Abstract
High-quality medical care is the result of clinical decisions based upon scientific principles garnered from basic, translational, and clinical research. Information regarding the natural history of diseases and their responses to various treatments is introduced into the medical literature through the approximately one million PubMed journal articles published each year. Pharmaceutical and device companies, universities, departments, and researchers all stand to gain from research publication. Basic and translational research is highly competitive. Success in obtaining research funding and career advancement requires scientific publication in the medical literature. Clinical research findings can lead to changes in the pattern of orthopaedic practice and have implications for the utilization of pharmaceuticals and orthopaedic devices. Research findings can be biased by ownership of patents and materials, funding sources, and consulting arrangements. The current high-stakes research environment has been characterized by an increase in plagiarism, falsification or manipulation of data, selected presentation of results, research bias, and inappropriate statistical analyses. It is the responsibility of the orthopaedic community to work collaboratively with industry, universities, departments, and medical researchers and educators to ensure the integrity of the content of the orthopaedic literature and to enable the incorporation of best practices in the care of orthopaedic patients.
The misrepresentation of natural observation has existed for as long as scientific research has been recorded1,2. Ptolemy, the renowned second-century Egyptian astronomer, recorded astronomical measurements that he could not have made. Ptolemy’s work, purporting to prove that Earth was the center of the universe, influenced science and philosophy for centuries. Copernicus, who revolutionized our understanding of both Earth and man’s place in the universe, was accused of heresy when he reported a conflicting celestial configuration based on appropriate scientific methods and accurate measurements. The legendary physicist and Nobel laureate Robert Millikan (1868-1953), who discovered the negative charge of the electron, selected only fifty-eight of 140 observations for inclusion in his scientific presentations. While this selective use of data likely improved precision and the credibility of his claims, it did not truly represent his actual scientific findings. Sir Cyril Burt (1883-1971), a noted British psychologist, fabricated (extrapolated) data to show that human intelligence is 75% inherited. His work influenced educational programs and policies for generations.
Defining Scientific Misconduct: From the Obvious to the Subtle and Insidious
The U.S. Office of Research Integrity defines misconduct as “fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results” (http://ori.hhs.gov/definition-misconduct). Fabrication involves the presentation of observations or events that in fact never occurred; the experiments were never performed. Fabrication continues to occur and in some cases has influenced the treatment of patients with musculoskeletal disease. From 1996 to 2008, Dr. Scott Reuben published a series of articles that examined the potential role of cyclooxygenase-2 (COX-2) specific inhibitors in controlling postoperative pain following orthopaedic surgery. In a series of carefully designed and double-blind placebo-controlled studies, Dr. Reuben established that Celebrex (celecoxib; Pfizer), Bextra (valdecoxib; Pfizer), and Vioxx (rofecoxib; Merck) dramatically improved pain management for patients undergoing joint replacement, spine fusion, and anterior cruciate ligament reconstruction and decreased the complications associated with the standard use of opiates3. Dr. Reuben, a Professor of Anesthesiology and Pain Medicine at Tufts and the Chief of Acute Pain at Baystate Medical Center, was widely recognized for revolutionizing pain management for orthopaedic patients. A 2007 editorial in Anesthesia & Analgesia stated that Reuben had been at the “forefront of redesigning pain management protocols” through his “carefully planned” and “meticulously documented studies4.”
In 2008, it was discovered that two abstracts submitted by Dr. Reuben for Baystate Medical Center’s Annual Research Week lacked institutional review board approval. Investigation showed that Dr. Reuben had never enrolled patients or performed the studies described in the manuscripts. Further review resulted in Baystate requesting medical journals to retract a combined total of twenty-one of Dr. Reuben’s papers. Dr. Reuben’s advocacy for COX-2 inhibitors to treat postoperative pain appeared in reviews, textbooks, and practice guidelines. Beginning in 2000, Reuben advocated that physicians should shift from the use of first-generation nonsteroidal anti-inflammatory drugs to the use of Vioxx, Celebrex, and Bextra to treat musculoskeletal pain3. Reuben urged the United States Food and Drug Administration (FDA) not to restrict use of the drugs he studied, citing their efficacy and safety. Drug companies organized educational programs and symposia on the basis of Reuben’s reports. Various editorials noted that “millions of orthopaedic patients’ pain management has been affected by Dr. Reuben’s research” and “Reuben’s studies led to the sale of billions of dollars of Celebrex and Vioxx.”5
In 2010, Reuben, who had research supported by Pfizer and who served on a speaker’s panel for the company, was sentenced to six months in prison, was ordered to pay a $5000 fine, and was required to provide $360,000 in restitution to drug companies. To many, it was surprising that Reuben could perform such extensive fabrication of high-impact research for so long. Reuben had numerous coauthors and worked in an academic institution where he successfully progressed through the promotion process5. These accounts demonstrate the high level of trust afforded to scientists and reveal that, although institutions approve research, they rarely monitor the findings associated with that research5.
