Abstract
OBJECTIVE:
To determine whether provision of vaccine-health-literacy-promoting information in text message vaccine reminders improves receipt and timeliness of the second dose of influenza vaccine within a season for children in need of 2 doses.
METHODS:
During the 2012–2013 season, families of eligible 6-month through 8-year-old children were recruited at the time of their first influenza vaccination from 3 community clinics in New York City. Children (n = 660) were randomly assigned to “educational” text message, “conventional” text message, and “written reminder-only” arms. At enrollment, all arms received a written reminder with next dose due date. Conventional messages included second dose due date and clinic walk-in hours. Educational messages added information regarding the need for a timely second dose. Receipt of second dose by April 30 was assessed by using χ2 tests. Timeliness was assessed by receipt of second dose by 2 weeks after due date (day 42) using χ2 and over time using a Kaplan-Meier analysis.
RESULTS:
Most families were Latino and publicly insured with no significant between-arm differences between groups. Children in the educational arm were more likely to receive a second dose by April 30 (72.7%) versus conventional (66.7%) versus written reminder-only arm (57.1%; P = .003). They also had more timely receipt by day 42 (P < .001) and over time (P < .001).
CONCLUSIONS:
In this low-income, urban, minority population, embedding health literacy information improved the effectiveness of text message reminders in promoting timely delivery of a second dose of influenza vaccine, compared with conventional text messages and written reminder only.
Keywords: immunizations, influenza, reminder-recall, text message, mobile health
What’s Known on This Subject:
Influenza vaccine coverage is low, and young children in need of 2 doses in a given season are at particular risk, with less than half receiving both doses. Text message vaccine reminders increase receipt of first dose of influenza vaccine.
What This Study Adds:
Little is known about what types of text message reminders are most effective, including embedding educational information. We demonstrate that text message reminders increase timely receipt of the second dose of influenza vaccine and embedding health literacy information improves effectiveness.
Influenza is a significant and largely preventable source of morbidity and mortality; however, vaccine coverage remains low.1–5 Nationally, only 56.6% of children 6 months to 17 years receive at least 1 needed dose in a given influenza season.6 Young children <9 years old are at particular risk for undervaccination because, depending on previous influenza vaccination status, they may need to receive 2 doses in a given season.7,8 Nonetheless, even among those who initiate vaccination, only 40% to 60% receive a second needed dose.9–11 Timeliness of vaccination is also key, as a child in need of 2 doses is not fully protected until 2 weeks after receipt of the second dose.8 Even in children who ultimately receive 2 doses in a season, the time interval between doses is often beyond the recommended 28 days.9 This leaves many unprotected when the virus begins circulating.12
Although reminder-recall systems that notify families that their child needs a vaccine are widely recommended,13,14 traditional strategies implemented via mail or telephone have had limited to no efficacy in urban, low-income, and minority populations15–17 who are also at high risk for undervaccination.18,19 We recently demonstrated the effectiveness of using text message reminders for first influenza vaccine doses in this population.20 However, the impact of such reminders on return for a second needed dose is unknown. Receipt of this dose is an important and likely fruitful target because families have already “accepted” vaccination.
Furthermore, it is unknown which type of text messaging is most effective. Two potential factors associated with failure to receive this second dose are failure to remember to return for the dose21 and limited health literacy regarding influenza vaccination. The latter includes lack of knowledge and understanding about influenza and influenza vaccine and confusion regarding need for multiple doses and timing of doses.22–25 Conventional text message reminders can notify a family that a vaccine is due and are helpful for those who solely forget to return. However, they presuppose that a family or patient already has the requisite knowledge, attitudes, and motivation to act on them. The inclusion of health literacy-promoting information in a text message second dose reminder could potentially increase its efficacy by providing specific information that may compel a family to act overall and in a more timely fashion. It is important to understand, as text message reminders become more widely used, whether adding this type of information is beneficial; this has not been directly assessed.
Therefore, the objective of this study was to compare the effectiveness of text message vaccine reminders with and without vaccine education versus written reminder-only on receipt of second dose of influenza vaccine in young, low-income children.
Methods
This randomized controlled trial was conducted during the 2012–2013 influenza season in 3 community-based pediatric clinics affiliated with New York-Presbyterian Hospital/ Columbia University Medical Center in Northern Manhattan in New York City (NYC). The study sites are part of an ambulatory-care network staffed by 1 centrally administered pediatric group practice. These sites serve a primarily Latino population, nearly all of whom speak English or Spanish. Most (95%) are eligible for free vaccines through the Vaccines-for-Children program. The sites do not routinely conduct influenza vaccine reminder-recalls, but have vaccination walk-in hours. The study was approved by the Medical Center’s Institutional Review Board.
