Abstract
Rationale
Positive allosteric modulators (PAMs) of type 5 metabotropic glutamate receptors (mGluR5) exert pro-cognitive effects in animal models of various neuropsychiatric diseases. However, few studies to date have examined ability of mGluR5 PAMs to reverse cognitive deficits in operant delayed matching/non-matching-to-sample (DMS/DNMS) tasks.
Objectives
To determine the ability of the mGluR5 PAM 3-cyano-N-1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) to reverse set-shifting deficits induced by the NMDA receptor antagonist MK-801.
Methods
Male Sprague-Dawley rats were initially trained to lever press for sucrose reinforcement under either DMS or DNMS conditions. Following successful acquisition of the task, reinforcement conditions were reversed (DNMS→DMS or DMS→DNMS). In Experiment 1, rats were treated daily prior to each session with either vehicle/vehicle, vehicle/MK-801 (0.06 mg/kg) simultaneously, CDPPB (20 mg/kg)/MK-801 simultaneously, or CDPPB 30 min prior to MK-801. In Experiment 2, rats were treated with either vehicle/vehicle, vehicle/MK-801, or CDPPB 30 min prior to MK-801 only prior to sessions that followed task reversal.
Results
In Experiment 1, no group differences in initial task acquisition were observed. Rats treated with vehicle+MK−801 showed significant set-shifting impairments following task reversal, which were partially attenuated by simultaneous administration of CDPPB/MK-801, and completely precluded by administration of CDPPB 30 min prior to MK-801. In Experiment 2, MK-801 did not impair reversal learning and no other group differences were observed.
Conclusions
MK-801 induced deficits in operant set-shifting ability were prevented by pretreatment with CDPPB. MK-801 did not produce deficits in initial task learning or when treatment was initiated following task reversal.
Keywords: operant set-shifting, type 5 metabotropic glutamate receptor, N-methyl-D-aspartate receptor, cognitive impairment, match-to-sample, schizophrenia
Introduction
Cognitive impairment is a core feature of many chronic illnesses such as schizophrenia, Huntington’s disease, Alzheimer’s disease, and drug addiction. Cognitive impairments can affect working memory, reference memory, attention, cognitive flexibility, and various other aspects of executive functioning (Green et al. 2004; Silver et al., 2003. Secondary effects of cognitive impairments can affect a patient’s ability to maintain relationships, employment, or appropriate daily hygiene practices, and often result in cognitive and behavioral perseveration. Although such impairments and perseveration occur across a range of neuropsychiatric diseases, the focus of the present study was to examine this phenomenon in the context of schizophrenia.
Schizophrenia affects approximately 1% of the population and is typically diagnosed in males during their early 20s and in females during their later 20s. As a result, adequate treatment is necessary for the remainder of the affected individual’s life, making schizophrenia one of the most burdensome and costly neuropsychiatric diseases (Rowley et al. 2001). There are several hypotheses regarding the underlying pathophysiology of schizophrenia, one of which is hypofunctioning of the N-methyl-D-aspartate (NMDA) glutamate receptor subtype (Lin et al., 2012; Olney et al., 1999; Snyder and Gao, 2013). As a result, glutamate-based therapies are currently being explored for the treatment of schizophrenia (Snyder and Gao 2013; Krystal et al., 2010; Nisewender and Conn, 2010; Menniti et al. 2013). Direct pharmacological targeting of the NMDA receptor via the orthosteric glutamate binding site is generally regarded as a nonviable approach since NMDA receptor agonists produce CNS hyperactivity, seizures, and excitotoxicity. An alternative to directly targeting the orthosteric glutamate binding site is to directly or indirectly target binding sites for obligatory endogenous co-agonists such as glycine and D-serine. For example, inhibition of glycine transporters such as GlyT1 increases extracellular levels of glycine and thereby facilitates NMDA receptor activity (Vandenberg & Aubrey, 2001). Furthermore, various GlyT1 inhibitors have shown efficacy in improving cognitive impairments in schizophrenia (Javitt 2012).
A third possible approach to increase NMDA receptor function is via activation of type 5 metabotropic glutamate receptors (mGluR5). Facilitation of mGluR5 activity indirectly increases NMDA receptor function through structural and biochemical coupling of these two receptor subtypes. mGluR5 and NMDA receptors are structurally linked via proteins such as postsynaptic density 95 (PSD-95), Shank, and the Homer family of proteins. Localization of these two receptors in close proximity facilitates their bidirectional coupling via signaling intermediates such as phospholipase C and intracellular calcium (Gerber et al., 2007; Hermans and Challiss, 2001 Piers et al., 2012), which increases NMDA receptor activity including increased probability of receptor channel opening (Menniti et al. 2013; Niswender and Conn 2010). Currently, the most advantageous approach to increasing mGluR5 receptor function is via positive allosteric modulators (PAMs), which increase mGluR5 signaling only in the presence of endogenous glutamate. The use of orthosteric mGluR5 agonists has proved to be a less viable approach, since these ligands exhibit limited brain penetrance following systemic administration, poor selectivity for specific mGluR subtypes, and induce rapid desensitization of the receptor (Chen and Conn, 2008; Krystal et al., 2010; Menniti et al. 2013; Niswender and Conn 2010).
Results from several studies indicate that the mGluR5 PAM 3-cyano-N-1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) reverses experimentally induced cognitive impairments in animal models of schizophrenia. Stefani and Moghaddam (2010) found that CDPPB at doses of 10 and 30 mg/kg attenuated dizocilpine (MK-801) induced cognitive deficits in a T-maze based set-shifting task. Clifton et al. (2013) found that acute administration of CDPPB and the mGluR5 PAM ADX47273 reversed phencyclidine (PCP) and MK-801 induced impairment in social novelty discrimination in adult rats, and when administered during adolescence reversed early postnatal MK-801 and PCP-induced impairments. Alternatively, Horio and colleagues showed that when PCP was administered chronically, acute administration of CDPPB was unable to attenuate deficits in novel object recognition in mice; however, subchronic (14 days) of administration of CDPPB was effective in rescuing such impairments (Horio et al. 2013). Uslaner et al. (2010) also showed that CDPPB was able to ameliorate MK-801 induced cognitive impairments in novel object recognition, and Vales et al. (2010) found that CDPPB was effective in attenuating MK-801 induced impairments in active allothetic place avoidance.
