Abstract
Toxoplasma gondii is an obligate intracellular parasite that causes serious opportunistic infections, birth defects, and blindness in humans. Microtubules are critically important components of diverse structures that are used throughout the Toxoplasma life cycle. As in other eukaryotes, spindle microtubules are required for chromosome segregation during replication. Additionally, a set of membrane-associated microtubules is essential for the elongated shape of invasive “zoites,” and motility follows a spiral trajectory that reflects the path of these microtubules. Toxoplasma zoites also construct an intricate, tubulin-based apical structure, termed the conoid, which is important for host cell invasion and associates with proteins typically found in the flagellar apparatus. Last, microgametes specifically construct a microtubule-containing flagellar axoneme in order to fertilize macrogametes, permitting genetic recombination. The specialized roles of these microtubule populations are mediated by distinct sets of associated proteins. This review summarizes our current understanding of the role of tubulin, microtubule populations, and associated proteins in Toxoplasma; these components are used for both novel and broadly conserved processes that are essential for parasite survival.
INTRODUCTION
Microtubules are a universal component of eukaryotic organisms, including humans and unicellular protozoans. These polymers are constructed by assembly of α-β-tubulin heterodimers and represent an important target for small molecules that are used to treat cancers and helminth infections (1–3). Specialized properties of distinct microtubule populations can be mediated by small differences in the sequences of specific α- and β-tubulin isotypes, posttranslational modifications to both α- and β-tubulin subunits, and interactions with specific microtubule-associated proteins (MAPs) (4–8). These factors permit microtubules to coordinate diverse and essential roles in mitosis and meiosis, cytoplasmic architecture, and motility. Microtubules are indispensable components of a number of structures in Toxoplasma gondii, an obligate intracellular parasite that infects nucleated cells in a wide variety of vertebrates (9–11). Infection with this protozoan causes birth defects and blindness in humans and other host organisms (12). T. gondii has a complex life cycle, during which it develops into distinct forms which occupy discrete niches in specific host organisms. The morphology of the microtubule cytoskeleton is largely conserved in four “zoite” stages which share the properties of host cell invasion and asexual replication. Zoite forms have five distinct tubulin-containing structures: spindle microtubules, the centriole, subpellicular microtubules, the conoid, and intraconoid microtubules (described below and illustrated in Fig. 1B). The organization of microtubules in the gametocyte, gamete, and zygote stages is less well characterized but is distinct from that of zoites (13). In particular, male gametes elaborate flagella, which are important for motility and macrogamete fertilization (14).
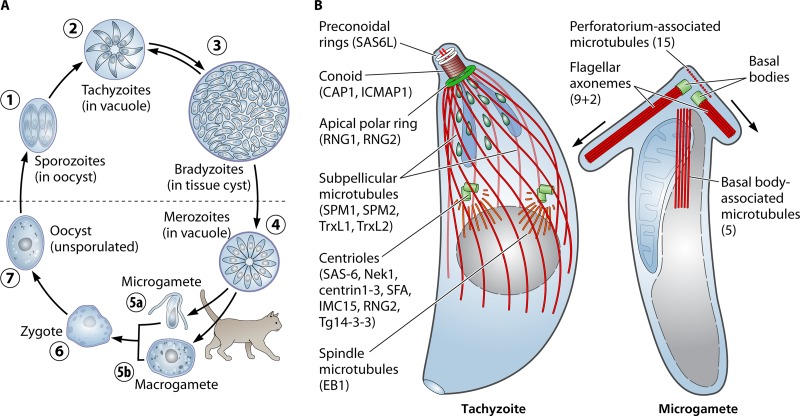
FIG 1.
(A) Organization of the microtubule cytoskeleton during the Toxoplasma life cycle. Infection of diverse vertebrate hosts occurs by ingestion of oocysts harboring sporozoites (1) or tissue cysts harboring bradyzoites (3). The released zoites infect and replicate in cells of the intestine to create tachyzoites (2), which disseminate throughout the host to infect diverse cell types. After development of an immune response to infection, tachyzoites differentiate into bradyzoites, a slow-growing form that persists for the life of the host organism. Bradyzoites can differentiate back into tachyzoites if host immunity wanes. In cats, tachyzoites and bradyzoites form merozoites (4), and these give rise to microgametes (5a) and macrogametes (5b). A transiently diploid zygote (6) is produced by fertilization, and becomes an unsporulated oocyst (7) after formation of an exterior cell wall. Oocysts are shed in feces and sporulate at ambient temperatures, creating two sporocysts, each containing four sporozoites (1). (B) A schematic of microtubule populations in tachyzoites and microgametes. (Left) The zoite stages have conserved microtubule structures which are best defined in tachyzoites. The spindle and subpellicular microtubule populations (red) associate with centrioles and the APR MTOCs (green). Defined components of these structures are noted. An unusual tubulin-based structure, the conoid, is located above the APR and can be withdrawn into it. There are two preconoidal rings above the conoid and two intraconoid microtubules within its circumference. (Right) Toxoplasma microgametes have two flagella, which originate at basal bodies in the apical region. Above these basal bodies is a pointed perforatorium structure which contains 15 short microtubules. A set of 5 peripheral microtubules extends from the basal body region toward the posterior with a longitudinal alignment. Due to the difficulty in obtaining the gamete stages, specific protein markers of these microtubules have not been identified. The images in panel B are based on previous figures in references 77, 138, and 139).
TUBULIN FAMILY MEMBERS IN TOXOPLASMA
Microtubules consist of α-β-tubulin heterodimers which assemble into a hollow cylinder-shaped polymer. Most typically, microtubules contain 13 protofilaments, a substructure reflecting the longitudinal head-to-tail association of α-β heterodimers (15). Protofilaments associate laterally to form the microtubule, which is a polar structure. The slow-growing “minus” end of the microtubule (terminating in α subunits) is typically embedded in a microtubule organizing center (MTOC), whereas the fast-growing “plus” end (terminating in β subunits) is free to shrink and grow. Tubulin heterodimers also assemble into other polymer forms, such as “doublet microtubules,” which consist of an incomplete C-shaped 10-protofilament microtubule fused to the wall of a 13-protofilament microtubule, and “triplet microtubules,” which consist of two incomplete C-shaped 10-protofilament microtubules fused to a 13-protofilament microtubule (16, 17). Doublet microtubules are essential to cilia and flagella, while triplet microtubules are typically found in centrioles and basal bodies.
Eukaryote genomes generally contain more than one gene for closely related α- or β-tubulin proteins, termed isotypes. Isotypes have small variations in their amino acid sequences and consequently exhibit subtly different biochemical properties. In multicellular organisms, specific cell types can modify properties such as the microtubule assembly rate by changing the relative expression levels of individual isotypes that contribute to the pool of available subunits (4, 5, 18, 19). Moreover, specific isotypes can be required for specialized structures, such as the flagellar axoneme (20). The Toxoplasma genome contains genes for three α and three β isotypes (21, 22). The Toxoplasma α1 isotype is abundantly represented in tachyzoite and oocyst proteomes and is mutated in all dinitroaniline-resistant lines described to date (23–25). Protein mass spectrometry (MS) and mRNA data deposited at ToxoDB.org suggest that α2-tubulin may be expressed at low levels in tachyzoites and is expressed in oocysts while α3-tubulin is expressed in both tachyzoites and oocysts. Protein and mRNA data deposited at ToxoDB.org suggest that all three β isotypes are expressed in tachyzoites and oocysts. Although the β isotypes are quite similar (∼98% identity), the α isotypes have dramatically distinct amino acid sequences (40 to 70% identity), suggesting that they have specialized functions. This is particularly evident in the H1-S2 (N) and M loops that coordinate lateral subunit interactions. α2-Tubulin has a 4-amino-acid insert in the H1-S2 loop which could increase the flexibility of α2-containing microtubules. Most strikingly, amino acids of the H1-S2 loop and M loop are poorly conserved between α1- and α3-tubulins, which may suggest that these isotypes do not copolymerize efficiently. Last, tubulin tails are typically acidic and unstructured, extending away from the microtubule to promote interactions with MAPs (8, 26, 27). The carboxy-terminal tail of α3-tubulin is uncharacteristically long, encompassing a much less acidic 35-amino-acid extension to the portion that is closer to the microtubule surface, which suggests that it may coordinate atypical interactions. The unusual features of α2- and α3-tubulin may be critical to specialized tubulin structures, such as the conoid and flagellar axoneme.
