Abstract
We report on a patient with an abdominal wall abscess that developed after an inguinal hernia repair that utilized synthetic mesh. Mycobacterium goodii, a recently recognized, rapidly growing mycobacterium related to M. smegmatis, was isolated both from the abdominal wall aspirate and from surgically drained material. Infection resolved following thorough debridement, mesh removal, and prolonged antimicrobial therapy. This case report extends our understanding of the spectrum of M. goodii infection.
CASE REPORT
A 65-year-old former rancher and current restaurateur underwent ventral abdominal and right indirect inguinal hernia repairs in January 2003 at a Colorado hospital. Prosthetic mesh material was used for the surgical repair. Eighteen days later he noticed redness and swelling at the operative site. One day later, because of fever, chills, rigors, and increasing wound swelling and pain, he was admitted to a community hospital. A computerized tomogram (CT) demonstrated an abdominal wall fluid collection, and the patient was transferred to our facility for further care.
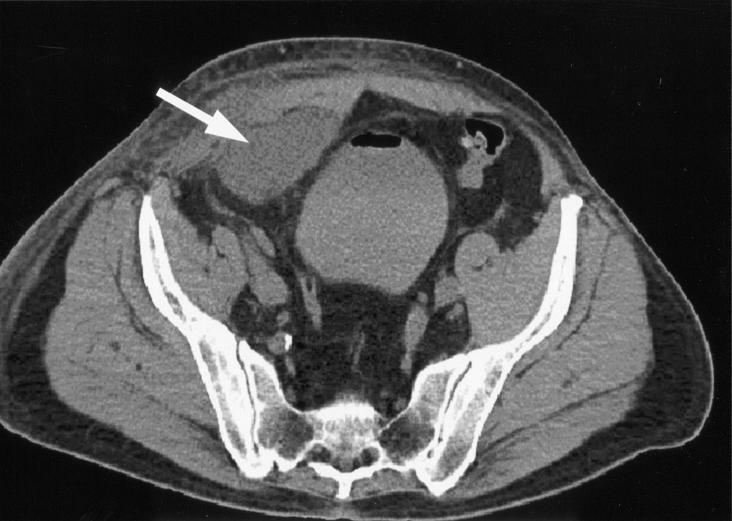
The patient was febrile upon admission (temperature, 38.8°C). The right inguinal herniorraphy site was erythematous and tender. Purulent material was draining from the wound. Cellulitic changes extended from the right thigh into the groin and scrotum. Laboratory data demonstrated an increased total white blood cell count (11.0 × 103 cells/μl) with neutrophilia (88%). An abdominal CT scan showed a 9.0- by 7.5- by 4.0-cm fluid collection in the right lower quadrant of the abdomen, in the abdominal wall, and in preperitoneal space (Fig. 1). Piperacillin-tazobactam therapy was initiated. On the second hospital day, 120 ml of thick brown material was aspirated percutaneously from the abscess cavity. The patient underwent open surgical exploration of the wound on the same day. A large amount of pus was encountered immediately upon entering the subcutaneous space. Extensive debridement of the abdominal wall and preperitoneal space was performed, and the prosthetic mesh was removed. A small, recurrent indirect inguinal hernia was repaired by using the Bassini method, and the wound was closed with staples. Gram stains of both the percutaneously aspirated material and the surgically debrided tissue showed gram-positive bacilli and many polymorphonuclear neutrophils. Staining of the material to detect acid-fast bacilli was not performed. On the third day of incubation, cultures revealed acid-fast bacilli, identified as Mycoplasma goodii by 16S rRNA sequencing (testing was performed at the Mycobacteriology Laboratory, Mayo Medical Laboratories, Rochester, Minn., and used MicroSeq ID 16S ribosomal DNA reagents and version 1.4.2 software [Applied Biosystems, Foster City, Calif.]), with a similarity index of 100%. Microtiter broth dilution antimicrobial susceptibility testing (Sensititre Customplate TLS CLM2DMAY; Trek Diagnostic Systems, Inc., Cleveland, Ohio) indicated that the isolate was sensitive to imipenem (MIC, 2 μg/ml), tobramycin (MIC, 2 μg/ml), ciprofloxacin (MIC, <0.25 μg/ml), amikacin (MIC, <2 μg/ml), doxycycline (MIC, <0.12 μg/ml), and sulfamethoxazole (MIC, 8 μg/ml). Resistance to cefoxitin (MIC, >64 μg/ml) and clarithromycin (MIC, >32 μg/ml) was demonstrated.
FIG. 1.
CT scan showing preperitoneal abdominal abscess (arrow) with surrounding inflammatory changes.
