Abstract
The growing number of bacterial pathogens that are resistant to numerous antibiotics is a cause for concern around the globe. There have been no new broad-spectrum antibiotics developed in the last 40 years, and the drugs we have currently are quickly becoming ineffective. In this article, we explore a range of therapeutic strategies that could be employed in conjunction with antibiotics and may help to prolong the life span of these life-saving drugs. Discussed topics include antiresistance drugs, which are administered to potentiate the effects of current antimicrobials in bacteria where they are no longer (or never were) effective; antivirulence drugs, which are directed against bacterial virulence factors; host-directed therapies, which modulate the host's immune system to facilitate infection clearance; and alternative treatments, which include such therapies as oral rehydration for diarrhea, phage therapy, and probiotics. All of these avenues show promise for the treatment of bacterial infections and should be further investigated to explore their full potential in the face of a postantibiotic era.
Keywords: antimicrobial peptide, antivirulence, efflux pump, immunomodulatory peptide, outer membrane permeabilizer, phage therapy, probiotic, quorum sensing, type III secretion system, β-lactamase
Man has interacted with pathogens throughout human history, but the manner in which we have treated infections over the millennia has changed drastically, especially over the past 100 years with the advent of modern antibiotics. This class of pharmaceuticals encompasses drugs that act to either directly kill bacteria (bactericidal agents) or to inhibit their growth (bacteriostatic agents). They ushered in a golden age which has allowed the successful treatment of millions of individuals who might not have survived prior to antibiotic use. However, as the twentieth century progressed, bacteria emerged that were immune to these new weapons. The age of resistance had begun. No matter how many novel antibiotic agents are developed to act on diverse targets (protein synthesis, DNA/RNA synthesis, cell wall synthesis, folate synthesis or membrane potential), resistance always ensues (1). While some groups are working to develop novel antibiotics that work against multidrug-resistant bacteria [see, for example (2)\, the success rate appears to be declining and over time, and it is likely that these drugs will also elicit resistance.
Over the years, we have become highly reliant on antibiotics and these drugs are heavily entrenched in our culture. Antimicrobials are not just restricted to those who are ill; they are utilized prophylactically to prevent the onset of infections, present in consumer goods such as hand soap and toothpaste and fed to livestock to increase growth rates. Unfortunately, this widespread use has increased antibiotic resistance in both human and animal reservoirs and in the environment (3), even among bacteria that were not targets of the drugs. This ensures that pathogens have a vast and readily available pool of resistance genes from which to draw and pressure from antibiotic usage provides positive selection for the spread of these resistance genes and mutations.
A 2013 report by the American Centers for Disease Control estimates that more than 2 million illnesses and 23 thousand deaths are caused by drug-resistant microbes in the USA annually (4). Such statistics have prompted health organizations to institute stricter policies for antibiotic use to try to curb the emergence of resistance. These policies are undoubtedly helping to extend the usage of antibiotics but are likely to be insufficient to fix this emerging situation.
Unfortunately, the pace of antibiotic development has also slowed over the past several decades. In contrast to the 1940s, 50s, and 60s, when many novel antibiotics were developed in a relatively short time period, no new chemical classes of broad-spectrum antibiotics and few narrow spectrum drugs have appeared in the last 40 years. This reflects at least in part the limited number of potential targets available in bacteria and difficulties inherent in creating molecules with no or limited toxicity in man. It should also be noted that the drug regulation process has evolved substantially in the past half century. Increasingly, more rigorous clinical trials and safety checks have been required before a drug is introduced to the market. Such legislation is aimed to protect the consumer; however, certain antimicrobials prescribed today would likely not meet current standards. In addition to the lack of recent development success, emerging antibiotic resistance and the requirement for prudence in prescribing novel antibiotics (limiting usage) are collectively depressing antibiotic development by Pharma (5). It is simply not profitable to develop drugs that may have a short life span (due to antibiotic resistance), are usually used only a single time in any given customer, and that are cautiously prescribed by physicians (to slow the development of antibiotic resistance). Government incentives to fill the gap, such as the American GAIN act of 2012 (which provides benefits such as fast track FDA review and 5 additional years of market exclusivity), are likely to help over time. For example, the new drugs dalvance [approved by the FDA in May 2014 (6)\ and oritavancin (7) were both subject to this new legislation. They are both administered intravenously and combat skin infections caused by Gram-positive bacteria including multidrug-resistant strains, such as methicillin resistant Staphylococcus aureus (MRSA). Oritavancin and dalvance were both in the pipeline long before the GAIN act was passed. It remains to be seen whether the act will incentivize the development of novel drugs. Nevertheless, novel therapies for Gram-negative bacteria are notoriously more difficult to develop due to the additional outer membrane barrier that limits efficacy. Unfortunately, many of the most recalcitrant multidrug-resistant bacteria that we are facing today are Gram-negative species. According to the 2013 report on antibiotic resistance threats by the CDC, more than 730 000 infections and over 3400 deaths annually are caused by Gram-negative bacteria in the USA alone (4). We would also be wise to formally consider the possibility that we have nearly exhausted our supply of discoverable non-toxic antibiotic drugs or at least our list of targets. Given current trends, our effective arsenal against an increasing multidrug-resistant bacterial population is bound to decrease.
It is perhaps time to rethink the overall strategy before we find ourselves in an era where infectious disease becomes as major a cause of mortality in the developed world as it is in the developing world. We must learn to use the antibiotics that we have wisely. One major way forward is to develop compounds (termed here adjuvants) that act in concert with the known conventional antibiotics, thus enhancing their activity, especially against resistant isolates. One possible reason that it has become increasingly difficult to develop novel antimicrobials is that there are a limited number of direct protein targets. An antimicrobial target must be an essential protein, enable the development of drugs that are able to get taken up without excessive efflux, and when inhibited must lead to bactericidal or at least bacteriostatic action. Thus the same handful of targets (the ribosome, dihydrofolate reductase, RNA polymerase, cell wall biosynthesis including penicillin-binding proteins, etc.) have been extensively studied and exploited for decades and many others have been attempted without notable successes, leading one to question whether there are many new exploitable targets. The advantage of developing adjuvants is that one does not need to find an essential target but rather one that when inhibited enhances the activity of one of the antibiotics that hits these targets (with the classical example of exploited adjuvants being β-lactamase inhibitors). In the following article, we discuss innovative possibilities for antimicrobial adjuvants including antiresistance drugs, antivirulence drugs, host-directed therapies, and alternative treatments. Such adjunctive treatments could help prolong the lives of our existing antibiotics and forestall the arrival of a postantibiotic era.
Antiresistance Drugs
The global increase in multidrug-resistant pathogenic bacteria presents a particular challenge to translational medicine. This is especially due to clear difficulties in the design of new drugs coupled with the remarkable rise in mortality and morbidity in the developed world. In particular, the dissemination of multidrug-resistant ‘ESKAPE’ organisms (Enterococcus spp., Staphylococcus aureus,Klebsiellaspp., Acinetobacter baumannii,Pseudomonas aeruginosa,and Enterobacterspp.) is an enormous challenge (8). Nowadays, it is possible to find Gram-negative bacterial strains with enhanced resistance to all available antibiotics (9,10). The Infectious Diseases Society of America (IDSA) has identified antimicrobial resistance as the greatest global threat to human health (11). If, on one hand, bacteria are becoming more lethal and dangerous, on the other, the scientific community is formulating novel adjuvants for antibiotic compounds to stave off bacterial resistance. The following members of this class of compounds will be discussed: β-lactamase inhibitors (12), efflux pump inhibitors (13), and outer membrane permeabilizers (14).
β-lactamase inhibitors
β-lactam antibiotics have been utilized therapeutically for more than 70 years to manage a wide range of conditions caused by bacterial pathogens. These bactericidal compounds are valuable agents that are generally harmless to humans. They act by inhibiting cell wall synthesizing enzymes called penicillin-binding proteins (PBPs), which lack specific mammalian homologs (15). Despite the fact that new β-lactam-containing analogues have occupied the pharmaceutical pipelines for several years, scarcely any of these compounds have progressed to clinical trials as singular agents (16). This has likely been due to the extensive proliferation of β-lactamases, which collectively hydrolyze an extensive array of β-lactam drugs including the carbapenem family (17). Building on the successes of clavulanic acid, it is now a well-established principle that the combination of a β-lactamase inhibitor (as an adjuvant to suppress enzymatic resistance) with a β-lactam can increase the efficacy and spectrum of the antibiotic. For this reason, a great deal of research has been focused on the development of novel (usually non-antibiotic) β-lactamase inhibitors from a variety of different families for co-administration with β-lactams (Table1). To date, the primary targets for lactamase inhibitors have been the class A β-lactamases, which can be inactivated by various inhibitors following different reaction sequences but increasingly the class C inducible chromosomal cephalosporinases and the plasmid borne carbapenemases are being addressed.
Table 1.
β-Lactamase inhibitors promoting β-lactam activity against resistant bacteria
| Name | Compound | Use in combination with | Sources | References |
|---|---|---|---|---|
| Clavulanic acid | (2R,5R,Z)-3-(2-hydroxyethylidene)-7-oxo-4-oxa-1-aza-bicyclo[3.2.0\heptane-2-carboxylic acid | Amoxicillin Ticarcillin |
Streptomyces clavuligerus | (165) |
| Sulbactam | (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0\heptane-2-carboxylic acid 4,4-dioxide | Amoxicillin Cefoperazone |
Synthetic | (165) |
| Tazobactam | (2S,3S,5R)-3-methyl-7-oxo-3-(1H-1,2,3-triazol-1-ylmethyl)-4-thia-1-azabicyclo[3.2.0\heptane-2-carboxylic acid 4,4-dioxide | Piperacillin | Synthetic derivated from penicillin | (166) |
| Avibactam | (2S,5R)-7-Oxo-6-(sulfooxy)-1,6-diazabicyclo[3.2.1\octane-2-carboxamide | Ceftazidime Ceftaroline Aztreonam |
Synthetic | (167–169) |
| Cobaltocenium-containing polymers | Hexafluorophosphate (PF6−)-paired cobaltocenium-containing polymer, poly(2-(methacrylolyoxy)ethyl cobaltoceniumcarboxylate hexafluorophosphate) | Penicillin-G Amoxicillin Ampicillin Cefazolin |
Synthetic | (22) |
| MK-7655 | [(2S,5R)-7-oxo-2-(piperidin-4-ylcarbamoyl)-1,6-iazabicyclo[3.2.1\octan-6-yl\ hydrogen sulfate | Imipenem | Synthetic | (23) |
| Phthalic acid and derivatives | Benzene-1,2-dioic acid; phthalic acid; ortho-phthalic acid | Biapenem Carbapenem |
Synthetic | (170) |
| Succinic acid derivatives | Compound 1, compound 11 | Imipenem | Synthetic | (171) |
| RPX7009 | Boron-based lactamase inhibitors | Biapenem Meropenem Doripenem Ertapenem |
Synthetic | (172) |
| NagZ inhibitor | 3-N-acyl azepanes | Ceftazidime | Synthetic | (26) |
| BAL30072 | Siderophore monosulfactam | Meropenem | Synthetic | (30) |
| SA2-13 | Penam sulfones | Ampicillin | Synthetic | (27) |
| Compounds 1 and 2 | Polyketides | Meropenem | Penicilliumsp. | (28) |
| Metallopolymer | Cobaltocenium-containing polymers | Penicillin-G Amoxicillin Ampicillin Cefazolin |
Synthetic | (34) |
| ME1071 | Maleic acid derivative | Biapenem | Synthetic | (29) |
| Aspergillomarasmine A | Natural fungal extract | Meropenem | Aspergillus versicolor | (33) |
| FPI-1465 | Unknown class of β-lactamase inhibitor | Meropenem Ceftazidime Aztreonam | Synthetic | http://www.fedorapharma.com/site/rd_pipeline |
One classical example of combined β-lactam and β-lactamase inhibitor therapy is the administration of penicillins with the β-lactamase inhibitors clavulanic acid, sulbactam or tazobactam. These three compounds have been used successfully in combination for three decades in both parenteral and oral therapies (18) (See Figure1). In addition to these three drugs, several pharmaceutical companies have developed novel solutions for bacterial resistance. Among these is avibactam (NXL104; AstraZeneca) (Table1), which was originally developed by Novexel (16). Avibactam is a non-β-lactam bicyclic diazabicyclooctane, has no antibacterial activity, and forms reversible covalent bonds with several β-lactamases (19,20). Its mechanism of action involves covalent acylation of its β-lactamase targets. Avibactam displays activity against a wide variety of class A and C β-lactamase synthesizing strains, including those that are poorly inhibited by clavulanic acid and tazobactam such as plasmid borne KPC (Klebsiella pneumoniacarbapenemase), ESBL (extended spectrum β-lactamase), and AmpC-overexpressing strains (21). These properties are making avibactam one of the most promising antiresistance drugs in the USA (22). Another diazibicyclooctane compound recently developed by Merck is MK-7655. MK-7655 is a piperidine analogue that is used together with imipenem. It displays functional similarities to avibactam and has the ability to inhibit both class A and C lactamases (23).
Figure 1.
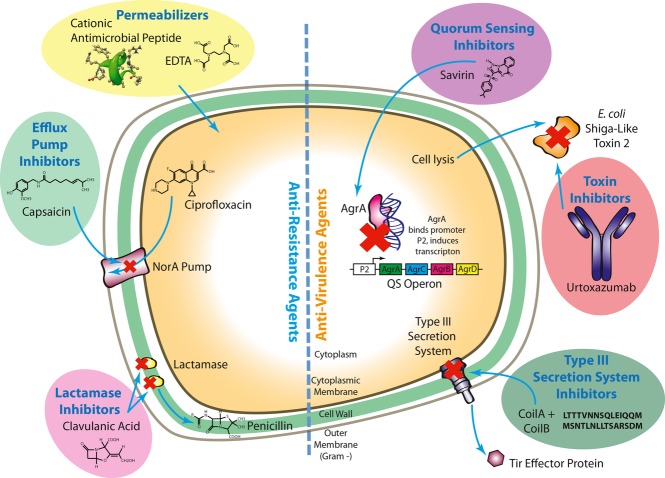
Selected Antiresistance and Antivirulence Agents and their Mechanisms of Action. Cationic peptides (54) and EDTA (55) are outer membrane permeabilizing agents that make the outer membrane more amenable to the penetration of antibiotics. Capsaicin (175) is an efflux pump inhibitor that acts on the NorA pump of S. aureusto impede the efflux of the antibiotic ciprofloxacin from inside the cell. Clavulanic acid (165) is a lactamase inhibitor that inhibits the action of lactamase on penicillin, therefore increasing penicillin's effectiveness. Savirin (86) is a quorum-sensing inhibitor that arrests the binding of AgrA to the P2 promoter, which is upstream of a S. aureusquorum-sensing operon. Binding of AgrA to the P2 promoter induces transcription of the quorum-sensing operon, so savirin's action prevents quorum-sensing gene expression. Urtoxazumab (97) is a toxin inhibitor that inactivates E. coliShiga-like toxin 2. Shiga-like toxin 2 is produced by late cycle phage genes and then released from the cell upon lysis. The toxin is then bound and inactivated by the urtoxazumab antibody. CoilA and CoilB (113,114) are peptides that inhibit the assembly of the type III secretion system apparatus. By preventing the formation of the type III secretion system, secretion of effector proteins (such as Tir) is effectively stopped.
