Abstract
BACKGROUND
Familial restrictive cardiomyopathy (FRCM) has a poor prognosis due to diastolic dysfunction and restrictive physiology (RP). Myocardial stiffness, with or without fibrosis, underlie the RP, but the mechanism(s) of restrictive remodeling is unclear. Myopalladin (MYPN) is a messenger molecule that links structural and gene regulatory molecules via translocation from the Z-disk and I-bands to the nucleus in cardiomyocytes. Expression of N-terminal MYPN peptide results in a severe disruption of sarcomere.
OBJECTIVES
A nonsense MYPN-Q529X mutation previously identified in the FRCM family was studied here in an animal model to explore the molecular and pathogenic mechanisms of FRCM.
METHODS
Functional (echocardiography, cardiac magnetic resonance [CMR] imaging, electrocardiography), morphohistological, gene expression, and molecular studies were performed in knock-in heterozygote (MypnWT/Q526X) and homozygote mice harboring the human MYPN-Q529X mutation.
RESULTS
At 12 weeks of age, echocardiographic and CMR imaging signs of diastolic dysfunction with preserved systolic function were identified in MypnWT/Q526X mice. Histology revealed interstitial and perivascular fibrosis without overt hypertrophic remodeling. Truncated MypnQ526X protein was found to translocate to the nucleus. Levels of total and nuclear cardiac ankyrin repeat protein (Carp/Ankrd1) and phosphorylation of Mek1/2, Erk1/2, Smad2, and Akt were reduced. Up-regulation was evident for muscle LIM protein (Mlp), desmin, and heart failure (Nppa, Nppb, and Myh6) and fibrosis (Tgfβ1, αSma, Opn, and Postn) markers.
CONCLUSIONS
Heterozygote MypnWT/Q526X knock-in mice develop RCM due to persistence of mutant Mypn-Q526X protein in the nucleus. Down-regulation of Carp and up-regulation of Mlp and desmin appear to augment fibrotic restrictive remodeling, and reduced Erk1/2 blunts a hypertrophic response in MypnWT/Q526X hearts.
Keywords: CARP/ANKRD1, ERK1/2, fibrosis, remodeling
Introduction
Restrictive cardiomyopathy (RCM) accounts for ~5% of diagnosed cardiomyopathies and is characterized by diastolic dysfunction and restrictive physiology (RP), while systolic function typically remains normal or near normal (1). The volume and wall thickness of the ventricles is usually normal or small, while atrial or bi-atrial enlargement occurs due to impaired ventricular filling during diastole (2). Particularly in children, RCM maintains the poorest prognosis among all types of heart muscle diseases with 2- and 5-year mortality of 50% and 70%, respectively, and the highest rate of sudden cardiac death (SCD) (3). Survivors ultimately develop heart failure (HF) due to RP, as well as pulmonary hypertension; however, the mechanistic basis of restrictive physiology with diastolic dysfunction, myocardial fibrosis, and cardiac stiffness is unclear.
A history of familial RCM (FRCM) is reported in approximately 30% of RCM cases, with autosomal dominant inheritance most commonly noted (4). Several genes, typically encoding proteins of the sarcomere, Z-disk, cytoskeleton, or intermediate filament network, have been associated with autosomal dominant FRCM (4). The myopalladin (MYPN) gene, which is located at chromosome 10q21.3, encodes a 145-kDa protein that participates in linking regulatory molecules involved in sarcomeric I-band and Z-disk assembly and muscle gene expression (5). The N-terminal MYPN interacts with cardiac ankyrin repeat protein (CARP/ANKRD1), a transcriptional coinhibitor of genes involved in the development of HF and hypertrophy (6). MYPN has dual localization, sarcoplasm, and nucleus similar to that seen with CARP (5). At the Z-disk, MYPN interacts with α-actinin (ACTN2) and with SH3-domain of nebulette (NEBL) (7). Mutations in the MYPN gene cause diverse phenotypes in humans, including dilated cardiomyopathy, hypertrophic cardiomyopathy, and restrictive cardiomyopathy (8,9). We have previously reported a nonsense autosomal dominant mutation (MYPN-Q529X) that resulted in FRCM in siblings via disturbed myofibrillogenesis and sarcomeric Z-disk disruption (9).
In this study, knock-in mice carrying a heterozygous and homozygous Mypn-Q526X mutation in exon 10 of murine Mypn gene, homologous to the human MYPN-Q529X mutation, were analyzed to determine the pathophysiology and molecular mechanism(s) of FRCM.
METHODS
GENERATION OF KNOCK-IN MICE
The study conformed to the protocols approved by the Institutional Animal Care and Use Committee at Cincinnati Children's Hospital Medical Center. To generate a murine Mypn-Q526X mutation, we targeted exon 10 in the Mypn gene (Supplemental Figure 1A), using a homologous recombination method as described previously (10) and detailed in Supplemental Materials.
EVALUATION OF HEART FUNCTION IN MICE
Serial echocardiography and electrocardiography (ECG) was performed in mice at 6 and 12 weeks of age (12 animals/group). Cardiac magnetic resonance (CMR) imaging was performed in 12-week-old animals when mice showed markedly increased E/A (early [E] and late [A] diastolic velocities) ratio, signs of “restrictive filling,” or diastolic dysfunction by echocardiography. See Supplemental Materials for experimental details.
HISTOPATHOLOGY, IMMUNOHISTOCHEMISTRY, QUANTITATIVE REAL-TIME PCR, AND ELECTRON MICROSCOPY
Histopathology including H&E, Masson's trichrome, immunohistochemical, transcriptional, and Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) analysis was performed to assess structural, fibrotic, hypertrophic, and/or apoptotic remodeling in the heart. Transmission electron microscopy (TEM) was performed on glutaraldehyde-perfused hearts as previously described (11). After isolation of total ribonucleic acid (RNA) from ventricular tissues, quantitative real-time polymerase chain reaction (PCR) was performed as described in Supplemental Materials. Six 12-week-old animals/group were used. See experimental details in Supplemental Materials.
