Abstract
Nuclear dynamics can vary widely between fungal species and between stages of development of fungal colonies. Here we compared nuclear dynamics and mitotic patterns between germlings and mature hyphae in Fusarium oxysporum. Using fluorescently labeled nuclei and live-cell imaging, we show that F. oxysporum is subject to a developmental transition from a uninucleate to a multinucleate state after completion of colony initiation. We observed a special type of hypha that exhibits a higher growth rate, possibly acting as a nutrient scout. The higher growth rate is associated with a higher nuclear count and mitotic waves involving 2 to 6 nuclei in the apical compartment. Further, we found that dormant nuclei of intercalary compartments can reenter the mitotic cycle, resulting in multinucleate compartments with up to 18 nuclei in a single compartment.
INTRODUCTION
Cellular growth and the dynamics of organelles in ascomycetous fungi have been studied extensively in different model organisms, and several common properties have been described. For example, it is well understood that filamentous fungi grow by hyphal tip extension and that the vesicle-rich Spitzenkörper is the main coordinator of tip growth (1, 2). Further, it has been shown that hyphal compartments are separated from each other by septa, which can be perforated, ensuring cytoplasmic continuity (3–5). Vegetative hyphae have the ability to branch and fuse with each other to form an interconnected mycelial network, which also can have cytoplasmic continuity (6, 7). In some fungi, such as Neurospora crassa, free nuclear movement between hyphal compartments throughout the interconnected mycelium has been observed (8).
A further common characteristic of the most intensively studied ascomycete species is that hyphal compartments can be multinucleate. For example, compartments of Ashbya gossypii can harbor 8 to 10 nuclei (9). Aspergillus nidulans has been shown to have compartments with 10 to 60 nuclei (10). The number of nuclei can go up to hundreds of nuclei (in N. crassa) or even thousands of nuclei (in aseptate glomeromycete fungi [11, 12; for a review, see reference 13]). Multinucleated hyphae exhibit different modes of mitotic divisions. In synchronous mitosis, all nuclei of a hyphal compartment divide at the same time, as is for instance is the case in apical cells of Ceratocystis fagacearum (14). An alternative is parasynchronous mitosis, a wave of nuclear divisions that travels along the hypha or hyphal compartment, which has been extensively studied in A. nidulans (15). Finally, nuclei of the same compartment can undergo asynchronous mitosis independently of their neighboring nuclei, as has been observed in N. crassa and A. gossypii (16, 17; for a review, see reference 18). Colletotrichum lindemuthianum provides an interesting case, in which different mitotic patterns at different developmental stages were observed. Apical compartments of hyphae of mature colonies exhibit synchronous, parasynchronous, and asynchronous mitoses, whereas in subapical compartments, only synchronous and asynchronous mitoses were observed (19). The mycelium of multinucleate fungi has the potential to contain genetically different nuclei, leading to phenotypic plasticity as well as potentially contributing to fungal virulence (20–23).
Nuclear dynamics in Fusarium oxysporum has not been fully resolved. In studies from the 1960s, using microscopic methods, including phase-contrast microscopy and fixed-cell staining, F. oxysporum was described as a multinucleate fungus undergoing waves of mitotic nuclear divisions involving several compartments (4, 24, 25). However, in a recent study using modern live-cell imaging techniques and fluorescent labeling, Ruiz-Roldán et al. observed F. oxysporum as a uninucleate fungus, in which only the nucleus of the apical compartment is mitotically active (26).
The aim of this study was to resolve these apparent contradictions and to obtain a fuller understanding of nuclear dynamics of F. oxysporum using fluorescently labeled nuclei and live-cell imaging. We found the following. (i) After completion of colony initiation, specialized hyphae with a higher growth rate start to explore the surrounding medium. The higher growth rate is associated with a higher number of nuclei in the apical compartment and mitotic waves involving all nuclei of this compartment. (ii) In intercalary compartments, dormant nuclei can be reactivated to enter the mitotic cycle. Apparently, F. oxysporum is subject to a developmental change from a uninucleate state in germlings and newly branched hyphae to at least two alternative multinucleate states in hyphae of the mature colony.
MATERIALS AND METHODS
Strains and culture conditions.
Fusarium oxysporum f. sp. lycopersici strain 4287 (FGSC9935), Fusarium oxysporum f. sp. melonis strain 001 (FGSC10441), and the nonpathogenic Fusarium oxysporum strain 47 (FGSC 10445) were used as the parent strains for fungal transformation. They were stored as a monoconidial culture at −80°C and revitalized on potato dextrose agar (PDA) (Difco) at 25°C. Agrobacterium tumefaciens EHA105 (27) was used for Agrobacterium-mediated transformation of F. oxysporum and was grown in either Luria broth (LB) or 2YT medium (28) containing 20 μg/ml rifampin at 28°C. Introduction of the plasmids into the Agrobacterium strain was performed as previously described (29). Escherichia coli DH5α (Invitrogen) was used for construction, propagation, and amplification of the plasmid and was grown at 37°C in LB medium containing 50 μg/ml kanamycin.
For microscopy, the fungus was grown on either PDA supplemented with 2% xylose or on low-nutrient or minimal medium (0.17% yeast nitrogen base [YNB; Difco] without amino acids and ammonium sulfate, 100 mM KNO3, 2% xylose, 1.2% agarose) at room temperature. Unless otherwise indicated, the medium was prepared in the shape of a microscope slide. Spores were collected from either PDA plates or NO3 medium (0.17% YNB, 100 mM KNO3, 3% sucrose), filtered with one layer of sterile Miracloth (Calbiochem), and washed with sterile water prior to mounting on agarose slides. These were incubated spore phase down in a microscope chamber (Nunc) and observed for up to 3 days. To visualize cell walls and septa, 1 μM calcofluor white (Fluka) was added to the medium (30). To counterstain DNA, the fungus was treated for 1 min with 1 mg/ml Hoechst 33342 (Life Technologies) and washed with water before microscopy.
Construction of histone H1::GFP fusion protein-expressing vector.
