Abstract
PURPOSE
The purpose of this prospective study was to evaluate the effectiveness of newly developed autogenous tooth bone graft material (AutoBT)application for sinus bone graft procedure.
MATERIALS AND METHODS
The patients with less than 5.0 mm of residual bone height in maxillary posterior area were enrolled. For the sinus bone graft procedure, Bio-Oss was grafted in control group and AutoBT powder was grafted in experimental group. Clinical and radiographic examination were done for the comparison of grafted materials in sinus cavity between groups. At 4 months after sinus bone graft procedure, biopsy specimens were analyzed by microcomputed tomography and histomorphometric examination for the evaluation of healing state of bone graft site.
RESULTS
In CT evaluation, there was no difference in bone density, bone height and sinus membrane thickness between groups. In microCT analysis, there was no difference in total bone volume, new bone volume, bone mineral density of new bone between groups. There was significant difference trabecular thickness (0.07 µm in Bio-Oss group Vs. 0.08 µm in AutoBT group) (P=.006). In histomorphometric analysis, there was no difference in new bone formation, residual graft material, bone marrow space between groups. There was significant difference osteoid thickness (8.35 µm in Bio-Oss group Vs. 13.12 µm in AutoBT group) (P=.025).
CONCLUSION
AutoBT could be considered a viable alternative to the autogenous bone or other bone graft materials in sinus bone graft procedure.
Keywords: Tooth, Bio-Oss, Sinus
INTRODUCTION
It is generally accepted that autogenous bone is the gold standard among the many different types of bone graft materials for hard tissue defect restoration in maxillofacial area, although there are several shortcomings such as secondary defect of donor site and limited amount of available bone. Therefore, in order to overcome such shortcomings of autogenous bone, homogeneic bone, xenogeneic bone and alloplastic materials were developed and widely used in clinics. However, there are still unsolved problems related with graft materials such as unpredictable bony healing, immune response, risk of inflammation and high cost.
Anorganic bovine bone was used for a long time and confirmed as osteoconductive material. Bio-Oss (Geistlich Pharma AG, Wolhusen, Switzerland) is the representative commercial product. Anorganic bovine bone supplies scaffold for de novo bone formation. It was reported that anorganic bovine bone showed better bone healing properties in comparison to other hydroxyapatite materials.1,2,3,4
In 2008, autogenous tooth bone graft material which was made from the extracted patient's own teeth was developed in Korea, and the commercial product was named as AutoBT (Korea Tooth Bank Co., Seoul, Korea). In previous experimental study, AutoBT was proved as biocompatible material showing both osteoinductive and osteoconductive healing process.5,6,7 Since 2009, successful clinical outcomes of AutoBT were reported when used in cases of sinus bone graft, ridge augmentation, guided bone regeneration, tooth transplantation, and extraction socket graft.8,9,10,11,12,13,14 Also, from the retrospective clinical study, it was confirmed that there was no immune reaction and low risk of infection in AutoBT graft site.15
However, until now, there was no systematic clinical study for the evaluation of clinical efficacy and safety of AutoBT. The purpose of this study was to evaluate effectiveness of AutoBT application in comparison with Bio-Oss for sinus bone graft procedure.
MATERIALS AND METHODS
This study and the consent forms were approved by Seoul National University Bundang Hospital Institutional Review Board (IRB Number: E-1110-067-003), and the guidelines for Good Clinical Practice were respected. Clinical and radiographic examination of the participants was done for the initial screening procedure to confirm inclusion and exclusion criteria. Inclusion criteria were 1) minimum age requirement of 18 years, 2) generally healthy or with controlled systemic disease, 3) less than 5.0 mm of residual bone height (RBH), 4) existing occluding dentition, 5) nor temporomandibular joint symptom neither occlusion problems. Exclusion criteria were 1) any pathologic condition of maxilla including acute maxillary sinusitis, 2) previous radiation therapy history on maxilla, 3) any psychological problem, 4) accidental occurrence of large perforation during sinus lift procedure.
According to the sample size calculation on the focus of new bone formation, total 43 participants (21 in control and 22 in experimental group) were enrolled. The allocation of the participants was done by random sequence generator. The participants were allocated either in the control group (Bio-Oss group, group 1) or experimental group (AutoBT group, group 2). The first computed tomography (CT) was taken pre-operatively to all the participants for the determination of baseline characteristics. After the allocation, two participants in Bio-Oss group and three participants in AutoBT group were declined to participate. Finally, data from 38 participants (19 in Bio-Oss group and 19 in AutoBT group) were collected and analyzed.
Considering the preparation time of autogenous tooth for the participants allocated in experimental group, the teeth needed to be extracted (such as hopeless teeth involved in advanced periodontal disease, non-restorable decayed teeth or non-functional third molars) were extracted 2 weeks before sinus bone graft procedure. Extracted teeth were put into a storage container and kept refrigerated or frozen. The material was produced in the form of powder (0.5-1.0 mm) through the process of removing the soft tissue attached to the collected teeth. Tooth was sectioned as crown and root portion and pulp tissue was removed. The ground teeth powder was washed to remove adhering contaminants and remaining soft tissues. The washed AutoBT is then dehydrated, defatted, and freeze-dried. Next, it was sterilized using ethylene oxide gas, packed, and sent back to the hospital.
We are investigating the written documents of screening test every quarter for the quality control such as the particle test on facility to keep Class 10,000 as well as measurements of remained reagent after processing, i.e. demineralization, defatting, sterilization and remained water content after freeze drying on the processed tooth. In addition, documents of the microbiologic test are conducting on random samples of tooth materials after the processing. For non-contamination, each tooth should be enrolled into the process separately and independently. Microbiologic culture test on tooth materials after processing are also performed and documented as a tool for quality control and assurance according to Technical Manual of Korea Tooth Bank. The safety of the AutoBT is guaranteed through proper quality assurance procedures.16
All patients who underwent surgery took antibiotics (Augmentin, Ilsung Pharmaceuticals Co., Seoul, Korea) and a non-steroidal anti-inflammatory drug (Somalgen, Kunwha Pharmaceutical Co., Seoul, Korea) starting from 1 day before surgery to 5-7 days after surgery. They were made to gargle with 0.1% chlorhexidine solution (Hexamedine, Bukwang Pharm. Co., Ltd., Seoul, Korea) right before surgery and were instructed to gargle 3 times a day for 5 days after surgery. Surgery was performed under local anesthesia. Crestal incision with anterior releasing and mucoperiosteal flap elevation was done on edentulous area. A small oval-shaped window was prepared in the sinus wall. After the meticulous elevation of sinus membrane, Bio-Oss 1.5-2.0 cc in control group and AutoBT powder 1.5-2.0 cc was grafted in experimental group. The suture was removed 10 days after surgery.
