Abstract
Symbiotic association of epichloae endophytes (Epichloë/Neotyphodium species) with cool-season grasses of the subfamily Pooideae confers bioprotective benefits to the host plants against abiotic and biotic stresses. While the production of fungal bioprotective metabolites is a well-studied mechanism of host protection from insect herbivory, little is known about the antibiosis mechanism against grass pathogens by the mutualistic endophyte. In this study, an Epichloë festucae mutant defective in antimicrobial substance production was isolated by a mutagenesis approach. In an isolated mutant that had lost antifungal activity, the exogenous DNA fragment was integrated into the promoter region of the vibA gene, encoding a homologue of the transcription factor VIB-1. VIB-1 in Neurospora crassa is a regulator of genes essential in vegetative incompatibility and promotion of cell death. Here we show that deletion of the vibA gene severely affected the antifungal activity of the mutant against the test pathogen Drechslera erythrospila. Further analyses showed that overexpressing vibA enhanced the antifungal activity of the wild-type isolate against test pathogens. Transformants overexpressing vibA showed an inhibitory activity on test pathogens that the wild-type isolate could not. Moreover, overexpressing vibA in a nonantifungal E. festucae wild-type Fl1 isolate enabled the transformant to inhibit the mycelial and spore germination of D. erythrospila. These results demonstrate that enhanced expression of vibA is sufficient for a nonantifungal isolate to obtain antifungal activity, implicating the critical role of VibA in antifungal compound production by epichloae endophytes.
INTRODUCTION
Epichloae endophytes (holomorphic Epichloë and anamorphic Neotyphodium) are clavicipitaceous fungi that maintain a systemic and constitutive symbiotic relationship with a broad spectrum of cool-season grasses of the subfamily Pooideae (1). In the mutualistic relationship between epichloae endophytes and cool-season grasses, the reported benefits include protection of the host plant from insect and vertebrate herbivores (2–5), resistance to diseases (6–9), and an increase in tolerance to abiotic stresses, such as drought (10, 11). The protective ability of some epichloae species makes them suitable agents of biological plant protection against economically important grass diseases and insect and small animal herbivores (12). This encouraged breeders to develop and eventually led to the release of “endophyte-enhanced” turfgrass and perennial ryegrass cultivars (13).
The production and release of anti-insect metabolites are among the known mechanisms for how epichloae endophytes protect their hosts from insect herbivory. Epichloae endophytes in association with their grass hosts are noted to synthesize bioprotective alkaloids, such as peramine and lolines, and another class of compound, the janthitrems, which increase resistance of the plant hosts to insect feeding (2, 14–16). Moreover, the genetic basis of the biosynthesis of endophyte-derived anti-insect metabolites was recently elucidated (5).
Several studies have demonstrated that some strains of Epichloë/Neotyphodium species inhibit the growth of several grass pathogens in culture medium (17, 18). Yue et al. (19) identified indole-3-acetic acid, a sesquiterpene, and indole-3-ethanol, a diacetamide, from cultures of epichloae isolates as antifungal metabolites against grass pathogens. Seto et al. (20) reported that the cyclic peptide epichlicin, produced by Epichloë typhina, inhibits the spore germination of Clasdosporium phlei. Thus far, however, the association of disease resistance with these chemical components has not been established in planta.
Similar to the insect-deterrent activity of endophytes, which varies between the insect species and the endophyte strains producing different anti-insect metabolites (16, 21), differences in the magnitude and range of microbial inhibitory activities were also reported among individual strains of a single species of epichloae endophyte and the infecting pathogen species (17, 22–24). This observation suggests that some epichloae strains have the potential to produce greater quantities of one or more antifungal compounds and that the inhibitory role of endophyte-derived metabolites depends on the endophyte isolate-grass pathogen species combination.
In contrast to the recent advances in the genetic basis of biosynthesis of endophyte-derived anti-insect metabolites, so far there has been no report on the genes involved in the biosynthesis and regulation of epichloae endophyte-derived antimicrobial substances. Unlocking the genetic basis of the differences in the production of antibiotic substances among epichloae strains will provide a deeper understanding of the role of endophytes in plant protection and their promise for further practical use in biological control.
Previously, the antifungal activity of a geographically diverse collection of Epichloë festucae isolates was assessed against several grass pathogens. We identified isolate E437, which showed antifungal activity against several grass pathogens, including Drechslera erythrospila, Drechslera siccans, Drechslera dictyoides, Colletotrichum graminicola, and Bipolaris sorokiniana (9). Perennial ryegrass infected with isolate E437 exhibited reduced disease symptoms caused by D. erythrospila (9), suggesting that the antifungal compound produced by E. festucae isolate E437 could be involved in the protection of the host plant.
The objective of this study was to identify genes involved in the production of the antifungal compound in E. festucae isolate E437. We employed plasmid insertion mutagenesis to isolate mutants that lost antifungal activity against the leaf spot pathogen D. erythrospila. Functional analysis of the disrupted gene in an isolated mutant was performed, and the potential involvement of the identified gene, vibA, encoding a homologue of a transcription factor for fungal heterokaryon incompatibility, in expression of antifungal activity in symbiotic fungi is discussed.
MATERIALS AND METHODS
Fungal strains and growth conditions.
Epichloë festucae strains E437 and Fl1 were kindly provided by Christopher L. Schardl (University of Kentucky) and Barry Scott (Massey University, New Zealand). The cultures of E. festucae (see Table S1 in the supplemental material) were grown on 2.4% potato dextrose agar (PDA) and maintained at 23°C or kept at 4°C until use. The test fungal grass pathogens, namely, Drechslera erythrospila (isolate 638; MAFF no. 305378), D. siccans (isolate 962; MAFF no. 305397), D. dictyoides (isolate 963; MAFF no. 305398), Colletotrichum graminicola (isolate PR-1; MAFF no. 306600), Sclerotinia homoeocarpa (isolate SU16-3; MAFF no. 236941), and Rhizoctonia solani (isolate 1374; MAFF no. 511374), were acquired from the collection of the National Institute of Agrobiological Sciences (NIAS), Japan. A Magnaporthe grisea isolate (WK3-1) was kindly provided from the collection of Yukio Tosa (Kobe University, Japan). All test fungal grass pathogens were grown on PDA at 23°C and maintained at 4°C until use.
Nucleic acid isolation, Southern blot hybridization, PCR, and quantitative reverse transcription-PCR (qRT-PCR) analysis.
Fungal genomic DNA was isolated from freeze-dried mycelium by a previously described method (25) or by using an Extract-N-Amp plant PCR kit (Sigma) according to the manufacturer's instructions. Genomic digests were transferred to positively charged nylon membranes (Hybond-N+; GE Healthcare, United Kingdom) by capillary transfer and fixed by UV cross-linking in a UV cross-linker (CL-1000; Ultra-Violet Products Ltd., United Kingdom). The filter was probed with [α-32P]dCTP-labeled probes (3,000 Ci/mmol) (MP Biomedicals). Probe labeling, hybridization, and detection conditions were described previously (26). Standard PCR amplifications of genomic or plasmid DNA templates were performed with PrimeStar HS DNA polymerase (TaKaRa, Japan). Sequences of PCR primers used in this study are provided in Table S2 in the supplemental material.
Total RNA was isolated from frozen mycelium by use of TRIzol reagent (Invitrogen) and reverse transcribed with ReverTra Ace (Toyobo, Japan). Quantitative RT-PCR was performed in a LightCycler Quick System 350S instrument (Roche Applied Science, Germany), using Thunderbird SYBR qPCR mix (Toyobo, Japan) with gene-specific primers (see Table S2 in the supplemental material). The thermal cycler conditions used were described previously (27).
Preparation of deletion, complementation, and overexpression constructs.
