Abstract
This case series evaluated the clinical efficacy of autogenous tooth bone graft material (AutoBT) in alveolar ridge preservation of an extraction socket. Thirteen patients who received extraction socket graft using AutoBT followed by delayed implant placements from Nov. 2008 to Aug. 2010 were evaluated. A total of fifteen implants were placed. The primary and secondary stability of the placed implants were an average of 58 ISQ and 77.9 ISQ, respectively. The average amount of crestal bone loss around the implant was 0.05 mm during an average of 22.5 months (from 12 to 34 months) of functional loading. Newly formed tissues were evident from the 3-month specimen. Within the limitations of this case, autogenous tooth bone graft material can be a favorable bone substitute for extraction socket graft due to its good bone remodeling and osteoconductivity.
Keywords: Transplantation, Autologous, Extraction socket, Dental implants, Bone substitutes
INTRODUCTION
The dimensional change of an alveolar ridge is inevitable over time after tooth extraction. Bone resorption is more rapid in cases of existing periodontal disease, inflammatory periapical lesions, or serious previous bone wall defects after the extraction.1 The soft tissue collapses into the defect hindering normal and natural healing.1,2
Bone resorption after tooth extraction can make the implant placement difficult. It also makes oral hygiene care around the prosthesis difficult. Therefore, various methods were attempted to minimize alveolar bone resorption. Different types of bone substitutes such as demineralized freeze-dried bone allograft (DFDBA), bioglass, and/or hydroxyapatite were used either with resorbable or nonresorbable membrane. And the reported cases showed the reduced alveolar bone resorption.3,4,5,6,7
Here, a ridge preservation using the recently developed autogenous tooth bone graft material (AutoBT, Korea Tooth Bank, Seoul, Korea) at an extraction socket with osseous defects was performed for an implant site development. AutoBT is made from patient's own extracted non-restorable tooth or third molars. The processed AutoBT is grafted back to the same patient when bone substitue is necessary in procedures such as guided bone regeneration for implant site development. Previous studies demonstrated favorable clinical results with osteoconductive and osteoinductive potentials.8,9,10 We clinically and histologically evaluated cases wherein implant had been placed successfully after a certain healing period.
MATERIALS AND METHODS
This prospective case series was conducted after obtaining an approval from the Seoul National University Bundang Hospital Institutional Review Board (No.: B-0905/076-010). Each patient received sufficient explanations regarding study purpose, AutoBT fabrication process, treatment procedure, histological evaluation, and possible complications. The surgery was performed once the patient signed the informed consent.
This study was conducted to evaluate the patients who had received AutoBT grafting at an extraction socket for delayed implant placement from November 2008 to August 2010. A total of thirteen patients were recruited. Ten patients were men and three were women with an average age of 54 (from 44 years old to 66 years old). Although five patients had hypertension and diabetes mellitus, they were receiving adequate medical treatment and were well controlled.
The inclusion criteria were as below:
Extraction cases of single premolar or molar
Osseous destruction after tooth extraction
When the residual bone height is less than 4 mm to the sinus floor or inferior alveolar canal after tooth extraction
Patients who are healthy overall or who have controlled systemic disease (ASA I or II)
The Exclusion criteria was as below:
Smoker
Patient who has received bone graft on the site to be operated
Patient who has received radiation therapy
Pateient who has history of sinus disease or symptoms
Patient who has acute infection
When the decision was made to extract a non-restorable tooth, procedures of socket graft using his or her own tooth were explained to the patient. Once the patient agreed, he or she signed the request form allowing to fabricate his or her tooth into AutoBT. Extracted tooth was stored in 75% alcohol sample bottle and kept refrigerated or frozen. The dentist could request either AutoBT powder form (from 0.5 to 1 mm particle size) or block form depend on the purpose of procedures. In order to process into AutoBT, an anatomical crown portion of the tooth was dissected after removal of attached soft tissues. Remaining soft tissues and contaminants were removed with distilled water. The washed AutoBT was then dehydrated, defatted, and freeze-dried. Next, it was sterilized using ethylene oxide gas, packed, and sent to the hospital that requested the treatment. A block form was fabricated in the same process as processing powder form without being crushed, thus maintaining the original tooth root shape.
The tooth extraction was performed by one oral and maxillofacial surgeon at Seoul National University Bundang Hospital. Complete curettage and wound irrigation were performed after tooth extraction. Extracted tooth was fabricated into powder or block type graft material and stored until the next operation. The implant site developement at an extraction socket was performed an average of 1.2 months (from 0.5 to 4 months) after tooth extraction. AutoBT block and/or powder was grafted (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). Depend on the size of bony defects, the clinician decided to use additional other types of bone graft material and to cover the site with a barrier membrane. Primary wound closure was performed using 4-0 vicryl. After a healing period of an average of 3.7 months (from 2 to 7 months), an implant was placed by one periodontist and one oral and maxillofacial surgeon at Seoul National University Bundang Hospital Institutional Review Board (No.: B-0905/076-010). Histologic specimen was taken in patients who consented the biopsy procedure. An average implant healing period was 3.3 months (from 2 to 5 months). Prosthodontic treatment was performed by one prosthodontist at the same hospital (Fig. 6, Fig. 7, Fig. 8).