Falsification involves the modification of scientific data so that it supports a particular hypothesis. In 1998, Dr. Andrew Wakefield and coauthors published a study in The Lancet of twelve children, suggesting a link between the measles, mumps, and rubella (MMR) vaccine and autism6. The results were widely reported by the media, were popularized on a variety of web sites, resulted in the refusal of vaccination by many parents, and led to lawsuits by parents of autistic children against vaccine manufacturers. The Lancet and the press later learned that Wakefield had received a $110,000 payment from the Legal Aid Board prior to publishing the paper. The Legal Aid Board was seeking evidence that could be used in lawsuits against vaccine manufacturers and, following publication of the article, provided an additional $674,000 payment to Wakefield. A retrospective review of the data used by Wakefield revealed that the diagnosis and/or dates of records were changed for all twelve children in the publication report so as to support the author’s conclusions7,8.
The Lancet partially retracted Wakefield’s paper in 2004, and later issued a full retraction. The General Medical Council of the United Kingdom (U.K.) found Wakefield guilty of professional misconduct and revoked his medical license. However, public suspicion that vaccinations can cause autism persists. Vaccination rates have dropped sharply in many countries, including the United States, and this drop in vaccinations is a major contributor to the increased incidence of measles and mumps, resulting in outbreaks of the diseases and deaths in multiple countries9. Subsequent studies have demonstrated no link between the MMR vaccine and autism. Position statements supporting vaccination and the absence of a link with autism have been released by the Centers for Disease Control and Prevention (CDC), the American Academy of Pediatrics, the Institute of Medicine, the National Academy of Sciences, and the U.K. National Health Service. Nonetheless, the general public maintains a widespread belief in such an association. In 2012, the CDC reported a U.S. outbreak of whooping cough that infected 41,000 children—the largest outbreak since 1955. This is an instance in which falsification has compromised public health worldwide.
Plagiarism is the inappropriate use of previously published information without attribution and with representation that the work is original. In 2011, an investigator reviewing the published literature (the National Library of Medicine’s PubMed) related to osteoarthritis discovered an article that had been published twice in its entirety. The first instance of publication was in 2006, when the Journal of Orthopaedic Research published the paper with the title, “Chondrocyte Gene Expression in Osteoarthritis: Correlation with Disease Severity10.” In 2011, that same article was published as “Alterations in Expression of Cartilage-Specific Genes for Aggrecan and Collagen Type II in Osteoarthritis” in the Romanian Journal of Morphology and Embryology11. The articles were identical except for the titles and list of authors. Dr. Mogoanta, Editor of the Romanian Journal of Morphology and Embryology, withdrew the article, notified PubMed, banned the authors from future publication, ceased collaboration with the reviewers, notified the Dean of Medicine and Chair of the Ethics Committee, and coauthored an editorial for the Journal of Orthopaedic Research12.
While the case of plagiarism is clearly against the rules, stretching the boundaries of research ethics can be a more subtle and insidious process. More subtle events include an intentional failure to acknowledge previous work, intentionally incomplete or inaccurate description of methods, and repeat publication of similar work. In 2011, the Journal of Orthopaedic Research published an editorial that was titled “Publishing the Results of Multiple Experiments Using the Same Methods and Outcome Measures.”13 The editorial described a scenario that occurred in 2002 and 2003 in which one author conducted a series of experiments using the same methods, and a single control group, to examine the effects of twenty-four different materials on bone formation. The author subsequently reported positive results in eleven publications, in ten different journals, over a period of seven years. In some manuscripts, the author failed to cite the previous publications, used the same description of the methods, and published similar or nearly identical figures. The same control group was used in each study, but this fact was not reported in the multiple publications.
Ensuring Accuracy in Research
The disclosure of conflicts of interest in publications and presentations has become standard. However, standards at different journals vary and typically do not involve detailed financial information. A study of disclosure at the 2012 meeting of the American Academy of Orthopaedic Surgeons revealed that 90% of the presentations had the required disclosure slide, but 10% did not14. The average amount of time dedicated to the disclosure slide was 3.1 seconds, permitting only a cursory review of the information. Only 45% of the disclosure slides had conflict-of-interest information that included coauthors. Only 15% of the disclosure slides had information regarding institutional conflicts14.