At 4 months after sinus bone graft procedure, implant surgery was planned.17,18 Under the additional consent of participants, biopsy was preceded using 3.0 mm diameter trephine bur (Dentium Co., Suwon, Korea) at the site of implant placement to evaluate the grafted site for the microcomputed tomography (microCT) and histomorphometric analysis. All implants were placed according to the manual that was recommended by the manufacturer.
For the baseline, pre-operative CT (Brilliance 64, Philips Medical, Cleveland, OH, USA) was taken in all the patients. At the time of patient screening process, panoramic radiography (Orthoceph OC 100 CR, Instrumentarium Imaging, Tuusula, Finland) was taken. At 4 months after sinus bone graft procedure, post-operative CT follow-up was done for the evaluation of grafted bone density, grafted bone height, and sinus membrane thickness.
Pre-operative and post-operative bone density and bone height were measured using Simplant™ (Columbia Scientific Inc., Columbia, MD, USA) software. Sinus membrane thickness was evaluated using INFINITT PACS (INFINITT Healthcare Co., Seoul, Korea) software.
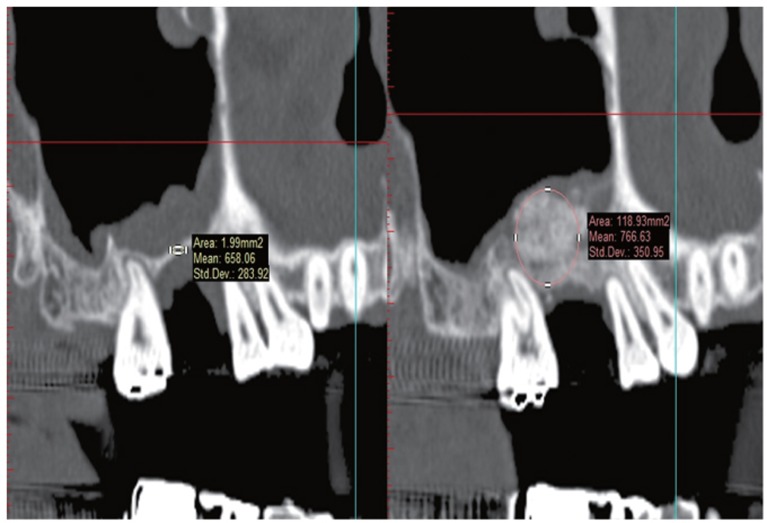
For the bone density evaluation, mean Hounsfield unit (HU) of residual bone was measured at the sinus lift and implant placement planned site from pre-operative CT. Then mean HU of grafted bone site was measured from post-operative CT and compared (Fig. 1). Based on the guideline of bone quality suggested in the software (HU ≥ 1250 = D1, 850-1249 = D2, 350-849 = D3, 150-349 = D4), bone density was calculated. This measurement was performed by one oral and maxillofacial surgeon who does not know the control/experimental group.
Fig. 1.
Measurement of bone density by Simplant software.
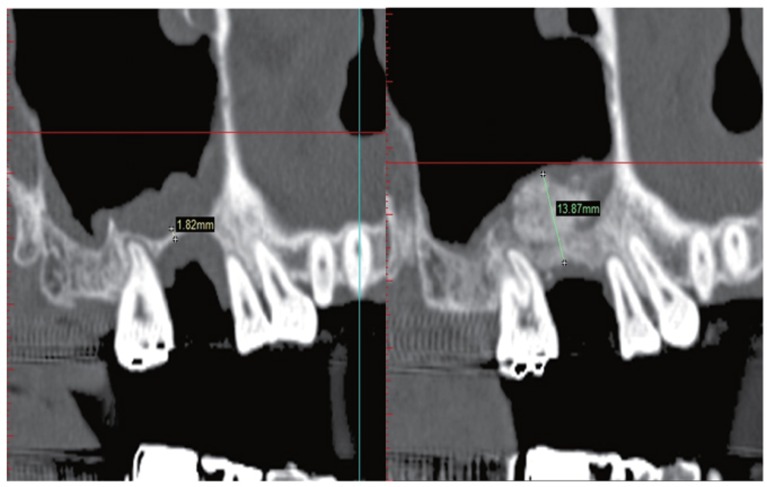
For the evaluation of bone height, RBH was measured at the sinus bone graft and implant placement planned site from pre-operative CT. Then grafted bone height (the distance between alveolar crest and the most upper part of bone graft) was measured from post-operative CT and compared with pre-operative height at the same location (Fig. 2).
Fig. 2.
Measurement of bone height by Simplant software.
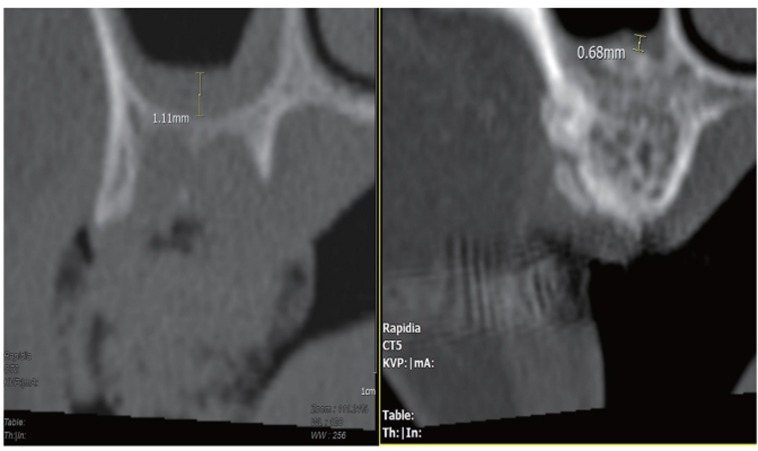
For the evaluation of sinus membrane thickness, the thickest area of sinus membrane of pre-operative bone graft planned site was measured in sagittal view.19,20 Then the thickest area of sinus membrane of post-operative bone graft site was measured and compared (Fig. 3).
Fig. 3.
Measurement of sinus membrane thickness by INFINITT PACS software.