A group of lactamase inhibitors that have been a focus of the pharmaceutical industry in recent years are the boronic acid-containing β-lactamase inactivators, or BAs. In 2012, Rempex published the structure of RPX7009 (24), a BA that has inhibitory activity toward class A and class C serine β-lactamases (Table1). It is interesting to note that although there are or have been a large number of BAs in development, only RPX7009 has been in phase I clinical trials ((25) and NCT01897779). In addition to these, other small molecules have been utilized to reduce β-lactamase effectiveness including trihydroxyazepane NagZ inhibitors (26), penam sulfones (27), polyketides (28), maleic acid derivatives (29), BAL30072, (which is a siderophore monosulfactam similar to aztreonam and is currently in phase I clinical trials) (30) and BAL30376, (which combines three β-lactams including the bridged monobactam class C β-lactamase inhibitor BAL29880, the siderophore monobactam BAL19764 and clavulanic acid) (31). Recently, O-acyl and O-phosphyl hydroxamates were described as novel classes of lead stage β-lactamase inhibitors. One example is related to the N-acyl derivative of a cyclic O-acyl hydroxamic acid, 3H-benzo[d\[1,2\oxazine-1,4-dione, while another is related to the N-tertbutoxycarbonyl derivative (32). Such compounds are prodrugs rather than β-lactamase inhibitors per se; however, they spontaneously hydrolyze in aqueous solutions to produce an O-phthaloyl hydroxamic acid, which is a β-lactamase inhibitor. This compound can cyclize in solution to yield phthalic anhydride which is also a β-lactamase inhibitor.
King et al. (33) have recently shown that a fungal derivative called aspergillomarasmine A resensitizes NDM and VIM-expressing Pseudomonas,Acinetobacterand the Enterobacteriaceaeto meropenem. In addition, the compound is well tolerated at therapeutic doses by mice and significantly increases murine survival when used in combination with meropenem upon challenge with K. pneumoniaeN11-2218. A compound called FPI-1465 is currently being developed by Fedora Pharmaceuticals (http://www.fedorapharma.com/site/rd_pipeline). This molecule has synergistic action in vitrowith meropenem, ceftazidime, and aztreonam in multiple strains of bacteria expressing carbapenamase and ESBL. FPI-1465 has also been tested with promising results in animal infection models and is expected to proceed to clinical trials shortly.
On the other hand, macromolecular inhibitors of β-lactams have also been described. Zhang et al. (34) introduced a novel class of charged metallopolymers, named cobaltocenium-containing polymers, which effectively kill bacterial cells and have additive effects with many β-lactam antibiotics. The IC90 values for these polymers alone are in the 3–5 μm range for MRSA, and the polymers do not cause hemolysis, but have yet to be tested in animal models. Polypeptides called BLIPs (β-lactamase inhibitory proteins) bind and inhibit class A β-lactamases (35).
In addition to the compounds described above, several platforms have been developed to screen and develop novel inhibitors with high affinity to β-lactamase. Among them, an ultrafiltration LC/MS-based assay for identification of inhibitors of NDM-1 (New Delhi metallo-β-lactamase) has been applied (36) with high reproducibility. This strategy led to the identification of a potent inhibitor named ligand 14 from a small-molecule fragment mixture. Ligand 14 has an IC50 of 1.81 μm, but has not yet been tested for its ability to permeate cell membranes or toxicity. Molecular modeling indicated a mechanism of action whereby ligand 14 interacts directly with the zinc atom in the β-lactamase active site. Another unusual approach being applied is phage display technology, which was used to screen single-domain antibody fragments (also named nanobodies) that were able to inhibit β-lactamases (37). In this context, fifty nanobodies were identified as inhibitors, but only one, called NbVIM_38 showed allosteric inhibitory activity. The inhibitory activity was present at micromolar concentrations for all β-lactams evaluated. This lead stage compound has not yet been tested for toxicity.
In addition to demonstrating inhibition of β-lactamases, members of this class of drugs must demonstrate synergistic action with β-lactams both in vitroand in vivo. Doses of both the β-lactam and the β-lactamase inhibitor must also be carefully titrated to achieve the optimal synergy with minimal toxicity. Clinical trials can therefore pose a substantial hurdle for this type of drug. It is important to note that although the myriad of compounds described here have the ability to inhibit several kinds of β-lactamases, resistance to β-lactamase inhibitors has appeared. For example, a decrease in the susceptibility of E. colistrains to β-lactamase inhibitors was observed when these agents were used in combination with cephalosporins (38). Similarly, ESBL enzymes with resistance to the more traditional β-lactamase inhibitors are very widespread. These data teach us that coevolution never stops. Bacteria have a strong capacity to find a way to survive this particular adjuvant strategy, thus providing the necessity to constantly attempt to find novel and more potent antimicrobial drugs and adjuvants.
Pump inhibitors
Efflux pump overexpression is an important mechanism of bacterial resistance that results in antibiotics being expelled from bacterial cells. In Gram-negative bacteria, the slow rate of uptake of antibiotics through the semi-permeable outer membrane acts to make these organisms prohibitively resistant to drugs that are good efflux pump substrates and considerably less susceptible to even poor efflux pump substrates. This issue has been a major limitation in attempts to develop new antibiotics. Pump inhibition is thus a strategy that could re-establish the potency of current antibiotics against resistant bacteria and perhaps drive the development of new antibiotics.
RND efflux pumps are involved in intrinsic resistance in many Gram negatives, and when derepressed lead to multidrug-resistance phenotypes in the Enterobacteriaceaeand Pseudomonas aeruginosa. Thus, they are potential targets for novel agents that could restore susceptibility to different antibiotics (39). Among possible inhibitors is the dual permeabilizer and efflux pump inhibitor, phenylalanine-arginine ß-naphthylamide (PAßN). This compound inhibits the efflux action of many RND family pumps and is able to reduce intrinsic and mutational resistance to multiple antibiotics (Table2) (40,41). However, despite the fact that its activity has been known for more than a decade, it has not yet progressed to the clinic.
Table 2.
Pump inhibitors promoting antimicrobial activity against resistant bacteria
| Compound | Used in combination with | Sources | Pumps | Target | References |
|---|---|---|---|---|---|
| Boronic acid derivatives | Ciprofloxacin | Synthetic | NorA | S. aureus | (173) |
| (Z)-N-benzylidene-2-(tert-butoxycarbonylamino)-1-(5-iodo-1H-indol-3-yl)ethanamine | Ciprofloxacin | Synthetic | NorA | S. aureus | (174) |
| Capsaicin | Ciprofloxacin | Capsicumspp. | NorA | S. aureus | (175) |
| Pyrazolo[4,3-c\[1,2\benzothiazine 5,5-dioxide analogues | Ciprofloxacin | Synthetic | NorA | S. aureus | (176) |
| Flavones and 2-(4-Propoxyphenyl)quinoline derivatives | Ciprofloxacin | Synthetic | NorA | S. aureus | (177) |
| 4-methyl-N-[2-(1-methyl-1H-pyrrol-2-yl)-1H-benzimidazol-5-yl\benzenesulfonamide (16), 2-{[3-(benzyloxy)benzyl\amino}-1-phenylpropan-1-ol (21), 4-({[3-cyano-6-ethyl-4-(trifluoromethyl)-5,6,7,8-tetrahydroquinolin-2-yl\thio}methyl)benzoic acid (23), and 3-{5-[(Z)-(3-sec-butyl-2,4-dioxo-1,3-thiazolidin-5-ylidene)methyl\-2-furyl}-4-chlorobenzoic acid (28) | Ciprofloxacin | Synthetic | NorA | S. aureus | (178) |
| 3-(substituted-3,4-dihydronaphthyl)-2-propenoic acid amides | Ciprofloxacin | Synthetic | NorA | S. aureus | (179) |
| Homoisoflavonoid | Ethidium bromide | Caesalpinia digyna | EPs | M. smegmatis | (46) |
| Phenylalanine-arginine ß-naphthylamide | Cyclines Quinolones Piperacillin Cefotaxime Ceftazidime Ciprofloxacin |
Synthetic | RND |
B. thailandensis P. aeruginosa |
(39–41) |
| Peptide nucleic acids | Ciprofloxacin Erythromycin | Synthetic | CmeABC | C. jejuni | (48–50) |
Another target that has been a focus of considerable research is the NorA efflux pump from S. aureus,which confers resistance to several antimicrobial agents including the fluoroquinolones (42), resulting in a multidrug-resistance phenotype. Many compounds from a variety of sources and different classes have been tested for their ability to deactivate the NorA pump and restore antibiotic activity versus resistant S. aureus(Table2; Figure1). Research has also been conducted to engineer fluoroquinolones to avoid efflux via NorA pumps to improve their antimicrobial efficacy. This approach has led to the development of such drugs as garenoxacin (a dual topoisomerase IV and DNA gyrase inhibitor) (43) and the lead stage compound DX-619 (an inhibitor of type II topoisomerase) (44).
Multidrug efflux pumps have also been described as a resistance mechanism in Mycobacteria (45). Natural products have been a central focus in the search for inhibitors of these pumps, in contrast to the situation with β-lactamase (Table1) and NorA (Table2) inhibitors, which are mainly produced synthetically. Among these natural derivatives is bonducellin, a homoisoflavonoid that is purified from Caesalpinia digynaroots. As a proof of principle, this chemical is able to synergize with ethidium bromide against resistant Mycobacterium smegmatis(46), although this agent is not a commercial antibiotic.
Another possibility for reducing the deleterious effects of efflux pumps involves the utilization of antisense peptide nucleic acids, also known as PNAs. PNAs are synthetic nucleic acid homologs in which the polynucleotide phosphate backbone is replaced by a flexible pseudopeptide polymer. PNAs act as antisense mediators by binding with high specificity to complementary DNA and RNA sequences and inhibit gene expression and translation (47). A PNA compound was utilized to sensitize Campylobacter jejuniby decreasing the expression of the CmeABC efflux pump, which commonly confers resistance to several antimicrobials including ciprofloxacin and erythromycin (48–50).
Finally, traditional medicine has also isolated several plant extracts with the ability to decrease the activity of pump inhibitors (51,52). This demonstrates that exploring new sources for adjuvants might also contribute to the reduction of bacterial resistance by improving the possibilities of finding novel and useful compounds.
Outer membrane permeabilizers
Gram-negative bacteria are intrinsically resistant to most antibiotics due to the permeability barrier provided by the outer membrane (53,54). The outer membrane is a semi-permeable barrier comprising an asymmetric bilayer perforated by channel-forming proteins called porins. The area of channels through which hydrophilic antibiotics like, for example, β-lactams can pass is quite small (<1% of the surface area) and therefore restricts the rate of uptake into the cell, leading to greater effectiveness of other resistance mechanisms such as β-lactamases and efflux pumps. The outer layer of the outer membrane is occupied by the unusual polyanionic molecule lipopolysaccharide (LPS), which is stabilized by the cross-bridging of divalent cations. This serves to restrict the passage of hydrophobic drugs which cannot partition easily into the membrane but also provides an opportunity. Agents that extract or displace divalent cations from this membrane cause it to become increasingly permeable to both hydrophobic and hydrophilic substances and even small proteins like lysozyme (55,56). There are indeed several polycations, for example, polymyxins, aminoglycosides, cationic antimicrobial peptides, and dibasic macrolides (azithromycin) (54), that interact at this site on the outer membrane causing the outer membrane to become locally destabilized and thus permeable to the interacting polycation, a process termed ‘self-promoted uptake’. At the same time, other compounds, including antibiotics, can more easily penetrate the permeabilized membrane (56).
Thus, permeabilizers represent a method by which the activity of antibiotics, severely limited by the presence of the outer membrane, can be increased. These compounds are typically cationic and amphiphilic or chelators, which can be developed from peptides, peptide-like compounds, polymers or lipids, such as, for example, antimicrobial peptides (54–56) and cholic acid (57,58). These physicochemical properties are quite general, and consequently, some agents are much more effective permeabilizers than others (59). One study surveyed the ability of various compounds to permeabilize the outer membrane of P. aeruginosastrains and demonstrated the effectiveness of citric acid, poly-L-lysine, EDTA and polymyxin B nonapeptide (PMBN; a deacylated version of polymyxin without antibiotic activity but retaining the outer membrane permeabilizing activity of polymyxin B) (55). Other investigations have shown that cationic peptides are taken up by self-promoted uptake (54) and consequently can act as permeabilizers showing synergy in P. aeruginosaefflux pump over expressing stains with ciprofloxacin, carbenicillin, and nalidixic acid (56) (see Figure1). Improving the bactericidal activity of highly muralytic bacteriophage endolysin EL188 has also been investigated, and synergy has been demonstrated with EDTA, citric acid, poly-L-lysine, and PMBN permeabilizer (60). In another example, diamines were utilized to improve membrane permeabilization, showing an improvement of bactericidal effects caused by novobiocin and tyrocidine and an induction of K+ leakage from the bacterial cytoplasm (61).
Exogenous natural polyamines have been shown to enhance P. aeruginosasusceptibility to different antibiotics, including nalidixic acid, trimethoprim, β-lactams, and chloramphenicol (62) (Figure1). Nevertheless, these same compounds were unable to improve the efficiency of novobiocin, erythromycin, and fusidic acid. This caused the authors to propose that the improvement of antibiotic susceptibility caused by polyamines is different from that associated with other compounds such as EDTA and PMBN.