PROTEIN EXPRESSION, PULL-DOWN AND WESTERN BLOTTING
Human embryonic kidney (HEK293) cells were transfected with different chimeras of MYPN-GFP and CARP-V5 complementary deoxyribonucleic acids (cDNAs) to evaluate MYPN and CARP interactions using immunoprecipitation (IP) and co-immunoprecipitation (Co-IP). Cellular fractionation was performed using the NE-PER kit (Pierce, Rockford, Illinois). Western blotting was used for protein evaluation and levels of proteins were quantified in relative density units using ImageJ software as described in Supplemental Materials.
STATISTICAL ANALYSIS
Statistical analysis reported as mean ± structural equation modeling was performed with Student t test or 1-way analysis of variance using GraphPad5 software (GraphPad Software, Inc., La Jolla, California). A probability value of p ≤ 0.05 was considered significant.
RESULTS
MANIFESTATION OF DIASTOLIC DYSFUNCTION
Given that persistence of 65-kDa-MypnQ526X peptide in vivo may potentially cause a “poison peptide” effect, levels of the Mypn protein were confirmed by Western blotting; the MypnWT/Q526X line #3 with the highest 65-kDa-MypnQ526X protein expression was selected for further breeding (Supplemental Figure 1B). Heterozygous and homozygous recombination of the Mypn-Q526X mutation was confirmed by sequencing of Mypn exon 10 in genomic DNA obtained from mouse tails (Supplemental Figure 1C). We verified Mypn messenger RNA (mRNA) transcription levels in the heart, skeletal muscle, liver, and kidneys by reverse transcription PCR. As expected, homozygotes had impaired Mypn mRNA transcription in heart and skeletal muscle compared to wild-type (WT) and heterozygote littermates (Supplemental Figure 1D-E). Thus, homozygous MypnQ526X mice were also generated to utilize as a model of Mypn gene ablation.
Both MypnWT/Q526X and MypnQ526X mice were born and grew normally, with no skeletal myopathy observed up to 12 weeks of age. At 6 weeks, increased E/A ratios, features of RP in humans (12), were detected in MypnWT/Q526X mice compared to WT and homozygotes. The same cohort of mice underwent a serial echocardiography at 12 weeks of age and an increase in the E/A ratio became significantly profound (Figure 1A). While systolic function and chamber dimensions were preserved, MypnWT/Q526X mice showed signs of impaired diastolic filling of the left ventricle (LV), including decreased end-diastolic volume (LVEDV) and internal dimensions compared to WT and homozygotes (Supplemental Table 1).
FIGURE 1. Phenotypic Characterization of Mypn Mice.
(A) M-mode images of mitral valve movement by 2-dimensional echocardiography indicate an increase of E/A ratios in MypnWT/Q526X compared to WT or homozygotes, which became significant in 12-week-old heterozygote mutants (grey column) compared to WT and homozygote mice. (B) Cardiac magnetic resonance (CMR) images in the 4-chamber view demonstrate enlarged left atria in MypnWT/Q526X mice (middle columns) compared to WT (left columns) and MypnQ526X (right columns) mice. (C) Electrocardiography in mice. MypnWT/Q526X mice display arrhythmias (arrows), including (b) premature atrial contractions, (c) premature ventricular contractions, and 2° atrioventricular block (d; 1:7 Wenkebach).
*p < 0.05.
E/A = early [E] and late [A] diastolic velocities; Mypn = myopalladin; WT = wild-type.
Further, CMR imaging was utilized in the same cohort of 12-week-old mice to precisely evaluate cardiac anatomy and diastolic function. No significant differences between groups were found in circumferential strain, strain rate, torsion, or torsion rate (data not shown). The left atrial cross-sectional area of MypnWT/Q526X mice (3.8 ± 0.5 mm2) was larger compared to WT mice (3.5 ± 0.6 mm2) and homozygotes (3.0 ± 0.3 mm2, p = 0.02) as measured in the 4-chamber view (Figure 1B and Supplemental Table 2). The LVEDV was significantly lower in the MypnWT/Q526X mutants compared to WT mice (p = 0.03). The sphericity index, the ratio of the short- to long-axis dimensions of the LV, was also lower in the MypnWT/Q526X mice (0.51 ± 0.03) compared to WT animals (0.56 ± 0.03; p = 0.02), consistent with LV remodeling in heterozygotes only.
Arrhythmias and conduction abnormalities are reportedly present in 15% to 30% of RCM patients, especially in pediatric patients (13). ECGs revealed that heart rate, P, PR, and QRS durations were similar among mutant and WT mice, while T wave duration was decreased in MypnWT/Q526X versus WT or homozygotes (Supplemental Table 3). Arrhythmias including premature atrial contractions, premature ventricular contractions, and type II second-degree atrioventricular block (Figure 1C) were observed in heterozygotes only.