To our knowledge, only two previous studies have examined the ability of mGluR5 PAMs to reverse pharmacologically induced cognitive deficits in an operant set-shifting paradigm (Darrah et al., 2008; Gilmour et al., 2013). Operant-based paradigms offer the advantage of modeling tasks used to assess cognitive flexibility and perseveration in humans, such as the Wisconsin Card Sorting Task (Franke et al., 1992; Goldberg et al., 1987; Joel et al., 1997; Silver et al., 2003). In a study by Darrah and colleagues (2008), rats were trained to respond for food reinforcement based on discrimination between two perceptual stimuli dimensions (spatial location of the nosepoke aperture and illumination of a stimulus light), and effects of CDPPB on MK-801 induced deficits in this task were examined. However, this study did not employ a delayed matching/non-matching-to-sample component, which increases working memory load and thus increases the difficulty of the task (Dudchenko, 2004, Dudchenko et al., 2013). In a study by Gilmour and colleagues (2013), rats were trained to respond for food in a delayed non-matching-to-position nosepoke paradigm, and the efficacy of the novel mGluR5 PAMs LSN2463359 and LSN2814617 on reversal of set-shifting performance deficits induced by the competitive (closed channel) NMDA receptor antagonist SDZ 220,581 were assessed. However, in this latter study, the efficacy of these mGluR5 PAMs against the effects of more widely used non-competitive (open channel) NMDA antagonists such as MK-801 were not assessed. In addition, both of these studies utilized acute dosing procedures which can lead to difficulty in interpretation of the potential antipsychotic and pro-cognitive effects of these ligands when administered daily in a clinical setting (Varvel et al., 2002; Hagan and Jones, 2005).
Therefore, the purpose of the present study was to assess the ability of CDPPB to reverse MK-801 induced cognitive deficits in an operant set-shifting task incorporating delayed matching/non-matching-to-sample procedures (Experiment 1). Chronic drug administration was utilized in order to more closely resemble daily intake patterns that are generally required for therapeutic purposes in humans. However, to control for the possibility that daily administration of CDPPB and/or MK-801 during the first phase of testing might have carryover effects on performance following task reversal, separate groups of animals received drug administration only during the post-reversal phase of the procedures (Experiment 2). Finally, many of the aforementioned studies have assessed the ability of CDPPB to reverse MK-801 induced cognitive deficits utilizing vastly different drug administration paradigms, with some studies administering CDPPB prior to MK-801, some administering the two drugs simultaneously, and others administering MK-801 prior to CDPPB. Therefore, a tertiary purpose of the present study was to establish whether pretreatment with CDPPB prior to MK-801 would produce more robust effects on set-shifting ability deficits as compared to simultaneous administration.
Methods and Materials
Subjects
Subjects were male Sprague-Dawley rats (Harlan Laboratories, Livermore, CA) weighing 250-300 g upon arrival. Rats were pair-housed throughout the duration of the study. The vivarium was held at a temperature of 22±1 °C, and a 12 hour reversed light/dark cycle (lights off 7 am) was used. Animals had free access to water throughout the experiment except during behavioral testing. Rats were food restricted to 80% of their body weight by allowing them to free-feed for one hour after each daily session. Food restriction was necessary since it has previously been demonstrated that MK-801 decreases the reinforcing properties of sucrose in free feeding animals (Vardigan et al., 2010), which would adversely affect task performance in the present study. In addition, food reinforcement was not used since it has previously been demonstrated that MK-801 can actually increase food intake and meal size under certain conditions (Burns and Ritter, 1997; Treece et al., 2000; Gillespie et al., 2005), thus confounding the use of food as a reinforcer in during behavioral testing. Animals were maintained in accordance with the guidelines described in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) and all procedures and facilities were approved by the Institutional Animal Care and Use Committee at Arizona State University.
Drugs
3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamide (CDPPB) was synthesized by Chemir Analytical Services (Maryland Heights, MO) according to published methods (Kinney et al., 2005) and suspended in a vehicle consisting of 10% v/v Tween 80 (Sigma-Aldrich, St. Louis, MO). MK-801 was purchased from Sigma-Aldrich and dissolved in sterile saline (0.9% w/v NaCl). Doses for CDPPB (20 mg/kg) and MK-801 (0.06 mg/kg) were based on previous studies (Ayala et al., 2009; Horio et al., 2013; Stefani and Moghaddam, 2010; Uslaner et al., 2009) and pilot work performed in our laboratory. All drugs were administered via the subcutaneous (s.c.) route in a volume of 0.5 ml.
