Abstract
In the United States the principal environmental exposure to mercury is through dietary consumption of sea food. Although the mechanism by which low levels of mercury affect the nervous system is not well established, epidemiological studies suggest that low level exposure of pregnant women to dietary mercury can adversely impact cognitive development in their children, but that Docosahexaenoic acid (DHA), the most prominent n-polyunsaturated fatty acid (n-PUFA) present in fish may counteract negative effects of mercury on the nervous system. Aside from effects on the nervous system, epidemiological and animal studies have also suggested that low level mercury exposure may be a risk factor for autoimmune disease. However unlike the nervous system where a mechanism linking mercury to impaired cognitive development remains elusive, we have previously suggested a potential mechanism linking low level mercury exposures to immune system dysfunction and autoimmunity. In the immune system it is well established that disruption of CD95 mediated apoptosis leads to autoimmune disease. We have previously shown in vitro as well as in vivo that in lymphocytes burdened with low levels of mercury, CD95 mediated cell death is impaired. In this report we now show that DHA counteracts the negative effect of mercury on CD95 signaling in T lymphocytes. T cells which have been pre-exposed to DHA are able to cleave pro-caspase 3 and efficiently signal programmed cell death through the CD95 signaling pathway, whether or not they are burdened with low levels of mercury. Thus DHA may lower the risk of autoimmune disease after low level mercury exposures.
Keywords: Docosahexaenoic acid, mercury, CD95, T cell
INTRODUCTION
Exposure to high or even moderate levels of mercury is associated with adverse effects in humans. At high to moderate cellular burdens, mercury is toxic and damages the nervous and immune systems (Gerstner et al., 1977; Clarkson, 1997; Risher et al., 2002). The problem is with evaluating the risk of exposure to current low-level environmental sources of mercury, in that despite more than 35 years of searching, no clear answer has yet emerged as to where the danger point for chronic low level exposure lies (Clarkson et al., 2006). For the most part, interest in low-level mercury exposures has centered on the nervous system. The current EPA RfD (lowest exposure level that is likely to be without risk of harmful effects) for mercury is to a large extent based on data from just two epidemiological studies, one in the Faroe Islands, and the other in New Zealand. Both studies found a correlation between consumption of sea mammals with high mercury levels and defects in cognitive development of children (Budtz-Jorgensen et al., 2000; Crump et al., 1998).
On the other hand a large and ongoing study in the Seychelles where individuals consume large amounts of ocean fish, and mercury exposures are similar to the Faroes and New Zealand cohorts, has so far generally failed to confirm the Faroes and New Zealand findings (Myers et al., 2003; Myers et al., 2009). At the present time it is unclear why the results differ, but it has been suggested that in the Seychelles, high levels of dietary n-3 polyunsaturated fatty acids (n-3 PUFA) found in fish, but not mammals, may serve to ameliorate deleterious effects of mercury (Clarkson and Magos, 2006; Strain et al., 2008). Although diets high in n-3 PUFA are thought by many to be beneficial, unfortunately it has never been directly shown that dietary n-3 PUFA will directly counter any toxic effects associated with low-level mercury exposures. Nor is there currently any understanding of a mechanism which would support such an interaction between n-3 PUFA and mercury in the nervous system.
In recent years, aside from an ongoing concern of the effect of mercury on the nervous system, there has been growing interest in the effect of low-level environmental mercury exposures on the immune system. The evidence suggests that following low-level exposure mercury cooperates with other factors, such as genetic predisposition, exposure to antigens and other toxicants, or infection, in order to exacerbate autoimmune diseases (Silbergeld et al., 2005). Although as in the nervous system the mechanism has not yet been fully elucidated, we have suggested that one way in which mercury may be associated with autoimmune disease is through interference with the CD95 signaling pathway. Disruption of CD95 signaling is well known to have detrimental pro-inflammatory and autoimmune consequences (Strasser et al., 2004; Barreiro et al., 2004; Krueger et al., 2003), and we have reported that both in vitro and in vivo, low level exposures to mercury leading to environmentally relevant mercury burdens on T cells result in diminished CD95 signaling (Whitekus et al., 1999; McCabe, Jr. et al., 2005a; Laiosa et al., 2007). While the targets of mercury are not necessarily the same in the immune and nervous systems, we conjectured that in general n-3 PUFAs might counter the negative effect of low level Hg in the immune system as they seem to do in the nervous system. In particular we hypothesized that the n-3 PUFA docosahexaenoic acid (DHA), might affect Hg's ability to interfere with CD95 signaling.
Materials and Methods
Cell line
The Jurkat human T cells (Clone E6-1) were obtained from the American Type Culture Collection (ATCC), Rockville, MD. They were maintained in RPMI 1640 supplemented with 10% FBS (Hyclone), 2 mM L-glutamine ( Hyclone) and 1X antibiotic antimycotic (Hyclone) at 37°C in humidified atmosphere of 5 % CO2. The cells were passaged twice a week.
Anti-CD95 (Fas) killing of Jurkat cells
Jurkat cells were resuspended in complete medium at 2X106 cells per ml. 100 μl of cells were added to each well of 96 well tissue culture plates. Anti-Fas activating antibody (Anti-Fas clone CH11; Millipore) was added to the appropriate wells to final concentrations of 0, 12.5, 25, 50 or 100 ng/ml. The treated cells were incubated for 2 to 24 hr at 37°C. At the end of the incubation period, cell viability was determined using the MTT assay according to the manufacturer's instructions. The level of killing was determined as percent of untreated cells.