Once the infecting microorganism was found to be an acid-fast bacterium, antimicrobial therapy was changed to a combination of cefoxitin and amikacin. When antimicrobial susceptibility data became available, treatment was changed to oral trimethoprim-sulfamethoxazole. A 1-month course was prescribed. The patient's surgical wound healed but erythema and purulent drainage recurred several months later. The patient resumed trimethoprim-sulfamethoxazole therapy briefly, and the wound healed rapidly without additional recurrence.
In 1885, Lustgarten, a first assistant of Moricz Kaposi at the Vienna General Hospital dermatological wards, first described an acid-fast, “undulatory” bacillus in the syphilitic lesions of 16 patients (7). Its role as a cause of syphilis was later disproven, and the organism was named Mycobacterium smegmatis in recognition of its presence in normal penile secretions (6). Although rarely a cause of human disease, M. smegmatis was reported by Wallace and associates to be a pathogen in a number of human cases, nearly all associated with skin and soft tissue infection (13). Others have reported individual cases of M. smegmatis posttraumatic skin and soft tissue infection (9), vascular catheter-related bacteremia (11), pleuropulmonary infection (5, 12), and fatal, disseminated infection in a child with inherited interferon gamma receptor deficiency (10). Other rapidly growing nontuberculous mycobacteria associated with human disease include M. fortuitum, M. chelonae, and M. abscessus (4).
Differences in antimicrobial susceptibility among M. smegmatis isolates have recently led to the realization that these bacteria might comprise at least three groups of rapidly growing mycobacteria. Using high-pressure liquid chromatography and PCR-restriction enzyme patterns, two newly named species, M. wolinskyi and M. goodii, can be distinguished from M. smegmatis (1). In the initial microbiological report of M. goodii, Brown et al. noted that most of the 28 isolates were recovered from nonpulmonary sources, including skin and soft tissue infections and osteomyelitis following penetrating trauma or surgery (1). Additional isolates were associated with nosocomial infection (catheter-related sepsis, pacemaker site infection, and other surgical wounds), and other isolates were from patients with lipoid pneumonia or other types of pulmonary infection. After Brown et al.'s index tabulation of cases, only one additional report of human M. goodii has appeared: Friedman and Sexton described a case of olecranon bursitis in a patient with type 2 diabetes mellitus and benign monoclonal gammopathy (3). Their patient did not have any history of penetrating trauma, but the possibility of bacterial introduction with intrabursal injections or subsequent surgery was raised.
We hypothesize that our patient acquired M. goodii infection at the time of his hernia repair. He presented with abdominal wall and preperitoneal abscesses 3 weeks after surgery. To our knowledge, this is the first reported case of postoperative abdominal wall abscess with M. goodii.
M. goodii grows on trypticase soy agar and Middlebrook 7H10 agar within 7 days at 30, 35, and 45°C. Nitrate reductase and iron uptake reactions are positive, and the 3-day arylsulfatase reaction is negative. Colonies grow on MacConkey agar without crystal violet. Colonies are usually smooth and off-white to cream colored. Yellow to orange pigmentation occurring after 10 to 14 days of incubation on 7H10 agar is a characteristic feature that helps to differentiate M. goodii from nonpigmented M. fortuitum strains. Unique high-pressure liquid chromatography mycolic acid patterns, 16S rRNA sequencing, and PCR-restriction fragment length polymorphism typing analysis facilitate identification (1, 2). In contrast to some of the other rapidly growing mycobacteria, M. goodii organisms have not been recovered from the environment (2).
Antimicrobial susceptibility data are sparse. Brown et al. reported that almost all of their M. goodii isolates were susceptible to amikacin, doxycycline, ciprofloxacin, and sulfamethoxazole and were variably susceptible to cefoxitin and clarithromycin (MIC values at which 50% of the isolates tested were inhibited [MIC50] were 64 and 32 μg/ml, respectively) (1). They reported intermediate susceptibility to tobramycin, with MIC50 and MIC90 values of 2 and 8 μg/ml, respectively, and believed the reduced susceptibility to tobramycin to be an important difference between M. smegmatis and the newly described species, M. goodii and M. wolinskyi. However, in both the present case and the case reported by Friedman and Sexton (3), isolates were susceptible to tobramycin.
We elected to treat our patient with trimethoprim-sulfamethoxazole because of its favorable safety and pharmacokinetic profile as well as its low cost and uncomplicated outpatient administration. For treatment to have the greatest chance of success, we assume that antimicrobial therapy, directed by susceptibility testing, must be accompanied by removal of any foreign body that is associated with the infection. An experience with a case of hernia repair mesh-associated infection due to M. fortuitum, treated successfully with mesh removal and a course of sulfamethoxazole (8), was previously reported, and Wallace et al. reported a treatment failure with a similar infection when the mesh could not be removed (14). In the present case, aggressive surgical debridement was combined with removal of the entire hernia repair synthetic mesh.