Delayed matching/non-matching to sample paradigm
Delayed match-to-sample (DMS) or non-match-to-sample (DNMS) tasks were based on previously published procedures (Joel et al., 1997) and were conducted in daily sessions (approx. 45 min in length) consisting of 30 trials per session. Each trial was comprised of three components: a sample component, choice component and an inter-trial interval (ITI). At the beginning of each daily session, animals were placed into standard operant conditioning chambers (model ENV-007, Med Associates, St. Albans, VT) containing a house light, food receptacle and two retractable levers. Each chamber was interfaced to a PC computer, and MED-PC IV software (Med Associates) was used to control experimental parameters and record responses. The house light was illuminated to signal the beginning of the first trial. During the sample component, either the left or right lever, designated the sample lever, was randomly inserted into the chamber. Pressing the sample lever resulted in delivery of a sucrose pellet reinforcer (45 mg, Test Diet, St. Louis, MO) and a 30 sec delay. Following the delay, the choice component of the task commenced wherein both levers were inserted into the chamber. During the choice component, if the animal was assigned to the DMS condition as the initial task and pressed the same lever that was presented during the sample component, a sucrose pellet was delivered followed by a 30 sec inter-trial interval (ITI). An incorrect lever press resulted in the house-light turning off and initiation of a 60 sec time-out period. Alternatively, if the animal was assigned to the DNMS condition for the initial task, and pressed the lever that was not presented during the sample component, a sucrose pellet was delivered followed by a 30 sec ITI. To control for task-specific learning, half of the subjects in each group were assigned to the DMS condition as the initial task and the other half the DNMS. Animals were considered to have met acquisition criteria when they exhibited at least 80% correct responses for 4 consecutive days, and were allowed a maximum of 21 days of testing to meet this criteria. In the event animals reached this criteria prior to 21 days of testing, they continued in the same testing conditions through the 21st day of testing. Animals that did not reach the 80% correct acquisition criteria by the 21st day of testing were removed from the study. On Day 22 of testing, animals were switched from their previously assigned task to the opposite task (i.e., DNMS→DMS, or DMS→DNMS) and subsequently tested for 21 additional days. Animals were then euthanized by anesthesia with isoflurane followed by decapitation and brain removal.
Testing Procedures
In Experiment 1, animals were randomly assigned to one of the following 4 treatment groups and were treated 20 min prior to each daily session with the following drug combinations: vehicle/vehicle, vehicle/MK-801 (0.06 mg/kg) simultaneously, CDPPB (20 mg/kg)/MK-801 simultaneously, or CDPPB 30 min prior to MK-801. In order to control for the possibility that daily administration of CDPPB and/or MK-801 during the first phase of testing (prior to task reversal) might have carryover effects on performance following task reversal, Experiment 2 was conducted, in which separate groups of animals underwent similar initial testing, but received either vehicle/vehicle, vehicle/MK-801, or CDPPB 30 min prior to MK-801 only during the post-reversal phase of the procedures (days 22-42).
Data analyses
For each daily session, the proportion of correct responses (calculated as the total number of correct responses divided by the overall total number of responses) were analyzed using a mixed two-way ANOVA with Treatment as the between-subjects factor and Day (session) as the within-subjects factor. Since initial analyses revealed no group differences during the acquisition of the initial task prior to task reversal for both Experiments 1 and 2, only data from sessions following task reversal were included in subsequent analyses. Multivariate ANOVAs were used to determine differences between specific treatment groups on each day of testing following task reversal. Statistical analyses were performed using Prism (version 5.00, GraphPad Software, La Jolla, CA) and SPSS (version 21.0, IBM, Armonk, NY). p-values less than 0.05 were considered statistically significant for all tests.