Preparation of DHA-EE complexed BSA
Docosahexaenoic acid ethyl ester (DHA-EE) at a concentration of 500 mg/ml (1.4 M) in ethanol was obtained from Cayman Chemical, Ann Arbor, MI. The DHA-EE was diluted to 4.5 μM with degassed RPMI under N2. An equal volume of fatty acid-free Bovine Serum Albumin (BSA; A3803; Sigma) at a concentration of 1.5 μM in degassed DPBS was added to the DHA-EE to give a molar ratio of 3:1. The tube was flushed with N2, covered with aluminum foil and vortexed for 10 minutes. The DHA-EE/BSA was then incubated for 2 hours at 37°C. The DHA-EE/BSA was stored at 4°C under N2 until used. The DHA-EE/BSA was diluted to the desired concentration in complete RPMI medium prior to addition to cells.
DHA-EE treatment of cells
Cells at between 2X106 and 1.4X106 cells/ml were treated with DHA-EE/BSA or BSA overnight at 37°C. The cells were then treated with dH2O or HgCl2 to a final concentration of 5 μM for 10 minutes at 37°C. Following the incubation with HgCl2, 100 μl of cells were added to wells of a 96-well tissue culture plate. The cells were then treated with varying concentrations anti-CD95 (Anti-Fas clone CH11; Millipore) or DPBS for varying periods of time. At the end of the anti-CD95 treatment, cell viability was determined using the MTT assay
MTT Assay
At the end of treatment, 25 μl of MTT (5 μg/ml in DPBS) was added to the wells and the plate was incubated for 2 hours at 37°C. 100 μl of 10% SDS/0.01 N HCl solution was added to each well and the plate was incubated for 37°C overnight. Absorbance values were determined by reading the plate was at 570 nm and 630 nm on a SpectraFluor Plus plate reader (Tecan) running Magellan software. The difference between the two wavelengths was calculated and used to determine the percent viability.
Caspase-3 Plate Assay
Jurkat cells were resuspended in 10 ml complete medium at 0.62X106 cells per ml. The cells were treated DHA-EE complexed to BSA or BSA alone. The cells were then incubated over night at 37°C. The cells were then counted and resuspended at 2X106 cells per ml. One ml of cells was added to 12X75 mm polystyrene tubes. The cells were treated with HgCl2 to a final concentration of 5 μM or ddH2O for 10 minutes at 37°C. Anti-Fas antibody (Millipore) was added to the appropriate tubes to a final concentration of 250 ng/ml. Cells were incubated for 6 h or 2 h at 37°C. Cells were pelleted and lysed according to the caspase-3 kit (Millipore) instructions. The caspase-3 assay was performed according to kit instructions. Caspase-3 activity was determined using a SpectraFluor Plus plate reader at 405 nm.
Caspase Activity determination by Flow Cytometry
Jurkat cells were suspended in complete medium at 1.5X106 cells per ml. The cells were treated with DHA-EE complexed to BSA or BSA alone at a final concentration of 10 μM DHA-EE and then incubated over night at 37°C. The cells were then counted and resuspended at 1X106 cells per ml and treated with HgCl2 to a final concentration of 5 μM or ddH2O for 10 minutes at 37°C. One ml of cells was added to 12X75 mm polystyrene tubes. Anti-Fas antibody (Millipore) was added to the appropriate tubes to a final concentration of 100 ng/ml. Cells were incubated for 5 hr. at 37°C. The cells were pelleted and carefully resuspended in caspase 3 substrate (PhiPhiLux-G1D2) as described by manufacturer (OncoImmunin, Inc.). Briefly, cell pellets were mixed with substrate by pipetting and incubated for 1 hour at 37°C. Cells were pelleted and resuspended in dilution buffer and analyzed by flow cytometry. Cells were read using a Beckman Coulter Cyan ADP flow cytometer. The cells were excited with a 488 nm laser line and detected in the FITC channel. Data was analyzed using Summit software, which included a provision for the Overton histogram subtraction algorithm (Beckman Coulter).
Statistical Analysis
ANOVA analysis was accomplished utilizing the Kruskal-Wallis One Way Analysis of Variance. Statistical significance between population means was established utilizing the two-tailed t test embedded in the excel program (Microsoft) assuming an alpha value of 0.05.