Families were recruited between August 29, 2012, and March 31, 2013. Children were eligible for inclusion if (1) they were 6 months through 8 years old at vaccination; (2) received their first influenza dose of the season at a study site; (3) were in need of 2 doses that season by using the first option described below, as this was standard policy at the sites; and (4) had a cellular phone with text message capabilities. During the 2012–2013 season, nationally, there were 2 acceptable options for determining who needed a second dose of influenza vaccine.7 The first included any child who had not received 2 doses of vaccine since July 2010 (the first season the 2009 H1N1-strain was included in the seasonal vaccine). The second included any child who had not received 2 previous seasonal influenza vaccinations plus at least 1 2009 H1N1-containing vaccination, either as a seasonal or monovalent pandemic vaccine. All eligible children seen when research assistants were on-site were approached.
At recruitment, families signed a consent form and texted an enrollment message into the text message platform, which automatically sent a confirmation message. All families received a written reminder with the date the next influenza vaccine dose was due. They also were verbally administered a demographic and attitudes survey and took the Short Test of Functional Health Literacy in Adults (S-TOFHLA).26 Parents received $10 compensation.
Subjects were randomly assigned centrally with a 1:1:1 allocation at an individual level by using a permuted block design with a block size of 9, stratified by age and clinic site. Arms included usual care (written reminder-only), conventional text messages (plus written reminder), and educational text messages (plus written reminder). The study analyst was blinded to individual group assignment. With the analyzed sample size of 660 and equal allocation, we had 80% power to detect a 13% difference between groups, allowing for 5% type I error.
Intervention
Parents of children randomly assigned to receive usual care did not receive any further intervention beyond the written reminder. Those randomly assigned to receive text message reminders additionally received a reminder on 3 dates before the dose was due (day 7, day 21, and day 25 after first influenza vaccine dose), on the day it was due (day 28), and 2 weeks after it was due (day 42). Five messages were selected based on our previous studies.20 Messages were sent in English or Spanish based on the participant’s request at enrollment. Parents of children randomly assigned to the conventional text message group received messages with the date after which the next dose was due and clinic specific walk-in hours (Supplemental Table 4). Those randomly assigned to educational text messages additionally received educational information that included that the child was not protected until he or she received the second dose, that reaching full protection can take 2 weeks after second dose administration, and that doctors recommend a second dose. In addition, in 1 interactive message, parents could select to receive more information via text message (Supplemental Table 4). The text messages had a third grade Flesch-Kincaid readability statistic.
The messages were sent by using a customized software platform that is integrated with the hospital’s immunization information system (IIS). This system automatically collects vaccine administrations from the sites’ common electronic health record (EHR) as well as from the New York Citywide Immunization Registry (CIR), allowing inclusion of vaccines administered to clinic patients at outside practices in NYC. NYC Public Health Law requires documentation for all vaccinations administered to <19 year-olds be submitted to the CIR,27 and ∼94% of facilities that vaccinate children report regularly.28 Messages were discontinued when a second influenza dose was received.
The primary end point was receipt of a second influenza vaccine dose by the end of the influenza season, April 30, 2013. We also secondarily assessed timely vaccination as indicated by receipt of second vaccine dose by day 42, and by the proportion of children receiving a second dose over time since their first dose. Day 42 was selected as it was 2 weeks after the due date as well as the final message date. Children were given until day 42 to be vaccinated even if that date was past April 30. Doses given before day 24 post vaccination were excluded from timeliness analyses based on the 28-day recommended interval minus the Advisory Committee on Immunization Practices-approved 4 day window.29 Vaccination information was retrieved from the hospital IIS, and included vaccines from the CIR.
Postintervention, we conducted a telephone satisfaction survey by using both closed and open-ended questions.
Statistical Analyses
All analyses used the individual child as the unit of analysis. Differences in proportions of end points between randomized groups were calculated by using χ2 tests, with confidence limits on the differences and relative rates (RRs) reported. The number needed to text was calculated based on the absolute risk reduction of remaining unvaccinated by April 30 in the educational versus the written reminder-only groups. A secondary analysis was performed stratifying by receipt of dose 1 before versus on or after November 15.9,30 Kaplan-Meier analyses were used to compare the cumulative proportions of children in each arm who received the second dose over time. Individual children were censored at April 30 or when they received the second dose. Sensitivity analyses were performed to assess the impact of the intervention in those children in need of 2 doses regardless of which of the 2 options for determining need for a second dose was employed. Analyses were conducted with SPSS 20.0 (IBM SPSS Statistics, IBM Corporation).