Natural products have also been evaluated as agents for sensitizing Gram-negative bacteria to different antibiotics(63). In this context, the utilization of outer membrane permeabilizers from different sources combined with antibiotics would theoretically provide additional means of controlling the growth of resistant bacteria. Alternatively, known polycationic antibiotics that interact directly with the outer membrane should demonstrate excellent synergy in combination.
Although outer membrane permeabilizers have been a focus of research for many years, none have been successful in making it to the market. Prokaryotic and eukaryotic membranes are composed of different lipids, therefore there have been problems with certain permeabilizers (particularly cholic acid and its derivatives) showing a lack of bacterial specificity while polymyxin B nonapeptide demonstrated toxicity in early clinical trials. In addition, some of these compounds have also been shown to alter lipid metabolism in eukaryotic cells, making them unsuitable for use in humans (64). Perhaps a better way forward would be to destabilize the outer membrane by using antimicrobial/immunomodulatory peptides (discussed below) or by inhibiting essential lipopolysaccharide biosynthetic steps (e.g. LpxC, LpxH).
Adjuvants directed against adaptively resistant biofilms
In about 65% of infections, bacteria grow as biofilms, which are structured communities of organisms growing on surfaces. In this growth state, typical of chronic and device-related infections, bacteria become adaptively 10- to 1000-fold more resistant to antibiotics. As a new class of adjuvants, it was demonstrated that peptide 1018 not only had broad-spectrum antibiofilm activity (65) but also strongly synergized with highly utilized antibiotics (ceftazidime, tobramycin, imipenem, and ciprofloxacin) (66). It has also been previously shown that both the human peptide LL-37 and the synthetic peptide 1037 prevent biofilms from forming at concentrations that are only fractions of their MICs (67,68). Many other antibiofilm agents are in development and were recently discussed (69).
Antivirulence Drugs
Traditional antimicrobial drugs act in a bacteriostatic or bactericidal manner to eliminate microbial pathogens. They target gene products that act in processes that are essential to bacterial survival such as cell wall synthesis and folate metabolism, although intriguingly they are usually developed for their activities against bacteria growing in vitroin free solution rather than, for example, in vivoin the arguably more natural biofilm growth state. The species selectivity of these drugs can be broad or somewhat narrower in spectrum, but is never targeted solely against pathogenic species. In contrast, antivirulence drugs target gene products called virulence factors that are expressed in a bacterium-specific manner under infection conditions, and which are not essential for bacterial viability but rather are required for pathogenesis. Certain virulence factors that play offensive roles, such as toxins and host cell destroying enzymes (cf. more general host interaction factors like pili), are mostly absent from non-pathogenic species (70). Virulence factors are integral to the disease process, and in their absence, bacteria are generally unable to cause a pathological infection in their human hosts. The host immune system can work more effectively against any potential pathogens in the absence of virulence factors, and local flora may be more likely to outcompete pathogens as well. There are clear precedents that targeting virulence factors works, and, for example, many bacterial vaccines are directed in whole or in part at raising antibodies to neutralize toxins or other virulence factors. An attractive feature of this strategy is that drugs directed against virulence factors may be less likely to elicit resistance phenotypes as they do not disrupt pathways that are essential for viability, and they are unlikely to disrupt the normal flora as such species usually lack virulence factors (71). Although most antivirulence drugs are developed independently of their ability to act with antimicrobials, it seems likely that they would be used in combination therapies. It will be interesting to observe what types of combinatorial effects occur when antivirulence and antimicrobial compounds are used together. Targets of anti-infective drugs include quorum sensing, type II/III secretion systems, toxins, and biofilms to name a few (72) (Table3).
Table 3.
Antivirulence compounds with activity against bacterial pathogens
| Virulence system | Compound name(s) | Bacterium | Function | References |
|---|---|---|---|---|
| Quorum Sensing (QS) | Meta-bromo-thiolactone (mBTL) | P. aeruginosa | Binds and inhibits rhlR and lasR, inhibits pyocyanin production, reduces host killing in C. elegansinfection model | (84) |
| QS | 6-hydro-3H-1,2,3-triazolo[5,4-d\pyrimidin-7-one (C1), 2-amino-3-(3-fluorophenyl)propanoic acid (F1), 5-imino-4,6-dihydro-3H-1,2,3-triazolo[5,4-d\pyrimidin-7-one (G1), 2-amino-3-hydroxy-3-phenylpropanoic acid (H1) and indole-3-carboxylic acid (F2) | P. aeruginosa | All inhibit QS gene production, but only G1 competitively inhibits lasR receptor (expected target) | (85) |
| QS | Savirin | S. aureus | Binds agrA, inhibits production of agr QS system regulated genes, modulates host defense | (86) |
| QS | Halogenated furanone | P. aeruginosa | Inhibits biofilm formation, acts synergistically with tobramycin to disrupt biofilms and protects mice against chronic infections | (87,88) |
| QS | Catalytically enhanced E101G/R230C mutant of Geobacillus kaustophiluslactonase (GKL) | A. baumannii | Hydrolyzes C-3-hydroxylated acyl homoserine lactones, decreases biomass of biofilms | (91) |
| QS | Compound 1, compound 2 and compound 3 | B. mallei, Y. pestis | Inhibit acyl homoserine lactone synthase Bmal1 | (92) |
| Toxin | MEDI4893 | S. aureus | Alpha-toxin antibody | NCT01769417 |
| Toxin | Urtoxazumab | E. coli | Shiga-like toxin 2 antibody | (97) |
| Toxin | CDA1–CDB1 | C. difficile | Monoclonal antibodies against tcdA and tcdB toxins used with metronidazole or vancomycin | (98), NCT00350298 |
| Type II secretion system | Compounds 1,2,4,5,6,8 and 9 | P. aeruginosa | Inhibit secretion of phospholipase C and elastase, unknown mechanism | (104) |
| Type III Secretion System (T3SS) | TS027 and TS101 | P. aeruginosa | Decrease rsmY and rsmZ transcription, reduce exoS production | (109) |
| T3SS | Salicylidene acylhydrazides | Y. pseudotuberculosis,E. coliO157:H7 | Decrease T3SS production in culture, prevent host cell attachment, interact with wrbA, tpx, and folX. Increase activity of tpx and wrbA, which decrease T3SS expression | (110–112) |
| T3SS | CoilA and CoilB | E. coli, C. rodentium | Bind to C-terminal domain of espA, prevent espA polymerization, T3SS effector secretion | (113,114) |
| T3SS | Aurodox | E. coli, C. rodentium | Mechanism of action unclear, decreases T3SS-mediated hemolysis | (115) |
Quorum-sensing inhibitors
Quorum sensing (QS) is a process through which microbes are able to sense when cells reach a certain population density (quorum) via the production, secretion into their environment, uptake and receptor binding of specific diffusible molecules. QS systems were first discovered in light-producing Vibriospecies, but have since been identified in a broad range of both Gram-negative and Gram-positive bacteria. QS is of primary importance in certain pathogenic species, as many genes that control the production of virulence factors are regulated by signaling cascades initiated by the binding of QS ligands to their receptors (73). QS has also been shown to play a role in biofilm formation. The QS systems of Pseudomonas aeruginosaare perhaps the best studied of any bacterium, and this organism has been broadly employed as a model for QS system studies. Pseudomonas aeruginosahas two acyl homoserine lactone systems, which together control the transcription of nearly 9% of the genes in this organism (74–76). The Las system produces the signaling molecule N-3-oxododecanoyl-homoserine lactone, which can bind to both its own receptor (LasR) and the orphan receptor QscR (77,78). The second homoserine lactone system is the Rhl system, which synthesizes N-butyryl-homoserine lactone. This molecule binds to the RhlR receptor. These two systems are hierarchical as expression of the RhlR receptor is controlled by the Las system (79). In addition to the two homoserine lactone QS systems, P. aeruginosaalso produces 2-heptyl-3-hydroxyl-4-quinolone, which binds to the receptor PqsR (80).
Binding of QS molecules to their receptors in P. aeruginosaspecifically induces the expression of genes coding for various virulence factors including lectins, hydrogen cyanide, alkaline protease, exotoxin A, elastase, phenazine, pyocyanin, and rhamnolipids (81). Mutation of lasI(the protein that synthesizes N-3-oxododecanoyl-homoserine lactone) has been shown to impair (but not prevent) the formation of biofilms, which are important in the colonization of certain patients, such as individuals with cystic fibrosis (82). Moreover, lasIand lasRmutations have also been shown to decrease virulence in a mouse burn wound infection model (83).
As QS lies upstream of the expression of many virulence factor genes (some of which are directly toxic to animals) and is important for biofilm formation, compounds that inhibit these processes are a class of therapeutics that may work well as antimicrobial adjuvants. It should be noted that QS systems are also present in several non-pathogenic species. Therefore, care should be taken to ensure that inhibitors of these systems are pathogen specific. There are several ways in which QS can be inhibited (Table3). One method is to interfere with the binding of QS signaling molecules to their receptors. Bassler and colleagues (84) demonstrated that meta-bromo-thiolactone not only prevents virulence factor expression and biofilm formation, but also protected C. elegansand human A549 lung cells from killing by P. aeruginosa. Tan and colleagues conducted virtual screening on a library of natural compounds and were able to identify five that bound to the LasR protein in P. aeruginosaand altered downstream gene expression (85). Notably, levels of several lasR-regulated virulence factors were reduced in cells. The most promising inhibitor was also able to decrease the amount of extracellular DNA released by P. aeruginosain biofilms. Another group discovered an inhibitor of the S. aureusAgr QS system that they named savirin (86) (Figure1). This compound was found during a virtual screen and is able to significantly reduce the expression of genes regulated by the Agr QS system. In addition, it enhances murine macrophage-mediated bacterial killing, increases murine host defense to bacterial challenge, and elicits less resistance response from bacteria than traditional antibiotics (86). A natural product furanone was able to inhibit P. aeruginosabiofilm formation, modestly protect mice versus chronic Pseudomonasinfections and act synergistically versus biofilms with the antibiotic tobramycin (87,88).
Another method of inhibiting QS in bacteria is to destroy quorum-sensing molecules themselves. It has been shown that multiple enzymes are capable of cleaving both acyl homoserine lactones and quinolones produced by various species into molecules that cannot bind to QS receptors (89,90). Chow et al. (91) recently engineered a lactonase and showed that its action significantly decreased the thickness and mass of biofilms formed by Acinetobacter baumannii. A third method to disrupt bacteria QS is to halt the production of QS molecules in the first place by inhibiting the enzymes that synthesize them, such as lasI and rhlI. A high-throughput screen for such inhibitors revealed two that were active against the acyl homoserine lactone synthases of both Burkholderia malleiBmal1 and Yersinia pestisYspl (92). These inhibitors also showed activity in a cell-based assay and the most potent compound appeared to bind the enzyme in a non-competitive manner.
Bacterial toxin inhibitors
Toxins are virulence factors that are capable of killing host cells and/or modulating a variety of eukaryotic cell systems including cell signaling, transport, membrane integrity, and the cytoskeleton (93). For example, Shiga toxin from Shigella dysenteriaeand Shiga-like toxins from Escherichia colicause dysentery and food-borne illness that can lead to kidney failure. These toxins are composed of two types of subunits (A and B). The B subunits are responsible for binding to the host cell surface, while the A subunit is the active toxin. The A subunit is endocytosed into the cell and works by cleaving an N-glycosidic bond in the 28S rRNA (94). This stops protein synthesis. Clostridium difficile(which causes severe diarrhea) also secretes two toxins while in the human gut. These toxins (TcdA and TcdB) are internalized by cells via clathrin-coated vesicles. The toxins self-cleave inside the host cell and their glucosyltransferase domains disable Rac, Rho, and other GTPases (95). Staphylococcus aureusalpha-toxin forms pores in host cell membranes that allow certain cations, ATP and small molecules to pass through (96). This process leads to cell lysis.
Regardless of the method of toxin action, most current pharmaceutical endeavors to stop the effects of bacterial toxins are focused on toxin-specific antibodies (Table3). A S. aureusalpha-toxin antibody is currently undergoing phase I clinical trials (NCT01769417), while the safety and pharmacokinetics of an E. coliShiga-like toxin 2 antibody called urtoxazumab were recently positively evaluated in a human study (97) (Figure1). Toxin antibodies have also been used as an adjunctive therapy with antibiotic treatment for C. difficileinfections. A dose of two monoclonal antibodies against C. difficiletoxins TcdA and TcdB together with metronidazole or vancomycin decreased the re-infection rate by 31% (98). Re-infection following treatment with antibiotics is extremely common among C. difficilepatients (38% of patients treated with only antibiotics in this study became re-infected); therefore, this dual treatment comprised of antibiotics and antibodies is particularly promising.
Type II/III secretion system inhibitors
Type II secretion systems (T2SS) are utilized by a range of pathogenic and non-pathogenic Gram-negative bacteria to export folded proteins to the exterior of the cell. In addition, T2SS can be used in the assembly of cell surface organelles such as flagella and pili ((103,100)). The system itself is composed of 12 or more types of protein subunits, depending on the species ((103,100)). Proteins that are secreted by the T2SS first reach the periplasm via the Sec or Tat pathway. The T2SS then transports the proteins across the outer membrane. Some of these secreted proteins are involved in bacterial virulence, such as the metalloprotease elastase, hemolytic phospholipase C [both secreted by P. aeruginosa(101,102)\, and the outer membrane lipoprotein SslE of enteropathogenic E. colithat is required for biofilm formation (103).
As T2SS are not solely involved in virulence, and many non-virulent bacteria have them, there has been less research into methods of inhibition of this system than of other virulence determinants, such as toxins. However, there has been some work in this area as several T2SS substrates are virulence factors. For example, Moir and colleagues developed a high-throughput bioluminescent screening assay for T2SS inhibitors. Although none of the compounds were able to inhibit Sec-mediated β-lactamase translocation into the periplasm, nine compounds suppressed the secretion of elastase by P. aeruginosa(104). Seven of these compounds also suppressed the secretion of phospholipase C. Most research in the T2SS area has been focused on Sec inhibitors rather than inhibitors of the type II secretion apparatus itself. This may be due to the fact that the Sec pathway is present in both Gram-negative and Gram-positive bacteria, exports a broad range of proteins from the cytoplasm, and some of the genes involved are essential for viability (105). Therefore, compounds inhibiting the Sec machinery fall into the category of traditional antimicrobials rather than antivirulence drugs.