RESULTS
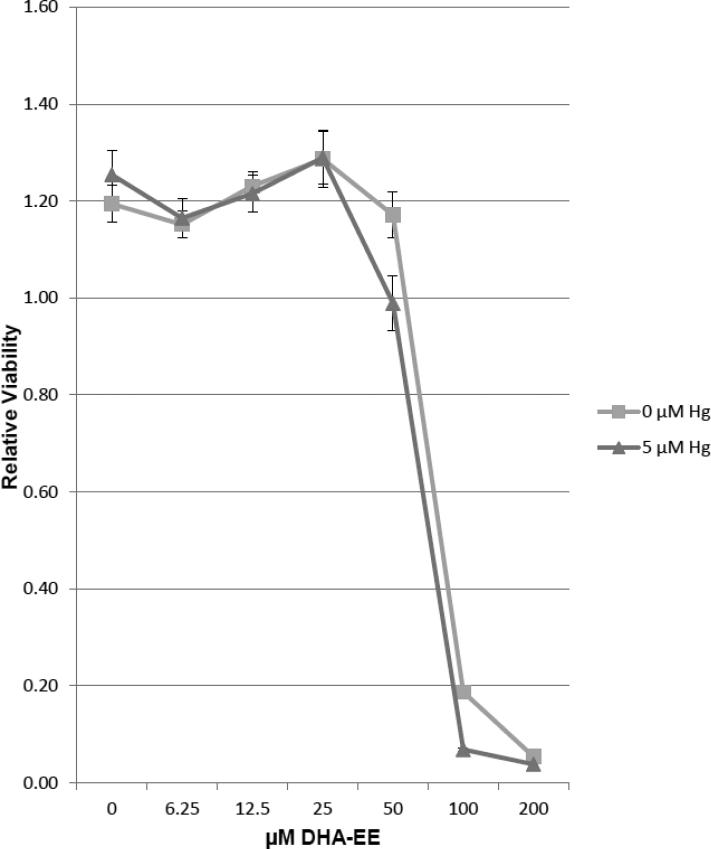
Cell viability is unaffected by exposure to concentrations of DHA-EE below 100 μM in the presence or absence of 5 μM Hg2+
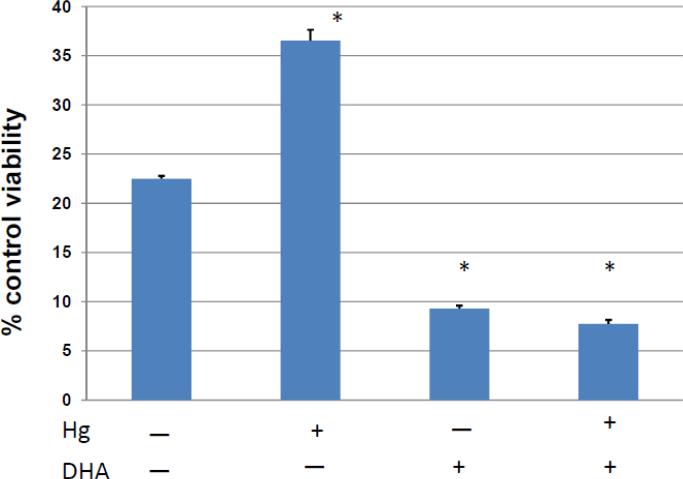
We have previously found that under some conditions that high levels of DHA could be toxic (Church et al., 2010). In order to assure that this was not the case under the conditions employed in these experiments, in figure 1 Jurkat T cells were exposed to 5 μM Hg2+ or vehicle control . After 10 minutes all cells were placed into 96 well culture plates. Two-fold serial dilutions of BSA complexed with DHA-EE starting with 200 μM, or BSA alone was added to cells which were exposed to mercury, as well as to cells which were not. All cells were then cultured overnight and then assayed for viability with the MTT assay. We find that in the absence of Hg2+, concentrations of DHA-EE at or below 50 uM have no statistically different effect on cell viability from cells which have not been exposed to DHA-EE. On the other hand, for concentrations of DHA-EE at or greater than 100 μM, we find a dose dependent decrease in cell viability. For cells cultured with concentrations of DHA-EE of 50 μM and above, we find a tendency to a slight decrease in viability of cells which have also been exposed to Hg2+. However with the exception of cells exposed to 100 μM DHA-EE, the differences are not statistically significant.
Figure 1.
Cell viability is unaffected by exposure to low levels of DHA, either in the presence or absence of mercury. Jurkat T cells were incubated overnight with various concentrations of DHA. Cell viability was then assessed for each culture with the MTT assay. OD570nm-630nm was determined and represented as a measure of relative viability. Error bars represent the SEM of 6 replicate samples.
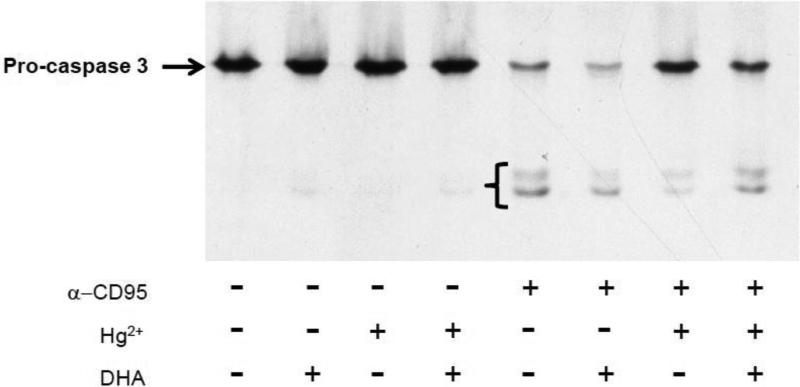
DHA ameliorates mercury's ability to interfere with CD95 mediated cleavage of caspase 3
Activated (cleaved) caspase 3 is a key player in CD95 mediated cell death, and during CD95 signaling cleavage/activation of caspase 3 normally follows downstream of ligand binding to the CD95 receptor (Abbas, 1996). We have previously shown that upon exposure to mercury, activation of caspase 3 in T cells is compromised and correlated with a diminished ability of CD95 to signal apoptosis (McCabe, Jr. et al., 2005b;McCabe, Jr. et al., 2003a). In figure 2 Jurkat T cells were incubated overnight with DHA-EE conjugated BSA (27 μM DHA-EE) or DHA-EE free BSA. Cells were then incubated for 10 minutes with or/ without 5 μM Hg2+. Each of these groups were then in turn treated (or not treated) with anti-CD95 (50 ng/ml) antibody to activate (or not) CD95/Fas signaling. Cell lysates were then prepared, and western blotting for caspase 3 performed with an antibody recognizing intact zymogen pro-caspase 3 (arrow) as well as the cleaved/activated forms (bracket). Confirming our previous findings, we find that in cells which have not been pretreated with DHA-EE, Hg2+ interferes with the ability of CD95 to signal cleavage of caspase 3. However figure 2 also shows that in cells which have been pretreated with DHA-EE, CD95 is able to signal caspase 3 cleavage in cells which have been burdened with Hg2+.