As described, at 4 months after sinus bone graft procedure, biopsy specimen were taken at the site of implant placement using 3.0 mm trephine bur. Five participants in Bio-Oss group and one participant in AutoBT group were declined to take out trephine biopsy before implant placement. So, 14 specimens from Bio-Oss group and 18 specimens from AutoBT group were taken. After fixation of trephine bur in 10% buffered formalin solution, bony specimens (including trephine bur) were sent to Department of Dental Biomaterials Science and Dental Research Institute, School of Dentistry, Seoul National University for microCT analysis. Bone tissue contained in trephine bur was embedded in methyl-methacrylate resin. Trephine bur and outer resin embedding was removed using diamond disk without tissue damage. Cylindrical specimen was fabricated. Cylindrical samples embedded in resin were imaged with a microCT system (SKYscan 1172, Bruker-Microct, Kontich, Belgium) in high resolution scanning mode (pixel matrix: 683×2000×1048, pixel size: 10.89 µm). X-ray source was set at 70 kV and 141 µA with the aid of 0.5 mm thick aluminum filter to optimize the contrast, a 360° rotation step of 0.3° and 590 ms exposure time. Before microCT taking of specimens, two phantoms (0.25 and 0.75 BMD) were taken for the calibration. NRECON reconstruction software (NRecon v.1.4.4, SkyScan) was used to create two-dimensional, 2000×1048 pixel images. After image reconstruction, the region of interest (ROI) was selected within the reconstructed images of water to calibrate the standard unit of X-ray CT density (HU) using CTAn Ver. 1.6.0 (SkyScan, Bruker-Microct, Kontich, Belgium) analysis software. Firstly, total core height composed of residual bone and new bone portion was measured, and then image analysis was performed after setting up graft material and new bone area as ROI. To distinguish new bone from graft material, graft material was taken and range of HU was analyzed. Minimum HU value of new bone was set as 350 considering Tajima et al.21's suggestion and HU of D3 bone. Maximum HU value of new bone was calculated by subtraction of HU of graft material. The area higher than low ranked 5% of HU value distribution of binary image was considered as HU of graft material. HU value of Bio-Oss was more than 1900. In the case that the range of HU value was 350-1900 in Bio-Oss specimen, it was considered as new bone and analyzed. As the same concept, the HU of AutoBT was more than 2100. Applying HU guideline as described, total bone amount and new bone amount of Bio-Oss and AutoBT specimen were calculated. Also, mean HU value, BMD and mean trabecular thickness of new bone were calculated.
After microCT analysis, in the process of specimen preparation for histologic evaluation, 2 specimens in Bio-Oss group and 2 specimens in AutoBT group were excluded due to bad quality. These specimens were not homogenous enough for proper analysis, resulting in analysis of a total of 28 biopsy specimens (12 in Bio-Oss group, 16 in AutoBT group). After microCT taking, secondary embedding of core sample was done using Technovit 7200 (Heraeus Kulzer, Wehrheim, Germany). Block type specimen were trimmed and then attached on acrylic slide using EXAKT 4230 attach machine. After middle cutting and grinding of attached block, acrylic slide was attached. Slide cutting and grinding was done on the slide attached side and 20-30 µm thickness histologic specimens were fabricated. Slide review for histologic evaluation was done by two authors (Lee & Jun) with two different staining techniques. Masson's trichrome (MT) and toluidine blue (TB) stains were used. For the histomorphometric analysis, the tissue stained with MT was examined at high magnification (×100). Representative area of graft material and new bone were selected, and then using KAPPA mage base program, area of newly-formed bone, area of graft material, area of bone marrow space was calculated and expressed as a percentage to total area. The tissue stained with TB was examined at high magnification (×200). Five sites of osteoid forming site surrounding graft material were selected and measured and mean osteoid thickness surrounding graft material was calculated.
Statistical analysis was performed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed using mean ± SD. Differences in proportions were tested with the chi square test or Fisher's exact test. Independent t-test or Mann-Whitney U test were used for the comparison between groups of clinical, radiological and histological evaluation on bone volume-related, bone density-related or bone architecture-related parameters. Paired t-test was used for the evaluation of time dependent changes such as sinus membrane thickness. The univariate normality assumptions were verified with the Shapiro-Wilk test and the Brown and Forsythe's variation of Levene's test statistics were used to verify the homogeneity of variances. Mann Whitney U test was performed when these assumptions were not fulfilled. P<.05 was considered statistically significant.
RESULTS
There were 24 male participants (11 in Bio-Oss group and 13 in AutoBT group) and 14 female participants (8 in Bio-Oss group and 6 in AutoBT group). There was no group difference of gender distribution in this study (P=.501).
The mean age of participants was 58.21 years in Bio-Oss group (n=19) and 53.15 years in AutoBT group (n=19). There was no age difference between groups (P=.158).
Implant primary stability measured using Osstell Mentor was in the range of 41-88 ISQ (mean=70.59 ISQ) (n=28 implants) in Bio-Oss group and 39-85 ISQ (mean=64.92 ISQ) (n=29 implants) in AutoBT group. There was no significant difference between groups (P=.166).
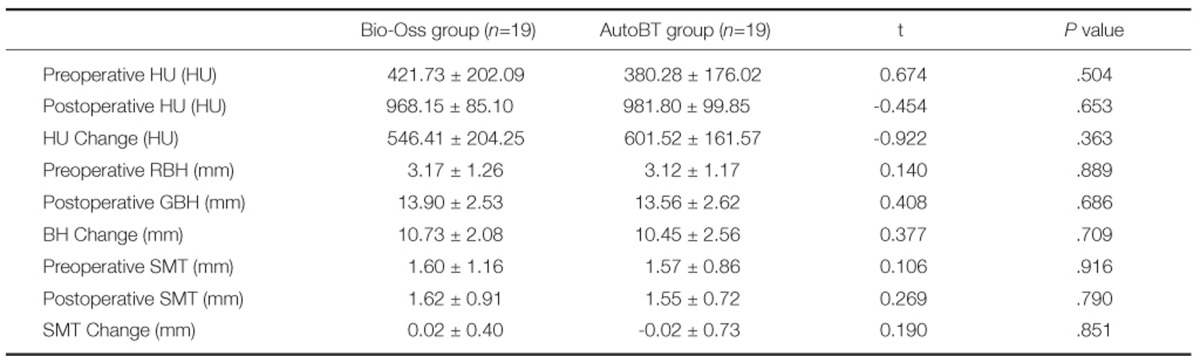
Pre-operative mean bone density of residual alveolar bone area was 421.73 HU in Bio-Oss group and 380.28 HU in AutoBT group. Mean pre-operative bone density of residual alveolar bone area was included in the category of D3 in both groups. Post-operative mean bone density of grafted bone area was 968.15 HU in Bio-Oss group and 981.80 HU in AutoBT group. Mean post-operative bone density of grafted bone area was included in the category of D2 in both groups.