The base vector for deletion constructs, pNPP150, was prepared as follows. The 1.1-kb SpeI/NotI fragment of the herpes simplex virus thymidine kinase (HSVtk) gene was amplified from the pGKO2 vector (28) by use of primers Spe-HSVtk-F and HSVtk-Nt-R and then cloned into SpeI/NotI sites of pPN94 (29) to generate pPN94-HSVtk. The 2.5-kb TEF promoter-HSVtk-trpC terminator cassette was amplified with primers 94-pTEF-F and TrpC-94-R and cloned into the HpaI site of pSF15.15 (29) to generate pNPP150. The E. festucae vibA deletion construct pNPP151 was prepared as follows. A 1.0-kb fragment 5′ of vibA was amplified from E437 genomic DNA by use of primers IF830KO5-F2 and IF830KO5-R2. A 1.2-kb fragment 3′ of vibA was amplified from the genomic DNA of E. festucae strain E437 by use of primers IF830KO3-F and IF830KO3-R. A 1.5-kb trpC promoter-hph cassette was amplified from pNPP150 by use of primers hph-F and hph-R. These three PCR products were then cloned into a linearized pNPP150 vector (amplified with primers 94-pTEF-F and pNPP150-R) by use of an In-Fusion HD cloning kit (Clontech). To reintroduce the E. festucae vibA gene into the ΔvibA deletion strain, complementation construct pNPP152 was prepared as follows. The 5.0-kb E. festucae vibA gene, including the 2-kb upstream and 1.1-kb downstream regions of its vibA coding sequence (see Fig. 2A), was amplified from E437 genomic DNA by use of primers EI-830comp-F1 and 830comp-EV-R1, digested with XbaI and EcoRV, and subsequently cloned into the XbaI/EcoRV sites of pBlueScript II KS(+) (Stratagene). The vibA overexpression construct pNPP154 was prepared by ligating an XbaI/EcoRI digest of a 1.8-kb vibA fragment, amplified from cDNA of E. festucae strain E437 by use of primers OEx-vib1-XbaI-F1 and OEx-vib1-EcoRI-R1, into the XbaI/EcoRI sites of pPN94. To express green fluorescent protein (GFP) under the control of the vibA promoter, a BamHI/NotI 0.7-kb enhanced GFP (EGFP) fragment was prepared by digesting a PCR product that was amplified with primers B-EGFP-F and EGFP-NI-R from pNPP1 (30). An EcoRI/BamHI 2.0-kb vibA promoter region was prepared by digesting a PCR product amplified with primers 830-promoter-EcoRI-F1 and 830-promoter-BamHI-R1. Both DNA fragments were cloned into the EcoRI/NotI sites of pNPP1 to generate pNPP153. The base vector for the expression of GFP fusion proteins, pNPP140, was prepared as follows. A BamHI/NotI 0.7-kb 3GA-EGFP fragment, amplified with primers BI-3GA and EGFP-NI-R from pNPP9 (31), was cloned into the BamHI/NotI sites of pNPP94. Three copies of glycine-alanine (GA) at the N terminus of EGFP acted as a spacer between GFP and the test protein. To express vibA tagged with the GFP gene under the control of the TEF promoter, the 1.8-kb vibA fragment was amplified by use of primers IF94GFP-Vib1-F and IF94GFP-Vib1-R. The purified PCR product was ligated into the BamHI site of pNPP140 by use of an In-Fusion HD cloning kit to generate pNPP155. Constructs used in this study are listed in Table S3 in the supplemental material.
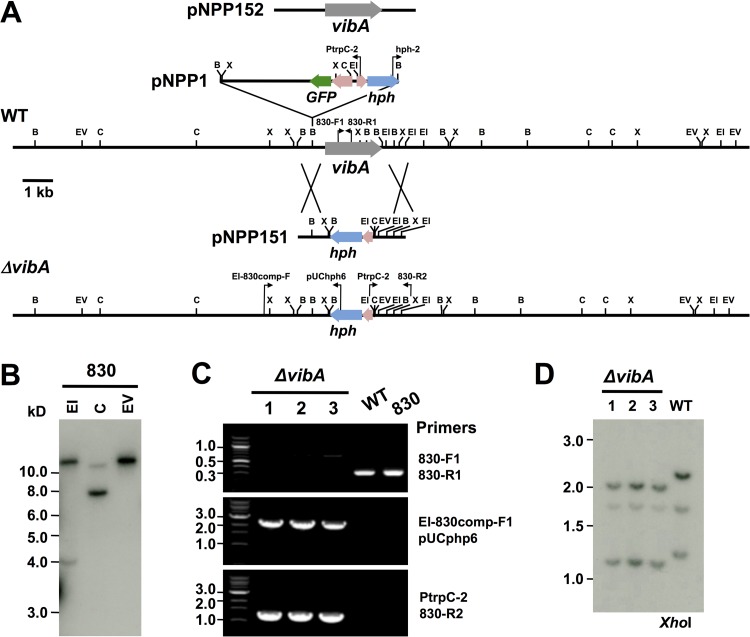
FIG 2.
Deletion of E. festucae vibA. (A) Physical maps of the E. festucae wild-type vibA genomic region, the integration site of pNPP1 in mutant 830, the linear insert of the replacement construct pNPP151, the E. festucae vibA mutant genomic region, and the insert of the complementation construct pNPP152, showing restriction enzyme sites for BamHI (B), EcoRI (EI), EcoRV (EV), ClaI (C), and XhoI (X). (B) Autoradiograph of a DNA gel blot of EcoRI (EI), ClaI (C), and EcoRV (EV) genomic digests of mutant 830 of E. festucae E437, probed with 32P-labeled pNPP1. Sizes (in kilobases) of markers are indicated at left. (C) PCR-based verification of vibA mutants. Primer pairs used are indicated to the right. The locations of primers are indicated in panel A. (D) Autoradiograph of a Southern blot of XhoI genomic digests of E. festucae wild-type E437 (WT) and vibA deletion strains (ΔvibA), probed with 32P-labeled pNPP151. Sizes (in kilobases) of markers are indicated at left.
E. festucae transformation and molecular analysis of transformants.
Protoplasts of E. festucae were prepared according to a previously described method (32). Protoplasts were transformed with 5 μg of either a circular (pSF17, pNPP152, pNPP153, pNPP154, and pNPP155) or linear (pNPP151, amplified with primers pII99-2 and pII99-3) PCR-amplified product, as described previously (33). For restriction enzyme-mediated integration (REMI) mutagenesis, 5 μg of BamHI-linearized pNPP1, 12 units of BamHI, and 20 μl of K buffer for restriction enzymes were added to 100 μl of protoplast mixture. Transformants were selected on YPS-PDA medium containing hygromycin (150 μg/ml) or Geneticin (400 μg/ml). For the selection of vibA knockout transformants, 1 μM 5-fluoro-2-deoxyuridine (F2dU) was added to PDA, and the HSVtk gene was used as a negative-selection marker against ectopic transformants (28). The putative transformants for E. festucae vibA gene replacement were confirmed by amplifying the vibA gene by using internal vibA gene primers 830-F1 and 830-R1 (see Table S2 in the supplemental material).
DNA sequencing and bioinformatics.
DNA fragments were sequenced by the dideoxynucleotide chain termination method, using BigDye ver. 3 chemistry (Applied Biosystems). Products were separated on an ABI 3130 analyzer (Applied Biosystems). Sequence data were analyzed and annotated in the MacVector program (ver. 11; MacVector Inc.). The sequence of the vibA locus was obtained from E. festucae E2368 genome sequences kindly provided by Christopher L. Schardl (University of Kentucky) (http://csbio-l.csr.uky.edu/endophyte/). Deduced protein sequences of fungal VibA (VIB-1) were collected from the fungal genome resources of the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov) and the Broad Institute (http://www.broad.mit.edu/annotation/fungi/fgi/). Protein sequences were aligned using ClustalW ver. 2.1 (34) with default settings.
Microscopy.
Confocal fluorescence images were captured using a confocal laser scanning microscope (FV1000-D; Olympus, Japan) with a 405-nm (DAPI [4′,6-diamidino-2-phenylindole]) or 488-nm excitation source. Fluorescence images were recorded between 425 and 475 nm (DAPI) or between 495 and 520 nm (GFP).
Mycelial and conidial germination inhibitory assay.
A dual-culture assay using mycelial blocks was performed to determine the antifungal activity of E. festucae transformants against the grass pathogens. Mycelial blocks of E. festucae strains were placed on quarter positions of a PDA plate and allowed to grow for 7 days before a mycelial plug of grass pathogen (3-mm diameter) was placed in the center of the PDA plate. The culture was incubated at 23°C until either a clear zone of inhibition was observed or the colonies of the two fungi made contact. Inhibition was determined by measuring the clear zone between the E. festucae and grass pathogen colonies. The conidial germination inhibitory assay was performed as follows. Mycelia of E. festucae transformants were inoculated into PD broth and cultured in an orbital shaker (100 rpm) at 23°C. After 7 days of incubation, the culture filtrate was harvested and then sterilized using a filter membrane unit (Millex-HA filter unit with a 45-μm pore diameter; Millipore). Twenty microliters of endophyte culture filtrate was added to a sterile biconcave microscope slide and mixed with 5 μl of conidial suspension of D. erythrospila (1 × 104 spores/ml). In the control test, the conidial suspension was mixed with sterile PD broth. All treatments were replicated at least three times. The slides were placed in petri dishes and kept at 25°C. After 12 h, the germination rate of conidia was scored from at least 150 conidia. The replicated experiment was repeated at least three times.
Detection of extracellular protease activity of epichloae isolates.
Extracellular protease activity of the epichloae isolates was detected as follows (35). A 3-mm mycelial plug from each isolate was separately placed in the center of a PDA plate supplemented with 1% (wt/vol) gelatin (Becton, Dickinson) and incubated at 23°C. Extracellular protease activity was detected as a visible halo around the colonies of endophyte isolates after staining with 0.1% amido black in methanol-acetic acid-water (30:10:60 [vol/vol/vol]) for 30 min (see Fig. S5 in the supplemental material).
Inoculation of perennial ryegrass with D. erythrospila.
Inoculation of perennial ryegrass with D. erythrospila was performed as previously described (9). Leaves of perennial ryegrass were stained with lactophenol trypan blue 7 days after inoculation, using a previously reported method (36).
RESULTS
Isolation of an E. festucae mutant that had lost antifungal activity against temperate grass pathogens.