Results
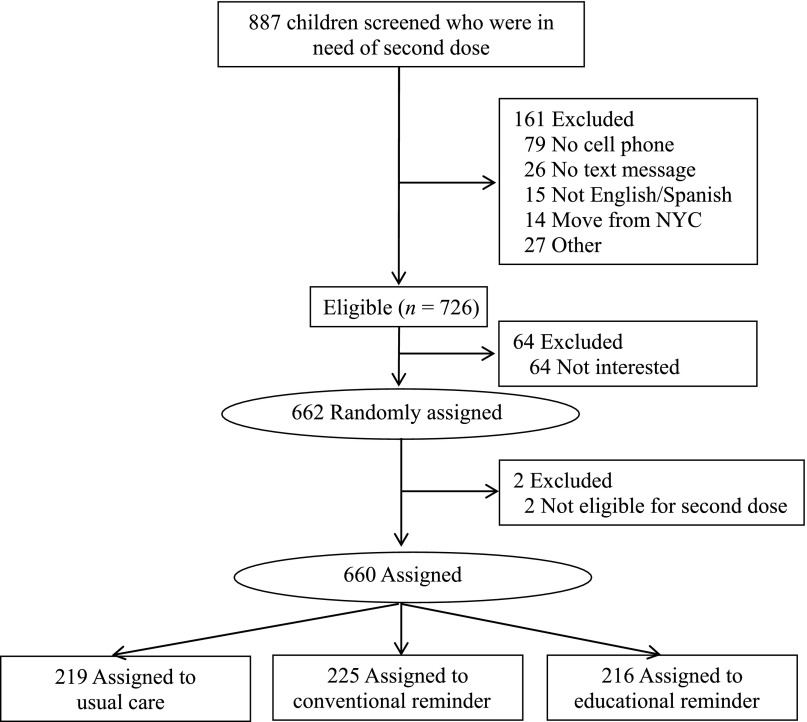
A total of 726 families were eligible among 887 screened who were in need of a second dose of influenza vaccine, and 662 were randomly assigned; 2 participants were found to be ineligible to receive a second dose immediately after randomization but before messages were sent; they were removed (Fig 1). Most families were Latino and publicly insured; 51.7% preferred Spanish (Table 1). Most (85.9%) had adequate health literacy. Nearly all (88.3%) had unlimited texting plans and 92.3% texted at least weekly. A small number (12.3%) had previously received a health care-related text message. There were no significant baseline differences (Table 1). The majority of parents thought the influenza vaccine was safe (92.0%) and/or effective (92.3%). Nearly three-quarters (71.9%) thought that their child was at least somewhat protected from influenza after 1 dose. There were no differences in perceptions of safety, effectiveness overall, or protection of 1 dose by group.
FIGURE 1.
Study flow diagram.
TABLE 1.
Characteristics of Study Population
| Total | Written Reminder, n = 219 | Conventional Text Message, n = 225 | Educational Text Message, n = 216 | P | |
|---|---|---|---|---|---|
| Age | .91 | ||||
| 6–23 mo | 484 (73.3) | 161 (73.5) | 163 (72.4) | 160 (74.1) | |
| 24–59 mo | 66 (10.0) | 21 (9.6) | 26 (11.6) | 19 (8.8) | |
| 5–8 y | 110 (16.7) | 37 (16.9) | 36 (16.0) | 37 (17.1) | |
| Gender | .66 | ||||
| Girl | 327 (49.5) | 114 (52.1) | 108 (48.0) | 105 (48.6) | |
| Boy | 333 (50.5) | 105 (47.9) | 117 (52.0) | 111 (51.4) | |
| Race/ethnicity | .57 | ||||
| Latino | 586 (88.8) | 199 (90.9) | 201 (89.3) | 186 (86.1) | |
| African American | 58 (8.8) | 14 (6.4) | 19 (8.4) | 25 (11.6) | |
| White | 2 (0.3) | 1 (0.5) | 0 (0) | 1 (0.5) | |
| Other | 14 (2.1) | 5 (2.3) | 5 (2.2) | 4 (1.9) | |
| Language most comfortable speaking with health care provider | .47 | ||||
| Spanish | 341 (51.7) | 121 (55.3) | 112 (49.8) | 108 (50.0) | |
| English | 318 (48.2) | 98 (44.7) | 112 (49.8) | 108 (50.0) | |
| Other | 1 (0.2) | 0 (0) | 1 (0.4) | 0 (0) | |
| Insurance | .15 | ||||
| Medicaid/State Children’s Health Insurance Program | 638 (96.7) | 208 (95.0) | 219 (97.3) | 211 (97.7) | |
| Commercial | 14 (2.1) | 7 (3.2) | 2 (0.9) | 5 (2.3) | |
| Uninsured | 8 (1.2) | 4 (1.8) | 4 (1.8) | 0 (0) | |
| Education | .56 | ||||
| < High school | 110 (16.7) | 33 (15.1) | 38 (16.9) | 39 (18.1) | |
| High school | 230 (34.8) | 83 (37.9) | 70 (31.1) | 77 (35.6) | |
| At least some college | 320 (48.5) | 103 (47.0) | 117 (52.0) | 100 (46.3) | |
| Text message plan type | .65 | ||||
| Unlimited plan | 583 (88.3) | 190 (86.8) | 200 (88.9) | 193 (89.4) | |
| Limited plan | 74 (11.2) | 27 (12.3) | 24 (10.7) | 23 (10.6) | |
| Did not know | 3 (0.5) | 2 (0.9) | 1 (0.4) | 0 (0) | |
| Text message frequency | .18 | ||||
| At least weekly | 609 (92.3) | 207 (94.5) | 201 (89.3) | 201 (93.1) | |
| Less often than weekly | 39 (5.9) | 11 (5.0) | 17 (7.6) | 11 (5.1) | |
| Never | 12 (1.8) | 1 (0.5) | 7 (3.1) | 4 (1.9) | |
| S-TOFHLAa | .99 | ||||
| Adequate | 434 (85.9) | 140 (86.4) | 149 (85.6) | 145 (85.8) | |
| Marginal | 27 (5.3) | 8 (4.9) | 10 (5.7) | 9 (5.3) | |
| Inadequate | 44 (8.7) | 14 (8.6) | 15 (8.6) | 15 (8.9) |
Data are presented as n (%).