Figure 2.
DHA allows mercury burdened T cells to retain the ability to cleave pro-caspase 3 after CD95 signaling. Jurkat T cells were incubated overnight with or without DHA. HgCl2 was then added to half the cells, and then cells were treated or not with anti-CD95 in order to stimulate CD95 signaling. Cells were then lysed and lysates analyzed by western blotting. Procaspase 3 is indicated by the arrow, while lower molecular weight cleaved (activated) caspase 3 is indicated by the bracket. The figure is a representative example from three independent experiments.
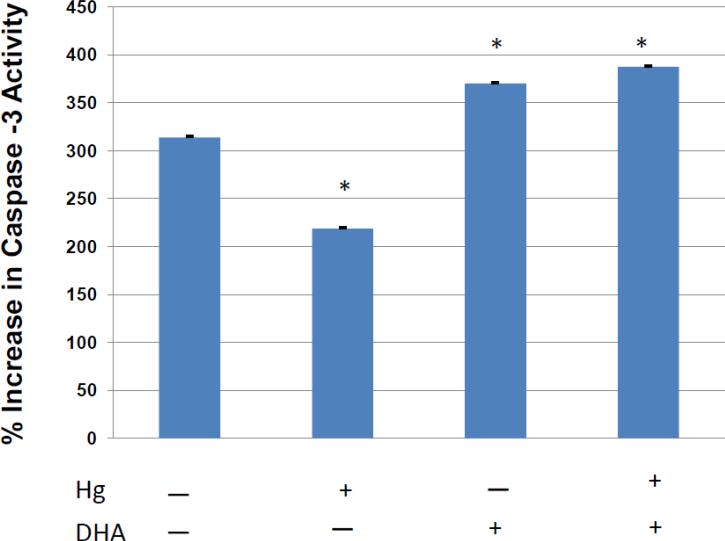
The qualitative western blot findings in figure 2 were confirmed and expanded upon with a quantitative assay for caspase-3 activation. As in figure 2, Jurkat T cells were or were not pretreated with DHA-EE and /or Hg2+, after which half of the cells were treated with activating anti-CD95. Cells from each of the differentially treated cell populations were placed into eight wells of a 96 well plate which was incubated for 5 hours, and then assayed for activated caspase 3 using a commercial (Millipore) fluorescence based microtiter plate assay. In figure 3 the caspase 3 activity level of anti-CD95 activated cells (in the presence or absence of Hg2+ and / or DHA-EE) was compared to untreated cells, and the results expressed as percentage increases in caspase 3 activity. We find in agreement with the western blotting results in figure 3, that Hg2+ interferes with CD95 mediated induction of caspase-3 activation, in that active caspase is reduced by about 30% after exposure to mercury. However, this effect of Hg2+ is abrogated in DHA treated cells.
Figure 3.
Quantitative assessment of the effect of DHA on CD95 mediated caspase 3 activation in mercury burdened T cells. Jurkat T cells were incubated overnight with or without DHA. HgCl2 was then added to half the cells, and then cells were treated with anti-CD95 in order to stimulate CD95 signaling. After an additional culture period cells were assayed for active caspase 3, and the % increase in caspase 3 activity determined by comparison with control cells which were not stimulated with anti-CD95. Error bars represent the SEM of 6 replicate samples. The (*) symbol represents a statistically significant difference of the mean compared to the mean of Hg (−) and DHA (−) cells.