Results
Each experimental group had an initial sample size of n=10 animals per group, half of which were initially assigned to the DMS condition and the other half to the DNMS condition. However, in Experiment 1, the following number of animals failed to meet acquisition criteria by the 21st day of the study, and were therefore removed from the experiment: vehicle/vehicle (n=2), vehicle/MK-801 (n=4), CDPPB/MK-801 simultaneously (n=3), and CDPPB 30 min prior to MK-801 (n=1). Therefore, the final group sample sizes were as follows: vehicle/vehicle (n=8), vehicle/MK-801 (n=6), CDPPB/MK-801 simultaneously (n=7), and CDPPB 30 min prior to MK-801 (n=9). There were no apparent group biases in failure to acquire the task, such that roughly equal numbers of animals in each group acquired the initial DMS or DNMS task. In Experiment 2, all 10 animals per group reached acquisition criteria during the initial phase of the experiment.
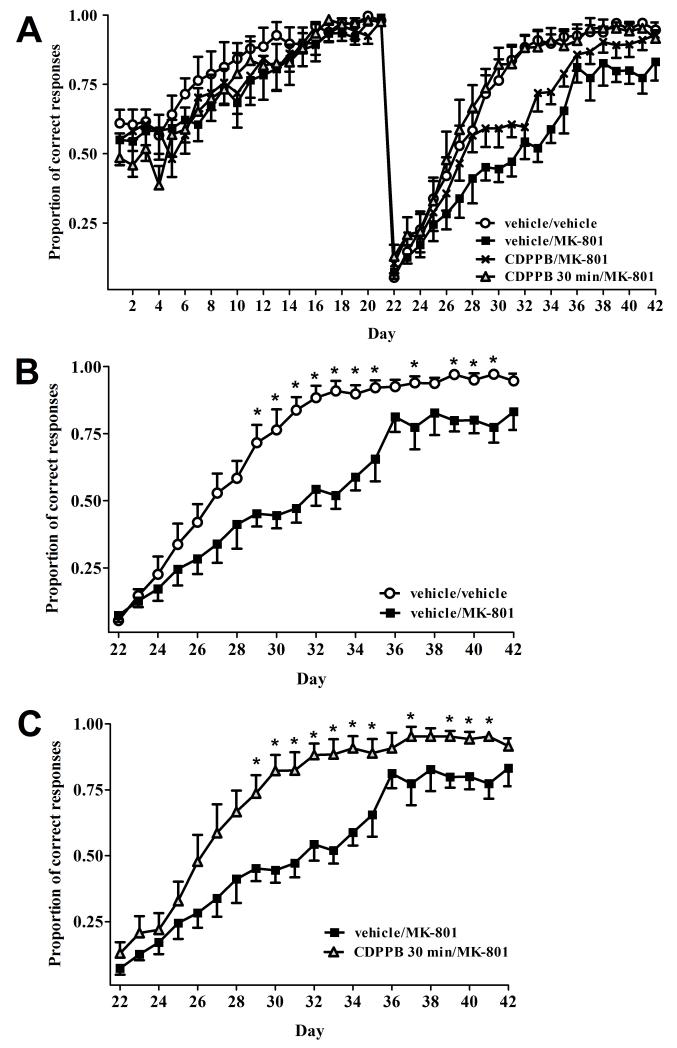
Data from all 4 treatment groups in Experiment 1 are shown in Fig. 1A. For ease of visualization of inter-group comparisons, data from various treatment groups in Fig. 1A are re-plotted in Figs. 1B and 1C. No group differences were observed during the initial task acquisition (days 1-21) (all p-values >0.05). Following task reversal (days 22-42), a mixed two-way ANOVA revealed significant effects of Treatment (F3,26=5.21, p<0.01), Day (F20,520=170.95, p<0.001) and a Treatment × Day interaction (F60,520=2.04, p<0.001). Post hoc analysis revealed that animals in the vehicle/MK-801 group exhibited a significant decrease in proportion of correct responding as compared with the vehicle/vehicle group on days 29-35, 37, and 39-41 (all p’s<0.05), indicating that MK-801 induced a significant impairment in set-shifting ability (Fig. 1B). Animals pre-treated with CDPPB 30 min prior to MK-801 (CDPPB 30 min/MK-801, Fig. 1C) showed a significantly increased proportion of correct responding as compared with animals treated with vehicle/MK-801 group on days 29-35, 37, and 39-41 (all p’s<0.05), indicating a reversal of MK-801 induced deficits. Furthermore, pre-treatment with CDPPB prior to MK-801 resulted in performance equivalent to vehicle/vehicle treated rats (all p’s >0.05, see Fig. 1A). However, simultaneous treatment with CDPPB and MK-801 produced only partial attenuation of MK-801 induced deficits in set-shifting ability, as compared to the vehicle/MK-801 group (Fig. 1A), as evidenced by a significantly increased proportion of correct responding only on day 33 (p<0.05), and a trend towards significant differences on days 31, 34, and 41 (p’s=0.08, 0.07, and 0.06, respectively).
Figure 1.
A) Performance in the operant DMS/DNMS set-shifting task for all 4 treatment groups in Experiment 1. Task reversal occurred on day 22. B) Depiction of data from panel A showing that rats treated with vehicle/MK-801 exhibited significant set-shifting impairments following task reversal. *p<0.05 vs. vehicle/MK-801 on the corresponding day. C) Depiction of data from panel A showing that MK-801 induced set-shifting impairments are precluded by administration of CDPPB 30 min prior to MK-801. Samples sizes are as follows: vehicle/vehicle (n=9), vehicle/MK-801 (n=6), CDPPB/MK-801 (n=7), and CDPPB 30 min/MK-801 (n=8) *p<0.05 vs. vehicle/MK-801 on the corresponding day. All data values represent mean ± SEM.
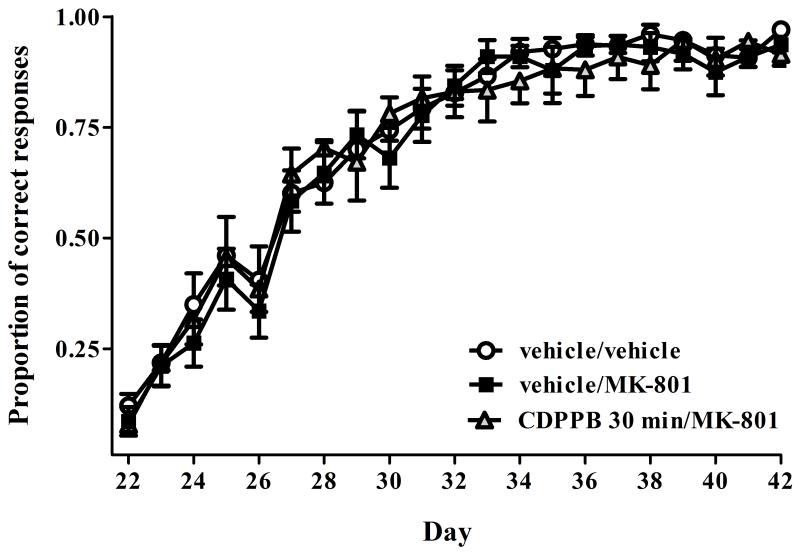
In Experiment 2, no group differences were observed the acquisition of the initial task (all p’s>0.05, data not shown). Following task reversal (Fig. 2), a mixed two-way ANOVA revealed a significant effect of Day (F20,567=70.87, p<0.001) but no significant effects of Treatment (p>0.05) or a Treatment × Day interaction (p>0.05). These results suggest that MK-801 does not induce impairments in set-shifting ability when treatment is initiated following task reversal, and that are no significant effects of CDPPB on set-shifting ability.
Figure 2.
Post-task reversal performance in the operant DMS/DNMS set-shifting task for all 3 treatment groups in Experiment 2: vehicle/vehicle, vehicle/MK-801, and CDPPB 30 min/MK-801. Task reversal occurred on day 22. As in Experiment 1, not group differences in the initial task acquisition were observed (data not shown). All data values represent mean±SEM. n=10 animals per group.