FIBROSIS IN HETEROZYGOUS MYPNWT/Q526X HEARTS
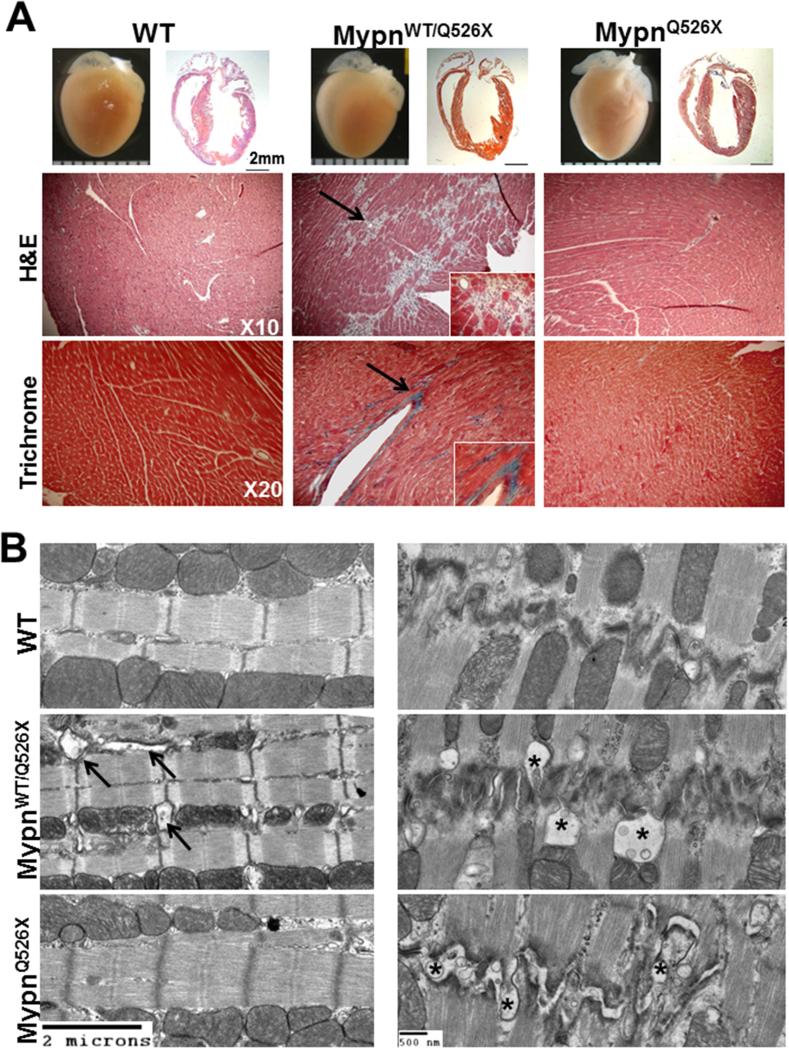
Morphologic evaluation of cardiac muscle revealed no hypertrophy in any animal (Figure 2A). Histologically, diffuse interstitial and perivascular fibrosis was found in MypnWT/Q526X ventricular myocardium only. Dystrophin staining revealed intact sarcolemmal integrity and normal cardiomyocyte size among the different groups, confirming absence of cardiomyocyte hypertrophy (Supplemental Figure 2A). No positive TUNEL staining was seen in any group, excluding the involvement of apoptosis. Ultrastructurally, t-tubules enlargement and mild intercalated disk disruption was seen in heterozygous and homozygous animals (Figure 2B).
FIGURE 2. Morphohistology of Hearts from 12-week-old Mice.
(A) No significant chamber enlargement or cardiac muscle hypertrophy is seen in all groups (upper panels). Sections stained with hematoxylin and eosin (H&E; 10X magnification) demonstrate significant interstitial and perivascular infiltration in the MypnWT/Q526X myocardium (middle panel) with focal loss of myofibrils (arrow) compared to WT (left panel) and homozygotes (right panel). Masson trichrome staining (X20 magnification) reveals interstitial and perivascular fibrosis in MypnWT/Q526X myocardium. Right lower panel displays enlarged details. (B) Transmission electron microscopy demonstrates no difference in the sarcomeric and Z-disk organization between groups (left panels, 24,000X magnification). Enlarged t-tubules (arrows) and widened cell-cell junctions with excess convolutions (asterisks) in both mutants are shown. Abbreviations as in Figure 1.
Taken together, these results show that heterozygote MypnWT/Q526X mutants demonstrated fibrotic remodeling without an overt hypertrophic response, accurately recapitulating the human RCM phenotype. Homozygous MypnQ526X mice had no or a minimal phenotype as result of MYPNQ526X ablation. Therefore, further comparative molecular studies were carried out in MypnWT/Q526X and WT littermates.
PUTATIVE Z-DISK STRESS-SENSOR DYSREGULATION IN MYPNWT/Q526X HEARTS
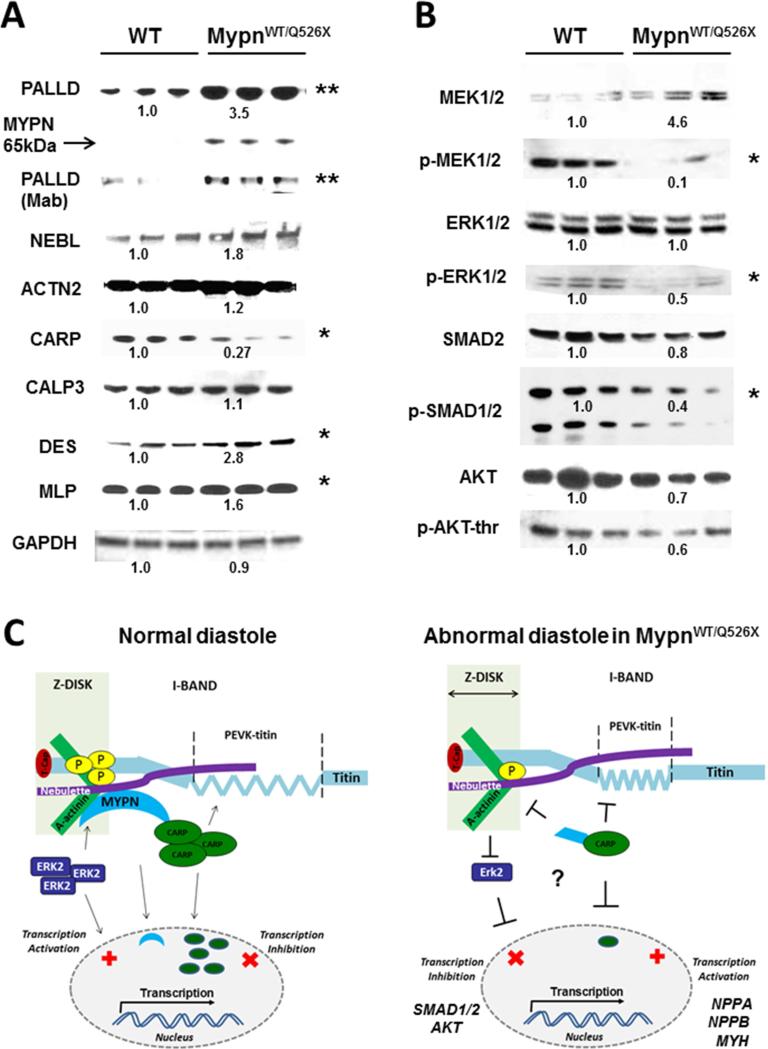
Immunohistochemical analysis revealed no differences in MYPN at the Z-disks. Notably, coincident de-localization of Carp and desmin to the periphery was found in MypnWT/Q526X cardiomyocytes only (Figure 3, arrows). On a protein level, while no MypnWT was seen, the 65kDa-MypnQ526X was detected in the hearts of heterozygous mice as expected (Figure 4A). A significant increase in the expression of the Mypn's homolog, palladin (90kDa), was evident in MypnWT/Q526X hearts and confirmed by monoclonal antibody and immunohistochemical staining (Supplemental Figure 2B). Nebulette and α-actinin2 were mildly up-regulated, whereas Carp was considerably down-regulated in MypnWT/Q526X (p = 0.03). Levels of calpain3 were similar in mutants and WT, excluding the likelihood of Carp degradation due to increased protease activation. Desmin and muscle LIM protein (Mlp/Csrp3), the putative Z-disk stress-sensors, were significantly up-regulated in heterozygotes versus WT (p <0.05). Mild down-regulation of α-tubulin, caveolin-3, and vinculin occurred in MypnWT/Q526X compared to WT, suggesting an early recruitment of t-tubules and adherens junctions, respectively (Supplemental Figure 2B). Intercalated disk proteins such as desmoplakin2, connexin43, or N-cadherin were not affected. Levels of cardiac troponin I (cTnI) and phospho-cTnI were normal, consistent with intact sarcomeres.