Microtubule polymerization in Toxoplasma tachyzoites is inhibited by dinitroanilines, a class of small molecules that selectively bind to plant and protozoan tubulin but not vertebrate tubulins (28, 29). Computational modeling indicates that dinitroanilines bind to the Toxoplasma α1-tubulin subunit but not to vertebrate α-tubulin (25, 30), with the binding site situated beneath the H1-S2 (N) loop such that dinitroanilines interfere with protofilament interactions, causing microtubule disruption. Diverse single point mutations in Toxoplasma α1-tubulin can confer resistance to dinitroanilines (23–25). Substitutions are clustered in a core region and in areas of subunit contact. Biochemical studies indicate that mutations reduce dinitroaniline affinity and increase heterodimer affinity within the microtubule lattice (31). Lines with resistance mutations have an increased incidence of replication defects due to the failure of spindle and subpellicular microtubules to work in synchrony. This is most clearly seen in lines harboring mutations that cause tachyzoites to have longer subpellicular microtubules, indicating that increased microtubule stability shifts the dimer-polymer equilibrium toward assembled microtubules. In the absence of dinitroaniline selection, compensatory mutations subsequently arise in the genes for α1- or β1-tubulin (23, 32). Under nonselecting conditions, lines with compensatory mutations have increased fitness relative to resistant parental strains but are less fit than wild-type parasites. The secondary mutations also lower the resistance conferred by the primary α1-tubulin mutations, indicating that resistance is associated with a fitness disadvantage and increasing fitness occurs at the cost of resistance. To date, all dinitroaniline resistance mutations are located in the α1-tubulin gene. Several residues in α2-tubulin (P165, V238, and M378) and α3-tubulin (F136 and L235) correspond to α1-tubulin substitutions that confer dinitroaniline resistance, perhaps indicating that α2- and α3-tubulins are less sensitive to these compounds (29). Since compensatory mutations that modulate defects conferred by α1-tubulin mutations are identified in β1-tubulin but not the β2- or β3-tubulin gene, it is likely that the α1- and β1-tubulin isotypes form a prominent population of tubulin heterodimers (32).
In all eukaryotes studied to date, tubulin dimers can be altered by posttranslational modifications that differentially mark distinct microtubule subpopulations (33, 34). Toxoplasma α1-tubulin can be modified by acetylation of lysine 40 and detyrosination (removal of the carboxy-terminal tyrosine 453) (22). Notably, only α1-tubulin is susceptible to both modifications: α2-tubulin lacks a lysine 40 and ends in a valine, while α3-tubulin ends with an asparagine. Both α1- and β1-tubulins are polyglutamylated, a modification observed on axoneme, centriole, and spindle microtubules. Significantly, tachyzoite tubulin exhibits novel tubulin modifications: the carboxy-terminal tails of α1- and β1-tubulin can be methylated, and the last 5 amino acids of α1-tubulin can be truncated (ΔYGDEY). Work in other organisms has demonstrated that posttranslational modifications alter how microtubules interact with associated proteins, and this in turn influences the relative sensitivity of microtubules to stabilizing or destabilizing drugs (33, 34). Deletion of enzymes that acetylate or glycylate tubulin in the related alveolate Tetrahymena is associated with decreased microtubule stability indicated by increased resistance to paclitaxel and increased sensitivity to dinitroanilines (35, 36).
In addition to α-β dimers, other members of the tubulin superfamily are critically important in many eukaryotes (37, 38). Centrioles and yeast spindle pole bodies contain γ-tubulin, which is essential for microtubule nucleation (37, 39). The Toxoplasma genome contains a single-copy gene for γ-tubulin. When endogenous Toxoplasma γ-tubulin is tagged with an in-frame fusion to yellow fluorescent protein (YFP), the fusion protein localizes to centrioles (J. de Leon and N. Morrissette, unpublished observations). Additional δ- and ε-tubulins are found in protozoan and vertebrate genomes, including the Toxoplasma genome. These tubulins are implicated in construction of doublet and triplet microtubules found in the flagellar axoneme, basal bodies, and centrioles (40–42). Curiously, γ-tubulin but not δ- and ε-tubulin are represented in MS and RNA-Seq data deposited at ToxoDB.org, suggesting that not all family members are expressed in tachyzoites.
MICROTUBULE-BASED STRUCTURES ARE REORGANIZED DURING THE PARASITE LIFE CYCLE
Toxoplasma is often called a “cosmopolitan” parasite because its asexual forms can invade and replicate in diverse nucleated cell types in a wide variety of vertebrate host organisms throughout the world (43). Acute infection is caused by the rapidly proliferating tachyzoite form, which causes host cell death and tissue damage. Upon an immune response to infection, tachyzoites differentiate into bradyzoites, a slow-growing, “tissue cyst” form that evades host defenses to persist for the life of the host organism. Bradyzoites can differentiate back into tachyzoites if host immunity wanes. Bradyzoites are also orally infectious when tissues from an infected animal are consumed by a carnivore (Fig. 1A). In contrast to zoite proliferation, the sexual cycle is restricted to the intestinal epithelium of felids (43, 44). Tachyzoites and bradyzoites form merozoites, and these give rise to male and female gametocytes. Gametocytes produce microgametes and macrogametes and fertilization generates a transiently diploid zygote. A cell wall forms around the zygote, producing an oocyst. Sporulation after oocyst excretion creates two sporocysts, each containing four sporozoites. New infections are initiated after ingestion of sporozoite-containing oocysts or bradyzoite-containing tissue cysts. The four zoite stages have conserved microtubule structures which are distinct from microtubule organization in gametocytes, gametes, and the zygote.
Microtubules in gametocytes, gametes, and zygotes.
During the sexual cycle, a subset of asexually replicating zoites differentiates to form gametocytes, gametes, and fertilized zygotes in cats. Although the ultrastructural features of these forms have been described (45–47), the requirements for differentiation in a felid gut have impeded detailed characterization of specific cytoskeletal proteins in these stages. Macrogametes contain numerous mitochondria and wall-forming bodies: elements which promote formation of the oocyst wall and drive creation of sporozoites (45, 48, 49). There is no specific information about the macrogamete cytoskeleton. Microgametes are elongated and consist primarily of a nucleus and mitochondrion (Fig. 1B). Two flagella extend from basal bodies at the apex and project toward the posterior (13, 14, 46). Five cytoplasmic microtubules also originate in this region and extend longitudinally within the microgamete body. A row of 15 short microtubules associates with the apical perforatorium above the basal bodies. These last two microtubule structures may reflect vestiges of subpellicular microtubules and a conoid, features of zoite forms. Microgametes use flagella to reach macrogametes, with fertilization leading to formation of zygotes. Again, nothing is known of the organization of or requirements for cytoskeletal components in the zygote and oocyst.
Although there is not much direct information about the composition of microgametes, we can infer some features of the microtubule cytoskeleton because basal bodies and flagella have a conserved architecture and protein components (50). Flagella are flexible membrane-enclosed microtubule-containing protrusions that extend from the cell body. They often function in motility, through creation of an undulating waveform by the flagellar axoneme. Most axonemes have a conserved “9+2” structure consisting of 9 doublet microtubules which surround an internal “central pair” of microtubules (51). Bending occurs when microtubule motor activity slides a subset of the doublet microtubules along the central pair microtubules. The “9+2” organization of axonemes is also found in organisms with cilia, a term that is generally used when organisms exhibit more than two axonemes. More recently, it has been recognized that flagella and cilia also function in detecting and sending signals (52–54).
Axonemes emerge from the cell body as an extension to basal bodies, which are structurally identical to centrioles. Basal bodies and centrioles typically consist of nine triplet microtubule blades arranged into a turbine shape (55). Duplication of these structures is usually semiconservative, with a new “daughter” centriole emerging at a right angle to the older “mother” centriole. There are exceptions to both of these traits: centrioles can form de novo under certain circumstances (56, 57), and centrioles from some species contain singlet or doublet microtubules rather than triplets (58). Centrioles in nonflagellated Toxoplasma zoite forms contain nine singlet microtubules (9). Although Toxoplasma microgametes have a 9+2 axoneme, the structure of the associated basal bodies remains undefined (13, 14, 46). If microgamete basal bodies are formed from triplets, either the singlet microtubule centrioles inherited from zoites mature into triplets (perhaps by gamete-specific expression of δ- and ε-tubulin) or triplet basal bodies form de novo. Asexual-stage merozoites of Plasmodium spp. lack centrioles; basal bodies are thought to form de novo during microgametogenesis (59).
Although centrioles and basal bodies have the same underlying structure, additional components enable the basal body to extend an axoneme and to orient this protrusion in order to coordinate a motile response to external cues. The filaments that orient the axoneme are termed the flagellar apparatus (60–62). The transition zone, located at the distal end of the centriole, serves as the entry site for intraflagellar transport (IFT) machinery in order to move components to the distal end of the axoneme and to return excess or damaged materials to the cytoplasm. IFT machinery is essential for construction of most axonemes. However, a few organisms assemble axonemes in the cell cytoplasm rather than at the cell periphery (63). For example, Drosophila lack a morphologically distinct transition zone and genes for IFT components. Although they share an ancestor, many features of flagellar assembly have diverged in the interval that separates the Toxoplasma and Plasmodium lineages. Plasmodium spp. lack IFT machinery and asexual stage centrioles; the microgamete axoneme is constructed in the cytoplasm after de novo assembly of basal bodies (59, 64, 65). Asexual-stage Toxoplasma zoites have centrioles (9), and there are IFT homologs encoded in the genome (64, 66). Additionally, the centriole components CEP164 and VFL1 are present in the Toxoplasma genome but are missing from Plasmodium (50). As CEP164 mediates vesicle docking for IFT transport (67), its disappearance is consistent with the loss of IFT from Plasmodium. VFL1 is less well characterized; it is thought to play a role in establishing rotational orientation in Chlamydomonas basal bodies (68). The retention of asexual centrioles and IFT machinery in Toxoplasma may be tied to the conoid, a tubulin-based structure found in zoites.
Microtubule populations in asexual zoite stages.
There are four invasive “zoite” forms of Toxoplasma (sporozoites, tachyzoites, bradyzoites, and merozoites). Although zoites lack flagella, they are motile, requiring actin and myosin to efficiently invade host cells (69). Since the tachyzoite stage is most amenable to experimental manipulation, we know most about the properties of microtubules in this stage. It is likely that most aspects of the microtubule cytoskeleton will be conserved with other zoite forms.