Binary vector pRW2h suitable for Agrobacterium-mediated fungal transformations was used as a backbone for vector construction (31). We constructed a new vector, pRW2h + GFP, in a way that any protein of interest can be expressed as a green fluorescent protein (GFP) fusion protein. For this we introduced the GFP gene under the control of a promoter and a terminator with a multiple-cloning site between the promoter and the GFP gene. This construct was introduced in the multiple-cloning site between the left border and the hph resistance cassette. To be able to control expression of the GFP fusion protein, an inducible xylanase promoter from Penicillium chrysogenum (32) was used. The promoter region was PCR amplified with the primer combination FP2875 (5′-AAAATTAATTAACTGATGCGAGCAACAGTATG-3′) and FP3528 (5′-GATATCTGGTTACCAGATCTTGTTAACAGGGATGGAGGCGATACTTA-3′), using pXPcFLPnatFRT vector (33) as the template. The resulting amplicon was cloned in the PacI/EcoRV site in PRW2h. The terminator region of Six1 (34) was PCR amplified with the primer combination FP2877 (5′-AAAAGGTAACCATTATAACCTGCAGGGGGCCCGTTGCGATCCA-3′) and FP3704 (5′-TTTTGATATCGGCGCGCCATACCTACGGCATCGAGTTTC-3′), using F. oxysporum f. sp. lycopersici 4287 genomic DNA (gDNA) as the template, and cloned in the BstEII/EcoRV site of the vector resulting from the previous step, thus introducing five additional restriction sites. Next, the GFP gene was PCR amplified with primer combination FP3510 (5′-AAAAGGTAACCAGCCCGGGCAATTTAAATATGAGTAAAGGAGAAGAACTTTT-3′) and FP3513 (5′-TTTTTTATAATTTATTTGTATAGTTCATCCATGC-3′), using pGWB451 vector (35) as the template, and cloned in the BstEII/PsiI site between the promoter and terminator regions. To generate an F. oxysporum f. sp. lycopersici histone H1-GFP fusion protein (HH01::GFP), HH01 (FOXG_12732; http://www.broadinstitute.org/annotation/genome/fusarium_group/) without a stop codon was PCR amplified with the primer combination FP3516 (5′-AAAAAGATCTAATGCCTCCCAAAGCCGCT-3′) and FP3517 (5′-TTTTGGTTACCTTCGCCTTGGCAGCGGCC-3′) from F. oxysporum f. sp. lycopersici 4287 gDNA, and the amplicon was cloned in the BglII/BstEII site in-frame with the GFP gene. The obtained plasmid, pRW2h + HH01::GFP was transformed into Agrobacterium tumefaciens EHA105 and used for subsequent A. tumefaciens-mediated Fusarium transformation. (For the plasmid map, see Fig. S1 in the supplemental material.).
Agrobacterium-mediated Fusarium transformation.
Agrobacterium-mediated transformation of F. oxysporum f. sp. was performed as previously described (36), with minor adjustments (37). Transformants were selected on Czapek Dox agar (CDA; Oxoid) containing 100 μg/ml hygromycin (Duchefa). Fluorescence was tested on CDA containing 2% xylose.
Microscopic analysis.
Successful transformation and DNA counterstaining were tested by localization of fluorescent signal using the AMG Evos FL digital inverted microscope equipped with transmitted light, GFP (470/22 to 510/42 nm) or DAPI (4′,6-diamidino-2-phenylindole) (357/44 to 447/60 nm) light cubes, and driven by built-in software for image acquisition and the inverted agar block method (30).
For confocal microscopy, an Eclipse Ti inverted microscope (Nikon) with a FN1 spinning disk and electron microscope–charge-coupled device (EM-CCD) camera, iXon DU897 (Andor), was used with a plan apo VC 40× 1.4 oil objective (Nikon). GFP was excited with a 488-nm light (emission 505- to 530-nm-pass filter) and calcofluor with a 405-nm light (emission 420- to 470-nm-pass filter). Pictures were analyzed with the Nikon NIS and Fiji software from imageJ (http://fiji.sc/Fiji).
RESULTS
The number of nuclei per compartment varies in Fusarium oxysporum.
Studies on nuclear dynamics are often performed with germlings. To obtain a fuller picture of nuclear dynamics, we compared germlings with hyphae of a mature colony using three different Fusarium oxysporum strains (F. oxysporum f. sp. lycopersici 4287, F. oxysporum f. sp. melonis 001, and F. oxysporum 47) expressing histone H1 tagged with a green fluorescent protein (HH01::GFP). We observed nuclear dynamics and number in germlings after 10 to 15 h and in mature hyphae after 2 days.