FIGURE 3. Immunohistochemistry of Mouse Hearts.
MYPN (red) was co-immunostained with CARP (A) and DES (B), both shown in green. Right panels represent the merged images with DAPI (blue). Although no changes in MYPN localization are noted, the striated co-staining pattern in yellow was lost in MypnWT/Q526X hearts, indicating de-localization of CARP and DES from the cytosol to the periphery of cardiomyocytes (arrows).
CARP = cardiac ankyrin repeat protein; DAPI = diamidino-2-phenylindole; DES = desmin; other abbreviations as in Figure 1.
FIGURE 4. Protein Analysis of Mouse Hearts.
(A-B) Western blotting images of WT (left lanes) and MypnWT/Q526X (right lanes) hearts. Significant changes in protein expression from WT were indicated in fold changes in relative density. Expression of 65kDa-MypnQ526X peptide is indicated by the arrow. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a reference protein. (C) Hypothetical pathogenic mechanisms of diastolic dysfunction caused by the heterozygous Mypn-Q526X mutation. The left panel depicts an idealized model of normal diastolic function. MYPN-CARP at “the central I-band” mechano-transmits the titin-PEVK-based myofibrillar stress/strain signals to the nucleus. MYPN connects with nebulette-SH3 and α-actinin (ACTN2), further interacting with actin, γ-filamin and titin-Z-repeats, which are dependent on ERK2 phosphorylation. In MypnWT/Q526X mouse (right panel), levels of mechanosensory molecules are perturbed. CARP and MEK1/2-ERK1/2 are reduced; MLP and DES are up-regulated. CARP-dependent genes such as NPPA, NPPB, and MYH are up-regulated, while ERK1/2-downstream genes, SMAD1/2 and AKT, are down-regulated.
*p <0.05; **p <0.01.
CALP3 = calpain3; ERK = extracellular-signal-regulated kinase; MEK = mitogen-activated protein kinase; MLP = muscle LIM protein; NEBL = nebulette; PALLD = palladin; other abbreviations as in Figures 1 and 3.
ANALYSIS OF DOWNSTREAM SIGNALING PATHWAYS
As cardiac stretch is regulated mainly at the Z-disks by extracellular-signal-regulated kinase (ERK)2-dependent phosphorylation of titin at the Zis1/Z-repeats (14), we sought to determine whether the ERK cascade is altered. Reduced phosphorylated-MAPK/ERK1/2 (p-Mek1/2) and p-Erk1/2 was seen in MypnWT/Q526X hearts compared to WT (Figure 4B), while other potential mitogen-activated protein kinase (MAPK)-associated targets, including p-53, c-Jun N-terminal kinase, p-38, focal adhesion kinase, nuclear factor kappa-light-chain-enhancer of activated B cells, signal transducer and activator of transcription 3, ERBB4, and transforming growth factor-β1, were not affected (Supplemental Figure 2C). Phosphorylation of Smad2 and Akt was reduced in MypnWT/Q526X animals, however. Further, CARP-dependent genes including Nppa, Nppb, and Myh6 mRNA (Supplemental Table 4) were elevated in MypnWT/Q526X hearts, supporting the idea of diminished inhibitory effects of CARP due to reduced levels of CARP protein (Figure 4C). Significant increases in fibrotic (αSma, Tgf-β1, Postn and Opn), inflammatory (Vcam, Icam andIl-1β), and antiapoptotic (Bcl-2) genes were identified in MypnWT/Q526X hearts.
