Abstract
Although rotavirus vaccines are available, rotaviruses remain the major cause of childhood diarrheal disease worldwide. The Rotarix (GlaxoSmithKline Biologicals Rixensart, Belgium) and RotaTeq (Merck and Co., Inc. Whitehouse Station, New Jersey, USA) vaccines are effective for reducing the morbidity and mortality of rotavirus infection. This article aims to assess the epidemiology of rotaviral gastroenteritis and the efficacy and effectiveness of licensed rotavirus vaccines. This review concludes by presenting challenges in the field that require further exploration by and perspectives from basic and translational research in the future.
Keywords: Effectiveness, efficacy, rotavirus, rotavirus vaccine.
INTRODUCTION
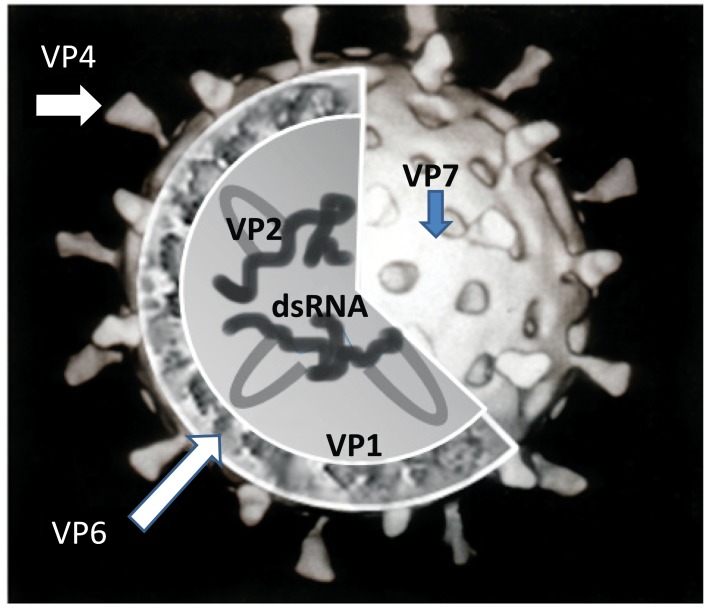
Despite declining child mortality and morbidity resulting from rotaviral gastroenteritis, rotavirus infection persists as the most common cause of hospitalization and mortality in children due to the severe diarrhea and dehydration that result from infection [1]. Rotaviruses were first identified in animals in the 1960s and then were subsequently discovered in humans via electron microscopic examination of the duodenums of children who had severe diarrhea [2]. Rotavirus is a member of the family Reoviridae and the genus Rotavirus. By electron microscopy, the virus is observed to have a 70 nm, non-enveloped, icosahedral structure that surrounds a double-stranded RNA genome (Fig. 1). The genomic RNA of rotavirus is enclosed in a triple-layered capsid [1, 2]. The rotavirus genome is composed of 11 RNA segments that encode for the VP1-VP4, VP6, and VP7 structural and NSP1-NSP6 non-structural viral proteins. The VP6 protein forms the middle capsid layer and is responsible for the group-specific antigenic determinants. Serotypes of rotavirus are determined by the VP7 and VP4 proteins, which are the major outer capsid surface proteins and act as independent neutralizing antigens. NSP4 is also antigenic and plays a role as an enterotoxin that is capable of causing diarrhea. Rotaviruses are categorized into different genotypes according to the particular NSP4 proteins expressed [1].
Fig. (1).
Cartoon structure of rotavirus.
VP7 and VP4 are post-translationally modified; VP7 is glycosylated, whereas VP4 protein is cleaved by a protease. The G and P serotypes of rotavirus are determined by VP7 and VP4, respectively. P serotypes are difficult to define by traditional methods using virus neutralization assays. Instead, molecular methods have been used to determine the genotype of those “P serotypes” based on sequence analysis [3]. The genotypes are tentatively designated in brackets (e.g., P1B [8]), as these genotypes are closely related to known serotypes. The VP7 and VP4 proteins are the targets for neutralizing antibodies, which might provide serotype-specific and, in some instances, cross-reactive protection against acute gastroenteritis; therefore, they are main targets for vaccine development [4].
Human rotaviruses are remarkably diverse. Thus far, at least 42 different P-G serotype combinations have been identified due to the independent assortment and segregation of the G and P proteins, resulting in the production of different strains [5]. Fortunately, only a small number of the different rotavirus strains circulating worldwide are capable of causing human illness.
This review aims to assess the global epidemiology of rotavirus disease and to summarize the current status of rotavirus vaccine use and effectiveness worldwide. This study includes findings from the rotavirus literature collected from January 2005 through May 2014. Previous work was found using the search terms ‘epidemiology of rotavirus’ and ‘vaccines of rotavirus’ in MEDLINE and PubMed, resulting in 283 and 368 reviews, respectively. Articles that were not published in the English language, manuscripts without an abstract, and opinion articles were excluded from the review. After preliminary screening, a total of 121 articles were considered to be relevant for inclusion in this review.
