Abstract
The ability to make behavioural inferences from skeletal remains is critical to understanding the lifestyles and activities of past human populations and extinct animals. Muscle attachment site (enthesis) morphology has long been assumed to reflect muscle strength and activity during life, but little experimental evidence exists to directly link activity patterns with muscle development and the morphology of their attachments to the skeleton. We used a mouse model to experimentally test how the level and type of activity influences forelimb muscle architecture of spinodeltoideus, acromiodeltoideus, and superficial pectoralis, bone growth rate and gross morphology of their insertion sites. Over an 11-week period, we collected data on activity levels in one control group and two experimental activity groups (running, climbing) of female wild-type mice. Our results show that both activity type and level increased bone growth rates influenced muscle architecture, including differences in potential muscular excursion (fibre length) and potential force production (physiological cross-sectional area). However, despite significant influences on muscle architecture and bone development, activity had no observable effect on enthesis morphology. These results suggest that the gross morphology of entheses is less reliable than internal bone structure for making inferences about an individual’s past behaviour.
Keywords: Muscle fibre, Physiological cross-sectional area, Periosteal growth, Entheses, Exercise, Functional anatomy
Introduction
A principal focus of bioarchaeology and palaeontology is to glean information about habitual activities of past populations from skeletal material (e.g., Hawkey and Merbs, 1995; Hawkey, 1998; Wilczak, 1998a,b; Knüsel, 2000; Eshed et al., 2004; Molnar, 2006, 2010; Villotte, 2006; Weiss, 2007, 2010; Havelková et al., 2011; Jurmain et al., 2012). Muscle attachment sites (entheses) are used by researchers in fields as diverse as bioarchaeology and palaeontology to infer cultural behaviour (Weiss, 2007; Molnar, 2010), technology use (Eshed et al., 2004; Marzke et al., 2007; Drapeau, 2008), subsistence strategies (Hawkey, 1998), labour differences (Churchill and Morris, 1998; Villotte et al., 2010; Weiss, 2010; Havelková et al., 2011; Niinimäki, 2011; Niinimäki and Sotos, 2013), social stratification (Molnar, 2006; Havelková et al., 2011, 2013; Henderson et al., 2013; Nolte and Wilczak, 2013), locomotor patterns (Davis, 1964; McGowan, 1979; Eliot and Jungers, 2000; Wang et al., 2004; Zumwalt, 2005, 2006), and evolutionary pathways (Davis, 1964; McGowan, 1979; Eliot and Jungers, 2000; Wang et al., 2004; Marzke and Shrewsbury, 2006; Drapeau, 2008).
Entheses (i.e., topography of muscle attachment) vary intra- and inter-specifically (e.g., differences in size, more or less rugose surface area, differences in location; Zumwalt, 2005, 2006). This variation in muscle attachment sites has been assumed to reflect differences in behaviour and researchers have functionally linked increased bone robusticity and bony landmark size with enlarged muscle mass (e.g., Trinkaus, 1976; Rathburn, 1987; Churchill and Morris, 1998) and/or elevated muscle use in habitual daily behaviours (e.g., Hawkey and Merbs, 1995; Hawkey, 1998; Jurmain et al., 2012). Despite this potential, however, surprisingly little is understood about the relationship between the gross appearance of bony features and the structure and function of the associated attaching soft-tissues.
While the relationship between muscle activity/size/force and muscle attachment size may make intuitive sense, the relationship between behaviour and entheseal morphology may not be as straightforward as posited (Hoyte and Enlow, 1966; Antón 1994; Eliot and Jungers, 2000; Marzke and Shrewsbury, 2006; Schoenau and Fricke, 2008; Cardoso and Henderson, 2010; Green et al., 2012; Jurmain et al., 2012; Schlecht, 2012). To date, there has only been one experimental study that directly tested the relationship between entheseal morphology and normal activity. Zumwalt (2005, 2006) quantified and compared enthesis morphology and the masses of muscles attached to them between an exercise (running on a treadmill for one hour a day, five days a week for 90 days) and sedentary control group of adult female sheep. Despite significant differences in muscle masses between the experimental and control groups, she found no effect of exercise on any measure of enthesis morphology across six attachment sites. She concluded that there is no direct causal relationship between muscle size or activity and attachment site morphology. Zumwalt (2005, 2006) noted, however, that the absence of a bony response could be related to the use of skeletally mature animals and suggested that future studies should focus on growing subadults, when the structural properties of bone are much more malleable (e.g., Biewener and Bertram, 1993, Mosley and Lanyon, 2002).
It has been assumed that during growth, muscles and tendons attach primarily to the periosteum, and that their Sharpey’s fibres - extrinsic coarse collagen fibres that are continuous with the connective tissue of muscles that also anchor them to bone - pass through the periosteum and firmly attach to the underlying bone only after longitudinal bone growth is complete (e.g., Wilczak, 1998b; Zumwalt, 2005, 2006). Accordingly, most entheseal research has focused on adult morphology as it has been presumed that the morphology of juvenile muscle attachment sites does not reflect the size or activity of the attaching muscle. Contrary to previous assumptions, there is empirical evidence that Sharpey’s fibres can be found anchoring the muscle to bone before complete fusion of the growth plates (Hoyte and Enlow, 1966; McFarlin et al., 2008; Rabey, 2014).
Prior studies reveal the complexity of influences on muscle attachment site morphology. Indeed a number of studies have failed to detect the most basic of relationships, namely, the one between a muscle and the presence and/or absence of its attachment site (Bryant and Seymour, 1990; Eliot and Jungers, 2000; Marzke and Shrewsbury, 2006; Marzke et al., 2007). To date, no studies have examined the effects of muscle activity on muscle fibre architecture and attachment site morphology. Muscular anatomy is highly labile during an individual’s lifetime, particularly in response to changes in exercise regime (Salmons and Henriksson, 1981; Asfour et al., 1984; Ishihara et al., 1998; Allen et al., 2001; Lieber, 2002; Harber et al., 2012). Likewise, physical activity influences the morphology of bones (e.g., Rubin and Lanyon, 1984a), particularly during growth (e.g., Parfitt, 2004; Pearson and Lieberman, 2004; Robling et al., 2006). Yet, the relationship between architectural parameters of muscle that influence function (e.g., fibre length [Lf,] and physiological cross-sectional area [PCSA]) and the morphology of associated muscle attachment sites is poorly understood. The purpose of this study is to examine how physiologically normal variation in activity influences the development of muscle fibre architecture (mass, Lf, PCSA), periosteal bone growth, and muscle attachment sites. We therefore tested the hypothesis that habitual exercise (running and climbing) influences muscle fibre architecture, bone growth, and entheseal morphology during development in mice.
Experimental model
We used an experimental approach to test the influence of exercise regime on the development of muscular fibre architecture, periosteal bone growth, and enthesis morphology in subadult laboratory mice. Mice are useful models for this study because they develop rapidly and the internal architecture of their upper limb muscles is similar to that of humans (Mathewson et al., 2012). Subadult mice were chosen because they reach peak bone density at approximately four months of age, prior to the timing of epiphyseal closure (Beamer et al., 1996; Kilborn et al., 2002). The experimental treatment in this study occurred during the most rapid phase of skeletal growth and maturity. Mice also reach sexual maturity and adult body mass size at one and a half months of age (Kilborn et al., 2002); therefore, the mice in this study were reproductively mature and reached adult body mass. The well-documented response of bone tissue to elevated loading environments has made mice and other small mammals important models for understanding general mammalian bone biology, including bone biology relevant to human health (Enlow and Brown, 1958; Kimes et al., 1981; Robling et al., 2006; Elkasrawy and Hamrick, 2010; Byron et al., 2011; Burr and Allen, 2013).
Predictions regarding the effect of exercise on muscle fibre architecture, bone growth and enthesis morphology
The objective of this study is to determine the effect of exercise on the muscles that attach directly to bone, periosteal bone growth, and the attachment site of the muscles. We draw on theoretical and empirical relationships to generate a series of predictions regarding the effects of exercise and activity level on muscle size and force, on bone growth and modeling, and the mechanical link between muscle activity, bone growth, and enthesis size and shape. We frame these predictions in terms of the expected differences between exercise and control groups. We use this opportunity to explore differences between running and climbing groups, as we have no a priori expectations for how these two groups should differ.
Prediction 1. Exercise mice have larger muscle masses and muscle physiological cross-sectional areas (PCSA), and altered fibre lengths compared with controls
Fibre architecture refers to the arrangement of muscle fibres relative to the force-generating axis of the muscle (Gans and Bock, 1965; Gans and Gaunt, 1991; Lieber and Ward, 2011). Two muscle architectural variables that are important determinants of muscle function are fibre length (Lf) and physiological cross-sectional area (PCSA) (Gans, 1982; Lieber, 2002). Fibre length represents the sum of the lengths of the serially arranged sarcomeres within a fibre and has been empirically shown to be proportional to a muscle’s maximum excursion (range of motion) (Bang et al., 2006; Gokhin et al., 2009) and, by extension, velocity of contraction (Bodine et al., 1982; Winters et al., 2011). The PCSA represents the sum of the cross-sectional areas of all fibres within a given muscle, and is empirically proportional to the maximum force a muscle can generate (Powell et al., 1984). Muscle mass is not a good proxy for muscle force. This is because mass does not account for the angulation of fibres relative to the muscle’s force-generating axis (pennation angle; Gans, 1982; Bryant and Seymour, 1990; Anapol and Barry, 1996; Lieber, 2002), which can influence muscle PCSA. Therefore, in addition to muscle mass, we estimated Lf and PCSA as these provide the best architectural estimates of a whole muscle’s maximum potential excursion/contraction velocity and force. We expect that increased activity will be associated with more powerful muscles (Williams et al., 2008), in terms of potential maximum force generation or potential excursion/contraction velocity of each individual muscle.
Prediction 2. Exercise mice have faster rates of periosteal bone growth compared with controls
Bone remodelling during growth entails differential rates of bone deposition across different region of the same bone, as well as local destruction of tissues deposited during earlier growth stages. These local variations in bone growth and remodelling processes are thought to produce changes in whole bone structure, which are mechanically appropriate (Enlow, 1962). Bone growth and remodelling are primary mechanisms by which bones adapt to increase mechanical loading, such as the loads experienced during exercise (although the responsiveness declines after skeletal maturity) (e.g., Martin et al., 1998; Pearson and Lieberman, 2004). Many studies show that (human and non-nonhuman) juveniles who exercise build more bone than others who are less active, and the mechanical loading due to exercise stimulates periosteal growth prior to skeletal maturity (e.g., Pearson and Lieberman, 2004). Fluorescent vital labelling of bone in the sample allowed this study to obtain quantitative measurements of periosteal bone depositional rates. Given differences in activity among the subadult mice, it is expected that the bone growth in the exercise mice will have faster growing cortices, especially underlying the deltoid crest, compared with controls.
Prediction 3. Exercise mice have relatively larger deltoid crests (entheses) compared with controls
Fibrous entheses are mainly found on the shafts of long bones where there is a large surface for the muscles to attach. These muscles attach directly to the bone or periosteum and are anchored by Sharpey’s fibres (Hems and Tillmann, 2000; Benjamin et al., 2002). In this manner, blood vessels from the soft tissue may anastomose with those of the bone (Dörfl, 1969). Since mechanical stress (e.g., muscle force) experienced by a surface area (e.g., enthesis) is proportional to the force applied in each unit area of that surface (Biewener, 1992), hypertrophy of bony attachments for larger and/or more active muscles is a theoretically advantageous mechanism to reduce stress or maintain acceptable stress magnitudes. When entheses are subjected to the force of a contracting muscle, blood flow to periosteal bone increases, which can stimulate bone growth and increase the size of the attachment site to strengthen it (Chamay and Tschantz, 1972; Woo et al., 1981; Hawkey and Merbs, 1995; Montgomery et al., 2005). Therefore, increased activity in the exercise groups is expected to result in larger entheses. Because the rugosity and shape of entheses have also been linked to differences in behaviour and activity level, we also explored differences between exercise and controls in enthesis shape. Anthropologists often presume that morphological variation of long bone entheses result from frequent, strenuous, habitual activity, where muscle contractions are frequently recruited for limb movements (Schlecht, 2012), but experimental tests are lacking.