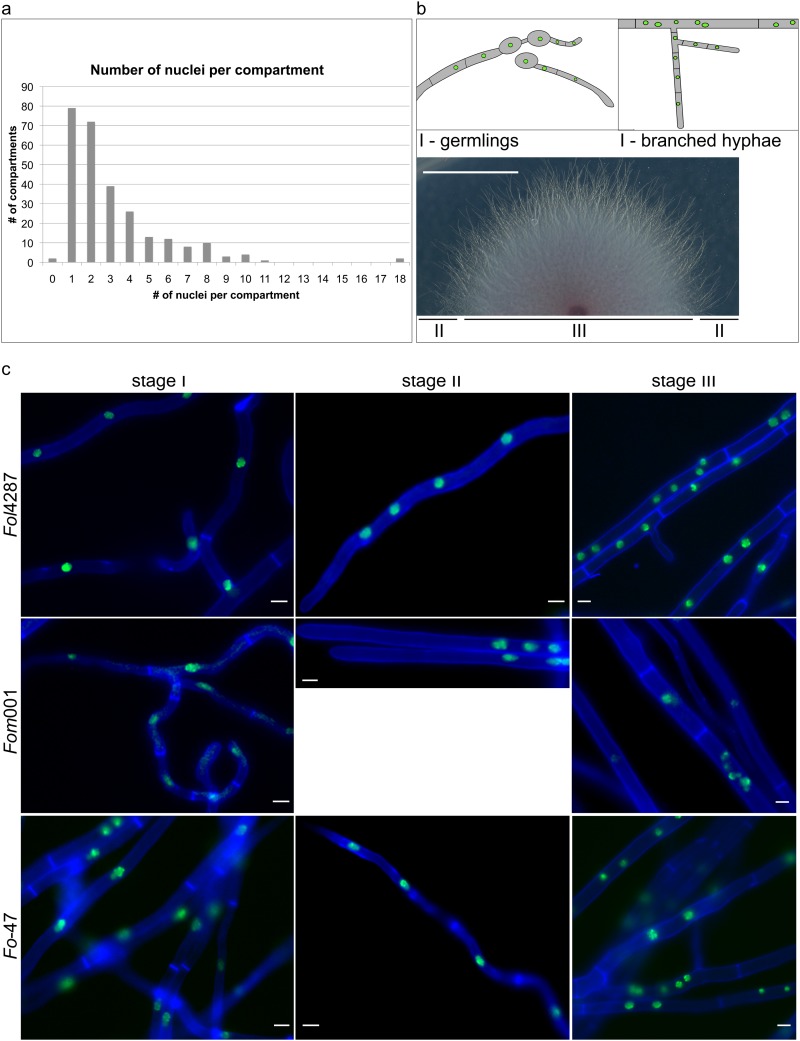
As previously described (26), we saw that during early colony development, germlings of F. oxysporum have uninucleate compartments (see Fig. S3 in the supplemental material). This situation changed drastically after 2 days, when the number of nuclei per compartment became highly variable. In addition to compartments with a single nucleus, compartments with two or more nuclei were observed (Fig. 1). In F. oxysporum f. sp. lycopersici 4287, we recorded a distribution in the range of 0 to 18 with an average of 3.1 ± 2.6 (mean ± standard deviation [SD]) nuclei per compartment after 2 days on minimal medium (Fig. 1a and Table 1). Depending on the nuclear count, the length of the compartments also varied greatly (48 ± 49 μm, mean ± SD) (Table 1; see Fig. S2 in the supplemental material). Both odd and even numbers of nuclei per compartment were observed. Multiple nuclei were found in apical and subapical compartments of hyphal tips as well as in intercalary compartments of mature hyphae, in the center as well as at the edge of the colony. Nonetheless, we could classify hyphae into three distinct developmental stages based on several characteristics. First, newly branched hyphae showed the same nuclear dynamics as germlings and usually contained 1 or 2 nuclei per compartment (Fig. 1b, upper panel, and c, left panel). Second, we discovered a specialized hyphal form or developmental stage in F. oxysporum that is relatively thin (2 to 3 μm in diameter compared to 4 to 6 μm for mature hyphae) (Fig. 1c, middle panel) and showed a higher growth rate than germlings or newly branched hyphae (Table 1). This newly described hyphal form, referred to here as “fast-growing hyphae,” was observed after 2 days but not during early colony initiation and was mostly encountered in the growth front of the colony (Fig. 1b, lower panel). With hyphae 176 ± 150 μm (mean ± SD), apical compartments of fast-growing hyphae were longer than those of germlings and contained 2 to 6 nuclei (Table 1). Hyphae of the third developmental stage displayed a higher nuclear density, manifested in either smaller compartments with a single nucleus or larger compartments with multiple nuclei (up to 18 nuclei were observed) (Fig. 1c, right panel). Stage III was mostly observed behind the growth front of the colony and represents hyphae of the mature colony (Fig. 1b, lower panel).
FIG 1.
The number of nuclei per compartment is dependent on the hyphal type. (a) Range of number of nuclei per compartment. Calculations were based on 65 hyphae from 5 biological replicates. Both odd and even numbers were found. (b) Different stages of colony development in F. oxysporum. The upper panel shows a schematic representation of stage I, in which each compartment harbors 1 nucleus. This stage was found in germlings and in newly branched hyphae. The lower panel shows colony phenotype after 2 days. Stage II, in which fast-growing hyphae with multinucleate apical compartments are frequently encountered, can be found in the growth front. Stage III with multinucleate intercalary compartments can usually be found in mature hyphae behind the growth front, where aerial hyphae start to emerge. Scale bar, 1 cm. (c) The three developmental stages were found in all three strains tested: F. oxysporum f. sp. lycopersici strain 4287 (Fol4287), F. oxysporum f. sp. melonis strain 001 (Fom001), and nonpathogenic F. oxysporum strain 47 (Fo-47). All strains expressed HH01::GFP and were stained with 1 μM calcofluor white. Scale bars, 10 μm.
TABLE 1.
Emergence and characteristics of multinucleate compartments in F. oxysporum f. sp. lycopersici 4287 on different media
| Parameter | Result (n) ona: |
|
|---|---|---|
| Minimal medium | PDA | |
| No. of nuclei per compartment on day 2 | 3.1 ± 2.6 (271) | NDb |
| Size of compartment on day 2, μm | 47.5 ± 48.6 (271) | ND |
| Size of apical compartment in fast-growing hyphae on day 2, μm | 176.2 ± 149.8 (30) | ND |
| Germination start, h | 10 | 10 |
| Growth rate at 15 h, μm/h | 19.8 ± 7.6 (6) | 30.0 ± 7.8 (5) |
| Appearance of fast-growing hyphae, h after germination | 6 | 10 |
| Growth rate of fast-growing hyphae, μm/h | 164.3 ± 64.9 (5) | 119.4 ± 35.2 (5) |
| Appearance of intercalary mitosis, h after germination | 23 | 26 |
Values with “±” are means ± SD.
ND, not determined.
These distinct developmental stages with their characteristic nuclear dynamics were observed in all three tested strains, suggesting this phenomenon is common in F. oxysporum (Fig. 1c). To rule out an effect of histone tagging and microscopic setup, we performed DNA counterstaining of hyphae grown on PDA plates. After 2 days, stages II and III were clearly distinguishable in colonies of F. oxysporum f. sp. lycopersici 4287 (see Fig. S4 in the supplemental material).
Fusarium oxysporum shows various patterns of mitosis.