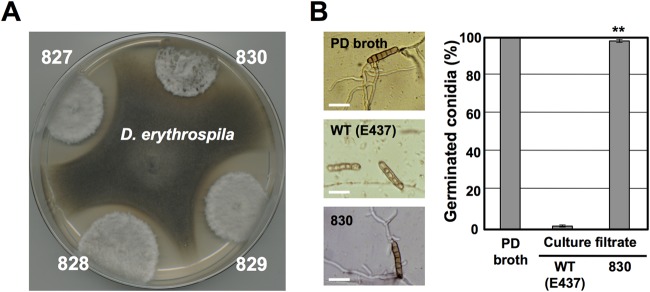
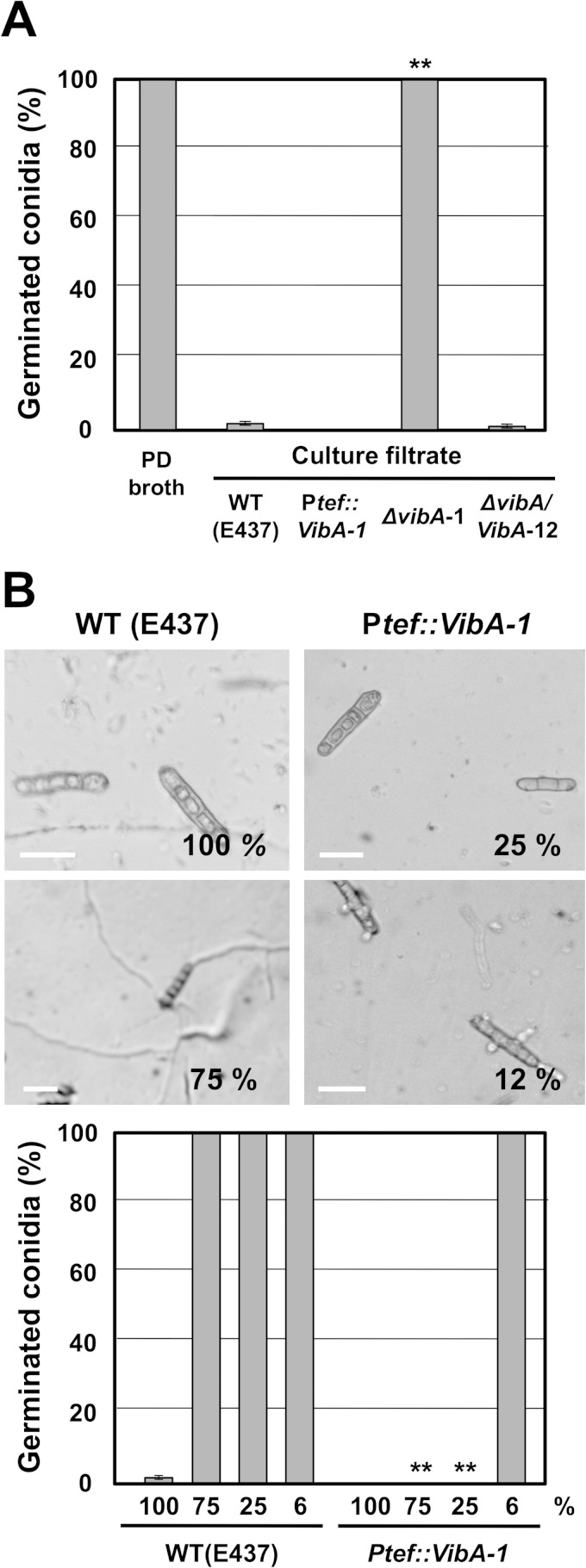
Previously, we identified E. festucae isolate E437 as an isolate exhibiting antifungal activity against grass pathogens, including Drechslera erythrospila, D. siccans, D. dictyoides, Colletotrichum graminicola, and Bipolaris sorokiniana (9). To isolate E437 mutants with reduced antifungal activity, we employed plasmid insertion mutagenesis (restriction enzyme-mediated integration [REMI]) (37). Protoplasts of E. festucae E437 were transformed with the pNPP1 plasmid linearized with BamHI. From in vitro dual-culture assays of 1,200 independent plasmid insertion mutants against D. erythrospila, we isolated one mutant, designated mutant 830, which had lost the ability to inhibit the mycelial growth of the pathogen (Fig. 1A). Mutant 830 also could not inhibit the colony growth of D. siccans, D. dictyoides, C. graminicola, and B. sorokiniana (see Fig. S1 in the supplemental material). We previously showed that a culture filtrate of wild-type E437, but not those of other nonantifungal E. festucae isolates, had inhibitory activity toward conidial germination of D. erythrospila (9). The culture filtrate of mutant 830 had no inhibitory effect on the germination of pathogen conidia (Fig. 1B).
FIG 1.
Isolation of mutant 830, which had lost inhibitory activity toward mycelial growth of grass pathogens. (A) Loss of inhibitory activity of mutant 830 toward mycelial growth of D. erythrospila. Colonies of REMI transformants were grown to a diameter of 10 to 12 mm and then inoculated with a mycelial plug of D. erythrospila. The culture was incubated at 23°C until either a clear zone of inhibition was observed or the colonies of the two fungi had made contact. (B) Percentages and morphologies of germinated conidia of D. erythrospila 12 h after incubation in culture filtrate of E. festucae wild-type (WT) E437 or mutant 830. Potato dextrose (PD) broth served as a control. The frequency of germinated conidia was calculated from at least 150 spores. Data are means and standard errors from three biological replicates (>50 spores/experiment). Data marked with asterisks are significantly different from the control (WT E437) as assessed by two-tailed Student's t test: **, P < 0.01. Bars = 30 μm.
Mutant 830 contains a plasmid insertion in the promoter region of the vibA gene.
Genomic DNA of mutant 830 was digested with the restriction enzyme EcoRV that was absent from the transformation vector pNPP1 because the vector lacked the relevant restriction site, and Southern blot analysis was performed with pNPP1 as the probe. Only one hybridizing band was observed, indicating the presence of a single vector integration site in the genome of this mutant (Fig. 2A and B). To identify the site of pNPP1 insertion, the genomic DNA of mutant 830 was digested with EcoRI or ClaI. Two hybridized bands were detected in either EcoRI-digested (over 12 kb and approximately 4 kb) or ClaI-digested (approximately 11 kb and 8 kb) genomic DNA of mutant 830 (Fig. 2B). The weakly hybridizing 4-kb EcoRI fragment, which was expected to have a hygromycin resistance (hph) gene cassette (Fig. 2A), was isolated from the gel and self-ligated. The chromosomal DNA flanking the hph cassette was PCR amplified using the primers hph2 and Ptrpc-2 and then sequenced. Analysis of this sequence and the genome sequence of E. festucae isolate E2638 showed that pNPP1 was integrated at the BamHI site of an expected promoter region of a gene encoding a transcription factor homologous to VIB-1 of Neurospora crassa (38). Single insertion of the pNPP1 vector at this BamHI site was further confirmed by a series of PCR analyses with various primer combinations (see Fig. S3 in the supplemental material). The deduced protein encoded by this gene shared 47% sequence identity with VIB-1 (accession no. Q9C2N1) of N. crassa, which regulates expression of genes involved in nonself recognition, since it was shown to differentially regulate het-6 alleles in N. crassa (Fig. 3) (38–40). The vib-1 homologue in E. festucae is referred to here as vibA. E. festucae VibA is a putative transcription factor containing an NDT80/PhoG DNA binding domain (Fig. 3A). VibA-GFP expressed under the control of the TEF promoter showed obvious nuclear localization (Fig. 3B), further supporting the hypothesis that VibA acts as a regulator of gene expression. VibA gene homologues can be found as single-copy genes in the genomes of Ascomycota fungi, except for some species of hemiascomycetous yeasts, but obvious homologues are absent from the Basidiomycota and Zygomycota (Fig. 3A; see Fig. S2 in the supplemental material).
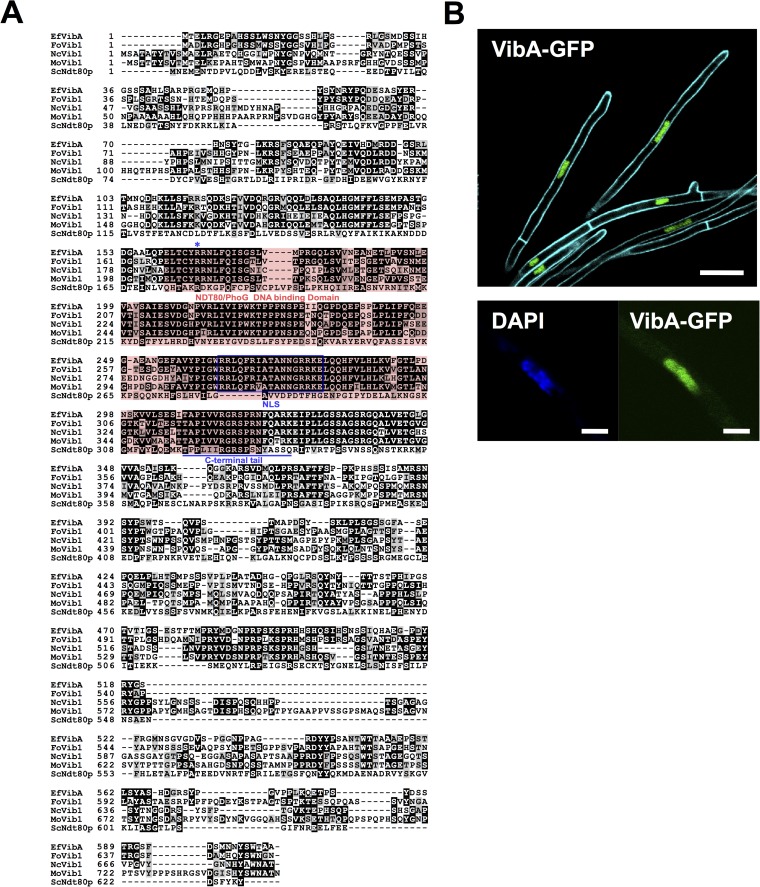
FIG 3.