Type III secretion systems (T3SS) are virulence factors of certain Gram-negative pathogens including P. aeruginosa,Yersinia pestis,Salmonellaspp., Chlamydiaspp., E. coli, and Vibriospp. They comprise 14 or more proteins (depending on the species) that assemble in a stepwise manner into complex structures that span both the inner and outer bacterial membranes and can extend to the eukaryotic host cell's membrane (106). They act as molecular syringes that transfer first their own mammalian host cell receptor and subsequently bacterial effector molecules directly into the host cell's cytoplasm. Effector molecules target multiple host cell types including those of the innate immune system to promote host colonization. Effectors from various bacteria have been shown to modify protein export from the golgi, tight junctions between cells, depolymerization of actin, mitochondrial membrane polarity, membrane integrity, cell division, phagocytosis, cell migration, and cause cell death (107,108).
There are several mechanisms that could be used to target the T3SS. Such strategies fall into two broad categories: preventing the expression of genes encoding the molecular syringe or effectors and interfering with the assembly/activity of the syringe (Table3). Yamazaki et al. (109) identified two phenolic compounds that fall into the first category. These compounds caused almost no growth inhibition of P. aeruginosa, but significantly decreased the production of the effector exoS. This was due to a decrease in transcription of rsmY and rsmZ, which are small RNAs that act post-transcriptionally in P. aeruginosa. A class of compounds called the salicylidene acylhydrazides has also been a recent focus of research. Kauppi et al. (109) performed a screen of a chemical library with a reporter gene assay for inhibitors of the Yersinia pseudotuberculosisT3SS. They identified three compounds that had either mild or no effects on bacterial growth, but reduced T3SS expression to 20% or less of control at concentrations of between 10 and 50 μm. Tree et al. (110) examined the effects of four members of the same class of compounds on E. coliO157:H7 and a range of other E. colioutbreak isolates. The presence of the salicylidene acylhydrazides prevented the E. coliO157:H7 T3SS expression in culture and inhibited bacterial attachment to bovine cells. These compounds affected the expression of genes associated with virulence in the isolates to various degrees. Using affinity chromatography, Wang et al. (111) identified three bacterial proteins that bind salicylidene acylhydrazides, namely Tpx (a thiol peroxidase), WrbA (an NAD(P)H quinone oxidoreductase), and FolX (a dihydroneopterin-tri-P-epimerase). They proposed a mechanism whereby the salicylidene acylhydrazides bolstered the repressive action of Tpx and WrbA on the T3SS.
Larzabal et al. designed two 15-amino-acid peptides (CoilA and CoilB) that interact with the C-terminal domain of EspA, a component of the T3SS (113,114) (Figure1). Administration of these peptides prevented hemolysis of red blood cells by enteropathogenic and enterohemorrhagic E. colidue to the inhibition of the T3SS. Both the polymerization of EspA and effector secretion into eukaryotic cells were decreased. In addition, in a mouse model of infection with Citrobacter rodentium(the enteropathogenic E. coliequivalent in mice), the presence of the peptides blocked colon damage. Another compound that was identified as an inhibitor of the T3SS in a screening study was aurodox (115). Aurodox is produced naturally by some Streptomycesspecies and is an antibiotic that inhibits EF-TU in certain bacteria (116). In one screening study, the compound did not affect bacterial growth at concentrations below 40 μg/mL but at only 1.5 μg/mL, a decrease in T3SS-mediated hemolysis by enteropathogenic E. coliwas observed in vitro. In addition, a boost in survival was detected in a mouse infection model of C. rodentiumusing aurodox compared to tetracycline (115). The mechanism by which aurodox affects type III secretion is, however, unclear.
Antivirulence drugs in perspective
Antivirulence drugs can pose more hurdles for development than standard antibiotics, as they are ideally targeted uniquely toward pathogens (and sometimes individual genera of bacteria) rather than large groups of prokaryotes and can be quite specific due to the massive heterogeneity in virulence systems in bacteria. Their effectiveness can also be more difficult to ascertain. For example, the major test for conventional antibiotic action is bacterial growth inhibition. However, inhibiting virulence factors should not cause a decrease in growth of bacteria outside their hosts. Standard MIC measurements are not possible for these compounds. Therefore, costly animal infection models are required sooner in development to test which of a panel of antivirulence drugs are most effective. Efficacy measures must rely on the drugs' ability to clear infections in animals. This complicates the determination of an optimal dose. However, many researchers believe that these therapies are much less likely to exert evolutionary pressure to develop resistance on bacteria than antibiotics (71). This is due to the fact that antivirulence drugs do not impair microbial growth under most conditions and only act when virulence factors are being expressed. Virulence factors are usually not necessary for bacterial survival, but rather serve in pathogenesis.
Additional hurdles to the development of antivirulence drugs exist, including the issue of whether such therapies can be given after an infection has already been established, or whether they should be taken prophylactically in high-risk situations. Where these drugs synergize with existing antimicrobials, one could envision a combination antivirulence and antimicrobial treatment for infections. However, in order for antivirulence drugs to make it to this stage, they must be rigorously evaluated in clinical trials, where combination doses would have to be titrated. For certain antivirulence drugs, entry into bacterial cells is also a hurdle to clear on the path to development. This is not an issue for toxin inhibitors that act outside the cell. However, quorum-sensing inhibitors that work via repression of quorum-induced gene expression or drugs that act to block the assembly of the T3SS would be required to clear the outer membrane of Gram negatives.
One clinical trial (NCT00610623) aimed to test the effects of inhibiting quorum sensing in hospital patients with ventilators that were colonized with P. aeruginosa(117). They used the macrolide antibiotic azithromycin that can act as QS inhibitor and also has little anti-Pseudomonasantibiotic activity. Before treatment with azithromycin, P. aeruginosapopulations were composed of both wild-type cells and less virulent QS mutants and the latter increased over time in the absence of treatment. Azithromycin inhibited QS and actually increased the proportion of more virulent wild-type cells as without extra QS molecules in the environment, the QS mutants stopped replicating as efficiently. Another study was conducted that examined the ability of P. aeruginosaQS mutants to propagate in cultures grown in minimal media in the presence of brominated furanone C-30 (a QS inhibitor), where adenosine was used as a carbon source to mimic infection conditions (118). It was found that the QS mutants increased in frequency and that they were more virulent in a C. elegansinfection model. For an antivirulence drug to have a decreased chance of eliciting bacterial resistance, it must not negatively affect bacterial growth or fitness. These studies suggest that selection pressure dynamics should be further examined for antivirulence drugs to determine which antivirulence strategies have lowered resistance potential. This is especially true in the case of chronic infections, which may be more difficult to clear and where the possibility of enriching the proportion of more virulent phenotypes over a long treatment period is possible.
Host-Directed Therapies
The innate immune system is the body's first line of defense against bacterial infections. As opposed to the adaptive immune system, this branch of our immune systems is primed to respond immediately to pathogens. Pathogens possess distinct chemical signatures (also termed pathogen associated molecular patterns) that are recognized by an assortment of pattern recognition receptors (PRRs) located on the cell surface, in the cytoplasm and inside the endosomes of dendritic cells and macrophages. PRRs fall into three main categories: Toll-like receptors (TLRs), RIG-I-like receptors (RLRs), and the NOD receptors.
There are ten TLRs in humans, each of which has an affinity for a specific set of signature molecules. Most TLRs recognize bacteria and their signature proteins, lipids, nucleotides, and other components (119,120). Of the TLRs that recognize bacterial signatures, all are located on the cell surface except 3, 7, and 9, which are associated with endosomes. Binding of a bacterial signature molecule to a TLR initiates a signaling cascade instigated by the activation of MyD88-dependent and/or MyD88-independent and many other signal transduction pathways and leading to numerous effector functions including the transcription of pro-inflammatory cytokines and/or type I interferons (119,120).
RLRs are located in the cytoplasm and are devoted to sensing viruses, intracellular bacteria and parasites via their genetic material/polynucleotides. Activation of RLR signaling leads to a TRAF-3-mediated cascade that leads to the production of type I interferons (119,120). NOD receptors are a large family of proteins that form multiprotein complexes in the cytoplasm. Their ligands range from bacterial-derived muramyl dipeptide to fungi, viruses, and flagellin (119,120). The inflammasome complex, which is induced by this and other PRR-directed pathways, activates caspase upon ligand binding, which in turn cleaves pro-IL-1βinto its active (pro-inflammatory) form. Other NOD receptors initiate signaling cascades upon ligand binding that directly result in the production of pro-inflammatory cytokines (119,120).
Thus the key effector functions of innate immunity include protective functions, such as recruitment of immune cells, their activation by triggering of signal transduction pathways or differentiation, and enhancement of microbial clearance primarily by phagocytosis, as well pro-inflammatory cytokines and processes that can be supportive when moderately induced but potentially harmful when excessively produced. Ultimately the key to manipulating innate immunity for therapeutic benefit involves stimulating protective immunity while avoiding excessive and potentially harmful inflammatory responses.
Innate immune system agonists as vaccine adjuvants
As bacterial signatures are strong stimulants of the immune system, many are being explored as adjuvants to enhance immunogenicity in a wide range of vaccines. Natural TLR agonists and robust synthetic agonists such as polyinosinic: polycytidylic acid (poly I:C; TLR3 agonist) and CpG oligonucleotides (TLR9 agonist) have been studied in this capacity. These agonists set off a cascade that triggers the maturation of dendritic cells and antigen presentation and activates immune cells to secrete cytokines (121) (Table3).
Another example involves a phase I clinical trial that is currently being conducted on a vaccine against Yersinia pestis, the causative agent of bubonic and pneumonic plague (NCT01381744). The vaccine is composed of flagellin (a potent TLR5 agonist) and Y. pestisF1 and V antigens. It is hoped that the vaccine will protect inoculated individuals against pneumonic plague. In another recent study conducted on mice by Orr et al., it was concluded that the use of both glucopyranosyl lipid adjuvant-stable emulsion (GLA-SE), (a TLR4 ligand), and CpG-containing DNA (a TLR9 ligand) as adjuvants in a M. tuberculosissubunit vaccine had a synergistic effect in increasing immunity to bacterial challenge (122). The authors propose that this synergy may be due to the activation of both MyD88 and TRIF signaling pathways. Multiple PRRs are also stimulated by the YF-17D (yellow fever) and Infanrix vaccines (diphtheria, tetanus, pertussis, polio and influenza) (as well as others), to achieve a greater immune response (123,124).
Immunomodulatory peptides
Another method of immune system modulation is the use of immunomodulatory peptides to control the immune response to infections (Table4). Immunomodulatory peptides are naturally occurring components of our innate immune system that assist the body in the recognition and clearance of pathogens. Such peptides are short (under 50 amino acids in length), amphipathic, cationic and may also have direct bactericidal action against a broad range of bacteria. For example, the activity of the innate defense peptide LL-37 has been well characterized. This peptide is naturally present in the human body at concentrations of up to 5 μg/mL. LL-37 displays faint antimicrobial activity but is able to exercise a broad influence on the innate immune system (125). LL-37's activity includes the upregulation of the neutrophil antimicrobial response and the downregulation of pro-inflammatory cytokines and IFN-gamma (126–128). This peptide elevates angiogenesis (129), S. aureuscutaneous infection clearance, wound healing (130,131), and promotes rat survival in an E. colisepsis model (132). Many synthetic peptides have also been tested for their ability to aid in the clearance of infections. For instance, IDR-1018 has been evaluated in a variety of circumstances for its immunomodulatory capabilities. It is capable of increasing wound healing (and is superior to LL-37 in this respect) and augments the speed of S. aureuscutaneous infection clearance (131). Administration of IDR-1018 in a mouse M. tuberculosisinfection model significantly decreased CFU counts in the lung (133), while giving malaria-infected mice a combination of standard antimalarial drugs and IDR-1018 significantly improved their survival rates and decreased signs of inflammation (134). In addition to its immunomodulatory activity, IDR-1018 also has some antimicrobial properties and the ability to act synergistically with many antibiotics to clear biofilms formed by several bacteria including P. aeruginosaand S. aureus(66).
Table 4.
Host-directed therapies with targets in the innate immune system
| Therapy type | Compound name(s) | Efficacy data | References |
|---|---|---|---|
| Vaccine adjuvant | Flagellin/F1/V (flagellin and Y. pestisF1 and V antigens) | Phase I clinical trial data not yet available | NCT01381744 |
| Vaccine adjuvant | ID93+GLA-SE+CpG (M. tuberculosissubunit vaccine + GLA-SE+CpG) | Addition of CpG as 2nd adjuvant had synergistic effect in increasing immunity to bacteria | (122) |
| Immunomodulatory peptide | LL-37 | Increases angiogenesis, wound healing, S. aureuscutaneous infection clearance and rat survival in an E. colisepsis model | (129–132) |
| Innate defense regulator peptides | IDR-1018 IDR-1 IDR-1002 IDR-HH2 | Protects in murine models versus MDR M. tuberculosis,E. coli, Salmonella, S. aureusincluding MRSA, VRE, HSV virus; LPS/hypoxia–ischemia; cerebral malaria; leads to enhanced wound healing in mice and pigs; component of adjuvant combinations | (131,133,134) |
| Immunomodulatory peptide | hLF1-11 (lactoferritin derivative) | Similar ability to gentamicin to clear MRSA infection in rabbit osteomyelitis infection model, well tolerated in phase I clinical trial | (135,136) |
| Immunomodulatory peptide | EDC34 (tissue factor pathway inhibitor 2 derivative) | Enhances mouse survival of E. coliand P. aeruginosainfections in combination with ceftazidime | (137) |
hLF1-11, an 11-amino-acid peptide derivative of the human protein lactoferritin demonstrated similar infection clearing capabilities to gentamycin in a rabbit osteomyelitis infection model (135). The peptide was then tested in a phase I clinical trial for safety in healthy volunteers and hematopoietic stem cell transplant patients (136). Some patients had a slight increase in levels of transaminases, but it was not determined whether this was directly caused by the peptide or not. Otherwise, the peptide was well tolerated. In another study, a peptide from the C-terminus of tissue factor pathway inhibitor 2 (TFPI-2), EDC34, was effective at enhancing mouse survival of E. coliand P. aeruginosainfections when used in combination with ceftazidime (137). The peptide also proved to be bactericidal on its own.
Immunomodulatory drugs can pose a substantial hurdle in their creation. They are generally modeled on factors that are produced by the mammalian immune system, and as such, have to be tested extensively for efficacy in vivo, which drives up costs. Mammalian immune systems differ from one another, and drugs that are efficacious in mice may not work similarly in humans (138). The immune system can be a minefield to navigate and drugs can cause a myriad of problems if over stimulation occurs.