EPIDEMIOLOGY
Disease Burden
Rotavirus infection causes severe gastroenteritis in infants and young children worldwide. Globally, there are at least 600,000 children < 5 years old who die from diarrhea with severe dehydration and electrolyte and acid-base disturbances each year [6]. The majority of rotavirus-related deaths (> 80%) are found in resource-limited countries, such as those found in southern Asia and sub-Saharan Africa [6]. Most childhood rotavirus infections occur by 5 years of age and are unrelated to community sanitary conditions, home location of the infected children, or the greater socioeconomic status of the affected countries [7]. Therefore, the overall incidence of rotavirus infection would not change even if improvements in water supplies, sanitation, personal nutrition, housing, and public health education were made, suggesting that viral transmission might occur via non-fecal routes [8]. Vaccines are an effective and available measure for combating rotavirus disease and for preventing rotavirus infection [9].
Acute gastroenteritis is one of the leading causes of childhood mortality worldwide and accounts for 15% of all deaths in younger children [10]. Most of these deaths occur in malnourished infants from countries of lower socioeconomic status and from the disadvantaged rural regions of Africa and Asia [10, 11].
Whereas the mortality from rotavirus in young children is rare in industrialized countries with higher socioeconomic status, rotavirus disease incidence is similar in countries from both higher and lower socioeconomic levels [12]. Before the rotavirus vaccine was licensed, it had been estimated that rotavirus infection and disease resulted in 220,000 hospitalizations, 1.8 million outpatient visits, and more than 7.1 million children who had episodes of rotavirus-related gastroenteritis annually in industrialized countries [6, 12]. A previous study from the United States showed that approximately 60 deaths, 410,000 outpatient visits, 272,000 emergency department visits, and 70,000 hospitalizations were caused by rotavirus infection each year during the 1990s and early 2000s [13], indicating that approximately 1.2% - 1.5% of younger children in the United States had been hospitalized for rotavirus disease [14]. In Taiwan, using data from the National Health Insurance Data Bank, at least 150,000 cases of rotavirus gastroenteritis occurred each year between 2000 and 2005, before the rotavirus vaccine was introduced [15-19]. Among these, 106,000 were outpatient visits, 12,800 were emergency department visits, 15,000 were hospitalizations, and approximately 7 deaths were caused by rotavirus infection during this study period [15-19].
The societal costs resulting from rotavirus infection have been calculated for different countries. In the United States, it was estimated that the annual societal cost from rotavirus infection in 2004 was 900 million US dollars [13]; in Taiwan in 2005, rotavirus-related costs were approximately 21.5 million US dollars [20]; in the European Union (EU) in 2002, the societal costs tallied almost 550 million euros annually [21]; and, in developing countries in 2007, rotavirus was estimated to cost 423 million US dollars [22]. According to these data, rotavirus gastroenteritis requires a huge expenditure of societal health care resources.
Global Surveillance of Rotavirus
As the promotion of rotavirus vaccination continues to increase worldwide, it has become an important tool for global rotavirus surveillance. The usefulness of rotavirus surveillance stems from the monitoring of serotype distribution across different regions and the prediction of newly emergent rotavirus strains. Another role of the rotavirus surveillance programs is to assess the impact of vaccination on the reduction of rotavirus disease occurrence and death as well as to identify causes of diarrheal disease other than rotavirus. Starting from 2008, the World Health Organization (WHO) has collected information on the clinical characteristics and laboratory testing data of rotavirus infection in younger children with acute diarrhea. In 2013, the WHO assessed the surveillance network performance and recommended that all WHO members using the network should utilize the system as a bridge to also perform surveillance for other vaccine-preventable diseases [23, 24]. This protocol was first implemented in Asia [25] and was then expanded to many countries and regions, including Latin America, the Mediterranean region, Europe, and Africa [24]. During 2011 and 2012, informative data from 37 countries were collected by these global systems [26]. The data collected from various regional surveillance systems have been standardized, which has increased its reliability and perceived validity. Moreover, the sustainability of the rotavirus surveillance system has been improved by the implementation of this model.
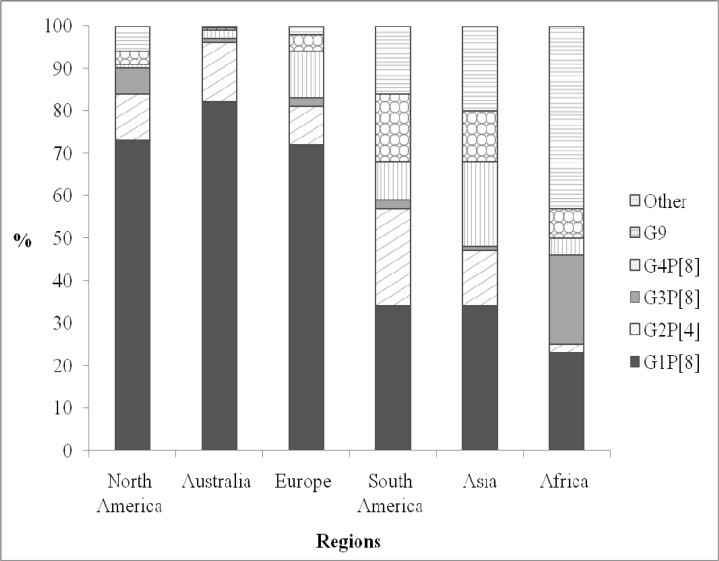
The information regarding circulating rotavirus strains that has been collected as part of rotavirus surveillance also provides a valuable resource for vaccine development, the identification of novel viral strains and the evaluation of vaccine effectiveness. Additionally, these data are useful to monitor the circulating rotavirus strains after the implementation of a vaccination program. (Fig. 2) Previous studies show that the most prevalent, globally circulating rotavirus strains of the VP7 serotypes are G1, G2, G3, G4, and G9; whereas, P[8], P[4] and P[6] and G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8] are the most (70-90%) common circulating VP4 genotypes [27-31]. In contrast to previous studies, the most common VP7 serotypes identified in Taiwan are G1 (40%) followed by G3 (27%), G9 (18%), and G2 (8%) [32-38].