PERSISTENCE OF NUCLEAR 65KDA-MYPN PEPTIDE ASSOCIATED WITH RCM PHENOTYPE
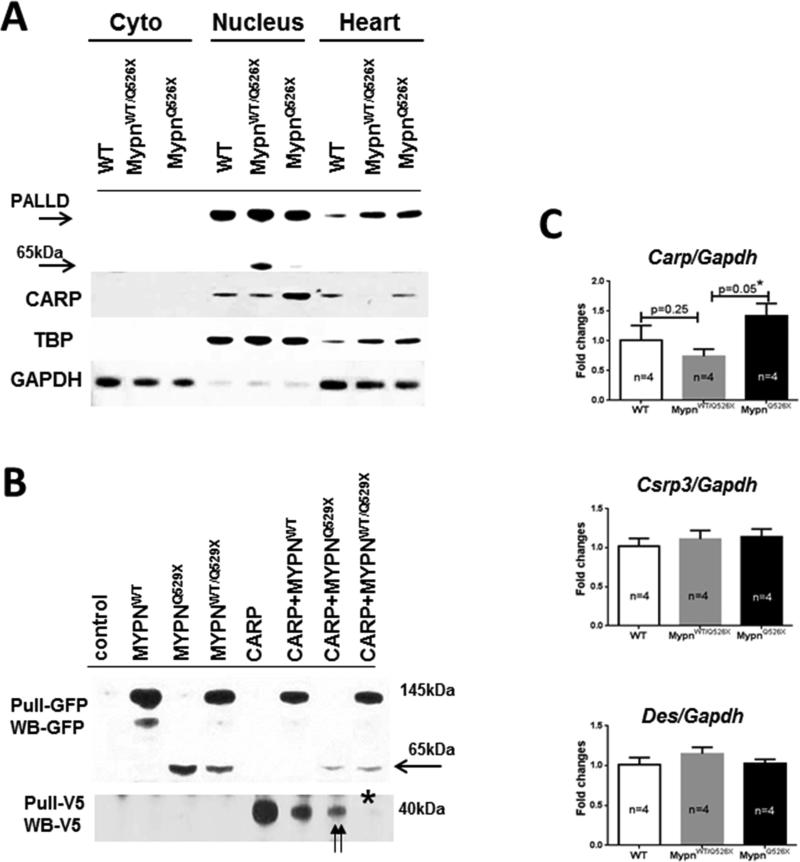
To determine whether the location and expression levels of proteins are associated with the RCM phenotype in vivo, mouse hearts from all groups (WT and heterozygote and homozygote mutants) were fractionated and levels of total, nuclear, and cytoplasmic Mypn, palladin, and Carp were quantitated. Comparable expression of nuclear palladin was documented in all groups (Figure 5A), excluding the likelihood of pathologic effects of increased palladin in the nucleus. Interestingly, the 65kDa-MypnQ526X peptide was detected in the nuclear fraction of heterozygote hearts and very little amount in homozygotes, suggesting a potential cause of the RCM phenotype. A concordant decrease in total Carp levels was detected in MypnWT/Q526X hearts.
FIGURE 5. Expression and Interaction of MYPN and CARP.
(A) Levels of MYPN and CARP in cytoplasmic and nuclear fractions and whole lysates of mouse hearts. GAPDH was used as a cytosolic reference, and TATA-box binding protein (TBP) as a nuclear reference protein. The 65kDa-MypnQ526X peptide was detected (arrow) in the nucleus and total CARP decreased in MypnWT/Q526X hearts. The 65kDa-MypnQ526X peptide is degraded, while levels of nuclear CARP are increased in MypnQ526X samples. (B) Immunoprecipitation studies in HEK293 cells. The expressed human proteins were pulled down with anti-GFP or anti-V5 antibodies and detected by Western blotting with anti-GFP (upper panel) or with anti-V5 (lower panels). Reduced levels of pulled-down 65kDa-MYPNQ529X compared to 145kDa-MYPNWT protein in MYPNQ529X cells and MYPNWT/Q529X mixtures are indicated by arrow. Double arrows indicate reduced levels of 40kDa-CARP-V5 in MYPNQ529X cells compared to MYPNWT cells suggestive for suppressive effects of mutant MYPNQ529X on CARP levels. Asterisks indicate a failure of anti-V5 in pulling the 40kDa-CARP-V5 in the MYPNWT/Q529X mixture. (C) Levels of mRNA of Carp, Mlp/Csrp3, and Des in mouse hearts expressed in fold changes relative to levels of Gapdh in WT mice. Results are obtained in a triple from n = 4 mice/group.
Csrp = cysteine and glycine-rich protein 3; HEK = human embryonic kidney; mRNA = messenger ribonucleic acid; other abbreviations as in Figures 1, 3, and 4.
To further clarify MYPN-CARP interactions, different combinations of human MYPNWT and MYPNQ529X cDNAs fused with green fluorescent protein (GFP) or mixtures of both MYPNWT and MYPNQ529X (MYPNWT/Q529X) were co-transfected with human CARP-V5 construct in HEK293 cells and pulled down. As shown in Figure 5B, GFP antibody pulled down the 65kDa-MYPNQ529X as did the 145kDa-MYPNWT in all transfected cells, except control and CARP-transfected ones. In contrast, levels of V5-pulled 40kDa-CARP were reduced in MYPNQ529X cells compared to MYPNWT. Further, the 40kDa-CARP-V5 was undetectable in MYPNWT/Q529X mixed lysates presumably due to greater levels in reduction of CARP expression compared to mutant MYPNQ529X cells. These effects support the in vivo data of reduced murine Carp levels in heterozygous hearts and the idea that nuclear 65kDa-MypnQ526X may suppress Carp expression.