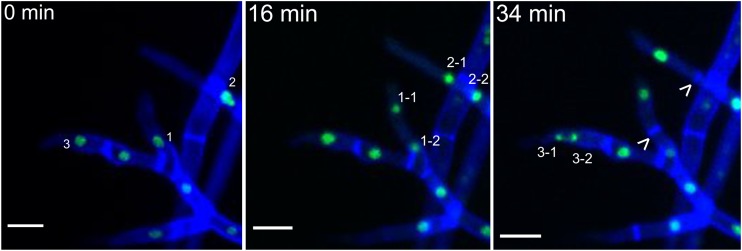
Next, we used live-cell imaging to observe nuclear dynamics after 10 to 15 h in germlings and after 2 days in hyphae of mature colonies. In germlings, only the nucleus of the apical compartment was active and underwent mitosis. This mitosis, then, led to a mitotically inactive nucleus residing in the newly formed first subapical compartment and a mitotically active nucleus in the apical compartment, which entered the next mitotic cycle (see Fig. S3 and Movie S1 in the supplemental material). Again, newly branched hyphae behaved similarly to germlings, where only the apical nucleus was mitotically active (Fig. 2; see Movie S2 in the supplemental material). This stage of uninucleate compartments we refer to as stage I. After 2 days this uniform mitotic pattern changed and became more complicated; hyphae of developing colonies showed two additional mitotic patterns.
FIG 2.
In newly branched hyphae, only the apical nucleus is mitotically active. Shown are time-lapse sequences of mitosis of uninucleated apical compartments of newly branched hyphae. The numbers indicate nuclei that will undergo mitosis, and arrowheads indicate newly formed septa. Shown is F. oxysporum f. sp. lycopersici 4287 HH01::GFP stained with 1 μM calcofluor white. Scale bars, 10 μm.
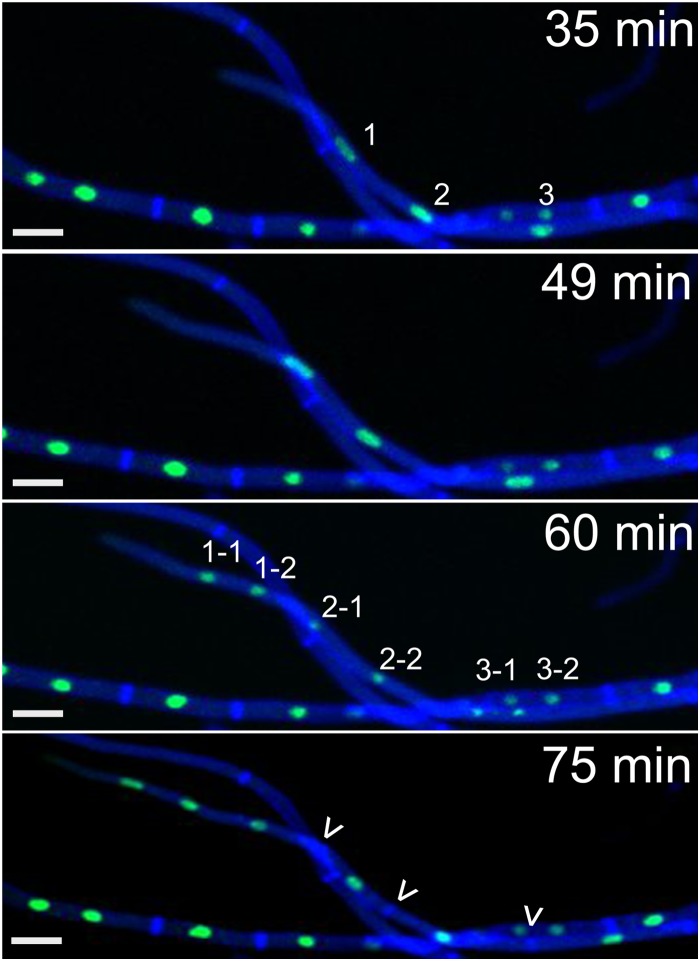
In stage II, apical compartments of fast-growing hyphae contained several nuclei, and these were all mitotically active. Fast-growing hyphae show a rapid parasynchronous mitotic wave of up to 6 nuclei coupled to fast growth, which we refer to as “hyphal growth spurts” from here on. Typically, a hyphal growth spurt started by extension of the apical compartment and fast migration of the resident nuclei along the growth vector in the direction of the hyphal tip (Fig. 3, top 2 panels). During this first phase, nuclei appear to be elongated, which we attribute to the rapid movement, probably caused by pulling of the nuclei toward the hyphal tip by the cytoskeleton machinery. Next, a mitotic wave, including all nuclei of the apical compartment (usually 2 to 4) occurs, starting with the most apical nucleus. This is followed by formation of the same number of septa resulting mostly in uninucleate subapical compartments, leaving again the original number of nuclei (2 to 4) in the apical compartment (Fig. 3, bottom 2 panels). In some cases, the first subapical compartment with 1 to 3 nuclei was included in the mitotic wave. Interestingly, the same number of nuclei that entered the first mitotic cycle reenter the next cycle. Always the most apical nuclei of the apical compartment enter the next mitotic cycle, independent of their individual ancestry (see Movie S3 in the supplemental material).
FIG 3.
Spurts of mitotic waves occur in fast-growing hyphae. Shown are time-lapse sequences of mitotic waves in fast-growing hyphae. In fast-growing hyphae (164 ± 65 μm/h on minimal medium), nuclei migrate toward the hyphal tip. A mitotic wave follows, starting from the apical nucleus. This mitotic wave can include several compartments and up to six nuclei. The number of septa formed is the same as the number of mitoses, and the same number of apical nuclei will enter the next mitotic cycle. Numbers indicate nuclei that will undergo mitosis, and arrowheads indicate newly formed septa. Shown is F. oxysporum f. sp. lycopersici 4287 HH01::GFP stained with 1 μM calcofluor white. Scale bars, 10 μm.