Fig. (2).
Geographic distribution of rotavirus serotypes.
The highest diversity of rotavirus strains is found in the regions of Africa and Asia. Possible reasons for this high diversity include the prevalence of cases of mixed infections with multiple rotavirus strains and/or housing in close proximity to domestic animals from which the shedding of virus occurs [29]. In addition to differences of geography, dominant circulating strain genotypes can also differ according to the season [39]. The new G9 and G12 rotavirus strains have occurred and spread globally over the past few years [40]. It is possible that in the same period, different serotypes of rotavirus could circulate within the same area. Persistent monitoring of circulating rotavirus strains is still a critical tool for determining the molecular characteristics of rotavirus disease and the effectiveness of rotavirus vaccination.
ROTAVIRUS VACCINES
Bishop et al. first found that infants infected with rotavirus would become immune to later rotavirus re-infection [41]. According to their observations, children infected with rotavirus were protected against rotavirus gastroenteritis after re-infection [41]. Oral delivery of live attenuated rotavirus has been the predominant guiding concept for the development of a rotavirus vaccine. Using the "Jennerian" concept, experts have considered that immunization with animal rotaviruses will result in naturally attenuated strains in humans [42]. Attenuated human rotaviruses have also recently been generated by passage in cell culture and have been used in experimental studies [43]. Furthermore, rotaviruses isolated from asymptomatic infants might be naturally less virulent and may make good candidates for the development of an oral vaccine [44].
Monovalent Animal Strain Vaccines
Both rotaviruses strains RIT4237 (P6[1] G6) and Wistar Calf 3 (WC3, serotype P7[5]G6) are of bovine origin. The RIT4237 bovine strain was obtained from a calf and attenuated by passage in cell culture. MMU18006 (P5B[3]G3) is a bovine-simian (rhesus) reassortant rotavirus vaccine (RRV) strain [45-49]. The efficacy of the vaccines derived from these strains has been studied. Initially, the efficacy trials in Finland showed satisfactory results; however, the follow-up studies were disappointing due to the lack of protection observed against rotaviral gastroenteritis [47, 48].
The WC3 bovine strain was discovered in 1981 when it was obtained from a calf. Field trials revealed that vaccines generated from this strain had variable efficacy and finally yielded particularly disappointing results for protection against rotavirus infection [45, 46]
Between 2000 and 2001, China introduced the Lanzhou lamb rotavirus vaccine (LLR) as a childhood vaccine for protection against rotaviral diarrheal disease [50]. The LLR vaccine is a monovalent (P[12]G10) live attenuated oral vaccine. This vaccine strain was derived from a lamb rotavirus and was developed and produced by the Lanzhou Institute of Biological Products. The LLR efficacy trial was conducted in the Guangzhou province. In this case controlled study, 838 hospitalized children with rotavirus infections, aged 2 months to 5 years, were enrolled as cases, and 838 healthy children were enrolled as controls [50]. The study showed that the efficacy against hospitalization due to rotaviral gastroenteritis after receiving one dose of LLR was 73% (95% CI: 61-82%). Immunological studies have shown that the neutralizing antibody activity specific for all G serotypes among children aged 6-24 months was 40% and 70%, before and after vaccination, respectively [51]. However, the actual efficacy of the vaccine remains undetermined because a strictly randomized, placebo-controlled phase III clinical trial to test the LLR was never performed [50]. According to the reported trials, the majority of children were vaccinated at an older age than would be typical for initial infection and acute rotavirus gastroenteritis. It was not determined whether the children who had been previously vaccinated had previously been exposed to rotavirus, in which case the LLR could have improved the status of the pre-existing antibody response [51]. In China, children aged 2 to 36 months are vaccinated with this formulation and receive yearly boosters [51]. It is estimated that 10,000,000 doses of LLR were administered in China from 2001 to 2008 [51]; however, the vaccine has not been recommended for use in national immunization programs in China or elsewhere until now.
Quadrivalent Human-Rhesus Reassortant Vaccine
RotaShield was the first multivalent live oral reassortant vaccine. It belongs to a group that includes the rhesus rotavirus tetravalent [RRV--TV] vaccine. This tetravalent vaccine is a combination of the G1 to G4 virus strains. It includes three rhesus-human reassortant strains, including human serotype strains (G1, G2, and G4) and the rhesus RRV serotype (G3) [52]. The primary efficacy study demonstrated that the vaccine-conferred protection against all cases of rotavirus-related gastroenteritis was 57-76%, and protection against severe rotavirus disease was 82-96% [53]. An adverse reaction of fever was observed after the first vaccination with Rotashield; however, no other important side effects have been found to occur as a result of Rotashield vaccination at the time of licensing [54].