In a recent study in The Journal of Bone & Joint Surgery, a significant association was found between the funding source and the qualitative conclusions in publications examining the prevention of deep vein thrombosis following total joint arthroplasty15. From 2004 to 2010, sixty-six studies published in PubMed-cited literature with identified industry or nonindustry funding were reviewed to determine whether a treatment was classified as favorable, neutral, or unfavorable in the prevention of clotting. Only two of the fifty-two industry-sponsored studies had negative results, in contrast to negative findings being observed in three of the fourteen studies without industry support15. A study of articles published in the MEDLINE database between 1980 and 2002 revealed a significant relationship between industry sponsorship and pro-industry conclusions16. The odds ratio was 3.6. Khan et al. reported that in five major journals, between 2002 and 2004, there existed a strong statistical link between industry funding and favorable outcomes17. While these reports do not necessarily indicate wrongdoing, they suggest possible increased risk of bias when interpreting results from industry-sponsored clinical trials.
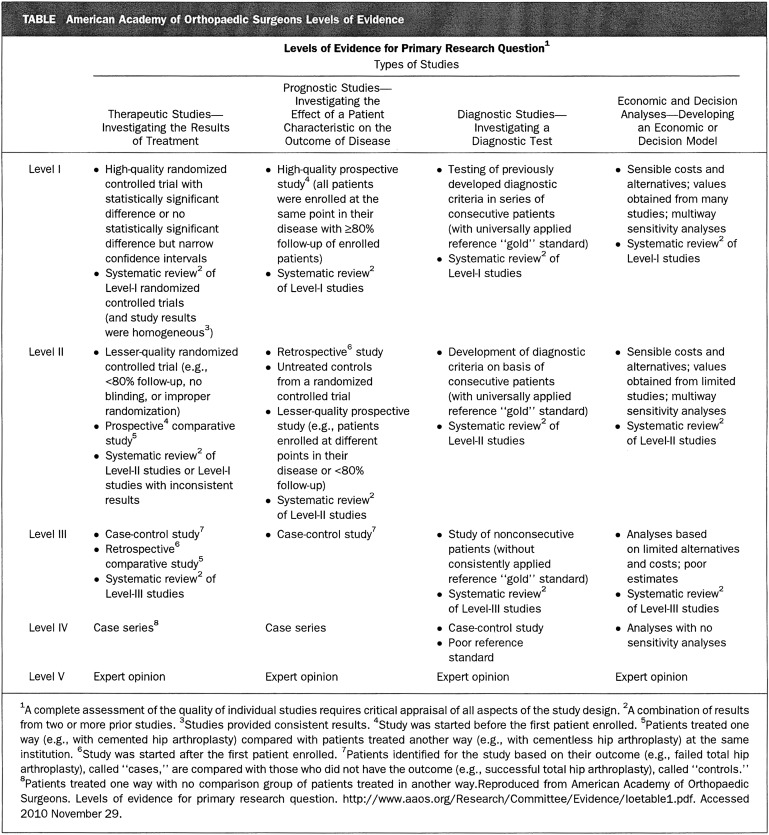
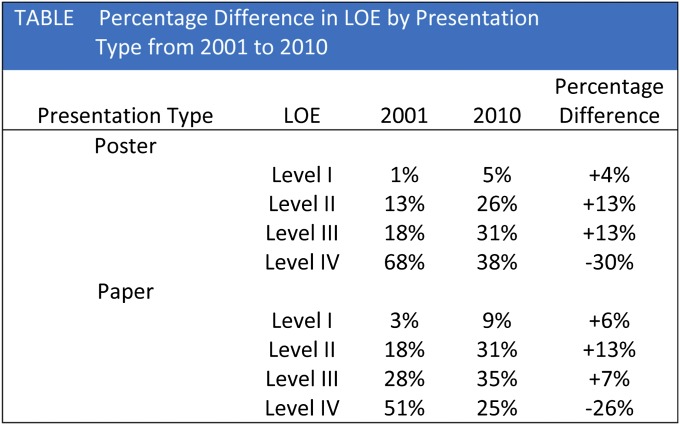
Many journals now include information about levels of evidence based on trial design, and there has been a shift toward more publications being conducted with a higher level of evidence. The highest level of evidence is the placebo-controlled double-blind clinical trial with appropriate power analysis and statistical support. The lowest level of evidence involves the editorial or expert-opinion type of article (Fig. 1). The level of evidence supporting presentations at the American Academy of Orthopaedic Surgery meeting progressively increased from 2001 to 201018. The number of studies utilizing Level-I or Level-II evidence has increased from 17% to 36% over that time (Fig. 2)18. In an article published in 2008, Okike et al. stated that their review of more than 1100 articles revealed that the major scientific factor influencing acceptance for publication in The Journal of Bone & Joint Surgery involved the level of evidence of the article19.