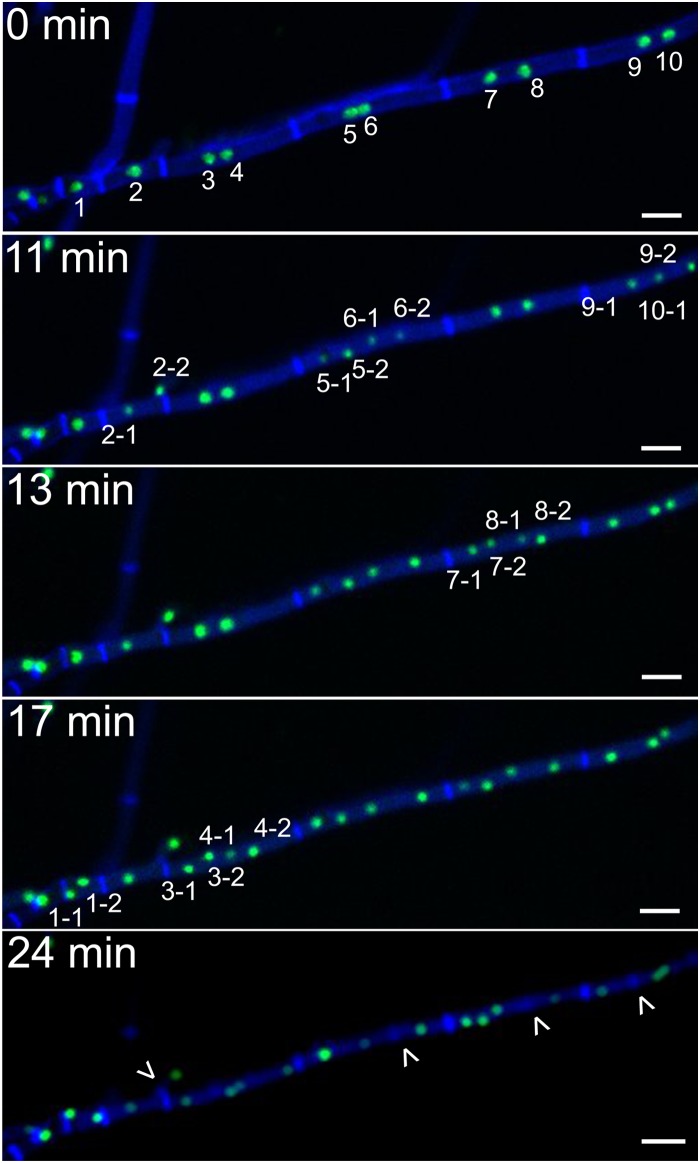
Intercalary compartments represented a further intriguing case. Dormant nuclei of older compartments (so-called “intercalary compartments”) were in some cases reactivated and underwent mitosis. However, this was not associated with hyphal growth in the form of elongation, extension, or branching, resulting in the high nuclear density described above. Intercalary mitosis included one or several compartments, each with one or more nuclei. All nuclei involved showed a mitotic wave that was asynchronous within the compartment as well as between different compartments (Fig. 4, top 4 panels). It was previously reported that mitosis is followed by septum formation between the two daughter nuclei (26). However, after intercalary mitoses, septum formation took place between some sister nuclei but not all, explaining the occurrence of higher and odd numbers of nuclei per compartment (Fig. 4, bottom panel; see Movie S4 in the supplemental material). Compartments with a very high nuclear density may result from a progressively lower frequency of septum formation as nuclei multiply within a compartment, which is in accordance with the limited number of cases that we have observed. Additionally, an intercalary mitotic wave can include some compartments while excluding their neighboring compartments (see Movies S3 and S4 in the supplemental material). Within the time frame of our studies, we did not observe the continuation of an intercalary mitotic wave to adjacent compartments. This indicates a strict regulation of the entry point of individual compartments into mitosis, which does not necessarily travel along the hypha.
FIG 4.
Intercalary compartments undergo asynchronous mitoses. Time-lapse sequences of asynchronous mitoses in intercalary compartments. Nuclei of intercalary compartments can be activated from their dormant state and undergo a mitotic wave, which can include several compartments and a number of nuclei, varying from 1 to 8 or more per compartment. The mitotic wave is asynchronous within and between compartments. Formation of septa does not always occur between daughter nuclei. Numbers indicate nuclei that will undergo mitosis, and arrowheads indicate newly formed septa. Shown is F. oxysporum f. sp. lycopersici 4287 HH01::GFP stained with 1 μM calcofluor white. Scale bars, 10 μm.
Emergence and characteristics of fast-growing hyphae during colony development depend on medium composition.
For a better understanding of the development from a uninucleate to a multinucleate state, we monitored nuclear dynamics and mitotic patterns for 48 h. For our microscope studies, we usually use minimal medium, since in this medium hyphal growth is less dense and there is less production of aerial hyphae compared to with PDA medium (see Fig. S5 in the supplemental material). Additionally, aromatic compounds in PDA medium show a strong autofluorescence in the GFP channel, leading to more background signal. However, to exclude malnutrition as a cause for the distinct nuclear behavior during the different stages of development, we tested nuclear dynamics of hyphae grown on PDA as well as on minimal medium (Table 1).
The different media did not affect germination of microconidia, the most frequently occurring conidia in F. oxysporum cultures, and after 10 h, most spores were germinated on both minimal medium and PDA. In general, the hyphal growth rate on PDA (30 ± 8 μm/h) was higher than on minimal medium (20 ± 8 μm/h). Fast-growing hyphae first emerged after completion of colony initiation—i.e., germination, conidial anastomosis tube (CAT) fusion, and maturation of germ tubes into vegetative hyphae (38). The medium composition influenced the timing of the appearance of fast-growing hyphae. On minimal medium, the first fast-growing hyphae were observed 6 h after germination, whereas on PDA the first fast-growing hyphae were seen 10 h after germination. The medium composition also influenced the growth rate of fast-growing hyphae: at 164 ± 65 μm/h, they grew faster on minimal medium than on PDA (119 ± 35 μm/h). Compared to the growth rate of regular hyphae on the respective media, fast-growing hyphae grew approximately 8 times faster on minimal medium and 4 times faster on PDA, indicating a role of medium composition and perhaps nutrient availability in the development of fast-growing hyphae. At this stage, namely, where fast-growing hyphae appeared on a regular basis on both minimal medium and PDA, multinucleate compartments were almost exclusively observed in the apical compartment of fast-growing hyphae. The occurrence of intercalary mitosis was not dependent on medium composition, and the first multinucleate intercalary compartments were observed 23 or 26 h after germination on minimal medium or PDA, respectively.