Alternative Treatments for Bacterial Infections
Enormous efforts have been invested in the discovery of novel antimicrobial compounds and antimicrobial adjuvants for the control of bacterial infections with only modest success to date. Therefore, many alternative treatments are in use. Some of these treatments are extremely modest like simply avoiding bacteria that are able to cause infections by cooking fresh foods at proper temperatures, washing any raw fruits and vegetables that could contain potential hazardous pathogens, and keeping the hands clean. Other unusual and more complex treatments have also been applied including phage therapy or the use of competitive beneficial micro-organisms. There is evidence that these methods provide at least a modicum of effectiveness and must be considered as a possible substitute in the complete absence of useful antibiotics.
Oral rehydration systems
One real problem in several poor regions is dehydration caused by bacterial and viral infections. In fact, dehydration is a major cause of pediatric morbidity and mortality through the world. More than 750 000 deaths occur worldwide in children younger than 5 years due to diarrheal diseases each year (139). One of the oldest methods that has been utilized for centuries to treat diarrheal diseases caused by many pathogens is to improve patient hydration with oral rehydration solutions (ORS). ORS can be water, saline, a homemade isosmotic solution prepared with a glass of water, a tea spoon of salt and a dessert spoon of sugar or a plant-derived fluid-like green coconut water (139,140). Treatment with ORS has made the difference between life and death in many cases. The large cholera outbreak in Haiti in 2010 and 2011 after the catastrophic earthquake is an example of such a case. This outbreak saw the largest cohort of pregnant women with cholera hospitalized to date, and they were treated using standard cholera treatment guidelines, which include erythromycin and rehydration via IV if the patient is dehydrated and continued hydration with ORS throughout the course of the illness to replace fluids lost through diarrhea. The repeated administration of ORS helped to prevent the severe dehydration that is the main risk factor for fetal death, especially in large epidemics (141), and saved the lives of hundreds of people.
Phage therapy
Rehydration is a simple measure taken to ameliorate the symptoms of bacterial infections, but other more complex treatments have also been used, such as phage therapy. Bacteriophages are viruses with specific host ranges that attack bacteria (142). Bacteriophage utilization is not new, having been applied for the first time in 1917, using an oral phage preparation to treat bacterial dysentery (143). Moreover, phages were broadly used in Soviet Union countries and companies in the USA and Europe developed bacteriophage products in the 1930s until the discovery of antibiotics lead to a decrease their utilization (144). Bacteriophages can act in either a lytic or lysogenic manner, but affect bacterial growth primarily during lytic cycles. When infected by a lytic phage, the viral DNA does not insert into the bacterial (host) genome and replicates separately from the host DNA. In this circumstance, phages replicate in high numbers inside the bacterial cell, leading to cell lysis. At the completion of the cycle, newly formed phage particles are released from the lysed cell. Other phages act in a lysogenic manner, whereby the phage genome integrates into the bacterial host genome (as a prophage), but the bacterium continues to replicate normally. In this case, the virus stays in a dormant (unexpressed) state for lengthy time periods and becomes activated by adverse environmental conditions. Activation results in the replication of phage particles and host cell lysis (145).
An important characteristic of bacteriophages is that their host ranges are extremely specific for certain bacteria, and therefore, they do not disturb the host organism and intestinal microflora. Treatment with antibiotics often destroys host microbial communities (146). Bacteriophages may transport virulence factors or toxic genes (147). Therefore, the genomes of phages to be used as antimicrobials should be sequenced so that genes with similarity to known virulence factors or toxins can be identified (148). Due to these limitations, bacteriophages have been much more utilized in the treatment of animal infections as veterinary products (149,150) or as anticontaminants for medical supplies such as antibiofilm catheter protection (151). Although this type of therapy has only been approved in Russia, Georgia, and a few other countries for extreme infections (152), bacteriophages are a clear and useful strategy to control bacteria that no longer respond to conventional antibiotics (153). In such cases, genetically modified phages have yielded enhanced activity against antibiotic-resistant bacteria, persistent cells, and biofilm cells, and also act as robust adjuvants for antibiotics (154,155). Phase I/II clinical trials are scheduled or underway for a number of phage preparations. One preparation is being tested as a topical agent for burn wound infections (NCT02116010), while another group of phage cocktails is being assayed via both topical and oral delivery systems for the treatment of persistent postoperative, upper respiratory tract and GI tract infections (NCT00945087). Overall, the oral and IV administration of phages for the eradication of bacterial infections poses a much higher safety risk than topical application, and there is the additional concern of uncertain immune responses to these large antigenic cocktails. Therefore, there are considerable regulatory hurdles that must be cleared for such therapies.
Probiotics and prebiotics
Another option for the control of bacterial pathogens involves directly or indirectly increasing the beneficial micro-organisms inside the body. To achieve this aim, prebiotics and probiotics have been commonly utilized and could be considered an interesting strategy to control-resistant bacteria through interspecific competition. Probiotics are live non-pathogenic micro-organisms that are commonly derived from gastrointestinal microbiota. They offer clear benefits to human health when present at specific concentrations. Prebiotics are foods, such as specialized plant fiber that nourish helpful bacteria already present in the digestive tract. In this case, the host's body does not digest the fibers, but the fibers promote the growth of beneficial bacteria. Both probiotics and prebiotics work to effectively increase the population of harmless micro-organisms in the gut to compete with and supplant resident drug-resistant bacteria (156).
The use of probiotics and prebiotics for improving intestinal health was recommended many years ago (157). Nowadays, most probiotics are bile-resistant Gram-positive bacterial strains from the Lactobacillusgroup including the genera Lactobacillus, Enterococcus, Streptococcus, Lactococcus, Pediococcus, Bifidobacterium,and Leuconostoc(158). Administration of prebiotics and probiotics have been directly linked to human health, such as the improvement of the epithelial barrier, the ability to digest lactose, pH-lowering capacity, adhesion of probiotic bacteria to the intestinal mucosa and concomitant inhibition of pathogen adhesion, and immune system modulation by inducing immune cell recruitment and triggering suitable inflammatory and immune responses (159). Some probiotics are also able to inhibit the growth of pathogenic strains via the synthesis of antimicrobial substances such as volatile fatty and modified bile acids as well bacteriocins (160). Bacteriocins can be extremely selective for pathogens and utilized to control them. These compounds kill cells by pore formation and/or by inhibition of cell wall synthesis. Several studies have revealed that certain bacteriocins show potential as therapeutic agents (161) and should be explored further. A pioneering strategy has been proposed that includes the administration of probiotics expressing antimicrobial peptides (AMPs) for patients with severe and resistant bacterial infections (162). Such a dual therapy with the combination benefits of direct AMP antibacterial activity and the probiotic bacteria's ability to inhibit pathogenic bacterial adhesion to host cells could be advantageous. This combined therapy would have the added benefit of the immunomodulatory activities of both the AMP and the probiotic. The joint utilization of probiotics and exogenous AMPs has not yet been tested in humans (163).
The translation of prebiotics, probiotics (164), and the other alternative therapies described in this section is still under debate, especially for the treatment of resistant and serious infections. In all cases, they should be considered as reasonable strategies to control the most dangerous bacteria that become less controllable each day.
Concluding Remarks
To effectively combat bacterial infections, we must work to extend the life span of our current repertoire of antibiotics. There are many strategies to do this, including restricting the quantity of antibiotics that are used in agriculture, reducing the number of antibiotic prescriptions that are given for non-microbial diseases (such as the flu), better educating the public so they are aware they must finish their entire antibiotic prescription (even if they are feeling better), and employing antibiotic adjuvants. Employing adjuvants will enable us to use extant drugs on microbes that have long since developed resistance or were never susceptible to certain drugs in the first place. In addition, by utilizing an adjuvant and an antimicrobial concurrently, the odds of developing resistance are decreased. By modulating the host's innate immune system, we can improve the body's ability to eliminate infections via multiple mechanisms. Greater reliance on immunomodulatory therapies may equate with decreased reliance on traditional antimicrobial drugs as our bodies will be able to clear more virulent bacteria. Employing alternative treatment methods such as those discussed above for bacterial diseases would help to decrease the amount of antibiotics being used and may lengthen the time required for antimicrobial resistance to develop in some species.
Other points that should be taken into consideration include the life span of these new anti-infective and immunomodulatory drugs in the environment. Many antibiotics persist in the environment for an extended period of time after they have been excreted. This can lead to augmented levels of antibiotic resistance genes in areas where antibiotics are present (3) and poses concerns regarding the spread of these genes into pathogenic species or potential pathogens. Although anti-infective and immunomodulatory drugs are believed to have a reduced potential to elicit resistance from bacteria, it would be wise to ensure that they do not accumulate in the environment in the same way as antibiotics.
Acknowledgments
We acknowledge funding for our own adjuvants research from the Canadian Institutes for Health Research MOP-123477 and from the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number 4R33AI098701-03. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. R.E.W.H. holds a Canada Research Chair in Health and Genomics.
References
- 1.Clatworthy AE, Pierson E, Hung DT. Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol. 2007;3:541–548. doi: 10.1038/nchembio.2007.24. [DOI] [PubMed] [Google Scholar]
- 2.Liu YC, Li YS, Lyu SY, Hsu LJ, Chen YH, Huang YT, Chan HC, Huang CJ, Chen GH, Chou CC, Tsai MD, Li TL. Interception of teicoplanin oxidation intermediates yields new antimicrobial scaffolds. Nat Chem Biol. 2011;7:304–309. doi: 10.1038/nchembio.556. [DOI] [PubMed] [Google Scholar]
- 3.Kristiansson E, Fick J, Janzon A, Grabic R, Rutgersson C, Weijdegard B, Soderstrom H, Larsson DG. Pyrosequencing of antibiotic-contaminated river sediments reveals high levels of resistance and gene transfer elements. PLoS ONE. 2011;6:e17038. doi: 10.1371/journal.pone.0017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. 2013. Antibiotic resistance threats in the United States, 2013 http://www.cdc.gov/drugresistance/threat-report-2013/
- 5.Hogberg LD, Heddini A, Cars O. The global need for effective antibiotics: challenges and recent advantages. Trends Pharmacol Sci. 2010;31:509–515. doi: 10.1016/j.tips.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 6.FDA. 2014. FDA news release: FDA approves dalvance to treat skin infections http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm398724.htm.