Short Test of Functional Health Literacy in Adults (S-TOFHLA) not available on all participants because of family’s time availability at enrollment.
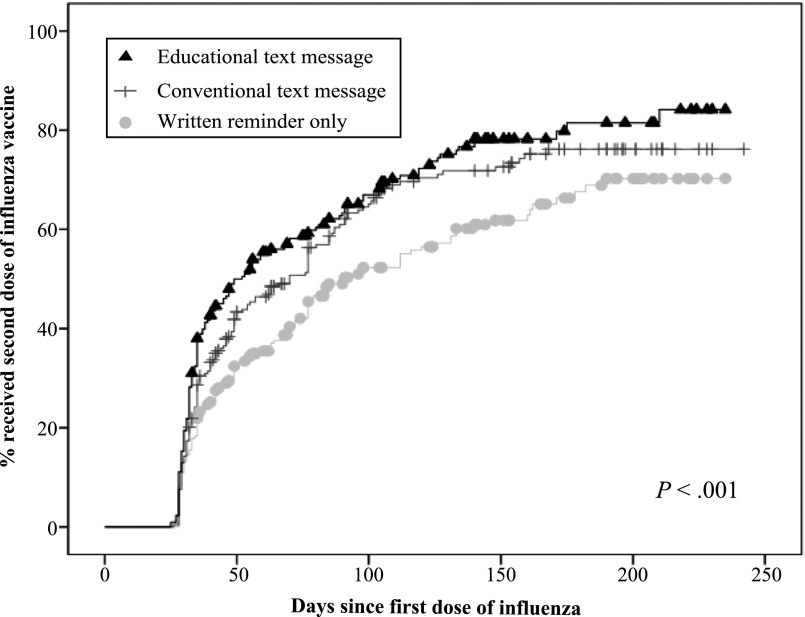
Children in the educational text message reminder group were significantly more likely to receive a second dose of influenza vaccine by April 30 (72.7%) than both those in the conventional text message reminder group (66.7%) and written reminder-only group (57.1%; P = .003; Table 2). Differences accrued early and were sustained (P < .001; Fig 2). The number needed to text an educational reminder for 1 additional child to receive a needed second dose by April 30 compared with written reminder-only was 6.4. Children in the educational reminder group were also more likely to receive their second dose within 2 weeks of its due date (day 42 post vaccination; educational reminder group 43.5% versus conventional reminder 33.8% versus written reminder-only 25.6%; P < .001; Table 2). Overall, the largest differences observed between children in the educational reminder versus conventional text message groups were for children receiving their first dose before November 15. Conventional reminders had a greater effect compared with written reminder-only for the children who were first vaccinated on or after November 15 (Table 3). Tests for interaction between clinic site and intervention group, and age and intervention group were nonsignificant both for receipt by April 30 and by day 42.
TABLE 2.