(i) Centrioles and spindle microtubules.
Microtubule-containing spindles coordinate mitosis in asexual-stage Toxoplasma zoites. Centrioles are found at the poles of the mitotic spindle (9); while most centrioles consist of nine triplet microtubule blades with mothers and daughters arranged in orthogonal pairs (70, 71), Toxoplasma zoite centrioles consist of nine singlet microtubules and are arranged in a parallel configuration. This unusual appearance and organization is conserved in related coccidian species (72, 73). During mitosis, chromosomes are partitioned into opposite sides of a horseshoe-shaped nucleus, which then divides. The nuclear envelope remains intact during mitosis such that spindle microtubules traverse the centrocone, an electron-dense region of the nuclear membrane, to contact the chromosome centromeres (Fig. 1B). Centromeres and associated kinetochores are retained at the centrocone throughout the cell cycle (74, 75).
(ii) APR and subpellicular microtubules.
The elongated shape of zoites is conferred by a set of nondynamic subpellicular microtubules that closely underlie the pellicle (76, 77). The pellicle is formed by association of the plasma membrane with an underlying patchwork of flattened vesicles, termed the inner membrane complex (IMC). The IMC originates at the apical polar ring (APR), such that the apical region of the parasite is solely enclosed by the plasma membrane while the region below the APR is defined by the pellicle. Toxoplasma tachyzoites construct a corset of 22 evenly spaced subpellicular microtubules using the APR as an MTOC to define the number and spacing of microtubules. Work in related parasites indicates that the minus ends of subpellicular microtubules are inserted into cogwheel-like projections of the APR (78). Freeze-fracture studies of Toxoplasma reveal regular arrays of uniformly sized intramembranous particles in the IMC membranes (76, 79). These likely represent transmembrane domains of receptors for a filament network that can be observed after pellicle extraction (80). The network is formed by a family of alveolin proteins (81–83) that provide tensile strength, akin to the function of intermediate filaments in vertebrate cells. Isolated subpellicular microtubules are decorated with an unidentified MAP that binds with a 32-nm periodicity, and a similar periodicity is observed in the particles of the IMC membranes, suggesting that the intimate association of microtubules and the pellicle is coordinated in part by an interaction between these elements (76).
(iii) Conoid and intraconoid microtubules.
In addition to the spindle and subpellicular microtubules, Toxoplasma and other coccidian apicomplexans build an unusual tubulin-containing structure at the zoite apex (77). The conoid is constructed of 10 to 14 curved tubulin sheets that form a hollow cone (84). It is topped by two fibrous preconoidal rings, while two ∼400-nm-long microtubules are located within its circumference (76, 77, 84). A complex of conoid, intraconoid microtubules, and preconoidal rings can extend beyond the APR or withdraw to be surrounded by the subpellicular microtubules. Conoids are found in Toxoplasma and other coccidians and some gregarines but are missing from other apicomplexans, such as Plasmodium, most likely because they were lost from or considerably reduced in these lineages (85). Similar structures, termed “incomplete conoids” or “pseudoconoids,” are found adjacent to basal bodies and flagella in organisms from several nonapicomplexan alveolate lineages. Incomplete conoids or pseudoconoids are built from a set of nearly vertical apical microtubules that create an open-sided cone. Recent work on two marine alveolates which contain an adjacent conoid and flagellar apparatus suggests that these structures are intimately associated (66, 86). Tomography of Psammosa pacifica (a dinoflagellate) shows that the pseudoconoid associates with rootlet microtubules that originate in the flagellar apparatus; work on Chromera velia (a chromerid) reveals filamentous connections between the pseudoconoid and rootlets of the flagellar apparatus. These observations are consistent with the finding that the Toxoplasma conoid and centriole retain connections mediated by proteins that typically associate with the base of flagella (87–89).
MICROTUBULE-ASSOCIATED PROTEINS
Unlike the apicomplexan actin cytoskeleton, where conserved actin binding proteins have evolved distinct properties to function in a monomer-rich environment, with the exception of microtubule motors and centriole components, many Toxoplasma MAPs are novel proteins.
Microtubule motors in Toxoplasma.
Microtubule motors are conserved multisubunit machines that convert energy from ATP hydrolysis to move along microtubules. Dyneins move toward the microtubule minus end, while kinesins typically move toward the plus end. The Toxoplasma genome has a number of genes that encode kinesin and dynein subunits, as well as some components (Arp1, p25, p27, and p62) of dynactin, a protein complex that regulates microtubule motor activity (90). Kinesins are usually composed of two heavy and two light chains: each heavy chain contains a motor domain, while light chains coordinate cargo association (91). These motors function in mitosis and meiosis and in transport of cellular cargo. The relative position of the motor domain in the heavy chain is predictive of kinesin activity: motors located in the center (KinI motors) disrupt microtubules; motors at the amino terminus (KinN) are plus-end directed, while motors at the carboxy terminus (KinC) are minus-end directed. The Toxoplasma genome contains ∼19 heavy-chain genes, which appear to represent all three subtypes. To date, Toxoplasma kinesins have not been studied, although a Plasmodium falciparum KinI kinesin has been used for structural studies (92, 93). Dyneins contain ∼12 subunits and function as motors either to power axoneme beating or in organelle transport, spindle function, and centrosome assembly in the cytoplasm (94). Although some subunits are shared by flagellar and cytoplasmic dyneins, others, including the motor domain-containing heavy chains (DHCs), are specific for each subset. The Toxoplasma genome contains annotated genes for ∼10 DHCs that likely represent both flagellar and cytoplasmic types. The genome also contains annotated genes for several types of intermediate and light chains, including light chain type 1, Tctex-1, Roadblock/LC7, the axonemal assembly factor, axonemal light chain, and axonemal intermediate chain (69). Although overexpressed dynein light chain 1 (DLC1) fused to green fluorescent protein (GFP) localizes to the spindle poles, centrioles, the basal end, and the conoid region of tachyzoites (95), a subsequent study of a shortened version of the same protein (minus 49 amino-terminal residues, termed T. gondii DLC8a [TgDLC8a]) localizes it only to the apical region of tachyzoites (96). The latter study used a small epitope tag that was introduced as an in-frame fusion at the carboxy terminus of the endogenous locus. The absence of labeling of the spindle poles, centrioles, and basal end suggests that localization at these sites may be a consequence of overexpression in the initial study. The later study also localized three other members of the dynein LC8 subfamily (TgDLC8b, TgDLC8c, and TgDLC8d) by endogenous tagging, indicating that all have a cytosolic distribution in tachyzoites.
MAPs in the microgamete.
Although some proteins that associate with the microtubule cytoskeleton are likely used in all parasite forms, specialized flagellar components may be required in a stage-specific fashion. Since many centrosome, basal body, and flagellar proteins are widely conserved, computational genomics can be used to identify orthologs in diverse eukaryotes. Although both “centriole” and “centrosome” have been used to describe the Toxoplasma organelle, the term “centrosome” was coined to identify a type of MTOC that consists of core centrioles surrounded by a pericentriolar matrix (PCM) characterized by distinct markers (97). There are robust homologs of “core” centriole components (δ- and ε-tubulin, centrin 2, SAS-4, SAS-6, CEP164, DIP13, VFL1, and CEP76) but not PCM proteins (PCM1, CP110, CEP97, ninein, rootletin, asterless, NAP1, CEP68, and CEP55) in the Toxoplasma genome (50). Therefore, the Toxoplasma structure is strictly a centriole. Surprisingly, several core components (CEP135, POC5, and centriolin) are present in ciliate genomes but missing from Toxoplasma and other apicomplexans. Since ciliates and apicomplexans share an ancestor, genes for these components were likely lost from the apicomplexan lineage.
Zoite MAPs.
Tachyzoites (and other zoite forms) contain five distinct tubulin-containing structures. Each likely contains unique MAPs as well as shared elements. Proteins that associate with specific populations are beginning to be identified, in part by MS surveys of tachyzoite fractions, which are enriched in APR, conoid, and subpellicular microtubules (95, 98).
(i) Centriole and spindle proteins.
The complement of MAPs localizing to spindle microtubules remains largely uncharacterized, though several proteins have been identified on centrioles located at the spindle poles. Since DLC1/TgDLC8a localizes to the spindle only as an overexpressed GFP fusion protein (95, 96), the plus-end binding protein EB1 (J. de Leon and N. Morrissette, unpublished observations) is the sole marker of mitotic microtubules. It is quite likely that dyneins and kinesins are spindle components, in addition to other, yet-to-be-characterized MAPs. Both novel and conserved proteins localize to zoite centrioles. SAS-6, Nek1, and centrin are conserved centriole components: SAS-6 is required for centriole biogenesis (99), and Nek1 is required for centriole separation (100). The EF-hand-containing centrins are well-established centriole markers (101, 102). Toxoplasma SAS-6 is found at the centrioles during tachyzoite replication (87). Nek1 is recruited to tachyzoite centrioles prior to duplication and is essential to their subsequent separation (100). Toxoplasma expresses three centrin isoforms: all localize to centrioles, and centrin 2 and centrin 3 are found at additional subcellular sites (95, 103, 104). One critical role of the Toxoplasma centriole is likely to be as a signaling platform. Three novel proteins move from the Toxoplasma centrioles to emerging daughter buds during zoite replication. RNG2 first appears at newly replicated centrioles and is subsequently a marker of the nascent APR of daughter parasites (89), while IMC15, an alveolin family protein (81), and T. gondii 14-3-3 (Tg14-3-3), one of four homologs in the Toxoplasma genome (105), associate with centrioles early in replication and then relocate to daughter buds. The Toxoplasma genome encodes three homologs of striated fiber assemblin (SFA). SFA is a component of striated fibers which associate with basal bodies in the flagellar apparatus of green algae (106, 107). SFA2 and SFA3 localize to filaments that tether centrioles to emerging daughter buds at the APR and conoid during tachyzoite replication (88).