Materials and methods
Sample
Thirty female outbred wild-type mice (CD-1, Charles River Laboratory derived, purchased from Harlan Laboratories, Indianapolis, IN, USA) consisting of two age groups were included in the study. One group comprised newly weaned mice that were 25 days old, and the other group comprised mice that were 46 days old. Five mice from both age categories were randomly chosen for each of the three experimental groups (10 mice per group) to examine the effects of activity and age on muscle fibre architecture and attachment morphology. Mice belonging to both age groups were 103 and 124 days old, respectively, at the end of the experiment. The mice reached full adult body mass over the duration of the experiment and the experimental treatment occurred throughout the most rapid phase of skeletal growth and maturity (Beamer et al., 1996; Kilborn et al., 2002). The two age groups were combined in the analyses reported below, in part because there were no significant differences between the two in average body mass.
Experimental design
The mice were separated into three groups: (1) control mice (CON) were housed in cages with no exercise apparatus, (2) running mice (WHL) were housed in cages containing two activity wheels, and (3) climbing mice (CLB) were housed in cages outfitted with a 1 m tall wire-mesh tower where water sources were positioned at the top. Experimental mice were free to use the exercise wheels or climb up the meshed cages at will, such that muscle activity could be investigated within normal, non-pathological limits. The amount of activity from the running and climbing groups was recorded during the initial experiment (Green, 2010; Green et al., 2012). Mice were given three subcutaneous injections containing fluorescent bone-labelling dyes at two week intervals during the experiment: 1) Alizarin-red (25 mg/kg), 2) DCAF-green (15 mg/kg), and 3) Xylenol-orange (80 mg/kg). These labels are incorporated into the mineralizing front of growing bone and represent known time points during the duration of the experiment. Thus, they enable the calculation of osteogenesis rate as the distance between two adjacent labels divided by the time interval (Castanet et al., 2004) (Fig. 1).
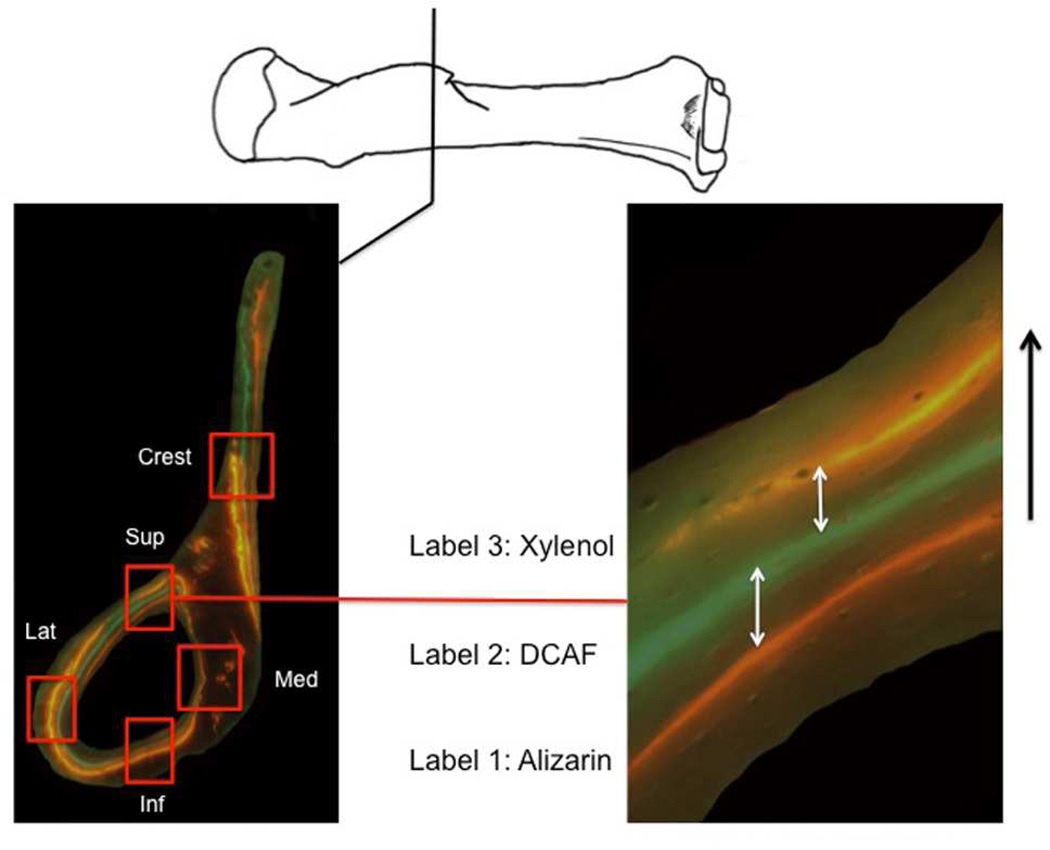
Figure 1.
Representation of the superimposed microscopy images showing the fluorescent labelling used for bone growth rate calculations. The cross-section on the left is of the distal end of the deltoid crest (climbing mouse). Each red square represents location of growth calculations (Crest = deltoid crest; Sup = superior; Med = medial; Inf = inferior; Lat = lateral quadrant). The image on the right represents the measurements taken on the cranial quadrant of the cross section (white arrows). Measurements of distances between all pairs of consecutive labels were taken. Individual measurements were then averaged across section levels to derive a mean daily growth rate calculated for each individual. Black arrow (right) indicates the direction of periosteal bone growth.
The experiment lasted a total of 78 days. On average, CLB climbed approximately 140 m per night, while WHL engaged in substantially more exercise, running approximately 1900 m per night. Anecdotally, the total number of mice climbing at any one time did not appear to limit opportunities for other mice, while the number of wheels per cage may very well have prevented some mice from running (Green, 2010, Green et al., 2012). Thus, the total distance covered by each running mouse may not represent the upper limit of their running distance abilities, but still represents a significant amount of activity relative to the climbing and control groups. Movements (kinematics) of the forelimb differed significantly between the climbing and the running activities (Green, 2010; Green et al., 2012). All experimental protocols and procedures received prior approval by The George Washington University Institutional Animal Care and Use Committee (IACUC #16-12,7).
Muscles and muscle attachment sites
We analysed spinodeltoideus, acromiodeltoideus, and superficial pectoralis muscles that all have a fibrous attachment directly to the periosteum of the deltoid crest. The deltoid crest is a clearly defined and prominent ridge on the humerus. The spinodeltoideus inserts along the entire lateral surface of the deltoid crest, while the acromiodeltoideus and the superficial pectoralis share the medial surface of the prominent ridge (Fig. 2). These muscles are important in weight bearing and are critical for stabilization and force production during locomotor activities known to be regularly performed by mice (Clarke and Still, 1999). They function as the principal protractors and retractors of the humerus, enabling the animal to propel itself forward and resist gravitational forces (e.g., to maintain glenohumeral joint flexion when standing, walking, climbing or running). These muscles are essential to the habitual gaits experienced by all mice in this study. We chose the deltoid crest because it is one of the most commonly studied fibrous attachment sites (e.g., Benjamin et al., 1986; Dysart et al., 1989; Bryant and Seymour, 1990; Hawkey and Merbs, 1995; Churchill and Morris, 1998; Wilczak, 1998a,b; Eshed et al., 2004; Montgomery et al., 2005; Mariotti et al., 2007; Drapeau, 2008; Cardoso and Henderson, 2010; Villotte et al., 2010; Niinimäki, 2011; Davis et al., 2013; Niinimäki et al., 2013) and rates of osteogenesis in the underlying bone can be easily documented (Greene, 1935; Chiasson, 1975).
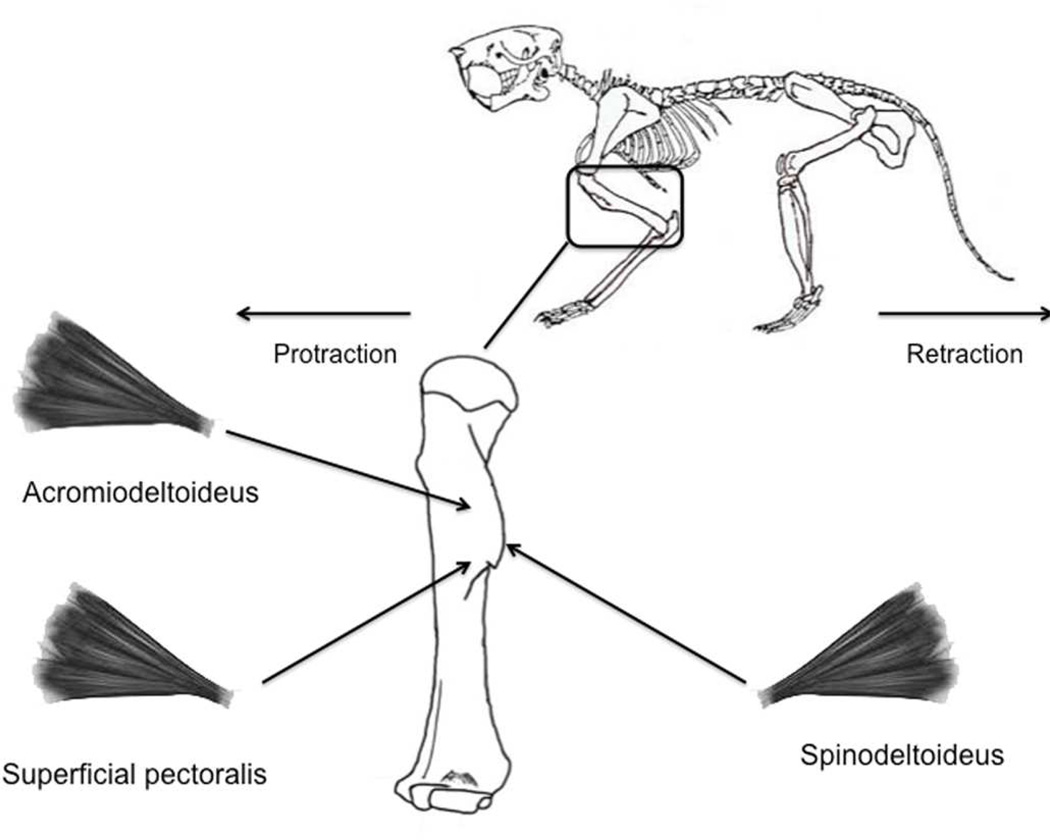
Figure 2.
Schematic representation of the spinodeltoideus, acromiodeltoideus, and the superficial pectoralis muscles measured in the sample along with their attachment location on the deltoid crest of the humerus.
Dissections and muscle architectural variables
The left forelimb of each individual was dissected under an Olympus S2×12 stereomicroscope. After removing the skin and superficial fascia, surface pennation angles (θ) of the spinodeltoideus, superficial pectoralis, and acromiodeltoideus were measured using a reticle accurate to the nearest 0.01 mm (Mathewson et al., 2012). The muscles then were removed, cleaned of excess fat and fascia, and submerged in 10% buffered formalin for at least 48 hours to ensure complete fixation and a constant specific muscle density (Ward and Lieber, 2005). After complete fixation, muscles were weighed to the nearest 0.001 g.