Fig. 1.
Levels of evidence in clinical trials. The highest level of evidence is the double-blind placebo controlled research design (Level I). The lowest level of evidence is the statement of expert opinion (Level V). (Table reproduced from Voleti PB, Donegan DJ, Baldwin KD, Lee GC. Level of evidence of presentations at American Academy of Orthopaedic Surgeons annual meetings. J Bone Joint Surg Am. 2012;94:e50[1-5].)
Fig. 2.
Differences in level of evidence in support of clinical research studies presented at the Annual Meeting of the American Academy of Orthopaedic Surgeons, 2001 to 2010. LOE = level of evidence. (Table reproduced from Voleti PB, Donegan DJ, Baldwin KD, Lee GC. Level of evidence of presentations at American Academy of Orthopaedic Surgeons annual meetings. J Bone Joint Surg Am. 2012;94:e50[1-5].)
Journals now use information technology to help ensure the accurate and appropriate publication of research findings. Many instances of dual publication and/or plagiarism are identified by plagiarism-checking software. Similarities in language can be cross-referenced with all of the previously published materials and scientific journals. When a certain threshold of similarity is identified, a secondary review can be conducted.
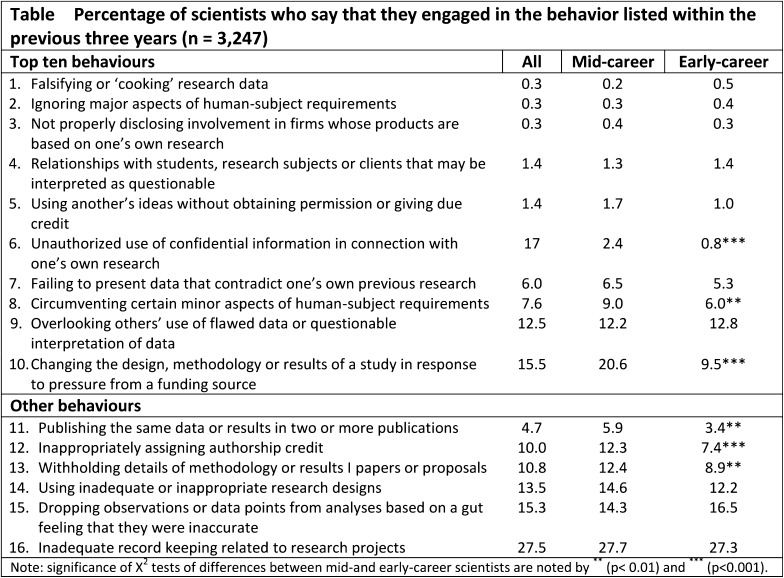
Since 1975, there has been a tenfold increase in the number of journal articles that are retracted20. The likelihood of retraction is greatest in high-impact journals that publish articles with substantial clinical relevance20. A high-profile retraction of an article published in The Journal of Bone & Joint Surgery (British volume—now known as The Bone & Joint Journal) occurred in 200921,22. The manuscript showed that the use of bone morphogenetic protein-2 (BMP-2) in open tibial fractures in military service members improved healing and reduced the need for subsequent surgery21,22. An inquiry arose after the journal received a report that the coauthors had not previously seen or approved the manuscript and that much of the paper was essentially false. Further investigation by the journal and by Walter Reed Army Medical Center (now known as the Walter Reed National Military Medical Center) led to retraction of the paper21,22. Fraud is the leading cause of journal retractions; approximately 68% of the cases of retraction due to misconduct are the result of fraud, 43% are from duplicate publication, and 14% are due to plagiarism20. Although the number of retractions has markedly increased, these figures still likely tremendously underestimate fraudulent behavior. A confidential analysis of research behaviors published in Nature in 2005 showed extensive questionable ethical behavior23 (Fig. 3). Surprisingly, senior scientists were more likely to engage in questionable behaviors23.
Fig. 3.
Percentage of scientists admitting engaging in various questionable ethical behaviors at various career stages. (Reprinted by permission from Macmillan Publishers Ltd: [Nature]; table reproduced from Martinson BC, Anderson MS, de Vries R. Scientists behaving badly. Nature. 2005 Jun 9;435[7043]:737-8.)