(ii) Subpellicular MAPs and APR proteins.
Although subpellicular microtubules emanate from the APR, it does not contain γ-tubulin, which is a marker of diverse MTOCs. However, two proteins, RNG1 and RNG2, have recently been characterized as APR constituents (89, 108). RNG1 is a small, low-complexity, detergent-insoluble protein (108). It is a late component of the APR, appearing only as daughters emerge, similar to glideosome-associated proteins, which associate only with the mature pellicle (109–111). There are RNG1 orthologs in Neospora caninum and Sarcocystis neurona but no obvious homologs in other apicomplexans. The small size and low complexity may make RNG1 homologs difficult to identify in more distantly related apicomplexans. Alternately, RNG1 may coordinate interactions between the APR and the conoid and have been discarded from other lineages in association with loss of the conoid. RNG2 is related to a family of charged repeat motif proteins identified in the pellicle of Tetrahymena, a distantly related ciliate (112). The Tetrahymena proteins appear filamentous and underlie rows of cilia. Toxoplasma RNG2 is a large protein; when the amino and carboxy termini are uniquely tagged, they localize in discrete rings, indicating that RNG2 forms a cuff of vertically organized subunits (89). During conoid extrusion, the relative position of the rings is inverted, revealing that the cuff connects the APR to the conoid. Moreover, loss of RNG2 alters signaling, blocks microneme secretion, and inhibits host cell entry, connecting it to other essential events in invasion.
The subpellicular microtubules which extend from the APR are nondynamic and have unusual stability in cold and in the presence of detergents, conditions that typically induce disassembly. The SPM1 MAP has six tandem copies of a 32-amino-acid repeat and localizes along the subpellicular microtubules (113). Loss of SPM1 reduces tachyzoite fitness, and the subpellicular microtubules become sensitive to detergent extraction. SPM1 is essential for localization of several other proteins to the subpellicular microtubules, including TrxL1 and TrxL2, two novel proteins that contain a thioredoxin fold (114). A second MAP, SPM2, associates with the central region of the subpellicular microtubules independent of SPM1 (113). Significantly, the subpellicular microtubules continue to associate with the pellicle in the absence of SPM1 or SPM2, indicating that other, yet-to-be-identified MAPs provide this function.
(iii) Proteins associated with the conoid complex.
As described above, the conoid is an unusual organelle found in a subset of apicomplexans. To date, it is unclear which tubulin isoforms contribute to the novel polymers that form this organelle (84). A number of proteins localize to the conoid, intraconoid microtubules, and fibrous preconoidal rings. Centrin 3 localizes to the conoid in addition to the tachyzoite centrioles (95, 103, 104). A dynein light chain (DLC1/DLC8a) is found in the conoid region (95, 96). Toxoplasma and other simple centriole-containing eukaryotes have homologs of a SAS-6-like (SAS6L) protein (87). SAS6L consists of a conserved domain that is also found in the amino-terminal region of the centriole protein SAS-6, and it localizes to the preconoidal rings in tachyzoites. Since Trypanosoma brucei SAS6L localizes to the basal plate, it is probable that SAS6L is similarly localized at the base of the axoneme in Toxoplasma microgametes, which lack a conoid structure. One role for SAS6L may be to anchor SFA, a cell cycle-regulated filament system that links the conoid and APR to the centriole in replicating tachyzoites (88). Two novel proteins have been localized to the conoid region. ICMAP1 is a novel SMC-like domain-containing protein that localizes to the intraconoid microtubules in tachyzoites (115). Homologs are restricted to conoid-containing apicomplexans (Neospora and Eimeria), consistent with the distribution of the intraconoid microtubules in the coccidian lineage. A small conoid-associated protein 1 (CAP1) was identified in a genetic screen for mutants that have a compromised ability to survive in activated macrophages (116).
MICROTUBULE-DEPENDENT PROCESSES
Microtubule function is crucial to Toxoplasma motility, invasion, growth, and sexual recombination, indicating that tubulin and MAPs are excellent drug targets. Inhibition of microtubule polymerization or microtubule-associated functions would disrupt essential processes, including chromosome segregation, cytokinesis, motility, and fertilization.
Mitosis and cytokinesis.
Toxoplasma replication couples one or more cycles of nuclear division to bud formation to synchronously create two daughters by endodyogeny or multiple progeny by endopolygeny (117–120). Both processes use microtubules to segregate duplicated chromosomes, though endodyogeny is better understood, since it is characteristic of in vitro tachyzoite replication. Given that a centromere-kinetochore complex permanently tethers chromosomes to the centrocone region of the nuclear envelope, the spindle may function primarily to mechanically separate the nucleus (74, 75). The kinetochore protein Nuf2 is essential for chromosome segregation and for association of the centrocone with centrioles (75). Plastid segregation requires the centrioles and spindle (121), as well as the MyoF myosin and parasite actin (122, 123). As centrioles at the spindle poles separate, they are tethered by actin and MyoF. Consequently, the hemi-spindles are held at an acute angle so that each half of the “horseshoe-shaped” nucleus is drawn into an apical daughter bud accompanied by the plastid. Regardless of the number of nuclear divisions preceding cytokinesis, centriole abundance reflects the number of daughter buds required to complete replication. SFA2 and SFA3 filaments connect centrioles to emergent daughters; conditional depletion of either protein blocks bud formation (88). A temperature-sensitive Nek1 mutation demonstrates that centriole separation is vital to nuclear division and counting daughters (100, 124). Loss of Nek1 activity prevents completion of mitosis at the restrictive temperature. In these circumstances, centrioles duplicate but do not separate. Unseparated centrioles direct formation of a single daughter bud harboring a polyploid nucleus.
In contrast to metazoan cells, where cytokinesis is driven by an actomyosin ring, completion of endodyogeny or endopolygeny is driven by extension of daughter IMC and associated subpellicular microtubules to enclose the apical organelles, mitochondria, plastid, and nucleus in daughter buds (10, 125, 126). Following centriole separation, several centriole-associated proteins relocate to emerging daughters. RNG2 shifts from the centrioles to the APR (89), and both IMC15 and Tg14-3-3 move from the centrioles to the IMC of emergent daughters (81, 105). Although the timing of bud formation in endodyogeny versus that in endopolygeny is distinct, the cytoskeletal components are likely to be quite similar, with assembly regulated by initiation of key structures, such as the conoid, APR, IMC, and underlying microtubules (69, 127). IMC-delineated daughters emerge from the mother by adopting her plasma membrane (9). Some cytoskeletal components, including the APR marker RNG1, only associate with mature pellicle structures consisting of IMC in close association with the plasma membrane (108–111).
Gliding motility and invasion.
Toxoplasma tachyzoites (as well as many other apicomplexan zoites) move and invade cells using an immobilized, IMC-localized myosin motor to move short actin filaments linked to the cytoplasmic tails of secreted adhesins (69). Although most studies have focused on the fundamental role of myosin and actin filaments in this novel process, microtubules are also critically important. Zoite motility in a three-dimensional matrix follows a spiral trajectory imposed by underlying subpellicular microtubules (128). Since cytoplasmic dyneins can generate membrane tension (129), microtubule motors may keep the IMC taut against the subpellicular microtubules in order to restrain the IMC-associated MyoA myosin so that its motor activity translocates actin filaments. Intracellular tachyzoites treated with low levels of the dinitroaniline oryzalin can assemble short microtubules that are sufficient to complete nuclear division and budding; these parasites have shortened subpellicular microtubules and are noninvasive, likely because they fail to adequately immobilize MyoA to power host cell entry (9). Other cytoskeletal proteins that influence shape also alter movement: tachyzoites lacking the IMC proteins PHIL1, SIP, and CBAP are shorter and wider than wild-type parasites and have reduced motility (128, 130, 131). Conoid extrusion is specifically inhibited by a small molecule, conoidin A, which was identified in a screen for invasion inhibitors (132, 133). Extrusion is also blocked by agents that interfere with Ca2+ signaling, inhibit kinases, or interfere with the actin and myosin machinery (134, 135). There are indications that conoid motility is linked to signal transduction and secretion: depletion of the APR protein RNG2 blocks microneme release and inhibits host cell invasion (89).
Gametogenesis and zygote formation.
The least experimentally accessible portion of the Toxoplasma life cycle is the sexual cycle: forms which develop only in the cat gut have proven difficult to study. Development of methods to induce this process in vitro would facilitate genetic studies and permit characterization of the microtubule cytoskeleton in the macrogamete, microgamete, and zygote stages. Information present in the genome of Toxoplasma can be used to predict properties of the basal bodies and flagella, since these are conserved organelles which have been extensively studied in other systems (50). In addition, since gametogenesis is more easily studied in Plasmodium, we can look to findings in this apicomplexan lineage (136, 137). Although it is a reasonable assumption that many aspects of the microtubule cytoskeleton will be similar between Toxoplasma and Plasmodium, observations that there are significant differences in the genomic complement of IFT and centriole components indicate that not everything will be conserved (50, 64, 66).