This vaccine was approved by the U.S. FDA in 1998; however, it was suspended from use and withdrawn from the market in 1999 due to the risk of intussusception. Investigations indicated that the use of this vaccine had a significant association with a higher risk of intussusception [55], which was quantified at an incidence of 1 per 10,000 individuals vaccinated. Most cases occurred within 3-14 days following the first dose, with the children who received their first dose after 3 months of age showing the highest risk [56]. The cause of this association remains unclear; however, these adverse effects have shown that large field trials should be conducted for future rotavirus vaccine candidates. The safety criteria for rotavirus vaccines are required to be less than a 1 in 10,000 risk of intussusception in large field trials [39]. As further precaution, strict vaccination programs have been established in which the first vaccine dose is administered at an age of 6-14 weeks and no ‘catch-up’ schedules are allowed. Post-marketing surveillance for intussusception has been established in several countries [57].
Human-Bovine Rotavirus Reassortant Vaccine
The RotaTeq vaccine contains 4 human and 1 bovine live reassortant rotaviruses, including the G1-G4 common VP7 types from a human rotavirus parent strain and the P7[5] attachment protein from the WC3 bovine rotavirus parent strain [41, 42]. The fifth reassortant virus expresses the attachment protein (P1A[8]) from the human rotavirus parent strain and the G6 outer capsid protein from the bovine rotavirus parent strain [35, 58]. RotaTeq is an oral vaccine that is administered at 1- to 2-month intervals beginning at 6 to 12 weeks of age and given in three doses [58, 59].
The efficacy of RotaTeg against all diarrheal diseases and severe gastroenteritis was 74% and 98% in a large efficacy trial, respectively [60]. In addition, a large safety trial has been conducted with more than 70,000 infants, and various subgroups were analyzed [58, 61]. RotaTeq has demonstrated efficacy against each of the common circulating rotavirus serotypes. It also has been found that RotaTeq has high efficacy for protecting against the risk for rotavirus infection-related hospitalization and emergency department and physician visits. Compared with the risk of intussusception following treatment with a placebo, no evidence of an increased risk of intussusception following vaccination has been found. Additionally, efficacy and safety trials have been conducted in developing countries. These studies showed that the vaccine reduces the healthcare resource utilization attributable to rotavirus diarrhea in infants, but there was no evidence of an increased risk of intussusception or other serious adverse events [62]. Moreover, no associations have been found between intussusception or Kawasaki disease and the safety of RotaTeq, as reported by the post-marketing surveillance data in the United States [63]. The effectiveness of the RotaTeq vaccine against rotavirus gastroenteritis-related (RGE) hospitalizations and deaths was tested in Asian countries by Khoury et al. using a simple mathematical model [64]. Their study showed that the overall effectiveness against RGE-related hospitalizations and the substantial reduction in RGE-related deaths was 82% to 89% in the region.
This vaccine has been licensed in the United States, and in 2006, the vaccine was recommended by the Advisory Committee on Immunization Practices (ACIP) for inclusion in the routine infant immunization schedule [65].
Monovalent Human G1 Rotavirus Vaccine
Another live-attenuated human rotavirus vaccine (strain 89-12) was developed by tissue culture passage of a wild-type human rotavirus isolate [66]. This vaccine used the P1A [8] G1 strain as a parent strain. The P1A [8] G1 strain contains the most common of the human rotavirus VP7 and VP4 antigens. Originally, Avant Immunotherapeutics used and modified this vaccine. Then, GlaxoSmithKline Biologicals further modified it by cloning and tissue culture passaging of the parent 89-12 vaccine strain. Finally, this vaccine was licensed by GlaxoSmithKline Biologicals [67]. The vaccine is known as RIX4414 (Rotarix). The efficacy trials conducted in the United States and Finland showed that Rotarix had high efficacy [67]. In addition, this vaccine was tested in Latin American countries. Overall, the efficacy of Rotarix against severe rotavirus disease was 86% [68]. Other clinical trials involving 63,000 children have been conducted. These studies showed that the Rotarix efficacy against hospitalization was 85%, and the efficacy against non-G1 serotypes was 75% [68]. Another study involving more than 15,000 healthy infants, aged 6-13 weeks and living in Latin American countries, was performed to establish the efficacy and safety of Rotarix. This study showed that the efficacy of the vaccine against the G1 wild-type was 81% to 82%, against the pooled non-G1 strains was 78%, against the pooled non-G1P[8] strain was 81%, against hospitalization with severe diarrhea was 83%, and against hospitalization with any cause of diarrhea was 39% [69]. No evidence of increasing adverse events or risk of intussusception were observed in individuals after receiving the vaccine [69]. This vaccine was first licensed in Mexico and the Dominican Republic in 2004, and it was approved by the United States in 2008. ACIP immediately recommended it for inclusion in the routine immunization schedule for infants [65]. In recent years, Rotarix has been introduced in more than 90 countries worldwide.