DISCUSSION
In this study, we showed that within the first 48 h of colony formation by Fusarium oxysporum, hyphal characteristics and nuclear dynamics change, and these changes can be grouped into three developmental stages. The first stage is colony initiation, including germination, CAT fusion, and maturation of germ tubes into vegetative hyphae, which at this stage contain exclusively uninucleate compartments. Depending on the medium composition, this stage can last 16 to 20 h after seeding of spores. The second stage is marked by the emergence of fast-growing hyphae that fan out with a 4 to 8 times higher growth rate, presumably to sample the surrounding environment for nutrient availability. At this stage, the first multinucleate compartments, the apical compartments of fast-growing hyphae, emerge. Fast-growing hyphae are present throughout further colony development, in line with a role of these specialized hyphae as scouts. Supporting this idea, we observed that on nutrient-limited medium, fast-growing hyphae are formed earlier and show a higher growth rate. In addition to the proposed function as scouts, fast-growing hyphae might also play a role in pathogenicity of F. oxysporum. The very thin hyphal tips of these specialized hyphae might facilitate the penetration of the host's root surface in the absence of appressoria, as was suggested by Ruiz-Roldán et al. (26, 39). The third stage is characterized by a dramatic change in nuclear dynamics in intercalary compartments of mature hyphae: dormant nuclei of intercalary compartments are reactivated and undergo mitoses. Not all of these mitoses are followed by septum formation, resulting in highly multinucleated compartments and hyphae with a high nuclear density.
Newly branched hyphae, which appear first during stage II, behave much like germlings. In the cases where we observed the transition from a uninucleate stage to a multinucleate stage was shorter, it was reached after a few mitoses rather than many hours after germination, as is the case for germlings (Table 1).
One question emerging from these observations is what the benefit could be, if any, of the transition from a uninucleate to a multinucleate state. The answer to this question may well be different for fast-growing hyphae versus intercalary compartments. Although in their final form (smaller diameter, long apical compartment, and mitotic wave), fast-growing hyphae were only found after completion of colony initiation, initial steps toward their formation appear already shortly after germination. As described by Ruiz-Roldán et al., the size of apical compartments increases after each mitosis, which could be a consequence of accelerating growth (26) (Fig. 1 and Table 1). To maintain a certain nuclear density in these fast-growing apical compartments, we propose that multiple nuclei have to undergo mitosis. A similar model has been suggested for other filamentous fungi with a high growth rate, like N. crassa, in which the nuclear population in the growing tip is supported by multiple mitoses and rapid migration of the newly formed nuclei through interconnected hyphae (13, 40).
The advantages of multinuclearity and multiple mitoses in intercalary compartments have been investigated in several fungi, for example in N. crassa and Fusarium moniliforme. These studies revealed that the same colony contained different nuclear populations (20, 41), demonstrating the potential importance of multinuclearity for fungal diversification and evolution. This is important for two reasons. (i) If only the apical nucleus is mitotically active, propagation of a spontaneous mutation would be limited to few points within a fungal colony, and the generation of diversity would mostly occur at the edges of the colony (13). (ii) In previous studies, the important role of horizontal gene and chromosome transfer in generating genomic diversity in filamentous fungi was demonstrated (22, 23, 42, 43). Horizontal gene and chromosome transfer requires at least a temporary tolerance for a multinucleate state in the mycelium. To have an effect on genetic diversity, new hyphae and/or spores should emerge from multinucleate compartments to produce offspring. We have not observed this in our study. As far as production of microconidia goes, in F. oxysporum this takes place in phialides in which nuclei of all spores originate from a single nucleus (26) (see Movie S5 in the supplemental material).
Storage of nitrogen and phosphorus could represent a further functional advantage for the transition to multinuclearity in compartments of older mycelium (20). Under starvation conditions, the filamentous fungus Aspergillus oryzae is capable of degrading nuclei from compartments of older mycelium through macroautophagy and utilizing the released nutrients to support colony survival and growth (44). Conversely, in F. oxysporum, compartments of older mycelium might reactivate dormant nuclei to undergo mitosis as a way to store nutrients in the form of DNA.
Another interesting question is how the transitions discussed above are regulated. A strict regulation must be in place to control entry into mitosis in some compartments and exclude the neighboring compartments. Aspergillus nidulans displays an elaborate system to regulate cell-to-cell connectivity during the cell cycle. Cytosolic continuity during interphase but not during mitosis is achieved by localization of the NIMA (i.e., “never in mitosis A”) kinase to septal pores from the time of septum formation throughout interphase. During mitosis, NIMA transiently locates to nuclei, where it plays an important role in entry into mitosis as well as in nuclear pore complex disassembly (45–47). A similar system could be in place in F. oxysporum, facilitating the inclusion of some compartments and exclusion of others from entry into the next mitotic cycle.
One of the challenges of a multinucleate fungal lifestyle is potential nuclear competition during reproduction and spore dispersal. We propose a model in which F. oxysporum essentially follows a multinucleate lifestyle. This multinucleate state would be repressed during sporulation to overcome nuclear competition and is again derepressed after colony initiation, when a multinucleate lifestyle is advantageous. It will be interesting to see what happens during fusion between older hyphae and whether the postfusion nuclear degradation described by Ruiz-Roldán (26) is indeed the result of a repression of tolerance of the multinucleate state.
It stands to reason that a similar system as described here for F. oxysporum is also in place for other fungi. For example, Magnaporthe grisea, which was described as uninucleate, sometimes also exhibits multinucleate compartments in older mycelium (48). A future challenge could be to determine the advantages of this lifestyle, perhaps by finding a way to suppress either the multinucleate state or the uninucleate state through specific mutations.
Supplementary Material
ACKNOWLEDGMENTS
This work was made possible by a Vici grant from the Netherlands Organization for Scientific Research (NWO) to M.R.
We thank U. Kück for plasmid pXPcFLPnatFRT, which served as the template for amplification of the Penicillium chrysogenum xylanase P promoter. Special thanks goes to Ronald Breedijk for technical support.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00200-14.