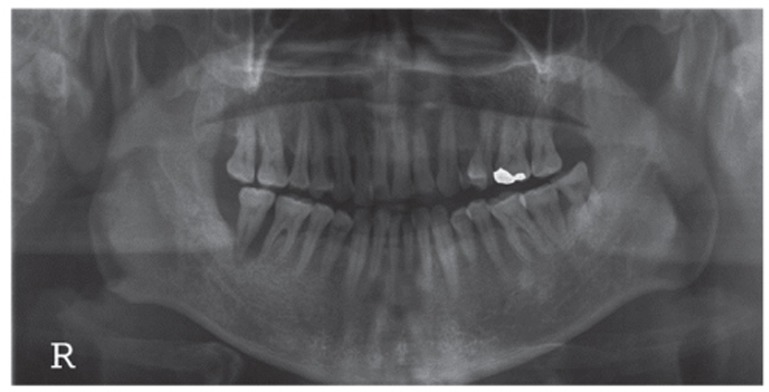
Fig. 1.
A panoramic radiograph of 45 years old male patient. The right mandibular second molar was assigned to be extracted. The extracted tooth was fabricated into autogenous tooth block and powder were made. Due to insufficient bone level near the inferior alveolar canal, delayed implant placement was planned after AutoBT grafting.
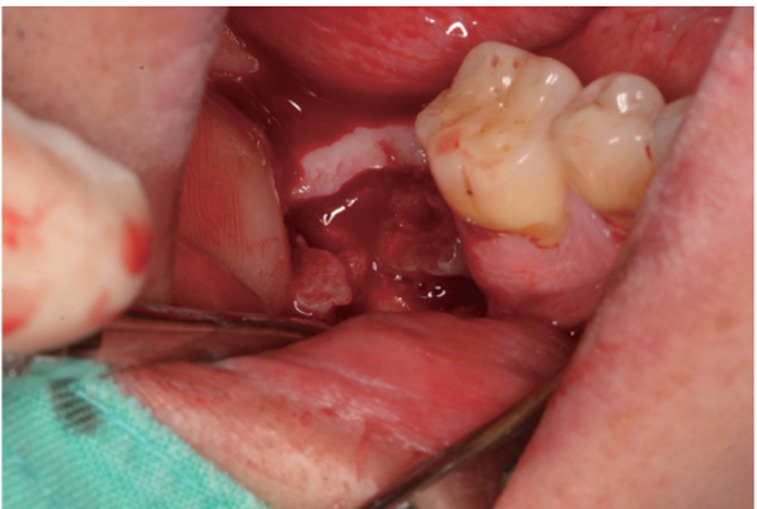
Fig. 2.
