Abstract
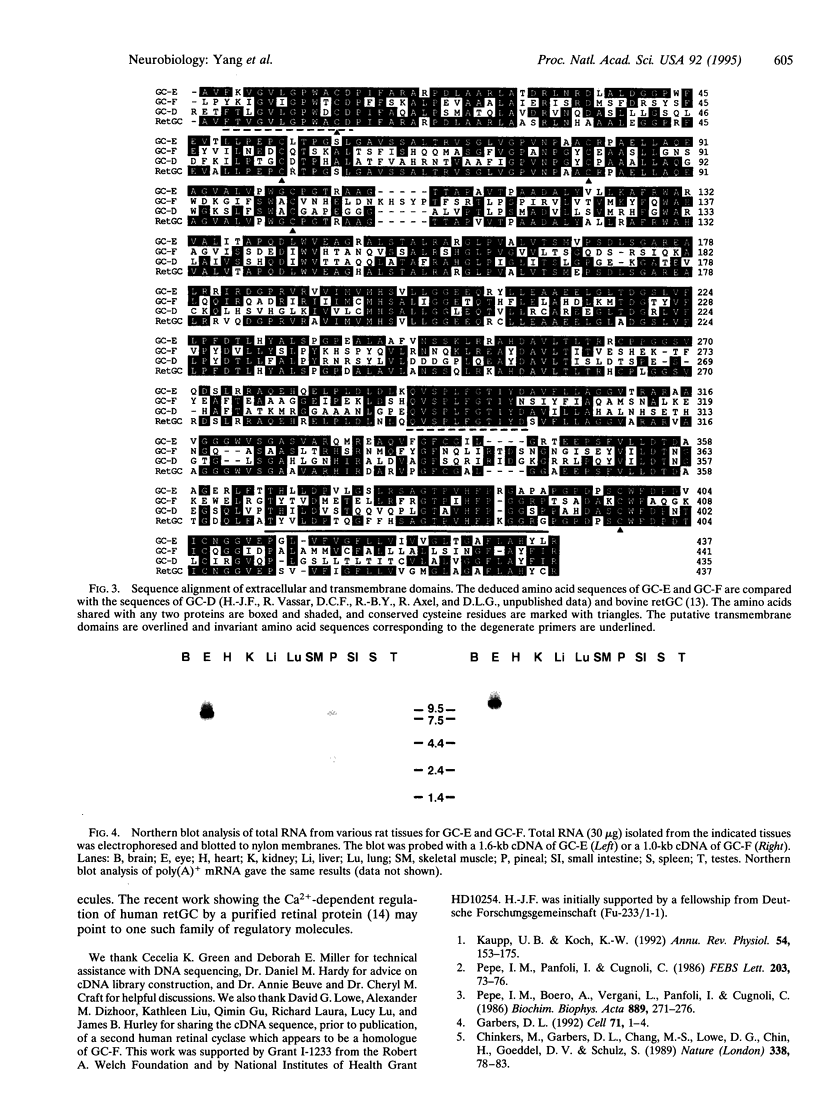
The cDNAs for two membrane guanylyl cyclases, designated E (GC-E) and F (GC-F, were isolated from a rat eye cDNA library. Their deduced topographic structures correspond to known members of the guanylyl cyclase receptor family, containing an extracellular domain, a single membrane-spanning domain, a protein kinase-like domain, and a cyclase catalytic domain. GC-E was expressed in the eye and the pineal gland, whereas GC-F expression was confined to the eye. Overproduction of GC-E and GC-F in COS cells resulted in expression of guanylyl cyclase activity, but ligands known to activate other guanylyl cyclase receptors failed to stimulate enzyme activity. Thus, both GC-E and GC-F remain orphan receptors. Amino acid sequence similarity between GC-E and GC-F in the extracellular region and homology with a cyclase expressed in olfactory neurons and retGC, a rod outer-segment-specific cyclase, suggest that there is another subfamily of guanylyl cyclase receptors, possibly restricted to sensory tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad I., Barnstable C. J. Differential laminar expression of particulate and soluble guanylate cyclase genes in rat retina. Exp Eye Res. 1993 Jan;56(1):51–62. doi: 10.1006/exer.1993.1008. [DOI] [PubMed] [Google Scholar]
- Andersson S., Davis D. L., Dahlbäck H., Jörnvall H., Russell D. W. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J Biol Chem. 1989 May 15;264(14):8222–8229. [PubMed] [Google Scholar]
- Chinkers M., Garbers D. L., Chang M. S., Lowe D. G., Chin H. M., Goeddel D. V., Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989 Mar 2;338(6210):78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Use of eukaryotic expression technology in the functional analysis of cloned genes. Methods Enzymol. 1987;152:684–704. doi: 10.1016/0076-6879(87)52074-2. [DOI] [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizhoor A. M., Lowe D. G., Olshevskaya E. V., Laura R. P., Hurley J. B. The human photoreceptor membrane guanylyl cyclase, RetGC, is present in outer segments and is regulated by calcium and a soluble activator. Neuron. 1994 Jun;12(6):1345–1352. doi: 10.1016/0896-6273(94)90449-9. [DOI] [PubMed] [Google Scholar]
- Domino S. E., Tubb D. J., Garbers D. L. Assay of guanylyl cyclase catalytic activity. Methods Enzymol. 1991;195:345–355. doi: 10.1016/0076-6879(91)95179-n. [DOI] [PubMed] [Google Scholar]
- Dryer S. E., Henderson D. A cyclic GMP-activated channel in dissociated cells of the chick pineal gland. Nature. 1991 Oct 24;353(6346):756–758. doi: 10.1038/353756a0. [DOI] [PubMed] [Google Scholar]