Pre-operative mean residual bone height was 3.17 mm in Bio-Oss group and 3.12 mm in AutoBT group. Also, post-operative mean grafted bone height was 13.90 mm in Bio-Oss group and 13.56 mm in AutoBT group. Pre-operative mean sinus membrane thickness was 1.60 mm in Bio-Oss group and 1.57 mm in AutoBT group. Post-operative mean sinus membrane thickness was 1.62 mm in Bio-Oss group and 1.55 mm in AutoBT group. There was no significant differences between groups (Table 1).
Table 1.
CT evaluation on bone density, bone height, and sinus membrane thickness
RBH: residual bone height, GBH: grafted bone height, BH: bone height, SMT: sinus membrane thickness.
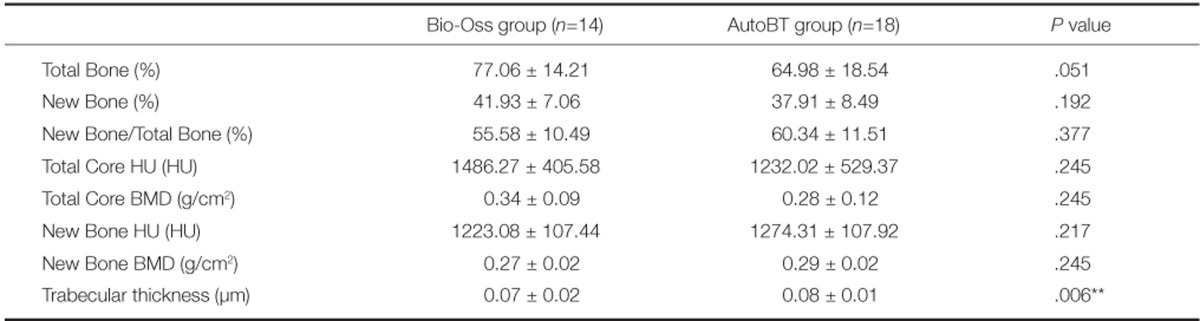
In volume-related parameters, mean total bone volume (new bone + graft material) was 77.06% in Bio-Oss group and 64.98% in AutoBT group. There was no significant difference between groups (P=.051). Mean new bone volume was 41.93% in Bio-Oss group and 37.91% in AutoBT group. There was no significant difference between groups (P=.192). The proportion of new bone volume to total bone volume (new bone/total bone) was 55.58% in Bio-Oss group and 60.34% in AutoBT group. There was no significant difference between groups (P=.377).
In density-related parameters, mean total core density was 1486.27 HU in Bio-Oss group and 1232.02 in AutoBT group. There was no significant difference between groups (P=.245). Also, mean bone mineral density (BMD: volumetric density of calcium hydroxyapatite) of total core was 0.34 g/cm2 in Bio-Oss group and 0.28 g/cm2 in AutoBT group. There was no significant difference between groups (P=.245). Mean new bone density was 1223.08 HU in Bio-Oss group and 1274.31 in AutoBT group. There was no significant difference between groups (P=.217). Also, mean BMD of new bone was 0.27 g/cm2 in Bio-Oss group and 0.29 g/cm2 in AutoBT group. There was no significant difference between groups (P=.245).
In architecture related parameter, trabecular thickness was 0.07 µm in Bio-Oss group and 0.08 µm in AutoBT group. There was significant difference between groups (P=.006) (Table 2).
Table 2.
MicroCT evaluation on volume-related, density-related and architecture-related parameters
P value: ** <.01 by Mann-Whitney U test.
BMD: Bone mineral density.
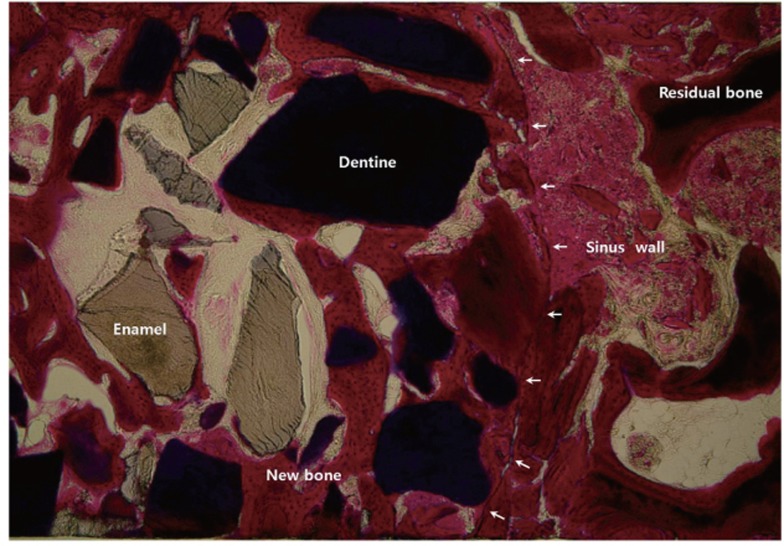
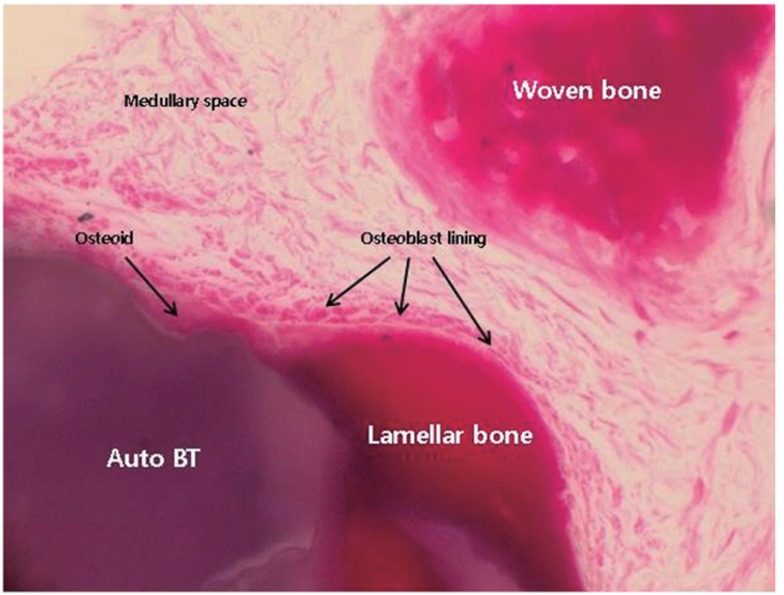
In low magnification (×35) microscopic findings of AutoBT sample, the border between residual bone and new bone of sinus graft site was identified. Matured lamellar bone showing bony integration between residual bone and maxillary sinus was detected. Bone formation was found surrounding both enamel and dentin portion of graft material. New bone bridges between graft materials were also detected (Fig. 5). In high magnification (×400) microscopic findings of AutoBT sample, newly-formed and matured lamellar bone was detected surrounding graft material, osteoblasts covering newly-formed bone accumulated osteoid. Active woven bone formation was also detected. Medullary space formation composed of well vascularized connective tissue was also detected within new bone (Fig. 6).