(A) The deduced protein sequence of E. festucae VibA (EfVibA) was aligned with those of Fusarium oxysporum Vib1 (FoVib1; accession no. FOXB_17342), Magnaporthe oryzae Vib1 (MoVib1; accession no. MGG_00729), Neurospora crassa Vib1 (NcVib1; accession no. NCU03725.5), and Saccharomyces cerevisiae Ndt80p by ClustalW ver. 2.1 (34) with default settings. The NDT80/PhoG DNA binding domain is indicated with a red box. A predicted bipartite nuclear localization signal (NLS) is indicated in a blue box. The C-terminal tail of Ndt80p predicted to make contact with the DNA major groove (54) is underlined, and the arginine residue required for the function of Ndt80p in Candida albicans (55) is indicated by an asterisk. (B) Localization of VibA-GFP in hyphae of E. festucae. VibA-GFP was expressed under the control of a TEF promoter in E. festucae E437 and then monitored by confocal laser scanning microscopy. (Bottom) Hypha of E. festucae expressing VibA-GFP and stained with DAPI. Bars = 10 μm (upper panel) and 2 μm (lower panel).
Deletion of E. festucae vibA causes a loss of antifungal activity.
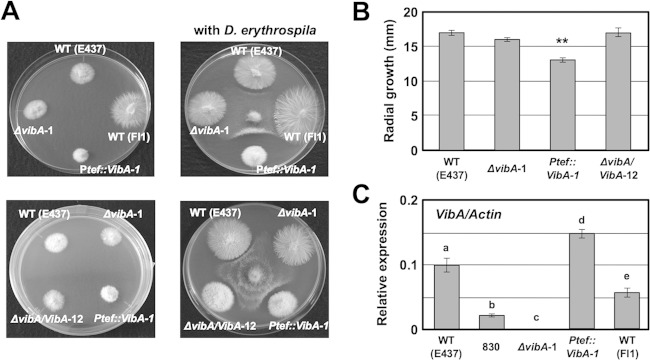
To confirm that the plasmid insertion in the promoter region of vibA was responsible for the reduced antifungal activity of mutant 830, a vibA deletion mutant was generated by replacing the vibA gene with the hph gene via homologous recombination (Fig. 2A). Transformants obtained were subjected to in vitro dual-culture assay against D. erythrospila, from which candidate vibA mutants that failed to inhibit the mycelial colony growth of the pathogen were further selected. Ten of 15 transformants tested had lost the ability to inhibit the colony growth of the pathogen, a phenotype similar to that of mutant 830. Three of the putative vibA deletion mutants were then confirmed by PCR and Southern hybridization to verify the integrity of the target gene replacement events (Fig. 2C and D). The 340-bp band amplified with the internal vibA gene primers (830-F1 and 830-R1) was detected in wild-type E437 and mutant 830, but not in vibA deletion mutants, while 2.5-kb and 1.4-kb bands, amplified with primers targeting the flanking region and the deletion cassette (EI-830comp-F1 plus pUCphp6a and PtrpC-2 plus 830-R2), were detected only in vibA mutants (Fig. 2A and C). Moreover, DNA gel blot analysis of XhoI genomic digests of the transformants and wild-type E437 probed with the deletion construct pNPP151 confirmed that these transformants contained a replacement at the vibA locus, without any extra integration of the vector (Fig. 2D). Both E. festucae E437 and the vibA mutant have three XhoI restriction sites, located within the vibA coding sequence and its regulatory regions, but they differ in the locations of XhoI recognition sites. This explains the difference in generated band sizes in DNA gel blots (Fig. 2A and D). Deletion of vibA did not have any significant effect on mycelial growth (Fig. 4A and B; see Fig. S4 in the supplemental material), but like mutant 830, the vibA mutants could not inhibit the mycelial growth of D. erythrospila (Fig. 4A). Likewise, vibA deletion mutants also could not inhibit the mycelial growth of D. siccans, D. dictyoides, C. graminicola, and B. sorokiniana (data not shown). Moreover, the culture filtrates of vibA mutants could not inhibit the conidial germination of D. erythrospila (Fig. 5A).
FIG 4.
Effects of vibA gene deletion and overexpression on colony morphology, radial growth, and antimicrobial activity against D. erythrospila. (A) Colony morphologies of E. festucae wild-type strain E437 [WT (E437)], wild-type strain Fl1 [WT (Fl1)], the vibA deletion mutant (ΔvibA-1), a vibA-complemented transformant (ΔvibA/VibA-12), and a vibA-overexpressing strain (Ptef::VibA-1) on PDA before (left) and after (right) pathogen inoculation. The E. festucae strains were allowed to grow on PD agar for 7 days before a mycelial block of the pathogen was placed on the culture plate. (B) Radial growth of E. festucae strains 7 days after inoculation onto PDA. Error bars indicate the standard deviations. Data marked with asterisks are significantly different from the control (WT E437) as assessed by two-tailed Student's t test (**, P < 0.01). (C) Relative expression of vibA in E. festucae strains in axenic culture. Total RNAs were isolated from mycelia of E. festucae strains grown in PD broth for 7 days, and relative expression levels of vibA in endophyte strains were normalized against the actin gene. Different letters indicate significant differences as assessed by two-tailed Student's t test (P < 0.05).
FIG 5.
Inhibitory activities of culture filtrates of E. festucae wild-type E437 [WT (E437)], a vibA-overexpressing strain (Ptef::VibA-1), a vibA deletion mutant (ΔvibA-1), and a vibA-complemented transformant (ΔvibA/VibA-12) on conidial germination of D. erythrospila. (A) Percentages of germinated conidia after 12 h of incubation in PD broth or culture filtrate of E. festucae strains. The frequency of germinated conidia was calculated from at least 150 spores. Data are means ± standard errors from 3 biological replicates (>50 spores/experiment). Data marked with asterisks are significantly different from the control (WT E437) as assessed by two-tailed Student's t test (**, P < 0.01). (B) Comparison of inhibitory activities of culture filtrates of E. festucae wild-type E437 and the vibA-overexpressing transformant on conidial germination of D. erythrospila. Morphologies of conidia are shown for D. erythrospila after 12 h of incubation in 100% or diluted culture filtrate of WT E437 or the vibA-overexpressing transformant. Values in the image panels refer to concentrations of endophyte culture filtrate. Percentages of germinated conidia after 12 h of incubation in different concentrations of culture filtrates of E. festucae strains are shown. The frequency of germinated conidia was calculated from at least 150 spores. Data are means ± standard errors from three biological replicates (>50 spores/experiment). Data marked with asterisks are significantly different from the control (WT E437) as assessed by two-tailed Student's t test (**, P < 0.01).
To further confirm that E. festucae vibA is essential for the antifungal activity of isolate E437, plasmid pNPP152 (Fig. 2A), containing the full-length vibA gene along with its regulatory regions, was transformed into protoplasts of the ΔvibA knockout mutant. The candidate complement transformants were then subjected to dual-culture assay against D. erythrospila. Geneticin-resistant complement transformants inhibited the mycelial colony growth and conidial germination of D. erythrospila (Fig. 4A and 5A).
A previous report indicated that N. crassa VIB-1 is required for the production of downstream effectors associated with heterokaryon incompatibility, including the production of extracellular proteases (39). To investigate the role of E. festucae VibA in the production of proteases, the ability of the E. festucae strains to produce extracellular proteases was detected on PDA medium, with gelatin as the substrate. Extracellular protease activity was detected as a visible halo around each colony of endophyte isolates (35). Within 10 days after inoculation, a visible halo (cleared area) around the colony was obvious for wild-type E437, while no halo was detected around the colony of the vibA mutant. Recovery of the protease activity was observed for the complement transformant. These results indicated that E. festucae VibA is involved in the production of extracellular proteases (see Fig. S5 in the supplemental material).
Expression analysis of the vibA gene in mutant 830 and the nonantifungal wild-type Fl1 isolate.
Expression levels of the vibA gene in E. festucae isolate E437, mutant 830, the ΔvibA mutant, and the nonantifungal wild-type isolate Fl1 in axenic culture were investigated. The relative expression level of the vibA gene in mutant 830 was significantly decreased compared with that in wild-type E437 (approximately 20% of the wild-type level), indicating that the loss of antifungal activity in mutant 830 was a consequence of the compromised function of the vibA promoter by vector insertion (Fig. 4C). The relative vibA expression level in Fl1, an E. festucae isolate with no inhibitory activity against any of the test pathogens (9), was lower (approximately 60%) than that in isolate E437. As expected, no expression of the vibA gene was detected in the vibA deletion mutant (Fig. 4C).
vibA expression is enhanced when E. festucae is challenged by a pathogen.
