Abstract
Repetitive-sequence-based PCR (rep-PCR) is useful for generating DNA fingerprints of diverse bacterial and fungal species. Rep-PCR amplicon fingerprints represent genomic segments lying between repetitive sequences. A commercial system that electrophoretically separates rep-PCR amplicons on microfluidic chips, and provides computer-generated readouts of results has been adapted for use with Mycobacterium species. The ability of this system to type M. tuberculosis and M. avium complex (MAC) isolates was evaluated. M. tuberculosis strains (n = 56) were typed by spoligotyping with rep-PCR as a high-resolution adjunct. Results were compared with those generated by a standard approach of spoligotyping with IS6110-targeted restriction fragment length polymorphism (IS6110-RFLP) as the high-resolution adjunct. The sample included 11 epidemiologically and genotypically linked outbreak isolates and a population-based sample of 45 isolates from recent immigrants to Seattle, Wash., from the African Horn countries of Somalia, Eritrea, and Ethiopia. Twenty isolates exhibited unique spoligotypes and were not analyzed further. Of the 36 outbreak and African Horn isolates with nonunique spoligotypes, 23 fell into four clusters identified by IS6110-RFLP and rep-PCR, with 97% concordance observed between the two methods. Both approaches revealed extensive strain heterogeneity within the African Horn sample, consistent with a predominant pattern of reactivation of latent infections in this immigrant population. Rep-PCR exhibited 89% concordance with IS1245-RFLP typing of 28 M. avium subspecies avium strains. For M. tuberculosis as well as M. avium subspecies avium, the discriminative power of rep-PCR equaled or exceeded that of RFLP. Rep-PCR also generated DNA fingerprints from M. intracellulare (n = 8) and MACx (n = 2) strains. It shows promise as a fast, unified method for high-throughput genotypic fingerprinting of multiple Mycobacterium species.
Genotypic analysis of M. tuberculosis isolates has been used to confirm tuberculosis outbreaks, to investigate tuberculosis transmission and reactivation in communities, to confirm cross-contamination in the laboratory, and to study clonal expansion of strains (3, 5, 12, 13, 15, 18, 23). Genotypic analysis of atypical mycobacteria, most notably the M. avium complex (MAC), has been similarly useful for investigating routes of acquisition from environmental sources, confirming contamination of laboratory cultures, and studying clonal expansion (2, 11, 29).
DNA fingerprinting of most M. tuberculosis isolate populations can be accomplished by a combination of PCR-based methods, usually spoligotyping and mycobacterial interspersed repetitive unit-variable-number tandem repeat (MIRU-VNTR) typing. PCR-based methods require relatively small cell mass to generate results, a considerable advantage given the low growth rates of pathogenic mycobacteria. Spoligotyping uses a dot blot apparatus to detect variability within the direct repeat locus (12, 14), while MIRU-VNTR assesses repeating units at multiple loci in the M. tuberculosis genome based on product size after PCR amplification (8). The discriminative power of MIRU-VNTR approaches that of IS6110-targeted restriction fragment length polymorphism (IS6110-RFLP), a more-labor-intensive high-resolution approach (3, 16, 21). MIRU-VNTR may eventually replace IS6110-RFLP for routine high-resolution typing; however, for the time being the latter remains the “gold standard” for evaluating new strain typing methods for M. tuberculosis.
There are no consensus PCR-based methods for typing Mycobacterium species other than M. tuberculosis. RFLP is widely used for M. avium subspecies avium strains, most of which carry the insertion element IS1245. A consensus protocol for IS1245-RFLP (24) has good resolving power when the element is present in moderate copy number (5 to 30 copies), however like IS6110-RFLP it requires large cell mass and is labor intensive. Pulsed-field gel electrophoresis (2, 29) also requires a large cell mass, and few laboratories have the required equipment. Not surprisingly given the limited molecular epidemiological tools available for MAC and other environmental mycobacteria, our understanding of the routes of acquisition of these opportunistic pathogens remains limited. Similarly, it remains difficult to use strain typing to investigate contamination of clinical laboratory cultures with environmental mycobacteria.
For laboratories interested in typing multiple Mycobacterium species, a unified approach that is simple to use, PCR based, and capable of high throughput, would clearly be desirable. Recently, a commercially available repetitive-sequence-based PCR (rep-PCR) system was adapted for use on mycobacteria. Rep-PCR was initially developed for DNA fingerprinting of nosocomial pathogens such as Staphylococcus aureus (10), Streptococcus pneumoniae (26), and enteric gram-negative rods (25, 27). The method takes advantage of repetitive elements interspersed throughout bacterial genomes. When amplified by PCR, these elements generate highly discriminative genomic fingerprints (6, 9, 20, 27, 28). The DiversiLab System, a commercial rep-PCR system marketed for use on a number of bacterial and fungal species (Bacterial Barcodes, Houston, Tex.), is claimed to require 5 h for the entire process of DNA extraction, PCR, electrophoretic separation, detection of fluorescently labeled amplicons on automated microfluidic chips, and computer-generated printout of results (Bacterial Barcodes). Prior to the commercial release of the DiversiLab Mycobacterium strain typing kit at the end of 2003, we evaluated the performance of a beta version of the kit on a panel of 94 M. tuberculosis and MAC isolates.
MATERIALS AND METHODS
Study design for M. tuberculosis typing.
The analysis was conducted on 56 isolates of M. tuberculosis each derived from a separate patient in Seattle, Washington, from 1998 to 2003. This group included a population-based sample of 45 M. tuberculosis isolates derived from recent immigrants from the African Horn countries of Eritrea, Ethiopia, and Somalia. In order to protect donor privacy, isolates were submitted by Seattle area clinical laboratories without patient identifiers, according to human subjects protection protocols approved by the Seattle Biomedical Research Institute and University of Washington Institutional Review Boards. The study also included 11 epidemiologically linked clinical isolates archived from a recent tuberculosis outbreak among U.S.-born and foreign-born homeless people in Seattle (18). These 11 isolates were known to share common spoligotypes and IS6110-RFLP banding patterns. They were used to generate a cutoff value that defines identical isolates analyzed by rep-PCR.