Subsequently, levels of Carp, Mlp, and Des mRNA were evaluated in the myocardial tissues of heterozygote and homozygote mice to further elucidate whether differential phenotypes are associated with nuclear MypnQ526X-mediated alteration in transcriptional activities of mechanosensitive molecules. As shown in Figure 5C, divergent changes in CARP mRNA levels were detected in MypnWT/Q529X and MypnQ529X hearts compared to WT controls. Carp transcription was reduced in MypnWT/Q529X, while MypnQ529X mice presented Carp up-regulation, and this variation between mutants was statistically significant. In contrast, no significant difference in transcription of Mlp and Des is revealed in any groups.
DISCUSSION
The characteristic features of autosomal dominant familial RCM result in a high-risk disorder with poor clinical outcomes including HF, arrhythmias, and SCD (2). These features include diastolic dysfunction and restrictive ventricular filling, with limited ability to augment cardiac stroke volume due to increased myocardial stiffness and interstitial fibrosis; however, a mechanistic etiology remains unclear (4). MYPN is a sarcomeric protein localized at the Z-disk, I band, and nucleus. Expression of the N-terminal region of MYPN in cardiomyocytes resulted in severe disruption of the sarcomere (5), while N-terminal mutation MYPN-Y20C reduced CARP-MYPN binding and caused hypertrophic cardiomyopathy in vivo (Central Illustration) via perturbed MYPN nuclear shuttling and abnormal assembly of terminal Z-disks within intercalated disks (9). Mutations in MYPN, therefore, cause diverse cardiomyopathic phenotypes within a critical “final common pathway” (9,15).
CENTRAL ILLUSTRATION. MYPN-mutation Induced Abnormal Response to Physiological and Pathological Stimuli.
Illustrated model of MYPN-mutation induced abnormal response to physiological and pathological stimuli. In this model, the middle panel represents normal, balanced MYPN interactions that regulate adequate response to various stresses. In the MYPN-Q529X-mutation induced RCM, MYPNQ529X peptide (right panel) translocates to the nucleus, decreases CARP expression, and increases MLP and DES on a protein level, leading to fibrosis via up-regulated POSTN and OPN. The hypertrophic response is abolished via reduced ERK1/2, SMADs, and AKT. Limited or loss of adaptive responses to various stimuli results in the development of RCM. Conventional cardiovascular drugs, including ACE inhibitors, ARBs, and β-blockers, thus, have limited effect in patients with RCM. The left panel represents the mechanism of MYPN-Y20C-mutation induced HCM as result of reduced binding of CARP to MYPNY20C peptide and impaired nuclear translocation of MYPN. Subsequent alterations in DES, ·-actinin, and vinculin, desmoplakin and connexin43 were evident.
ACTN2 = ·-actinin; ACE = angiotensin-converting enzyme; AngII=angiotensin II; ARB = angiotensin II receptor blocker; β-AR = alpha- and beta-adrenergic receptors; CARP = cardiac ankyrin repeat protein; Cx43 = connexin43; DES = desmin; Dox=doxorubicin; DSP = desmoplakin; HCM = hypertrophic cardiomyopathy; MYPN = myopalladin; RCM = restrictive cardiomyopathy; TGF-β = transforming growth factor beta; VIN = vinculin.
The objectives of this study were to uncover the molecular pathogenetic mechanism(s) and discover the associated key signaling molecules in MYPN-Q529X mutation-induced restrictive remodeling using a knock-in mouse model. We demonstrated that homozygous MypnQ526X mice display no phenotype due to ablation of mutant protein. Heterozygous MypnWT/Q526X mutants, corresponding to a human heterozygous MYPN-Q529X mutation, recapitulated the clinical and pathologic features of human FRCM, including diastolic dysfunction with preserved systolic function by 12 weeks of age. Interstitial and perivascular fibrosis was seen before overt cardiac contractile dysfunction, while myocyte hypertrophy, apoptosis, and necrosis were absent.
From these data, we hypothesize that the RCM phenotype results from persistence of dysfunctional truncated MypnQ526X protein and consequent multiple pathological “hits.” First, MypnQ526X translocates to the nucleus, probably perturbing levels of Carp expression (Central Illustration). Second, MypnQ526X causes alterations in Mlp/cysteine-rich protein 3(Csrp3) and desmin and blunts the phosphorylation of Mek1/2-Erk1/2, likely, via negative nuclear feedback. Further, activation of Smad2 and of Akt are reduced, likely to compensate the mutation-induced “final common pathway” of RCM (15).
Balanced levels of CARP are reported to be essential for proper adaptive responses to different stresses in striated muscle (Central Illustration), including exercise (16), stretch (17), cytokines (18), hypoxia (19), adrenergic stress (20), and anthracycline (doxorubicin) toxicity (21). Multiple mechanisms, including 26S-proteosome degradation, TGF-β-SMADs, and caspase3 and calpain3 proteases, are reported as CARP regulators (22). Ablation of all forms of muscle ankyrin repeat proteins (MARPs), including CARP, enhances the MyoD and MLP expression in skeletal muscle (23). While ablation of CARP or MARPs has been shown to not affect the cardiac function similar to our MypnQ526X homozygotes (24), cardiac-restricted CARP over-expression results in an inhibition of hypertrophic and fibrotic remodeling via reduced TGF-β and ERK1/2 (18). Our previous MypnY20C transgenic mouse model (Central Illustration) suggested a crucial role of nuclear WT-Mypn by demonstrating the development of hypertrophic cardiomyopathy in vivo as a result of perturbed nuclear shuttling of Mypn (9). In the present MypnWT/Q526X model, the mutant MypnQ526X translocates to the nucleus, reducing the expression of Carp. Transcription of key fibrotic molecule, Tgf-β1 was up-regulated, but Tgf-β1 protein levels were not changed, and Smad2 was down-regulated, suggesting an alternative TGF-β-SMAD-independent fibrotic pathway. Increased 90kDa-palladin was shown to induce alpha-smooth muscle actin (aSma) through Tgf-β1 and Erk1/2 activation (25), whereas decreased Ankrd2 and Mlp/Csrp3 levels were reported in forced skeletal muscle inactivity in mice (26). Therefore, decreased Carp and elevated Mlp/Csrp3 and Des likely led to fibrosis via Postn and Opn in our model (17). Moreover, up-regulation of Icam, Vcam, and Il-1b in mutants may contribute to perivascular fibrosis (27).