Flow cytometry provides further insight into the effect of DHA on caspase 3 cleavage in mercury burdened T cells
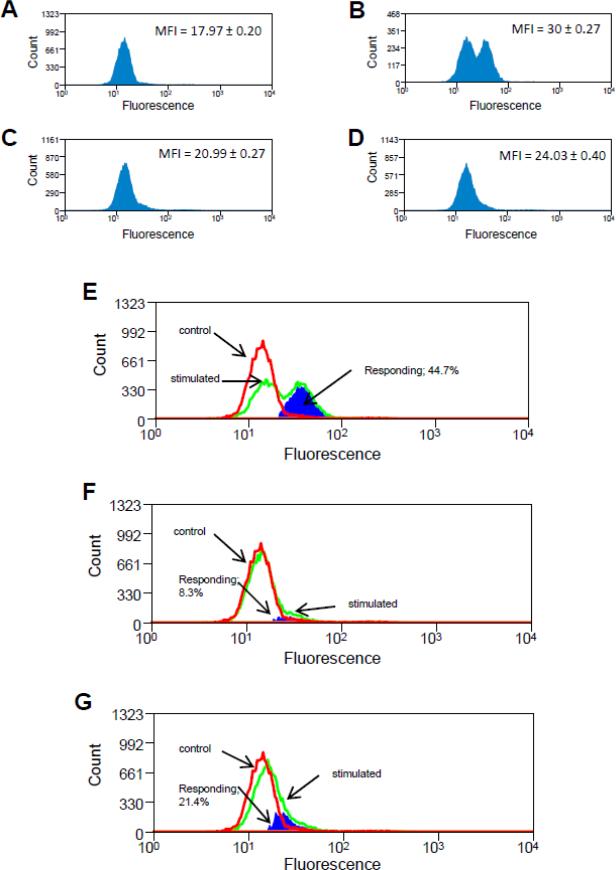
In figure 4, Jurkat T cells were treated with DHA-EE, Hg2+ and anti-CD95 as in figure 3, and then again as in figure 3 incubated for 5 hours before being assayed for activated caspase 3. However instead of utilizing a microtiter plate assay to measure caspase 3 activity, cells were incubated with a lipophillic cell permeable fluorogenic substrate possessing the DEVDGI target peptide sequence for caspase 3 (Komoriya et al., 2000). After substrate incubation, for cells in which an active caspase 3 enzyme was present the target peptide sequence was cleaved, releasing the fluorophore that is detectable in the FITC channel of the flow cytometer. Histograms representing the (active caspase 3) fluorescence of cells which were untreated; cells which have been treated with anti-CD95, cells which have been treated with combined anti-CD95 and Hg2+; and cells which have been treated with the combination of DHA-EE, Hg2+ and anti-CD95 are presented in figures 4 A-D respectively. (The histogram for cells which were treated with DHA-EE alone was obtained, and found to be similar to the untreated cells, but is not shown) For each histogram shown, the mean fluorescence index (MFI) of the population (defined as the median total FITC signal of the histogram) is indicated.
Figure 4.
Flow cytometry confirms that mercury burdened T cells which have been exposed to DHA retain the ability to activate caspase 3 in response to CD95 signaling. Jurkat T cells were treated with DHA, HgCl2, anti-CD95 or vehicle controls. Cells were then treated with a lipophillic cell permeable fluorogenic substrate possessing the DEVDGI target peptide sequence for caspase 3, and fluorescence (indicative of active caspase 3), was measured by flow cytometry and the results displayed with the histograms which are graphed. The mean fluorescence Index (MFI) of each histogram, defined as the median of the distribution is displayed to the upper right of each figure. Where indicated, subtraction of histograms was accomplished utilizing the Overton algorithm. (A) Histogram for untreated control cells; (B) Histogram for cells which have been treated only with anti-CD95; (C) Histogram for cells which were treated with Hg2+ prior to anti-CD95 stimulation; (D) Histogram for cells which were treated with Hg2+ prior to anti-CD95 stimulation, but which were also exposed to DHA. (E) Subtraction of histogram representing cells treated only with anti-CD95 (figure 4B) from control cell histogram (figure 4A) (F) Subtraction of histogram for cells which were treated with Hg2+ prior to anti-CD95 stimulation (figure 4C) from control cell histogram (figure 4A). (G) Subtraction of histogram for cells which were treated with Hg2+ prior to anti-CD95 stimulation, but which were also exposed to DHA (figure 4D) from control cell histogram (figure 4A).
We find that the results are in agreement with those of figure 3. In particular figure 4A represents the population distribution of the background signal obtained from untreated cells. This signal arises from an assortment of sources, including auto fluorescence, nonspecific cleavage of the fluorogenic substrate, as well as the low level of endogenous apoptosis inherent in cultured cell populations. Figure 4B represents the distribution of the signal obtained from the population of T cells which have been stimulated with anti-CD95. While the control histogram (A) contains a single low intensity peak, the histogram for the anti-CD95 activated cells (B) depicts a high intensity peak in addition to a low intensity peak. The high intensity peak arises as a result of active caspase 3 in CD95 stimulated cells and its presence explains the higher MFI attributed to the total stimulated cell population (30.00) vs. that attributed to the control population (17.97). We assume that the difference in MFI (30.00 - 17.97 = 12.03) between the stimulated and control populations is due to and can be taken as a specific measure of CD95 mediated activation of caspase 3.
For cells which have been exposed to Hg prior to activation, we find that after CD95 mediated stimulation the population is characterized by a single peak with an MFI of 20.99. As for figure B, the difference in MFI from control cells (20.99 – 17.97 = 3.02) is taken as a measure of caspase 3 activation. In agreement with figure 3, we interpret these results to indicate that Hg interferes with, but does not completely block the ability of CD95 to stimulate activation of caspase 3. Finally, figure 4D represents the population of cells which have been stimulated with anti-CD95 after exposure to both Hg and DHA. Here the population is again characterized by a single peak, but with an MFI of 24.03. As the difference in MFI from controls (30.00-17.97 = 12.03) is greater than that of figure 4C, we interpret this finding to support the results of figure 3 showing that DHA ameliorates mercury's ability to interfere with CD95 mediated cleavage of caspase 3.
Although by comparing the MFIs of differently treated cell populations, the flow cytometer can be used interchangeably with an ELISA plate assay to determine changes in caspase 3 activation on a population level, (as the caspase 3 plate assay does essentially the same thing), the real power of the instrument in this application lies in its ability to determine caspase activation differences on a cellular level. For instance, consider figure 4A representing the population distribution of the background signals obtained from control cells, and figure 4B representing the population distribution of active caspase 3 in cells which have been stimulated with anti-CD95. The fact that the control histogram (A) contains a single low intensity peak, while the histogram for the activated cells (B) depicts a high intensity peak in addition to a low intensity peak, implies that after CD95 mediated activation there are two distinct cell populations, cells which are activated and cells which are not. Put another way, CD95 mediated caspase 3 activation seems to be an all or none event and the high intensity peak represents the cells with activated caspase 3.