The 45 African Horn immigrant strains were spoligotyped following internationally standardized protocols (14). Strains with orphan spoligotypes (n = 20) were assumed to be unrelated to each other and were not analyzed further. Strains which initially appeared to have nonunique spoligotypes (n = 25) (Table 1) were analyzed by IS6110-RFLP following the standardized protocol (22). Subsequent confirmatory analysis determined that the last three strains in Table 1 were in fact members of separate orphan spoligotypes.
TABLE 1.
M. tuberculosis isolates derived from African Horn immigrants and analyzed by IS6110-RFLP and rep-PCR
| Strain | Code | Spoligotype (spoDB3.0)a | Octal codea | IS6110-RFLP cluster |
|---|---|---|---|---|
| 254 | MTB-01 | 21 | 703377400001771 | B |
| 182 | MTB-15 | 21 | 703377400001771 | B |
| 188 | MTB-16 | 21 | 703377400001771 | B |
| 162 | MTB-20 | 21 | 703377400001771 | B |
| 152 | MTB-30 | 21 | 703377400001771 | B |
| 177 | MTB-31 | 25 | 703777740003171 | A |
| 231 | MTB-34 | 25 | 703777740003171 | A |
| 148 | MTB-26 | 25 | 703777740003171 | A |
| 139 | MTB-33 | 25 | 703777740003171 | A |
| 150 | MTB-13 | 25 | 703777740003171 | A |
| 245 | MTB-32 | 43 | 777777747413771 | No match |
| 149 | MTB-36 | 43 | 777777747413771 | No match |
| 246 | MTB-23 | 53 | 777777777760771 | No match |
| 244 | MTB-25 | 53 | 777777777760771 | No match |
| 229 | MTB-28 | 53 | 777777777760771 | No match |
| 252 | MTB-06 | 149 | 777000377760771 | Not typeable |
| 146 | MTB-17 | 149 | 777000377760771 | Not typeable |
| 160 | MTB-24 | 149 | 777000377760771 | Not typeable |
| 224 | MTB-27 | 429 | 703777740003731 | C |
| 248 | MTB-29 | 429 | 703777740003731 | C |
| 151 | MTB-22 | ND | 777777775720731 | No match |
| 263 | MTB-35 | ND | 777777775720731 | No match |
| 232 | MTB-19 | 37b | 777737777760771 | No match |
| 262 | MTB-02 | 777b | 777777777420771 | No match |
| 261 | MTB-37 | 804b | 477777777760771 | No match |
Spoligotype nomenclature identical to that of spoDB3.0 database (12). ND, not designated in spoDB3.0.
Three strains were initially thought to belong to a single shared spoligogroup, and were submitted for rep-PCR analysis. Subsequent confirmatory analysis showed that they were in fact orphan spoligogroups.
DNA samples from the 25 African Horn strains in Table 1 and the 11 outbreak strains (total n = 36) were assigned random code numbers and mailed to Bacterial Barcodes for blinded analysis on the beta version DiversiLab Mycobacterium kit. Results were returned in the form of a rep-PCR dendrogram. Linkages inferred from the experimental combination of spoligotyping and rep-PCR were compared to linkages determined by the “gold standard” combination of spoligotyping and IS6110-RFLP. A limitation of this strategy is that numerical percent similarity values were automatically applied to rep-PCR, whereas RFLP clusters were identified by visual analysis of results. This was necessary because the numerical output of RFLP gel analysis software remains problematic.
Study design for MAC typing.
Rep-PCR analysis was conducted on 38 archived MAC isolates originating from anonymous patients or environmental sources in Washington, California, Arkansas, and Massachusetts (Table 2). Isolates were mycobactin independent and were determined to be M. avium subspecies avium, M. intracellulare, or intermediate forms (MACX) by small-subunit ribosomal DNA PCR as described previously (7, 19). The sample set submitted for rep-PCR analysis included 28 M. avium subspecies avium isolates, 3 of which were submitted in duplicate without informing the testers, for a total of 31 M. avium subspecies avium isolates tested by rep-PCR. The duplicate strains were included to test the reproducibility of the method. Eight M. intracellulare and two MACx isolates were also tested.
TABLE 2.