There were virtually no open-access journals in 1993. By 2009, more than 4500 open-access journals were in operation24. The open-access journals are digital, online, free of charge, and in most cases lack copyright restriction. While advertised as “peer-reviewed” publications, the review process in many journals is cursory, and in some cases the review period is after publication. Publication in open-access journals is costly for authors. The Public Library of Science (PLOS) journals are leaders in the open-access publishing environment and have credibility. However, even the PLOS journals are very expensive, with publication cost to the author ranging from $1300 to $2900 per article, depending on the subspecialty journal. In 2011, the PLOS, BioMed Central, and Hindawi open-access journals collectively published over 50,000 articles24. Thus, the open-access publishing business has become a multimillion-dollar business operation.
Despite safeguards, the likelihood that information entering the publication process will later be disproved is quite high. Dr. John Ioannidis has been a leader in evaluating the tendency for published materials to be later proven incorrect, incomplete, or inaccurate25. He has published these findings both in scientific journals as well as in the popular press. His work suggests that essentially all Level-IV evidence reports will be proven false or inaccurate over time25. Up to 40% of articles with Level-I evidence will also be disproved. He found that randomized clinical trials, when repeated, show similar results only two of every five times25.
A New Paradigm: The Burden of Proof
There has been general advocacy for stronger actions and increased punishment for investigators participating in malfeasance in their research26. The implications of research findings have amplified over time. The expenditure for drugs in 1990 was $40 billion. In 2008, the cost of prescription drugs in the United States was $234 billion, accounting for approximately 10% of the cost of U.S. health care27. A recent publication has noted that $3.6 billion was recovered in thirteen pharmaceutical fraud cases. Most of these cases were initiated by whistleblowers, and the pharmaceutical fraud cases collectively accounted for approximately 40% of all federal fraud whistleblower recoveries27. As of July 2012, nine of the ten largest drug companies were bound by corporate integrity agreements under civil and criminal settlements or judgments in the United States28. The British Medical Journal (now known as The BMJ) reported that companies systematically planned complex marketing campaigns to increase drug sales, including active promotion of off-label drugs or otherwise inappropriate use of drugs, despite knowledge that such use could seriously harm patients28.
Positive research findings in the high-stakes pharmaceutical industry result in enormous financial rewards and offer new medical treatments. Successful drug research and medical treatments are innovative and at times push the boundary of public policy. An example is the potential for stem-cell therapy to result in improvements in tissue regeneration, to avert the process of aging, or to treat inflammatory diseases. Several highly notable cases of stem-cell fraud have gained public attention29,30. Fraudulent reports can slow the progress of research, decrease the willingness of the public to fund scientific discovery, and greatly enhance general skepticism of the research community and its findings. Academic medical centers also bear increased risk in this era of scrutiny. After a recent case of fraud was discovered at Cornell Medical Center and Weill College of Medicine, Cornell University agreed to pay the government $4.4 million to settle a Justice Department investigation31.
Technologies that are capable of examining large data sets have increased the amount of scientific scrutiny that is being performed. Search algorithms are available as a commercial contracting enterprise, in some cases are publicly available, and are sometimes proprietary research tools32. Both subspecialty journals and high-impact scientific journals now publish original articles that focus on large data sets that implicate individual investigators in research fraud33-35. Similarly, individual web sites make accusations and speculate about research fraud, and often publish this information on public web sites36.
While “whistleblower” activities can enhance integrity in research, they are not foolproof. Investigators wrongly accused of research fraud or malfeasance face an extraordinarily adversarial environment. The burden of proof is not on the accuser but is on the investigator to validate their research findings and integrity. In many cases, the initial accusation is high profile with widespread coverage and interest. When the investigator is vindicated, the announcements are lower profile and frequently escape public notice. A claim of fraud for any investigator typically results in a formal review in an academic medical center, including the appointment of a committee to review the allegations. The investigator is required to retain and make available all of the original data and must report on the original data to the investigative committee. Offices of public relations and legal departments also review these cases. A straightforward review process, given the time required, the number of senior officials involved in the process, and the multiple reports generated, typically costs between $20,000 and $50,000.