Receipt of Second Dose Influenza Vaccine by April 30, 2013, and by Day 42 Post Vaccination
| % Receipt Second Dose | Difference Versus Written Text Message Reminder | Difference Versus Conventional Text Message Reminder | ||||
|---|---|---|---|---|---|---|
| Absolute Difference, % (95% CI) | RR (95% CI) | Absolute Difference, % (95% CI) | RR (95% CI) | |||
| Receipt by April 30 | ||||||
| Primary analytic sample | ||||||
| Educational text message (n = 216) | 72.7% (157) | P = .003 | 15.6% (15.1–16.1) | 1.27 (1.11–1.47) | 6.0% (5.6–6.5) | 1.09 (0.96–1.23) |
| Conventional text message (n = 225) | 66.7% (150) | 9.6% (9.1–10.0) | 1.17 (1.01–1.35) | — | — | |
| Written reminder only (n = 219) | 57.1% (125) | — | — | — | — | |
| Receipt by day 42 post vaccination | ||||||
| Primary analytic sample | ||||||
| Educational text message (n = 216) | 43.5% (94) | P < .001 | 17.9% (17.5–18.4) | 1.70 (1.30–2.24) | 9.6% (9.1–10.0) | 1.28 (1.01–1.63) |
| Conventional text message (n = 224) | 33.9% (76) | 8.4% (7.9–8.8) | 1.33 (0.99–1.77) | — | — | |
| Written reminder only (n = 219) | 25.6% (56) | — | — | — | — | |
Receipt by day 42 does not include participant who received the second dose too early and was not revaccinated. CI, 95% confidence interval; RR, relative rate.
FIGURE 2.
Kaplan–Meier analyses of time from receipt of first influenza vaccine dose to receipt of second influenza vaccine dose. The x-axis represents time in days since receipt of the first influenza vaccine dose until receipt of second influenza vaccine dose. The y-axis represents the cumulative proportion of children vaccinated. End date is April 30, 2013.
TABLE 3.
Impact of Intervention Based on Enrollment Date: Receipt of Second Dose Influenza Vaccine by April 30, 2013, and by Day 42 Post Vaccination
| % Receipt Second Dose | Difference Versus Written Text Message Reminder | Difference Versus Conventional Text Message Reminder | ||||
|---|---|---|---|---|---|---|
| Absolute Difference, % (95% CI) | RR (95% CI) | Absolute Difference, % (95% CI) | RR (95% CI) | |||
| Receipt by April 30 | ||||||
| Receipt of first dose before November 15 | ||||||
| Educational text message (n = 111) | 89.2% (99) | P = .034 | 12.6% (11.7–13.5) | 1.17 (1.03–1.32) | 10.6% (9.7–11.4) | 1.13 (1.01–1.27) |
| Conventional text message (n = 117) | 78.6% (92) | 2.1% (1.2–2.9) | 1.03 (0.89–1.18) | — | — | |
| Written reminder only (n = 111) | 76.6% (85) | — | — | — | — | |
| Receipt of first dose after or on November 15 | ||||||
| Educational text message (n = 105) | 55.2% (58) | P = .013 | 18.2% (17.3–19.1) | 1.49 (1.11–2.01) | 1.5% (0.6%–2.5%) | 1.03 (0.81–1.32) |
| Conventional text message (n = 108) | 53.7% (58) | 16.7% (15.7–17.6) | 1.45 (1.07–1.96) | — | — | |
| Written reminder only (n = 108) | 37.0% (40) | — | — | — | — | |
| Receipt by day 42 post vaccination | ||||||
| Receipt of first dose before November 15 | ||||||
| Educational text message (n = 111) | 49.5% (55) | P = .004 | 20.7% (19.8–21.6) | 1.72 (1.21–2.43) | 15.1% (14.2–15.9) | 1.44 (1.05–1.97) |
| Conventional text message (n = 116) | 34.5% (40) | 5.7% (4.8–6.5) | 1.20 (0.81–1.76) | — | — | |
| Written reminder only (n = 111) | 28.8% (32) | — | — | — | — | |
| Receipt of first dose after or on November 15 | ||||||
| Educational text message (n = 105) | 37.1% (39) | P = .049 | 14.9% (14.0–15.9) | 1.67 (1.09–2.57) | 3.8% (2.9–4.7) | 1.11 (0.77–1.61) |
| Conventional text message (n = 108) | 33.3% (36) | 11.1% (10.2–12.0) | 1.50 (0.96–2.33) | — | — | |
| Written reminder only (n = 108) | 22.2% (24) | — | — | — | — | |
Receipt by day 42 does not include participant who received the second dose too early and was not revaccinated. CI, 95% confidence interval; RR, relative rate.
There was no difference among groups for the proportion of children (n = 81) who no longer needed an additional dose if the second 2-dose eligibility option was employed (educational reminder group [13.4%] versus conventional reminder [13.3%] versus written reminder-only [10.0%]; P = .47). In sensitivity analyses, we removed these children as well as 4 others who were found to not need a second dose after messages were sent. Findings were similar with slightly larger effect sizes overall with the exception of vaccination by April 30 for conventional versus written reminder-only (relative rate, 1.14 [95% confidence interval: 0.99–1.31]).
Only 2 participants asked to stop further messages (0.3%). No messages were undeliverable. Thirty-four (15.7%) of those in the educational reminder group responded to the interactive message; many requested information on more than 1 topic. The most common request was for information regarding why timing of the second dose is important (n = 22), followed by why 2 doses are needed (n = 18), and adverse reactions (n = 14).