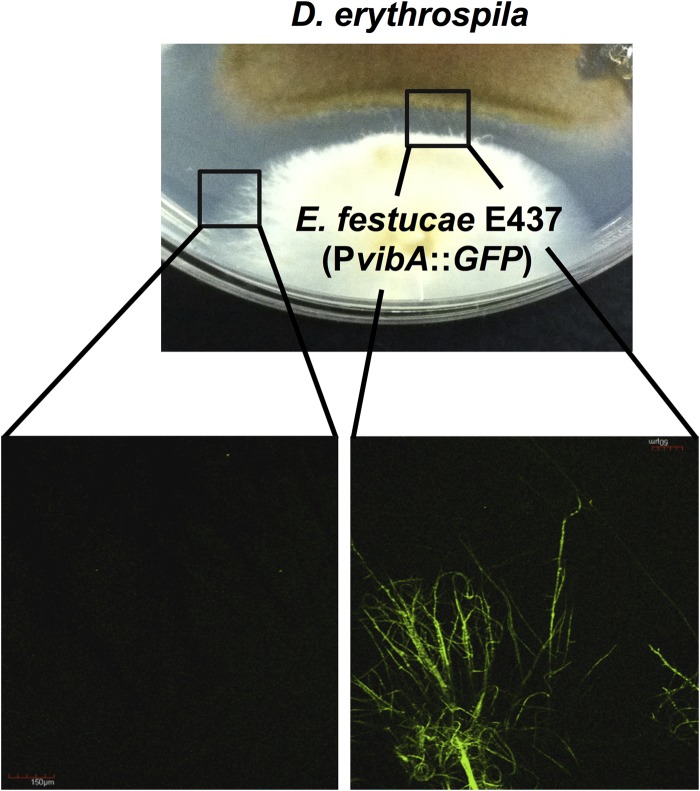
To determine whether vibA in E. festucae is differentially expressed in the absence of or when cocultured with a grass pathogen, transformants expressing GFP under the control of the vibA promoter were subjected to an inhibition assay with D. erythrospila. Confocal microscopy of transformants showed that the expression of GFP was enhanced when the mycelia of the endophyte were challenged by a pathogen. Enhanced expression of GFP was obvious despite the 5-mm average distance between the two fungal species. On the other hand, mycelia of the transformant that were not confronted by the pathogen showed low GFP fluorescence signals (Fig. 6), suggesting that expression of endophyte vibA was enhanced by the encounter with other fungal species.
FIG 6.
vibA expression is enhanced when E. festucae is challenged by the pathogen. A transformant expressing GFP under the control of the vibA promoter (PvibA::GFP) was subjected to an inhibition assay with D. erythrospila. The micrographs show GFP fluorescence of endophyte hyphae not confronted (left) and challenged (right) by the pathogen.
Overexpression of E. festucae vibA enhances its inhibitory activity against grass pathogens.
The vibA gene under the control of the TEF promoter was introduced into isolate E437 to investigate the effect of enhanced vibA expression on antifungal activity. The transformation vector pNPP154, containing a Ptef::vibA cassette, was introduced into E437 protoplasts. Ten vibA-overexpressing transformants were then subjected to in vitro inhibitory assay against grass pathogens. Overexpression of vibA reduced radial growth of the colony and enhanced production of aerial hyphae, but hyphal morphology and production of protease around the colony were not significantly affected (Fig. 4A and B; see Fig. S4 and S5 in the supplemental material). All the tested vibA-overexpressing transformants showed enhanced inhibitory activities against D. erythrospila, D. siccans, D. dictyoides, and C. graminicola. Enhanced inhibitory activity was manifested as wider clear zones between colonies of vibA-overexpressing transformants and the grass pathogens (Fig. 4A and 7). Moreover, transformants overexpressing vibA could inhibit the mycelial growth of Rhizoctonia solani, Magnaporthe grisea, and Sclerotinia homoeocarpa, but wild-type E437 could not (Fig. 7).
FIG 7.
Inhibitory activity of vibA-overexpressing transformant (Ptef::VibA-1) toward mycelial growth of grass pathogens. Colonies of E. festucae wild-type E437 (left side) and a vibA-overexpressing transformant (right side) were grown for 7 days before a mycelial plug of grass pathogen was inoculated. Each culture was incubated at 23°C for 7 to 14 days, until a clear zone of inhibition was observed.
To compare the inhibitory activity of the culture filtrate of the vibA-overexpressing mutant with that of wild-type E437, conidia of D. erythrospila were incubated in serially diluted culture filtrates of the vibA-overexpressing transformant and wild-type E437. Culture filtrate of E437 diluted to 75% did not inhibit the conidial germination of D. erythrospila. In contrast, conidial germination of the pathogen was not observed even in the culture filtrate of the vibA-overexpressing transformant diluted to 25%, and short germ tubes of the pathogen were seen in the 12% culture filtrate (Fig. 5B). These results showed that the vibA-overexpressing transformant produced approximately 10 times more inhibitory compound than wild-type E437 did.
The nonantifungal wild-type Fl1 isolate gains inhibitory activity by overexpression of vibA.
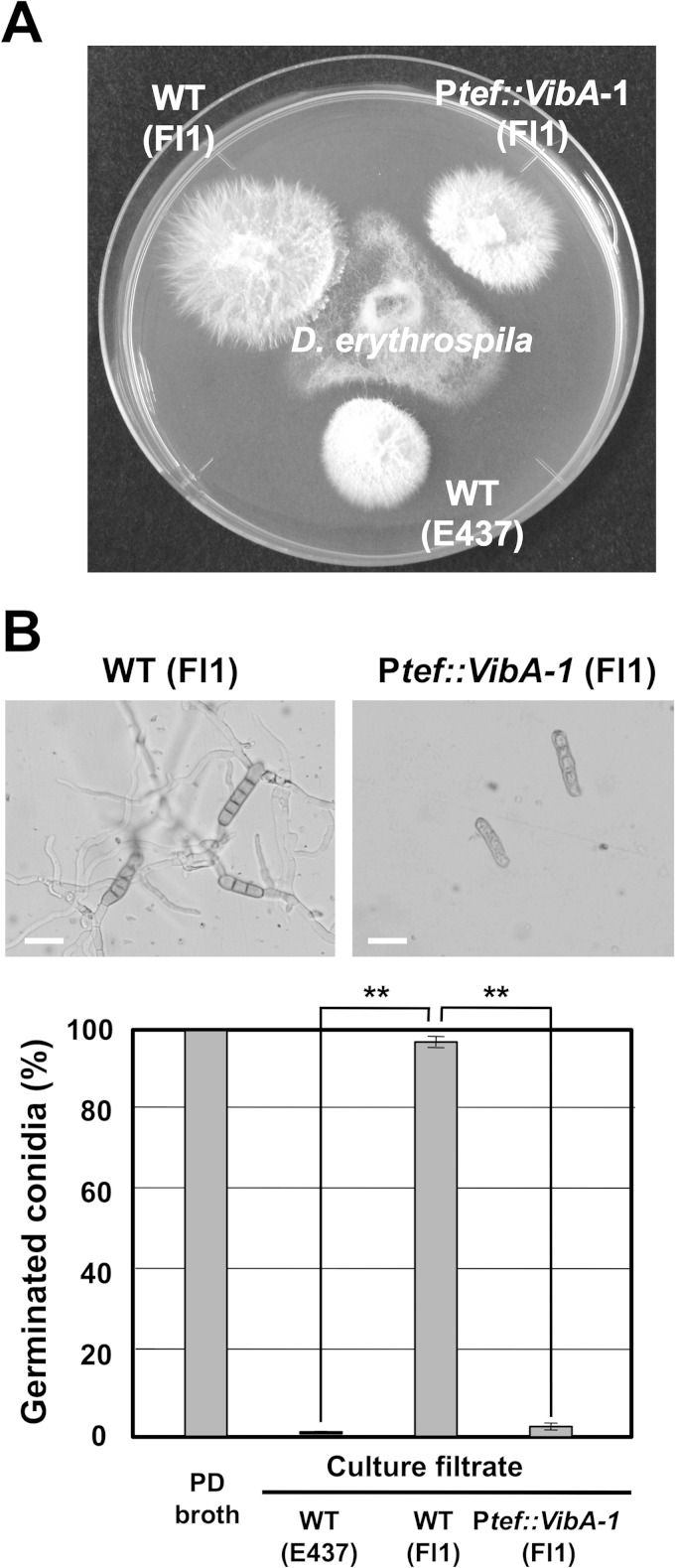
The transformation vector pNPP154, containing the Ptef::vibA cassette, was introduced into the wild-type Fl1 isolate, which has no antifungal activity (9). Transformants of Fl1 expressing vibA showed reduced radial growth, i.e., the same as that of E437 overexpressing vibA (Fig. 4 and 8). The vibA-overexpressing Fl1 transformants showed inhibitory activity against D. erythrospila (Fig. 8A). The culture filtrate of wild-type Fl1 had no inhibitory activity toward conidial germination of D. erythrospila, but the culture filtrate of Fl1 overexpressing vibA significantly inhibited the germination of pathogen conidia (Fig. 8B). These results indicate that enhanced expression of vibA is sufficient for a nonantifungal isolate to obtain antifungal activity. Thus, VibA is a master transcription factor for the production of antifungal compounds of symbiotic fungi.