Indian Neonatal Strain Vaccines
In India, candidate rotavirus vaccines are being developed using two strains (116E strain and I321 strain) isolated from newborns. The 116E strain is a P8[121] G9 natural reassortant between a human parent strain and a VP4 gene of bovine origin. This strain was isolated in 1985 from an outbreak of asymptomatic rotavirus infections in New Delhi [70]. The sequence of the VP4 gene is homologous to that of P[11], a genotype commonly found in cattle. In addition, the I321 strain was identified from an outbreak of a nosocomial infection at a maternity center in Bangalore and was identified to be a bovine-human reassortant strain [71]. The genome of the I321 strain is different from the genome of the 116E strain. The I321 strain includes nine bovine gene segments. Among them, only gene segments five and seven, which encoded nonstructural proteins 1 and 3, are of human origin. A new strain with the identical G and P segments as the I321 strain has emerged in Vellore, India [72] as a cause of gastroenteritis in children.
Recently published results from a phase 3 clinical trial of an oral, attenuated rotavirus vaccine (ROTAVAC, Bharat Biotech International, Limited, of India), manufactured in India and based on the natural human-bovine reassortant strain G10P [73], which causes asymptomatic infection in neonates, demonstrated that the vaccine effectiveness for protection from severe rotavirus gastroenteritis in the first year of children’s lives was 56% [74]. Several live oral rotavirus vaccines are in development in India. The results from the Indian multicenter trial showed that this vaccine efficacy was comparable to that of the 2 internationally licensed rotavirus vaccines in low-income settings, and alternate dosing schedules, including neonatal dosing schedules, are being evaluated as well [75].
CONCLUSION
The disease burden of rotavirus is substantial, and the economic burden from infection in infants and children is a threat worldwide. Rotavirus vaccination is a cost-effective measure to prevent rotavirus infection. The monovalent rotavirus vaccine (a G1P[8] human rotavirus strain) and the pentavalent vaccine exert similar degrees of effectiveness for protecting against infection with homotypic and heterotypic rotavirus strains [76]. There are no specific, persistent strains circulating in the world at this time, suggesting that vaccine-induced selective pressure did not occur. Expanding rotavirus surveillance networks to industrialized and developing countries as well as implementing persistent rotavirus surveillance are important for identifying newly emergent rotavirus strains and for evaluating the effectiveness of strain-specific vaccine responses in different countries.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Estes MK, Kapikian AZ, et al., editors. In nipe Fields virology. 5th ed. Philadelphia (PA): Lippincott, Williams and Wilkins, ; 2007. Rotaviruses. 94 pp. [Google Scholar]
- 2.Bishop RF, Davidson GP, Holmes IH , et al. Virus particles in epithelial cells of duodenal mucosa from children with acute non-bacterial gastroenteritis. Lancet. 1973;2:1281–3. doi: 10.1016/s0140-6736(73)92867-5. [DOI] [PubMed] [Google Scholar]
- 3.Desselberger U. Rotaviruses. Virus Res. 2014;190C:75–96. doi: 10.1016/j.virusres.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Hoshino Y, Kapikian AZ. Rotavirus serotypes classification and importance in epidemiology, immunity and vaccine development. J Health Popul Nutr. 2000;18: 5–14. [PubMed] [Google Scholar]
- 5.Gentsch Jr, Laird AR, Bielfelt B , et al. Serotype diversity and reassortment between human and animal rotavirus strains implications for rotavirus vaccine programs. J Infect Dis. 2005;192:S146–59. doi: 10.1086/431499. [DOI] [PubMed] [Google Scholar]
- 6.Parashar UD, Gibson CJ, Bresse JS , et al. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bilcke J, Van Damme P, Van Ranst M , et al. Estimating the incidence of symptomatic rotavirus infections a systematic review and meta-analysis. PLoS One. 2009;4:e6060. doi: 10.1371/journal.pone.0006060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanran A, Fitzwater S, Zhen A , et al. Prevention of rotavirus gastroenteritis in infants and children rotavirus vaccine safety, efficacy, and potential impact of vaccines. Biologics. 2010;4:213–29. doi: 10.2147/btt.s6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel MM, Steele D, Gentsch Jr , et al. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011;30:S1–5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- 10.Black RE, Cousens S, Johnson HL , et al. Global, regional, and national causes of child mortality in 2008,: a systematic analysis. Lancet. 2010;375:1969–87. doi: 10.1016/S0140-6736(10)60549-1. [DOI] [PubMed] [Google Scholar]