SUMMARY AND OUTLOOK
Microtubules in Toxoplasma and other apicomplexan pathogens are critically important to diverse structures and essential processes, including replication, invasion, and fertilization. Dinitroaniline compounds inhibit invasion and replication, indicating that tubulin is a robust target for antiparasitic therapies (29). Likewise, motors, MAPs, and MTOC components contribute to microtubule-based machines that are critical to parasite function. The ancestor of the apicomplexan parasites likely had both a conoid-containing apical complex and adjacent basal bodies with associated flagella (85). Toxoplasma flagella are restricted to male gametes, and a number of conserved centriole and flagellar components were apparently lost from the apicomplexan lineage (50). Nonetheless, the centriole serves as a signaling platform, with novel parasite-specific proteins that move from it to developing daughter buds during Toxoplasma zoite replication (81, 89, 105). Moreover, although zoites lack flagella, they retain several flagellar apparatus-associated proteins which may be important for diverse processes, including replication and invasion (87–89). Collectively, these studies reveal that there are key differences between the Toxoplasma microtubule cytoskeleton and microtubules in metazoan hosts, which represent robust targets for future therapeutic agents.
ACKNOWLEDGMENTS
This article was written while I was on sabbatical at the Walter and Eliza Hall Institute in Melbourne, Australia. I thank the Cowman lab for their hospitality and the University of California, Irvine, for their support. I also thank Ross Waller (University of Cambridge) and Bill Wickstead (University of Nottingham) for useful discussions of the content and Ross for critically reviewing the manuscript.
Biography

Naomi Morrissette earned her B.A. degree in biochemistry at Smith College and her Ph.D. degree in biology at the University of Pennsylvania. She first became interested in the unusual and baroque Toxoplasma cytoskeleton while doing her thesis research in the laboratory of David Roos. After postdoctoral studies of macrophage phagocytosis with Alan Aderem (Rockefeller University and the University of Washington), she returned to the topic of Toxoplasma microtubules as a research fellow in David Sibley's laboratory at Washington University. She is currently an Associate Professor in the Department of Molecular Biology and Biochemistry at the University of California, Irvine. Her fascination with tubulin and microtubules continues: she currently studies the interactions of small molecules with tubulin, tubulin genetics, and microtubule-associated proteins and the conoid in Toxoplasma.
REFERENCES
- 1.Amos LA. 2011. What tubulin drugs tell us about microtubule structure and dynamics. Semin Cell Dev Biol 22:916–926. doi: 10.1016/j.semcdb.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Jordan A, Hadfield JA, Lawrence NJ, McGown AT. 1998. Tubulin as a target for anticancer drugs: agents which interact with the mitotic spindle. Med Res Rev 18:259–296. doi:. [DOI] [PubMed] [Google Scholar]
- 3.Pellegrini F, Budman DR. 2005. Review: tubulin function, action of antitubulin drugs, and new drug development. Cancer Invest 23:264–273. doi: 10.1081/CNV-200055970. [DOI] [PubMed] [Google Scholar]
- 4.Lu Q, Luduena RF. 1994. In vitro analysis of microtubule assembly of isotypically pure tubulin dimers. Intrinsic differences in the assembly properties of alpha beta II, alpha beta III, and alpha beta IV tubulin dimers in the absence of microtubule-associated proteins. J Biol Chem 269:2041–2047. [PubMed] [Google Scholar]
- 5.Rezania V, Azarenko O, Jordan MA, Bolterauer H, Luduena RF, Huzil JT, Tuszynski JA. 2008. Microtubule assembly of isotypically purified tubulin and its mixtures. Biophys J 95:1993–2008. doi: 10.1529/biophysj.108.132233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutten T, Chan J, Lloyd CW. 1997. A 60-kDa plant microtubule-associated protein promotes the growth and stabilization of neurotubules in vitro. Proc Natl Acad Sci U S A 94:4469–4474. doi: 10.1073/pnas.94.9.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vera JC, Rivas CI, Maccioni RB. 1988. Heat-stable microtubule protein MAP-1 binds to microtubules and induces microtubule assembly. FEBS Lett 232:159–162. doi: 10.1016/0014-5793(88)80408-3. [DOI] [PubMed] [Google Scholar]
- 8.Wehenkel A, Janke C. 2014. Towards elucidating the tubulin code. Nat Cell Biol 16:303–305. doi: 10.1038/ncb2938. [DOI] [PubMed] [Google Scholar]
- 9.Morrissette NS, Sibley LD. 2002. Disruption of microtubules uncouples budding and nuclear division in Toxoplasma gondii. J Cell Sci 115:1017–1025. [DOI] [PubMed] [Google Scholar]
- 10.Shaw MK, Compton HL, Roos DS, Tilney LG. 2000. Microtubules, but not actin filaments, drive daughter cell budding and cell division in Toxoplasma gondii. J Cell Sci 113(Part 7):1241–1254. [DOI] [PubMed] [Google Scholar]
- 11.Stokkermans TJ, Schwartzman JD, Keenan K, Morrissette NS, Tilney LG, Roos DS. 1996. Inhibition of Toxoplasma gondii replication by dinitroaniline herbicides. Exp Parasitol 84:355–370. doi: 10.1006/expr.1996.0124. [DOI] [PubMed] [Google Scholar]
- 12.Halonen SK, Weiss LM. 2013. Toxoplasmosis. Handb Clin Neurol 114:125–145. doi: 10.1016/B978-0-444-53490-3.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson DJP, Dubremetz J-F. 2014. The ultrastructure of Toxoplasma gondii, p 19–59. In Weiss LM, Kim K (ed), Toxoplasma gondii, 2nd ed Academic Press, Boston, MA. [Google Scholar]
- 14.Pelster B, Piekarski G. 1971. Electron microscopical studies on the microgametogeny of Toxoplasma gondii. Z Parasitenkd 37:267–277. (In German.) [DOI] [PubMed] [Google Scholar]
- 15.Li H, DeRosier DJ, Nicholson WV, Nogales E, Downing KH. 2002. Microtubule structure at 8 A resolution. Structure 10:1317–1328. doi: 10.1016/S0969-2126(02)00827-4. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Fernandez JJ, Marshall WF, Agard DA. 2012. Three-dimensional structure of basal body triplet revealed by electron cryo-tomography. EMBO J 31:552–562. doi: 10.1038/emboj.2011.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sui H, Downing KH. 2006. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature 442:475–478. doi: 10.1038/nature04816. [DOI] [PubMed] [Google Scholar]
- 18.Dumontet C, Jordan MA, Lee FF. 2009. Ixabepilone: targeting betaIII-tubulin expression in taxane-resistant malignancies. Mol Cancer Ther 8:17–25. doi: 10.1158/1535-7163.MCT-08-0986. [DOI] [PubMed] [Google Scholar]
- 19.Tseng CY, Mane JY, Winter P, Johnson L, Huzil T, Izbicka E, Luduena RF, Tuszynski JA. 2010. Quantitative analysis of the effect of tubulin isotype expression on sensitivity of cancer cell lines to a set of novel colchicine derivatives. Mol Cancer 9:131. doi: 10.1186/1476-4598-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fackenthal JD, Turner FR, Raff EC. 1993. Tissue-specific microtubule functions in Drosophila spermatogenesis require the beta 2-tubulin isotype-specific carboxy terminus. Dev Biol 158:213–227. doi: 10.1006/dbio.1993.1180. [DOI] [PubMed] [Google Scholar]
- 21.Nagel SD, Boothroyd JC. 1988. The alpha- and beta-tubulins of Toxoplasma gondii are encoded by single copy genes containing multiple introns. Mol Biochem Parasitol 29:261–273. doi: 10.1016/0166-6851(88)90081-3. [DOI] [PubMed] [Google Scholar]
- 22.Xiao H, El Bissati K, Verdier-Pinard P, Burd B, Zhang H, Kim K, Fiser A, Angeletti RH, Weiss LM. 2010. Post-translational modifications to Toxoplasma gondii alpha- and beta-tubulins include novel C-terminal methylation. J Proteome Res 9:359–372. doi: 10.1021/pr900699a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma C, Li C, Ganesan L, Oak J, Tsai S, Sept D, Morrissette NS. 2007. Mutations in alpha-tubulin confer dinitroaniline resistance at a cost to microtubule function. Mol Biol Cell 18:4711–4720. doi: 10.1091/mbc.E07-04-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma C, Tran J, Gu F, Ochoa R, Li C, Sept D, Werbovetz K, Morrissette N. 2010. Dinitroaniline activity in Toxoplasma gondii expressing wild-type or mutant alpha-tubulin. Antimicrob Agents Chemother 54:1453–1460. doi: 10.1128/AAC.01150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrissette NS, Mitra A, Sept D, Sibley LD. 2004. Dinitroanilines bind alpha-tubulin to disrupt microtubules. Mol Biol Cell 15:1960–1968. doi: 10.1091/mbc.E03-07-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiken J, Sept D, Costanzo M, Boone C, Cooper JA, Moore JK. 2014. Genome-wide analysis reveals novel and discrete functions for tubulin carboxy-terminal tails. Curr Biol 24:1295–1303. doi: 10.1016/j.cub.2014.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirajuddin M, Rice LM, Vale RD. 2014. Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat Cell Biol 16:335–344. doi: 10.1038/ncb2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morejohn LC, Bureau TE, Mole-Bajer J, Bajer AS, Fosket DE. 1987. Oryzalin, a dinitroaniline herbicide, binds to plant tubulin and inhibits microtubule polymerization in vitro. Planta 172:252–264. doi: 10.1007/BF00394595. [DOI] [PubMed] [Google Scholar]