We surveyed 587 (88.9%) participants, including 89.4% of parents in the educational, 88.4% in the conventional, and 89.0% in the written reminder-only groups. Of those in either text message group, 91.1% remembered receiving messages. Of those, nearly all (98.0%) were very satisfied or satisfied with the messages; the remaining was a little satisfied. Similarly nearly all (96.0%) would be very likely or likely to recommend them to another parent, and 3.5% would be a little likely. There were no differences between messaging groups in terms of satisfaction and recommendation. Most (87.9%) felt the number of messages sent was the right amount, 4.6% thought it was too many, 6.9% too few, and 0.6% did not have an opinion. Most (87.8%) liked having an interactive option. The most common reasons that parents reported liking the text messages were that they acted as a reminder, provided information, were quick and did not require talking with anyone, and demonstrated someone “cared.” Nearly two-thirds (60.8%) of parents reported the reminder was either the main reason or part of the reason they brought their child for a second dose, and 70.1% that it affected bringing their child sooner. There were no differences between text message groups in terms of attitudes toward message number, timing, or perceived message impact.
Discussion
This randomized controlled trial provides information important for establishing best practices for influenza vaccine text message reminders. In the text messaging groups, receipt of the second dose ranged from 66.7% to 72.7% depending on which message type was used. This was higher than observed in both the written-reminder only group (57.1%) as well as previous studies in this community where the average second-dose rates over 5 seasons ranged from 31.6% to 47.5%.9 It is also higher than national studies, which indicate second-dose rates in 2012 in 8 IIS sites to be ∼60%.31 Parents also had high levels of satisfaction, would recommend the text messages to other parents, and perceived them to be helpful, potentially illustrating their general acceptance.
Text messages including educational information had a greater effect than conventional reminders, with the highest effects compared with conventional reminders observed in those first vaccinated earlier in the season. We previously demonstrated that families may be unaware that a child is in need of 2 doses or when to return.22 Families may also not understand that timely vaccination is important.8,12 According to the transtheoretical model of behavior change, an individual needs to move from lack of awareness to contemplation to adoption of a behavior.32 Applying this model, a parent must first know that their child needs a second dose. They then face the decision whether to have their child receive that dose. It may be that educational information is needed for some families to move along this continuum of decision-making. Embedding such information into reminders may be an important addition to influenza vaccine reminders that should be considered. Additionally, having an interactive component may engage some families. In this and previous studies, the majority of parents were interested in interactive messages, although in this study most did not request more information.33
Text message reminders may be particularly helpful for vaccines that require multiple doses in which families need to return in a timely fashion. For subsequent doses after initiation, reminders may need to be sent close to the due-date such that families present on or shortly after that date, but not too early. Due to their relatively inexpensive pricing and real-time delivery, multiple text messages can be sent in the amount of time it may take for 1 letter reminder to be delivered. They also allow precise timing of messages that cannot be controlled when using mailed reminders, and the timing can be tailored to an individual child’s due date. Text messages may work best as a cue to action so that pairing them with walk-ins for vaccination or same-day appointments may be helpful.
One of the strengths of text messaging is the scalability. Therefore, linking reminders with readily available vaccine data either from an EHR or an IIS may best capitalize on this strength. Most (78%) of office-based physicians use an EHR, and that number is growing.34 Additionally, there are currently IIS in all 50 states plus 5 additional cities, covering over 19 million children 0 to 6 years old nationally,35 including the majority of children in need of 2 doses. Studies have employed IIS to send traditional forms of influenza reminders.36 Linking IIS records to second dose text message reminders could be an important next step. In a recent survey, we found most parents owned a text message-enabled cell phone (89%) and used text messaging services (97%).33 Although 84% had never received health-related text messages, 88% were comfortable receiving them. In a national survey, 56% of parents surveyed were willing to register their cell number with their child’s usual immunization provider and 18% were undecided.37
There are limitations to this study. Vaccination records could be incomplete, although our EHR captures all vaccination events and transfers them automatically to our hospital IIS. Furthermore, any incomplete data should affect all arms similarly. Additionally, if a child received a vaccine elsewhere in NYC, the data would be captured through the synchronization of the hospital’s and city’s IIS. However, 4 participants were noted to not need a second dose of influenza vaccine after the intervention start; 3 of these had received influenza vaccines outside our medical system that caused the discrepancy with eligibility. Secondly, although we included the due dates of vaccination in the messages, one unintended consequence could be receipt of a second dose too early; however, we found that only 3 participants were vaccinated too early (2 in the written reminder group, 1 in the conventional text message group; 2 of the 3 were vaccinated outside of our medical system). Although we did not have any undeliverable messages, on the postsurvey some families did not remember receiving the text messages. We do not know if they in fact did not receive them or forgot. Finally, this study took place in one network of clinics affiliated with an academic medical center that serves a primarily minority and publicly insured population.
