAT25 | |
| BAT26 | BAT40 | ||
| Genetic testing | MSH6 | NA | MSH6 |
| c.2677_2678delCT | NA | NMD | |
| PREMM1,2,6 score | |||
| MLH1 | 4.56 | 0.70 | 3.24 |
| MSH2 | 13.12 | 0.88 | 3.31 |
| MSH6 | 2.99 | 0.99 | 1.45 |
NA, not assessed; NMD, no mutation detected. IHC, immunohistochemistry.
Two patients who met Amsterdam I criteria underwent comprehensive MSH6 genetic testing (one tested on a clinical basis and one on a research basis), and neither was found to carry a germline mutation or a variant of uncertain significance (VUS). The other 17 patients who underwent germline testing had negative results, with no mutations or VUS identified.
Discussion
In the current study, we report a cohort of 71 patients diagnosed with CRC displaying MSI-L with the primary objective of assessing the prevalence of Lynch syndrome due to mutations in MSH6 in this specific subgroup of CRCs. All patients had informative results for the MSI analysis and the IHC of the MMR proteins performed in the primary tumor. The majority of these patients were referred to genetic counseling for recommendations regarding the genetic work-up, and almost a third of the patients underwent genetic testing. Among these patients, only one was found to be a carrier of a mutation in the MSH6 gene. This is the first report of this specific mutation in the literature and causes a frameshift mutation in exon 4. This patient had a personal history of small bowel carcinoma, rectal and prostate cancer, as well as a maternal family history of late-onset ovarian cancer and breast cancer. The rectal cancer of this patient had MSI-L with instability restricted to mononucleotide alleles and loss of MSH6 expression, reflecting a deficiency in the MMR repair system secondary to this gene alteration. Strikingly, the PREMM1,2,6 score of this patient for the presence of mutations in MSH2 was higher than for MSH6.
Our results are in agreement with other groups’ previous reports showing a modest or absent association between the presence of MSI-L and germline mutations in MSH6 [12, 22]. Although it is biologically plausible that germline mutations in MHL1, MSH2 and PMS2 generate low levels of MSI or MSS tumors, this occurrence is rarely observed [23, 24]. In fact, only historical reports of the MSI-L phenotype and MSH6 mutations have been present in the literature since the late 1990s [8, 13]. In our opinion, the conflicting association between MSI-L and MSH6 mutations comes from the heterogeneity of the populations studied. Considering previous work, there is first a group of publications that analyzed the presence of germline mutations in those cases with a clinical presentation consistent with Lynch syndrome that had not been identified to have germline mutations in MLH1 and MSH2, and these studies found a high frequency of carriers of MSH6 alterations. This series of cases is mainly composed of patients with tumors that display MSI-H and MSI-L in a smaller proportion and present with a weak phenotype in terms of age at diagnosis and higher diversity in the type of tumors identified. These reports were published right after the initial identification of MSH6 as another gene associated with Lynch syndrome (known initially as GTBP) [8, 25]. A second group of articles analyzed cancer risk and phenotype of known MSH6 carriers [6, 7]. Finally, a third group of publications considered mixed populations of patients with “familial CRC”, “young-onset CRC”, and “MSI-L tumors” [12, 13, 15, 22]. To estimate the true frequency of MSH6 mutations in MSI-L cases and reframe the algorithm to guide the genetic work-up of these patients, more studies are needed from academic institutions with large cohorts of systematically identified CRCs displaying MSI-L. In this regard, our study is one of the most comprehensive series in the literature analyzing this clinical context.
The molecular basis for the association between MSI-L CRCs and MSH6 mutations has been clearly supported by basic research findings both in vitro [21] and in MSH6 knock-out mice [26]. At the molecular level, MSH6 partners with MSH2 to recognize single-base or small insertion/deletion mispairs, thus developing into a weaker MSI phenotype. In fact, patients and families diagnosed with Lynch syndrome and carrying germline MSH6 mutations have a later age of cancer diagnosis and have a lower incidence of CRC [6, 7, 25]. In addition, the fact that MSH6 detects single-base errors is consistent with the clinical observation of the presence of MSI-L in tumors from MSH6 mutation carriers. On the basis of this, some authors have advocated the use of panels of microsatellite markers that contain only mononucleotide tracts in order to maximize the detection rate of MSH6 carriers [27]. In this regard, we have explored the hypothesis that stratification of MSI-L patients by the presence of changes in dinucleotide versus mononucleotide markers would allow for the identification of distinct subgroups with clinicopathological characteristics, thus aiding in the prioritization of patients to undergo genetic testing. We observed no significant differences between the dinucleotide and mononucleotide groups, except for a trend in the mononucleotide group to be more frequently located in the left side of the colon, which is consistent with previous reports [13].