- 29.Morrissette N, Sept D. 2008. Dinitroaniline interactions with tubulin: genetic and computational approaches to define the mechanisms of action and resistance, p 327–349. In Blume Y, Baird WV, Yemets A, Breviario D (ed), The plant cytoskeleton: a key tool for agro-biotechnology. Springer, Dordrecht, Netherlands. [Google Scholar]
- 30.Mitra A, Sept D. 2006. Binding and interaction of dinitroanilines with apicomplexan and kinetoplastid alpha-tubulin. J Med Chem 49:5226–5231. doi: 10.1021/jm060472+. [DOI] [PubMed] [Google Scholar]
- 31.Lyons-Abbott S, Sackett DL, Wloga D, Gaertig J, Morgan RE, Werbovetz KA, Morrissette NS. 2010. Alpha-tubulin mutations alter oryzalin affinity and microtubule assembly properties to confer dinitroaniline resistance. Eukaryot Cell 9:1825–1834. doi: 10.1128/EC.00140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma C, Tran J, Li C, Ganesan L, Wood D, Morrissette N. 2008. Secondary mutations correct fitness defects in Toxoplasma gondii with dinitroaniline resistance mutations. Genetics 180:845–856. doi: 10.1534/genetics.108.092494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhey KJ, Gaertig J. 2007. The tubulin code. Cell Cycle 6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 34.Wloga D, Gaertig J. 2010. Post-translational modifications of microtubules. J Cell Sci 123:3447–3455. doi: 10.1242/jcs.063727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akella JS, Wloga D, Kim J, Starostina NG, Lyons-Abbott S, Morrissette NS, Dougan ST, Kipreos ET, Gaertig J. 2010. MEC-17 is an alpha-tubulin acetyltransferase. Nature 467:218–222. doi: 10.1038/nature09324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wloga D, Webster DM, Rogowski K, Bre MH, Levilliers N, Jerka-Dziadosz M, Janke C, Dougan ST, Gaertig J. 2009. TTLL3 is a tubulin glycine ligase that regulates the assembly of cilia. Dev Cell 16:867–876. doi: 10.1016/j.devcel.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Dutcher SK. 2001. The tubulin fraternity: alpha to eta. Curr Opin Cell Biol 13:49–54. doi: 10.1016/S0955-0674(00)00173-3. [DOI] [PubMed] [Google Scholar]
- 38.Gull K. 2001. Protist tubulins: new arrivals, evolutionary relationships and insights to cytoskeletal function. Curr Opin Microbiol 4:427–432. doi: 10.1016/S1369-5274(00)00230-7. [DOI] [PubMed] [Google Scholar]
- 39.Luders J, Stearns T. 2007. Microtubule-organizing centres: a re-evaluation. Nat Rev Mol Cell Biol 8:161–167. doi: 10.1038/nrm2100. [DOI] [PubMed] [Google Scholar]
- 40.Dutcher SK, Morrissette NS, Preble AM, Rackley C, Stanga J. 2002. Epsilon-tubulin is an essential component of the centriole. Mol Biol Cell 13:3859–3869. doi: 10.1091/mbc.E02-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutcher SK, Trabuco EC. 1998. The UNI3 gene is required for assembly of basal bodies of Chlamydomonas and encodes delta-tubulin, a new member of the tubulin superfamily. Mol Biol Cell 9:1293–1308. doi: 10.1091/mbc.9.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross I, Clarissa C, Giddings TH Jr, Winey M. 2013. Epsilon-tubulin is essential in Tetrahymena thermophila for the assembly and stability of basal bodies. J Cell Sci 126:3441–3451. doi: 10.1242/jcs.128694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubey JP. 2014. The history and life cycle of Toxoplasma gondii, p 1–17. In Weiss LM, Kim K (ed), Toxoplasma gondii, 2nd ed Academic Press, Boston, MA. [Google Scholar]
- 44.Dubey JP, Lindsay DS, Speer CA. 1998. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clin Microbiol Rev 11:267–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferguson DJ, Hutchison WM, Siim JC. 1975. The ultrastructural development of the macrogamete and formation of the oocyst wall of Toxoplasma gondii. Acta Pathol Microbiol Scand B Microbiol 83:491–505. [DOI] [PubMed] [Google Scholar]
- 46.Scholtyseck E, Mehlhorn H, Hammond DM. 1972. Electron microscope studies of microgametogenesis in Coccidia and related groups. Z Parasitenkd 38:95–131. doi: 10.1007/BF00329023. [DOI] [PubMed] [Google Scholar]
- 47.Scholtyseck E, Mehlhorn H, Hammond DM. 1971. Fine structure of macrogametes and oocysts of Coccidia and related organisms. Z Parasitenkd 37:1–43. doi: 10.1007/BF00259543. [DOI] [PubMed] [Google Scholar]
- 48.Ferguson DJ, Birch-Andersen A, Siim JC, Hutchison WM. 1979. Ultrastructural studies on the sporulation of oocysts of Toxoplasma gondii. I. Development of the zygote and formation of the sporoblasts. Acta Pathol Microbiol Scand B Microbiol 87B:171–181. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson DJ, Birch-Andersen A, Siim JC, Hutchison WM. 1979. Ultrastructural studies on the sporulation of oocysts of Toxoplasma gondii. II. Formation of the sporocyst and structure of the sporocyst wall. Acta Pathol Microbiol Scand B Microbiol 87B:183–190. [DOI] [PubMed] [Google Scholar]
- 50.Hodges ME, Scheumann N, Wickstead B, Langdale JA, Gull K. 2010. Reconstructing the evolutionary history of the centriole from protein components. J Cell Sci 123:1407–1413. doi: 10.1242/jcs.064873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mitchell DR. 2004. Speculations on the evolution of 9+2 organelles and the role of central pair microtubules. Biol Cell 96:691–696. doi: 10.1016/j.biolcel.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avasthi P, Marshall W. 2013. Ciliary secretion: switching the cellular antenna to ‘transmit’. Curr Biol 23:R471–R473. doi: 10.1016/j.cub.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 53.Goetz SC, Anderson KV. 2010. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet 11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood CR, Huang K, Diener DR, Rosenbaum JL. 2013. The cilium secretes bioactive ectosomes. Curr Biol 23:906–911. doi: 10.1016/j.cub.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azimzadeh J, Marshall WF. 2010. Building the centriole. Curr Biol 20:R816–R825. doi: 10.1016/j.cub.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marshall WF, Vucica Y, Rosenbaum JL. 2001. Kinetics and regulation of de novo centriole assembly. Implications for the mechanism of centriole duplication. Curr Biol 11:308–317. doi: 10.1016/S0960-9822(01)00094-X. [DOI] [PubMed] [Google Scholar]
- 57.Mizukami I, Gall J. 1966. Centriole replication. II. Sperm formation in the fern, Marsilea, and the cycad, Zamia. J Cell Biol 29:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Preble AM, Giddings TM Jr, Dutcher SK. 2000. Basal bodies and centrioles: their function and structure. Curr Top Dev Biol 49:207–233. [DOI] [PubMed] [Google Scholar]
- 59.Sinden RE, Talman A, Marques SR, Wass MN, Sternberg MJ. 2010. The flagellum in malarial parasites. Curr Opin Microbiol 13:491–500. doi: 10.1016/j.mib.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 60.Melkonian M. 1982. The functional analysis of the flagellar apparatus in green algae. Symp Soc Exp Biol 35:589–606. [PubMed] [Google Scholar]
- 61.Okamoto N, Keeling P. 2014. A comparative overview of the flagellar apparatus of dinoflagellate, perkinsids and colpodellids. Microorganisms 2:73–91. doi: 10.3390/microorganisms2010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ringo DL. 1967. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J Cell Biol 33:543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riparbelli MG, Cabrera OA, Callaini G, Megraw TL. 2013. Unique properties of Drosophila spermatocyte primary cilia. Biol Open 2:1137–1147. doi: 10.1242/bio.20135355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Briggs LJ, Davidge JA, Wickstead B, Ginger ML, Gull K. 2004. More than one way to build a flagellum: comparative genomics of parasitic protozoa. Curr Biol 14:R611–R612. doi: 10.1016/j.cub.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 65.Sinden RE, Croll NA. 1975. Cytology and kinetics of microgametogenesis and fertilization in Plasmodium yoelii nigeriensis. Parasitology 70:53–65. doi: 10.1017/S0031182000048861. [DOI] [PubMed] [Google Scholar]