The fraction of activated cells included in the high signal intensity peak in figure 4B can be determined by utilization of the Overton subtraction algorithm (Overton, 1988). Aside from a “data smoothing” correction procedure primarily affecting lower signal intensities, the Overton algorithm essentially subtracts the control signal histogram (in this case figure 4A) from the histogram representing the signal recorded from activated cells (in this case figure 4B) on a channel by channel basis. This is demonstrated in figure 4E. The heavy (red) line represents the histogram obtained from untreated cells (originally plotted in figure 4A), and has here been labeled “control”, while the lighter (green) line represents the histogram obtained from cells which have been treated with anti-CD95 (originally plotted in figure 4B) and is here labeled “stimulated”. The darkly shaded (blue) area represents the difference between the two histograms as calculated with the Overton algorithm, and in this case corresponds to cells (44.7% of the total), which have responded to the anti-CD95 signal by activating caspase 3. In other words the Overton analysis shows that the difference in MFI (30.00-17.97 = 12.03) which we previously determined between the activated and control distributions is due to the fact that 44.7 % of the cells have responded to anti-CD95 by activating caspase 3.
In a similar manner, figure 4F utilizes the Overton algorithm to subtract the control histogram (figure 4A) from the histogram obtained from cells treated with the combination of anti-CD95 and Hg2+ (figure 4C). Again, the control histogram is represented by the heavy (red) line, the histogram representing CD95 stimulated cells is associated with the light (green) line, and the difference between the two is given by the (blue) shaded area. Figure 4F indicates that for Hg2+ treated cells only 8.3% of the total population responds to anti-CD95. This is down from 44.7% for cells treated with anti-CD95 in the absence of Hg2+, confirming that Hg2+ interferes with CD95 mediated activation of caspase 3. Finally figure 4G utilizes the Overton algorithm to show that after combined DHA and Hg2+ exposure, 21.4% of the cells now respond to anti-CD95 by cleaving caspase 3, reinforcing our thesis that DHA ameliorates mercury's ability to interfere with CD95 mediated cleavage of caspase 3.
The ability of DHA to preserve CD95 mediated caspase 3 cleavage in mercury treated T cells is correlated with the preservation of CD95 mediated death
Control experiments demonstrated that the effects of DHA with respect to both toxicity and CD95 mediated caspase 3 activation are dose dependent. In figure 5 changes in caspase 3 activity are correlated with the functional outcome of cell death. Jurkat T cells were pretreated with DHA-EE (67 μM, the highest DHA concentration for which we were confident DHA was not toxic) or vehicle control overnight. Cells were then treated with Hg2+ or vehicle control as in figures 2-4, after which half of the cells were treated with activating anti-CD95. Cells from each of the differentially treated cell populations were then placed into eight wells of a 96 well plate and incubated for 22 hours. Cell viability was then determined with the MTT assay. The results for each of the anti-CD95 treated populations were then compared to control populations which were identically treated with respect to DHA and Hg2+, but which were not exposed to anti-CD95. The results were then expressed as the % of the control viability.
Figure 5.
Quantitative assessment of the effect of DHA on CD95 mediated cell death in mercury burdened T cells. Jurkat T cells were incubated overnight with DHA or vehicle control. HgCl2 was then added to half the cells, and then cells were treated with anti-CD95 in order to stimulate CD95 signaling of cell death. After an additional culture period cells were assayed for viability, and the % decrease in viability determined by comparison with control cells which were not stimulated with anti-CD95. Error bars represent the SEM of 6 replicate samples. The (*) symbol represents a statistically significant difference of the mean compared to the mean of Hg (−) and DHA (−) cells.
We find that as expected, in cells which have not been exposed to either Hg2+ or DHA that after CD95 signaling, cell viability is reduced. In this case viability is reduced about 80% from control levels. However in cells which have been exposed to Hg2+, CD95 mediated cell death is attenuated, in that cell viability is only reduced about 65% from control levels. On the other hand, in cells which were pre-exposed to DHA, Hg2+ no longer attenuates CD95 mediated cell death. In fact DHA appears to slightly enhance CD95 mediated cell death.
Discussion
We have previously shown that exposure of lymphocytes to low levels of Hg2+ is not classically toxic in the sense that cell growth is maintained, but that signaling through the CD95 death pathway is significantly inhibited (Whitekus, Santini, Rosenspire, and McCabe, 1999;McCabe, Jr., Eckles, Langdon, Clarkson, Whitekus, and Rosenspire, 2005a;Ziemba et al., 2005;McCabe, Jr. et al., 2003b;Laiosa, Eckles, Langdon, Rosenspire, and McCabe, Jr., 2007). In the normally functioning immune system excessive lymphocyte proliferation is limited in part by CD95 mediated Activation Induced Cell Death (AICD). On the other hand, failure of CD95 signaling is well known to have detrimental autoimmune and pro-inflammatory consequences as AICD is responsible for maintaining peripheral tolerance to self-antigens (Zhang et al., 2004), as well as limiting bystander effects by terminating the immune response to a pathogen after its elimination (Strasser and Pellegrini, 2004;Barreiro, Luker, Herndon, and Ferguson, 2004;Krueger, Fas, Baumann, and Krammer, 2003). The implication of our previous work is that under circumstances where lymphocyte death is desirable, low level Hg2+ exposures, by interfering with CD95 signaling may promote lymphocyte viability, leading to pathological outcomes which contribute to autoimmune and inflammatory disorders.