M. goodii is an infrequently recognized cause of posttraumatic and nosocomial infection. However, increasing recognition of infection due to M. goodii and other rapidly growing nontuberculous mycobacteria likely will occur as improved methods for bacterial growth and identification become available. Clinicians should request that specimens be submitted for isolation of mycobacteria whenever routine bacterial cultures are nondiagnostic. Microbiology technologists should be aware of the potential importance of these bacteria and should pursue identification of any rapidly growing acid-fast bacillus. Improved understanding of M. goodii and other nontuberculous mycobacteria will require enhanced surveillance, identification, and reporting of clinical cases.
REFERENCES
- 1.Brown, B. A., B. Springer, V. A. Steingrube, R. W. Wilson, G. E. Pfyffer, M. J. Garcia, M. C. Menendez, B. Rodriguez-Salgado, K. C. Jost, Jr., S. H. Chiu, G. O. Onyi, E. C. Böttger, and R. J. Wallace, Jr. 1999. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: a cooperative study from the International Working Group on Mycobacterial Taxonomy. Int. J. Syst. Bacteriol. 49:1493-1511. [DOI] [PubMed] [Google Scholar]
- 2.Brown-Elliott, B. A., D. E. Griffith, and R. J. Wallace, Jr. 2002. Newly described emerging human species of nontuberculous mycobacteria. Infect. Dis. Clin. N. Am. 16:187-220. [DOI] [PubMed] [Google Scholar]
- 3.Friedman, N. D., and D. J. Sexton. 2001. Bursitis due to Mycobacterium goodii, a recently described, rapidly growing mycobacterium. J. Clin. Microbiol. 39:404-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffith, D. E., and R. J. Wallace, Jr. 15 July 2003, revision date. Microbiology of nontuberculous mycobacteria. UpToDate 11.3 [Online.] http://www.uptodate.com.
- 5.Kumar, K. J., J. Chandra, R. N. Mandal, R. Dutta, and N. K. Jain. 1995. Fatal pulmonary infection caused by Mycobacterium smegmatis in an infant. Indian J. Pediatr. 62:619-621. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann, K. B., and R. O. Neumann. 1931. Mycobacterium smegmatis, p. 755-757. In Bacteriology, especially determinative bacteriology. G. E. Stechert & Co., New York, N.Y.
- 7.Lustgarten, S. 1885. The bacillus of syphilis. Lancet i:609-610. [Google Scholar]
- 8.Matthews, M. R., D. M. Caruso, R. B. Tsujimura, J. D. Smilack, B. A. Pockaj, and J. M. Malone. 1999. Ventral hernia synthetic mesh repair infected by Mycobacterium fortuitum. Am. Surg. 65:1035-1037. [PubMed] [Google Scholar]
- 9.Newton, J. A., Jr., P. J. Weiss, W. A. Bowler, and E. C. Oldfield III. 1993. Soft-tissue infection due to Mycobacterium smegmatis: report of two cases. Clin. Infect. Dis. 16:531-533. [DOI] [PubMed] [Google Scholar]
- 10.Pierre-Audigier, C., E. Jouanguy, S. Lamhamedi, F. Altare, J. Rauzier, V. Vincent, D. Canioni, J.-F. Emile, A. Fischer, S. Blanche, J.-L. Gaillard, and J.-L. Casanova. 1997. Fatal disseminated Mycobacterium smegmatis infection in a child with inherited interferon γ receptor deficiency. Clin. Infect. Dis. 24:982-984. [DOI] [PubMed] [Google Scholar]
- 11.Skiest, D. J., and M. E. Levi. 1998. Catheter-related bacteremia due to Mycobacterium smegmatis. South Med. J. 91:36-37. [DOI] [PubMed] [Google Scholar]
- 12.Vonmoos, S., P. Leuenberger, V. Beer, and R. de Haller. 1986. Pleuropulmonary infection caused by Mycobacterium smegmatis. Case description and literature review. Schweiz Med Wochenschr/J. Suisse de Médecine 116:1852-1856. [PubMed] [Google Scholar]
- 13.Wallace, R. J., Jr., D. R. Nash, M. Tsukamura, Z. M. Blacklock, and V. A. Wilcox. 1988. Human disease due to Mycobacterium smegmatis. J. Infect. Dis. 158:52-59. [DOI] [PubMed] [Google Scholar]
- 14.Wallace, R. J., Jr., J. M. Swenson, V. A. Silcox, and M. G. Bullen. 1985. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J. Infect. Dis. 152:500-514. [DOI] [PubMed] [Google Scholar]