M. avium subspecies avium isolates analyzed by IS1245-RFLP and rep-PCR
| Strain | Species | IS1245-RFLP cluster | Additional informationb |
|---|---|---|---|
| 100 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 101 | M. avium subsp. avium | B | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 102a | M. avium subsp. avium | D | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 103a | M. avium subsp. avium | B | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 104a | M. avium subsp. avium | B | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 105 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 107 | M. avium subsp. avium | A | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 108 | M. avium subsp. avium | A | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 110 | M. avium subsp. avium | D | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 111 | M. avium subsp. avium | NT | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 113 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 116 | M. avium subsp. avium | NT | AIDS isolate; Los Angeles, Calif., 1982-1985 |
| 500 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 501 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 502 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 503 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 504 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 505 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| 506 | M. avium subsp. avium | AIDS isolate; Los Angeles, Calif., 1982-1985 | |
| MVH15 | MACX | Clinical isolate; Little Rock, Ark., 1996-2000 | |
| MVH21 | MACX | Clinical isolate; Little Rock, Ark., 1996-2000 | |
| MVH11 | M. intracellulare | Clinical isolate; Little Rock, Ark., 1996-2000 | |
| MVH16 | M. intracellulare | Clinical isolate; Little Rock, Ark., 1996-2000 | |
| MVH19 | M. intracellulare | Clinical isolate; Little Rock, Ark., 1996-2000 | |
| MVH06 | M. intracellulare | Clinical isolate; Little Rock, Ark., 1996-2000 | |
| MVH09 | M. intracellulare | Clinical isolate; Little Rock, Ark., 1996-2000 | |
| HMC02 | M. avium subsp. avium | A | Clinical isolate; Seattle, Wash., 1996-2000 |
| HMC38 | M. intracellulare | Clinical isolate; Seattle, Wash., 1996-2000 | |
| HMC40 | M. intracellulare | Clinical isolate; Seattle, Wash., 1996-2000 | |
| HMC43 | M. intracellulare | Clinical isolate; Seattle, Wash., 1996-2000 | |
| W2001 | M. avium subsp. avium | Environmental isolate; Boston, Mass., 1998 | |
| W2008 | M. avium subsp. avium | Environmental isolate; Boston, Mass., 1998 | |
| NWH196 | M. avium subsp. avium | C | Standard cluster; Seattle, Wash., 2002 |
| NWH197 | M. avium subsp. avium | C | Standard cluster; Seattle, Wash., 2002 |
| NWH198 | M. avium subsp. avium | C | Standard cluster; Seattle, Wash., 2002 |
| NWH199 | M. avium subsp. avium | C | Standard cluster; Seattle, Wash., 2002 |
| NWH200 | M. avium subsp. avium | C | Standard cluster; Seattle, Wash., 2002 |
| NWH201 | M. avium subsp. avium | C | Standard cluster; Seattle, Wash., 2002 |
Among the 28 M. avium subspecies avium isolates were 6 anonymous isolates that had previously been submitted to us by clinicians at a Seattle hospital. Because these isolates came from patients without known risk factors for MAC infection, it had been suspected that they were laboratory contaminants. IS1245-RFLP typing and an independent method, IS999 typing (17), confirmed that they were nearly identical to each other. This set of isolates was used to define the cutoff level of relatedness that defines a cluster by rep-PCR.
The gold standard for typing M. avium subspecies avium was IS1245-RFLP. A standardized protocol (24) was followed with one deviation: Restriction endonuclease SacII was used in place of PvuII. Side-by-side comparisons showed that SacII was less susceptible to inhibition by interfering substances that copurified with MAC DNA. This resulted in far more consistent results. Furthermore, the GC-rich SacII recognition sequence yielded IS1245-containing fragments that were smaller on average than PvuII fragments, allowing us to resolve high-copy-number patterns on standard gel electrophoresis apparatus. This change did not affect internal clustering within the study. In some cases, IS999-RFLP (17) was used as an independent test of linkage inferred by IS1245-RFLP. There are no standardized RFLP methods for typing M. intracellulare and MACX strains; however, it was considered useful to test whether rep-PCR can generate fingerprints of these strains. Coded DNA samples were sent to the manufacturer for blinded analysis on the rep-PCR system. Results were returned and analyzed as with M. tuberculosis results.
Rep-PCR-based DNA fingerprinting on the DiversiLab System.
Bacteria were grown on Middlebrook 7H10 slants with oleate-albumin-dextrose-catalase enrichment. For rep-PCR analysis, DNA was extracted from a 10-μl loop of each Mycobacterium colony using the UltraClean Microbial DNA Isolation Kit (Mo Bio Laboratories, Solana Beach, Calif.). Sample DNA was standardized to approximately 25 ng/μl by spectrophotometric analysis, and genomic integrity was visualized using agarose gel electrophoresis. Samples were assigned arbitrary code numbers and mailed to the manufacturer. Aliquots of 75 ng were amplified using the DiversiLab Mycobacterium typing kit (Bacterial Barcodes, Inc.) following the manufacturer's instructions. Thermal cycling parameters were as follows: initial denaturation at 95°C for 5 min; 35 cycles of denaturation at 95°C for 30 s, annealing at 66°C for 45 s, and extension at 72°C for 60 s; and a final extension at 72°C for 3 min. Detection and analysis of rep-PCR products were implemented using the DiversiLab System in which the amplified fragments of various sizes and fluorescent intensities were separated and detected using a microfluidics chip with the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, Calif.). Further analysis was performed with the Web-based DiversiLab software (version 2.1.66), which uses the Pearson correlation coefficient and unweighted pair group method with arithmetic means, to automatically compare the rep-PCR-based DNA fingerprints of unknown isolates. The report generated by the DiversiLab System contained a dendrogram and a scatter plot for sample comparison. Reports also included electropherograms, gel-like images, and selectable demographic fields.
RESULTS
Rep-PCR-based DNA fingerprinting of a known cluster of M. tuberculosis outbreak isolates.
After blinded analysis of M. tuberculosis DNA samples, the DiversiLab System generated a dendrogram which was returned to us for comparison to IS6110-RFLP results. The 11 epidemiologically linked Seattle outbreak isolates were known to belong to spoligogroup 1. They shared an identical 15-band IS6110 banding pattern designated SBRI9, along with additional common genotypic markers that placed them in the N subfamily of the W-Beijing clade (18). Ten of these isolates exhibited ≥98% similarity to each other by rep-PCR. The 11th outbreak isolate, strain 181, was 93% similar to the other 10 by rep-PCR (Fig. 1). This isolate was indistinguishable from the other SBRI9 cluster isolates by standard IS6110-RFLP (Fig. 2). However, in some RFLP experiments, strain 181 exhibited faint additional bands approximately 1.5 and 2.25 kb in size which were not visible in the other strains (not shown). Although the 11 SBRI9 isolates were correctly grouped with each other by rep-PCR analysis, strain 181 might have been considered unlinked to the others by rep-PCR were it not for the availability of independent genotypic and epidemiological information (18). Based on the similarity observed among these 11 SBRI9 isolates, 93% similarity was chosen as the cutoff value for clustered M. tuberculosis isolates.