AutoBT block was transplanted at the extraction socket 4 weeks after extraction.
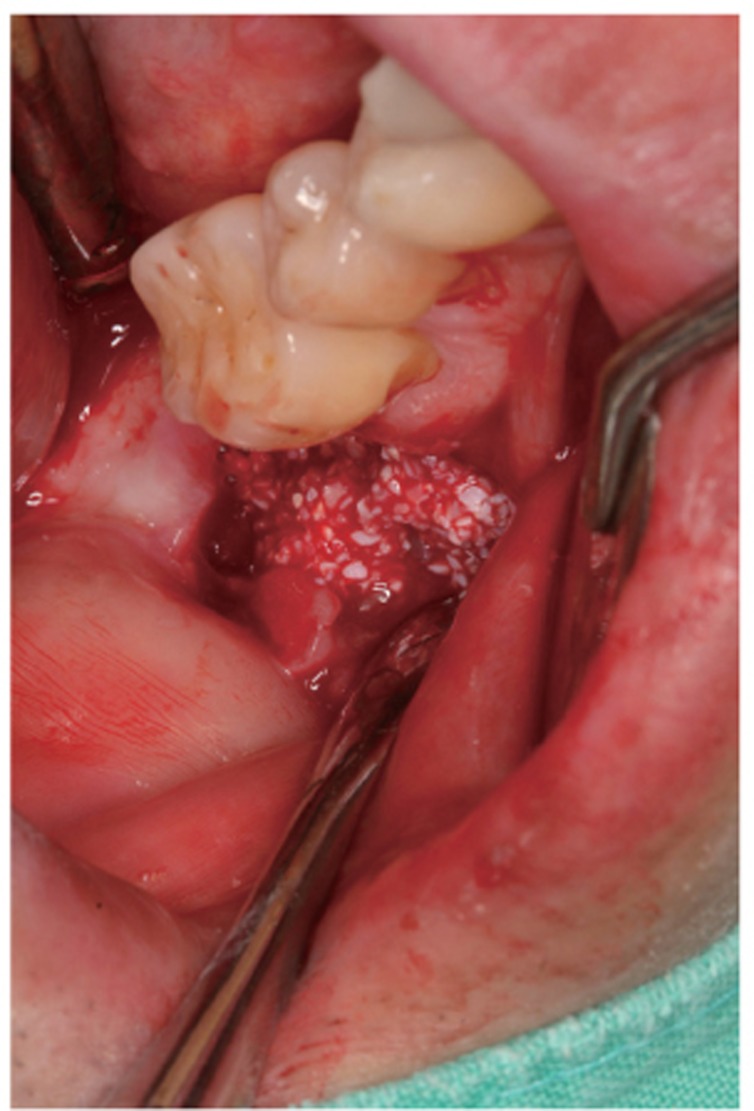
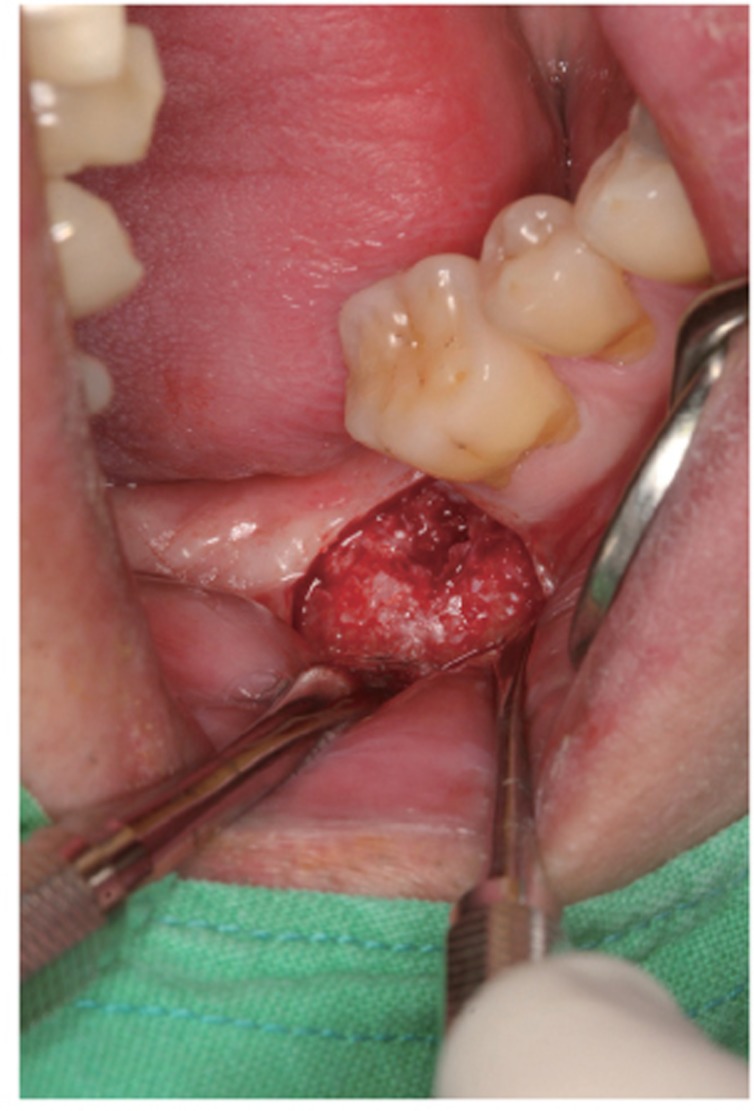
Fig. 3.
AutoBT powder was grafted around the block to fill the bony defect. The grafted site was covered with a resorbable collagen membrane. The primary closure was achieved.
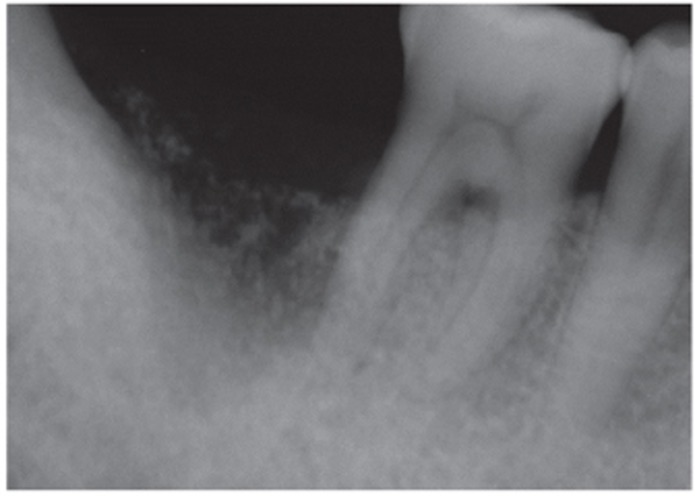
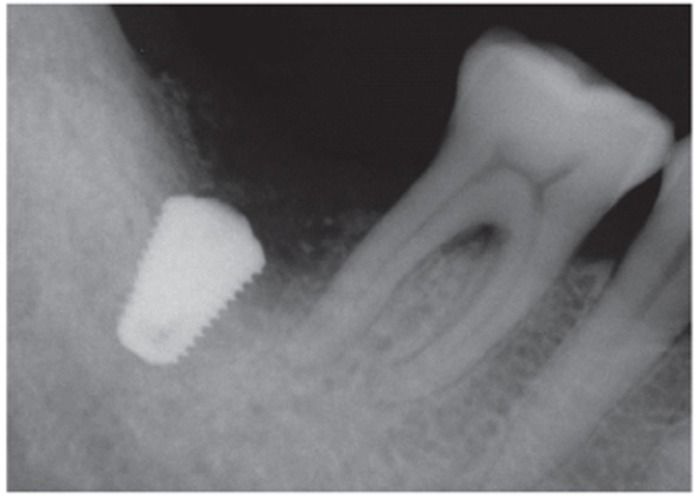
Fig. 4.
A periapical radiograph immediately after AutoBT socket grafting.