FIG 8.
Gain of function in nonantifungal isolate Fl1 by expression of vibA. (A) Inhibitory activities of mycelial colonies of E. festucae wild-type Fl1 [WT (Fl1)], wild-type E437 [WT (E437)], and a vibA-expressing Fl1 transformant (Ptef::VibA-1 Fl1) against D. erythrospila. (B) Percentages of germinated conidia after 12 h of incubation in culture filtrates of E. festucae strains. The frequency of germinated conidia was calculated from at least 150 spores. Data are means ± standard errors from three biological replicates (>50 spores/experiment). Data marked with asterisks indicate significant differences as assessed by two-tailed Student's t test (**, P < 0.01).
Deletion of E. festucae vibA cancels the protective effect of wild-type E437 infection in suppressing disease development caused by D. erythrospila.
Perennial ryegrass was inoculated with E. festucae E437, the vibA mutant, or the vibA-overexpressing transformant to investigate the effects of vibA knockout and overexpression on the severity of disease symptoms on host plants. All infected plants showed normal growth as endophyte-free plants, and there was no obvious difference in the growth patterns of endophyte strains in host plants (data not shown). Detached leaves of perennial ryegrass without endophyte infection or infected with E. festucae strains were inoculated with a conidial suspension of D. erythrospila. Seven days after the inoculation, the detached leaves of perennial ryegrass, regardless of endophyte status, exhibited leaf spot lesions over the inoculated area (see Fig. S7 in the supplemental material). As previously reported (9), lesion sizes on E437-inoculated ryegrass were smaller than those on endophyte-free leaves. In contrast, the severity of disease symptoms on leaves of vibA mutant-infected plants was comparable to that on endophyte-free plants. Plants infected with the vibA-overexpressing transformant showed a disease severity similar to that of E437-infected ryegrass.
DISCUSSION
Recently, we demonstrated that an E. festucae strain that produces an antibiotic substance in culture medium reduced grass leaf spot disease (D. erythrospila) development in perennial ryegrass (Lolium perenne) (9). Although the chemical component of the inhibitory compound produced by this endophyte isolate has not yet been identified, preliminary characterization of the endophyte culture filtrate showed that the candidate inhibitory substance is a low-molecular-weight bioactive compound (9). Using the aforementioned bioprotective isolate of E. festucae, we report here the involvement of a transcription factor in the biosynthesis and regulation of the inhibitory substance produced by the endophyte against grass pathogens.
In this study, we isolated an E. festucae E437 mutant, designated mutant 830, that had lost antifungal activity against the test pathogen D. erythrospila. Mutant 830 had a plasmid insertion in the promoter region of the vibA gene, which encodes a putative transcription factor homologous to N. crassa VIB-1. In N. crassa, VIB-1 is required for the expression of genes involved in nonself recognition, leading to heterokaryon incompatibility and cell death (39). In filamentous fungi, heterokaryon incompatibility as a consequence of nonself recognition is manifested by the rejection of heterokaryon formation among genetically different isolates of the same fungal species (41). While hyphal cell fusion and formation of a vegetative heterokaryon between different individuals can be beneficial for fungi, as functional diploidy and for formation of a hyphal network for nutrient transport and resource utilization (42), heterokaryon incompatibility can also be advantageous to organisms, as a mechanism to reduce the risk of transmission of infectious factors in cytoplasm and to avoid exploitation by aggressive strains (41). In N. crassa, heterokaryon incompatibility is genetically regulated by het loci, wherein genetic differences can constrain heterokaryon formation (41). A loss-of-function mutation in N. crassa vib-1 suppressed the het-c- and mat-associated heterokaryon incompatibility; hence, heterokaryosis by hyphal fusion occurred with strains with which the mutant was formerly incompatible (38, 43). In addition to vegetative incompatibility, N. crassa VIB-1 is also involved in the negative regulation of conidiation, formation of aerial hyphae, development of protoperithecia (female reproductive structures), and production of extracellular protease (38, 43, 44).
VibA (VIB-1) contains an NDT80/PhoG DNA binding domain. Several studies have shown that transcription factors with an NDT80/PhoG DNA binding domain have diverse functions in different Ascomycota species. In Saccharomyces cerevisiae, Ndt80p is a transcriptional regulator of meiosis and sporulation. S. cerevisiae Ndt80p binds to a 9-bp regulatory sequence in the promoter regions of target genes, called the middle sporulation element (MSE), and is expected to directly activate ∼150 genes, including those related to multiple processes for meiotic commitment and sporulation (45–47). On the other hand, in Candida albicans, Ndt80p was isolated as a regulator of the gene for a drug efflux pump: CDR1. Further analyses indicated that C. albicans Ndt80p is also involved in the regulation of a large number of genes for diverse biological functions, including sterol metabolism and drug resistance (48, 49). The N. crassa genome has three genes encoding putative transcription factors with an NDT80/PhoG DNA binding domain (44). None of them is required for meiosis, while VIB-1 and FSD-1 (the closest homologue of yeast Ndt80p) are both involved in the formation of protoperithecia (44).
Among the limited reports on functional analyses of vib-1 homologues in filamentous Ascomycota fungi, the vib-1 orthologue in Aspergillus nidulans, xprG (phoG), was shown to be involved in protease production in response to nutrient limitation. Deletion mutants of xprG cannot utilize proteins as a carbon or nitrogen source (50). More recently, Katz et al. (51) reported that XprG regulates a large number of genes in response to carbon starvation, including the genes required for the production of secondary metabolites, such as penicillin and sterigmatocystin. The E. festucae vibA mutant, like the N. crassa vib-1 and A. nidulans xprG mutants (39, 50), is also defective in the production of extracellular protease. It is therefore likely that the positive regulation of protease production is a conserved role of VIB-1-like transcription factors in filamentous Ascomycota fungi.
While no particular report has implicated the protease gene as essential in the execution of cell death during heterokaryon incompatibility, a strong increase in the cellular proteolytic activity has been observed during the incompatibility reaction in Podospora anserina (52, 53). After fusion of incompatible individuals, rapid cytological changes of hyphal cells, including vacuolization of the cytoplasm, were observed. This was followed by the destruction of cytosolic compartments, further implicating the involvement of cell lytic enzymes in the induction of cell death in heterokaryon incompatibility. In contrast, the E. festucae E437 isolate, as a fungal antagonist, did not cause hyphal cell lysis of the confronted pathogen but affected its active apical hyphal growth and differentiation activity (9). The antifungal compound produced by the E437 isolate in culture is a thermostable, low-molecular-mass (<3.5 kDa) compound (9). Additionally, the extracellular protease production ability of E. festucae wild-type isolates was not necessarily associated with antimicrobial activity in culture (9). Further supporting this deduction are the two E. festucae E437 nonantifungal mutants we isolated recently, T547 and T692, which show extracellular protease activity comparable to that of wild-type E437 (T. Hashikawa and D. Takemoto, unpublished data). Altogether, the data show that, given that NDT80/PhoG-type transcription factors of S. cerevisiae, C. albicans, and A. nidulans have large numbers of direct target genes (45, 49, 51), it is likely that the genes for the production of inhibitory metabolites in E. festucae, rather than the cell lytic enzymes, are critical targets of VibA for the antifungal activity of the endophyte.
Pathogen recognition is not essential for isolate E437 to produce the inhibitory compound in culture, but it is probably an important cue for enhanced synthesis of the inhibitory compound. Under our experimental conditions, despite the fact that we did not quantify the intensity of GFP fluorescence, an elevated fluorescence intensity of GFP under the control of the vibA promoter was obvious when the endophyte colony was cocultured with D. erythrospila. The increased expression of GFP was observed despite the 5-mm gap between colonies of the pathogen and the endophyte. Relative to the hyphal diameter of the two interacting fungal species, a 5-mm zone of inhibition can be an extremely long distance for the endophyte to directly recognize the presence of the pathogen. Diffusible secretory products from the pathogen might be recognized as extraneous substances by the endophyte. Alternatively, a few directly confronted endophytic hyphae might induce the response of the remaining cells via intracolony communication. As for the case of fungal heterokaryon incompatibility, induction of cell death occurred when cells of incompatible individuals fused and products of het loci directly interacted in continuous cytosol (41). Collectively, our results imply that while the phenomena of heterokaryon incompatibility and the antagonistic effect of the endophyte against pathogens are both consequences of nonself recognition, the factors affecting the induction of responses are apparently distinct from each other.
Overexpressing the vibA gene from the genome of an antifungal epichloae isolate increased the amount of inhibitory compound produced by the transformant. The antifungal activity of the culture filtrate of the vibA-overexpressing transformant was about 10 times stronger than that of the wild type. Consequently, compared with that for the wild-type isolate, a wider zone of inhibition was observed in the dual-culture assay involving the vibA-overexpressing transformant. Moreover, the ability of the vibA-overexpressing transformant to inhibit test pathogens that were not inhibited by the wild-type isolate indicates that the test pathogens have different levels of sensitivity to the inhibitory compound produced by the endophyte. R. solani and S. homoeocarpa are fast-growing fungi, so it seems likely that a higher concentration of inhibitory endophyte product is needed to inhibit the mycelial growth of these two pathogens. Furthermore, overexpressing vibA in the nonantifungal E. festucae wild-type isolate Fl1 seemingly increased the quantity of the synthesized inhibitory compound to a certain concentration that was effective to inhibit the mycelial growth and conidial germination of D. erythrospila. This apparent effective antifungal concentration threshold was also evident when the wild-type E437 culture filtrate diluted to 75% did not exhibit antifungal activity against D. erythrospila.