To adjust for differences in fibre length that occur because the mice were fixed in a variety of glenohumeral joint postures, fibre lengths were normalized to a standard sarcomere length (Felder et al., 2005). Muscles were chemically digested for up to six hours in 30% nitric acid solution and then submerged in saline for 24 hours to arrest the digestion process. Small fibre bundles then were manually dissected and isolated under a dissecting microscope and in situ fibre length (Lf) was measured using a reticle. Six to 10 fibre bundles per muscle were then mounted on glass slides, cover-slipped, and air-dried overnight (Taylor et al., 2009). Laser diffraction was used to measure in situ sarcomere length (Lieber et al., 1994). Three sarcomere length measurements per fibre bundle were obtained. To normalize fibre length (NLf), the following equation was used:
NLf = Lf * 2.4 µm / Ls
where Lf (mm) is the in situ measured fibre length, Ls the in situ measured sarcomere length (µm) and 2.4 µm is the experimentally determined optimal sarcomere length (length at which the maximum tetanic tension is generated) in mouse hind limb muscles (Edman, 2005). Using the above measurements, physiological cross-sectional area (PCSA) was calculated using the following formula:
PCSA = (muscle mass * cos θ) / (NLf * 1.0564)
where 1.0564 (g cm−3) is the specific density of muscle (Mendez and Keys, 1960).
Bone preparation and variables
After dissections, the forelimb of each mouse was skeletonized for measurement of the gross morphology of the deltoid crest using a 1% Terg-A-Zyme (Alconox, NY) solution, stored in a dark laboratory oven at 45°C with 24-hour solution changes over a period of one week. Once the bones were cleaned, humeri were digitally photographed and the following variables were measured using Image J software: 1) maximum length of the humerus, 2) maximum length and width of the deltoid crest on the lateral and medial side of the crest, 3) the area of both the lateral and medial side of the attachment, 4) crest thickness (at the histological section where growth rate measurements were taken), and 5) the distal angle of the deltoid crest (Fig. 3).
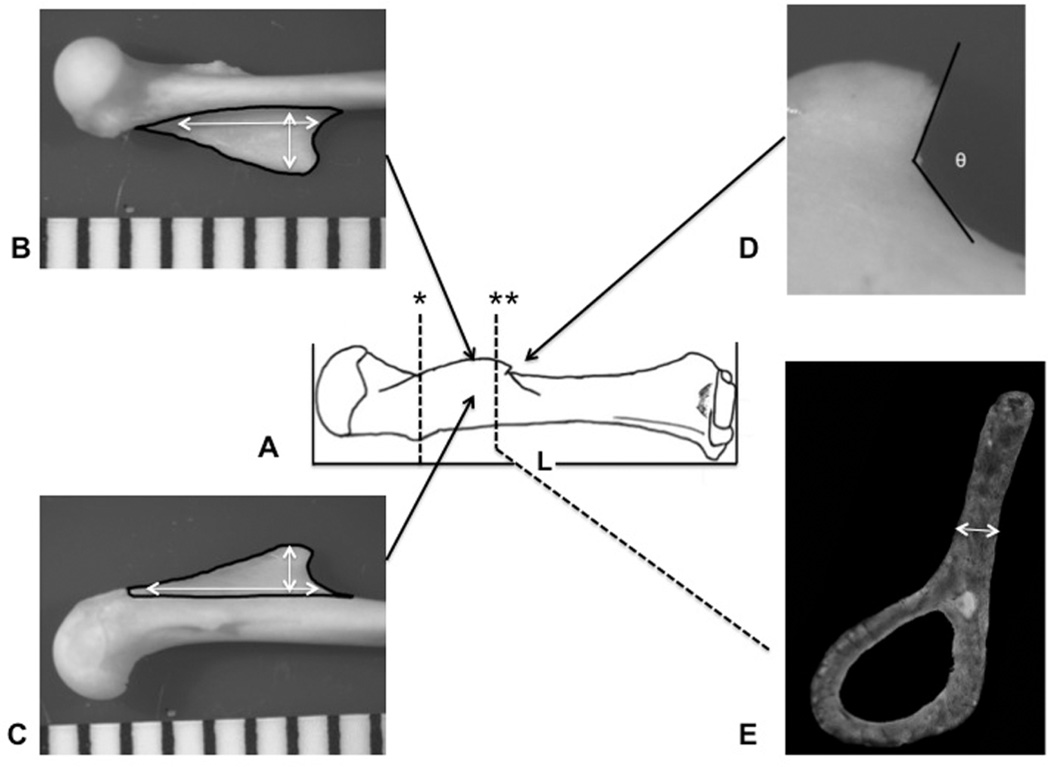
Figure 3.
Bony variables: A) maximum length of the humerus (L) was measured as the greatest distance between the top of the humeral head and the most distant point on the distal humerus parallel with the long axis of the bone; * represents histological cut at the proximal 25% diaphyseal length. Maximum length, width (white arrows), and area (black contours) of the deltoid crest, on the lateral (B) and medial side (C) of the crest; D) angle of the crest (θ) was measured at the distal edge of the prominent ridge; E) thickness of the crest measured in the distal margin of the crest cross-section (**).
Histological thin sections were prepared following the procedures in Goldman et al. (1998) with some modifications following McFarlin et al. (2008) and Cho (2012) for better specimen embedding and mounting. After skeletonization, the humerus was cut transversely just distal to the deltoid crest to improve infiltration of the bone during embedding. The proximal end of the humerus was then dehydrated in a series of graded ethanol changes, followed by clearing in two changes of Methyl Salicylate. The bones were then embedded in Caroplastic polyester resin (Carolina Biological Supply, NC) and two histological thin sections were prepared for analysis of bone growth rate: 1) at the distal margin of the deltoid crest, and 2) at the 25% diaphysis length (Fig. 3). Each embedded block was ground on a series of carbomide emery papers (Buehler Ltd., Lake Bluff, IL) to 1200 grit, and the prepared surface was mounted to a plastic slide using superglue. Mounted sections were ground to a final thickness of 100 ± 5 µm, and the imaging surface was prepared to a surface topography of 1200 grit and temporarily cover-slipped for imaging.
Digital montage images of entire bone cross-sections were collected on a Zeiss AxioImager microscope configured with an automated LUDL stage (0.1 µm accuracy in ‘X’ and ‘Y’). The same field of view images were collected under 40X magnification in brightfield (BF), circularly polarized light (CPL), and fluorescence illumination for imaging of Alizarin-red, DCAF-green, and Xylenol-orange labels. Images were captured using a Qimaging colour CCD camera (40X objective, 1 pixel = 1.346 µm) and an integrated MicroBrightField Stereo Investigator system with Virtual Slice, which automates the montaging of entire cross-sections. Images were then imported to Adobe Photoshop CS3, and superimposed using the ‘Layers’ function and their transparency adjusted to allow for visualization of relationships between fluorescent labels and bone features. The superimposed and flattened images were imported into Image J to calculate the rate of periosteal bone growth (Fig. 1). Rates of osteogenesis were calculated as the distance between two fluorescent labels over the time elapsed (Castanet et al., 2004). In instances where all three fluorochromes were present, a total of two measurements could be taken at one location. Not all groups displayed all three fluorescent labels (Fig. 4). Thus, we had a more limited sample for this analysis: WHL (n = 4), CON (n = 8), and CLB (n = 9). Maximum distance measurements were taken within each quadrant (anterior, posterior, medial, and lateral) throughout both cross-section levels (Fig. 1). These measurements from both cross-sections were averaged to generate an overall bone growth rate of the humeral shaft for each individual. In addition, the rate of osteogenesis for the bone region immediately underlying the deltoid crest was calculated from the cross-section at the distal margin of the deltoid crest.
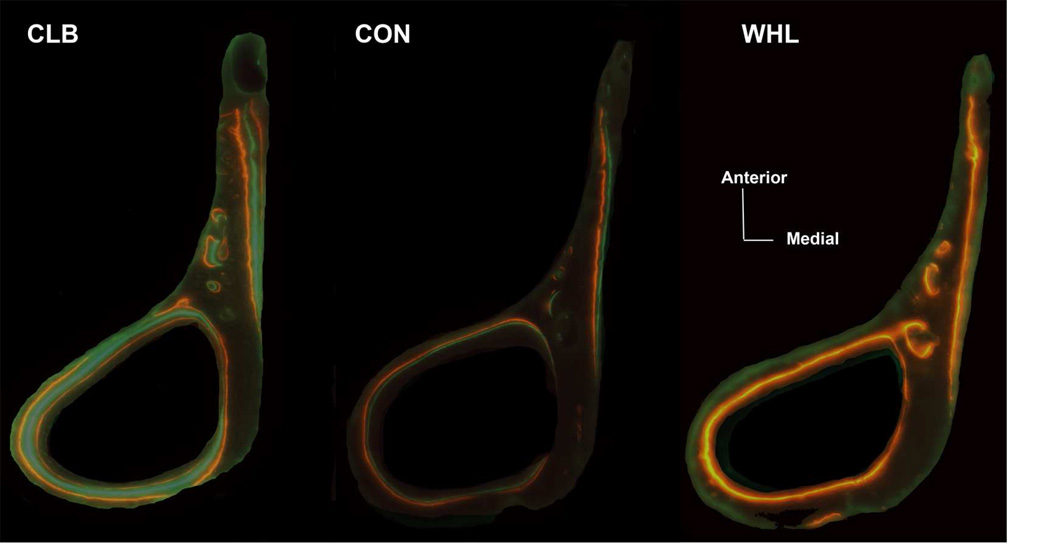
Figure 4.
Example of the fluorochrome labels at the distal margin of the deltoid crest cross-section for each activity group. Bone cross-sections from most wheel-running (WHL) mice preserved mainly the last administered Xylenol-orange label. Most climbing (CLB) mice preserved all three labels, and controls (CON) showed a more variable pattern in the labels present.
Statistical analysis
Prior to hypothesis testing, the data were tested for normality using a Shapiro-Wilk test (Sokal and Rohlf, 2012). Because the data were not normally distributed, we used nonparametric statistical tests to evaluate absolute and relative differences in muscle fibre architecture and bony variables among the groups. The nonparametric Kruskal-Wallis test was used to test for a significant effect of activity on all soft- and hard-tissue variables. A significant Kruskal-Wallis was followed by two-tailed, pairwise, non-parametric Mann-Whitney U-tests. Geometric scaling principles were used to evaluate the relationship between muscle mass, attachment size, and body size (Zumwalt, 2005, 2006; Payne et al., 2006; Michilsens et al., 2009): muscle mass was divided by body mass (Mb), lengths were divided by (Mb)1/3, and surface areas were divided by (Mb)2/3. Due to multiple comparisons, the Dunn-Šidák correction test was used (Sokal and Rohlf, 2012) to reduce the probability of making a type 1 error. This method was preferred to the Bonferroni correction, which tends to be overly conservative (Sokal and Rohlf, 2012). A total of four tests compared body mass (P-values ≤ 0.01 were considered significant at the adjusted Dunn-Šidák level for these comparisons), 48 tests compared the soft-tissue variables (P ≤ 0.001), 32 tests compared deltoid crest surface morphology variables (P ≤ 0.002), and eight tests compared bony histological variables (P ≤ 0.007). All statistical tests were performed using IBM SPSS Statistics (version 20.0 for Mac).