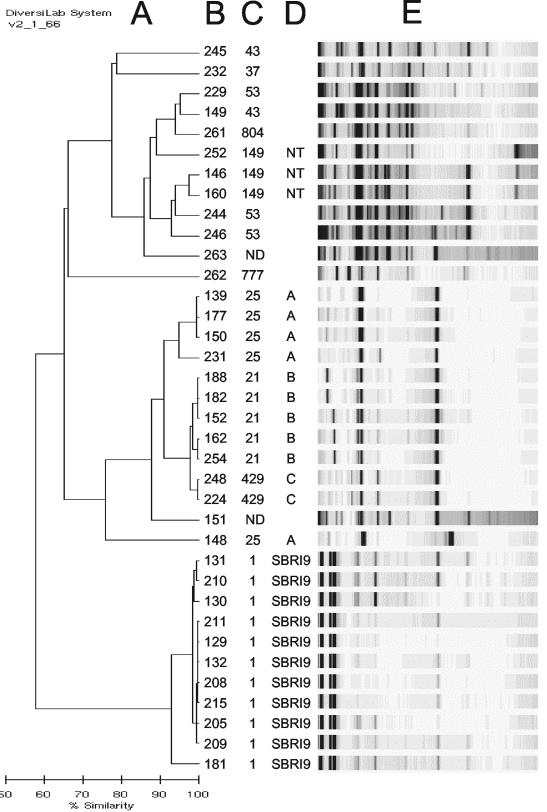
FIG. 1.
Rep-PCR analysis of 25 African Horn immigrant isolates and 11 Seattle outbreak isolates. A dendrogram (A) and gel-like images (E) were generated on coded DNA samples by Bacterial Barcodes, Inc. Correct strain identifications (B), spoligogroups (C), and IS6110-RFLP clusters (D), corresponding with nomenclature used in Table 1, were added by the authors. NT, not typeable by IS6110-RFLP. Strains without RFLP cluster designations had unique IS6110-RFLP patterns.
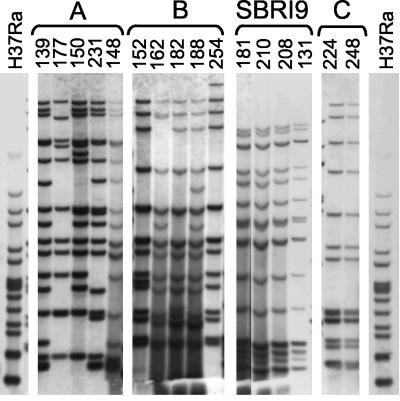
FIG. 2.
IS6110-RFLP patterns of the four major multistrain clusters of M. tuberculosis isolates in Fig. 1. The image was constructed from a single gel. Because some lanes exhibited stronger signals than others, a composite of long exposure and short exposure images was constructed. M. tuberculosis strain H37Ra patterns are shown on each end.
Rep-PCR-based DNA fingerprinting of isolates from recent African Horn immigrants to Seattle.
Among the 45 African Horn isolates in the study, 20 had unique spoligotype patterns. The remaining 25 isolates were assigned code numbers and submitted for blinded rep-PCR analysis (subsequent confirmatory analysis revealed that 3 of these 25 strains were also members of orphan spoligotypes). The 25 African Horn isolates tested by rep-PCR fell into 11 different spoligogroups (Table 1). Three of these spoligogroups were shown by IS6110-RFLP to constitute clusters labeled A, B, and C (Table 1; Fig. 2). Rep-PCR correctly grouped together the five strains within cluster B (98% similarity by rep-PCR) and the two strains within cluster C (99% similarity by rep-PCR). However, rep-PCR also linked these two clusters to each other (97% similarity) despite their clear separation by spoligotype. It appears that rep-PCR must be interpreted in combination with an independent method such as spoligotyping.
Four of the five strains in IS6110-RFLP cluster A were linked to each other by rep-PCR (94% similarity); however, the fifth strain, 148, was well isolated from the others (87% similarity). Cluster A was less homogeneous by IS6110-RFLP than clusters B, C, and SBRI9 (Fig. 2). Nonetheless, there were no obvious genotypic or epidemiological distinctions between strain 148 and the other cluster A strains, so this result was scored as a false separation relative to the gold standard.
The three spoligogroup 149 isolates, strains 146, 160, and 252, had fewer than four IS6110 copies and therefore were not typeable by RFLP (not shown). These isolates are referred to as NT isolates in Fig. 1. Rep-PCR linked two of these isolates (96% similarity). Although the validity of this linkage could not be confirmed, the ability to generate complex banding patterns from low-IS6110-copy-number strains constitutes a potential benefit of rep-PCR.
The remaining 10 isolates in Fig. 1 exhibited unique IS6110-RFLP patterns (not shown) and were also separated from each other by the combination of spoligotyping and rep-PCR (strains 244, 146, and 160 were 93% similar to each other by rep-PCR, as were strains 229, 149, and 261; however, strains within both groups were separated from each other by spoligotyping). Overall, of the 11 outbreak isolates and 25 African Horn isolates typed by rep-PCR in Fig. 1 (n = 36), 23 segregated into four clusters identified by IS6110-RFLP, 10 exhibited unique RFLP patterns, and 3 were not typeable by RFLP. When interpreted in combination with spoligotyping, rep-PCR agreed with 32 of the 33 typeable RFLP results (97% concordance).
Heterogeneity of M. tuberculosis isolates from Eritrean, Ethiopian, and Somali immigrants to Seattle, 1998 to 2003.
In recent years, immigrants from the African Horn countries of Eritrea, Ethiopia, and Somalia have become the single largest immigrant group in the Seattle, Wash., area with regard to tuberculosis cases (1). Strains from these countries are underrepresented in studies of M. tuberculosis clonal expansion (12). Therefore, we used this evaluation to characterize the genotypic properties of isolates derived from anonymous tuberculosis patients who had immigrated from this region. Forty-five isolates from subjects treated at the King County Tuberculosis Clinic during the period from 1998 to 2003 were collected and spoligotyped. None of the 36 major epidemic superfamilies of M. tuberculosis spoligotypes defined previously (12) were prevalent within this sample; only one of them (spoligotype 53, which belongs to the T clade) was represented by more than one isolate.