- 11.Glass RI. New hope for defeating rotavirus. Sci Am. 2006;294(4):46–51. doi: 10.1038/scientificamerican0406-46. [DOI] [PubMed] [Google Scholar]
- 12.Parashar UD, Burton A, Lanata C , et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200:S9–15. doi: 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). Delayed onset and diminished magnitude of rotavirus activity--United States November 2007-May 2008. Morb Mortal Wkly Rep (MMWR). 2008;57:697–700. [PubMed] [Google Scholar]
- 14.Malek MA, Curns AT, Holman RC , et al. Diarrhea- and rotavirus-associated hospitalizations among children less than 5 years of age United States 1997 and 2000. Pediatrics. 2006;117:1887–92. doi: 10.1542/peds.2005-2351. [DOI] [PubMed] [Google Scholar]
- 15.Chen KT, Fan SF, Tang RB , et al. Hospital-based study of economic burden associated rotavirus diarrhea in Taiwan. Vaccine. 2007;25:4266–72. doi: 10.1016/j.vaccine.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 16.Wu CL, Yang YC, Huang LM , et al. Cost-effectiveness of childhood rotavirus vaccination in Taiwan. Vaccine. 2009;27:1492–9. doi: 10.1016/j.vaccine.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 17.Chen KT, Chen PY, Tang RB , et al. Sentinel hospital surveillance for rotavirus diarrhea in Taiwan 2001-2003. J Infect Dis. 2005;92:S44–8. doi: 10.1086/431495. [DOI] [PubMed] [Google Scholar]
- 18.Lu CY, Lauderdale TL, Fang YH , et al. Disease burden and related medical costs of rotavirus infections in Taiwan. BMC Infect Dis. 2006;6:176. doi: 10.1186/1471-2334-6-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mast TC, Chen PY, Lu KC , et al. Epidemiology and economic burden of rotavirus gastroenteritis in hospitals and paediatric clinics in Taiwan 2005-2006. Vaccine. 2010;28:3008–13. doi: 10.1016/j.vaccine.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Widdowson MA, Meltzer MI, Zhang X , et al. Cost-effectiveness and potential impact of rotavirus vaccination in the United States. Pediatrics. 2007;119:684–97. doi: 10.1542/peds.2006-2876. [DOI] [PubMed] [Google Scholar]
- 21.Standaert B, Harlin O, Desselberger U. The financial burden of rotavirus disease in four countries of the European Union. Pediatr Infect Dis J. 2008;27:S20–7. [Google Scholar]
- 22.Rheingans RD, Antil L, Dreibelbis R , et al. Economic costs of rotavirus gastroenteritis and cost-effectiveness of vaccination in developing countries. J Infect Dis. 2009;200:S16–27. doi: 10.1086/605026. [DOI] [PubMed] [Google Scholar]
- 23.Agócs MM, Serhan F, Yen C , et al. WHO global rotavirus surveillance network a strategic review of the first 5 years 2008-2012. MMWR Morb Mortal Wkly Rep. 2014;63:634–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Widdowson MA, Steele D, Vojdani J , et al. Global rotavirus surveillance determining the need and measuring the impact of rotavirus vaccines. J Infect Dis. 2009;200:S1–8. doi: 10.1086/605061. [DOI] [PubMed] [Google Scholar]
- 25.Bresee J, Fang ZY, Wang B , et al. First report from the Asian Rotavirus Surveillance Network. Emerg Infect Dis. 2004;10:988–95. doi: 10.3201/eid1006.030519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Rotavirus vaccines. WHO position paper January 2013. Wkly Epidemiol Rec. 2013;88:49–64. [PubMed] [Google Scholar]
- 27.Wu FT, Liang SY, Tsao KC , et al. Hospital-based surveillance and molecular epidemiology of rotavirus infection in Taiwan,2005-2007. Vaccine. 2009;27:F50–4. doi: 10.1016/j.vaccine.2009.08.090. [DOI] [PubMed] [Google Scholar]
- 28.Santos N, Hoshino Y. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev Med Virol. 2005;15:29–56. doi: 10.1002/rmv.448. [DOI] [PubMed] [Google Scholar]
- 29.Sharma S, Paul VK, Bhan MK , et al. Genomic characterization of nontypeable rotaviruses and detection of a rare G8 strain in Delhi, India. J Clin Microbiol. 2009;47:3998–4005. doi: 10.1128/JCM.00809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Rotavirus surveillance-- worldwide 2001-2008. MMWR Morb Mortal Wkly Rep. 2008;57:1255–7. [PubMed] [Google Scholar]
- 31.Iturriza-Gómara M, Dallman T, Bányai K , et al. Rotavirus surveillance in Europe 2005-2008: web-enabled reporting and real-time analysis of genotyping and epidemiological data. J Infect Dis. 2009;200:S215–21. doi: 10.1086/605049. [DOI] [PubMed] [Google Scholar]
- 32.Wu FT, Bányai K, Huang JC , et al. Diverse origin of P[19] rotaviruses in children with acute diarrhea in Taiwan Detection of novel lineages of the G3 G5 and G9 VP7 genes. J Med Virol. 2011;83:1279–87. doi: 10.1002/jmv.22052. [DOI] [PubMed] [Google Scholar]
- 33.Wu FT, Bányai K, Huang JC , et al. Human infection with novel G3P[25] rotavirus strain in Taiwan. Clin Microbiol Infect. 2011;17:1570–3. doi: 10.1111/j.1469-0691.2011.03531.x. [DOI] [PubMed] [Google Scholar]
- 34.Hwang KP, Huang YC, Bányai K , et al. Severe gastroenteritis associated with G3P[9] rotavirus in Taiwan. Infection. 2011;39:271–5. doi: 10.1007/s15010-011-0098-4. [DOI] [PubMed] [Google Scholar]