Our study has some limitations. First, not all patients diagnosed with an MSI-L tumor included in this case series underwent germline testing. Also, the collection of family history data in the cohort of patients that did not receive genetic counseling was limited, as this information was extracted from the electronic medical record and therefore may be incomplete. Third, the germline testing in the vast majority of patients included in this cohort was restricted to the MSH6 gene.
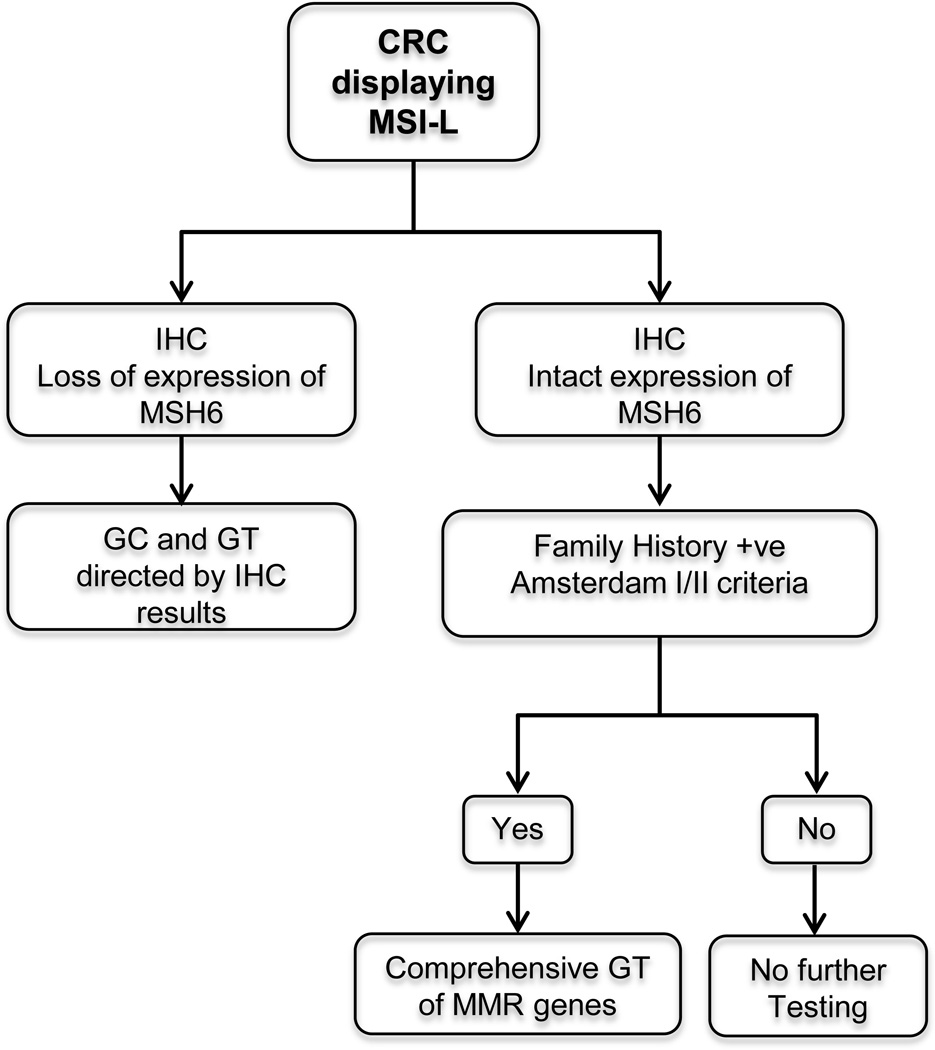
Finally, on the basis of the data reported by us here and by others in previously published series, and despite of the fact that our study did not identify mutations in MSH6 in two patients fulfilling Amsterdam I criteria and that MSH6 families tend to present with a weaker family history compared to families with MLH1 and MSH2 mutations, we propose a genetic work-up algorithm for patients with diagnosed CRC displaying MSI-L that supports genetic testing only in those individuals with family history (such as patients and families fulfilling Amsterdam I or II criteria) and/or lack of functioning of the MSH6 protein, as evidenced by a loss of expression shown in the IHC (Figure 2), in the context of clinical judgment. This algorithm assumes that institutions should continue testing tumors for both MSI analysis by PCR and IHC of the MMR proteins in order to identify the MSI-L subpopulation, either in parallel or sequentially, but favoring to perform first the MSI analysis or at least simultaneously. We acknowledge that currently is more convenient in clinical practice to perform IHC only or as the initial tumor testing; however the lack of MSI analysis would not allow to detect rare cases with MSI-L tumors that have strong family history (Amsterdam I or II) and have intact expression of MSH6, as we think that family history is still an important factor to be taken into consideration for recommending genetic testing among patients with MSI-L tumors.