REFERENCES
- 1.Riquelme M. 2013. Tip growth in filamentous fungi: a road trip to the apex. Annu Rev Microbiol 67:587–609. doi: 10.1146/annurev-micro-092412-155652. [DOI] [PubMed] [Google Scholar]
- 2.Steinberg G. 2007. Hyphal growth: a tale of motors, lipids, and the Spitzenkörper. Eukaryot Cell 6:351–360. doi: 10.1128/EC.00381-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gull K. 1978. Form and function of septa in filamentous fungi, vol III Edward Arnold, London, United Kingdom. [Google Scholar]
- 4.Mouriño-Pérez RR. 2013. Septum development in filamentous ascomycetes. Br Mycol Soc 27:1–9. doi: 10.1016/j.fbr.2013.02.002. [DOI] [Google Scholar]
- 5.Mouriño-Pérez RR, Riquelme M. 2013. Recent advances in septum biogenesis in Neurospora crassa. Adv Genet 83:99–134. doi: 10.1016/B978-0-12-407675-4.00003-1. [DOI] [PubMed] [Google Scholar]
- 6.Glass NL, Rasmussen C, Roca MG, Read ND. 2004. Hyphal homing, fusion and mycelial interconnectedness. Trends Microbiol 12:135–141. doi: 10.1016/j.tim.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Harris SD. 2008. Branching of fungal hyphae: regulation, mechanisms and comparison with other branching systems. Mycologia 100:823–832. doi: 10.3852/08-177. [DOI] [PubMed] [Google Scholar]
- 8.Roper M, Simonin A, Hickey PC, Leeder A, Glass NL. 2013. Nuclear dynamics in a fungal chimera. Proc Natl Acad Sci U S A 110:12875–12880. doi: 10.1073/pnas.1220842110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann A, Philippsen P. 2009. Of bars and rings: Hof1-dependent cytokinesis in multiseptated hyphae of Ashbya gossypii. Mol Cell Biol 29:771–783. doi: 10.1128/MCB.01150-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clutterbuck AJ, Roper JA. 1966. A direct determination of nuclear distribution in heterokaryons of Aspergillus nidulans. Camb J 7:185–194. [Google Scholar]
- 11.Namboodiri AN, Lowry RJ. 1967. Vegetative nuclear division in Neurospora. Am J Bot 54:735–748. doi: 10.2307/2440951. [DOI] [Google Scholar]
- 12.Giovannetti M, Sbrana C, Logi C. 2000. Microchambers and video-enhanced light microscopy for monitoring cellular events in living hyphae of arbuscular mycorrhizal fungi. Plant Soil 226:153–159. doi: 10.1023/A:1026415419193. [DOI] [Google Scholar]
- 13.Roper M, Ellison C, Taylor JW, Glass NL. 2011. Nuclear and genome dynamics in multinucleate ascomycete fungi. Curr Biol 21:R786–R793. doi: 10.1016/j.cub.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aist JR. 1969. The mitotic apparatus in fungi, Ceratocystis fagacearum and Fusarium oxysporum. J Cell Biol 40:120–135. doi: 10.1083/jcb.40.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clutterbuck AJ. 1970. Synchronous nuclear division and septation in Aspergillus nidulans. J Gen Microbiol 60:133–135. doi: 10.1099/00221287-60-1-133. [DOI] [PubMed] [Google Scholar]
- 16.Minke PF, Lee IH, Plamann M. 1999. Microscopic analysis of Neurospora ropy mutants defective in nuclear distribution. Fungal Genet Biol 28:55–67. doi: 10.1006/fgbi.1999.1160. [DOI] [PubMed] [Google Scholar]
- 17.Gladfelter AS, Hungerbuehler AK, Philippsen P. 2006. Asynchronous nuclear division cycles in multinucleated cells. J Cell Biol 172:347–362. doi: 10.1083/jcb.200507003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gladfelter AS. 2006. Nuclear anarchy: asynchronous mitosis in multinucleated fungal hyphae. Curr Opin Microbiol 9:547–552. doi: 10.1016/j.mib.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa FH, Souza EA, Read ND, Roca MG. 2013. Colletotrichum lindemuthianum exhibits different patterns of nuclear division at different stages in its vegetative life cycle. Mycologia 105:795–801. doi: 10.3852/12-298. [DOI] [PubMed] [Google Scholar]
- 20.Maheshwari R. 2005. Nuclear behavior in fungal hyphae. FEMS Microbiol Lett 249:7–14. doi: 10.1016/j.femsle.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 21.Jinks JL. 1952. Heterokaryosis; a system of adaption in wild fungi. Proc R Soc Lond B Biol Sci 140:83–99. doi: 10.1098/rspb.1952.0046. [DOI] [PubMed] [Google Scholar]
- 22.Rep M, Kistler HC. 2010. The genomic organization of plant pathogenicity in Fusarium species. Curr Opin Plant Biol 13:420–426. doi: 10.1016/j.pbi.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Ma LJ, van der Does HC, Borkovich KA, Coleman JJ, Daboussi MJ, Di Pietro A, Dufresne M, Freitag M, Grabherr M, Henrissat B, Houterman PM, Kang S, Shim WB, Woloshuk C, Xie X, Xu JR, Antoniw J, Baker SE, Bluhm BH, Breakspear A, Brown DW, Butchko RA, Chapman S, Coulson R, Coutinho PM, Danchin EG, Diener A, Gale LR, Gardiner DM, Goff S, Hammond-Kosack KE, Hilburn K, Hua-Van A, Jonkers W, Kazan K, Kodira CD, Koehrsen M, Kumar L, Lee YH, Li L, Manners JM, Miranda-Saavedra D, Mukherjee M, Park G, Park J, Park SY, Proctor RH, Regev A, Ruiz-Roldan MC, Sain D, Sakthikumar S, Sykes S, Schwartz DC, Turgeon BG, Wapinski I, Yoder O, Young S, Zeng Q, Zhou S, Galagan J, Cuomo CA, Kistler HC, Rep M. 2010. Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464:367–373. doi: 10.1038/nature08850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koenig R, Howard FL. 1962. Nuclear division and septum formation in hyphal tips of Fusarium oxysporum. Am J Bot 49:666. [Google Scholar]
- 25.Aist JR, Morris NR. 1999. Mitosis in filamentous fungi: how we got where we are. Fungal Genet Biol 27:1–25. doi: 10.1006/fgbi.1999.1146. [DOI] [PubMed] [Google Scholar]