Conclusions
In this low-income, urban, minority population, embedding health literacy information improved the effectiveness of text message reminders in promoting timely delivery of a second dose of influenza vaccine, compared with conventional text messages and written reminder only.
Supplementary Material
Acknowledgments
We thank Zuleika Parra-Valencia and Ameriangel Roman for their help in this study. We thank the EzVac team and New York-Presbyterian Hospital for its support of the EzVac Immunization Information System, and New York-Presbyterian Hospital Ambulatory Care Network.
Footnotes
Dr Stockwell conceptualized and designed the study, analyzed the data, and drafted the initial manuscript; Dr Hofstetter contributed to the conceptualization and design of the study, and reviewed and revised the manuscript; Ms DuRivage, Ms Barrett, and Drs Fernandez and Vargas aided in data collection and database management and critically reviewed the manuscript; Mr Camargo designed the text-messaging platform, aided in data collection, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the funding agency. The funding agency had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
This trial has been registered at www.clinicaltrials.gov (identifier: NCT01662583).
FINANCIAL DISCLOSURE: Dr Hofstetter receives support from the Pfizer Medical Education Group for a different investigator-initiated study; Dr Stockwell is a co-investigator but receives no financial support; Dr Hofstetter also received support through the Investigator-Initiated Studies Program of Merck, Sharp & Dohme Corp for a different study; the other authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was supported by an institutional career development grant (National Institutes of Health/National Cancer Institute grant number KM1 CA156709). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Moore DL, Vaudry W, Scheifele DW, et al. Surveillance for influenza admissions among children hospitalized in Canadian immunization monitoring program active centers, 2003-2004. Pediatrics. 2006;118(3). Available at: www.pediatrics.org/cgi/content/full/118/3/e610 [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292(11):1333–1340 [DOI] [PubMed] [Google Scholar]
- 3.Louie JK, Schechter R, Honarmand S, et al. Severe pediatric influenza in California, 2003-2005: implications for immunization recommendations. Pediatrics. 2006;117(4). Available at: www.pediatrics.org/cgi/content/full/117/4/e610 [DOI] [PubMed] [Google Scholar]
- 4.Neuzil KM, Zhu Y, Griffin MR, et al. Burden of interpandemic influenza in children younger than 5 years: a 25-year prospective study. J Infect Dis. 2002;185(2):147–152 [DOI] [PubMed] [Google Scholar]
- 5.Fiore AE, Uyeki TM, Broder K, et al. Centers for Disease Control and Prevention (CDC) . Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62 [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Seasonal Influenza (Flu) FluVaxView Influenza Vaccination Coverage 2012–2013 Flu Season. Available at: www.cdc.gov/flu/fluvaxview/1213season.htm. Accessed July 17, 2014
- 7.Centers for Disease Control and Prevention (CDC) . Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP)—United States, 2012-13 influenza season. MMWR Morb Mortal Wkly Rep. 2012;61(32):613–618 [PubMed] [Google Scholar]
- 8.Ritzwoller DP, Bridges CB, Shetterly S, Yamasaki K, Kolczak M, France EK. Effectiveness of the 2003-2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics. 2005;116(1):153–159 [DOI] [PubMed] [Google Scholar]
- 9.Hofstetter AM, Natarajan K, Martinez RA, Rabinowitz D, Vawdrey DK, Stockwell MS. Influenza vaccination coverage and timeliness among children requiring two doses, 2004–2009. Prev Med. 2013;56(3-4):165–170 [DOI] [PubMed] [Google Scholar]
- 10.Pabst LJ, Chaves SS, Weinbaum C. Trends in compliance with two-dose influenza vaccine recommendations among children aged 6 months through 8 years. Vaccine. 2013;31(31):3116–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt P, Block SL, Toback SL, Ambrose CS. A prospective observational study of US in-office pediatric influenza vaccination during the 2007 to 2009 influenza seasons: use and factors associated with increased vaccination rates. Clin Pediatr (Phila). 2010;49(10):954–963 [DOI] [PubMed] [Google Scholar]
- 12.Hofstetter AM, Natarajan K, Rabinowitz D, et al. Timeliness of pediatric influenza vaccination compared with seasonal influenza activity in an urban community, 2004–2008. Am J Public Health. 2013;103(7):e50–e58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briss PA, Rodewald LE, Hinman AR, et al. The Task Force on Community Preventive Services . Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. Am J Prev Med. 2000;18(suppl 1):97–140 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) . Recommendations of the Advisory Committee on Immunization Practices, the American Academy of Pediatrics, and the American Academy of Family Physicians: use of reminder and recall by vaccination providers to increase vaccination rates. MMWR Morb Mortal Wkly Rep. 1998;47(34):715–717 [PubMed] [Google Scholar]