Of the 45 isolates in the sample, 42 were typeable by the combination of spoligotyping and IS6110-RFLP (3 strains were not typeable due to low IS6110 copy number). Thirty-one of the 42 typeable isolates (74%) were placed into orphan groups by these modalities. The rest were divided into three clusters of two to five strains. Such heterogeneity is consistent with a predominant pattern of reactivation of past infections from heterogeneous sources.
As noted in the previous section, rep-PCR separated one of the cluster A strains from its siblings, and grouped two of the strains that were not typeable by RFLP. Other groupings were identical by rep-PCR and RFLP. When rep-PCR was used in place of IS6110-RFLP as the high-resolution adjunct to spoligotyping, 32 of the 45 strains in the African Horn cohort (71%) fell into orphan genotypes. When the three low-IS6110 copy number strains were removed from the sample set, orphan strains accounted for 32 of 42 (76%) of the sample set by rep-PCR, nearly identical to the 74% figure obtained by using IS6110-RFLP. Thus, within this sample the discriminative power of rep-PCR was equivalent to that of IS6110-RFLP.
Rep-PCR-based DNA fingerprinting of a M. avium subspecies avium isolates.
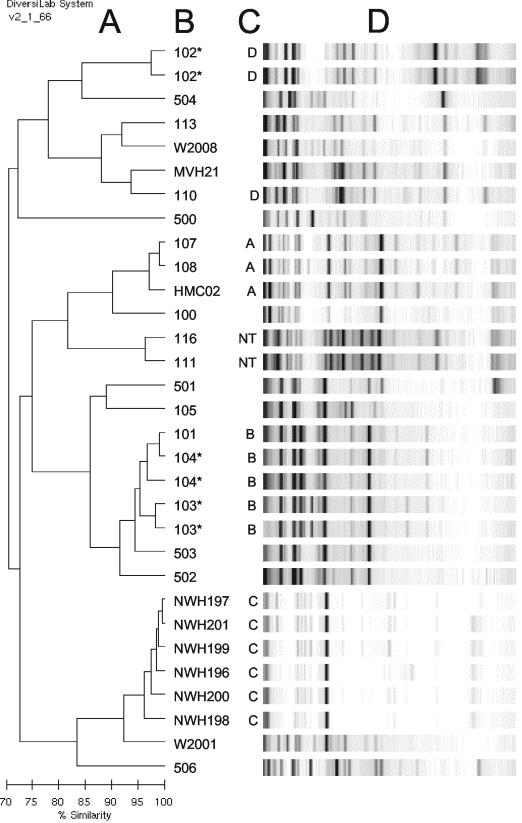
DNA samples from 28 M. avium subspecies avium strains were submitted for blinded rep-PCR typing (Table 2). Three of these samples were extracted and submitted in duplicate without informing the testers, bringing the total sample number to 31 (Fig. 3). The sample set included six genotypically linked isolates that were suspected to be contaminants of clinical laboratory cultures (see Materials and Methods). These six isolates, which exhibited similar but not identical IS1245-RFLP patterns (Fig. 4, cluster C), were used as a “standard cluster” to define a cutoff value of relatedness that we could expect to see among genotypically linked isolates. Rep-PCR correctly linked the cluster C isolates (≥92% similarity; Fig. 3). All three pairs of duplicate samples were also internally linked by rep-PCR (Fig. 3), the most divergent pair being the two strain 104 samples, which were 93% similar to each other.
FIG. 3.
Rep-PCR analysis of M. avium subspecies avium samples. The dendrogram (A) and gel-like images (D) were generated on coded DNA samples by Bacterial Barcodes, Inc. Correct strain identifications (B) and IS1245-RFLP clusters (C) were added by the authors. Duplicate samples are marked with asterisks. NT, not typeable by IS1245-RFLP.
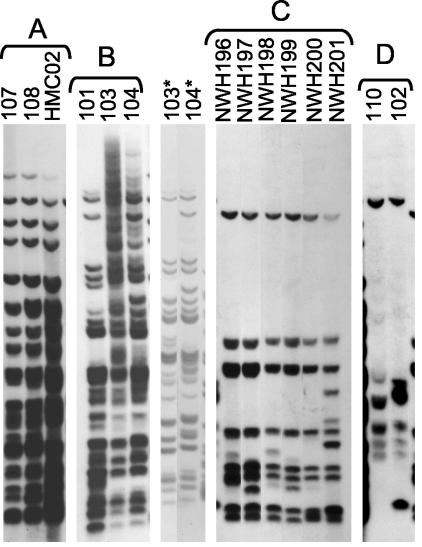
FIG. 4.
IS1245-RFLP patterns of the four major multistrain clusters of M. avium subspecies avium isolates in Fig. 3. The image was constructed from a single gel as in Fig. 2, except for the two lanes marked with asterisks. DNA from these two strains was incompletely digested in the main gel image of cluster B. Therefore, an image from a second gel was added to better document the similarity between these two strains.
IS1245-RFLP identified 3 additional clusters within this collection. Strains 101, 103, and 104 had highly similar IS1245-RFLP patterns (Fig. 4, cluster B); these strains came from separate adult AIDS patients at the same Los Angeles, Calif., hospital in the early 1980s. Strains 103 and 104 were 91% similar to each by rep-PCR, slightly below the cutoff value of 92% derived from cluster C. This was scored as a false separation relative to the gold standard. Strains HMC02, 107, and 108 were clustered by IS1245-RFLP (Fig. 4, cluster A), and were also linked to each other by rep-PCR (≥94% similarity). The linkage of the Seattle strain HMC02 with the Los Angeles strains 107 and 108 was unexpected but reproducible by an independent modality, IS999-RFLP (not shown).