Fig. 5.
Matured lamellar bone showing bony integration between residual bone and maxillary sinus was detected. Bone formation was found surrounding both enamel and dentin portion of AutoBT material. New bone bridges between graft materials were also detected (Masson's trichrome staining, ×35).
Fig. 6.
Newly-formed and matured lamellar bone was detected surrounding AutoBT material, osteoblasts covering newly-formed bone accumulated osteoid. Active woven bone formation was also detected. Medullary space formation composed of well vascularized connective tissue was also detected within new bone (Masson's trichrome staining, ×400).
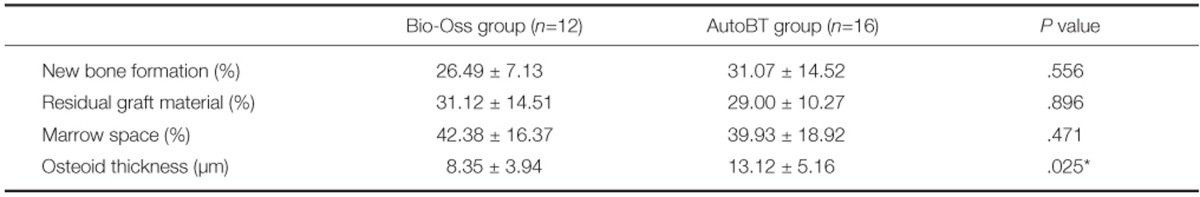
In histomorphometric analysis, the portion of new bone formation was 26.49% in Bio-Oss group and 31.07% in AutoBT group. There was no significant difference between groups (P=.556). The portion of residual graft material was 31.12% in Bio-Oss group and 29.00% in AutoBT group. There was no significant difference between groups (P=.896). The portion of bone marrow space was 42.38% in Bio-Oss group and 39.93% in AutoBT group. There was no significant difference between groups (P=.471). The mean osteoid thickness was 8.35 µm in Bio-Oss group and 13.12 µm in AutoBT group. There was significant difference between groups (P=.025) (Table 3).
Table 3.
Histomorphometric analysis
P value: * <.05 by Mann-Whitney U test.
DISCUSSION
Maxillary sinus cavity is a contained defect. However, maxillary sinus is not natural bone-forming area. These characteristics are why maxillary sinus is often selected for the evaluation of healing process of bone graft materials.22 So, in this study, as a model for the evaluation of healing process of two different bone graft material, maxillary sinus cavity was selected. In this study, there was no significant difference between groups in pre-operative RBH and bone density, which was the evidence that there was no selection bias. Also, implant primary stability measured at 4 months after graft was at favorable state, suggesting that the strength of bone graft site was excellent.
Transient swelling of the Schneiderian membrane occurred just after the sinus graft procedure post-operatively due to the inflammatory reaction. However, with the lapse of time, the change was recovered to normal thickness. In case that the biocompatibility of bone graft material was not good, there is a risk of post-operative infection and sinus membrane remained thickened state. In this study, although there was considerable increase of bone height, the thickness of sinus membrane maintained normally in both AutoBT and Bio-Oss groups post-operatively. The findings that sinus mucosa thickness was maintained stably over time could be an evidence of biocompatibility of graft materials. So, it was proposed that both AutoBT and Bio-Oss were biocompatible in application in the sinus graft procedure.
Kühl et al.23 reported that increased bone density after sinus bone graft procedure with autogenous bone or mixed with other bone graft material was detected in microCT. Huang et al.24 reported that BMD and bone volume of native bone (0.22 and 24.4%) were higher than BMD and bone volume of grafted bone (0.12 and 15.43%) at the time point of 4-5 months after iliac bone graft. Lundgren et al.25 evaluated bone volume using microCT analysis with core biopsy specimen at 6 months (40 ± 12%) and 12 months (48 ± 10%) respectively after sinus graft procedure with particulated mandibular symphysis bone. Chackartchi et al.26 performed microCT analysis with tissue specimen at 6-9 months after sinus graft procedure with bovine bone and reported that the volume of bone (7.99-14.64%) and bovine bone material (22.89-23.12%) seemed underestimated in comparison with histomorphometric evaluation. Chackartchi et al.26 suggested that partly due to the difficulty of distinguishment of exact borders showing direct connection between new bone and graft materials in microCT image, microCT technique had a chance to underestimate the volumes of the histological bone and graft materials.
In this study, all the same condition for CT taking was kept in detail. Under the guideline of HU value of graft material and previous study, the range of new bone was set up scientifically, bone graft material and new bone was analyzed separately. For the determination of lower limit of HU of new bone, bone quality guideline of previous study of Tajima et al.21 was referred. For the determination of upper limit of HU, using statistical method, microCT image of graft material were analyzed and the range of 95% HU was regarded as graft material. And equivalent to the lowest 5% HU was regarded as new bone.
In the microCT analysis of this study, though it was not statistically significant, total bone volume in the grafted area was higher in Bio-Oss group (77.06 ± 14.21) than in AutoBT group (64.98 ± 18.54). However, new bone portion in the total bone was higher in AutoBT group (60.34 ± 11.51%) than in Bio-Oss group (55.58 ± 10.49%). Furthermore, trabecular thickness in AutoBT group was significantly higher than Bio-Oss group, suggesting that the ability of new bone formation of AutoBT graft material was not inferior to Bio-Oss. Also, at 4 months, bone volume and bone density were relatively higher in comparison with other studies. HU and BMD of new bone at 4 months were corresponding with clinical findings showing D2 bone quality.
Histomorphometric researches related with bone graft evaluated and reported various parameters including total bone volume, new bone formation, woven/lamellar bone ratio, residual graft material amount, bone marrow including connective tissue. In this study, new bone formation, residual graft material ratio and bone marrow and osteoid thickness were measured. There have been many reports on histomorphometric analysis using tissue specimen harvested after sinus bone graft with various bone graft material.