Results
Body mass
Body mass varied by activity group (Table 1). The control group had the largest body mass, WHL had the lowest body mass, and CLB was intermediate. Post-hoc contrasts were significant between the CON and WHL groups (P = 0.001).
Table 1.
Results of Kruskal-Wallis and two-tailed Mann-Whitney contrasts between groups for body mass, soft-tissue, periosteal bone growth, and deltoid crest variables.
| Variable | Control (CON) |
Running (WHL) |
Climbing (CLB) |
Kruskal- Wallis |
CON-WHL | Mann-Whitney CON-CLB |
WHL-CLB |
|---|---|---|---|---|---|---|---|
| Body mass (g) | 33.93 ± 2.27 | 29.88 ± 1.82 | 31.94 ± 4.05 | 0.007 | 0.001 | NS | NS |
| Muscle variables | |||||||
| Acromiodeltoideus | |||||||
| Mass (µg) | 9.0 ± 0.67 | 8.4 ± 0.7 | 10.4 ± 0.7 | < 0.001 | NS | 0.001 | <0.001 |
| Lf (mm) | 4.29 ± 0.26 | 5.21 ± 0.32 | 3.54 ± 0.31 | < 0.001 | <0.001 | <0.001 | <0.001 |
| PCSA (mm2) | 1.61 ± 0.15 | 1.26 ± 0.14 | 2.49 ± 0.22 | < 0.001 | <0.001 | <0.001 | <0.001 |
| Pennation (°) | 34.73 ± 8.42 | 34.7 ± 3.89 | 26.07 ± 6.75 | < 0.001 | NS | 0.023* | 0.008* |
| Spinodeltoideus | |||||||
| Mass (µg) | 18.8 ± 2.9 | 17.9 ± 1.2 | 22.1 ± 3.96 | 0.05* | NS | 0.05* | 0.026* |
| Lf (mm) | 7.41 ± 0.77 | 8.47 ± 0.33 | 6.01 ± 0.75 | < 0.001 | <0.001 | 0.002* | <0.001 |
| PCSA (mm2) | 2.37 ± 0.29 | 1.96 ± 0.17 | 3.43 ± 0.63 | < 0.001 | 0.002* | <0.001 | <0.001 |
| Pennation (°) | 10.32 ± 1.68 | 11.45 ± 2.83 | 11.0 ± 3.67 | NS | NS | NS | <0.001 |
| Superficial pectoralis | |||||||
| Mass (µg) | 101.9 ± 6.15 | 101.0 ± 9.06 | 111.5 ± 6.45 | 0.009* | NS | 0.005* | 0.015* |
| Lf (mm) | 13.21 ± 0.31 | 14.84 ± 0.68 | 11.86 ± 0.77 | < 0.001 | <0.001 | <0.001 | <0.001 |
| PCSA (mm2) | 7.18 ± 0.4 | 6.33 ± 0.35 | 8.75 ± 0.49 | < 0.001 | <0.001 | <0.001 | <0.001 |
| Pennation (°) | 10.4 ± 2.39 | 10.15 ± 2.04 | 10.79 ± 2.01 | NS | NS | NS | NS |
| Periosteal bone growth | |||||||
| Crest (µm/day) | 2.46 ± 0.334 | 3.46 ± 0.405 | 2.99 ± 0.378 | 0.007 | 0.014* | 0.018* | NS |
| Mean (µm/day) | 2.1 ± 0.292 | 3.16 ± 0.3 | 2.8 ± 0.141 | < 0.001 | 0.007 | 0.001 | 0.014* |
| Muscle attachment site | |||||||
| Lateral | |||||||
| Max length (mm) | 1.44 ± 0.08 | 1.42 ± 0.06 | 1.4 ± 0.083 | NS | NS | NS | NS |
| Max width (mm) | 0.41 ± 0.04 | 0.42 ± 0.03 | 0.42 ± 0.01 | NS | NS | NS | NS |
| Area (mm2 ) | 0.38 ± 0.04 | 0.38 ± 0.034 | 0.37± 0.035 | NS | NS | NS | NS |
| Medial | |||||||
| Max length (mm) | 1.39 ± 0.11 | 1.41 ± 0.09 | 1.36 ± 0.09 | NS | NS | NS | NS |
| Max width (mm) | 0.36 ± 0.04 | 0.33 ± 0.03 | 0.33 ± 0.01 | NS | NS | NS | NS |
| Area (mm2) | 0.3 ± 0.037 | 0.3 ± 0.032 | 0.31± 0.04 | NS | NS | NS | NS |
| Angle (°) | 145.28 ± 18.34 | 147.21 ± 10.51 | 148.01 ± 9.6 | NS | NS | NS | NS |
| Thickness (mm) | 0.052 ± 0.004 | 0.05 ± 0.005 | 0.056 ± 0.006 | NS | NS | 0.023* | NS |
Lf = sarcomere adjusted fibre length; PCSA = sarcomere adjusted physiological cross-sectional area.
Bold = significant differences after the Dunn-Šidák correction method.
In all comparisons, n=10 for each group with the exception of analyses of periosteal bone growth, where n=4 for WHL, n=8 for CON and n=9 for CLB.
= non-significant after the Dunn-Šidák correction method
NS = non-significant.
Fibre architecture
The exercise and control groups differed in absolute (Table 1) and relative (Table 2) fibre architecture variables. Differences among groups were the same for absolute and relative variables, except for one exception: CLB mice had the absolutely greatest muscle masses, while the WHL group had the smallest muscle masses. These differences were only significant for the acromiodeltoideus muscle (P < 0.001). However, none of the relative muscle mass values were significantly different between groups (Table 2).
Table 2.
Results of Kruskal-Wallis and two-tailed Mann-Whitney contrasts between groups for body mass adjusted soft-tissue and deltoid crest variables.
| Variable | Control (CON) |
Running (WHL) |
Climbing (CLB) |
Kruskal- Wallis |
CON-WHL | Mann-Whitney CON-CLB |
WHL-CLB |
|---|---|---|---|---|---|---|---|
| Muscle variables | |||||||
| Acromiodeltoideus | |||||||
| Mass | 0.28 ± 0.05 | 0.29 ± 0.03 | 0.32 ± 0.05 | NS | NS | NS | <0.001 |
| Lf | 1.33 ± 0.1 | 1.68 ± 0.11 | 1.12 ± 0.11 | < 0.001 | <0.001 | 0.001 | <0.001 |
| PCSA | 0.15 ± 0.02 | 0.13 ± 0.02 | 0.25 ± 0.03 | < 0.001 | 0.005* | <0.001 | <0.001 |
| Spinodeltoideus | |||||||
| Mass | 0.56± 0.08 | 0.6 ± 0.04 | 0.69 ± 0.11 | 0.005* | 0.041* | 0.01* | 0.013* |
| Lf | 2.29 ± 0.23 | 2.73 ± 0.14 | 1.9 ± 0.24 | < 0.001 | <0.001 | 0.005* | <0.001 |
| PCSA | 0.23 ± 0.03 | 0.2 ± 0.01 | 0.34 ± 0.05 | < 0.001 | NS | <0.001 | <0.001 |
| Superficial pectoralis | |||||||
| Mass | 3.01 ± 0.23 | 3.38 ± 0.26 | 3.53 ± 0.38 | 0.006* | 0.013* | 0.004* | NS |
| Lf | 4.08 ± 0.13 | 4.78 ± 0.23 | 3.75 ± 0.29 | < 0.001 | NS | 0.008* | <0.001 |
| PCSA | 0.69 ± 0.04 | 0.66 ± 0.02 | 0.87 ± 0.06 | < 0.001 | <0.001 | <0.001 | <0.001 |
| Muscle attachment site | |||||||
| Lateral | |||||||
| Max length | 0.45 ± 0.029 | 0.46 ± 0.019 | 0.44 ± 0.03 | NS | NS | NS | NS |
| Max width | 0.13 ± 0.012 | 0.13 ± 0.008 | 0.13 ± 0.006 | NS | NS | NS | NS |
| Area | 0.04 ± 0.004 | 0.04 ± 0.004 | 0.04 ± 0.005 | NS | NS | NS | NS |
| Medial | |||||||
| Max length | 0.43 ± 0.04 | 0.45 ± 0.032 | 0.43 ± 0.038 | NS | NS | NS | NS |
| Max width | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.007 | NS | NS | NS | NS |
| Area | 0.03 ± 0.004 | 0.03 ± 0.003 | 0.03 ± 0.006 | NS | NS | NS | NS |
| Thickness | 0.015 ± 0.001 | 0.016 ± 0.002 | 0.018 ± 0.002 | NS | NS | 0.023* | NS |
Lf = sarcomere adjusted fibre length; PCSA = sarcomere adjusted physiological cross-sectional area.
Bold = significant differences after the Dunn-Šidák correction method.
In all comparisons, n=10 for each group with the exception of analyses of periosteal bone growth, where n=4 for WHL, n=8 for CON and n=9 for CLB.
= non-significant after the Dunn-Šidák correction method.
NS = non-significant.
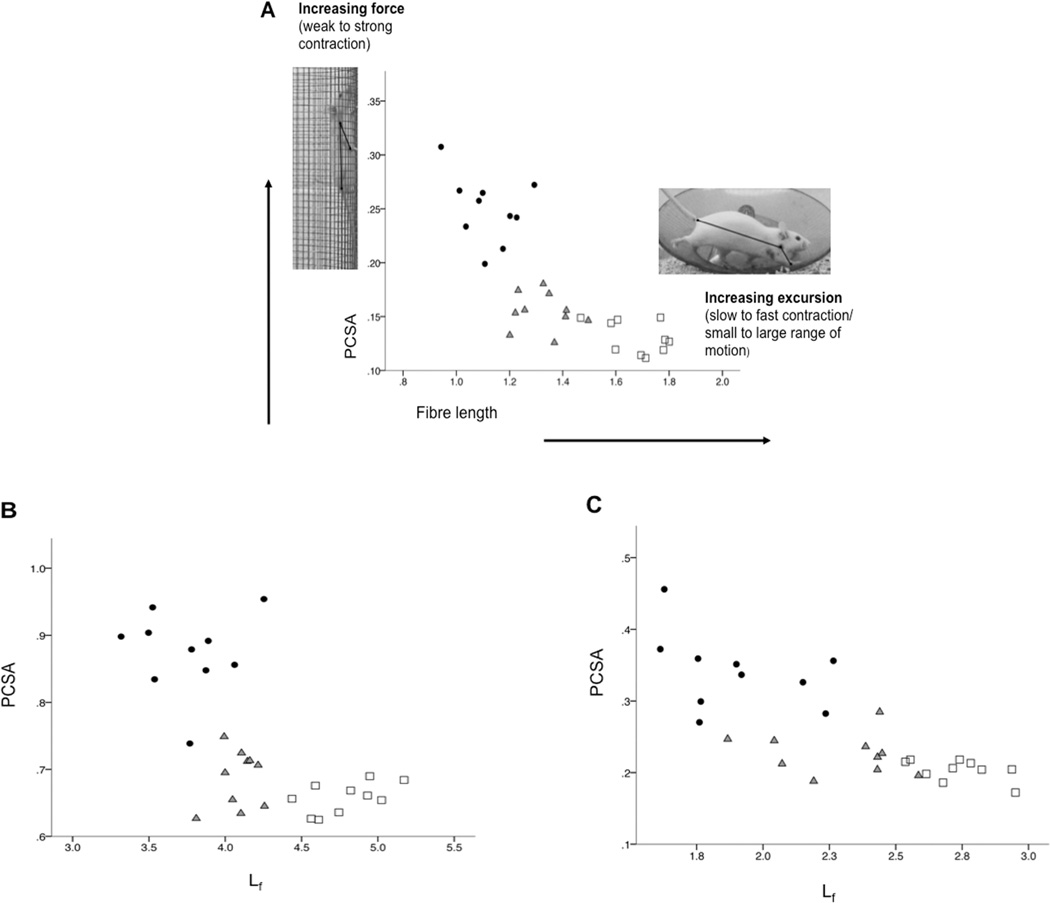
Muscle fibres (Lf; potential maximum excursion/contraction velocity) were relatively longer in the WHL group compared with the CON group (Table 2). The relatively shortest fibres were in the intermediate intensity CLB group, perhaps reflecting the shorter forelimb excursions involved in climbing (Green, 2010; Green et al., 2012) (Fig. 5). Relative muscle PCSAs (i.e., potential maximum force output) were greatest for the CLB group but contrary to our prediction, smallest for the WHL group (Table 2). Exercise had little effect on muscle pennation angles. Pennation did not differ for any muscle between the exercise and control groups and only acromiodeltoideus pennation angle differed significantly between exercise groups (Table 1).