Strains 102 and 110 had similar but not identical six- to seven-band IS1245-RFLP patterns (Fig. 4, cluster D). In view of their low IS1245 copy numbers, the genotypic relationship of these two strains is subject to interpretation. However, they also exhibited identical four-band IS999-RFLP patterns (not shown), and their epidemiological linkage supports their genotypic relationship. Rep-PCR did not link these strains (78% similarity), so this was scored as a false separation relative to the gold standard. Strains 111 and 116 were also linked to each other by rep-PCR (92% similarity) but generated no signal in IS1245-RFLP (NT isolate). The ability of rep-PCR to generate complex DNA fingerprints of these strains constitutes a potential advantage of the method.
Other isolates in Fig. 3 exhibited diverse IS1245-RFLP patterns in agreement with their diverse rep-PCR patterns (not shown). When the six standard cluster strains are included in the count, and the three redundant samples are excluded, the M. avium subspecies avium sample set totaled 28 isolates. Of these, 25 were clustered or isolated in identical ways by using rep-PCR or IS1245-RFLP (89% concordance). If the low-copy-number strains in cluster D are excluded from the count, the concordance increases to 25 of 26 (96%).
Rep-PCR-based DNA fingerprinting of a M. intracellulare and MACX isolates.
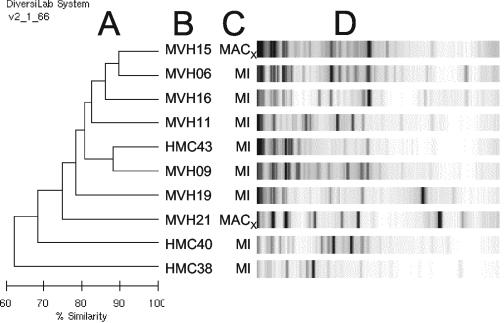
Eight M. intracellulare isolates and two MACX isolates were submitted for rep-PCR analysis. The rep-PCR system generated unique fingerprints of each strain (Fig. 5). Without consensus standard methods for comparison, it was impossible to validate these results. However, the ability of rep-PCR to generate complex fingerprint of these species constitutes a potential advantage.
FIG. 5.
Rep-PCR analysis of Mycobacterium intracellulare (MI) and MACX samples. The dendrogram (A) and gel-like images (D) were generated on coded DNA samples by Bacterial Barcodes, Inc. Correct strain identifications (B) and species (C) were added by the authors.
DISCUSSION
This study was designed to test the advantages of a new commercial rep-PCR system for typing M. tuberculosis and MAC strains. Foremost among the hypothetical strengths of rep-PCR is the ability to generate DNA fingerprints of any Mycobacterium species that is likely to be encountered in a clinical laboratory. With the exception of the technically demanding and nonportable pulsed-field gel electrophoresis method, other methods for mycobacteria are species specific. A second advantage of rep-PCR is its ability to generate fingerprints from small samples in real time, similar to spoligotyping and MIRU typing of M. tuberculosis. Its third advantage is its discriminative power, which we hypothesized would equal or exceed that of RFLP.
The DiversiLab System generated high-resolution DNA fingerprints from all isolates of M. tuberculosis, M. avium subspecies avium, M. intracellulare, and MACX that were examined. The sample included isolates that were not typeable by RFLP due to low copy number of the target insertion elements. The system has also generated fingerprints of multiple isolates of M. chelonae, M. simiae, M. mageritense, M. gordonae, M. peregrinum, and M. terrae (M. Healy [Bacterial Barcodes, Inc.], personal communication). Thus, rep-PCR is broadly applicable to Mycobacterium species.
Coded samples of ∼4 μg of DNA, each extracted from a loopful of cells, were delivered to the manufacturer for analysis on a prerelease rep-PCR system. Only 75 ng of each sample was actually used for each reaction. The protocol was identical to the manufacturer's other DiversiLab tests, which require 5 h from DNA extraction through dendrogram readout. In contrast to MIRU, which requires 4 to 12 separate PCRs per sample, rep-PCR requires only one PCR per sample. Thus, in terms of time to result, the DiversiLab System appears to match or surpass existing high-resolution PCR methods. It is far superior to existing RFLP and pulsed-field gel electrophoresis methods in this regard.
Within the M. tuberculosis and M. avium subspecies avium sample sets in this study, rep-PCR exhibited resolving power that equaled or exceeded that of RFLP. However, M. tuberculosis fingerprints generated by rep-PCR had to be interpreted in combination with spoligotype results, without which false linkages relative to RFLP appeared (e.g., strains 229 and 149 in column B near the top of Fig. 1). Moreover, one of the 11 SBRI9 strains of M. tuberculosis, strain 181, was separated from its siblings to a greater extent than expected from RFLP analysis. Examination of additional samples may reveal additional disagreements between rep-PCR and other methods. As with any strain typing method, rep-PCR should be interpreted in the context of independent modalities and epidemiological information. Because the present study encompassed four Mycobacterium species, the number of samples of each species was limited, and analysis of additional isolates is needed.
As one test of discriminative power, we used rep-PCR combined with spoligotyping to quantify the diversity of M. tuberculosis strains isolated from a cohort of recent immigrants to Seattle from African Horn countries. Spoligotyping was used as a low-resolution “first cut” and rep-PCR was used as a high-resolution adjunct to differentiate strains with nonunique spoligotypes. Of the 45 strains in the cohort, 32 were placed into orphan genotypes by spoligotyping alone or by spoligotyping in combination with rep-PCR. The remaining 13 clones segregated into 4 rep-PCR clusters that corresponded with RFLP clusters A, B, C, and NT (Fig. 1). This is nearly identical to the outcome obtained by using IS6110-RFLP as the high-resolution adjunct, the only two differences being that Cluster A contained four strains by RFLP and three strains by rep-PCR, and that the two-strain NT cluster identified by rep-PCR was not typeable by RFLP. Differences between the two outcomes could be resolved by adding a third modality such as MIRU. A combination of spoligotyping, MIRU, and rep-PCR would be entirely PCR based, such that complete results could be obtained from a single loopful of cells.