We hypothesize that the net result of decreased Erk1/2, Smads, and Akt signaling may blunt the hypertrophic response in heterozygotes (Figure 5C). As Carp has been shown to regulate the Erk2-dependent titin-PEVK spring force at the I-band, we assume that reduction in Mek1/2-Erk1/2 phosphorylation might have a negative feedback origin through the nuclear MypnQ526X-induced perturbation of mechanosensitive proteins: Carp, Mlp, and Des (28).
In summary, we demonstrate that the 65kDa-MypnQ526X peptide causes diastolic dysfunction without contractile impairment via net changes in the mechanosensory proteins. To date, no pharmacologic therapies have been shown to clearly improve outcomes in RCM patients, with heart transplantation being the definitive treatment option for many, especially in childhood (29). If our murine findings mirror the pathologic hallmarks of RCM in humans, it could explain why the TGF-β1 suppressor angiotensin-converting enzyme (ACE) inhibitors, β-blockers, and angiotensin receptor blockers (ARBs) are ineffective in RCM patient treatment (3). Further studies on time-dependent expression changes in CARP, MLP, DES, and ERK1/2 in RCM patients may provide useful information for discovering diagnostic and therapeutic targets.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE: Restrictive myocardial remodeling is responsible for the diastolic ventricular dysfunction that characterizes familial restrictive cardiomyopathy (FRCM), which carries a poor prognosis, particularly in affected children. No pharmacological therapies have been found that clearly improve clinical outcomes in patients with this disorder, leaving few alternatives to cardiac transplantation.
TRANSLATIONAL OUTLOOK: This study discovered early molecular markers of restrictive remodeling in a murine model of FRCM that cause diastolic dysfunction without contractile impairment. This points the way toward future studies by which the expression of genes regulating mechanosensory proteins could be modified as potential therapeutic targets.
Acknowledgements
We thank Dr. Siegfried Labeit (Klinikum Mannheim, Mannheim, Germany) for providing antibodies against myopalladin and nebulette.
Funding sources: This work was supported in part by a research grants from the American Heart Association, Children's Cardiomyopathy Foundation (EP, JAT), the John Patrick Albright Foundation and NIH-NHLBI (R01 HL53392 and R01 HL087000) (JAT).
ABBREVIATIONS
- CARP
cardiac ankyrin repeat protein
- CMR
cardiac magnetic resonance
- ECG
electrocardiography
- HF
heart failure
- MLP
muscle LIM protein
- MYPN
myopalladin
- RCM
restrictive cardiomyopathy
- SCD
sudden cardiac death
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
REFERENCES
- 1.Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Maskatia SA, Decker JA, Spinner JA, et al. Restrictive physiology is associated with poor outcomes in children with hypertrophic cardiomyopathy. Pediatr Cardiol. 2012;33:141–9. doi: 10.1007/s00246-011-0106-6. [DOI] [PubMed] [Google Scholar]
- 3.Webber SA, Lipshultz SE, Sleeper LA, et al. Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: a report from the Pediatric Cardiomyopathy Registry. Circulation. 2012;126:1237–44. doi: 10.1161/CIRCULATIONAHA.112.104638. [DOI] [PubMed] [Google Scholar]
- 4.Sen-Chowdhry S, Syrris P, McKenna WJ. Genetics of restrictive cardiomyopathy. Heart Fail Clin. 2010;6:179–86. doi: 10.1016/j.hfc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Bang ML, Mudry RE, McElhinny AS, et al. Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol. 2001;153:413–27. doi: 10.1083/jcb.153.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mikhailov AT, Torrado M. The enigmatic role of the ankyrin repeat domain 1 gene in heart development and disease. Int J Dev Biol. 2008;52:811–21. doi: 10.1387/ijdb.082655am. [DOI] [PubMed] [Google Scholar]
- 7.Ma K, Wang K. Interaction of nebulin SH3 domain with titin PEVK and myopalladin: implications for the signaling and assembly role of titin and nebulin. FEBS Lett. 2002;532:273–8. doi: 10.1016/s0014-5793(02)03655-4. [DOI] [PubMed] [Google Scholar]
- 8.Duboscq-Bidot L, Xu P, Charron P, et al. Mutations in the Z-band protein myopalladin gene and idiopathic dilated cardiomyopathy. Cardiovasc Res. 2008;77:118–25. doi: 10.1093/cvr/cvm015. [DOI] [PubMed] [Google Scholar]
- 9.Purevjav E, Arimura T, Augustin S, et al. Molecular basis for clinical heterogeneity in inherited cardiomyopathies due to myopalladin mutations. Hum Mol Genet. 2012;21:2039–53. doi: 10.1093/hmg/dds022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu P, Jenkins NA, Copeland NG. A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 2003;13:476–84. doi: 10.1101/gr.749203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maiellaro-Rafferty K, Wansapura JP, Mendsaikhan U, et al. Altered regional cardiac wall mechanics are associated with differential cardiomyocyte calcium handling due to nebulette mutations in preclinical inherited dilated cardiomyopathy. J Mol Cell Cardiol. 2013;60C:151–60. doi: 10.1016/j.yjmcc.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jean-Charles PY, Li YJ, Nan CL, Huang XP. Insights into restrictive cardiomyopathy from clinical and animal studies. J Geriatr Cardiol. 2011;8:168–83. doi: 10.3724/SP.J.1263.2011.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh MA, Grenier MA, Jefferies JL, Towbin JA, Lorts A, Czosek RJ. Conduction abnormalities in pediatric patients with restrictive cardiomyopathy. Circ Heart Fail. 2012;5:267–73. doi: 10.1161/CIRCHEARTFAILURE.111.964395. [DOI] [PubMed] [Google Scholar]