- 26.Ruiz-Roldán MC, Köhli M, Roncero MI, Philippsen P, Di Pietro A, Espeso EA. 2010. Nuclear dynamics during germination, conidiation, and hyphal fusion of Fusarium oxysporum. Eukaryot Cell 9:1216–1224. doi: 10.1128/EC.00040-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hood E, Gelvin S, Melchers S, Hoekema A. 1993. New Agrobacterium helper plasmids for gene transfer to plants (EHA105). Transgenic Res 2:208–218. doi: 10.1007/BF01977351. [DOI] [Google Scholar]
- 28.Sambrook J, Russel D. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 29.Mattanovich D, Rüker F, Machado AC, Laimer M, Regner F, Steinkellner H, Himmler G, Katinger H. 1989. Efficient transformation of Agrobacterium spp. by electroporation Nucleic Acids Res 17:6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hickey PC, Swift SR, Roca GM, Read ND. 2004. Live-cell imaging of filamentous fungi using vital fluorescent dyes and confocal microscopy. Methods Microbiol 34:63–87. doi: 10.1016/S0580-9517(04)34003-1. [DOI] [Google Scholar]
- 31.Houterman PM, Cornelissen BJ, Rep M. 2008. Suppression of plant resistance gene-based immunity by a fungal effector. PLoS Pathog 4:e1000061. doi: 10.1371/journal.ppat.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zadra I, Abt B, Parson W, Haas H. 2000. xylP promoter-based expression system and its use for antisense downregulation of the Penicillium chrysogenum nitrogen regulator NRE. Appl Environ Microbiol 66:4810–4816. doi: 10.1128/AEM.66.11.4810-4816.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopke K, Hoff B, Kück U. 2010. Application of the Saccharomyces cerevisiae FLP/FRT recombination system in filamentous fungi for marker recycling and construction of knockout strains devoid of heterologous genes. Appl Environ Microbiol 76:4664–4674. doi: 10.1128/AEM.00670-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Does HC, Duyvesteijn RG, Goltstein PM, van Schie CC, Manders EM, Cornelissen BJ, Rep M. 2008. Expression of effector gene SIX1 of Fusarium oxysporum requires living plant cells. Fungal Genet Biol 45:1257–1264. doi: 10.1016/j.fgb.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa T, Suzuki T, Murata S, Nakamura S, Hino T, Maeo K, Tabata R, Kawai T, Tanaka K, Niwa Y, Watanabe Y, Nakamura K, Kimura T, Ishiguro S. 2007. Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci Biotechnol Biochem 71:2095–2100. doi: 10.1271/bbb.70216. [DOI] [PubMed] [Google Scholar]
- 36.Mullins ED, Chen X, Romaine P, Raina R, Geiser DM, Kang S. 2001. Agrobacterium-mediated transformation of Fusarium oxysporum: an efficient tool for insertional mutagenesis and gene transfer. Phytopathology 91:173–180. doi: 10.1094/PHYTO.2001.91.2.173. [DOI] [PubMed] [Google Scholar]
- 37.Takken FL, Van Wijk R, Michielse CB, Houterman PM, Ram AF, Cornelissen BJ. 2004. A one-step method to convert vectors into binary vectors suited for Agrobacterium-mediated transformation. Curr Genet 45:242–248. doi: 10.1007/s00294-003-0481-5. [DOI] [PubMed] [Google Scholar]
- 38.Roca MG, Kuo HC, Lichius A, Freitag M, Read ND. 2010. Nuclear dynamics, mitosis, and the cytoskeleton during the early stages of colony initiation in Neurospora crassa. Eukaryot Cell 9:1171–1183. doi: 10.1128/EC.00329-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivain C, C A. 1999. Process of tomato root colonization by a pathogenic strain of Fusarium oxysporum f. sp. lycopersici in comparison with a non-pathogenic strain. New Pathologist 141:497–510. doi: 10.1046/j.1469-8137.1999.00365.x. [DOI] [Google Scholar]
- 40.Kasuga T, Glass NL. 2008. Dissecting colony development of Neurospora crassa using mRNA profiling and comparative genomics approaches. Eukaryot Cell 7:1549–1564. doi: 10.1128/EC.00195-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sidhu GS. 1983. Genetics of Gibberella fujikuroi. III. Significance of heterokaryosis in naturally occurring corn isolates. Can J Bot 61:3320–3325. [Google Scholar]
- 42.Miao VP, Covert SF, VanEtten HD. 1991. A fungal gene for antibiotic resistance on a dispensable (“B”) chromosome. Science 254:1773–1776. doi: 10.1126/science.1763326. [DOI] [PubMed] [Google Scholar]
- 43.Friesen TL, Stukenbrock EH, Liu Z, Meinhardt S, Ling H, Faris JD, Rasmussen JB, Solomon PS, McDonald BA, Oliver RP. 2006. Emergence of a new disease as a result of interspecific virulence gene transfer. Nat Genet 38:953–956. doi: 10.1038/ng1839. [DOI] [PubMed] [Google Scholar]
- 44.Shoji JY, Kikuma T, Arioka M, Kitamoto K. 2010. Macroautophagy-mediated degradation of whole nuclei in the filamentous fungus Aspergillus oryzae. PLoS One 5:e15650. doi: 10.1371/journal.pone.0015650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Souza CP, Osmani AH, Hashmi SB, Osmani SA. 2004. Partial nuclear pore complex disassembly during closed mitosis in Aspergillus nidulans. Curr Biol 14:1973–1984. doi: 10.1016/j.cub.2004.10.050. [DOI] [PubMed] [Google Scholar]
- 46.De Souza CP, Osmani SA. 2007. Mitosis, not just open or closed. Eukaryot Cell 6:1521–1527. doi: 10.1128/EC.00178-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen KF, Osmani AH, Govindaraghavan M, Osmani SA. 2014. Mitotic regulation of fungal cell-to-cell connectivity through septal pores involves the NIMA kinase. Mol Biol Cell 25:763–775. doi: 10.1091/mbc.E13-12-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaegashi H, Hebert TT. 1976. Perithecial development and nuclear behavior in Pyricularia. Phytopathology 66:122–126. doi: 10.1094/Phyto-66-122. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.