Discussion
In this study, we demonstrated that pharmacological blockade of NMDA receptors with MK-801 produced deficits in a delayed matching/non-matching-to-sample operant set-shifting task, and these effects were reversed when the mGluR5 PAM CDPPB was administered 30 minutes prior to, but not simultaneously with, MK-801. While other studies have reported similar positive effects of mGluR5 PAMs on the reversal of deficits in operant set-shifting paradigms induced by NMDA receptor antagonists (Darrah et al., 2008; Gastambide et al., 2013), it should be emphasized that the current study, as well as that conducted by Gastambide and colleagues (2013), employed a delayed matching/non-matching-to-sample component, which increases working memory load and can be considered to be of increased translational value in the assessment of the efficacy of novel antipsychotic medications (Dudchenko, 2004; Dudchenko et al., 2013). In addition, most other studies (summarized below) examining mGluR5 PAM-induced reversal of the cognitive impairing effects of MK-801 have used either acute or subchronic dosing regimens, whereas in the present study animals received pharmacological treatments either throughout the course of the study (Experiment 1) or only during the post-reversal phase (Experiment 2). As suggested by others (Varvel et al., 2002; Hagan and Jones, 2005), we assert that chronic dosing regimens such as those used in the present study have a higher translational value for assessing the efficacy of novel cognition-enhancing or antipsychotic medications, which would most likely require daily dosing in humans.
The ability of CDPPB to reverse MK-801 induced deficits in set-shifting ability is consistent with other findings indicating reversal of pharmacologically induced cognitive effects of mGluR5 PAMs using non-delay based operant tasks (Darrah et al., 2008; Gastambide et al., 2013); spatial alternation tasks (Stefani and Moghaddam, 2010; Fowler et al., 2013); reversal learning digging paradigms (Gastambide et al., 2012) social novelty discrimination (Clifton et al., 2013); novel object recognition (Horio et al., 2013; Reichel et al, 2011; Uslaner et al., 2009); and active allothetic place avoidance (Vales et al., 2009). mGluR5 PAMs have also been shown to reverse MK-801 induced impairments in sucrose preference (Vardigan et al., 2010), taste aversion and inhibitory avoidance tasks (Fowler et al., 2011), and alterations in prefrontal cortex neuronal firing patterns (Lecourtier et al., 2007; Homayoun et al., 2008; Homayoun and Moghaddam, 2010). In unimpaired animals, mGluR5 PAMs have been shown to improve spatial and recognition memory abilities (Ayala et al., 2009; Balschun et al., 2006; Uslaner et al., 2009). Thus, mGluR5 PAMs appear to be a promising class of cognition-enhancing agents, although recent reports of neurotoxicity induced by high dose exposure to these compounds requires further investigation (Parmentier-Batteur et al., in press).
The more robust efficacy of CDPPB pretreatment 30 min prior to MK-801 as compared with simultaneous administration of these two ligands is likely attributable to one or more factors. One possibility is that CDPPB may be slower to enter the brain than MK-801. In support of this, Kinney and colleagues (2005) demonstrated that acute CDPPB administration produces a brain-to-plasma area under the curve (AUC) ratio of 1.7, while Vezzani and colleagues (1989) showed that MK-801 produces a brain-to-plasma AUC of 12.5, suggesting significantly improved brain penetration of MK-801 vs. CDPPB. Another possibility is that CDPPB is less able to reverse the cognitive effects of MK-801 when this latter ligand is already bound to the NMDA receptor. This latter possibility may seem unlikely in light of findings by Stefani and Moghaddam (2010), who showed that treatment of rodents with similar doses of MK-801 prior to CDPPB prevented MK-801 induced deficits on cognitive set-shifting ability in a spatial plus maze task. However, in this study, both drugs were administered acutely rather than chronically as in the present study, and thus the order of ligand administration may become more important when these ligands are given repeatedly. Another possible explanation for the improved efficacy of CDPPB when administered 30 min prior to MK-801 as opposed to simultaneously may lie within the mechanism of action of MK-801. Since MK-801 is a non-competitive (open channel) NMDA receptor antagonist, prior potentiation of mGluR5 receptor function by CDPPB would result in increased probability of NMDA receptor channel opening (Zito and Scheuss, 2009), thus providing increased access of MK-801 to the channel pore. In theory, this phenomenon would be less likely to occur without prior activation of mGluR5 receptors. Clearly, additional studies would be needed to confirm this or any of the other aforementioned possibilities.
Worthy of discussion is the fact that recent findings suggest that there are different functional classes of mGluR5 PAMs that can exert differential effects on mGluR5 receptor function and the ability to reverse cognitive or behavioral deficits induced by NMDA receptor antagonists. For example, it has been reported that newer mGluR5 PAMs such as LSN2463359 and LSN2814617 are able to reverse decrements in instrumental responding for food as well as reversal learning in a digging-based and delayed match-to-position food seeking tasks induced by the competitive (closed channel) NMDA receptor antagonist SDZ 220,581 (Gastambide et al., 2013; Gilmour et al., 2013). Surprisingly, however, LSN2463359 failed to reverse performance decrements in these tasks induced by the non-competitive (open channel) NMDA receptor antagonists MK-801 and PCP (Gastambide et al., 2013). However, it should be noted that these studies only evaluated the acute effects of these mGluR5 PAMs. Ligand binding and pharmacokinetic experiments in these studies revealed very different profiles of these newer mGluR5 PAMs as compared to CDPPB, such that increased brain penetrance and receptor affinity, and binding to an allosteric site on the mGluR5 receptor different from that of CDPPB. Importantly, it has been suggested that mGluR5 PAMs acting on separate allosteric binding sites on the receptor recruit different signal transduction mechanisms, with some allosteric sites inducing increased intracellular calcium mobilization as compared to activation of extracellular signal-related kinase 1/2 (ERK1/2), and vice versa (Zhang et al., 2005). These different binding profiles and subsequent engagement of different cellular signaling mechanisms may ultimately influence their ability to indirectly potentiate NMDA receptor function when the receptor is in an open or closed state. Thus, the ability of mGluR5 PAMs to attenuate or reverse cognitive or behavioral impairments induced by NMDA receptor blockade may be highly dependent on the molecular profile of each ligand used, as well as the dosing regimen and behavioral paradigm employed. Future studies are needed to determine the precise cellular signaling mechanisms underlying the effects observed in the present study.