For nontuberculous mycobacteria, rep-PCR appears superior to any other existing method in terms of speed and ease of use. The discriminative power of rep-PCR for resolving M. avium subspecies avium isolates slightly exceeded that of IS1245-RFLP. A limitation of our evaluation was that a numerical cutoff was automatically applied to rep-PCR, whereas RFLP clusters were identified by visual analysis of results. This strategy was deemed necessary because the reproducibility and numerical output of RFLP gel analysis software remains problematic. When gel images generated by rep-PCR and RFLP were both assessed visually, rep-PCR appeared to be at least as discriminative as RFLP results.
Rep-PCR has some hypothetical limitations. It is a commercial system available from a single source. The “portability” of rep-PCR results, that is, the ability to compare strain fingerprints generated by different laboratories, remains untested for Mycobacterium species. The manufacturer compiles and offers to its customers a centralized, Web-based library of rep-PCR fingerprints, which can be searched for matches with any strain of interest (9). In view of the labor, expertise, and expense required for consistent generation and digitization of RFLP results, the DiversiLab System has the potential of being more portable than RFLP. However, by their nature rep-PCR results are likely to be less portable than those of MIRU and spoligotyping, which are easily expressed in numerical terms. The present study made no attempt to evaluate the cost of using the DiversiLab System, which requires an automated processor, relative to existing methods.
In summary, rep-PCR shows promise as a general tool for DNA fingerprinting of Mycobacterium isolates. Its most significant advantage is its broad applicability. It is also fast, PCR based, semiautomated, and highly discriminative. Further analysis may indicate that it can replace IS6110-RFLP as the high-resolution final adjunct to existing PCR-based methods for typing M. tuberculosis. It will be especially useful to laboratories that also work with nontuberculous Mycobacterium species.
Acknowledgments
We thank Maricel Lising and Mimi Healy of Bacterial Barcodes, Inc., for performing rep-PCR on their prerelease system and for providing helpful scientific input. The following sources provided or assisted with the acquisition of the Mycobacterium isolates: Linda Haba, Jeanette Frazier, Carolyn Wallis, Christy Huynh, the Washington State Department of Health (especially Trang Kuss, Kim Field, and Joseph Aharchi), Clark Inderlied, Marjorie Beggs, and Tim Ford.
This work was supported by a grant from Puget Sound Partners for Global Health, the Seattle-King County Department of Public Health, grant AI25767 from The National Institutes of Health (to G.A.C.), and equipment gifts from M. J. Murdock Charitable Trust and the Firland Foundation. K.L. was supported by NIH scientist scholar award HD07233 to the University of Washington, and D.L.-R. received support from the Mary Gates Endowment for Undergraduate Research.
K.S. is an independent consultant employed by Bacterial Barcodes, Inc.; the company provided no additional support for this study.
REFERENCES
- 1.Anonymous. 2002. Increase in African immigrants and refugees with tuberculosis—Seattle-King County, Washington, 1998-2001. Morbid. Mortal. Wkly. Rep. 51:882-883. [PubMed] [Google Scholar]
- 2.Arbeit, R. D., A. Slutsky, T. W. Barber, J. N. Maslow, S. Niemczyk, J. O. Falkinham III, G. T. O'Connor, and C. F. von Reyn. 1993. Genetic diversity among strains of Mycobacterium avium causing monoclonal and polyclonal bacteremia in patients with AIDS. J. Infect. Dis. 167:1384-1390. [DOI] [PubMed] [Google Scholar]
- 3.Barnes, P. F., and M. D. Cave. 2003. Molecular epidemiology of tuberculosis. N. Engl. J. Med. 349:1149-1156. [DOI] [PubMed] [Google Scholar]
- 4.Bermudez, L. E., P. Kolonoski, M. Wu, P. A. Aralar, C. B. Inderlied, and L. S. Young. 1999. Mefloquine is active in vitro and in vivo against Mycobacterium avium complex. Antimicrob. Agents Chemother. 43:1870-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bifani, P. J., B. Mathema, Z. Liu, S. L. Moghazeh, B. Shopsin, B. Tempalski, J. Driscoll, R. Frothingham, J. M. Musser, P. Alcabes, and B. N. Kreiswirth. 1999. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. JAMA 282:2321-2327. [DOI] [PubMed] [Google Scholar]
- 6.Bruijn, F. J. D., J. R. Lupski, and G. M. Weinstock. 1998. Bacterial genomes: physical structure and analysis. Chapman & Hall, New York, N.Y.