Figure 5.
Bivariate plot showing that for a given muscle (A: acromiodeltoideus, B: superficial pectoralis, and C: spinodeltoideus), there is an architectural trade-off between potential maximum force output (PCSA) and muscle excursion/contraction velocity (Lf). Climbing mice (dark circles) are at the top left quadrant of the plots with the shortest fibre length (Lf), but largest physiological cross-sectional area (PCSA). The running mice (white squares) are usually at the bottom right of the plots with the longest Lf and the smallest PCSA. The control mice (grey triangles) are between the two exercise groups and closest to the running group. All graphs represent body mass adjusted data.
Periosteal bone growth rate
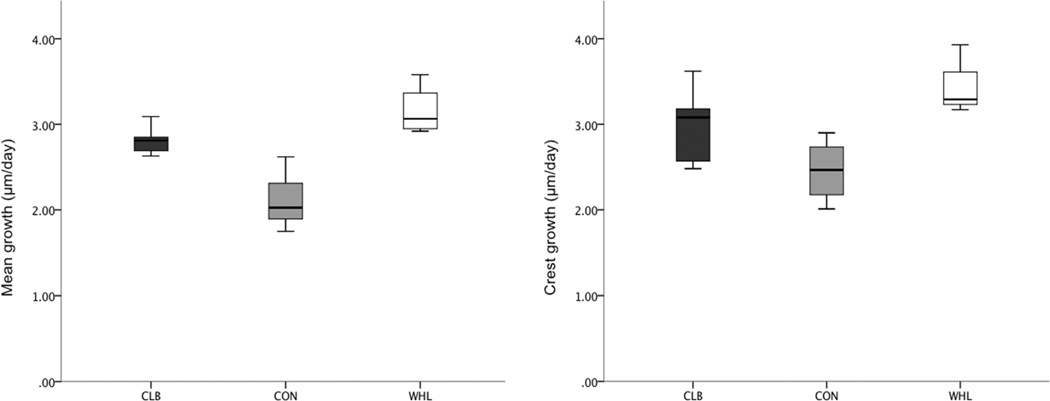
As predicted, when growth rate measurements collected across all cortices and labels were averaged for both histological section levels, the two exercise groups had significantly faster periosteal bone growth than the controls (Table 1 and Fig. 6). The WHL group had faster rates of bone growth compared with CLB but this difference was not significant following the Dunn-Šidák test (Table 1). Bone growth rates at the deltoid crest (Figs. 4 and 6) were significantly faster than growth rates averaged across cortices and cross-sections. The exercise mice had faster bone growth rates than the controls under the deltoid crest, but these differences were not significant following post-hoc pairwise comparisons (Table 1).
Figure 6.
Box-plot showing per day bone growth rate differences underlying the deltoid crest only (right) and the mean differences across both cross-sections (left) for each activity group. The running (WHL) group (white boxes) had on average faster rates of osteogenesis than the climbing (CLB) group (dark grey boxes) and the control (CON) group (light grey boxes) had the slowest periosteal bone growth of all groups. The center vertical line in each box marks the median of the sample; the length of each box shows the range within which the central 50% of the values fall. Whiskers indicate 10th and 90th percentiles.
Gross deltoid crest morphology
There were no significant differences (absolute or relative to body mass) among groups in any measure of the gross morphology of the muscle attachment site, including length, width, area, thickness, and shape (Tables 1 and 2).
Discussion
The results of this study demonstrate that differences in physiologically normal activity patterns and levels can influence muscle fibre architecture (e.g., Lf and PCSA) and rate of bone growth, yet have no observable effect on the external size and shape of the muscle attachment site. Therefore, our findings do not provide empirical support for comparative studies linking muscle size and attachment site surface morphology (e.g., Hawkey and Merbs, 1995; Hawkey, 1998; Wilczak, 1998a,b; Knüsel, 2000; Eshed et al., 2004; Molnar, 2006, 2010; Villotte, 2006; Weiss, 2007, 2010; Havelková et al., 2011). Instead, our experimental results in mice are consistent with experimental evidence in adult sheep that show no effect of exercise on enthesis morphology (Zumwalt, 2005, 2006). Taken together, the absence of experimental data in support of the effect of activity on enthesis morphology advises caution in attempting to infer activity level and pattern from muscle attachment sites.
There are a number of factors besides muscle size and activity that may contribute to the development of attachment sites. Bone does not respond to all stimuli, and some stimuli lead to different bony responses (Turner, 1998; Burr et al., 2002; Currey, 2002). The extent of sex, age, hormone level, and genetics on entheseal response to muscular activity is highly debated (e.g., Zumwalt, 2005, 2006; Hamrick et al., 2006; Plochocki et al., 2008; Plochocki, 2009; Jurmain et al., 2012). The perception of an attachment site itself (i.e., being faint or well-developed) can be biased if the observer does not control for normal variation between populations or the relative robusticity of the underlying bone (Robb, 1998; Weiss, 2003; Zumwalt, 2005, 2006). Further challenges include methodological consistency across studies in defining the boundaries of attachment areas, and determining which aspects of the muscle attachment site are the most meaningful for the interpretation (Wilczak, 1998b).
Activity and muscle architecture
Our prediction that in comparison to controls, the exercise groups would have relatively larger muscle PCSAs, and thus relatively greater force-generating capacity, is supported for the climbing group but not the wheel runners. Rather the WHL mice showed a significant increase in fibre length, and thus greater excursion (and contraction velocity) potential of the muscles. Differences in gait between the exercise and control groups were met by changes in the contractile behaviour of individual muscles. The running mice had the relatively longest fibres, while the climbing mice had the shortest. Thus, compared with the climbing and control groups, runners had deltopectoral fibres that can shorten (and lengthen) over a greater distance (and at higher velocities). These findings are consistent with significant kinematic differences observed between the climbing and running groups, including significantly more flexed (protracted) shoulders during touchdown and lift-off during running (Green, 2010; Green et al., 2012).
It has been theoretically argued (Stern, 1974; Gans, 1982; Biewener and Gills, 1999; Lieber, 2002, Williams et al., 2008) and empirically demonstrated (e.g., Taylor et al., 2009) that a muscle cannot be optimized for both maximum force generation (PCSA) and excursion/contraction velocity (fibre length). We observed this architectural trade-off among our experimental and control groups as activity type significantly influenced the potential maximum force-generating capacity of the muscles analysed in this study (Tables 1 and 2; Fig. 5). The climbers had muscles comprising relatively larger PCSAs but relatively shorter fibres compared with the running and control groups, as might be predicted with the vertical activity, where mice regularly moved up and down the cage mesh. In the absence of differences in pennation angles, climbing appears to have resulted in an increase in the potential force output of the muscles largely by increasing muscle mass (and therefore PCSA) without altering the orientation of the fibres relative to the muscle’s force generating axis. Overall, fibre architecture data indicate that increased activity was associated with more powerful muscles (Williams et al., 2008), in terms of potential maximum force-generating capacity (climbers) and potential maximum excursion/contraction velocity (runners). Control mice had the least powerful muscles among the groups studied here.
Activity and muscle attachment sites
Bone has been shown to be responsive to mechanical stresses, such that elevated amounts of activity are expected to influence bone morphology (e.g., Currey, 2002), including the muscle attachment site (e.g., Frost, 2003; Thomopoulos et al., 2010, 2011). Tension exerted by muscles is presumed to increase blood flow, stimulate local osteogenesis, and increase the mass of bone beneath the muscle, thereby producing an elevated area of insertion at a fibrous enthesis (Chamay and Tschantz, 1972; Woo et al., 1981; Hawkey and Merbs, 1995; Montgomery et al., 2005; Cardoso and Henderson, 2010; Jurmain et al., 2012; Niinimäki et al., 2013). Indeed, elevated activity influenced differential periosteal growth in the humerus and throughout the deltoid crest (Table 1; Figs. 4 and 6). However, our prediction that activity level would have an effect on size of the deltoid crest was not borne out by the data (Tables 1 and 2). Although the deltoid crest serves as the attachment site for three large powerful muscles, all of which attach directly to the periosteum and anastomose with the vessels of the bone (Dörfl, 1969), we found no evidence of entheseal surface hypertrophy nor alterations in enthesis shape in response to increased activity. This is despite significantly more powerful muscles in both exercise groups, relatively larger PCSAs in the CLB and relatively longer fibres in the WHL. Our results concur with Green and colleagues’ (2012) data that further highlighted the complexity between muscle mass and the size and morphology of the scapular fossae in subadult mice. All of these results call into question whether differences in entheseal morphologies among individuals in a species can be linked to variation in function and activity (e.g., Hawkey and Merbs, 1995; Hawkey, 1998; Wilczak, 1998a,b; Knüsel, 2000; Eshed et al., 2004; Molnar, 2006, 2010; Villotte, 2006; Weiss, 2007, 2010; Havelková et al., 2011), at least for the level of physiological activity employed in this study.
It is important to note that our experimental protocol involved voluntary activity designed to produce physiologically normal variation in the level and type of activity, and to avoid activities that might induce pathology. Some (e.g., Zumwalt, 2005, 2006; Jurmain et al., 2012) have argued that muscle attachments develop as a protective response to bone injury during muscle contraction, and that forceful muscle activity may not impact enthesis morphology until they reach pathological levels. If muscle attachment sites develop only in response to pathological activity levels, this would limit the usefulness of enthesis morphology for inferring differences in normal behaviour or for reconstructing daily activity patterns. Whether enthesis morphology changes only in response to pathological activity levels requires further investigation and evidence from controlled experiments.
It is well appreciated that bone does not grow by adding new bone to all outer (periosteal) surfaces with corresponding removal from all inner (endosteal) surfaces. Rather, bone growth is accomplished through a combination of deposition and resorption on the periosteal and endosteal surfaces (e.g., Enlow, 1962; Ruff et al., 1994; Currey, 2002). As a result, tension from muscles can be associated with simultaneous bone deposition and removal, though it may not necessarily change the size or shape of the bony protuberance or crest. However, as Hoyte and Enlow (1966) demonstrated, Sharpey’s fibres may be exempt from the type of destruction that occurs during bone resorption and remain unaltered even though superficial bone tissues are removed. Therefore, Sharpey’s fibres provide an important mechanism for anchoring muscle attachments to bone during growth and remodelling of the bone surface. This may partially explain why entheseal surface morphology did not change in response to activity over time, despite faster periosteal bone growth rate in both exercise groups. Further research should focus on examinations of the microstructure of the entheseal surface, such as bone modeling and remodelling, as this anatomy has the potential to be more labile in response to variations in muscle activity during bone development.