- 7.Corey GR, Kabler H, Mehra P, Gupta S, Overcash JS, Porwal A, Giordano P, Lucasti C, Perez A, Good S, Jiang H, Moeck G, O'Riordan W SOLO I Investigators. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370:2180–2190. doi: 10.1056/NEJMoa1310422. [DOI] [PubMed] [Google Scholar]
- 8.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlet J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 9.Higgins PG, Dammhayn C, Hackel M, Seifert H. Global spread of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2010;65:233–238. doi: 10.1093/jac/dkp428. [DOI] [PubMed] [Google Scholar]
- 10.van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant enterobacteriaceae: a review of treatment and outcomes. Diagn Microbiol Infect Dis. 2013;75:115–120. doi: 10.1016/j.diagmicrobio.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, Gerding D, Lynfield R, Reller LB, Rex J, Schwartz D, Septimus E, Tenover FC, Gilbert DN. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52:S397–S428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shlaes DM. New β-lactam-β-lactamase inhibitor combinations in clinical development. Ann NY Acad Sci. 2013;1277:105–114. doi: 10.1111/nyas.12010. [DOI] [PubMed] [Google Scholar]
- 13.Abuzaid A, Hamouda A, Amyes SG. Klebsiella pneumoniaesusceptibility to biocides and its association with cepA, qacΔE and qacE efflux pump genes and antibiotic resistance. J Hosp Infect. 2012;81:87–91. doi: 10.1016/j.jhin.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Urakawa H, Yamada K, Komagoe K, Ando S, Oku H, Katsu T, Matsuo I. Structure-activity relationships of bacterial outer-membrane permeabilizers based on polymyxin B heptapeptides. Bioorg Med Chem Lett. 2010;20:1771–1775. doi: 10.1016/j.bmcl.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 15.Bush K. Improving known classes of antibiotics: an optimistic approach for the future. Curr Opin Pharmacol. 2012;12:527–534. doi: 10.1016/j.coph.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Pucci MJ, Bush K. Investigational antimicrobial agents of 2013. Clin Microbiol Rev. 2013;26:792–821. doi: 10.1128/CMR.00033-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palzkill T. Metallo-β-lactamase structure and function. Ann N Y Acad Sci. 2013;1277:91–104. doi: 10.1111/j.1749-6632.2012.06796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bush K, Jacoby GA, Medeiros AA. A functional classification scheme for β-lactamases and its correlation with molecular structure. Antimicrob Agents Chemother. 1995;39:1211–1233. doi: 10.1128/aac.39.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stachyra T, Pechereau MC, Bruneau JM, Claudon M, Frere JM, Miossec C, Coleman K, Black MT. Mechanistic studies of the inactivation of TEM-1 and P99 by NXL104, a novel non-beta-lactam beta-lactamase inhibitor. Antimicrob Agents Chemother. 2010;54:5132–5138. doi: 10.1128/AAC.00568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Kern G, Walkup GK, Fisher SL. Avibactam is a covalent, reversible, non-beta-lactam beta-lactamase inhibitor. Proc Natl Acad Sci USA. 2012;109:11663–11668. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levasseur P, Girard AM, Claudon M, Goossens H, Black MT, Coleman K, Miossec C. In vitroantibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosaclinical isolates. Antimicrob Agents Chemother. 2012;56:1606–1608. doi: 10.1128/AAC.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flamm RK, Farrell DJ, Sader HS, Jones RN. Ceftazidime/avibactam activity tested against gram-negative bacteria isolated from bloodstream, pneumonia, intra-abdominal and urinary tract infections in US medical centres (2012) J Antimicrob Chemother. 2014;69:1589–1598. doi: 10.1093/jac/dku025. [DOI] [PubMed] [Google Scholar]
- 23.Blizzard TA, Chen H, Kim S, Wu J, Bodner R, Gude C, Imbriglio J, et al. Discovery of MK-7655, a β-lactamase inhibitor for combination with Primaxin®. Bioorg Med Chem Lett. 2014;24:780–785. doi: 10.1016/j.bmcl.2013.12.101. [DOI] [PubMed] [Google Scholar]
- 24.Hecker SJ, Reddy KR, Totrov M, Hirst GC, Lomovskaya O, Griffith DC, King P, Tsivkovski R, Sun D, Sabet M, Taraz Z, Dudley MN. 2012. Discovery of RPX7009, a broad-spectrum beta-lactamase inhibitor with utility versus class A serine carbapenemases. Abstr 52nd Intersci Conf Antimicrob Agents Chemother: http://www.icaac.org/
- 25.Winkler ML, Rodkey EA, Taracila MA, Drawz SM, Bethel CR, Papp-Wallace KM, Smith KM, Xu Y, Dwulit-Smith JR, Romagnoli C, Caselli E, Prati F, van den Akker F, Bonomo RA. Design and exploration of novel boronic acid inhibitors reveals important interactions with a clavulanic acid-resistant sulfhydryl-variable (SHV) β-lactamase. J Med Chem. 2013;56:1084–1097. doi: 10.1021/jm301490d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondon M, Hur S, Vadlamani G, Rodrigues P, Tsybina P, Oliver A, Mark BL, Vocadlo DJ, Blériot Y. Selective trihydroxyazepane NagZ inhibitors increase sensitivity of Pseudomonas aeruginosato β-lactams. Chem Commun (Camb) 2013;49:10983–10985. doi: 10.1039/c3cc46646a. [DOI] [PubMed] [Google Scholar]
- 27.Rodkey EA, Winkler ML, Bethel CR, Pagadala SR, Buynak JD, Bonomo RA, van den Akker F. Penam sulfones and β-lactamase inhibition: SA2-13 and the importance of the C2 side chain length and composition. PLoS ONE. 2014;9:e85892. doi: 10.1371/journal.pone.0085892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gan M, Liu Y, Bai Y, Guan Y, Li L, Gao R, He W, You X, Li Y, Yu L, Xiao C. Polyketides with New Delhi metallo-β-lactamase 1 inhibitory activity from Penicilliumsp. J Nat Prod. 2013;76:1535–1540. doi: 10.1021/np4000944. [DOI] [PubMed] [Google Scholar]
- 29.Yamada K, Yanagihara K, Kaku N, Harada Y, Migiyama Y, Nagaoka K, Morinaga Y, Nakamura S, Imamura Y, Miyazaki T, Izumikawa K, Kakeya H, Hasegawa H, Yasuoka A, Kohno S. In vivoefficacy of biapenem with ME1071, a novel metallo-β-lactamase (MBL) inhibitor, in a murine model mimicking ventilator-associated pneumonia caused by MBL-producing Pseudomonas aeruginosa. Int J Antimicrob Agents. 2013;42:238–243. doi: 10.1016/j.ijantimicag.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 30.Higgins PG, Stefanik D, Page MG, Hackel M, Seifert H. In vitroactivity of the siderophore monosulfactam BAL30072 against meropenem-non- susceptible Acinetobacter baumannii. J Antimicrob Chemother. 2012;67:1167–1169. doi: 10.1093/jac/dks009. [DOI] [PubMed] [Google Scholar]
- 31.Bush K, Macielag MJ. New β-lactam antibiotics and β-lactamase inhibitors. Expert Opin Ther Pat. 2010;20:1277–1293. doi: 10.1517/13543776.2010.515588. [DOI] [PubMed] [Google Scholar]
- 32.Tilvawala R, Pratt RF. Kinetics of action of a two-stage pro-inhibitor of serine β-lactamases. Biochemistry. 2013;52:7060–7070. doi: 10.1021/bi400873r. [DOI] [PubMed] [Google Scholar]
- 33.King AM, Reid-Yu SA, Wang W, King DT, De Pascale G, Strynadka NC, Walsh TR, Coombes BK, Wright GD. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature. 2014;510:503–506. doi: 10.1038/nature13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang JI, Chen YP, Miller KP, Ganewatta MS, Bam M, Yan Y, Nagarkatti M, Decho AW, Tang C. Antimicrobial metallopolymers and their bioconjugates with conventional antibiotics against multidrug-resistant bacteria. J Am Chem Soc. 2014;136:4873–4876. doi: 10.1021/ja5011338. [DOI] [PubMed] [Google Scholar]
- 35.Yuan J, Chow DC, Huang W, Palzkill T. Identification of a β-lactamase inhibitory protein variant that is a potent inhibitor of StaphylococcusPC1 β-lactamase. J Mol Biol. 2011;406:730–744. doi: 10.1016/j.jmb.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Li L, Chen S, Xu Y, Xia Q, Guo Y, Liu X, Tang Y, Zhang T, Chen Y, Yang C, Shui W. Identification of inhibitors of the antibiotic-resistance target New Delhi metallo-β-lactamase 1 by both nanoelectrospray ionization mass spectrometry and ultrafiltration liquid chromatography/mass spectrometry approaches. Anal Chem. 2013;85:7957–7965. doi: 10.1021/ac401732d. [DOI] [PubMed] [Google Scholar]
- 37.Sohier JS, Laurent C, Chevigné A, Pardon E, Srinivasan V, Wernery U, Lassaux P, Steyaert J, Galleni M. Allosteric inhibition of VIM metallo-β-lactamases by a camelid nanobody. Biochem J. 2013;450:477–486. doi: 10.1042/BJ20121305. [DOI] [PubMed] [Google Scholar]
- 38.Ripoll A, Galán JC, Rodríguez C, Tormo N, Gimeno C, Baquero F, Martínez-Martínez L, Cantón R SEIMC Quality Control Study Group. Detection of resistance to beta-lactamase inhibitors in strains with CTX-M beta-lactamases: a multicenter external proficiency study using a well-defined collection of Escherichia colistrains. J Clin Microbiol. 2014;52:122–129. doi: 10.1128/JCM.02340-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biot FV, Valade E, Garnotel E, Chevalier J, Villard C, Thibault FM, Vidal DR, Pagès JM. Involvement of the efflux pumps in chloramphenicol selected strains of Burkholderia thailandensis: proteomic and mechanistic evidence. PLoS ONE. 2011;6:e16892. doi: 10.1371/journal.pone.0016892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biot FV, Lopez MM, Poyot T, Neulat-Ripoll F, Lignon S, Caclard A, Thibault FM, Peinnequin A, Pagès JM, Valade E. Interplay between three RND efflux pumps in doxycycline-selected strains of Burkholderia thailandensis. PLoS ONE. 2013;8:e84068. doi: 10.1371/journal.pone.0084068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamers RP, Cavallari JF, Burrows LL. The efflux inhibitor phenylalanine-arginine beta-naphthylamide (PAβN) permeabilizes the outer membrane of gram-negative bacteria. PLoS ONE. 2013;8:e60666. doi: 10.1371/journal.pone.0060666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar A, Schweizer HP. Bacterial resistance to antibiotics: active efflux and reduced uptake. Adv Drug Deliv Rev. 2005;57:1486–1513. doi: 10.1016/j.addr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Ince D, Zhang X, Silver LC, Hooper DC. Dual targeting of DNA gyrase and topoisomerase IV: target interactions of garenoxacin (BMS-284756, T-3811ME), a new desfluoroquinolone. Antimicrob Agents Chemother. 2002;46:3370–3380. doi: 10.1128/AAC.46.11.3370-3380.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strahilevitz J, Truong-Bolduc QC, Hooper DC. DX-619, a novel des-fluoro(6) quinolone manifesting low frequency of selection of resistant Staphylococcus aureusmutants: quinolone resistance beyond modification of type II topoisomerases. Antimicrob Agents Chemother. 2005;49:5051–5057. doi: 10.1128/AAC.49.12.5051-5057.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.da Silva PEA, Von Groll A, Martin A, Palomino JC. Efflux as a mechanism for drug resistance in Mycobacterium tuberculosis. FEMS Immunol Med Microbiol. 2011;63:1e9. doi: 10.1111/j.1574-695X.2011.00831.x. [DOI] [PubMed] [Google Scholar]
- 46.Roy SK, Kumari N, Gupta S, Pahwa S, Nandanwar H, Jachak SM. 7-Hydroxy-(E)-3-phenylmethylene-chroman-4-one analogues as efflux pump inhibitors against Mycobacterium smegmatismc² 155. Eur J Med Chem. 2013;66:499–507. doi: 10.1016/j.ejmech.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 47.Paulasova P, Pellestor F. The peptide nucleic acids (PNAs): a new generation of probes for genetic and cytogenetic analyses. Ann Genet. 2004;47:349–358. doi: 10.1016/j.anngen.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Jeon B, Zhang Q. Sensitization of Campylobacter jejunito fluoroquinolone and macrolide antibiotics by antisense inhibition of the Cme- ABC multidrug efflux transporter. J Antimicrob Chemother. 2009;63:946–948. doi: 10.1093/jac/dkp067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mu Y, Shen Z, Jeon B, Dai L, Zhang Q. Synergistic effects of anti-CmeA and anti-CmeB peptide nucleic acids on sensitizing Campylobacter jejunito antibiotics. Antimicrob Agents Chemother. 2013;57:4575–4577. doi: 10.1128/AAC.00605-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oh E, Zhang Q, Jeon B. Target optimization for peptide nucleic acid (PNA)-mediated antisense inhibition of the CmeABC multidrug efflux pump in Campylobacter jejuni. J Antimicrob Chemother. 2014;69:375–380. doi: 10.1093/jac/dkt381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Noumedem JA, Mihasan M, Kuiate JR, Stefan M, Cojocaru D, Dzoyem JP, Kuete V. In vitroantibacterial and antibiotic-potentiation activities of four edible plants against multidrug-resistant gram-negative species. BMC Complement Altern Med. 2013;13:190. doi: 10.1186/1472-6882-13-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lacmata ST, Kuete V, Dzoyem JP, Tankeo SB, Teke GN, Kuiate JR, Pages JM. Antibacterial activities of selected Cameroonian plants and their synergistic effects with antibiotics against bacteria expressing MDR phenotypes. Evid Based Complement Alternat Med. 2012;2012:623723. doi: 10.1155/2012/623723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hancock REW. The Pseudomonas aeruginosaouter membrane permeability barrier and how to overcome it. Antibiot Chemother. 1985;36:95–102. doi: 10.1159/000410475. [DOI] [PubMed] [Google Scholar]
- 54.Hancock REW. Peptide antibiotics. Lancet. 1997;349:418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 55.Hancock REW, Wong PGW. Compounds which increase the permeability of the Pseudomonas aeruginosaouter membrane. Antimicrob Agents Chemother. 1984;26:48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scott MG, Yan H, Hancock REW. Biological properties of structurally related α-helical cationic antimicrobial peptides. Infect Immun. 1999;67:2005–2009. doi: 10.1128/iai.67.4.2005-2009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savage PB. Multidrug-resistant bacteria: overcoming antibiotic permeability barriers of gram-negative bacteria. Ann Med. 2001;33:167–171. doi: 10.3109/07853890109002073. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt EJ, Boswell JS, Walsh JP, Schellenberg MM, Winter TW, Li C, Allman GW, Savage PB. Activities of cholic acid-derived antimicrobial agents against multidrug-resistant bacteria. J Antimicrob Chemother. 2001;47:671–674. doi: 10.1093/jac/47.5.671. [DOI] [PubMed] [Google Scholar]
- 59.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1991;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Briers Y, Walmagh M, Lavigne R. Use of bacteriophage endolysin EL188 and outer membrane permeabilizers against Pseudomonas aeruginosa. J Appl Microbiol. 2011;110:778–785. doi: 10.1111/j.1365-2672.2010.04931.x. [DOI] [PubMed] [Google Scholar]
- 61.Ando M, Kamei R, Komagoe K, Inoue T, Yamada K, Katsu T. In situpotentiometric method to evaluate bacterial outer membrane-permeabilizing ability of drugs: example using antiprotozoal diamidines. J Microbiol Methods. 2012;91:497–500. doi: 10.1016/j.mimet.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 62.Kwon DH, Lu CD. Polyamines increase antibiotic susceptibility in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50:1623–1627. doi: 10.1128/AAC.50.5.1623-1627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guha A, Choudhury A, Unni BG, Roy MK. Effect of outer-membrane permeabilizers on the activity of antibiotics and plant extracts against Pseudomonas aeruginosa. Folia Microbiol (Praha) 2002;47:379–384. doi: 10.1007/BF02818694. [DOI] [PubMed] [Google Scholar]
- 64.Chatterjee S, Bijsmans IT, van Mil SW, Augustijns P, Annaert P. Toxicity and intracellular accumulation of bile acids in sandwich-cultured rat hepatocytes: role of glycine conjugates. Toxicol In Vitro. 2014;28:218–230. doi: 10.1016/j.tiv.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 65.de la Fuente-Núñez C, Reffuveille F, Haney EF, Strauss SK, Hancock REW. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014;10:e1004152. doi: 10.1371/journal.ppat.1004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reffuveille F, de la Fuente-Núñez C, Mansour S, Hancock REW. A broad-spectrum anti-biofilm peptide enhances action against bacterial biofilms. Antimicrob Agents Chemother. 2014;58:5363–5371. doi: 10.1128/AAC.03163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Overhage J, Campisano A, Bains M, Torfs ECW, Rehm BHA, Hancock REW. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun. 2008;76:4176–4182. doi: 10.1128/IAI.00318-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.de la Fuente-Núñez C, Korolik V, Bains M, Nguyen U, Breidenstein EBM, Horsman S, Lewenza S, Burrows L, Hancock REW. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother. 2012;56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de la Fuente-Núñez C, Reffuveille F, Fernández L, Hancock REW. Bacterial biofilm development as a multicellular adaptation: antibiotic resistance and new therapeutic strategies. Curr Opinion Microbiol. 2013;16:580–589. doi: 10.1016/j.mib.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 70.Ho Sui SJ, Fedynak A, Hsiao WW, Langille MG, Brinkman FS. The association of virulence factors with genomic islands. PLoS ONE. 2009;4:e8094. doi: 10.1371/journal.pone.0008094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117–128. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 72.Fernebro J. Fighting bacterial infections – future treatment options. Drug Resist Update. 2011;14:125–139. doi: 10.1016/j.drup.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Antunes LC, Ferreira RB, Buckner MM, Finlay BB. Quorum sensing in bacterial virulence. Microbiology. 2010;156:2271–2282. doi: 10.1099/mic.0.038794-0. [DOI] [PubMed] [Google Scholar]