- 7.Chen, Z. H., W. R. Butler, B. R. Baumstark, and D. G. Ahearn. 1996. Identification and differentiation of Mycobacterium avium and M. intracellulare by PCR. J. Clin. Microbiol. 34:1267-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowan, L. S., L. Mosher, L. Diem, J. P. Massey, and J. T. Crawford. 2002. Variable-number tandem repeat typing of Mycobacterium tuberculosis isolates with low copy numbers of IS6110 by using mycobacterial interspersed repetitive units. J. Clin. Microbiol. 40:1592-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deak, T., J. Chen, and L. R. Beuchat. 2000. Molecular characterization of Yarrowia lipolytica and Candida zeylanoides isolated from poultry. Appl. Environ. Microbiol. 66:4340-4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Vecchio, V. G., J. M. Petroziello, M. J. Gress, F. K. McCleskey, G. P. Melcher, H. K. Crouch, and J. R. Lupski. 1995. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J. Clin. Microbiol. 33:2141-2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falkinham, J. O., III. 1996. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 9:177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Filliol, I., J. R. Driscoll, D. van Soolingen, B. N. Kreiswirth, K. Kremer, G. Valetudie, D. D. Anh, R. Barlow, D. Banerjee, P. J. Bifani, K. Brudey, A. Cataldi, R. C. Cooksey, D. V. Cousins, J. W. Dale, O. A. Dellagostin, F. Drobniewski, G. Engelmann, S. Ferdinand, D. Gascoyne-Binzi, M. Gordon, M. C. Gutierrez, W. H. Haas, H. Heersma, E. Kassa-Kelembho, H. M. Ly, A. Makristathis, C. Mammina, G. Martin, P. Mostrom, I. Mokrousov, V. Narbonne, O. Narvskaya, A. Nastasi, S. N. Niobe-Eyangoh, J. W. Pape, V. Rasolofo-Razanamparany, M. Ridell, M. L. Rossetti, F. Stauffer, P. N. Suffys, H. Takiff, J. Texier-Maugein, V. Vincent, J. H. de Waard, C. Sola, and N. Rastogi. 2003. Snapshot of moving and expanding clones of Mycobacterium tuberculosis and their global distribution assessed by spoligotyping in an international study. J. Clin. Microbiol. 41:1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jasmer, R. M., M. Roemer, J. Hamilton, J. Bunter, C. R. Braden, T. M. Shinnick, and E. P. Desmond. 2002. A prospective, multicenter study of laboratory cross-contamination of Mycobacterium tuberculosis cultures. Emerg. Infect. Dis. 8:1260-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurepina, N. E., S. Sreevatsan, B. B. Plikaytis, P. J. Bifani, N. D. Connell, R. J. Donnelly, D. van Sooligen, J. M. Musser, and B. N. Kreiswirth. 1998. Characterization of the phylogenetic distribution and chromosomal insertion sites of five IS6110 elements in Mycobacterium tuberculosis: non-random integration in the dnaA-dnaN region. Tuberc. Lung Dis. 79:31-42. [DOI] [PubMed] [Google Scholar]
- 16.Kwara, A., R. Schiro, L. S. Cowan, N. E. Hyslop, M. F. Wiser, S. Roahen Harrison, P. Kissinger, L. Diem, and J. T. Crawford. 2003. Evaluation of the epidemiologic utility of secondary typing methods for differentiation of Mycobacterium tuberculosis isolates. J. Clin. Microbiol. 41:2683-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurent, J. P., S. Faske, and G. A. Cangelosi. 2002. Characterization of IS999, an unstable genetic element in Mycobacterium avium. Gene 294:249-257. [DOI] [PubMed] [Google Scholar]
- 18.Milan, S. J., H. A. Hauge, N. E. Kurepina, K. H. Lofy, S. Goldberg, M. Narita, C. Nolan, P. McElroy, B. Kreiswirth, and G. A. Cangelosi. 2004. Expanded geographical distribution of the N family of Mycobacterium tuberculosis strains within the United States. J. Clin. Microbiol. 42:1064-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mukherjee, S., M. Petrofsky, K. Yaraei, L. E. Bermudez, and G. A. Cangelosi. 2001. The white morphotype of Mycobacterium avium-intracellulare is common in infected humans and virulent in infection models. J. Infect. Dis. 184:1480-1484. [DOI] [PubMed] [Google Scholar]
- 20.Redkar, R. J., M. P. Dube, F. K. McCleskey, M. G. Rinaldi, and V. G. Del Vecchio. 1996. DNA fingerprinting of Candida rugosa via repetitive sequence-based PCR. J. Clin. Microbiol. 34:1677-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sola, C., I. Filliol, E. Legrand, S. Lesjean, C. Locht, P. Supply, and N. Rastogi. 2003. Genotyping of the Mycobacterium tuberculosis complex using MIRUs: association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Infect. Genet. Evol. 3:125-133. [DOI] [PubMed] [Google Scholar]
- 22.van Embden, J. D., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, et al. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rie, A., R. Warren, M. Richardson, T. C. Victor, R. P. Gie, D. A. Enarson, N. Beyers, and P. D. van Helden. 1999. Exogenous reinfection as a cause of recurrent tuberculosis after curative treatment. N. Engl. J. Med. 341:1174-1179. [DOI] [PubMed] [Google Scholar]
- 24.van Soolingen, D., J. Bauer, V. Ritacco, S. C. Leao, I. Pavlik, V. Vincent, N. Rastogi, A. Gori, T. Bodmer, C. Garzelli, and M. J. Garcia. 1998. IS1245 restriction fragment length polymorphism typing of Mycobacterium avium isolates: proposal for standardization. J. Clin. Microbiol. 36:3051-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versalovic, J., V. Kapur, T. Koeuth, G. H. Mazurek, T. S. Whittman, J. M. Musser, and J. R. Lupski. 1995. DNA fingerprinting of pathogenic bacteria by fluorophore-enhanced repetitive sequence-based polymerase chain reaction. Arch. Pathol. Lab. Med. 119:23-29. [PubMed] [Google Scholar]
- 26.Versalovic, J., V. Kapur, E. O. Mason, U. Shah, T. Koeuth, J. R. Lupski, and J. M. Musser. 1993. Penicillin-resistant Streptococcus pneumoniae strains recovered in Houston: identification and molecular characterization of multiple clones. J. Infect. Dis. 167:850-856. [DOI] [PubMed] [Google Scholar]
- 27.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Versalovic, J., and J. R. Lupski. 2002. Molecular detection and genotyping of pathogens: more accurate and rapid answers. Trends Microbiol. 10:s15-s21. [DOI] [PubMed] [Google Scholar]
- 29.Von Reyn, C. F., J. N. Maslow, T. W. Barber, J. O. Falkinham III, and R. D. Arbeit. 1994. Persistent colonisation of potable water as a source of Mycobacterium avium infection in AIDS. Lancet 343:1137-1141. [DOI] [PubMed] [Google Scholar]