Many studies on allograft such as demineralized bone matrix with cancellous bone chips, human mineralized bone, cancellous block, and demineralized freeze-dried bone (DFDB) graft were performed. In the analysis of bone tissue specimens harvested at 6-11 months, wide range of new bone (24-75%) and residual bone graft material (9-25%) were measured and D3 bone density was detected with relatively high marrow portion.27,28,29,30 In the studies of autogenous bone graft for sinus bone graft procedure, it was reported that newly formed bone portion was 38-40% and residual graft material was about 18%.31,32
Histomorphometric study with bone tissue samples harvested after healing period was performed using various alloplastic materials for the sinus bone graft. In the studies of HA and TCP graft mixed at 6:4 ratio, it was reported that new bone formation was 26-40% and residual bone graft material was 27% at 4-9 months healing period.33,34 On the other hand, in studies of TCP graft at 6-8 months of healing period, 21-55% of new bone and 13-34% of residual graft material was detected.31,35,36 Whereas, in the studies of HA graft at 4-6 months, 20-35% of new bone formation and 30-45% of residual graft material were reported.31,37
From the studies on anorganic bovine bones graft, 12-50% of new bone formation and 20-30% of residual graft material were reported after 4-10 months of healing period.31,37,38,39 Orsini et al.40 performed the histologic and ultrastructural analysis of regenerated bone in maxillary sinus augmentation using a porcine bone-derived biomaterial and reported that the portion of newly formed bone was 36.0 ± 2.8% and residual grafted material was 31.0 ± 1.6%. The result of Orsini et al.'s study was relatively similar with the result of this study.
After mixing and grafting 2 different bone graft materials, many researches were reported on histomorphometric analysis. At 6-10 months after sinus bone graft with autogenous bone and xenograft, the proportion of new bone formation was reported as 27-46%, whereas residual bone graft material was 16-37%.41,42,43 Hanisch et al.44 measured new bone formation ratio after the graft with DFDBA and bovine HA at a one to one ratio, and reported 8.1 ± 3.0% at 6 months, 9.0 ± 3.8% at 8 months, 20.7 ± 8.3% at 12 months respectively.
Gonshor et al.45 performed histologic and histomorphometric evaluation of allograft stem cell-based matrix sinus augmentation procedure. After mean 3.7 months of healing period, allograft cellular bone matrix (ACBM), containing native mesenchymal stem cells and osteoprogenitors graft group showed that vital bone content was 32.5 ± 6.8% and residual graft content was 4.9 ± 2.4%. On the other hand, conventional allograft group showed that vital bone content was 18.3 ± 10.6% and residual graft content was 25.8 ± 13.4%.
Some researchers proposed that histologic features of grafted site were influenced by harvesting technique of biopsy specimen. In comparison with crestal approach, the less bone formation tendency was detected in lateral window biopsy.46,47 In this study, crestal approach with 3.0 mm trephine and surgical stent was selected for harvesting biopsy specimen.
A slowly resorbable biomaterial might be suitable in sub-sinusal bone augmentation for preventing the re-expansion process and for augmenting the density of the regenerated tissues. Bovine HA seems to be the most efficient fillers for 3 dimensional stability of sub-sinusal bone augmentation.48 In this study, AutoBT and Bio-Oss showed similar healing pattern, excellent graft volume maintenance and active new bone formation, in microCT and histomorphometric evaluation of biopsy specimen 4 months after graft procedure. So, if it was applied for sinus bone graft, it would be useful material struggling repneumatization against intra-sinus pressure. Bone formation was found surrounding both enamel and dentin portion of AutoBT material. New bone bridges between graft materials were also detected in AutoBT group. Also newly-formed and matured lamellar bone was detected surrounding AutoBT material, osteoblasts covering newly-formed bone accumulated osteoid.
Bone healing of human sinus cavity showed different features according to the types of graft materials and healing time. Also, vital bone formation ratio increased gradually over time.49,50 In histomorphometric evaluation, the mean osteoid thickness was higher in AutoBT group (13.12 µm) than in Bio-Oss group (8.35 µm). Also, though it was not statistically significant, portion of new bone formation was higher in AutoBT group (31.07%) than in Bio-Oss group (26.49%).
CONCLUSION
In this prospective clinical study, sinus membrane thickness maintained stable in both graft materials. Also, there was no significant difference between groups on CT, microCT or histomorphometric analysis, except thicker trabecular and osteoid thickness in AutoBT group. Conclusively, AutoBT was not inferior to Bio-Oss as bone graft material for sinus bone graft procedures. So, AutoBT could be considered a viable alternative to the autogenous bone or other bone graft materials in sinus bone graft procedure. In terms of recycling autogenous tissue that would be usually discarded as medical waste, AutoBT is the new concept of graft material. It is marvelous that there is no donor site morbidity in spite of using autogenous tissue, and that there is no risk of immune response or spread of disease. In the future, more studies will be succeeded for the evaluation of AutoBT application for other bone graft procedures.
Fig. 4.
MicroCT image.
Footnotes
This study was supported by a grant of the Korea Healthcare technology R&D Project, Ministry of Health and Welfare, Republic of Korea (A102065).