Fig. 5.
A flap was raised again for an implant placement 5 months after AutoBT socket grafting.
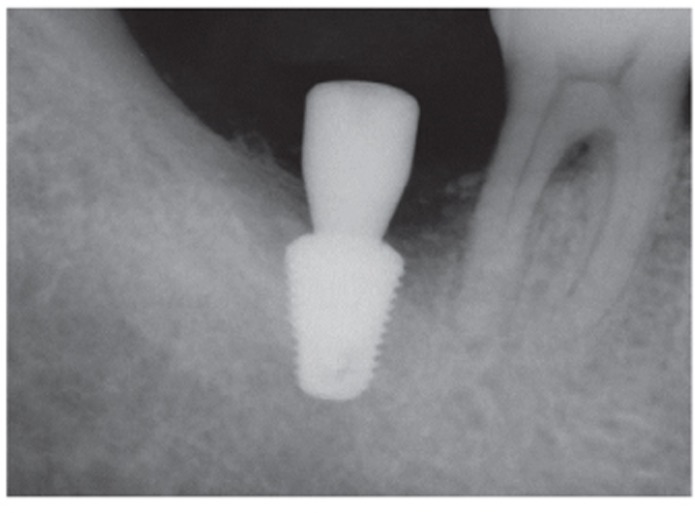
Fig. 6.
An implant was placed.
Fig. 7.
The second stage surgery was achieved 5 months after the implant placement.
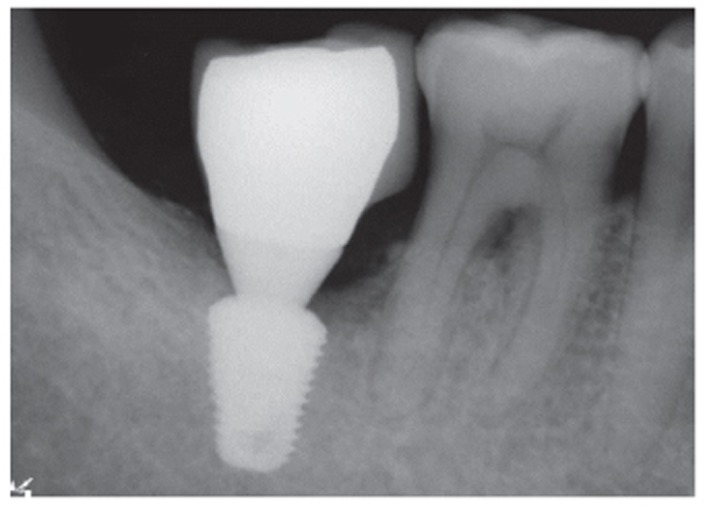
Fig. 8.
A follow-up periapical radiograph 14 months after the final prostheses.
The implant stability, adjunctive surgery, additional bone graft material and barrier membrane use, postoperative complication, implant success rate, and crestal bone loss of all subjected patients were carefully evaluated. Implant primary and secondary stability were measured by Osstell Mentor (Integration Diagnostics, Goteborg, Sweden).
The height of crestal bone was measured on the periapical radiograph taken immediately after the implant placement. Based on the length of the placed implant, the magnification rate of the periapical radiograph was calculated. Changes of marginal bone were evaluated by comparing the digital periapical radiographs at the first prosthetic loading and during a 1-, 2-, and 3-year follow up periods after the functioning of the final prosthesis as collected via PACS (INFINIT Co., Seoul, South Korea). After calibration, the amount of marginal bone resorption was measured vertically from an implant shoulder to the uppermost part where radiopacity was observed distinctly in mesio-distal crestal segment on the periapical radiograph taken with paralleling technique. For the minimum discrepancy, all measurements in this study were recorded by two clinicians who were not involved in any type of surgeries. Any of patient's medical or social history was not exposed to them, either. The amount of mesial and distal alveolar crest bone resorption was calculated considering the enlarged ratio of each individual radiograph.
Among the subjects in socket grafting, four patients who signed the additional informed consent on histological evaluation were subjected to bone biopsy. A biopsy was harvested by a 2 mm diameter trephine bur from the center of the extraction site. The time of bone biopsy depended upon the bony defected size, and the area of grafting site. The oral surgeon and periodontist who performed the surgery solely decided the time of implant placement surgeries. One specimen after three months and three specimens after four months of the bone graft surgery were harvested. The harvested specimens were immediately fixed with 10% formalin. The specimens were decalcified with Calci-Clear Rapid™ (National Diagnostics, Atlanta, GA, USA) for 12 hours. Next, the decalcified specimens were washed in running water and subjected to tissue treatment via the automatic tissue processor (Hypercentre XP, Shandon, Cheshire, UK) followed by Paraffin embedding. After paraffin embedding, the specimens were cut 5 µm sections and stained with Hematoxylin-Eosin for an examination under a bright-field microscope (Nikon Optical, Tokyo, Japan).