Body size, bone response, and implications for primates and other mammals
In the light of the potential effects of size and scale on bone biomechanics, our results based on mice may raise questions about how these findings are relevant to variation in enthesis morphology in larger-bodied primates (as well as larger-bodied non-primate mammals). Geometric similarity predicts that, due to their small size, mouse skeletons would be relatively strong (Rubin and Lanyon, 1984b; Biewener, 1990). Indeed, small mammals are often regarded as being overbuilt for their biomechanical loads. However, the relative strength of the skeleton allows small mammals to use greater peak forces involved in agile locomotion using crouched postures with flexed limbs (e.g., Rubin and Lanyon, 1982, 1984b; Biewener, 1989, 1990). Importantly, and despite their small size, mice experience strain magnitudes comparable to those of larger vertebrates during locomotion (Lee et al., 2002). The broad similarity in peak strain and bone safety factor in mammals across a large size range (Rubin and Lanyon, 1982, 1984b) is likely due to the strong relationship between body mass and limb posture (Biewener, 1990); in other words, larger mammals maintain comparable strain levels by adopting less flexed limb postures and engaging in less agile locomotion. The fact that small-bodied mammals maintain stress and strain levels comparable to large-bodied animals suggests that mice are a reasonable model for investigating the relationship between variation in muscle attachment sites and locomotor loads and drawing inferences from these results to larger-bodied mammals, including primates. Were this not the case, small mammals would likely not serve as such successful model organisms for understanding human bone biology (e.g., Robling et al., 2006).
In this study, the observed differences in voluntary exercise were sufficient to induce significant changes in muscle fibre architecture and bone growth rates. This indicates that mice were not too small for the elevated activity, and the elevated strains and stresses associated with it, to elicit a physiological response in the developing muscle and bone tissue. Yet, despite this response, activity did not alter the gross morphology (size or shape) of associated entheses. Comparative research on primates similarly found no clear relationship between muscle fibre architecture and size and shape of forelimb muscle attachment sites between species that differ in frequency of daily locomotor and postural behaviours (Rabey et al., 2011; Rabey, 2014).
Future research
Of course, the experimental results reported in this study leave open many questions. It remains unclear what magnitude or duration of activity may be required to induce alterations in enthesis morphology (i.e., is there a dose/response?). Nor is it known how these might vary at different attachment sites (e.g., fibrous versus fibrocartilaginous attachments), or how enthesis morphology might scale with muscle force or body size. Thus, future studies would benefit from experiments aimed at establishing the relationship between magnitude and frequency of load and changes in enthesis morphology in mice as well as other smaller- and larger-bodied animals. Studies are also needed to examine the scaling relationships of enthesis morphology with muscle force and body size in primates and non-primate mammals.
Our results indicate that further experiments are warranted to examine the effects of age (juveniles, subadults, adults, and aged individuals), sex, and size. Future research should include observations of gait parameters (e.g., ground reaction forces, velocity, loading rates) and metabolic demands to better interpret changes in the musculoskeletal anatomy. Taking together the results of this study and previous work, it is not clear what inferences about variation in activity, within a species, can be reliably inferred from the gross anatomy of entheses. Current evidence suggests instead that internal bone structure serves as a more reliable source of evidence about habitual activity. For example, there is abundant evidence, based on controlled experiments, demonstrating that activity influences cross-sectional geometry of long bones (e.g., Robling et al., 2006 and references therein) and the structure of trabecular bone in joints (e.g., Pontzer et al., 2006). Furthermore, data suggest that habitual activity levels also influence other characteristics of bone microstructure such as regional bone density (e.g., Zeininger et al., 2011) and the structure of Haversian systems and osteocyte lacunae (e.g., Sanchez et al., 2013). The organization of Sharpey’s fibres and bone cell lacunae are particularly promising avenues for research into muscle-bone interactions that may leave visible traces in skeletal tissue and fossil bones.
Conclusion
This study demonstrated that variation in activity of subadult mice resulted in differences in muscle fibre architecture and periosteal bone growth. These findings are consistent with results of previous studies demonstrating that normal variation in activity produces sufficient stimuli to cause significant differences in bone as well as muscle fibre architecture (e.g., Allen et al., 2001; Lee et al., 2002; Mori et al., 2003; Waters et al., 2004; Hamrick et al., 2006; Plochocki, 2009; Schmitt et al., 2010; Byron et al., 2011; Green et al., 2012; Mathewson et al., 2012). However, variation in activity failed to produce differences in the deltopectoral enthesis morphology. These findings suggest that enthesis morphology may provide little insight into activity type or level.
A major goal of musculoskeletal research is to understand how behaviour influences bone structure. Abundant evidence demonstrates that, both within and between species, the degree and nature of activity influences both cross-sectional (e.g., Hamrick et al., 2000; Currey, 2002; Ruff et al., 2006; Niinimäki et al., 2013; Rabey, 2014) and trabecular bone architecture (e.g., Pontzer et al., 2006; Griffin et al., 2010). However, the inter- and intra-specific relationships between activity and bone strength are complex. Based on the results of this study, it is clear that additional studies of how muscle influences enthesis development are needed. A lack of understanding of muscle attachment site development has led to oversimplified and unsubstantiated interpretations of entheseal morphology and activity patterns about past populations. Further knowledge of the development and functional significance of entheseal morphology is needed if one is to accurately reconstruct behaviour based on entheseal morphology in extant and fossil skeletal samples.
Acknowledgements
We would like to thank Amber MacKenzie, Cassandra Turcotte, and Drs. Timothy Bromage, Roxy Larsen, Charlotte Miller, Daniel Schmitt, and Angel Zeininger for their support and helpful comments. We are also very grateful to Mark Wagner and Will Morton from George Washington’s Institute from the Materials Science Machine Shop, and Mike Manion, Dr. Kathleen Gordon, Robert Bergen, and the rest of GWU’s Animal Research Facility, and Sara Miran for help with the experiments.
This study was funded by Wenner-Gren Foundation Dissertation Fieldwork Grants to KNR and DJG, NSF Doctoral Dissertation Improvement Grant BCS-0824552, NSF DGE-0801634, NSF BCS 0962677 and NIH R24 HD050837-01, GW Signature Program funding to the Center for the Advanced Study of Hominid Paleobiology, and the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David J. Green, Email: dgreen1@midwestern.edu.
Andrea B. Taylor, Email: andrea.taylor@duke.edu.
David R. Begun, Email: begun@chass.utoronto.ca.
Brian G. Richmond, Email: brichmond@amnh.org.
Shannon C. McFarlin, Email: mcfarlin@gwu.edu.
References
- Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J. Appl. Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- Anapol FC, Barry K. Fiber architecture of the extensors of the hindlimb in the semiterrestrial and arboreal guenons. Am. J. Phys. Anthropol. 1996;99:429–447. doi: 10.1002/(SICI)1096-8644(199603)99:3<429::AID-AJPA5>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Antón SC. Masticatory muscle architecture and bone morphology in primates. Ph.D. Dissertation, University of California; 1994. [Google Scholar]
- Asfour SS, Ayoub MM, Mital A. Effects of an endurance and strength training programme on lifting capability of males. Ergonomics. 1984;27:435–442. doi: 10.1080/00140138408963507. [DOI] [PubMed] [Google Scholar]
- Bang ML, Li X, Littlefield R, Bremner S, Thor A, Knowlton KU, Lieber RL, Chen J. Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. J. Cell Biol. 2006;173:905–916. doi: 10.1083/jcb.200603119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J. Anat. 1986;149:89–100. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Kumai T, Milz S, Boszcyk BM, Boszcyk AA, Ralphs JR. The skeletal attachment of tendons – tendons ‘entheses’. Comp. Biochem. Physiol. A. 2002;133:931–945. doi: 10.1016/s1095-6433(02)00138-1. [DOI] [PubMed] [Google Scholar]
- Biewener AA. Mammalian terrestrial locomotion and size: mechanical design principles define limits. BioScience. 1989;39:776–783. [Google Scholar]
- Biewener AA. Biomechanics of mammalian terrestrial locomotion. Science. 1990;250:1097–1103. doi: 10.1126/science.2251499. [DOI] [PubMed] [Google Scholar]
- Biewener AA. In vivo measurement of bone strain and tendon force. In: Biewener AA, editor. Biomechanics - Structures and Systems: A Practical Approach. New York: Oxford University Press; 1992. pp. 123–147. [Google Scholar]
- Biewener AA, Bertram LE. Skeletal strain patterns in relation to exercise training during growth. J. Exp. Biol. 1993;185:51–69. doi: 10.1242/jeb.185.1.51. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Gills GB. Dynamics of muscle function during locomotion: accommodating variable conditions. J. Exp. Biol. 1999;202:3387–3396. doi: 10.1242/jeb.202.23.3387. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Roy RR, Meadows DA, Zernicke RF, Sacks RD, Fournier M, Edgerton VR. Architectural, histochemical, and contractile characteristics of a unique biarticular muscle: the cat semitendinous. J. Neurophysiol. 1982;48:192–201. doi: 10.1152/jn.1982.48.1.192. [DOI] [PubMed] [Google Scholar]
- Bryant H, Seymour K. Observations and comments on the reliability of muscle reconstruction in fossil vertebrates. J. Morphol. 1990;206:109–117. doi: 10.1002/jmor.1052060111. [DOI] [PubMed] [Google Scholar]
- Burr DB, Allen MR. Basic and Applied Bone Biology. New York: Academic Press; 2013. [Google Scholar]
- Burr DB, Robling AG, Turner CH. Effects of biomechanical stress in animals. Bone. 2002;30:781–786. doi: 10.1016/s8756-3282(02)00707-x. [DOI] [PubMed] [Google Scholar]
- Byron C, Kunz H, Matuszek H, Lewis S, Van Valkinburgh D. Rudimentary pedal grasping in mice and implications for terminal branch arboreal quadrupedalism. J. Morphol. 2011;272:230–240. doi: 10.1002/jmor.10909. [DOI] [PubMed] [Google Scholar]
- Cardoso FA, Henderson CY. Enthesopathy formation in the humerus: data from known age-at-death and known occupation skeletal collections. Am. J. Phys. Anthropol. 2010;141:550–560. doi: 10.1002/ajpa.21171. [DOI] [PubMed] [Google Scholar]