References
- 1.Berglundh T, Lindhe J. Healing around implants placed in bone defects treated with Bio-Oss. An experimental study in the dog. Clin Oral Implants Res. 1997;8:117–124. doi: 10.1034/j.1600-0501.1997.080206.x. [DOI] [PubMed] [Google Scholar]
- 2.Piattelli M, Favero GA, Scarano A, Orsini G, Piattelli A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: a histologic long-term report of 20 cases in humans. Int J Oral Maxillofac Implants. 1999;14:835–840. [PubMed] [Google Scholar]
- 3.Carmagnola D, Adriaens P, Berglundh T. Healing of human extraction sockets filled with Bio-Oss. Clin Oral Implants Res. 2003;14:137–143. doi: 10.1034/j.1600-0501.2003.140201.x. [DOI] [PubMed] [Google Scholar]
- 4.Caubet J, Petzold C, Sáez-Torres C, Morey M, Iriarte JI, Sánchez J, Torres JJ, Ramis JM, Monjo M. Sinus graft with safescraper: 5-year results. J Oral Maxillofac Surg. 2011;69:482–490. doi: 10.1016/j.joms.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, Kim SY. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, Lim SY. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol. 2011;11:7442–7445. doi: 10.1166/jnn.2011.4857. [DOI] [PubMed] [Google Scholar]
- 7.Kim YK, Lee JK, Kim KW, Um IW, Murata M. Healing Mechanism and Clinical Application of Autogenous Tooth Bone Graft Material. In: Pignatello R, editor. Advances in Biomaterials Science and Biomedical Applications [Internet] Rijeka, Croatia: InTech; 2013. pp. 405–435. [Google Scholar]
- 8.Kim YK, Yi YJ. Horizontal ridge augmentation using ridge expansion and autogenous tooth bone graft: a case report. J Dent Rehabil Appl Sci. 2011;27:109–115. [Google Scholar]
- 9.Kim YK, Kim SG, Um IW. Vertical and horizontal ridge augmentation using autogenous tooth bone graft materials: case report. J Korean Assoc Maxillofac Plast Reconstr Surg. 2011;33:166–170. [Google Scholar]
- 10.Kim YK, Kim SG, Kim KW, Um IW. Extraction socket preservation and reconstruction using autogenous tooth bone graft: Case report. J Korean Assoc Maxillofac Plast Reconstr Surg. 2011;33:264–269. [Google Scholar]
- 11.Kim YK, Choi YH. Tooth autotransplantation with autogenous tooth- bone graft: a case report. J Korean Dent Sci. 2011;4:79–84. [Google Scholar]
- 12.Lee JY, Kim YK, Kim SG, Lim SC. Histomorphometric study of sinus bone graft using various graft material. J Dent Rehab App Sci. 2011;27:141–147. [Google Scholar]
- 13.Kim YK, Lee HJ, Kim KW, Kim SG, Um IW. Guide bone regeneration using autogenous teeth: case reports. J Korean Assoc Oral Maxillofac Surg. 2011;37:142–147. [Google Scholar]
- 14.Jeong KI, Kim SG, Kim YK, Oh JS, Jeong MA, Park JJ. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011;20:471–475. doi: 10.1097/ID.0b013e3182386d74. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Kim YK. Retrospective cohort study of autogenous tooth bone graft. Oral Biol Res. 2012;36:39–43. [Google Scholar]
- 16.Kim YK, Um IW, Murata M. Tooth bank system for bone regeneration-Safety report- J Hard Tissue Biol. 2014;23:371–376. [Google Scholar]
- 17.Jensen OT. The sinus bone graft. 2nd ed. Chicago: Quintessence Publ; 2006. pp. 103–125. [Google Scholar]
- 18.Kim YK, Kim SG, Park JY, Yi YJ, Bae JH. Comparison of clinical outcomes of sinus bone graft with simultaneous implant placement: 4-month and 6-month final prosthetic loading. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011;111:164–169. doi: 10.1016/j.tripleo.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Janner SF, Caversaccio MD, Dubach P, Sendi P, Buser D, Bornstein MM. Characteristics and dimensions of the Schneiderian membrane: a radiographic analysis using cone beam computed tomography in patients referred for dental implant surgery in the posterior maxilla. Clin Oral Implants Res. 2011;22:1446–1453. doi: 10.1111/j.1600-0501.2010.02140.x. [DOI] [PubMed] [Google Scholar]
- 20.Phothikhun S, Suphanantachat S, Chuenchompoonut V, Nisapakultorn K. Cone-beam computed tomographic evidence of the association between periodontal bone loss and mucosal thickening of the maxillary sinus. J Periodontol. 2012;83:557–564. doi: 10.1902/jop.2011.110376. [DOI] [PubMed] [Google Scholar]
- 21.Tajima N, Ohba S, Sawase T, Asahina I. Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. Int J Oral Maxillofac Implants. 2013;28:77–83. doi: 10.11607/jomi.2613. [DOI] [PubMed] [Google Scholar]
- 22.Nevins M, Kirker-Head C, Nevins M, Wozney JA, Palmer R, Graham D. Bone formation in the goat maxillary sinus induced by absorbable collagen sponge implants impregnated with recombinant human bone morphogenetic protein-2. Int J Periodontics Restorative Dent. 1996;16:8–19. [PubMed] [Google Scholar]
- 23.Kühl S, Götz H, Brochhausen C, Jakse N, Filippi A, d'Hoedt B, Kreisler M. The influence of substitute materials on bone density after maxillary sinus augmentation: a microcomputed tomography study. Int J Oral Maxillofac Implants. 2012;27:1541–1546. [PubMed] [Google Scholar]
- 24.Huang HL, Chen MY, Hsu JT, Li YF, Chang CH, Chen KT. Three-dimensional bone structure and bone mineral density evaluations of autogenous bone graft after sinus augmentation: a microcomputed tomography analysis. Clin Oral Implants Res. 2012;23:1098–1103. doi: 10.1111/j.1600-0501.2011.02273.x. [DOI] [PubMed] [Google Scholar]
- 25.Lundgren S, Moy P, Johansson C, Nilsson H. Augmentation of the maxillary sinus floor with particulated mandible: a histologic and histomorphometric study. Int J Oral Maxillofac Implants. 1996;11:760–766. [PubMed] [Google Scholar]
- 26.Chackartchi T, Iezzi G, Goldstein M, Klinger A, Soskolne A, Piattelli A, Shapira L. Sinus floor augmentation using large (1-2 mm) or small (0.25-1 mm) bovine bone mineral particles: a prospective, intra-individual controlled clinical, microcomputerized tomography and histomorphometric study. Clin Oral Implants Res. 2011;22:473–480. doi: 10.1111/j.1600-0501.2010.02032.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim DM, Nevins ML, Camelo M, Camelo JM, Schupbach P, Hanratty JJ, Uzel NG, Nevins M. The efficacy of demineralized bone matrix and cancellous bone chips for maxillary sinus augmentation. Int J Periodontics Restorative Dent. 2009;29:415–423. [PubMed] [Google Scholar]
- 28.Gapski R, Neiva R, Oh TJ, Wang HL. Histologic analyses of human mineralized bone grafting material in sinus elevation procedures: a case series. Int J Periodontics Restorative Dent. 2006;26:59–69. [PubMed] [Google Scholar]