RESULTS
After extractions of 15 teeth from 13 patients, AutoBT powder and/or block grafting was performed at an extraction socket for implant site development. AutoBT powder only was grafted in 10 sites. The combination of AutoBT powder and block were used in 4 sites. There was one case using only AutoBT block. There were two cases where additional allograft (DBX, Edison, NJ, USA) was used due to an insufficient amount of AutoBT only to completely fill a bony defect. A resorbable collagen membrane (BioGide: Geistlich Pharma AG, Wolhausen, Switzerland) was used in 13 sites out of 15 sites (Table 1). For postoperative complications, wound dehiscence developed in 2 cases, both of which exhibited favorable secondary healing. After an average healing period of 3.7 months (from 2 to 7 months), 14 implants were placed in 12 patients (Table 2). One patient became unreachable during the research period (Patient #3). The mean primary stability of placed implants was 58 ISQ (from 30 to 75) ISQ, and secondary stability was 77.9 ISQ (from 63 to 90 ISQ). One implant placed at left mandibular second molar site showed mobility after one month of prosthetic loading (Patient #5). Consequently, the implant was removed, and placed again with a 1 mm wider implant after 2 months of healing period. The average periimplant crestal bone loss during 22.5 months (from 12 to 34 months) of prosthetic loading was 0.05 mm. No crestal bone loss was observed except for 3 cases (Table 2). Patient #8 showed 0.2 mm, patient #12 showed 0.3 mm, and patient #13 showed 0.2 mm of crestal bone loss.
Table 1.
Summary of a ridge preservation of an extraction socket using autogenous tooth bone graft material (AutoBT) and its complications
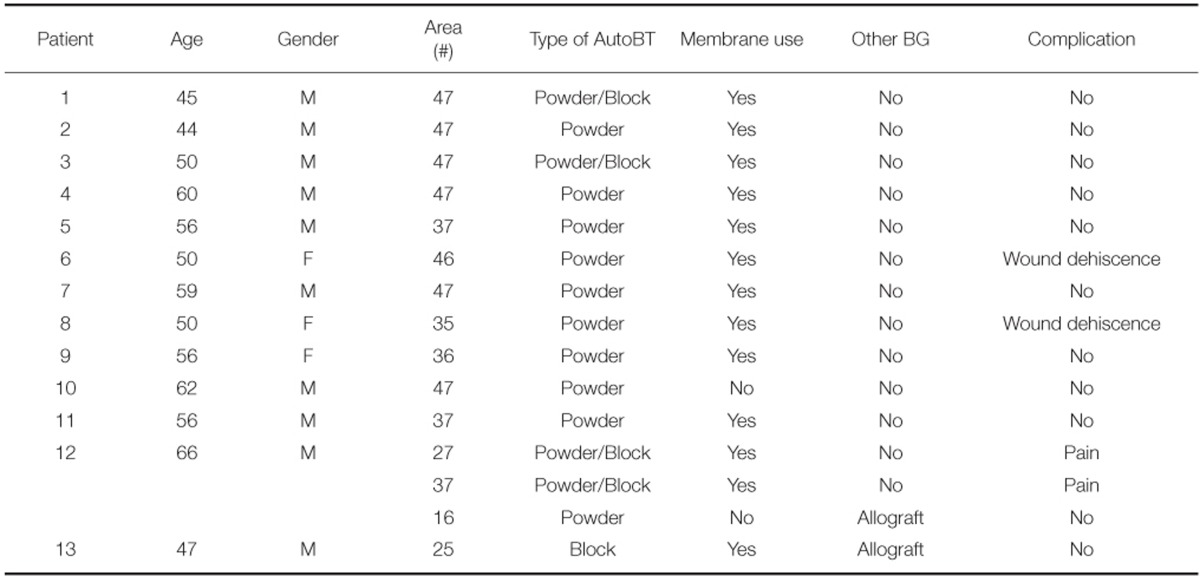
BG: bone graft material; M: male, F: female.
Table 2.
Summary of placed implants and crestal bone loss during follow up periods
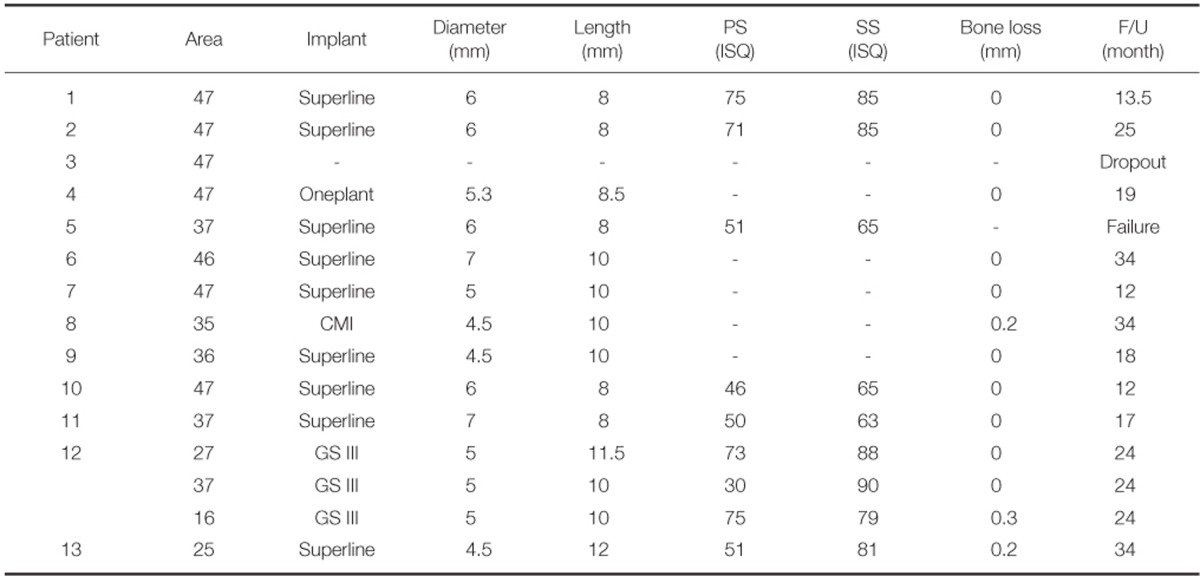
PS: primary stability; SS: secondary stability; F/U: follow-up; -: missing data.