Since epidemiological evidence has suggested that DHA might counteract the negative effects of low level mercury exposures on the nervous system, we hypothesized that DHA might also counteract negative effects of low level mercury exposures on the immune system. However unlike in the nervous system where the mechanism is essentially unknown, in the immune system we have a plausible mechanism for low level mercury exposures to negatively impact immune system functionality through interference with the well-established CD95 signaling pathway. This permitted us to utilize an in vitro model system to directly probe any effects of DHA on mercury dependent disruptions of CD95 mediated cell death.
Currently in the United States most environmental exposure to mercury is in the form of organic mercury, which generally occurs through the consumption of fish. However we chose not to directly utilize organic mercury species in this study, but rather inorganic Hg2+. The rationale for this decision was twofold. Initially there was a technical consideration. Under in vitro conditions organic mercury would be expected to cross the plasma membrane and affect multiple pathways besides the CD95 pathway. Unraveling the complicated results would be far from a trivial expansion of the analysis we have presented here. But most importantly, the results reported here with respect to inorganic mercury and DHA are by themselves directly relevant to current environmental exposures. First, it has been shown that as a result of metabolism, eventually about 70% of the total mercury burden in spleen and brain is in the form of inorganic mercury, regardless of the initial chemical form of the mercury during exposure (Sumino et al., 1975); Takizawa, 1986. Second, with respect to autoimmune disorders and inflammatory processes it has been shown that in animals exposed to organic mercury, that Hg2+ rather than organic mercury is the active species operating on the immune system (Havarinasab et al., 2005b;Havarinasab et al., 2005a). This being the case, there is no loss in generality in utilizing Hg2+, especially as we were working with an in vitro system.
Our main finding is that (at least in vitro) docosahexaenoic acid (DHA), the principle n-3 PUFA found in cold-water fish (Ackman, 1989), counteracts a negative immunological effect of mercury on T cells by restoring the functionality of the CD95 death signaling pathway in mercury burdened cells. The implication is that the incidence of mercury associated autoimmunity will be diminished in individuals who have been exposed to high levels of mercury, but who also have high levels of DHA. Considering that the major environmental exposure to mercury is through consumption of fish, which also coincidentally have high levels of DHA, it may be that some of the immunotoxic effects of environmental mercury may be blocked in individuals who consume large quantities of fish. In a general sense the situation is not too different from what has been proposed to occur with respect to neurotoxic effects of mercury being attenuated by DHA, but in this case we have identified a specific mechanism upon which further investigation can be targeted.
HIGHLIGHTS.
Inorganic mercury (Hg2+) interferes with CD95 mediated cell death in Jurkat T cells
DHA restores the ability of CD95 to signal cell death in Hg2+ intoxicated T cells
The restoration of CD95 mediated cell death by DHA is correlated with increased activation of Caspase 3.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Environmental Health Sciences grant R21ES021285 to AR
ABBREVIATIONS
- DHA
Docosahexaenoic acid
- n-PUFA
n-polyunsaturated fatty acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest
Reference List
- Abbas AK. Die and let live: Eliminating dangerous lymphocytes. Cell. 1996;84:655–657. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- Ackman RG. Nutritional composition of fats in seafoods. Prog. Food Nutr. Sci. 1989;13:161–289. [PubMed] [Google Scholar]
- Barreiro R, Luker G, Herndon J, Ferguson TA. Termination of antigen-specific immunity by CD95 ligand (Fas ligand) and IL-10. J.Immunol. 2004;173:1519–1525. doi: 10.4049/jimmunol.173.3.1519. [DOI] [PubMed] [Google Scholar]
- Budtz-Jorgensen E, Grandjean P, Keiding N, White RF, Weihe P. Benchmark dose calculations of methylmercury-associated neurobehavioural deficits. Toxicol.Lett. 2000;112-113:193–199. doi: 10.1016/s0378-4274(99)00283-0. [DOI] [PubMed] [Google Scholar]
- Church MW, Jen KL, Anumba JI, Jackson DA, Adams BR, Hotra JW. Excess omega-3 fatty acid consumption by mothers during pregnancy and lactation caused shorter life span and abnormal ABRs in old adult offspring. Neurotoxicol.Teratol. 2010;32:171–181. doi: 10.1016/j.ntt.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW. The toxicology of mercury. Crit.Rev.Clin.Lab.Sci. 1997;34:369–403. doi: 10.3109/10408369708998098. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev.Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18:701–713. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- Gerstner HB, Huff JE. Clinical Toxicology of Mercury. J.Toxicol.and.Environ.Health. 1977;2:491–526. doi: 10.1080/15287397709529452. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, Haggqvist B, Bjorn E, Pollard KM, Hultman P. Immunosuppressive and autoimmune effects of thimerosal in mice. Toxicol.Appl.Pharmacol. 