- 74.Gilbert KB, Kim TH, Gupta R, Greenberg EP, Schuster M. Global position analysis of the Pseudomonas aeruginosaquorum-sensing transcription factor LasR. Mol Microbiol. 2009;73:1072–1085. doi: 10.1111/j.1365-2958.2009.06832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schuster M, Lostroh CP, Ogi T, Greenberg EP. Identification, timing, and signal specificity of Pseudomonas aeruginosaquorum-controlled genes: a transcriptome analysis. J Bacteriol. 2003;185:2066–2079. doi: 10.1128/JB.185.7.2066-2079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deziel E, Gopalan S, Tampakaki AP, Lepine F, Padfield KE, Saucier M, Xiao G, Rahme LG. The contribution of MvfR to Pseudomonas aeruginosapathogenesis and quorum sensing circuitry regulation: multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol Microbiol. 2005;55:998–1014. doi: 10.1111/j.1365-2958.2004.04448.x. [DOI] [PubMed] [Google Scholar]
- 77.de Kievit TR, Iglewski BH. Bacterial quorum sensing in pathogenic relationships. Infect Immun. 2000;68:4839–4849. doi: 10.1128/iai.68.9.4839-4849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lequette Y, Lee JH, Ledgham F, Lazdunski A, Greenberg EP. A distinct QscR regulon in the Pseudomonas aeruginosaquorum-sensing circuit. J Bacteriol. 2006;188:3365–3370. doi: 10.1128/JB.188.9.3365-3370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Latifi A, Foglino M, Tanaka K, Williams P, Lazdunski A. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosalinks the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol Microbiol. 1996;21:1137–1146. doi: 10.1046/j.1365-2958.1996.00063.x. [DOI] [PubMed] [Google Scholar]
- 80.Diggle SP, Cornelis P, Williams P, Camara M. 4-quinolone signalling in Pseudomonas aeruginosa: old molecules, new perspectives. Int J Med Microbiol. 2006;296:83–91. doi: 10.1016/j.ijmm.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 81.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 83.Rumbaugh KP, Griswold JA, Iglewski BH, Hamood AN. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosain burn wound infections. Infect Immun. 1999;67:5854–5862. doi: 10.1128/iai.67.11.5854-5862.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.O'Loughlin CT, Miller LC, Siryaporn A, Drescher K, Semmelhack MF, Bassler BL. A quorum-sensing inhibitor blocks Pseudomonas aeruginosavirulence and biofilm formation. Proc Natl Acad Sci USA. 2013;110:17981–17986. doi: 10.1073/pnas.1316981110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tan SY, Chua SL, Chen Y, Rice SA, Kjelleberg S, Nielsen TE, Yang L, Givskov M. Identification of five structurally unrelated quorum-sensing inhibitors of Pseudomonas aeruginosafrom a natural-derivative database. Antimicrob Agents Chemother. 2013;11:5629–5641. doi: 10.1128/AAC.00955-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, Sklar LA, Horswill AR, Hall PR, Gresham HD. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureuspromotes host defense with minimal impact on resistance. PLoS Pathog. 2014;10:e1004174. doi: 10.1371/journal.ppat.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, Parsek MR, Rice SA, Eberl L, Molin S, Holby N, Kjelleberg S, Givskov M. Inhibition of quorum sensing in Pseudomonas aeruginosabiofilm bacteria by a halogenated furanone compound. Microbiology. 2002;148:87–102. doi: 10.1099/00221287-148-1-87. [DOI] [PubMed] [Google Scholar]
- 88.Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, Bagge N, Kumar N, et al. Attenuation of Pseudomonas aeruginosavirulence by quorum sensing inhibitors. EMBO J. 2003;22:3803–3815. doi: 10.1093/emboj/cdg366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pustenly C, Albers A, Buldt-Karentzopoulos K, Parschat K, Chhabra SR, Camara M, Williams P, Fetzner S. Dioxygenase-mediated quenching of quinolone-dependent quorum sensing in Pseudomonas aeruginosa. Chem Biol. 2009;12:1259–1267. doi: 10.1016/j.chembiol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 90.Uroz S, Chhabra SR, Camara M, Williams P, Oger P, Dessaux Y. N-Acylhomoserine lactone quorum-sensing molecules are modified and degraded by Rhodococcus erythropolisW2 by both amidolytic and novel oxidoreductase activities. Microbiol. 2005;151:3313–3322. doi: 10.1099/mic.0.27961-0. [DOI] [PubMed] [Google Scholar]
- 91.Chow JY, Yang Y, Tay SB, Chua KL, Yew WS. Disruption of biofilm formation by the human pathogen Acinetobacter baumanniiusing engineered quorum-quenching lactonases. Antimicrob Agents Chemother. 2014;58:1802–1805. doi: 10.1128/AAC.02410-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Christensen QH, Grove TL, Booker SJ, Greenberg EP. A high-throughput screen for quorum-sensing inhibitors that target acyl-homoserine lactone synthases. Proc Natl Acad Sci USA. 2013;110:13815–13820. doi: 10.1073/pnas.1313098110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schiavo G, van der Goot GF. The bacterial toxin toolkit. Nat Rev Mol Cell Biol. 2001;2:530–537. doi: 10.1038/35080089. [DOI] [PubMed] [Google Scholar]
- 94.Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a vero toxin (VT2) from Escherichia coliO157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur J Biochem. 1988;171:45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 95.Orth P, Xiao L, Hernandez LD, Reichert P, Sheth PR, Beaumont M, Yang X, et al. Mechanism of action and epitopes of Clostridium difficiletoxin B neutralizing antibody bezotoxumab revealed by x-ray crystallography. J Biol Chem. 2014;289:18008–18021. doi: 10.1074/jbc.M114.560748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berube BJ, Wardenburg JB. Staphylococcus aureusalpha-toxin: nearly a century of intrigue. Toxins. 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lopez EL, Contrini MM, Glatstein E, Gonzalez Ayala S, Sontoro R, Allende D, Ezcurra G, et al. Safety and pharmacokinetics of urtoxazumab, a humanized monoclonal antibody, against Shiga-like toxin 2 in healthy adults and in pediatric patients infected with shiga-like toxin producing Escherichia coli. Antimicrob Agents Chemother. 2010;54:239–243. doi: 10.1128/AAC.00343-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lowy I, Molrine DC, Leav BA, Blair BM, Baxter R, Berding DN, Nichol G, Thomas WD, Jr, Leney M, Sloan S, Hay CA, Ambrosino DM. Treatment with monoclonal antibodies against Clostridium difficiletoxins. N Engl J Med. 2010;362:197–205. doi: 10.1056/NEJMoa0907635. [DOI] [PubMed] [Google Scholar]
- 99.Nivaskumar M, Francetic O. Type II secretion system: a magic beanstalk or a protein escalator. Biochim Biophys Acta. 2014;1843:1568–1577. doi: 10.1016/j.bbamcr.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 100.Korotkov KV, Sandkvist M, Hol WG. The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol. 2012;10:336–351. doi: 10.1038/nrmicro2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Braun P, de Groot A, Bitter W, Tommassen J. Secretion of elastinolytic enzymes and their propeptides by Pseudomonas aeruginosa. J Bacteriol. 1998;180:3467–3469. doi: 10.1128/jb.180.13.3467-3469.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Voulhoux R, Ball G, Ize B, Vasil ML, Lazdunski A, Wu LF, Filloux A. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 2001;20:6735–6741. doi: 10.1093/emboj/20.23.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baldi DL, Higginson EE, Hocking DM, Praszkier J, Cavaliere R, James CE, Bennett-Wood V, Azzopardi KI, Turnbull L, Lithgow T, Robins-Browne RM, Witchurch CB, Tauschek M. The type II secretion system and its ubiquitous lipoprotein substrate, SslE, are required for biofilm formation and virulence of enteropathogenic Escherichia coli. Infect Immun. 2012;80:2042–2052. doi: 10.1128/IAI.06160-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moir DT, Di M, Wong E, Moore RA, Schweizer HP, Woods DE, Bowlin TL. Development and application of a cellular, gain-of-signal, bioluminescent reporter screen for inhibitors of type II secretion in Pseudomonas aeruginosaand Burkholderia pseudomallei. J Biomol Screen. 2011;16:694–705. doi: 10.1177/1087057111408605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Segers K, Anne J. Traffic jam at the bacterial sec translocase: targeting the SecA nanomotor by small-molecule inhibitors. Chem Biol. 2011;18:685–698. doi: 10.1016/j.chembiol.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 106.Chatterjee S, Chaudhury S, McShan AC, Kaur K, De Guzman RN. Structure and biophysics of type III secretion in bacteria. Biochemistry. 2013;52:2508–2517. doi: 10.1021/bi400160a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Engel J, Balachandran P. Role of Pseudomonas aeruginosatype III effectors in disease. Curr Opin Microbiol. 2009;12:61–66. doi: 10.1016/j.mib.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 108.Dean P. Functional domains and motifs of bacterial type III effector proteins and their roles in infection. FEMS Microbiol Rev. 2011;35:1100–1125. doi: 10.1111/j.1574-6976.2011.00271.x. [DOI] [PubMed] [Google Scholar]
- 109.Yamazaki A, Li J, Zeng Q, Khokhani D, Hutchins WC, Yost AC, Biddle E, Toone EJ, Chen X, Yang CH. Derivatives of plant phenolic compound affect the type III secretion system of Pseudomonas aeruginosavia a GacS-GacA two-component signal transduction system. Antimicrob Agents Chemother. 2012;56:36–43. doi: 10.1128/AAC.00732-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kauppi AM, Nordfelth R, Uvell H, Wolf-Watz H, Elofsson M. Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem Biol. 2003;10:241–249. doi: 10.1016/s1074-5521(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 111.Tree JJ, Wang D, McInally C, Mahajan A, Layton A, Houghton I, Elofsson M, Stevens MP, Gally DL, Roe AJ. Characterization of the effects of salicylidene acylhydrazide compounds on type III secretion in Escherichia coliO157:H7. Infect Immun. 2009;77:4209–4220. doi: 10.1128/IAI.00562-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang D, Zetterstrom CE, Gabrielsen M, Beckham KS, Tree JJ, Macdonald SE, Byron O, et al. Identification of bacterial target proteins for the salicylidene acylhydrazide class of virulence-blocking compounds. J Biol Chem. 2011;286:29922–29931. doi: 10.1074/jbc.M111.233858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Larzabal M, Zotta E, Ibarra C, Rabinovitz BC, Vilte DA, Mercado EC, Cataldi A. Effect of coiled-coil peptides on the function of the type III secretion system-dependent activity of enterohemorragic Escherichia coliO157:H7 and Citrobacter rodentium. Int J Med Microbiol. 2013;303:9–15. doi: 10.1016/j.ijmm.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 114.Larzabal M, Mercado EC, Vilte DA, Salazar-Gonzalez H, Cataldi A, Navarro-Garcia F. Designed coiled-coil peptides inhibit the type three secretion system of enteropathogenic Escherichia coli. PLoS ONE. 2010;5:e9046. doi: 10.1371/journal.pone.0009046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kimura K, Iwatsuki M, Nagai T, Matsumoto A, Takahashi Y, Shiomi K, Omura S, Abe A. A small-molecule inhibitor of the bacterial type III secretion system protects against in vivoinfection with Citrobacter rodentium. J Antibiot. 2011;64:197–203. doi: 10.1038/ja.2010.155. [DOI] [PubMed] [Google Scholar]
- 116.Hogg T, Mesters JR, Hilgenfeld R. Inhibitory mechanisms of antibiotics targeting elongation factor Tu. Curr Protein Pept Sci. 2002;3:121–131. doi: 10.2174/1389203023380855. [DOI] [PubMed] [Google Scholar]
- 117.Kohler T, Perron GG, Buckling A, van Delden C. Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog. 2010;6:e1000883. doi: 10.1371/journal.ppat.1000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maeda T, Garcia-Contreras R, Pu M, Sheng L, Garcia LR, Tomas M, Wood TK. Quorum quenching quandary: resistance to anti-virulence compounds. ISME J. 2012;6:493–501. doi: 10.1038/ismej.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 120.Regan T, Nally K, Carmody R, Houston A, Shanahan F, Macsharry J, Brint E. Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenesin intestinal epithelial cells and macrophages. J Immunol. 2013;191:6084–6092. doi: 10.4049/jimmunol.1203245. [DOI] [PubMed] [Google Scholar]
- 121.Schreibelt G, Tel J, Sliepen KH, Benitez-Ribas D, Figdor CG, Adema GJ, de Vries IJ. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol Immunother. 2010;59:1573–1582. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Orr MT, Beebe EA, Hudson TE, Moon JJ, Fox CB, Reed SG, Coler RN. A dual TLR agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen ID93. PLoS ONE. 2014;9:e83884. doi: 10.1371/journal.pone.0083884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Querec T, Bennouna S, Alkan S, Laouar Y, Gorden K, Flavell R, Akira S, Ahmed R, Pulendran B. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J Exp Med. 2006;203:413–424. doi: 10.1084/jem.20051720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schreibelt G, Benitez-Ribas D, Schuurhuis D, Lambeck AJ, van Hout-Kuijer M, Schaft N, Punt CJ, Figdor CG, Adema GJ, de Vries IJ. Commonly used prophylactic vaccines as an alternative for synthetically produced TLR ligands to mature monocyte-derived dendritic cells. Blood. 2010;116:564–574. doi: 10.1182/blood-2009-11-251884. [DOI] [PubMed] [Google Scholar]
- 125.De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zheng Y, Niyonsaba F, Ushio H, Nagaoka I, Ikeda S, Okumura K, Ogawa H. Cathelicidin LL-37 induces the generation of reactive oxygen species and release of human alpha-defensins from neutrophils. Br J Dermatol. 2007;157:1124–1131. doi: 10.1111/j.1365-2133.2007.08196.x. [DOI] [PubMed] [Google Scholar]
- 127.Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, Powers JP, Bryan J, Brinkman FS, Hancock REW. Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol. 2006;176:2455–2464. doi: 10.4049/jimmunol.176.4.2455. [DOI] [PubMed] [Google Scholar]
- 128.Nijnik A, Pistolic J, Wyatt A, Tam S, Hancock REW. Human cathelicidin peptide LL-37 modulates the effects of IFN-gamma on APCs. J Immunol. 2009;183:5788–5798. doi: 10.4049/jimmunol.0901491. [DOI] [PubMed] [Google Scholar]
- 129.Koczulla R, von Degenfeld G, Kupatt C, Krotz F, Zahler S, Gloe T, Issbrucker K, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shaykhiev R, Beisswenger C, Kandler K, Senske J, Puchner A, Damm T, Behr J, Bals R. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol. 2005;289:L842–L848. doi: 10.1152/ajplung.00286.2004. [DOI] [PubMed] [Google Scholar]
- 131.Steinstraesser L, Hirsch T, Schulte M, Kueckelhaus M, Jacobsen F, Mersch EA, Stricker I, Afacan N, Jenssen H, Hancock REW, Kindrachuk J. Innate defense regulator peptide 1018 in wound healing and wound infection. PLoS ONE. 2012;7:e39373. doi: 10.1371/journal.pone.0039373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cirioni O, Giacometti A, Ghiselli R, Bergnach C, Orlando F, Silvestri C, Mocchegiani F, Licci A, Skerlavaj B, Rocchi M, Saba V, Zanetti M, Scalise G. LL-37 protects rats against lethal sepsis caused by gram-negative bacteria. Antimicrob Agents Chemother. 2006;50:1672–1679. doi: 10.1128/AAC.50.5.1672-1679.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rivas-Santiago B, Castaneda-Delgado JE, Rivas Santiago CE, Waldbrook M, Gonzalez-Curiel I, Leon-Contreras JC, Enciso-Moreno JA, del Villar V, Mendez-Ramos J, Hancock REW, Hernandez-Pando R. Ability of innate defence regulator peptides IDR-1002, IDR-HH2 and IDR-1018 to protect against Mycobacterium tuberculosisinfections in animal models. PLoS ONE. 2013;8:e59119. doi: 10.1371/journal.pone.0059119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Achtman AH, Pilat S, Law CW, Lynn DJ, Janot L, Mayer ML, Ma S, Kindrachuk J, Finlay BB, Brinkman FS, Smyth GK, Hancock REW, Schofield L. Effective adjunctive therapy by an innate defense regulatory peptide in a pre-clinical model of severe malaria. Sci Trasl Med. 2012;4:135ra64. doi: 10.1126/scitranslmed.3003515. [DOI] [PubMed] [Google Scholar]
- 135.Faber C, Stallmann HP, Lyaruu DM, Joosten U, von Eiff C, van Nieuw Amerongen A, Wuisman PI. Comparable efficacies of the antimicrobial peptide human lactoferrin 1-11 and gentamicin in a chronic methicillin-resistant Staphylococcus aureusosteomyelitis model. Antimicrob Agents Chemother. 2005;49:2438–2444. doi: 10.1128/AAC.49.6.2438-2444.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Velden WJ, van Iersel TM, Blijlevens NM, Donnelly JP. Safety and tolerability of the antimicrobial peptide human lactoferrin 1-11 (hLF1-11) BMC Med. 2009;7:44. doi: 10.1186/1741-7015-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Papareddy P, Kalle M, Sorensen OE, Malmsten M, Morgelin M, Schmidtchen A. The TFPI-2 derived peptide EDC34 improves outcome of gram-negative sepsis. PLoS Pathog. 2013;9:e1003803. doi: 10.1371/journal.ppat.1003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Easton DM, Nijnik A, Mayer ML, Hancock REW. Potential of immunomodulatory host defense peptides as novel anti-infectives. Trends Biotechnol. 2009;27:582–590. doi: 10.1016/j.tibtech.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.World Health Organization. 2013. Diarrhoeal disease. Fact sheet No. 330. http://www.who.int/mediacentre/factsheets/fs330/en/
- 140.Mandal SM, Dey S, Mandal M, Sarkar S, Maria-Neto S, Franco OL. Identification and structural insights of three novel antimicrobial peptides isolated from green coconut water. Peptides. 2009;30:633–637. doi: 10.1016/j.peptides.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 141.Ciglenecki I, Bichet M, Tena J, Mondesir E, Bastard M, Tran NT, Antierens A, Staderini N. Cholera in pregnancy: outcomes from a specialized cholera treatment unit for pregnant women in Léogâne, Haiti. PLoS Negl Trop Dis. 2013;7:e2368. doi: 10.1371/journal.pntd.0002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sulakvelidze A, Alavidze Z, Morris JG., Jr Bacteriophage therapy. Antimicrob Agents Chemother. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.d'Herelle F. Surun microbe invisible antagoniste des bacteries dysenteriques. C R Acad Sci. 1917;165:373–375. [Google Scholar]
- 144.Hausler T. Viruses versus superbugs. A solution to the antibiotic crisis? 2006. New York Palgrave Macmillan.