The research climate is high risk, particularly for investigators involved in industry-supported research involving drugs with the potential for widespread clinical use. An example is the approval of BMP-2 for use in spine fusions37. During the FDA approval process, Medtronic supported seventeen clinical trials. These studies resulted in multiple publications and supported the use of BMP in spine fusion. However, additional evidence, accumulated over time, identified potential risk factors associated with BMP use in spine surgery. The original publications received ongoing analysis and scrutiny in the scientific literature and in the public press. Congressional investigation ensued and the orthopaedic surgeons involved in the studies were targeted in the press, in congressional hearings, and in the scientific literature37. In response to these pressures, Medtronic contracted with Yale University (Yale Open Data Access project; YODA)38,39. Yale was given complete access to all of the original data obtained in each of the Medtronic-sponsored clinical trials. It is believed to be the first time individual patient data were made available in aggregate38,39. To eliminate conflict of interest, Yale University served as a repository for the data but subcontracted two other universities, the Oregon Health & Sciences Center and the University of York in Great Britain, to perform an analysis of the individual patient data40,41.
These reports were published in June of 201340,41. Both groups independently performed an analysis that included an aggregate of all individual patient data—an analysis that typically is not performed during the FDA approval process, but that provides increased sensitivity for the identification of rare complications. The analysis of the aggregate individual patient data was compared with a meta-analysis of the published clinical trials, and to a meta-analysis of the confidential clinical trials reports from each of the seventeen Medtronic-sponsored clinical trials that were submitted to the FDA. The aggregate data suggested a higher incidence of complications40,41. The data further showed that while BMP is effective, it does not result in an incidence of spine fusion that is higher than what has been associated with traditional treatment, with use of autologous iliac crest bone graft40,41. The YODA initiative suggests that the original conclusions and the published studies likely overestimated the benefit of BMP. Because this developed into a several billion dollar per year industry, the research was high-stakes, and skepticism developed regarding the integrity of the company and of the involved investigators, many of whom had financial relationships with Medtronic.
The Medtronic case has several important lessons. First, investigators conducting high-profile drug-related trials are at risk for intense scrutiny; second, companies involved in such studies may benefit from providing open access to the data and/or having the individual patient data from the individual trials aggregated; and third, the YODA initiative may provide a new model whereby the accuracy of clinical trials can be ensured, and individuals and academic institutions can be shielded from accusations of research fraud39.
Conclusions
Why do authors engage in misconduct? Science, being a high-stakes enterprise, is based on the ability to produce new and important observations. An academic and/or industry scientific career is dependent on publication, which in turn has an impact on continued employment, promotion, grant support, personal recognition, and competition with other investigators. The current culture recognizes and celebrates discovery. However, the tortuous path to discovery with use of rigorous scientific principles, and the persistence required, are less appreciated. Good scientists are confident, and the burden of repeat experiments and the re-analysis of data can seem time-consuming and unnecessary. Research progress is sped up through deletion of “bad” data that can be rationalized and justified inappropriately. The victims are the scientific and clinical community and the patients whom they serve. Research integrity remains a critical issue for the medical profession and for orthopaedics, and it is not simply a problem for the research community (Table I). The trends showing increasing instances of malfeasance in research can be reversed only with the sustained and collaborative effort of departments, medical centers or institutions, professional societies, and journals. It is essential that we develop a strong culture of ethical awareness—one that celebrates the integrity of the medical profession and the knowledge that guides patient care.
TABLE I.
Activities to Develop a Culture of Research Integrity
| Department | Establish research committees to approve studies and track progress |
| Yearly department clinical research retreats | |
| Conferences, quality assurance, and grand rounds dedicated to research ethics | |
| Special review and approval process for industry-supported research | |
| Institution | Educational programs in the ethics of research and publication |
| Statistics cores to ensure accurate interpretation and presentation of data | |
| Masters and other degree-granting programs in clinical science designed for clinicians | |
| Societies | Develop Continuing Medical Education (CME) courses regarding ethics in research and clinical care |
| Symposia and conferences aimed at developing research expertise | |
| Develop strict criteria for the reporting of conflict of interest | |
| Journals | Provide level of evidence for all published articles |
| Reviewer training in publication ethics and statistical analysis | |
| Grading of statistics as a component of every article review | |
| Author and coauthor confirmation regarding the integrity of the data presented | |
| Development of an ethics board to provide final approval of manuscripts with conflict of interest |
Source of Funding
This work was funded in part through a Public Health Services Award (P50 AR 054041) from the National Institutes of Health (R.J.O.).