Superline (Dentium, Suwon, Korea), Oneplant (Warantec, Seoul, Korea), GS III (Osstem Implant Co., Busan, Korea), CMI (Neobiotec, Seoul, Korea).
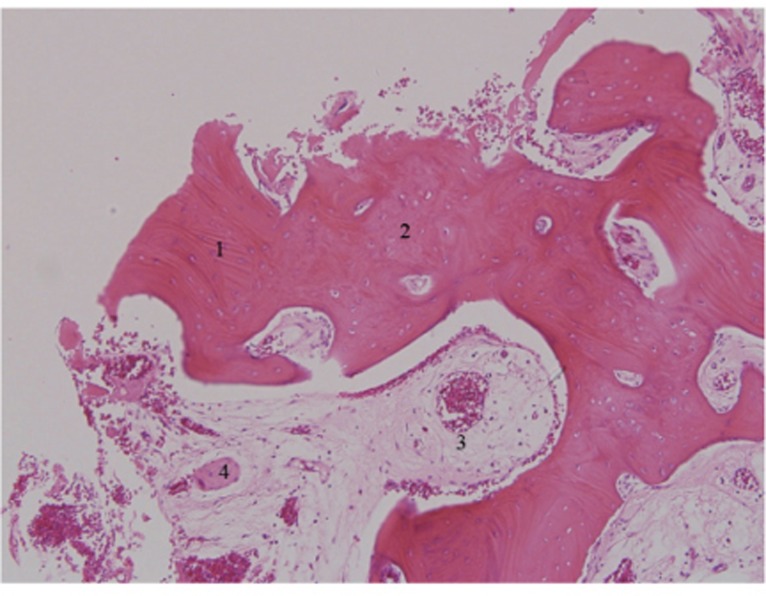
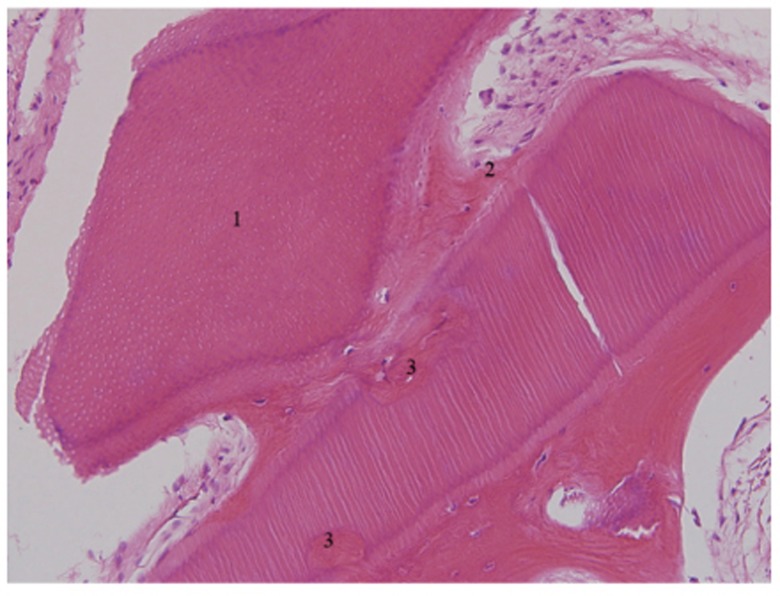
All histology specimens showed newly formed bone with loose fibrous tissue rich in angiogenesis. AutoBT particles were completely mixed with newly formed bone (Fig. 9). In 4-month histological specimens, the formation of osteoid was evident around AutoBT particles. AutoBT was agglutinated directly with newly formed bone, and high-quality loose areolar tissue rich in angiogenesis was well developed. Moreover, newly formed osteoid replaced spaces where AutoBT particles were absorbed (Fig. 10).
Fig. 9.
Histology of 3-month bone specimen. AutoBT particles were completely mixed with newly formed bone. Loose fibrous tissues rich in angiogenesis were observed as well (H-E stain, ×100). 1. Existence of dentin tubules in AutoBT particles; 2. Newly formed bone; 3. Loose fibrous tissue; 4. AutoBT particle.
Fig. 10.
Histology of 4-month bone specimen. It showed that the area where AutoBT had been resorbed was filled with newly formed osteoid (H-E stain, ×200).1. AutoBT particle; 2. Fibrous connective tissue; 3. Resorbed area filled with newly formed osteoid.