The production of antifungal compound is not proportional to the expression activity of VibA as a transcription factor. Compared with the vibA expression level in the antifungal wild-type isolate, the vibA expression level in mutant 830 was about 20%. However, the culture filtrate of mutant 830 had no inhibitory activity. Conversely, the expression level of vibA in the Ptef::vibA transformant was only 1.5 times more than that in the wild type, but the inhibitory activity of the culture filtrate of the same transformant was about 10 times stronger than that of the wild type. Nonetheless, the expression level of vibA in the Ptef::vibA transformant was surprisingly low considering that it was under the control of the highly expressive TEF promoter. One possible explanation for this is that increasing the expression of vibA incurs a developmental cost to the endophyte, as demonstrated by the reduced hyphal growth of the vibA-overexpressing transformants. On the other hand, the vibA expression level in the nonantifungal wild-type isolate Fl1 was about 60% that in the E437 wild-type isolate, but introduction of the Ptef::vibA cassette conferred antifungal activity to Fl1. This observed difference may have been due to the two amino acid substitutions between VibA proteins of the antifungal (E437) and nonantifungal (Fl1) E. festucae isolates (see Fig. S6 in the supplemental material). This amino acid difference could possibly affect the activity of VibA as the transcription factor to induce target genes required for the production of the antifungal compound.
In summary, we identified E. festucae VibA, a transcription factor containing an NDT80/PhoG DNA binding domain, as an essential factor for the antifungal activity of endophytic fungi against grass pathogens. A deletion mutant of vibA lost its antifungal activity against grass pathogens, whereas a nonantifungal endophyte isolate acquired antifungal activity by enhanced expression of vibA. Therefore, VibA could be a master transcription factor for the expression of antifungal activity of E. festucae. Except for the regulation of the gene expression of pin-c, tol, and het-6, which are involved in heterokaryon incompatibility in N. crassa, direct target genes and the promoter motif for the VIB-1-like transcription factor are largely unknown for filamentous Ascomycota fungi. To identify genes directly regulated by the VibA protein of endophytes, transcriptome analyses of vibA deletion mutant and overexpression transformants will be performed. Additionally, further analyses of nonantifungal mutants of E. festucae may reveal not only the molecular mechanisms for the production of antifungal compound by the endophytic fungus but also the overlapping and distinct mechanisms between intraspecies heterokaryon incompatibility and interspecies antagonistic interactions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Christopher Shardl (University of Kentucky) and Barry Scott (Massey University, New Zealand) for providing E. festucae isolates, Yukio Tosa (Kobe University, Japan) for providing isolates of M. grisea, and Masahiro Fujimori (The National Agriculture and Food Research Organization, Japan) for L. perenne seeds. We are grateful to Kazuhito Kawakita (Nagoya University, Japan) and Makoto Ojika (Nagoya University, Japan) for valuable suggestions, Joyce Cartagena and Rico Gamuyao for critical readings of the manuscript, and the Radioisotope Research Center, Nagoya University, for technical assistance.
This work was supported in part by a research grant from the Institute for Fermentation (Osaka, Japan), by the Toyoaki Scholarship Foundation, and by a Grant-in-Aid for Challenging Exploratory Research (grant 25660036) from the Japan Society for the Promotion of Science. The E. festucae E894 (Fl1) genome sequences were made available by Christopher Schardl, through grant EF-0523661 from the U.S. National Science Foundation (to Christopher Schardl, Mark Farman, and Bruce Roe) and grant 2005-35319-16141 from the USDA National Research Initiative (to Christopher Schardl).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00034-14.
REFERENCES
- 1.Schardl CL, Craven KD, Speakman S, Stromberg A, Lindstrom A, Yoshida R. 2008. A novel test for host-symbiont codivergence indicates ancient origin of fungal endophytes in grasses. Syst Biol 57:483–498. doi: 10.1080/10635150802172184. [DOI] [PubMed] [Google Scholar]
- 2.Rowan DD, Gaynor DL. 1986. Isolation of feeding deterrent against stem weevil from ryegrass infected with the endophyte Acremonium loliae. J Chem Ecol 12:647–658. doi: 10.1007/BF01012099. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson HH, Siegel MR, Blankenship JD, Mallory AC, Bush LP, Schardl CL. 2000. Contribution of fungal loline alkaloids to protection from aphids in a grass-endophyte mutualism. Mol Plant Microbe Interact 13:1027–1033. doi: 10.1094/MPMI.2000.13.10.1027. [DOI] [PubMed] [Google Scholar]
- 4.Shiba T, Sugawara K. 2005. Resistance to the rice leaf bug, Trigonotylus caelestialium, is conferred by Neotyphodium endophyte infection of perennial ryegrass, Lolium perenne. Entomol Exp Appl 115:387–392. doi: 10.1111/j.1570-7458.2005.00278.x. [DOI] [Google Scholar]
- 5.Tanaka A, Tapper BA, Popay A, Parker EJ, Scott B. 2005. A symbiosis expressed non-ribosomal peptide synthetase from a mutualistic fungal endophyte of perennial ryegrass confers protection to the symbiotum from insect herbivory. Mol Microbiol 57:1036–1050. doi: 10.1111/j.1365-2958.2005.04747.x. [DOI] [PubMed] [Google Scholar]
- 6.Bonos SA, Wilson MM, Meyer WA, Funk CR. 2005. Suppression of red thread in fine fescues through endophyte-mediated resistance. Appl Turfgrass Sci doi: 10.1094/ATS-2005-0725-01-RS. [DOI] [Google Scholar]
- 7.Clarke BB, White JF Jr, Hurley RH, Torres MS, Sun S, Huff DR. 2006. Endophyte-mediated suppression of dollar spot disease in fine fescues. Plant Dis 90:994–998. doi: 10.1094/PD-90-0994. [DOI] [PubMed] [Google Scholar]
- 8.Pańka D, Piesik D, Jeske M, Baturo-Cieśniewska A. 2013. Production of phenolics and the emission of volatile organic compounds by perennial ryegrass (Lolium perenne L.)/Neotyphodium lolii association as a response to infection by Fusarium poae. J Plant Physiol 170:1010–1019. doi: 10.1016/j.jplph.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Niones J, Takemoto D. An isolate of Epichloë festucae, an endophytic fungus of temperate grasses, shows growth inhibitory activity to selective grass pathogens. J Gen Plant Pathol, in press. doi: 10.1007/s10327-014-0521-7. [DOI] [Google Scholar]
- 10.Malinowski D, Leuchtmann A, Schmidt D, Nösberger J. 1997. Growth and water status in meadow fescue is affected by Neotyphodium and Phialophora species endophytes. Agron J 89:673–678. doi: 10.2134/agronj1997.00021962008900040021x. [DOI] [Google Scholar]
- 11.Malinowski DP, Belesky DP. 2000. Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40:923–940. doi: 10.2135/cropsci2000.404923x. [DOI] [Google Scholar]
- 12.Schardl CL, Phillips TD. 1997. Protective grass endophytes: where are they from and where are they going? Plant Dis 81:430–438. doi: 10.1094/PDIS.1997.81.5.430. [DOI] [PubMed] [Google Scholar]
- 13.Funk CR, White RH, Breen J. 1993. Importance of Acremonium endophytes in turfgrass breeding and management. Agric Ecosyst Environ 44:215–232. doi: 10.1016/0167-8809(93)90048-T. [DOI] [Google Scholar]
- 14.Patterson CG, Potter DA, Fanin FF. 1991. Feeding deterrence of alkaloids from endophyte-infected grasses to Japanese beetle grubs. Entomol Exp Appl 61:285–289. doi: 10.1111/j.1570-7458.1991.tb01561.x. [DOI] [Google Scholar]
- 15.Riedell WE, Kieckhefer RE, Petroski RJ, Powell RG. 1991. Naturally occurring and synthetic loline alkaloid derivatives: insect feeding behavior modification and toxicity. J Entomol Sci 26:122–129. [Google Scholar]
- 16.Tapper BA, Lane GA. 2004. Janthitrems found in a Neotyphodium endophyte of perennial ryegrass, p 301 In Kallenbach R, Rosenkrans CF Jr, Lock TR (ed), Proceedings of the 5th International Symposium on Neotyphodium/Grass Interactions. University of Arkansas, Fayetteville, AR. [Google Scholar]
- 17.Siegel MR, Latch GCM. 1991. Expression of antifungal activity in agar culture by isolates of grass endophytes. Mycologia 83:529–537. doi: 10.2307/3760368. [DOI] [Google Scholar]
- 18.White JF Jr, Cole GT. 1985. Endophyte-host associations in forage grasses. III. In vitro inhibition of fungi by Acremonium coenophialum. Mycologia 77:487–489. [Google Scholar]