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Broad W, Wade N. Betrayers of the truth: fraud and deceit in the halls of science. New York: Simon and Schuster; 1983. [Google Scholar]
- 2.Goldstein D. On fact and fraud: cautionary tales from the front lines of science. Princeton and Oxford: Princeton University Press; 2010. p 168. [Google Scholar]
- 3.Reuben SS, Buvanendran A. Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. J Bone Joint Surg Am. 2007June;89(6):1343-58 Paper officially retracted in: Heckman JD. Retractions. J Bone Joint Surg Am. 2009 Apr;91(4): 965. [DOI] [PubMed] [Google Scholar]
- 4.Goodman SB. Multimodal analgesia for orthopedic procedures. Anesth Analg. 2007July;105(1):19-20. [DOI] [PubMed] [Google Scholar]
- 5.White PF, Rosow CE, Shafer SL; Editorial Board of Anesthesia & Analgesia. The Scott Reuben saga: one last retraction. Anesth Analg. 2011March;112(3):512-5. [DOI] [PubMed] [Google Scholar]
- 6.Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P, Valentine A, Davies SE, Walker-Smith JA. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998February28;351(9103):637-41 Paper partially retracted in: Editors of The Lancet. Retraction of an interpretation. The Lancet. 2004 Mar 6;363(9411):750. Paper fully retracted in: Editors of The Lancet. Retraction—Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 2010 Feb 6;375(9713):445. [DOI] [PubMed] [Google Scholar]
- 7.Deer B. How the case against the MMR vaccine was fixed. BMJ. 2011;342:c5347. Epub 2011 Jan 5. [DOI] [PubMed] [Google Scholar]
- 8.Deer B. Secrets of the MMR scare. How the vaccine crisis was meant to make money. BMJ. 2011;342:c5258. Epub 2011 Jan 11. [DOI] [PubMed] [Google Scholar]
- 9.Cherry JD. Epidemic pertussis in 2012—the resurgence of a vaccine-preventable disease. N Engl J Med. 2012August30;367(9):785-7 Epub 2012 Aug 15. [DOI] [PubMed] [Google Scholar]
- 10.Eid K, Thornhill TS, Glowacki J. Chondrocyte gene expression in osteoarthritis: correlation with disease severity. J Orthop Res. 2006May;24(5):1062-8. [DOI] [PubMed] [Google Scholar]
- 11.Jalbă BA, Jalbă CS, Vlădoi AD, Gherghina F, Stefan E, Cruce M. Alterations in expression of cartilage-specific genes for aggrecan and collagen type II in osteoarthritis. Rom J Morphol Embryol. 2011;52(2):587-91. [PubMed] [Google Scholar]
- 12.Buckwalter JA, Wright T, Mogoanta L, Alman B. Plagiarism: an assault on the integrity of scientific research. J Orthop Res. 2012December;30(12):1867-8 Epub 2012 Aug 21. [DOI] [PubMed] [Google Scholar]
- 13.Buckwalter JA, Wright TM, Donahue HJ, Amadio PC. Publishing the results of multiple experiments using the same methods and outcome measures. J Orthop Res. 2011;29:155-6. [Google Scholar]
- 14.Sassoon AA, Trousdale RT. Podium disclosures at the 2012 AAOS meeting: an exercise in going through the motions. J Bone Joint Surg Am. 2013April17;95(8):e51. [DOI] [PubMed] [Google Scholar]
- 15.Lee YK, Chung CY, Koo KH, Lee KM, Ji HM, Park MS. Conflict of interest in the assessment of thromboprophylaxis after total joint arthroplasty: a systematic review. J Bone Joint Surg Am. 2012January4;94(1):27-33. [DOI] [PubMed] [Google Scholar]
- 16.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. JAMA. 2003January22-29;289(4):454-65. [DOI] [PubMed] [Google Scholar]
- 17.Khan SN, Mermer MJ, Myers E, Sandhu HS. The roles of funding source, clinical trial outcome, and quality of reporting in orthopedic surgery literature. Am J Orthop (Belle Mead NJ). 2008December;37(12):E205-12; discussion E212. [PubMed] [Google Scholar]
- 18.Voleti PB, Donegan DJ, Baldwin KD, Lee GC. Level of evidence of presentations at American Academy of Orthopaedic Surgeons annual meetings. J Bone Joint Surg Am. 2012April18;94(8):e50. [DOI] [PubMed] [Google Scholar]
- 19.Okike K, Kocher MS, Mehlman CT, Heckman JD, Bhandari M. Publication bias in orthopaedic research: an analysis of scientific factors associated with publication in the Journal of Bone and Joint Surgery (American Volume). J Bone Joint Surg Am. 2008March;90(3):595-601. [DOI] [PubMed] [Google Scholar]