- 145.Skurnika M, Strauch E. Phage therapy: facts and fiction. Intern J Med Microbiol. 2006;296:5–14. doi: 10.1016/j.ijmm.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 146.Inal JM. Phage therapy: a reappraisal of bacteriophages as antibiotics. Arch Immunol Ther Exp (Warsz) 2003;51:237–244. [PubMed] [Google Scholar]
- 147.Brüssow H, Canchaya C, Hardt WD. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68:560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mandal SM, Roy A, Ghosh AK, Hazra TK, Basak A, Franco OL. Challenges and future prospects of antibiotic therapy: from peptides to phages utilization. Front Pharmac. 2014;5:105. doi: 10.3389/fphar.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khairnar K, Raut MP, Chandekar RH, Sanmukh SG, Paunikar WN. Novel bacteriophage therapy for controlling metallo-beta-lactamase producing Pseudomonas aeruginosainfection in catfish. BMC Vet Res. 2013;26:264. doi: 10.1186/1746-6148-9-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Drilling A, Morales S, Boase S, Jervis-Bardy J, James C, Jardeleza C, Tan NC, Cleland E, Speck P, Vreugde S, Wormald PJ. Safety and efficacy of topical bacteriophage and ethylenediaminetetraacetic acid treatment of Staphylococcus aureusinfection in a sheep model of sinusitis. Int Forum Allergy Rhinol. 2014;4:176–186. doi: 10.1002/alr.21270. [DOI] [PubMed] [Google Scholar]
- 151.Lungren MP, Christensen D, Kankotia R, Falk I, Paxton BE, Kim CY. Bacteriophage K for reduction of Staphylococcus aureusbiofilm on central venous catheter material. Bacteriophage. 2013;3:e26825. doi: 10.4161/bact.26825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parfitt T. Georgia: an unlikely stronghold for bacteriophage therapy. Lancet. 2005;365:2166–2167. doi: 10.1016/S0140-6736(05)66759-1. [DOI] [PubMed] [Google Scholar]
- 153.Thiel K. Old dogma, new tricks—21st Century phage therapy. Nat Biotechn. 2004;22:31–36. doi: 10.1038/nbt0104-31. [DOI] [PubMed] [Google Scholar]
- 154.Lu TK, Collins JJ. Engineered bacteriophage targeting gene networks as adjuvants for antibiotic therapy. Proc Natl Acad Sci USA. 2009;106:4629–4634. doi: 10.1073/pnas.0800442106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Westwater C, Kasman LM, Schofield DA, Werner PA, Dolan JW, Schmidt MG, Norris JS. Use of genetically engineered phage to deliver antimicrobial agents to bacteria: an alternative therapy for treatment of bacterial infections. Antimicrob Agents Chemother. 2003;47:1301–1307. doi: 10.1128/AAC.47.4.1301-1307.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Walsh CJ, Guinane CM, O'Toole PW, Cotter PD. Beneficial modulation of the gut microbiota. FEBS Lett. 2014;588:4120–4130. doi: 10.1016/j.febslet.2014.03.035. [DOI] [PubMed] [Google Scholar]
- 157.Lilly DM, Stillwell RH. Probiotics: growth-promoting factors produced by microorganisms. Science. 1965;147:747–748. doi: 10.1126/science.147.3659.747. [DOI] [PubMed] [Google Scholar]
- 158.Volzing K, Borrero J, Sadowsky MJ, Kaznessis YN. Antimicrobial peptides targeting gram-negative pathogens, produced and delivered by lactic acid bacteria. ACS Synth Biol. 2013;2:643–650. doi: 10.1021/sb4000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu L, Walker WA. Pathologic and physiologic interactions of bacteria with the gastrointestinal epithelium. Am J Clin Nutr. 2001;73:1124–1130. doi: 10.1093/ajcn/73.6.1124S. [DOI] [PubMed] [Google Scholar]
- 160.Carr FJ, Chill D, Maida N. The lactic acid bacteria: a literature survey. Crit Rev Microbiol. 2002;28:281–370. doi: 10.1080/1040-840291046759. [DOI] [PubMed] [Google Scholar]
- 161.Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F. Natural antimicrobial peptides from bacteria: characteristics and potential applications to fight against antibiotic resistance. J Appl Microbiol. 2012;113:723–736. doi: 10.1111/j.1365-2672.2012.05338.x. [DOI] [PubMed] [Google Scholar]
- 162.Mandal SM, Silva ON, Franco OL. Recombinant probiotics with antimicrobial peptides: a dual strategy to improve immune response in immunocompromised patients. Drug Discov Today. 2014;19:1045–1050. doi: 10.1016/j.drudis.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 163.Petschow B, Doré J, Hibberd P, Dinan T, Reid G, Blaser M, Cani PD, et al. Probiotics, prebiotics, and the host microbiome: the science of translation. Ann N Y Acad Sci. 2013;1306:1–17. doi: 10.1111/nyas.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tahlan K, Park HU, Wong A, Beatty PH, Jensen SE. Two sets of paralogous genes encode the enzymes involved in the early stages of clavulanic acid and clavam metabolite biosynthesis in Streptomyces clavuligerus. Antimicrob Agents Chemother. 2004;48:930–939. doi: 10.1128/AAC.48.3.930-939.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Totir MA, Helfand MS, Carey MP, Sheri A, Buynak JD, Bonomo RA, Cary PR. Sulbactam forms only minimal amounts of irreversible acrylate-enzyme with SHV-1 β-lactamase. Biochemistry. 2007;46:8980–8987. doi: 10.1021/bi7006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carlier M, Carrette S, Stove V, Verstraete AG, De Waele JJ. Does consistent piperacillin dosing result in consistent therapeutic concentrations in critically ill patients? A longitudinal study over an entire antibiotic course. Int J Antimicrob Agents. 2014;14:S0924–S8579. doi: 10.1016/j.ijantimicag.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 167.Coleman K, Levasseur P, Girard AM, Borgonovi M, Miossec C, Merdjan H, Drusano G, Shlaes D, Nichols WW. The activity of ceftazidime and avibactam against β-lactamase-producing Enterobacteriaceae in a hollow-fiber pharmacodynamic model. Antimicrob Agents Chemother. 2014;58:3366–3372. doi: 10.1128/AAC.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Castanheira M, Williams G, Jones RN, Sader HS. Activity of ceftaroline-avibactam tested against contemporary Enterobacteriaceae isolates carrying β-lactamases prevalent in the United States. Microb Drug Resist. 2014;20:436–440. doi: 10.1089/mdr.2013.0181. [DOI] [PubMed] [Google Scholar]
- 169.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing enterobacteriaceae. Antimicrob Agents Chemother. 2011;55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hiraiwa Y, Morinaka A, Fukushima T, Kudo T. Metallo-β-lactamase inhibitory activity of 3-alkyloxy and 3-amino phthalic acid derivatives and their combination effect with carbapenem. Bioorg Med Chem. 2013;21:5841–5850. doi: 10.1016/j.bmc.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 171.Toney JH, Hammond GG, Fitzgerald PMD, Sharma N, Balkovec JM, Rouen GP, Olson SH, Hammond ML, Greenlee ML, Gao YD. Succinic acids as potent inhibitors of plasmid-borne IMP-1 metallo-β-lactamase. J Biol Chem. 2001;276:31913–31918. doi: 10.1074/jbc.M104742200. [DOI] [PubMed] [Google Scholar]
- 172.Goldstein EJ, Citron DM, Tyrrell KL, Merriam CV. In vitroactivity of Biapenem plus RPX7009, a carbapenem combined with a serine β-lactamase inhibitor, against anaerobic bacteria. Antimicrob Agents Chemother. 2013;57:2620–2630. doi: 10.1128/AAC.02418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fontaine F, Hequet A, Voisin-Chiret AS, Bouillon A, Lesnard A, Cresteil T, Jolivalt C, Rault S. First identification of boronic species as novel potential inhibitors of the Staphylococcus aureusNorA efflux pump. J Med Chem. 2014;57:2536–2548. doi: 10.1021/jm401808n. [DOI] [PubMed] [Google Scholar]
- 174.Hequet A, Burchak ON, Jeanty M, Guinchard X, Le Pihive E, Maigre L, Bouhours P, Schneider D, Maurin M, Paris JM, Denis JN, Jolivalt C. 1-(1H-Indol-3-yl)ethanamine derivatives as potent Staphylococcus aureusNorA efflux pump inhibitors. Chem Med Chem. 2014;9:1534–1545. doi: 10.1002/cmdc.201400042. [DOI] [PubMed] [Google Scholar]
- 175.Kalia NP, Mahajan P, Mehra R, Nargotra A, Sharma JP, Koul S, Khan IA. Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus. J Antimicrob Chemother. 2012;67:2401–2408. doi: 10.1093/jac/dks232. [DOI] [PubMed] [Google Scholar]
- 176.Sabatini S, Gosetto F, Serritella S, Manfroni G, Tabarrini O, Iraci N, Brincat JP, Carosati E, Villarini M, Kaatz GW, Cecchetti V. Pyrazolo[4,3-c\[1,2\benzothiazines 5,5-dioxide: a promising new class of Staphylococcus aureusNorA efflux pump inhibitors. J Med Chem. 2012;55:3568–3572. doi: 10.1021/jm201446h. [DOI] [PubMed] [Google Scholar]
- 177.Sabatini S, Gosetto F, Manfroni G, Tabarrini O, Kaatz GW, Patel D, Cecchetti V. Evolution from a natural flavones nucleus to obtain 2-(4-Propoxyphenyl)quinoline derivatives as potent inhibitors of the S. aureusNorA efflux pump. J Med Chem. 2011;54:5722–5736. doi: 10.1021/jm200370y. [DOI] [PubMed] [Google Scholar]
- 178.Brincat JP, Carosati E, Sabatini S, Manfroni G, Fravolini A, Raygada JL, Patel D, Kaatz GW, Cruciani G. Discovery of novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. J Med Chem. 2011;54:354–365. doi: 10.1021/jm1011963. [DOI] [PubMed] [Google Scholar]
- 179.Thota N, Reddy MV, Kumar A, Khan IA, Sangwan PL, Kalia NP, Koul JL, Koul S. Substituted dihydronaphthalenes as efflux pump inhibitors of Staphylococcus aureus. Eur J Med Chem. 2010;45:3607–3616. doi: 10.1016/j.ejmech.2010.05.006. [DOI] [PubMed] [Google Scholar]