- 66.Portman N, Foster C, Walker G, Slapeta J. 2014. Evidence of intraflagellar transport and apical complex formation in a free-living relative of the apicomplexa. Eukaryot Cell 13:10–20. doi: 10.1128/EC.00155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, Pereira G. 2012. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. J Cell Biol 199:1083–1101. doi: 10.1083/jcb.201202126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silflow CD, LaVoie M, Tam LW, Tousey S, Sanders M, Wu W, Borodovsky M, Lefebvre PA. 2001. The Vfl1 protein in Chlamydomonas localizes in a rotationally asymmetric pattern at the distal ends of the basal bodies. J Cell Biol 153:63–74. doi: 10.1083/jcb.153.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morrissette N, Gubbels M-J. 2014. The Toxoplasma cytoskeleton: structures, proteins and processes, p 455–503. In Weiss LM, Kim K (ed), Toxoplasma gondii, 2nd ed Academic Press, Boston, MA. [Google Scholar]
- 70.Azimzadeh J, Bornens M. 2007. Structure and duplication of the centrosome. J Cell Sci 120:2139–2142. doi: 10.1242/jcs.005231. [DOI] [PubMed] [Google Scholar]
- 71.Bornens M, Azimzadeh J. 2007. Origin and evolution of the centrosome. Adv Exp Med Biol 607:119–129. doi: 10.1007/978-0-387-74021-8_10. [DOI] [PubMed] [Google Scholar]
- 72.Dubremetz JF, Elsner YY. 1979. Ultrastructural study of schizogony of Eimeria bovis in cell cultures*. J Protozool 26:367–376. doi: 10.1111/j.1550-7408.1979.tb04639.x. [DOI] [PubMed] [Google Scholar]
- 73.Sheffield HG. 1966. Electron microscope study of the proliferative form of Besnoitia jellisoni. J Parasitol 52:583–594. doi: 10.2307/3276331. [DOI] [PubMed] [Google Scholar]
- 74.Brooks CF, Francia ME, Gissot M, Croken MM, Kim K, Striepen B. 2011. Toxoplasma gondii sequesters centromeres to a specific nuclear region throughout the cell cycle. Proc Natl Acad Sci U S A 108:3767–3772. doi: 10.1073/pnas.1006741108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farrell M, Gubbels MJ. 2014. The Toxoplasma gondii kinetochore is required for centrosome association with the centrocone (spindle pole). Cell Microbiol 16:78–94. doi: 10.1111/cmi.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morrissette NS, Murray JM, Roos DS. 1997. Subpellicular microtubules associate with an intramembranous particle lattice in the protozoan parasite Toxoplasma gondii. J Cell Sci 110(Part 1):35–42. [DOI] [PubMed] [Google Scholar]
- 77.Nichols BA, Chiappino ML. 1987. Cytoskeleton of Toxoplasma gondii. J Protozool 34:217–226. doi: 10.1111/j.1550-7408.1987.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 78.Russell DG, Burns RG. 1984. The polar ring of coccidian sporozoites: a unique microtubule-organizing centre. J Cell Sci 65:193–207. [DOI] [PubMed] [Google Scholar]
- 79.Porchet E, Torpier G. 1977. Freeze fracture study of Toxoplasma and Sarcocystis infective stages (author's transl). Z Parasitenkd 54:101–124. (In French.) doi: 10.1007/BF00380795. [DOI] [PubMed] [Google Scholar]
- 80.Morrissette NS, Sibley LD. 2002. Cytoskeleton of apicomplexan parasites. Microbiol Mol Biol Rev 66:21–38. doi: 10.1128/MMBR.66.1.21-38.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson-White BR, Ivey FD, Cheng K, Szatanek T, Lorestani A, Beckers CJ, Ferguson DJ, Sahoo N, Gubbels MJ. 2011. A family of intermediate filament-like proteins is sequentially assembled into the cytoskeleton of Toxoplasma gondii. Cell Microbiol 13:18–31. doi: 10.1111/j.1462-5822.2010.01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gould SB, Tham WH, Cowman AF, McFadden GI, Waller RF. 2008. Alveolins, a new family of cortical proteins that define the protist infrakingdom Alveolata. Mol Biol Evol 25:1219–1230. doi: 10.1093/molbev/msn070. [DOI] [PubMed] [Google Scholar]
- 83.Mann T, Beckers C. 2001. Characterization of the subpellicular network, a filamentous membrane skeletal component in the parasite Toxoplasma gondii. Mol Biochem Parasitol 115:257–268. doi: 10.1016/S0166-6851(01)00289-4. [DOI] [PubMed] [Google Scholar]
- 84.Hu K, Roos DS, Murray JM. 2002. A novel polymer of tubulin forms the conoid of Toxoplasma gondii. J Cell Biol 156:1039–1050. doi: 10.1083/jcb.200112086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leander BS, Keeling PJ. 2003. Morphostasis in alveolate evolution. Trends Ecol Evol 18:395–402. doi: 10.1016/S0169-5347(03)00152-6. [DOI] [Google Scholar]
- 86.Okamoto N, Keeling PJ. 2014. The 3D structure of the apical complex and association with the flagellar apparatus revealed by serial TEM tomography in Psammosa pacifica, a distant relative of the Apicomplexa. PLoS One 9:e84653. doi: 10.1371/journal.pone.0084653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Leon JC, Scheumann N, Beatty W, Beck JR, Tran JQ, Yau C, Bradley PJ, Gull K, Wickstead B, Morrissette NS. 2013. A SAS-6-like protein suggests that the Toxoplasma conoid complex evolved from flagellar components. Eukaryot Cell 12:1009–1019. doi: 10.1128/EC.00096-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Francia ME, Jordan CN, Patel JD, Sheiner L, Demerly JL, Fellows JD, de Leon JC, Morrissette NS, Dubremetz JF, Striepen B. 2012. Cell division in apicomplexan parasites is organized by a homolog of the striated rootlet fiber of algal flagella. PLoS Biol 10:e1001444. doi: 10.1371/journal.pbio.1001444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Katris NJ, van Dooren GG, McMillan PJ, Hanssen E, Tilley L, Waller RF. 2014. The apical complex provides a regulated gateway for secretion of invasion factors in Toxoplasma. PLoS Pathog 10:e1004074. doi: 10.1371/journal.ppat.1004074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gordon JL, Sibley LD. 2005. Comparative genome analysis reveals a conserved family of actin-like proteins in apicomplexan parasites. BMC Genomics 6:179. doi: 10.1186/1471-2164-6-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hirokawa N, Noda Y, Tanaka Y, Niwa S. 2009. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol 10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 92.Mulder AM, Glavis-Bloom A, Moores CA, Wagenbach M, Carragher B, Wordeman L, Milligan RA. 2009. A new model for binding of kinesin 13 to curved microtubule protofilaments. J Cell Biol 185:51–57. doi: 10.1083/jcb.200812052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shipley K, Hekmat-Nejad M, Turner J, Moores C, Anderson R, Milligan R, Sakowicz R, Fletterick R. 2004. Structure of a kinesin microtubule depolymerization machine. EMBO J 23:1422–1432. doi: 10.1038/sj.emboj.7600165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hirokawa N. 1998. Kinesin and dynein superfamily proteins and the mechanism of organelle transport. Science 279:519–526. doi: 10.1126/science.279.5350.519. [DOI] [PubMed] [Google Scholar]
- 95.Hu K, Johnson J, Florens L, Fraunholz M, Suravajjala S, DiLullo C, Yates J, Roos DS, Murray JM. 2006. Cytoskeletal components of an invasion machine–the apical complex of Toxoplasma gondii. PLoS Pathog 2:e13. doi: 10.1371/journal.ppat.0020013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qureshi BM, Hofmann NE, Arroyo-Olarte RD, Nickl B, Hoehne W, Jungblut PR, Lucius R, Scheerer P, Gupta N. 2013. Dynein light chain 8a of Toxoplasma gondii, a unique conoid-localized beta-strand-swapped homodimer, is required for an efficient parasite growth. FASEB J 27:1034–1047. doi: 10.1096/fj.11-180992. [DOI] [PubMed] [Google Scholar]
- 97.Rieder CL, Faruki S, Khodjakov A. 2001. The centrosome in vertebrates: more than a microtubule-organizing center. Trends Cell Biol 11:413–419. doi: 10.1016/S0962-8924(01)02085-2. [DOI] [PubMed] [Google Scholar]
- 98.Gomez de Leon CT, Diaz Martin RD, Mendoza Hernandez G, Gonzalez Pozos S, Ambrosio JR, Mondragon Flores R. 2014. Proteomic characterization of the subpellicular cytoskeleton of Toxoplasma gondii tachyzoites. J Proteomics 111:86–99. doi: 10.1016/j.jprot.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 99.Gonczy P. 2012. Towards a molecular architecture of centriole assembly. Nat Rev Mol Cell Biol 13:425–435. doi: 10.1038/nrm3373. [DOI] [PubMed] [Google Scholar]
- 100.Chen CT, Gubbels MJ. 2013. The Toxoplasma gondii centrosome is the platform for internal daughter budding as revealed by a Nek1 kinase mutant. J Cell Sci 126:3344–3355. doi: 10.1242/jcs.123364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Salisbury JL. 2004. Centrosomes: Sfi1p and centrin unravel a structural riddle. Curr Biol 14:R27–R29. doi: 10.1016/j.cub.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 102.Schiebel E, Bornens M. 1995. In search of a function for centrins. Trends Cell Biol 5:197–201. doi: 10.1016/S0962-8924(00)88999-0. [DOI] [PubMed] [Google Scholar]
- 103.Hartmann J, Hu K, He CY, Pelletier L, Roos DS, Warren G. 2006. Golgi and centrosome cycles in Toxoplasma gondii. Mol Biochem Parasitol 145:125–127. doi: 10.1016/j.molbiopara.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 104.Hu K. 2008. Organizational changes of the daughter basal complex during the parasite replication of Toxoplasma gondii. PLoS Pathog 4:e10. doi: 10.1371/journal.ppat.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lorestani A, Ivey FD, Thirugnanam S, Busby MA, Marth GT, Cheeseman IM, Gubbels MJ. 2012. Targeted proteomic dissection of Toxoplasma cytoskeleton sub-compartments using MORN1. Cytoskeleton (Hoboken) 69:1069–1085. doi: 10.1002/cm.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lechtreck KF, Frins S, Bilski J, Teltenkotter A, Weber K, Melkonian M. 1996. The cruciated microtubule-associated fibers of the green alga Dunaliella bioculata consist of a 31 kDa SF-assemblin. J Cell Sci 109(Part 4):827–835. [DOI] [PubMed] [Google Scholar]