- 35.Lin YP, Kao CL, Chang SY , et al. Determination of human rotavirus VP6 genogroups I and II by reverse transcription-PCR. J Clin Microbiol. 2008;46:3330–7. doi: 10.1128/JCM.00432-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SY, Chang YC, Lee YS , et al. Molecular epidemiology and clinical manifestations of viral gastroenteritis in hospitalized pediatric patients in Northern Taiwan. J Clin Microbiol. 2007;45:2054–7. doi: 10.1128/JCM.01519-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin YP, Chang SY, Kao CL , et al. Molecular epidemiology of G9 rotaviruses in Taiwan between 2000 and 2002. J Clin Microbiol. 2006;44:3686–94. doi: 10.1128/JCM.02107-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai HC, Lin SJ, Lin HR , et al. Phylogenetic analyses of human rotavirus in central Taiwan in 1996 2001 and 2002. J Clin Virol. 2005;32:199–217. doi: 10.1016/j.jcv.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Grimwood K, Lambert SB, Milne RJ. Rotavirus infections and vaccines burden of illness and potential impact of vaccination. Pediatr Drugs. 2010;12:235–56. doi: 10.2165/11537200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 40.Cunliffe NA, Bresee JS, Gentsch Jr , et al. The expanding diversity of rotaviruses. Lancet. 2002;359:640–2. doi: 10.1016/S0140-6736(02)07781-4. [DOI] [PubMed] [Google Scholar]
- 41.Bishop RF, Barnes GL, Cipriani E , et al. Clinical immunity after neonatal rotavirus infection.A prospective longitudinal study in young children. N Engl J Med. 1983;309:72–6. doi: 10.1056/NEJM198307143090203. [DOI] [PubMed] [Google Scholar]
- 42.Kapikian AZ, Hoshino Y, Chanock RM , et al. Jennerian and modified Jennerian approach to vaccination against rotavirus diarrhea using a quadrivalent rhesus rotavirus (RRV) and human-RRV reassortant vaccine. Arch Virol. 1996;12(Suppl. ):163–75. doi: 10.1007/978-3-7091-6553-9_18. [DOI] [PubMed] [Google Scholar]
- 43.Bernstein DI, Ward RL, editors. Rotaviruses. In Figin RD., Cherry, JD., 5th ed. Vol 2. Philadelphia, PA Saunders.: 2004. Textbook of Pediatric Infectious Diseases. pp. 2110–33. [Google Scholar]
- 44.Glass RI, Parashar UD, Bresee JS , et al. Rotavirus vaccines current prospects and future challenges. Lancet. 2006;368:323–32. doi: 10.1016/S0140-6736(06)68815-6. [DOI] [PubMed] [Google Scholar]
- 45.Bresee JS, Parashar UD, Widdowson MA , et al. Update on rotavirus vaccines. Pediatr Infect Dis J. 2005;24:947–52. doi: 10.1097/01.inf.0000186295.18969.e6. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Schael I, Garcia D, Gonzalez M , et al. Prospective study of diarrheal diseases in Venezuelan children to evaluate the efficacy of rhesus rotavirus vaccine. J Med Virol. 1990;30:219–29. doi: 10.1002/jmv.1890300315. [DOI] [PubMed] [Google Scholar]
- 47.De Mol P, Zissis G, Butzler JP , et al. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986;2:108. doi: 10.1016/s0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- 48.Santosham M, Letson GW, Wolff M , et al. A field study of the safety and efficacy of two candidate rotavirus vaccines in a Native American population. J Infect Dis. 1991;163:483–7. doi: 10.1093/infdis/163.3.483. [DOI] [PubMed] [Google Scholar]
- 49.Vesikari T, Isolauri E, D'Hondt E , et al. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984;1:977–81. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- 50.Fu C, Wang M, Liang J , et al. Effectiveness of Lanzhou lamb rotavirus vaccine against rotavirus gastroenteritis requiring hospitalization a matched case-control study. Vaccine. 2007;25(52):8756–61. doi: 10.1016/j.vaccine.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 51.Wang H. Research and progress of oral live reotavirus vaccine. Chinese J Virol. 2009;25 [Google Scholar]
- 52.Midthun K, Kapikian AZ. Rotavirus vaccines an overview. Clin Microbiol Rev. 1996;9:423–34. doi: 10.1128/cmr.9.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joensuu J, Koskenniemi E, Pang XL , et al. Randomised placebo-controlled trial of rhesus-human reassortant rotavirus vaccine for prevention of severe rotavirus gastroenteritis. Lancet. 1997;350:1205–9. doi: 10.1016/S0140-6736(97)05118-0. [DOI] [PubMed] [Google Scholar]
- 54.Centers for Disease Control and Prevention Rotavirus vaccine for the prevention of rotavirus gastroenteritis among children Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1999;48:1–20. [PubMed] [Google Scholar]
- 55.Intussusception among recipients of rotavirus vaccine - United State. . 48. MMWR Morb Mortal Wkly Rep. 1999,: 1998-1999. Centers for Disease Control and Prevention (CDC). pp. 577–81. [PubMed] [Google Scholar]
- 56.Simosen L, Viboud C, Elixhauser A , et al. More on RotaShield and intussusceptions the role of age at the time of vaccination. J Infect Dis. 2005;192: S36–43. doi: 10.1086/431512. [DOI] [PubMed] [Google Scholar]