Finally, another finding from the present study is that MK-801 does not induce impairments in the acquisition of learning of the initial DMS/DNMS task, nor does it impair set-shifting ability when MK-801 treatment is initiated following task reversal. These observations are consistent with various bodies of literature suggesting that impaired NMDA receptor function at low to moderate doses does not lead to deficits in initial task learning (Chadman et al., 2006; Harder et al., 1998; Murray et al., 1995; Palencia and Ragozzino, 2004; van der Meulen et al., 2003; van der Staay et al., 2011; Wozniak et al., 1990), but appears to have more deleterious effects on set-shifting which result in behavioral and cognitive perseveration (Braff et al., Egerton et al., 2005; Franke et al., 1992; Goldberg et al., 1987; Green et al., 2004; Silver et al., 2003). However, it could be argued that in Experiment 1 of the present study, the behavioral effects of CDPPB and/or MK-801 following task reversal could have been a result of accumulation of either drug during chronic drug administration prior to task reversal. The plasma elimination half-lives of CDPPB in rats are approximately 4 and 2 hrs, respectively (Kinney et al., 2005; Vezzani et al., 1989), and with chronic administration such an accumulation could indeed occur. However, several observations in the present study argue against this possibility. First, should any behavioral effects of CDPPB carried over from the pre-reversal to the post-reversal phase, these effects would likely have been observed in both the CDPPB/MK-801 and CDPPB 30 min/MK-801. However, this was not the case, since Experiment 1 demonstrated a reversal of the effects of MK-801 only in animals receiving CDPPB 30 min prior to MK-801 (Fig. 1). Also, initiation of all drug treatments following task reversal (Fig. 2) did not produce any deficits in acquiring the new task, suggesting a lack of carryover effects of MK-801 from the first phase of the experiment to the second phase. Finally, it has previously been demonstrated that long-term cognitive impairing effects of chronically administered MK-801 are predominantly observed at high doses (i.e., 0.2 mg/kg; Li et al., 2011) and not at the dose used in the present study (0.06 mg/kg). Taken together, these observations suggest that the ability of MK-801 to induce deficits in set-shifting ability, and its reversal by pretreatment with CDPPB, are mediated by continued drug administration following task reversal.
In summary, the present study lends further support to the notion that potentiation of mGluR5 function may be a novel therapeutic approach to improving cognitive deficits involving NMDA hypofunction, especially those involving higher working memory loads (Krystal et al., 2010; Niswender and Conn 2010; Menniti et al., 2013; Snyder and Gao, 2013). Our findings also support previous findings that low to moderate doses of MK-801 selectively impair reversal learning and behavioral perseveration, but do not impair initial task learning. This is consistent with the clinical literature where studies have shown that patients with schizophrenia or otherwise impaired prefrontal function are able to learn an initial cognitive task, but set-shifting ability and perseveration following a change in response contingency requirements is dramatically impaired (Braff et al., Egerton et al., 2005; Franke et al., 1992; Goldberg et al., 1987; Green et al., 2004; Silver et al., 2003). The present study also identifies the necessity of pretreatment with an mGluR5 PAM prior to administration of a non-competitive NMDA antagonist under chronic dosing conditions. Future studies examining the precise cellular signaling mechanisms downstream of mGluR5 receptor potentiation in producing the pro-cognitive effects are needed. Additional studies are also needed to explore ability of novel mGluR5 PAMs with improved brain penetrance and receptor affinity to reverse cognitive deficits induced by closed channel NMDA antagonists, as well as in other genetic or neurodevelopmental models of cognitive dysfunction. In addition, future studies should also employ additional behavioral paradigms that assess other cognitive domains such as spatial memory, attention, and sensorimotor gating.
Acknowledgments
This work was funded by NIH grant DA024355 to MFO.
References
- Akbarian S, Sucher NJ, Bradley D, Tafazzoli A, Trinh D, Hetrick WP, Potkin SG, Sandman CA, Bunney WE, Jones EG. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala JE, Chen Y, Banko JL, Sheffler DJ, Williams R, Telk AN, Watson NL, Xiang Z, Zhang Y, Jones PJ, Lindsley CW, Olive MF, Conn PJ. mGluR5 positive allosteric modulators facilitate both hippocampal LTP and LTD and enhance spatial learning. Neuropsychopharmacology. 2009;34:2057–2071. doi: 10.1038/npp.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balschun D, Zuschratter W, Wetzel W. Allosteric enhancement of metabotropic glutamate receptor 5 function promotes spatial memory. Neuroscience. 2006;142:691–702. doi: 10.1016/j.neuroscience.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, Zisook S. The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry. 1991;48:891–898. doi: 10.1001/archpsyc.1991.01810340023003. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns GA, Ritter RC. The non-competitive NMDA antagonist MK-801 increases food intake in rats. Pharmacol Biochem Behav. 1997;56:145–9. doi: 10.1016/S0091-3057(96)00171-2. [DOI] [PubMed] [Google Scholar]
- Chadman KK, Watson DJ, Stanton ME. NMDA receptor antagonism impairs reversal learning in developing rats. Behav Neurosci. 2006;120:1071–83. doi: 10.1037/0735-7044.120.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Conn PJ. mGluR5 positive allosteric modulators. Drugs Fut. 2008;33:355–360. [Google Scholar]