- 20.Fang FC, Steen RG, Casadevall A. Misconduct accounts for the majority of retracted scientific publications. Proc Natl Acad Sci U S A. 2012October16;109(42):17028-33 Epub 2012 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuklo TR, Groth AT, Anderson RC, Frisch HM, Islinger RB. Recombinant human bone morphogenetic protein-2 for grade III open segmental tibial fractures from combat injuries in Iraq. J Bone Joint Surg Br. 2008August;90(8):1068-72 Paper retracted in Scott J. J Bone Joint Surg Br. 2009 Mar;91(3):285-6. [DOI] [PubMed] [Google Scholar]
- 22.Scott J. Withdrawal of a paper. J Bone Joint Surg Br. 2009March;91(3):285-6. [DOI] [PubMed] [Google Scholar]
- 23.Martinson BC, Anderson MS, de Vries R. Scientists behaving badly. Nature. 2005June9;435(7043):737-8. [DOI] [PubMed] [Google Scholar]
- 24.Laakso M, Welling P, Bukvova H, Nyman L, Björk BC, Hedlund T. The development of open access journal publishing from 1993 to 2009. PLoS One. 2011;6(6):e20961 Epub 2011 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannidis JP. Why most published research findings are false. PLoS Med. 2005August;2(8):e124 Epub 2005 Aug 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Face up to fraud. Nature. 2012January19;481(7381):237-8 Epub 2012 Jan 18. [DOI] [PubMed] [Google Scholar]
- 27.Qureshi ZP, Sartor O, Xirasagar S, Liu Y, Bennett CL. Pharmaceutical fraud and abuse in the United States, 1996-2010. Arch Intern Med. 2011September12;171(16):1503-6. [DOI] [PubMed] [Google Scholar]
- 28.Davis C, Abraham J. Is there a cure for corporate crime in the drug industry? BMJ. 2013;346:f755. Epub 2013 Feb 6. [DOI] [PubMed] [Google Scholar]
- 29.Cyranoski D. Stem-cell fraud hits febrile field. Nature. 2012October18;490(7420):321. [DOI] [PubMed] [Google Scholar]
- 30.Snyder EY, Loring JF. Beyond fraud—stem-cell research continues. N Engl J Med. 2006January26;354(4):321-4. [DOI] [PubMed] [Google Scholar]
- 31.Ready T. Cornell University scientists face charges of fraud. Nat Med. 2005August;11(8):810. [DOI] [PubMed] [Google Scholar]
- 32.Butler D. Journals step up plagiarism policing. Nature. 2010July8;466(7303):167. [DOI] [PubMed] [Google Scholar]
- 33.Garner HR, McIver LJ, Waitzkin MB. Research funding: Same work, twice the money? Nature. 2013January31;493(7434):599-601. [DOI] [PubMed] [Google Scholar]
- 34.Errami M, Sun Z, George AC, Long TC, Skinner MA, Wren JD, Garner HR. Identifying duplicate content using statistically improbable phrases. Bioinformatics. 2010June1;26(11):1453-7 Epub 2010 May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garner HR. Combating unethical publications with plagiarism detection services. Urol Oncol. 2011Jan-Feb;29(1):95-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couzin-Frankel J. Image manipulation. Author of popular blog that charged fraud unmasked. Science. 2013January11;339(6116):132. [DOI] [PubMed] [Google Scholar]
- 37.Carragee EJ, Baker RM, Benzel EC, Bigos SJ, Cheng I, Corbin TP, Deyo RA, Hurwitz EL, Jarvik JG, Kang JD, Lurie JD, Mroz TE, Oner FC, Peul WC, Rainville J, Ratliff JK, Rihn JA, Rothman DJ, Schoene ML, Spengler DM, Weiner BK. A biologic without guidelines: the YODA project and the future of bone morphogenetic protein-2 research. Spine J. 2012October;12(10):877-80. [DOI] [PubMed] [Google Scholar]
- 38.Krumholz HM, Ross JS. A model for dissemination and independent analysis of industry data. JAMA. 2011October12;306(14):1593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krumholz HM, Ross JS, Gross CP, Emanuel EJ, Hodshon B, Ritchie JD, Low JB, Lehman R. A historic moment for open science: the Yale University Open Data Access project and Medtronic. Ann Intern Med. 2013June18;158(12):910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simmonds MC, Brown JV, Heirs MK, Higgins JP, Mannion RJ, Rodgers MA, Stewart LA. Safety and effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion: a meta-analysis of individual-participant data. Ann Intern Med. 2013June18;158(12):877-89. [DOI] [PubMed] [Google Scholar]
- 41.Fu R, Selph S, McDonagh M, Peterson K, Tiwari A, Chou R, Helfand M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: a systematic review and meta-analysis. Ann Intern Med. 2013June18;158(12):890-902. [DOI] [PubMed] [Google Scholar]