- 19.Yue Q, Miller CJ, White JF Jr, Richardson MD. 2000. Isolation and characterization of fungal inhibitors from Epichloë festucae. J Agric Food Chem 48:4687–4692. doi: 10.1021/jf990685q. [DOI] [PubMed] [Google Scholar]
- 20.Seto Y, Takahashi K, Matsuura H, Kogami Y, Yada H, Yoshihara T, Nabeta K. 2007. Novel cyclic peptide, epichlicin, from the endophytic fungus, Epichloë typhina. Biosci Biotechnol Biochem 71:1470–1475. doi: 10.1271/bbb.60700. [DOI] [PubMed] [Google Scholar]
- 21.Tintjer T, Rudgers JA. 2006. Grass-herbivore interactions altered by strains of a native endophyte. New Phytol 170:513–521. doi: 10.1111/j.1469-8137.2006.01720.x. [DOI] [PubMed] [Google Scholar]
- 22.Christensen MJ, Latch GCM, Tapper BA. 1991. Variation within isolates of Acremonium endophytes from perennial ryegrasses. Mycol Res 95:918–923. doi: 10.1016/S0953-7562(09)80087-7. [DOI] [Google Scholar]
- 23.Li CJ, Gao JH, Nan ZB. 2007. Interactions of Neotyphodium gansuense, Achnatherum inebrians, and plant-pathogenic fungi. Mycol Res 111:1220–1227. doi: 10.1016/j.mycres.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Ren AZ, Wang YH, Gao YB. 2009. Difference in antifungal activity of morphotypes of clavicipitaceous endophytes within and between species. Acta Ecol Sin 29:227–231. doi: 10.1016/j.chnaes.2009.08.005. [DOI] [Google Scholar]
- 25.Byrd AD, Schardl CL, Songlin PJ, Mogen KL, Siegel MR. 1990. The β-tubulin gene of Epichloë typhina from perennial ryegrass (Lolium perenne). Curr Genet 18:347–354. doi: 10.1007/BF00318216. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B. 2006. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic association. Plant Cell 18:1052–1066. doi: 10.1105/tpc.105.039263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka A, Cartwright GM, Saikia S, Yuka K, Takemoto D, Kato M, Tsuge T, Scott B. 2013. ProA, a transcriptional regulator of fungal fruiting body development, regulates leaf hyphal network development in the Epichloë festucae-Lolium perenne symbiosis. Mol Microbol 90:551–568. doi: 10.1111/mmi.12385. [DOI] [PubMed] [Google Scholar]
- 28.Khang CH, Park SY, Lee YH, Kang S. 2005. A dual selection based, targeted gene replacement tool for Magnaporthe grisea and Fusarium oxysporum. Fungal Genet Biol 42:483–492. doi: 10.1016/j.fgb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 29.Takemoto D, Tanaka A, Scott B. 2006. A p67Phox-like regulator is recruited to control hyphal branching in a fungal-grass mutualistic symbiosis. Plant Cell 18:2807–2821. doi: 10.1105/tpc.106.046169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayano Y, Tanaka A, Akano F, Scott B, Takemoto D. 2013. Differential roles of NADPH oxidases and associated regulators in polarized growth, conidiation and hyphal fusion in the symbiotic fungus Epichloë festucae. Fungal Genet Biol 56:87–97. doi: 10.1016/j.fgb.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Takemoto D, Kamakura S, Saikia S, Becker Y, Wrenn R, Tanaka A, Sumimoto H, Scott B. 2011. Polarity proteins Bem1 and Cdc24 are components of the filamentous fungal NADPH oxidase complex. Proc Natl Acad Sci U S A 108:2861–2866. doi: 10.1073/pnas.1017309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young CA, Bryant MK, Christensen MJ, Tapper BA, Bryan GT, Scott B. 2005. Molecular cloning and genetic analysis of a symbiosis-expressed gene cluster for lolitrem biosynthesis from a mutualistic endophyte of perennial ryegrass. Mol Gen Genomics 274:13–29. doi: 10.1007/s00438-005-1130-0. [DOI] [PubMed] [Google Scholar]
- 33.Itoh Y, Johnson R, Scott B. 1994. Integrative transformation of the mycotoxin-producing fungus, Penicillium paxilli. Curr Genet 25:508–513. doi: 10.1007/BF00351670. [DOI] [PubMed] [Google Scholar]
- 34.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 35.Vermelho AB, Meirelles MNL, Lopes A, Petinate SDG, Chaia AA, Branquinha MH. 1996. Detection of extracellular proteases from microorganisms on agar plates. Mem Inst Oswaldo Cruz 91:755–760. doi: 10.1590/S0074-02761996000600020. [DOI] [PubMed] [Google Scholar]
- 36.Takemoto D, Jones DA, Hardham AR. 2003. GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 33:775–792. doi: 10.1046/j.1365-313X.2003.01673.x. [DOI] [PubMed] [Google Scholar]
- 37.Kuspa A, Loomis WF. 1992. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc Natl Acad Sci U S A 89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang Q, Glass NL. 2002. Identification of vib-1, a locus involved in vegetative incompatibility mediated by het-c in Neurospora crassa. Genetics 162:89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dementhon K, Iyer G, Glass NL. 2006. VIB-1 is required for expression of genes necessary for programmed cell death in Neurospora crassa. Eukaryot Cell 5:2161–2173. doi: 10.1128/EC.00253-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lafontaine DL, Smith ML. 2012. Diverse interactions mediate asymmetric incompatibility by the het-6 supergene complex in Neurospora crassa. Fungal Genet Biol 49:65–73. doi: 10.1016/j.fgb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Glass NL, Kaneko I. 2003. Fatal attraction: nonself recognition and heterokaryon incompatibility in filamentous fungi. Eukaryot Cell 2:1–8. doi: 10.1128/EC.2.1.1-8.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleissner A, Simonin AR, Glass NL. 2008. Cell fusion in the filamentous fungus, Neurospora crassa. Methods Mol Biol 475:21–38. doi: 10.1007/978-1-59745-250-2_2. [DOI] [PubMed] [Google Scholar]
- 43.Xiang Q, Glass NL. 2004. The control of mating type heterokaryon incompatibility by vib-1, a locus involved in het-c heterokaryon incompatibility in Neurospora crassa. Fungal Genet Biol 41:1063–1076. doi: 10.1016/j.fgb.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 44.Hutchison EA, Glass NL. 2010. Meiotic regulators Ndt80 and ime2 have different roles in Saccharomyces and Neurospora. Genetics 185:1271–1282. doi: 10.1534/genetics.110.117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chu S, DeRisi J, Eisen M, Mulholland J, Botstein D, Brown PO, Herskowitz I. 1998. The transcriptional program of sporulation in budding yeast. Science 282:699–705. doi: 10.1126/science.282.5389.699. [DOI] [PubMed] [Google Scholar]
- 46.Chu S, Herskowitz I. 1998. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol Cell 1:685–696. doi: 10.1016/S1097-2765(00)80068-4. [DOI] [PubMed] [Google Scholar]
- 47.Winter E. 2012. The Sum1/Ndt80 transcriptional switch and commitment to meiosis in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 76:1–15. doi: 10.1128/MMBR.05010-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen CG, Yang YL, Shih HI, Su CL, Lo HJ. 2004. CaNdt80 is involved in drug resistance in Candida albicans by regulating CDR1. Antimicrob Agents Chemother 48:4505–4512. doi: 10.1128/AAC.48.12.4505-4512.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sellam A, Tebbji F, Nantel A. 2009. Role of Ndt80p in sterol metabolism regulation and azole resistance in Candida albicans. Eukaryot Cell 8:1174–1183. doi: 10.1128/EC.00074-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz ME, Gray K-A, Cheetham BF. 2006. The Aspergillus nidulans xprG (phoG) gene encodes a putative transcriptional activator involved in the response to nutrient limitation. Fungal Genet Biol 43:190–199. doi: 10.1016/j.fgb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 51.Katz ME, Braunberger K, Yi G, Cooper S, Nonhebel HM, Gondro C. 2013. A p53-like transcription factor similar to Ndt80 controls the response to nutrient stress in the filamentous fungus, Aspergillus nidulans. F1000Res 2:72. doi: 10.12688/f1000research.2-72.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bégueret J. 1972. Protoplasmic incompatibility: possible involvement of proteolytic enzymes. Nat New Biol 235:56–58. doi: 10.1038/newbio235056a0. [DOI] [PubMed] [Google Scholar]
- 53.Bégueret J, Bernet J. 1973. Proteolytic enzymes and protoplasmic incompatibility in Podospora anserina. Nat New Biol 243:94–96. doi: 10.1038/243094a0. [DOI] [PubMed] [Google Scholar]
- 54.Lamoureux JS, Stuart D, Tsang R, Wu C, Glover JN. 2002. Structure of the sporulation-specific transcription factor Ndt80 bound to DNA. EMBO J 21:5721–5732. doi: 10.1093/emboj/cdf572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang YL, Wang CW, Leaw SN, Chang TP, Wang IC, Chen CG, Fan JC, Tseng KY, Huang SH, Chen CY, Hsiao TY, Hsiung CA, Chen CT, Hsiao CD, Lo HJ. 2012. R432 is a key residue for the multiple functions of Ndt80p in Candida albicans. Cell Mol Life Sci 69:1011–1023. doi: 10.1007/s00018-011-0849-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.