- Duda T., Goraczniak R. M., Sitaramayya A., Sharma R. K. Cloning and expression of an ATP-regulated human retina C-type natriuretic factor receptor guanylate cyclase. Biochemistry. 1993 Feb 16;32(6):1391–1395. doi: 10.1021/bi00057a001. [DOI] [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Goraczniak R. M., Duda T., Sitaramayya A., Sharma R. K. Structural and functional characterization of the rod outer segment membrane guanylate cyclase. Biochem J. 1994 Sep 1;302(Pt 2):455–461. doi: 10.1042/bj3020455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F., Yamazaki A. Polymorphism in purified guanylate cyclase from vertebrate rod photoreceptors. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4746–4750. doi: 10.1073/pnas.88.11.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaupp U. B., Koch K. W. Role of cGMP and Ca2+ in vertebrate photoreceptor excitation and adaptation. Annu Rev Physiol. 1992;54:153–175. doi: 10.1146/annurev.ph.54.030192.001101. [DOI] [PubMed] [Google Scholar]
- Koch K. W., Stecher P., Kellner R. Bovine retinal rod guanyl cyclase represents a new N-glycosylated subtype of membrane-bound guanyl cyclases. Eur J Biochem. 1994 Jun 1;222(2):589–595. doi: 10.1111/j.1432-1033.1994.tb18901.x. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Fletcher R. T., Chader G. J., Krishna G. Expression of guanylate cyclase-A mRNA in the rat retina: detection using polymerase chain reaction. Biochem Biophys Res Commun. 1992 Jan 31;182(2):851–857. doi: 10.1016/0006-291x(92)91810-d. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lolley R. N., Craft C. M., Lee R. H. Photoreceptors of the retina and pinealocytes of the pineal gland share common components of signal transduction. Neurochem Res. 1992 Jan;17(1):81–89. doi: 10.1007/BF00966868. [DOI] [PubMed] [Google Scholar]
- Pepe I. M., Boero A., Vergani L., Panfoli I., Cugnoli C. Effect of light and calcium on cyclic GMP synthesis in rod outer segments of toad retina. Biochim Biophys Acta. 1986 Dec 19;889(3):271–276. doi: 10.1016/0167-4889(86)90189-8. [DOI] [PubMed] [Google Scholar]
- Pepe I. M., Panfoli I., Cugnoli C. Guanylate cyclase in rod outer segments of the toad retina. Effect of light and Ca2+. FEBS Lett. 1986 Jul 14;203(1):73–76. doi: 10.1016/0014-5793(86)81439-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S., Green C. K., Yuen P. S., Garbers D. L. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell. 1990 Nov 30;63(5):941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- Schulz S., Singh S., Bellet R. A., Singh G., Tubb D. J., Chin H., Garbers D. L. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989 Sep 22;58(6):1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- Shyjan A. W., de Sauvage F. J., Gillett N. A., Goeddel D. V., Lowe D. G. Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron. 1992 Oct;9(4):727–737. doi: 10.1016/0896-6273(92)90035-c. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]