- 29.Cammack GV, Nevins M, Clem DS, Hatch JP, Mellonig JT. Histologic evaluation of mineralized and demineralized freeze-dried bone allograft for ridge and sinus augmentations. Int J Periodontics Restorative Dent. 2005;25:231–237. [PubMed] [Google Scholar]
- 30.Chaushu G, Vered M, Mardinger O, Nissan J. Histomorphometric analysis after maxillary sinus floor augmentation using cancellous bone-block allograft. J Periodontol. 2010;81:1147–1152. doi: 10.1902/jop.2010.090751. [DOI] [PubMed] [Google Scholar]
- 31.Scarano A, Degidi M, Iezzi G, Pecora G, Piattelli M, Orsini G, Caputi S, Perrotti V, Mangano C, Piattelli A. Maxillary sinus augmentation with different biomaterials: a comparative histologic and histomorphometric study in man. Implant Dent. 2006;15:197–207. doi: 10.1097/01.id.0000220120.54308.f3. [DOI] [PubMed] [Google Scholar]
- 32.Szabó G, Huys L, Coulthard P, Maiorana C, Garagiola U, Barabás J, Németh Z, Hrabák K, Suba Z. A prospective multicenter randomized clinical trial of autogenous bone versus beta-tricalcium phosphate graft alone for bilateral sinus elevation: histologic and histomorphometric evaluation. Int J Oral Maxillofac Implants. 2005;20:371–381. [PubMed] [Google Scholar]
- 33.Kim YK, Yun PY, Lim SC, Kim SG, Lee HJ, Ong JL. Clinical evaluations of OSTEON as a new alloplastic material in sinus bone grafting and its effect on bone healing. J Biomed Mater Res B Appl Biomater. 2008;86:270–277. doi: 10.1002/jbm.b.31015. [DOI] [PubMed] [Google Scholar]
- 34.Kolerman R, Goshen G, Joseph N, Kozlovsky A, Shetty S, Tal H. Histomorphometric analysis of maxillary sinus augmentation using an alloplast bone substitute. J Oral Maxillofac Surg. 2012;70:1835–1843. doi: 10.1016/j.joms.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Kurkcu M, Benlidayi ME, Cam B, Sertdemir Y. Anorganic bovine-derived hydroxyapatite vs β-tricalcium phosphate in sinus augmentation: a comparative histomorphometric study. J Oral Implantol. 2012;38:519–526. doi: 10.1563/AAID-JOI-D-11-00061. [DOI] [PubMed] [Google Scholar]
- 36.Ozyuvaci H, Bilgiç B, Firatli E. Radiologic and histomorphometric evaluation of maxillary sinus grafting with alloplastic graft materials. J Periodontol. 2003;74:909–915. doi: 10.1902/jop.2003.74.6.909. [DOI] [PubMed] [Google Scholar]
- 37.Karabuda C, Ozdemir O, Tosun T, Anil A, Olgaç V. Histological and clinical evaluation of 3 different grafting materials for sinus lifting procedure based on 8 cases. J Periodontol. 2001;72:1436–1442. doi: 10.1902/jop.2001.72.10.1436. [DOI] [PubMed] [Google Scholar]
- 38.Wallace SS, Froum SJ, Cho SC, Elian N, Monteiro D, Kim BS, Tarnow DP. Sinus augmentation utilizing anorganic bovine bone (Bio-Oss) with absorbable and nonabsorbable membranes placed over the lateral window: histomorphometric and clinical analyses. Int J Periodontics Restorative Dent. 2005;25:551–559. [PubMed] [Google Scholar]
- 39.Zhang Y, Tangl S, Huber CD, Lin Y, Qiu L, Rausch-Fan X. Effects of Choukroun's platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: a histological and histomorphometric study. J Craniomaxillofac Surg. 2012;40:321–328. doi: 10.1016/j.jcms.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 40.Orsini G, Scarano A, Piattelli M, Piccirilli M, Caputi S, Piattelli A. Histologic and ultrastructural analysis of regenerated bone in maxillary sinus augmentation using a porcine bone-derived biomaterial. J Periodontol. 2006;77:1984–1990. doi: 10.1902/jop.2006.060181. [DOI] [PubMed] [Google Scholar]
- 41.Galindo-Moreno P, Avila G, Fernández-Barbero JE, Aguilar M, Sánchez-Fernández E, Cutando A, Wang HL. Evaluation of sinus floor elevation using a composite bone graft mixture. Clin Oral Implants Res. 2007;18:376–382. doi: 10.1111/j.1600-0501.2007.01337.x. [DOI] [PubMed] [Google Scholar]
- 42.Peleg M, Garg AK, Misch CM, Mazor Z. Maxillary sinus and ridge augmentations using a surface-derived autogenous bone graft. J Oral Maxillofac Surg. 2004;62:1535–1544. doi: 10.1016/j.joms.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 43.Artzi Z, Kozlovsky A, Nemcovsky CE, Weinreb M. The amount of newly formed bone in sinus grafting procedures depends on tissue depth as well as the type and residual amount of the grafted material. J Clin Periodontol. 2005;32:193–199. doi: 10.1111/j.1600-051X.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 44.Hanisch O, Lozada JL, Holmes RE, Calhoun CJ, Kan JY, Spiekermann H. Maxillary sinus augmentation prior to placement of endosseous implants: A histomorphometric analysis. Int J Oral Maxillofac Implants. 1999;14:329–336. [PubMed] [Google Scholar]
- 45.Gonshor A, McAllister BS, Wallace SS, Prasad H. Histologic and histomorphometric evaluation of an allograft stem cell-based matrix sinus augmentation procedure. Int J Oral Maxillofac Implants. 2011;26:123–131. [PubMed] [Google Scholar]
- 46.Tarnow DP, Wallace SS, Froum SJ, Rohrer MD, Cho SC. Histologic and clinical comparison of bilateral sinus floor elevations with and without barrier membrane placement in 12 patients: Part 3 of an ongoing prospective study. Int J Periodontics Restorative Dent. 2000;20:117–125. [PubMed] [Google Scholar]
- 47.Froum SJ, Tarnow DP, Wallace SS, Rohrer MD, Cho SC. Sinus floor elevation using anorganic bovine bone matrix (OsteoGraf/N) with and without autogenous bone: a clinical, histologic, radiographic, and histomorphometric analysis--Part 2 of an ongoing prospective study. Int J Periodontics Restorative Dent. 1998;18:528–543. [PubMed] [Google Scholar]
- 48.Lambert F, Léonard A, Drion P, Sourice S, Layrolle P, Rompen E. Influence of space-filling materials in subantral bone augmentation: blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin Oral Implants Res. 2011;22:538–545. doi: 10.1111/j.1600-0501.2010.02069.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee YM, Shin SY, Kim JY, Kye SB, Ku Y, Rhyu IC. Bone reaction to bovine hydroxyapatite for maxillary sinus floor augmentation: histologic results in humans. Int J Periodontics Restorative Dent. 2006;26:471–481. [PubMed] [Google Scholar]
- 50.De Leonardis D, Pecora GE. Prospective study on the augmentation of the maxillary sinus with calcium sulfate: histological results. J Periodontol. 2000;71:940–947. doi: 10.1902/jop.2000.71.6.940. [DOI] [PubMed] [Google Scholar]