2005a;204:109–121. doi: 10.1016/j.taap.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, Hultman P. Organic mercury compounds and autoimmunity. Autoimmun.Rev. 2005b;4:270–275. doi: 10.1016/j.autrev.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Komoriya A, Packard BZ, Brown MJ, Wu ML, Henkart PA. Assessment of caspase activities in intact apoptotic thymocytes using cell-permeable fluorogenic caspase substrates. J.Exp.Med. 2000;191:1819–1828. doi: 10.1084/jem.191.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger A, Fas SC, Baumann S, Krammer PH. The role of CD95 in the regulation of peripheral T-cell apoptosis. Immunol.Rev. 2003;193:58–69. doi: 10.1034/j.1600-065x.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Laiosa MD, Eckles KG, Langdon M, Rosenspire AJ, McCabe MJ., Jr. Exposure to inorganic mercury in vivo attenuates extrinsic apoptotic signaling in Staphylococcal aureus enterotoxin B stimulated T-cells. Toxicol.Appl.Pharmacol. 2007 doi: 10.1016/j.taap.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe MJ, Jr., Eckles KG, Langdon M, Clarkson TW, Whitekus MJ, Rosenspire AJ. Attenuation of CD95-induced apoptosis by inorganic mercury: caspase-3 is not a direct target of low levels of Hg2+. Toxicol.Lett. 2005a;155:161–170. doi: 10.1016/j.toxlet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- McCabe MJ, Jr., Eckles KG, Langdon M, Clarkson TW, Whitekus MJ, Rosenspire AJ. Attenuation of CD95-induced apoptosis by inorganic mercury: caspase-3 is not a direct target of low levels of Hg2+. Toxicol.Lett. 2005b;155:161–170. doi: 10.1016/j.toxlet.2004.09.013. [DOI] [PubMed] [Google Scholar]
- McCabe MJ, Jr., Whitekus MJ, Hyun J, Eckles KG, McCollum G, Rosenspire AJ. Inorganic mercury attenuates CD95-mediated apoptosis by interfering with formation of the death inducing signaling complex. Toxicol.Appl.Pharmacol. 2003;190:146–156. doi: 10.1016/s0041-008x(03)00159-5. [DOI] [PubMed] [Google Scholar]
- McCabe MJ, Jr., Whitekus MJ, Hyun J, Eckles KG, McCollum G, Rosenspire AJ. Inorganic mercury attenuates CD95-mediated apoptosis by interfering with formation of the death inducing signaling complex. Toxicol.Appl.Pharmacol. 2003b;190:146–156. doi: 10.1016/s0041-008x(03)00159-5. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Cox C, Shamlaye CF, Palumbo D, Cernichiari E, Sloane-Reeves J, Wilding GE, Kost J, Huang LS, Clarkson TW. Prenatal methylmercury exposure from ocean fish consumption in the Seychelles child development study. Lancet. 2003;361:1686–1692. doi: 10.1016/S0140-6736(03)13371-5. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Thurston SW, Pearson AT, Davidson PW, Cox C, Shamlaye CF, Cernichiari E, Clarkson TW. Postnatal exposure to methyl mercury from fish consumption: a review and new data from the Seychelles Child Development Study. Neurotoxicology. 2009;30:338–349. doi: 10.1016/j.neuro.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton WR. Modified histogram subtraction technique for analysis of flow cytometry data. Cytometry. 1988;9:619–626. doi: 10.1002/cyto.990090617. [DOI] [PubMed] [Google Scholar]
- Risher JF, Murray HE, Prince GR. Organic mercury compounds: human exposure and its relevance to public health. Toxicol.Ind.Health. 2002;18:109–160. doi: 10.1191/0748233702th138oa. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, Silva IA, Nyland JF. Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol. 2005;207:282–292. doi: 10.1016/j.taap.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Strain JJ, Davidson PW, Bonham MP, Duffy EM, Stokes-Riner A, Thurston SW, Wallace JM, Robson PJ, Shamlaye CF, Georger LA, Sloane-Reeves J, Cernichiari E, Canfield RL, Cox C, Huang LS, Janciuras J, Myers GJ, Clarkson TW. Associations of maternal long-chain polyunsaturated fatty acids, methyl mercury, and infant development in the Seychelles Child Development Nutrition Study. Neurotoxicology. 2008;29:776–782. doi: 10.1016/j.neuro.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Pellegrini M. T-lymphocyte death during shutdown of an immune response. Trends Immunol. 2004;25:610–615. doi: 10.1016/j.it.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Sumino K, Hayakawa K, Shibata T, Kitamura S. Heavy metals in normal Japanese tissues. Amounts of 15 heavy metals in 30 subjects. Arch.Environ.Health. 1975;30:487–494. doi: 10.1080/00039896.1975.10666759. [DOI] [PubMed] [Google Scholar]
- Takizawa Y. Mercury content in recognized patients and non-recognized patients exposed to methylmercury from Minamata bay in the last ten years. In: Tsubaki T, Takahashi H, editors. Recent advances in Minamata disease studies. Kodansha; Tokyo: 1986. pp. 24–39. [Google Scholar]
- Whitekus MJ, Santini RP, Rosenspire AJ, McCabe MJJ. Protection against CD95-mediated apoptosis by inorganic mercury in jurkat T cells. J Immunol. 1999;162:7162–7170. [PubMed] [Google Scholar]
- Zhang J, Xu X, Liu Y. Activation-induced cell death in T cells and autoimmunity. Cell Mol.Immunol. 2004;1:186–192. [PubMed] [Google Scholar]
- Ziemba SE, McCabe MJ, Jr., Rosenspire AJ. Inorganic mercury dissociated preassembled Fas/CD95 receptor oligomers in T lymphocytes. Toxicol Appl Pharmacol. 2005;206:333–342. doi: 10.1016/j.taap.2004.11.014. [DOI] [PubMed] [Google Scholar]