- Castanet J, Croci S, Aujard F, Perret M, Cubo J, de Margerie E. Lines of arrested growth in bone and age estimation in a small primate: Microcebus murinus . J. Zool. 2004;263:31–39. [Google Scholar]
- Chamay A, Tschantz P. Mechanical influences in bone remodelling. Experimental research on Wolff’s law. J. Biomech. 1972;5:173–180. doi: 10.1016/0021-9290(72)90053-x. [DOI] [PubMed] [Google Scholar]
- Chiasson RB. Laboratory Anatomy of the White Rat. Third edition. Iowa: WM.C. Brown Company Publishers; 1975. [Google Scholar]
- Cho H. The histology laboratory and principles of microscope instrumentation. In: Crowder C, Stout S, editors. Bone Histology: An Anthropological Perspective. Boca Raton: Taylor & Francis Group, LLC; 2012. pp. 341–359. [Google Scholar]
- Churchill SE, Morris AG. Muscle marking morphology and labour intensity in prehistoric Khoisan foragers. Int. J. Osteoarchaeol. 1998;8:390–411. [Google Scholar]
- Clarke KA, Still J. Gait analysis in the mouse. Physiol. Behav. 1999;66:723–729. doi: 10.1016/s0031-9384(98)00343-6. [DOI] [PubMed] [Google Scholar]
- Currey J. Bone: Structure and Mechanism. New Jersey: Princeton University Press; 2002. [Google Scholar]
- Davis CB, Shuler KA, Danforth ME, Hendron KE. Patterns of interobserver error in the scoring of entheseal changes. Int. J. Osteoarchaeol. 2013;23:147–151. [Google Scholar]
- Davis DD. The giant panda. A morphological study of evolutionary mechanism. Fieldiana Zool. Mem. 1964;3:1–339. [Google Scholar]
- Dörfl J. Vessels in the region of tendinous insertions. II Diaphysoperiosteal insertion. Folia Primatol. 1969;17:70–82. [PubMed] [Google Scholar]
- Drapeau M. Enthesis bilateral asymmetry in humans and African apes. HOMO J. Comp. Hum. Biol. 2008;59:93–109. doi: 10.1016/j.jchb.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Dysart PS, Harkness EM, Herbison GP. Growth of the humerus after denervation: an experimental study in the rat. J. Anat. 1989;167:147–159. [PMC free article] [PubMed] [Google Scholar]
- Edman KAP. Contractile properties of mouse single muscle fibers, a comparison with amphibian muscle fibers. J. Exp. Biol. 2005;208:1905–1913. doi: 10.1242/jeb.01573. [DOI] [PubMed] [Google Scholar]
- Eliot DJ, Jungers WL. Fifth metatarsal morphology does not predict presence or absence of fibularis tertius muscle in hominids. J. Hum. Evol. 2000;38:333–342. doi: 10.1006/jhev.1999.0337. [DOI] [PubMed] [Google Scholar]
- Elkasrawy MN, Hamrick MW. Myostatin (GDF-8) as a key factor linking muscle mass and bone structure. J. Musculoskelet. Neuronal Interact. 2010;10:56–63. [PMC free article] [PubMed] [Google Scholar]
- Enlow DH. Functions of the Haversian system. Am. J. Anat. 1962;110:269–305. doi: 10.1002/aja.1001100305. [DOI] [PubMed] [Google Scholar]
- Enlow DH, Brown SO. A comparative histological study of fossil and recent bone tissues. Part III. Texas J. Sci. 1958;10:187–230. [Google Scholar]
- Eshed V, Gopher A, Galili E, Israel H. Musculoskeletal stress markers in Natufian hunter gatherers and Neolithic farmers in the Levant: the upper limb. Am. J. Phys. Anthropol. 2004;123:303–315. doi: 10.1002/ajpa.10312. [DOI] [PubMed] [Google Scholar]
- Felder A, Ward SR, Lieber RL. Sarcomere length measurement permits high resolution normalization of muscle fiber length in architectural studies. J. Exp. Biol. 2005;208:3275–3279. doi: 10.1242/jeb.01763. [DOI] [PubMed] [Google Scholar]
- Frost HM. Bone’s mechanostat: a 2003 update. Anat. Rec. 2003;275:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- Gans C. Fiber architecture and muscle function. Exerc. Sport Sci. Rev. 1982;10:160–207. [PubMed] [Google Scholar]
- Gans C, Bock WJ. The functional significance of muscle architecture - a theoretical analysis. Adv. Anat. Embryol. Cell Biol. 1965;38:115–142. [PubMed] [Google Scholar]
- Gans C, Gaunt AS. Muscle architecture in relation to function. J. Biomech. 1991;24:53–65. doi: 10.1016/0021-9290(91)90377-y. [DOI] [PubMed] [Google Scholar]
- Gokhin DS, Bang ML, Zhang J, Chen J, Lieber RL. Reduced thin filament in length in nebulin-knockout skeletal muscle alters isometric contractile properties. Am. J. Physiol. 2009;296:1123–1132. doi: 10.1152/ajpcell.00503.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman HM, Kindsvater J, Bromage TG. Correlative light and backscattered electron microscopy of bone - Part I: Specimen preparation methods. Scanning. 1998;21:40–43. doi: 10.1002/sca.4950210106. [DOI] [PubMed] [Google Scholar]
- Green DJ. Shoulder functional anatomy and development: implications for interpreting early hominin locomotion. Ph.D. Dissertation, The George Washington University; 2010. [Google Scholar]
- Green DJ, Richmond BG, Miran SL. Mouse shoulder morphology responds to locomotor activity and kinematic differences in climbing and running. J. Exp. Zool. 2012;318:621–638. doi: 10.1002/jez.b.22466. [DOI] [PubMed] [Google Scholar]
- Greene EC. Anatomy of the Rat. New Jersey: Prentice Hall; 1935. [Google Scholar]
- Griffin NL, D’Août K, Ryan TM, Richmond BG, Ketcham RA, Postnov A. Comparative forefoot trabecular bone architecture in extant hominids. J. Hum. Evol. 2010;59:202–213. doi: 10.1016/j.jhevol.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, McPherron AC, Lovejoy CO, Hudson J. Femoral morphology and cross sectional geometry of adult myostatin-deficient mice. Bone. 2000;27:343–349. doi: 10.1016/s8756-3282(00)00339-2. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Ding K-H, Pennington C, Chao YJ, Wu Y-D, Howard B, Immel D, Borlongan C, McNeil PL, Bollag WB, Curl WW, Yu J, Isales CM. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39:845–853. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Harber MP, Konopka AR, Undem MK, Hinkley JM, Minchev K, Kaminsky LA, Trappe TA, Trappe S. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J. Appl. Physiol. 2012;113:1495–1504. doi: 10.1152/japplphysiol.00786.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelková P, Vilotte S, Velemínsk P, Polácek L, Dobisíková M. Enthesopathies and activity patterns in the Early Medieval Great Moravian population: evidence of division of labour. Int. J. Osteoarchaeol. 2011;21:487–504. [Google Scholar]
- Havelková P, Hladík M, Velemínský P. Entheseal changes: do they reflect socioeconomic status in the early medieval central European population? (Mikulčice - Kláteřisko, Great Moravian Empire, 9th-10th century) Int. J. Osteoarchaeol. 2013;23:237–251. [Google Scholar]
- Hawkey DE. Disability, compassion and the skeletal record: using musculoskeletal stress markers (MSM) to construct an osteobiography from early New Mexico. Int. J. Osteoarchaeol. 1998;8:326–340. [Google Scholar]
- Hawkey DE, Merbs CF. Activity induced musculoskeletal stress markers (MSM) and subsistence strategy changes among ancient Hudson Bay Eskimos. Int. J. Osteoarchaeol. 1995;5:324–338. [Google Scholar]
- Hems T, Tillmann B. Tendon entheses of the human masticatory muscles. Anat. Embryol. 2000;202:201–208. doi: 10.1007/s004290000107. [DOI] [PubMed] [Google Scholar]
- Henderson CY, Craps DD, Caffell AC, Millard AR, Gowland R. Occupational mobility in the 19th century rural England: the interpretation of entheseal changes. Int. J. Osteoarchaeol. 2013;23:197–210. [Google Scholar]
- Hoyte DAN, Enlow DH. Wolff’s law and the problem of muscle attachment on resorptive surfaces of bone. Am. J. Phys. Anthropol. 1966;24:205–214. doi: 10.1002/ajpa.1330240209. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Roy BB, Ohira Y, Ibata Y, Edgerton VR. Hypertrophy of rat plantaris muscle fibres after voluntary running with increasing loads. J. Appl. Physiol. 1998;84:2183–2189. doi: 10.1152/jappl.1998.84.6.2183. [DOI] [PubMed] [Google Scholar]
- Jurmain R, Cardoso FA, Henderson C, Villotte S. Bioarchaeology’s Holy Grail: The reconstruction of activity. In: Grauer AL, editor. A Companion to Paleopathology. Wiley-Blackwell: Massachusetts; 2012. pp. 531–552. [Google Scholar]
- Kilborn SH, Trudel G, Uhthoff H. Review of growth plate closure compared with age and sexual maturity and lifespan in laboratory animal. J. Am. Assoc. Lab. Anim. Sci. 2002;41:21–26. [PubMed] [Google Scholar]
- Kimes KR, Siegel MI, Sadler DL. Alteration of scapular morphology through experimental behavioural modification in the laboratory mouse (Mus musculus) Acta Anat. 1981;109:161–165. doi: 10.1159/000145379. [DOI] [PubMed] [Google Scholar]
- Knüsel C. Bone adaptation and its relationship to physical activity in the past. In: Cox M, Mays S, editors. Human Osteology in Archaeology and Forensic Science. London: Greenwich Medical Media Limited; 2000. pp. 381–402. [Google Scholar]
- Lee KCL, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31:1–9. doi: 10.1016/s8756-3282(02)00842-6. [DOI] [PubMed] [Google Scholar]
- Lieber RL. Skeletal Muscle Structure, Function, and Plasticity. Second edition. Baltimore: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- Lieber RL, Ward SR. Skeletal muscle design to meet functional demands. Phil. Trans. R. Soc. B. 2011;366:1466–1476. doi: 10.1098/rstb.2010.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber RL, Loren GJ, Fridén J. In vivo measurement of human wrist extensor muscle sarcomere length changes. J. Neurophysiol. 1994;71:874–881. doi: 10.1152/jn.1994.71.3.874. [DOI] [PubMed] [Google Scholar]
- Mariotti V, Facchini F, Belcastro MG. The study of entheses: proposal of a standardised scoring method for twenty-three entheses of the postcranial skeleton. Collegium Antropol. 2007;31:291–313. [PubMed] [Google Scholar]
- Martin RB, Burr DB, Sharkey NA. Skeletal Tissue Mechanics. Dordrecht: Springer-Verlag Inc; 1998. [Google Scholar]
- Marzke MW, Shrewsbury MM. The Oreopithecus thumb: pitfalls in reconstructing muscle and ligament attachments from fossil bones. J. Hum. Evol. 2006;51:213–215. doi: 10.1016/j.jhevol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Marzke MW, Shrewsbury MM, Horner KE. Middle phalanx skeletal morphology in the hand: can it predict flexor tendon size and attachments? Am. J. Phys. Anthropol. 2007;134:141–151. doi: 10.1002/ajpa.20646. [DOI] [PubMed] [Google Scholar]
- Mathewson MA, Chapman MA, Hentzen ER, Fridén J, Lieber RL. Anatomical, architectural, and biomechanical diversity of the murine forelimb muscles. J. Anat. 2012;221:443–451. doi: 10.1111/j.1469-7580.2012.01559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlin SC, Terranova CJ, Zihlman AL, Enlow DH, Bromage TG. Regional variability in secondary remodeling within long bone cortices of catarrhine primates: the influence of bone growth history. J. Anat. 2008;213:308–324. doi: 10.1111/j.1469-7580.2008.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan C. The hind limb musculature of the brown kiwi, Apteryx australis mantelli . J. Morphol. 1979;160:22–73. doi: 10.1002/jmor.1051600105. [DOI] [PubMed] [Google Scholar]
- Mendez RA, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Michilsens F, Vereecke EE, D’Août K, Aerts P. Functional anatomy of the gibbon forelimb: adaptations to a brachiating lifestyle. J. Anat. 2009;215:335–354. doi: 10.1111/j.1469-7580.2009.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar P. Tracing prehistoric activities: musculoskeletal stress marker analysis of a Stone-Age population on the island of Gotland in the Baltic Sea. Am. J. Phys. Anthropol. 2006;129:12–23. doi: 10.1002/ajpa.20234. [DOI] [PubMed] [Google Scholar]