DISCUSSION
Alveolar ridge preservation was desirable in cases where a buccal cortical bone plate was less than 1.5-2 mm thick.11 Many scholars introduced their unique ridge preservation techniques to maximize the alveolar ridge preservation result. Platelet-rich plasma, resorbable barrier membrane, acellular dermal matrix, collagen sponge, or calcium sulfate was mixed with a bone graft material for more favorable results.3,4,6,7,12,13 Wang and Tsao14 transplanted freeze-dried bone allograft (FDBA) for a socket grafting. Their 5 to 6-month histological specimens showed the formation and remodeling of trabecular bone in areas of mineralized cancellous allografts with no signs of inflammation. In addition, vital bone and connective tissues were seen in close contact with the remaining allograft. Another study also showed new bone formation in 9-month old biopsy sample when they used a deproteinized bovine bone mineral (DBBM) as a socket site filler material. Authors observed that DBBM particles adhered to a highly osteocyte-rich woven and lamellar-type bone.15
In spite of employing evidence-based ridge preservation techniques, minimal vertical bone resorption seemed to be unpredictable. Socket preservation could minimize the change of bone volume but could not prevent bone resorption.16,17 Studies further showed that the effect of preventing horizontal alveolar bone resorption was positive, but that of preventing vertical resorption was not predictable.17,18
In the present case series, we used the newly developed bone substitute as another grafting technique at an extraction socket for the future implant site development. AutoBT was developed and clinically applied from 2008 in Korea. In vitro and animal studies confirmed its biocompatibility, osteoinductive and osteoconductive potentials.19,20,21,22,23 Clinical studies provided clinical usefulness of AutoBT in reconstructive dentistry.8,9,10,17,24,25 Here, AutoBT grafting was performed in 15 extracted sites of 13 patients. There were no post-operative complications related to AutoBT grafting except wound dehiscence on two patients (Patient #6 & #8)(Table 1). But, both of them exhibited complete healings after all. There was one case of implant failure. The first implant placed to the patient #5 failed due to a lack of osseointegration (Primary stability: 51 ISQ, secondary stability: 65 ISQ). Although the first implant was removed due to mobility, the remained bone still retained its healthy status to place the second implant. As a result, the new implant with wider diameter (7 mm) was placed after 2 months of remodeling period. Yet, the data of patient #5 was excluded in bone resorption calculation. The patient #5 had a history of Type II diabetes mellitus. Although systemic health was in controlled state, the signs and symptoms of diabetes mellitus could have hindered the new bone formation and thus implant failed.
Except for the one implant failure case and a drop out case due to absence, AutoBT maintained a stable state around the implant with very minimal crestal bone loss (Table 2). Based on previous studies, AutoBT resorbed from 4 to 6 months after grafting. And the remodeling process with new bone formation continued up to 1 to 2 years.8,10,17 The bone resorption in presented cases was very minimal, because AutoBT was mainly made from roots of the extracted tooth. A root portion is consisted of low crystalline calcium phosphate, which is known to have good bony remodeling by osteoconduction.22 When AutoBT was processed including a crown portion of an extracted tooth, the resorption of AutoBT was delayed. Enamel in a crown mainly consists of high crystalline calcium phosphate, which is not easily decomposed by osteoclasts, thus inhibiting bone remodeling process.25
Histological evaluation conducted 3 to 4 months after socket grafting showed a favorable osteoconductive bone healing and remodeling as AutoBT particles started to be absorbed. Newly formed osteoid filled the absorbed area. Abundant blood vessels and active cellular activities were also observed in connective tissues around AutoBT particles, confirming an active remodeling and osteoconductive healing. Further, a loose areolar tissue was considered to form bone marrow in the future (Fig. 9, Fig. 10).
Some limitations still remain, such as small sample size (n=15), small number of histological specimens (n=4), and non-standardized type of implant. 2D X-ray evaluation also provided the limited information in marginal bone loss around implant. It can only provide the mesial and distal bone level. Additionally, randomized controlled clinical trials with comparison of different types of bone substitutes are needed for a full evaluation of AutoBT in hard tissue reconstructive dentistry. Yet, it is important to note that this is the first prospective case series demonstrating effectiveness of AutoBT in ridge preservation at an extraction socket for implant site development clinically and histologically.
CONCLUSION
Within the current studies so far, AutoBT can be a good alternative bone substitute due to its good bone remodeling and osteoconductivity in ridge preservation of an extraction socket for an implant site development. AutoBT can be an attractive option to patients who are in need of tooth extraction but afraid of bone substitute from different origin.