- 15.Irigoyen MM, Findley S, Wang D, et al. Challenges and successes of immunization registry reminders at inner-city practices. Ambul Pediatr. 2006;6(2):100–104 [DOI] [PubMed] [Google Scholar]
- 16.Hambidge SJ, Davidson AJ, Phibbs SL, et al. Strategies to improve immunization rates and well-child care in a disadvantaged population: a cluster randomized controlled trial. Arch Pediatr Adolesc Med. 2004;158(2):162–169 [DOI] [PubMed] [Google Scholar]
- 17.Daley MF, Steiner JF, Brayden RM, Xu S, Morrison S, Kempe A. Immunization registry-based recall for a new vaccine. Ambul Pediatr. 2002;2(6):438–443 [DOI] [PubMed] [Google Scholar]
- 18.Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J. 2011;30(2):100–106 [DOI] [PubMed] [Google Scholar]
- 19.Findley SE, Irigoyen M, Stockwell MS, Chen S. Changes in childhood immunization disparities between central cities and their respective states, 2000 versus 2006. J Urban Health. 2009;86(2):183–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA. 2012;307(16):1702–1708 [DOI] [PubMed] [Google Scholar]
- 21.Stockwell MS, Irigoyen M, Andres Martinez R, Findley SE. Failure to return: parental, practice, and social factors affecting missed immunization visits for urban children. Clin Pediatr (Phila). 2014;53(5):420–427 [DOI] [PubMed] [Google Scholar]
- 22.Hofstetter AM, Barrett A, Stockwell MS. Factors Impacting Influenza Vaccination of Urban Low-Income Latino Children Under Nine Years Requiring Two Doses in the 2010-2011 Season. J Community Health. 2014; (Aug):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125(4):654–659 [DOI] [PubMed] [Google Scholar]
- 24.Nowalk MP, Zimmerman RK, Lin CJ, et al. Parental perspectives on influenza immunization of children aged 6 to 23 months. Am J Prev Med. 2005;29(3):210–214 [DOI] [PubMed] [Google Scholar]
- 25.Grant VJ, Le Saux N, Plint AC, et al. Factors influencing childhood influenza immunization. CMAJ. 2003;168(1):39–41 [PMC free article] [PubMed] [Google Scholar]
- 26.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38(1):33–42 [DOI] [PubMed] [Google Scholar]
- 27.Statewide Immunization Registry, Pub Health Law, Article 21, Title 6, §2168.
- 28.New York City Department of Health and Mental Hygiene, Immunization Information System Annual Report 2013. Submitted to Program Operations Branch, Immunization Services Division, Centers for Disease Control and Prevention, March 31, 2014 [Google Scholar]
- 29.National Center for Immunization and Respiratory Diseases . General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60(2):1–64 [PubMed] [Google Scholar]
- 30.Jackson LA, Neuzil KM, Baggs J, et al. Compliance with the recommendations for 2 doses of trivalent inactivated influenza vaccine in children less than 9 years of age receiving influenza vaccine for the first time: a Vaccine Safety Datalink study. Pediatrics. 2006;118(5):2032–2037 [DOI] [PubMed] [Google Scholar]
- 31.Lu PJ, Santibanez TA, Williams WW, et al. Centers for Disease Control and Prevention (CDC) . Surveillance of influenza vaccination coverage—United States, 2007-08 through 2011-12 influenza seasons. MMWR Surveill Summ. 2013;62(4):1–28 [PubMed] [Google Scholar]
- 32.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12(1):38–48 [DOI] [PubMed] [Google Scholar]
- 33.Hofstetter AM, Vargas CY, Kennedy A, Kitayama K, Stockwell MS. Parental and provider preferences and concerns regarding text message reminder/recall for early childhood vaccinations. Prev Med. 2013;57(2):75–80 [DOI] [PubMed] [Google Scholar]
- 34.Hsiao C-J, Hing E. Use and Characteristics of Electronic Health Record Systems Among Office-Based Physician Practices: United States, 2001–2013. NCHS data brief, no 143. Hyattsville, MD: National Center for Health Statistics; 2014 [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) . Progress in immunization information systems—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(3):48–51 [PMC free article] [PubMed] [Google Scholar]
- 36.Dombkowski KJ, Harrington LB, Dong S, Clark SJ. Seasonal influenza vaccination reminders for children with high-risk conditions: a registry-based randomized trial. Am J Prev Med. 2012;42(1):71–75 [DOI] [PubMed] [Google Scholar]
- 37.Clark SJ, Butchart A, Kennedy A, Dombkowski KJ. Parents’ experiences with and preferences for immunization reminder/recall technologies. Pediatrics. 2011;128(5). Available at: www.pediatrics.org/cgi/content/full/128/5/e1100 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.