Figure 2.
Proposed genetic work-up algorithm for patients diagnosed with CRC displaying MSI-L. IHC, immunohistochemistry; GC, genetic counseling; GT, genetic testing; +ve, positive; MMR, mismatch repair.
Acknowledgements
This work was supported in part by the Conquer Cancer Foundation of the American Society of Clinical Oncology, Young Investigator Award (to E. Vilar), the Janice Davis Gordon Memorial Postdoctoral Fellowship in Colorectal Cancer Prevention from The University of Texas MD Anderson Cancer Center (to E. Borras), and by The University of Texas MD Anderson Cancer Center Core Support Grant – Sequencing and Microarray Facility Core (P30 CA016672). We thank Michael Worley and the Department of Scientific Publications for editorial assistance with the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors disclose no potential conflicts of interest.
Contributorship statement:
Conception and design: EV, MEM, AC, MRB, PML, YNY
Financial support: EV, YNY
Provision of study materials or patients: MEM, SAB, MWT, RRB, RL, MRB, PML, YNY
Pathology assessment: MWT, RRB
Molecular analysis: EV, EB, RL
Collection and assembly of data: EV, MEM, AC, YNY
Data analysis and interpretation: EV, MEM, AC, YNY, JY
Manuscript writing: All authors
Final approval of manuscript: All authors
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348:919–932. doi: 10.1056/NEJMra012242. [DOI] [PubMed] [Google Scholar]
- 3.Aaltonen LA, Peltomaki P, Leach FS, Sistonen P, Pylkkanen L, Mecklin JP, et al. Clues to the pathogenesis of familial colorectal cancer. Science. 1993;260:812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- 4.Bonadona V, Bonaiti B, Olschwang S, Grandjouan S, Huiart L, Longy M, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA. 2011;305:2304–2310. doi: 10.1001/jama.2011.743. [DOI] [PubMed] [Google Scholar]
- 5.Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendriks YM, Wagner A, Morreau H, Menko F, Stormorken A, Quehenberger F, et al. Cancer risk in hereditary nonpolyposis colorectal cancer due to MSH6 mutations: impact on counseling and surveillance. Gastroenterology. 2004;127:17–25. doi: 10.1053/j.gastro.2004.03.068. [DOI] [PubMed] [Google Scholar]
- 7.Plaschke J, Engel C, Kruger S, Holinski-Feder E, Pagenstecher C, Mangold E, et al. Lower incidence of colorectal cancer and later age of disease onset in 27 families with pathogenic MSH6 germline mutations compared with families with MLH1 or MSH2 mutations: the German Hereditary Nonpolyposis Colorectal Cancer Consortium. J Clin Oncol. 2004;22:4486–4494. doi: 10.1200/JCO.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Berends MJW, Mensink RGJ, Kempinga C, Sijmons RH, van der Zee AGJ, et al. Association of Hereditary Nonpolyposis Colorectal Cancer–Related Tumors Displaying Low Microsatellite Instability with MSH6 Germline Mutations. Amer J Hum Genet. 1999;65:1291–1298. doi: 10.1086/302612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Screening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer) N Engl J Med. 2005;352:1851–1860. doi: 10.1056/NEJMoa043146. [DOI] [PubMed] [Google Scholar]
- 10.Vilar E, Tabernero J. Molecular dissection of microsatellite instable colorectal cancer. Cancer Discov. 2013;3:502–511. doi: 10.1158/2159-8290.CD-12-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlinson I, Halford S, Aaltonen L, Hawkins N, Ward R. Does MSI-low exist. J Pathol. 2002;197:6–13. doi: 10.1002/path.1071. [DOI] [PubMed] [Google Scholar]
- 12.Parc YR, Halling KC, Wang L, Christensen ER, Cunningham JM, French AJ, et al. hMSH6 Alterations in Patients with Microsatellite Instability-Low Colorectal Cancer. Cancer Res. 2000;60:2225–2231. [PubMed] [Google Scholar]
- 13.Verma L, Kane MF, Brassett C, Schmeits J, Evans DG, Kolodner RD, et al. Mononucleotide microsatellite instability and germline MSH6 mutation analysis in early onset colorectal cancer. J Med Genet. 1999;36:678–682. [PMC free article] [PubMed] [Google Scholar]