- 57.Patel MM, López-Collada VR, Bulhôes MM , et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364:2283–92. doi: 10.1056/NEJMoa1012952. [DOI] [PubMed] [Google Scholar]
- 58.Vesikari T, Matson DO, Dennehy P , et al. Safety and efficacy of a pentavalent human-bovine (WC3):reassortant rotavirus vaccine. N Engl J Med. 2006; 354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 59.Chang CC, Chang MH, Lin TY , et al. Experience of pentavalent human-bovine reassortant rotavirus vaccine among healthy infants in Taiwan. J Formos Med Assoc. 2009;108:280–5. doi: 10.1016/S0929-6646(09)60067-X. [DOI] [PubMed] [Google Scholar]
- 60.Plusher GL. Pentavalent rotavirus vaccine (RotaTeg): a review of its use in the prevention of rotavirus gastroenteritis in Europe. Drugs. 2010;70:1165–88. doi: 10.2165/11205030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 61.Vesikari T, Itzler R, Karvonen A , et al. RotaTeg, a pentavalent rotavirus vaccine efficacy and safety among infants in Europe. Vaccine. 2009;28:345–51. doi: 10.1016/j.vaccine.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 62.Christie CD, Duncan ND, Thame KA , et al. Pentavalent rotavirus vaccine in developing countries safety and health care resource utilization. Pediatrics. 2010;126:e1499–506. doi: 10.1542/peds.2010-1240. [DOI] [PubMed] [Google Scholar]
- 63.Haber P, Patel M, Izurieta HS , et al. Postlicensure monitoring of intussusception after RotaTeq vaccination in the United States February 1 2006 to September 25 2007. Pediatrics. 2008;121:1206–12. doi: 10.1542/peds.2007-3793. [DOI] [PubMed] [Google Scholar]
- 64.El Khoury A, Mast TC, Ciarlet M , et al. Projecting the effectiveness of RotaTeq against rotavirus-related hospitalizations and deaths in six Asian countries. Hum Vaccine. 2011;7:506–10. doi: 10.4161/hv.7.5.14620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parashar UD, Alexander JP, Glass R. Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC).Prevention of rotavirus gastroenteritis among infants and children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006;55:1–13. [PubMed] [Google Scholar]
- 66.Bernstein DI, Sack DA, Rothstein E , et al. Efficacy of live, attenuated, human rotavirus vaccine 89-12 in infants a randomized placebo-controlled trail. Lancet. 1999;354:287–90. doi: 10.1016/S0140-6736(98)12106-2. [DOI] [PubMed] [Google Scholar]
- 67.Bernstein DI, Sack DA, Reisinger K , et al. Second-year follow-up evaluation of live, attenuated human rotavirus vaccine 89-12 in healthy infants. J Infect Dis. 2002;186:1487–9. doi: 10.1086/344732. [DOI] [PubMed] [Google Scholar]
- 68.De Vos B, Vesikari T, Linhares AC , et al. A rotavirus vaccine for prophylaxis of infants against rotavirus gastroenteritis. Pediatr Infect Dis J. 2004;23:S179–82. doi: 10.1097/01.inf.0000142370.16514.4a. [DOI] [PubMed] [Google Scholar]
- 69.Linhares AC, Velázquez FR, Pérez-Schael I , et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants a randomized, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 70.Das BK, Gentsch Jr, Hoshino Y , et al. Characterization of the G serotype and genogroup of New Delhi newborn rotavirus strain 116E. Virology. 1993;197:99–107. doi: 10.1006/viro.1993.1570. [DOI] [PubMed] [Google Scholar]
- 71.Das M, Dunn SJ, Woode GN , et al. Both surface proteins (VP4 and VP7):of an asymptomatic neonatal rotavirus strain (I321):have high levels of sequence identity with the homologous proteins of a serotype 10 bovine rotavirus. Virology. 1993;194:374–9. doi: 10.1006/viro.1993.1271. [DOI] [PubMed] [Google Scholar]
- 72.Iturriza Gómara M, Kang G, Mammen A , et al. Characterization of G10P[11] rotaviruses causing acute gastroenteritis in neonates and infants in Vellore India. J Clin Microbiol. 2004;42:2541–7. doi: 10.1128/JCM.42.6.2541-2547.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kawamura N, Tokoeda Y, Oshima M , et al. Efficacy, safety and immunogenicity of RIX4414 in Japanese infants during the first two years of life. Vaccine. 2011;29:6335–41. doi: 10.1016/j.vaccine.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 74.Bhandari N, Rongsen-Chandola T, Bavdekar A , et al. Efficacy of a monovalent human-bovine (116E) rotavirus vaccine in Indian children in the second year of life. Vaccine. 2014;32:A110–6. doi: 10.1016/j.vaccine.2014.04.079. [DOI] [PubMed] [Google Scholar]
- 75.Armah GE, Kapikian AZ, Vesikari T , et al. Efficacy, immunogenicity, and safety of two doses of tetravalent rotavirus vaccine RRV-TV in Ghana with the first dose administered during the neonatal period. J Infect Dis. 2013;208:423–31. doi: 10.1093/infdis/jit174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cortese MM, Immergluck LC, Held M , et al. Effectiveness of monovalent and pentavalent rotavirus vaccine. Pediatrics. 2013;132:e25–33. doi: 10.1542/peds.2012-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]