Footnotes
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
References
- 1.Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32:212–218. doi: 10.1111/j.1600-051X.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 2.Lekovic V, Kenney EB, Weinlaender M, Han T, Klokkevold P, Nedic M, Orsini M. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997;68:563–570. doi: 10.1902/jop.1997.68.6.563. [DOI] [PubMed] [Google Scholar]
- 3.Camargo PM, Lekovic V, Weinlaender M, Klokkevold PR, Kenney EB, Dimitrijevic B, Nedic M, Jancovic S, Orsini M. Influence of bioactive glass on changes in alveolar process dimensions after exodontia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:581–586. doi: 10.1067/moe.2000.110035. [DOI] [PubMed] [Google Scholar]
- 4.Gross J. Ridge preservation using HTR synthetic bone following tooth extraction. Gen Dent. 1995;43:364–367. [PubMed] [Google Scholar]
- 5.Hämmerle CH, Chiantella GC, Karring T, Lang NP. The effect of a deproteinized bovine bone mineral on bone regeneration around titanium dental implants. Clin Oral Implants Res. 1998;9:151–162. doi: 10.1034/j.1600-0501.1998.090302.x. [DOI] [PubMed] [Google Scholar]
- 6.Iasella JM, Greenwell H, Miller RL, Hill M, Drisko C, Bohra AA, Scheetz JP. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74:990–999. doi: 10.1902/jop.2003.74.7.990. [DOI] [PubMed] [Google Scholar]
- 7.Lekovic V, Camargo PM, Klokkevold PR, Weinlaender M, Kenney EB, Dimitrijevic B, Nedic M. Preservation of alveolar bone in extraction sockets using bioabsorbable membranes. J Periodontol. 1998;69:1044–1049. doi: 10.1902/jop.1998.69.9.1044. [DOI] [PubMed] [Google Scholar]
- 8.Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA, Park JJ. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011;20:471–475. doi: 10.1097/ID.0b013e3182386d74. [DOI] [PubMed] [Google Scholar]
- 9.Jeong KI, Kim SG, Oh JS, Lim SC. Maxillary sinus augmentation using autogenous teeth: preliminary report. J Korean Assoc Maxillofac Plast Reconstr Surg. 2011;33:256–263. [Google Scholar]
- 10.Kim YK, Lee HJ, Kim KW, Kim SG, Um IW. Guide bone regeneration using autogenous teeth: case reports. J Korean Assoc Oral Maxillofac Surg. 2011;37:142–147. [Google Scholar]
- 11.Darby I, Chen S, De Poi R. Ridge preservation: what is it and when should it be considered. Aust Dent J. 2008;53:11–21. doi: 10.1111/j.1834-7819.2007.00008.x. [DOI] [PubMed] [Google Scholar]
- 12.Luczyszyn SM, Papalexiou V, Novaes AB, Jr, Grisi MF, Souza SL, Taba M., Jr Acellular dermal matrix and hydroxyapatite in prevention of ridge deformities after tooth extraction. Implant Dent. 2005;14:176–184. doi: 10.1097/01.id.0000165082.77499.41. [DOI] [PubMed] [Google Scholar]
- 13.Shi B, Zhou Y, Wang YN, Cheng XR. Alveolar ridge preservation prior to implant placement with surgical-grade calcium sulfate and platelet-rich plasma: a pilot study in a canine model. Int J Oral Maxillofac Implants. 2007;22:656–665. [PubMed] [Google Scholar]
- 14.Wang HL, Tsao YP. Histologic evaluation of socket augmentation with mineralized human allograft. Int J Periodontics Restorative Dent. 2008;28:231–237. [PubMed] [Google Scholar]
- 15.Artzi Z, Nemcovsky CE. The application of deproteinized bovine bone mineral for ridge preservation prior to implantation. Clinical and histological observations in a case report. J Periodontol. 1998;69:1062–1067. doi: 10.1902/jop.1998.69.9.1062. [DOI] [PubMed] [Google Scholar]
- 16.Ten Heggeler JM, Slot DE, Van der Weijden GA. Effect of socket preservation therapies following tooth extraction in non-molar regions in humans: a systematic review. Clin Oral Implants Res. 2011;22:779–788. doi: 10.1111/j.1600-0501.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim YK, Yun PY, Lee HJ, Ahn JY, Kim SG. Ridge preservation of the molar extraction socket using collagen sponge and xenogeneic bone grafts. Implant Dent. 2011;20:267–272. doi: 10.1097/ID.0b013e3182166afc. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Kim MJ, Kim YK, Yun PY. Extraction socket preservation using β-TCP coated with recombinant human BMP (rhBMP-2): randomized clinical prospective study. Oral Biol Res. 2011;35:110–115. [Google Scholar]
- 19.Jeong HR, Hwang JH, Lee JK. Effectiveness of autogenous tooth bone used as a graft material for regeneration of bone in miniature pig. J Korean Assoc Oral Maxillofac Surg. 2011;37:375–379. [Google Scholar]
- 20.Kim JY, Kim KW, Um IW, Kim YK, Lee JK. Bone healing capacity of demineralized dentin matrix materials in a minipig cranium defect. J Korean Dent Sci. 2012;5:21–28. [Google Scholar]
- 21.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, Kim SY. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, Lim SY. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotech. 2011;11:7442–7445. doi: 10.1166/jnn.2011.4857. [DOI] [PubMed] [Google Scholar]
- 23.Murata M, Akazawa T, Hino J, Tazaki J, Ito K, Arisue M. Biochemical and histo-morphometrical analyses of bone and cartilage induced by human decalcified dentin matrix and BMP-2. Oral Biol Res. 2011;35:9–14. [Google Scholar]
- 24.Murata M, Sato D, Hino J, Akazawa T, Tazaki J, Ito K, Arisue M. Acid-insoluble human dentin as carrier material for recombinant human BMP-2. J Biomed Mater Res A. 2012;100:571–577. doi: 10.1002/jbm.a.33236. [DOI] [PubMed] [Google Scholar]
- 25.Kim GW, Yeo IS, Kim SG, Um IW, Kim YK. Analysis of crystalline structure of autogenous tooth bone graft material: X-Ray diffraction analysis. J Korean Assoc Oral Maxillofac Surg. 2011;37:225–228. [Google Scholar]