- Clifton NE, Morisot N, Girardon S, Millan MJ, Louseau F. Enhancement of social novelty discriminationby positive allosteric modulators at metabotropic glutamate 5 receptors: adolescent administration prevents adult-onset deficits induced by neonatal treatment with phencyclidine. Psychopharmacology. 2013;225:579–594. doi: 10.1007/s00213-012-2845-3. [DOI] [PubMed] [Google Scholar]
- Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Talpos J, Young J, Baxter MG. Animal models of working memory: a review of tasks that might be used in screening drug treatments for the memory impairments found in schizophrenia. Neurosci Biobehav Rev. 2013;37:2111–2124. doi: 10.1016/j.neubiorev.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Egerton A, Reid L, McKerchar CE, Morris BJ, Prat JA. Impairment in perceptual attentional set-shifting following PCP administration: a rodent model of set-shifting deficits in schizophrenia. Psychopharmacology. 2005;179:77–84. doi: 10.1007/s00213-004-2109-y. [DOI] [PubMed] [Google Scholar]
- Fowler SW, Ramsey AK, Walker JM, Serfozo P, Olive MF, Schachtman TR, Simonyi A. Functional interaction of mGlu5 and NMDA receptors in aversive learning in rats. Neurobiol Learn Mem. 2011;95:73–79. doi: 10.1016/j.nlm.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler SW, Walker JM, Klakotskaia D, Will MJ, Serfozo P, Simonyi A, Schachtman TR. Effects of a metabotropic glutamate receptor 5 positive allosteric modulator, CDPPB, on spatial learning task performance in rodents. Neurobiol Learn Mem. 2013;99:25–31. doi: 10.1016/j.nlm.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Franke P, Maier W, Hain C, Klingler T. Wisconsin Card Sorting Test: an indicator of vulnerability to schizophrenia? Schizophrenia Res. 1992;6:243–9. doi: 10.1016/0920-9964(92)90007-r. [DOI] [PubMed] [Google Scholar]
- Gastambide F, Cotel MC, Gilmour G, O’Neill MJ, Robbins TW, Tricklebank MD. Selective remediation of reversal learning deficits in the neurodevelopmental MAM model of schizophrenia by a novel mGlu5 positive allosteric modulator. Neuropsychopharmacology. 2012;37:1057–66. doi: 10.1038/npp.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastambide F, Gilmour G, Robbins TW, Tricklebank MD. The mGlu5 positive allosteric modulator LSN2463359 differentially modulates motor, instrumental and cognitive effects of NMDA receptor antagonists in the rat. Neuropharmacology. 2013;64:240–247. doi: 10.1016/j.neuropharm.2012.07.039. [DOI] [PubMed] [Google Scholar]
- Gerber U, Gee CE, Benquet P. Metabotropic glutamate receptors: intracellular signaling pathways. Curr Opin Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Gillespie BR, Burns GA, Ritter RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1504–11. doi: 10.1152/ajpregu.00169.2005. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Broad LM, Wafford KA, Britton T, Colvin EM, Fivush A, Gastambide F, Getman B, Heinz BA, McCarthy AP, Prieto L, Shanks E, Smith JW, Taboada L, Edgar DM, Tricklebank MD. In vitro characterisation of the novel positive allosteric modulators of the mGlu5 receptor, LSN2463359 and LSN2814617, and their effects on sleep architecture and operant responding in the rat. Neuropharmacology. 2013;64:224–239. doi: 10.1016/j.neuropharm.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR, Berman KF, Pliskin MA, Podd MH. Further evidence for dementia of the prefrontal type in schizophrenia? A controlled study of teaching the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 1987;44:1008–1014. doi: 10.1001/archpsyc.1987.01800230088014. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72:41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Jones DN. Predicting drug efficacy for cognitive deficits in schizophrenia. Schizophr Bull. 2005;31:830–53. doi: 10.1093/schbul/sbi058. [DOI] [PubMed] [Google Scholar]
- Harder JA, Aboobaker AA, Hodgetts TC, Ridley RM. Learning impairments induced by glutamate blockade using dizocilpine (MK-801) in monkeys. Br J Pharmacol. 1998;125:1013–8. doi: 10.1038/sj.bjp.0702178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. Biochem J. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. Proc Natl Acad Sci U S A. 2008;105:18041–6. doi: 10.1073/pnas.0806669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Group 5 metabotropic glutamate receptors: role in modulating cortical activity and relevance to cognition. Eur J Pharmacol. 2010;639:33–9. doi: 10.1016/j.ejphar.2009.12.042. [DOI] [PubMed] [Google Scholar]
- Horio M, Fujita Y, Hashimoto K. Therapeutic effects of metabotropic glutamate receptor 5 positive allosteric modulator CDPPB on phencyclidine-induced cognitive deficits in mice. Fund Clin Pharmacol. 2013;27:483–488. doi: 10.1111/j.1472-8206.2012.01045.x. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glycine transport inhibitors in the treatment of schizophrenia. Handb Exp Pharmacol. 2012;213:367–99. doi: 10.1007/978-3-642-25758-2_12. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I, Feldon J. Electrolytic lesions of the medial prefrontal cortex in rats disrupt performance on an analog of the Wisconsin Card Sorting Test, but do not disrupt latent inhibition: implications for animal models of schizophrenia. Behav Brain Res. 1997;85:187–201. doi: 10.1016/s0166-4328(97)87583-3. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Brien JA, O’Lemaire W, Burno M, Bickel DJ, Clements MK, Chen TB, Wisnoski DD, Lindsley CW, Tiller PR, Smith S, Jacobson MA, Sur C, Duggan ME, Pettibone DJ, Conn PJ, Williams DL., Jr. A novel selective positive allosteric modulator of metabotropic glutamate receptor subtype 5 has in vivo activity and antipsychotic-like effects in rat behavioral models. J Pharmacol Exp Ther. 2005;313:199–206. doi: 10.1124/jpet.104.079244. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Mathew SJ, D’Souza DC, Garakani A, Gunduz-Bruce H, Charney DS. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2010;24:669–93. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Homayoun H, Tamagnan G, Moghaddam B. Positive allosteric modulation of metabotropic glutamate 5 (mGlu5) receptors reverses N-methyl-D-aspartate antagonist-induced alteration of neuronal firing in prefrontal cortex. Biol Psychiatry. 2007;62:739–746. doi: 10.1016/j.biopsych.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JT, Su YA, Guo CM, Feng Y, Yang Y, Huang RH, Si TM. Persisting cognitive deficits induced by low-dose, subchronic treatment with MK-801 in adolescent rats. Eur J Pharmacol. 2011;652:65–72. doi: 10.1016/j.ejphar.2010.10.074. [DOI] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29:243–54. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- Menniti FS, Lindsley CW, Conn PJ, Pandit J, Zagouras P, Volkmann RA. Allosteric modulators for the treatment of schizophrenia: targeting glutamatergic networks. Curr Top Med Chem. 2013;13:26–54. doi: 10.2174/1568026611313010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray TK, Ridley RM, Snape MF, Cross AJ. The effect of dizocilpine (MK-801) on spatial and visual discrimination tasks in the rat. Behav Pharmacol. 1995;6:540–549. [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatric Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem. 2004;82:81–9. doi: 10.1016/j.nlm.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Parmentier-Batteur S, Hutson PH, Menzel K, Uslaner JM, Mattson BA, O’Brien JA, Magliaro BC, Forest T, Stump CA, Tynebor RM, Anthony NJ, Tucker TJ, Zhang XF, Gomez R, Huszar SL, Lambeng N, Faure H, LePoul E, Poli S, Rosahl TW, Rocher JP, Hargreaves R, Williams TM. Mechanism based neurotoxicity of mGlu5 positive allosteric modulators - development challenges for a promising novel antipsychotic target. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2012.12.003. in press. [DOI] [PubMed] [Google Scholar]
- Piers TM, Kim DH, Kim BC, Regan P, Whitcomb DJ, Cho K. Translational concepts of mGluR5 in synaptic diseases of the brain. Front Pharmacol. 2012;3:199. doi: 10.3389/fphar.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–792. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley M, Bristow L, Hutson P. Current and novel approaches to the drug treatment of schizophrenia. J Med Chem. 2001;44:477–501. doi: 10.1021/jm0002432. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P, Bilker W, Gur RC. Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry. 2003;160:1809–1816. doi: 10.1176/appi.ajp.160.10.1809. [DOI] [PubMed] [Google Scholar]
- Snyder MA, Gao WJ. NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front Cell Neurosci. 2013;7:31. doi: 10.3389/fncel.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol. 2010;639:26–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treece BR, Ritter RC, Burns GA. Lesions of the dorsal vagal complex abolish increases in meal size induced by NMDA receptor blockade. Brain Res. 2000;872:37–43. doi: 10.1016/s0006-8993(00)02432-x. [DOI] [PubMed] [Google Scholar]
- Uslaner JM, Parmentier-Batteur S, Flick RB, Surles NO, Lam JS, McNaughton CH, Jacobson MA, Hutson PH. Dose-dependent effect of CDPPB, the mGluR5 positive allosteric modulator, on recognition is associated with GluR1 and CREB phosphorylation in the prefrontal cortex and hippocampus. Neuropharmacology. 2009;57:531–538. doi: 10.1016/j.neuropharm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Vales K, Svoboda J, Benkovicova K, Bubenikova-Valesova V, Stuchlik A. The difference in effect of mGlu2/3 and mGlu5 receptor agonists on cognitive impairment induced by MK-801. Eur J Pharmacol. 2010;639:91–98. doi: 10.1016/j.ejphar.2009.11.067. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Aubrey KR. Glycine transport inhibitors as potential antipsychotic drugs. Expert Opin Ther Targets. 2001;5:507–518. doi: 10.1517/14728222.5.4.507. [DOI] [PubMed] [Google Scholar]
- van der Meulen JA, Bilbija L, Joosten RN, Bruin JP, Feenstra MG. The NMDA-receptor antagonist MK-801 selectively disrupts reversal learning in rats. Neuroreport. 2003;14:2225–8. doi: 10.1097/00001756-200312020-00018. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Rutten K, Erb C, Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav Brain Res. 2011;220:215–29. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- Vardigan JD, Huszar SL, McNaughton CH, Hutson PH, Uslaner JM. MK-801 produces a deficit in sucrose preference that is reversed by clozapine, D-serine, and the metabotropic glutamate 5 receptor positive allosteric modulator CDPPB: relevance to negative symptoms associated with schizophrenia? Pharmacol Biochem Behav. 2010;95:223–229. doi: 10.1016/j.pbb.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Varvel SA, Vann RE, Wise LE, Philibin SD, Porter JH. Effects of antipsychotic drugs on operant responding after acute and repeated administration. Psychopharmacology. 2002;160:182–91. doi: 10.1007/s00213-001-0969-y. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Serafini R, Stasi MA, Caccia S, Conti I, Tridico RV, Samanin R. Kinetics of MK-801 and its effect on quinolinic acid-induced seizures and neurotoxicity in rats. J Pharmacol Exp Ther. 1989;249:278–83. [PubMed] [Google Scholar]
- Wozniak DF, Olney JW, Kettinger Lr, Price M, Miller JP. Behavioral effects of MK-801 in the rat. Psychopharmacology. 1990;101:47–56. doi: 10.1007/BF02253717. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rodriguez AL, Conn PJ. Allosteric potentiators of metabotropic glutamate receptor subtype 5 have differential effects on different signaling pathways in cortical astrocytes. J Pharmacol Exp Ther. 2005;315:1212–9. doi: 10.1124/jpet.105.090308. [DOI] [PubMed] [Google Scholar]
- Zito K, Scheuss V. NMDA receptor function and physiological modulation. In: Squire LR, editor. Encyclopedia of Neuroscience. Academic Press; Oxford: 2009. pp. 1157–1164. [Google Scholar]