- Molnar P. Patterns of physical activity and material culture on Gotland, Sweden, during the Middle Neolithic. Int. J. Osteoarchaeol. 2010;20:1–14. [Google Scholar]
- Montgomery E, Pennington C, Isales CM, Hamrick MH. Muscle-bone interactions in drystrophin-deficient and myostatin-deficient mice. Anat. Rec. 2005;286A:814–822. doi: 10.1002/ar.a.20224. [DOI] [PubMed] [Google Scholar]
- Mori T, Okimoto N, Sakai A, Okazaki Y, Nakura N, Notomi T, Nakamura T. Climbing exercise increase bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J. Bone Miner. Res. 2003;18:2002–2009. doi: 10.1359/jbmr.2003.18.11.2002. [DOI] [PubMed] [Google Scholar]
- Mosley JR, Lanyon LE. Growth rate rather than gender determines the size of the adaptive response of the growing skeleton to mechanical strain. Bone. 2002;30:314–319. doi: 10.1016/s8756-3282(01)00626-3. [DOI] [PubMed] [Google Scholar]
- Niinimäki S. What do muscle marker ruggedness scores actually tell us? Int. J. Osteoarchaeol. 2011;21:292–299. [Google Scholar]
- Niinimäki S, Sotos LB. The relationship between intensity of physical activity and entheseal changes on the lower limb. Int. J. Osteoarchaeol. 2013;23:221–228. [Google Scholar]
- Niinimäki S, Söderling S, Junno J-A, Finnilä M, Niskanen M. Cortical bone thickness can adapt locally to muscular loading while changing with age. HOMO - J. Comp. Hum. Biol. 2013;64:474–490. doi: 10.1016/j.jchb.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Nolte MLS, Wilczak C. Three-dimensional surface area of the biceps enthesis, relationship to body size, sex, age, and secular changes in a 20th century American sample. Int. J. Osteoarchaeol. 2013;23:163–174. [Google Scholar]
- Parfitt AM. The attainment of peak bone mass: what is the relationship between muscle growth and bone growth? Bone. 2004;34:767–770. doi: 10.1016/j.bone.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Payne RC, Crompton RH, Isler K, Savage R, Vereecke EE, Günther MM, Thorpe SKS, D’Août K. Morphological analysis of the hindlimb in apes and humans I. Muscle architecture. J. Anat. 2006;208:709–724. doi: 10.1111/j.1469-7580.2006.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson OM, Lieberman DE. The aging of Wolff’s "law": ontogeny and responses to mechanical loading in cortical bone. Yearb. Phys. Anthropol. 2004;47:63–99. doi: 10.1002/ajpa.20155. [DOI] [PubMed] [Google Scholar]
- Plochocki JH. Mechanically-induced osteogenesis in the cortical bone of pre- to peripubertal stage and peri- to postpubertal stage mice. J. Orthop. Surg. Res. 2009;4:22. doi: 10.1186/1749-799X-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plochocki JH, Rivera JP, Zhang C, Ebba SA. Bone modeling response to voluntary exercise in the hindlimb of mice. J. Morphol. 2008;269:313–318. doi: 10.1002/jmor.10587. [DOI] [PubMed] [Google Scholar]
- Pontzer H, Lieberman DE, Momin E, Devlin MJ, Polk JD, Hallgrímsson B, Cooper DML. Trabecular bone in the bird knee responds with high sensitivity to changes in load orientation. J. Exp. Biol. 2006;209:57–65. doi: 10.1242/jeb.01971. [DOI] [PubMed] [Google Scholar]
- Powell PL, Roy RR, Kanim P, Bello M, Edgerton VR. Predictability of skeletal tension from architectural determinations in guinea pig hindlimbs. J. Appl. Physiol. 1984;57:1715–1721. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- Rabey KN. Forelimb muscle and muscle attachment morphology. Ph.D. Dissertation, University of Toronto; 2014. [Google Scholar]
- Rabey KN, Mackenzie AE, McCormick S, Begun DR. Functional anatomy of forelimb muscles in captive Sumatran orangutans. Am. J. Phys. Anthropol. 2011;144(S52):S175. [Google Scholar]
- Rathburn T. Health and disease at a South Carolina plantation: 1840–1870. Am. J. Phys. Anthropol. 1987;74:239–253. doi: 10.1002/ajpa.1330740211. [DOI] [PubMed] [Google Scholar]
- Robb J. The interpretation of skeletal muscle sites: a statistical approach. Int. J. Osteoarchaeol. 1998;8:363–377. [Google Scholar]
- Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodelling. A. Rev. Biomed. Eng. 2006;8:455–498. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Limb mechanics as a function of speed and gait: a study of functional strains in the radius and tibia of horse and dog. J. Exp. Biol. 1982;101:187–211. doi: 10.1242/jeb.101.1.187. [DOI] [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Regulation of bone formation by applied dynamic loads. J. Bone Joint Surg. 1984a;66:397–402. [PubMed] [Google Scholar]
- Rubin CT, Lanyon LE. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. J. Theor. Biol. 1984b;107:321–327. doi: 10.1016/s0022-5193(84)80031-4. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Walker AC, Trinkaus E. Postcranial robusticity in Homo. III: Ontogeny. Am. J. Phys. Anthropol. 1994;93:35–54. doi: 10.1002/ajpa.1330930103. [DOI] [PubMed] [Google Scholar]
- Ruff CB, Holt BH, Trinkaus E. Who’s afraid of the big bad Wolff? Wolff’s law and bone functional adaptation. Am. J. Phys. Anthropol. 2006;129:484–498. doi: 10.1002/ajpa.20371. [DOI] [PubMed] [Google Scholar]
- Salmons S, Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981;4:94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- Sanchez S, Dupret V, Tafforeau P, Trinajstic KM, Ryll B, Gouttenoire P-J, Wretman L, Zylberberg L, Peyrin F, Ahlberg PE. 3D microstructural architecture of muscle attachments in extant and fossil vertebrates revealed by synchrotron microtomography. PLoS One. 2013;8:e56992. doi: 10.1371/journal.pone.0056992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht SH. Understanding entheses: bridging the gap between clinical and anthropological perspectives. Anat. Rec. 2012;295:1239–1251. doi: 10.1002/ar.22516. [DOI] [PubMed] [Google Scholar]
- Schmitt D, Zumwalt AC, Hamrick MW. The relationship between bone mechanical properties and ground reaction forces in normal and hypermuscular mice. J. Exp. Zool. 2010;313A:339–351. doi: 10.1002/jez.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenau E, Fricke O. Mechanical influences on bone development in children. Eur. J. Endocrinol. 2008;159:S27–S31. doi: 10.1530/EJE-08-0312. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: The Principles and Practice of Statistics in Biological Research. Fourth edition. New York: W.H. Freeman and Company; 2012. [Google Scholar]
- Stern JT., Jr Computer modelling of gross muscle dynamics. J. Biomech. 1974;7:411–428. doi: 10.1016/0021-9290(74)90004-9. [DOI] [PubMed] [Google Scholar]
- Taylor AB, Eng CM, Anapol FC, Vinyard CJ. The functional correlates of jaw-muscle fiber architecture in tree-gouging and nongouging callitrichid monkeys. Am. J. Phys. Anthropol. 2009;139:353–367. doi: 10.1002/ajpa.20991. [DOI] [PubMed] [Google Scholar]
- Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion: what development can teach us about healing. J. Musculoskelet. Neuronal Interact. 2010;10:35–45. [PMC free article] [PubMed] [Google Scholar]
- Thomopoulos S, Das R, Birman V, Smith L, Ku K, Elson E, Pryse KM, Marquez P, Genin GM. Fibrocartilage tissue engineering: the role of the stress environment on cell morphology and matrix expression. Tissue Eng. A. 2011;17:1039–1053. doi: 10.1089/ten.tea.2009.0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkaus E. The evolution of the hominid femoral diaphysis during the Upper Pleistocene in Europe and the Near East. Z. Morph. Anthropol. 1976;67:291–319. [PubMed] [Google Scholar]
- Turner CH. Three rules of bone adaption to mechanical stimuli. Bone. 1998;23:399–407. doi: 10.1016/s8756-3282(98)00118-5. [DOI] [PubMed] [Google Scholar]
- Villotte S. Connaissance médicales actuelles, cotation des enthésopathies. Bull. Mém. Soc. Anthropol. Paris. 2006;18:65–85. [Google Scholar]
- Villotte S, Churchill SE, Dutour OJ, Henry-Gambier D. Subsistence activities and the sexual division of labor in the European Upper Paleolithic and Mesolithic: evidence from the upper limb enthesopathies. J. Hum. Evol. 2010;59:35–43. doi: 10.1016/j.jhevol.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Wang W, Crompton RH, Carey TS, Günther MM, Li Y, Savage R, Sellers WI. Comparison of inverse-dynamics musculo-skeletal models of AL 288-1 Australopithecus afarensis and KNM-WT 15000 Homo ergaster to modern humans, with implications for the evolution of bipedalism. J. Hum. Evol. 2004;47:453–478. doi: 10.1016/j.jhevol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. J. Biomech. 2005;38:2317–2320. doi: 10.1016/j.jbiomech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces type-specific angiogenesis in mouse skeletal muscle. Am. J. Physiol. 2004;287:C1342–C1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- Weiss E. Understanding muscle markers: aggregation and construct validity. Am. J. Phys. Anthropol. 2003;121:230–240. doi: 10.1002/ajpa.10226. [DOI] [PubMed] [Google Scholar]
- Weiss E. Muscle markers revisited: Activity pattern reconstruction with controls in a Central California Amerind population. Am. J. Phys. Anthropol. 2007;133:931–940. doi: 10.1002/ajpa.20607. [DOI] [PubMed] [Google Scholar]
- Weiss E. Cranial muscle markers: A preliminary examination of size, sex, and age effects. J. Comp. Hum. Biol. 2010;61:48–58. doi: 10.1016/j.jchb.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Wilczak CA. Consideration of sexual dimorphism, age, and asymmetry in quantitative measurements of muscle insertion sites. Int. J. Osteoarchaeol. 1998a;8:311–325. [Google Scholar]
- Wilczak CA. A new method for quantifying musculoskeletal stress markers (MSM): a test of the relationship between enthesis size and habitual activity in archaeological populations. Ph.D. Dissertation, Cornell University; 1998b. [Google Scholar]
- Williams SB, Wilson AM, Rhodes L, Andrews J, Payne RC. Functional anatomy and muscle moment arms of the pelvic limb of an elite athlete: the racing greyhound (Canis familiaris) J. Anat. 2008;213:361–372. doi: 10.1111/j.1469-7580.2008.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters TM, Takahashi M, Lieber RL, Ward SR. Whole muscle length-tension relationships are accurately modeled as scaled sarcomeres in rabbit hindlimb muscles. J. Biomech. 2011;44:109–115. doi: 10.1016/j.jbiomech.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SL, Kuei SC, Amiel D, Gomez MA, Hayes WC, White FC, Akeson WH. The effect of prolonged physical training on the properties of bone: a study of Wolff’s law. J. Bone Joint Surg. 1981;63A:780–787. [PubMed] [Google Scholar]
- Zeininger A, Richmond BG, Hartman G. Metacarpal head biomechanics: A comparative backscattered electron image analysis of trabecular bone mineral density in Pan troglodytes, Pongo pygmaeus, and Homo sapiens . J. Hum. Evol. 2011;60:703–710. doi: 10.1016/j.jhevol.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Zumwalt A. The effect of endurance exercise on the morphology of muscle attachment sites: an experimental study in sheep (Ovis aries). Ph.D. Dissertation, Johns Hopkins University; 2005. [Google Scholar]
- Zumwalt A. The effect of endurance exercise on the morphology of muscle attachment sites. J. Exp. Biol. 2006;209:444–454. doi: 10.1242/jeb.02028. [DOI] [PubMed] [Google Scholar]