- 107.Weber K, Geisler N, Plessmann U, Bremerich A, Lechtreck KF, Melkonian M. 1993. SF-assemblin, the structural protein of the 2-nm filaments from striated microtubule associated fibers of algal flagellar roots, forms a segmented coiled coil. J Cell Biol 121:837–845. doi: 10.1083/jcb.121.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tran JQ, de Leon JC, Li C, Huynh MH, Beatty W, Morrissette NS. 2010. RNG1 is a late marker of the apical polar ring in Toxoplasma gondii. Cytoskeleton (Hoboken) 67:586–598. doi: 10.1002/cm.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Frenal K, Polonais V, Marq JB, Stratmann R, Limenitakis J, Soldati-Favre D. 2010. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe 8:343–357. doi: 10.1016/j.chom.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 110.Gaskins E, Gilk S, DeVore N, Mann T, Ward G, Beckers C. 2004. Identification of the membrane receptor of a class XIV myosin in Toxoplasma gondii. J Cell Biol 165:383–393. doi: 10.1083/jcb.200311137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gilk SD, Gaskins E, Ward GE, Beckers CJ. 2009. GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex. Eukaryot Cell 8:190–196. doi: 10.1128/EC.00201-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gould SB, Kraft LG, van Dooren GG, Goodman CD, Ford KL, Cassin AM, Bacic A, McFadden GI, Waller RF. 2011. Ciliate pellicular proteome identifies novel protein families with characteristic repeat motifs that are common to alveolates. Mol Biol Evol 28:1319–1331. doi: 10.1093/molbev/msq321. [DOI] [PubMed] [Google Scholar]
- 113.Tran JQ, Li C, Chyan A, Chung L, Morrissette NS. 2012. SPM1 stabilizes subpellicular microtubules in Toxoplasma gondii. Eukaryot Cell 11:206–216. doi: 10.1128/EC.05161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu J, Wetzel L, Zhang Y, Nagayasu E, Ems-McClung S, Florens L, Hu K. 2013. Novel thioredoxin-like proteins are components of a protein complex coating the cortical microtubules of Toxoplasma gondii. Eukaryot Cell 12:1588–1599. doi: 10.1128/EC.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heaslip AT, Ems-McClung SC, Hu K. 2009. TgICMAP1 is a novel microtubule binding protein in Toxoplasma gondii. PLoS One 4:e7406. doi: 10.1371/journal.pone.0007406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Skariah S, Bednarczyk RB, McIntyre MK, Taylor GA, Mordue DG. 2012. Discovery of a novel Toxoplasma gondii conoid-associated protein important for parasite resistance to reactive nitrogen intermediates. J Immunol 188:3404–3415. doi: 10.4049/jimmunol.1101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu K, Mann T, Striepen B, Beckers CJ, Roos DS, Murray JM. 2002. Daughter cell assembly in the protozoan parasite Toxoplasma gondii. Mol Biol Cell 13:593–606. doi: 10.1091/mbc.01-06-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Piekarski G, Pelster B, Witte HM. 1971. Endopolygeny in Toxoplasma gondii. Z Parasitenkd 36:122–130. (In German.) [PubMed] [Google Scholar]
- 119.Speer CA, Dubey JP. 2005. Ultrastructural differentiation of Toxoplasma gondii schizonts (types B to E) and gamonts in the intestines of cats fed bradyzoites. Int J Parasitol 35:193–206. doi: 10.1016/j.ijpara.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 120.Striepen B, Jordan CN, Reiff S, van Dooren GG. 2007. Building the perfect parasite: cell division in apicomplexa. PLoS Pathog 3:e78. doi: 10.1371/journal.ppat.0030078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Striepen B, Crawford MJ, Shaw MK, Tilney LG, Seeber F, Roos DS. 2000. The plastid of Toxoplasma gondii is divided by association with the centrosomes. J Cell Biol 151:1423–1434. doi: 10.1083/jcb.151.7.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Andenmatten N, Egarter S, Jackson AJ, Jullien N, Herman JP, Meissner M. 2013. Conditional genome engineering in Toxoplasma gondii uncovers alternative invasion mechanisms. Nat Methods 10:125–127. doi: 10.1038/nmeth.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jacot D, Daher W, Soldati-Favre D. 2013. Toxoplasma gondii myosin F, an essential motor for centrosomes positioning and apicoplast inheritance. EMBO J 32:1702–1716. doi: 10.1038/emboj.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gubbels MJ, Lehmann M, Muthalagi M, Jerome ME, Brooks CF, Szatanek T, Flynn J, Parrot B, Radke J, Striepen B, White MW. 2008. Forward genetic analysis of the apicomplexan cell division cycle in Toxoplasma gondii. PLoS Pathog 4:e36. doi: 10.1371/journal.ppat.0040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nishi M, Hu K, Murray JM, Roos DS. 2008. Organellar dynamics during the cell cycle of Toxoplasma gondii. J Cell Sci 121:1559–1568. doi: 10.1242/jcs.021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shaw MK, Roos DS, Tilney LG. 2001. DNA replication and daughter cell budding are not tightly linked in the protozoan parasite Toxoplasma gondii. Microbes Infect 3:351–362. doi: 10.1016/S1286-4579(01)01392-2. [DOI] [PubMed] [Google Scholar]
- 127.Anderson-White B, Beck JR, Chen CT, Meissner M, Bradley PJ, Gubbels MJ. 2012. Cytoskeleton assembly in Toxoplasma gondii cell division. Int Rev Cell Mol Biol 298:1–31. doi: 10.1016/B978-0-12-394309-5.00001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Leung JM, Rould MA, Konradt C, Hunter CA, Ward GE. 2014. Disruption of TgPHIL1 alters specific parameters of Toxoplasma gondii motility measured in a quantitative, three-dimensional live motility assay. PLoS One 9:e85763. doi: 10.1371/journal.pone.0085763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hook P, Vallee RB. 2006. The dynein family at a glance. J Cell Sci 119:4369–4371. doi: 10.1242/jcs.03176. [DOI] [PubMed] [Google Scholar]
- 130.Lentini G, Kong-Hap M, El Hajj H, Francia M, Claudet C, Striepen B, Dubremetz JF, Lebrun M. 30 August 2014. Identification and characterization of Toxoplasma SIP, a conserved apicomplexan cytoskeleton protein involved in maintaining the shape, motility and virulence of the parasite. Cell Microbiol. doi: 10.1111/cmi.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tilley LD, Krishnamurthy S, Westwood NJ, Ward GE. 2014. Identification of TgCBAP, a novel cytoskeletal protein that localizes to three distinct subcompartments of the Toxoplasma gondii pellicle. PLoS One 9:e98492. doi: 10.1371/journal.pone.0098492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Haraldsen JD, Liu G, Botting CH, Walton JG, Storm J, Phalen TJ, Kwok LY, Soldati-Favre D, Heintz NH, Muller S, Westwood NJ, Ward GE. 2009. Identification of conoidin as a covalent inhibitor of peroxiredoxin II. Org Biomol Chem 7:3040–3048. doi: 10.1039/b901735f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu G, Botting CH, Evans KM, Walton JA, Xu G, Slawin AM, Westwood NJ. 2010. Optimisation of conoidin A, a peroxiredoxin inhibitor. ChemMedChem 5:41–45. doi: 10.1002/cmdc.200900391. [DOI] [PubMed] [Google Scholar]
- 134.Del Carmen MG, Mondragon M, Gonzalez S, Mondragon R. 2009. Induction and regulation of conoid extrusion in Toxoplasma gondii. Cell Microbiol 11:967–982. doi: 10.1111/j.1462-5822.2009.01304.x. [DOI] [PubMed] [Google Scholar]
- 135.Mondragon R, Frixione E. 1996. Ca(2+)-dependence of conoid extrusion in Toxoplasma gondii tachyzoites. J Eukaryot Microbiol 43:120–127. doi: 10.1111/j.1550-7408.1996.tb04491.x. [DOI] [PubMed] [Google Scholar]
- 136.Guttery DS, Holder AA, Tewari R. 2012. Sexual development in Plasmodium: lessons from functional analyses. PLoS Pathog 8:e1002404. doi: 10.1371/journal.ppat.1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marques SR, Ramakrishnan C, Carzaniga R, Blagborough AM, Delves MJ, Talman AM, Sinden RE. 26 August 2014. An essential role of the basal body protein SAS-6 in Plasmodium male gamete development and malaria transmission. Cell Microbiol. doi: 10.1111/cmi.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hammond DM, Roberts WL, Youssef NN, Danforth HD. 1973. Fine structure of the intranuclear spindle poles in Eimeria callospermophili and E. magna. J Parasitol 59:581–584. doi: 10.2307/3278800. [DOI] [Google Scholar]
- 139.Scholtyseck E. 1973. Ultrastructure, p 81–144. In Hammond DM, Long P (ed), The Coccidia: Eimeria, Isospora, TOXOPLASMA, and related genera. University Park Press, Baltimore, MD. [Google Scholar]