- 14.Lucci-Cordisco E, Rovella V, Carrara S, Percesepe A, Pedroni M, Bellacosa A, et al. Mutations of the 'minor' mismatch repair gene MSH6 in typical and atypical hereditary nonpolyposis colorectal cancer. Fam Cancer. 2001;1:93–99. doi: 10.1023/a:1013872914474. [DOI] [PubMed] [Google Scholar]
- 15.Pinto C, Veiga I, Pinheiro M, Mesquita B, Jeronimo C, Sousa O, et al. MSH6 germline mutations in early-onset colorectal cancer patients without family history of the disease. Br J Cancer. 2006;95:752–756. doi: 10.1038/sj.bjc.6603318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balmana J, Stockwell DH, Steyerberg EW, Stoffel EM, Deffenbaugh AM, Reid JE, et al. Prediction of MLH1 and MSH2 mutations in Lynch syndrome. JAMA. 2006;296:1469–1478. doi: 10.1001/jama.296.12.1469. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Bigas MA, Boland CR, Hamilton SR, Henson DE, Jass JR, Khan PM, et al. A National Cancer Institute Workshop on hereditary nonpolyposis colorectal cancer syndrome: meeting highlights and Bethesda guidelines. J Natl Cancer Inst. 1997;89:1758–1762. doi: 10.1093/jnci/89.23.1758. [DOI] [PubMed] [Google Scholar]
- 18.Umar A, Boland CR, Terdiman JP, Syngal S, de la Chapelle A, Ruschoff J, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–268. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinh TA, Rosner BI, Atwood JC, Boland CR, Syngal S, Vasen HFA, et al. Health Benefits and Cost-Effectiveness of Primary Genetic Screening for Lynch Syndrome in the General Population. Cancer Prev Res. 2011;4:9–22. doi: 10.1158/1940-6207.CAPR-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulos N, Nicolaides NC, Liu B, Parsons R, Lengauer C, Palombo F, et al. Mutations of GTBP in genetically unstable cells. Science. 1995;268:1915–1917. doi: 10.1126/science.7604266. [DOI] [PubMed] [Google Scholar]
- 21.Marsischky GT, Filosi N, Kane MF, Kolodner R. Redundancy of Saccharomyces cerevisiae MSH3 and MSH6 in MSH2-dependent mismatch repair. Genes Dev. 1996;10:407–420. doi: 10.1101/gad.10.4.407. [DOI] [PubMed] [Google Scholar]
- 22.Kets CM, van Krieken JH, Hebeda KM, Wezenberg SJ, Goossens M, Brunner HG, et al. Very low prevalence of germline MSH6 mutations in hereditary non-polyposis colorectal cancer suspected patients with colorectal cancer without microsatellite instability. Br J Cancer. 2006;95:1678–1682. doi: 10.1038/sj.bjc.6603478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scartozzi M, Bianchi F, Rosati S, Galizia E, Antolini A, Loretelli C, et al. Mutations of hMLH1 and hMSH2 in patients with suspected hereditary nonpolyposis colorectal cancer: correlation with microsatellite instability and abnormalities of mismatch repair protein expression. J Clin Oncol. 2002;20:1203–1208. doi: 10.1200/JCO.2002.20.5.1203. [DOI] [PubMed] [Google Scholar]
- 24.Lagerstedt Robinson K, Liu T, Vandrovcova J, Halvarsson B, Clendenning M, Frebourg T, et al. Lynch syndrome (hereditary nonpolyposis colorectal cancer) diagnostics. J Natl Cancer Inst. 2007;99:291–299. doi: 10.1093/jnci/djk051. [DOI] [PubMed] [Google Scholar]
- 25.Kolodner RD, Tytell JD, Schmeits JL, Kane MF, Gupta RD, Weger J, et al. Germline msh6 mutations in colorectal cancer families. Cancer Res. 1999;59:5068–5074. [PubMed] [Google Scholar]
- 26.Edelmann W, Yang K, Umar A, Heyer J, Lau K, Fan K, et al. Mutation in the mismatch repair gene Msh6 causes cancer susceptibility. Cell. 1997;91:467–477. doi: 10.1016/s0092-8674(00)80433-x. [DOI] [PubMed] [Google Scholar]
- 27.You JF, Buhard O, Ligtenberg MJ, Kets CM, Niessen RC, Hofstra RM, et al. Tumours with loss of MSH6 expression are MSI-H when screened with a pentaplex of five mononucleotide repeats. Br J Cancer. 2010;103:1840–1845. doi: 10.1038/sj.bjc.6605988. [DOI] [PMC free article] [PubMed] [Google Scholar]