- 14.Gautel M, Goulding D, Bullard B, Weber K, Furst DO. The central Z-disk region of titin is assembled from a novel repeat in variable copy numbers. J Cell Sci. 1996;109:2747–54. doi: 10.1242/jcs.109.11.2747. [DOI] [PubMed] [Google Scholar]
- 15.Bowles NE, Bowles KR, Towbin JA. The “final common pathway” hypothesis and inherited cardiovascular disease. The role of cytoskeletal proteins in dilated cardiomyopathy. Herz. 2000;25:168–75. doi: 10.1007/s000590050003. [DOI] [PubMed] [Google Scholar]
- 16.Chen YW, Nader GA, Baar KR, Fedele MJ, Hoffman EP, Esser KA. Response of rat muscle to acute resistance exercise defined by transcriptional and translational profiling. J Physiol. 2002;545:27–41. doi: 10.1113/jphysiol.2002.021220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed JS, Boriek AM. Loss of desmin triggers mechanosensitivity and up-regulation of Ankrd1 expression through Akt-NF-kappaB signaling pathway in smooth muscle cells. Faseb J. 2012;26:757–65. doi: 10.1096/fj.10-160291. [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Xu J, Li Y, et al. Cardiac ankyrin repeat protein attenuates cardiac hypertrophy by inhibition of ERK1/2 and TGF-beta signaling pathways. PLoS One. 2012;7:e50436. doi: 10.1371/journal.pone.0050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee MJ, Kwak YK, You KR, Lee BH, Kim DG. Involvement of GADD153 and cardiac ankyrin repeat protein in cardiac ischemia-reperfusion injury. Mol Med. 2009;41:243–52. doi: 10.3858/emm.2009.41.4.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zolk O, Marx M, Jackel E, El-Armouche A, Eschenhagen T. Beta-adrenergic stimulation induces cardiac ankyrin repeat protein expression: involvement of protein kinase A and calmodulin-dependent kinase. Cardiovasc Res. 2003;59:563–72. doi: 10.1016/s0008-6363(03)00476-0. [DOI] [PubMed] [Google Scholar]
- 21.Chen B, Zhong L, Roush SF, et al. Disruption of a GATA4/Ankrd1 signaling axis in cardiomyocytes leads to sarcomere disarray: implications for anthracycline cardiomyopathy. PLoS One. 2012;7:e35743. doi: 10.1371/journal.pone.0035743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanai H, Tanaka T, Aihara Y, et al. Transforming growth factor-beta/Smads signaling induces transcription of the cell type-restricted ankyrin repeat protein CARP gene through CAGA motif in vascular smooth muscle cells. Circ Res. 2001;88:30–6. doi: 10.1161/01.res.88.1.30. [DOI] [PubMed] [Google Scholar]
- 23.Barash IA, Bang ML, Mathew L, Greaser ML, Chen J, Lieber RL. Structural and regulatory roles of muscle ankyrin repeat protein family in skeletal muscle. Am J Physiol Cell Physiol. 2007;293:C218–27. doi: 10.1152/ajpcell.00055.2007. [DOI] [PubMed] [Google Scholar]
- 24.Bang ML, Gu Y, Dalton ND, Peterson KL, Chien KR, Chen J. The Muscle Ankyrin Repeat Proteins CARP, Ankrd2, and DARP Are Not Essential for Normal Cardiac Development and Function at Basal Conditions and in Response to Pressure Overload. PLoS One. 2014;9:e93638. doi: 10.1371/journal.pone.0093638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronty MJ, Leivonen SK, Hinz B, et al. Isoform-specific regulation of the actin-organizing protein palladin during TGF-beta1-induced myofibroblast differentiation. J Invest Dermatol. 2006;126:2387–96. doi: 10.1038/sj.jid.5700427. [DOI] [PubMed] [Google Scholar]
- 26.Roberts MD, Childs TE, Brown JD, Davis JW, Booth FW. Early depression of Ankrd2 and Csrp3 mRNAs in the polyribosomal and whole tissue fractions in skeletal muscle with decreased voluntary running. J Appl Physiol. 2012;112:1291–9. doi: 10.1152/japplphysiol.01419.2011. [DOI] [PubMed] [Google Scholar]
- 27.Wilhelmi MH, Leyh RG, Wilhelmi M, Haverich A. Upregulation of endothelial adhesion molecules in hearts with congestive and ischemic cardiomyopathy: immunohistochemical evaluation of inflammatory endothelial cell activation. Eur J Cardiothorac Surg. 2005;27:122–7. doi: 10.1016/j.ejcts.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Witt SH, Labeit D, Granzier H, Labeit S, Witt CC. Dimerization of the cardiac ankyrin protein CARP: implications for MARP titin-based signaling. J Muscle Res Cell Motil. 2005;26:401–8. doi: 10.1007/s10974-005-9022-9. [DOI] [PubMed] [Google Scholar]
- 29.Denfield SW, Webber SA. Restrictive cardiomyopathy in childhood. Heart Fail Clin. 2010;6:445–52. viii. doi: 10.1016/j.hfc.2010.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.