Abstract
The US President’s Emergency Plan for AIDS Relief (PEPFAR) has supported a comprehensive package of care in which interventions to address HIV-related tuberculosis (TB) have received increased funding and support in recent years. PEPFAR’s TB/HIV programming is based on the World Health Organization 12-point policy for collaborative TB/HIV activities, which are integrated into PEPFAR annual guidance. PEPFAR implementing partners have provided crucial support to TB/HIV collaboration, and as a result PEPFAR-supported countries in sub-Saharan Africa have made significant gains in HIV testing and counseling of TB patients and linkages to HIV care and treatment, intensified TB case finding, and TB infection control. PEPFAR’s support of TB/HIV integration has also included significant investment in health systems, including improved laboratory services and educating and enlarging the workforce. The scale-up of antiretroviral therapy along with support of programs to increase HIV counseling and testing and improve linkage and retention in HIV care may have considerable impact on TB morbidity and mortality, if used synergistically with isoniazid preventive therapy (IPT), intensified case finding and infection control. Issues to be addressed by future programming include accelerating implementation of IPT, increasing access and ensuring appropriate use of new TB diagnostics, supporting early initiation of antiretroviral therapy for HIV-infected TB patients, and strengthening systems to monitor and evaluate program implementation.
Keywords: tuberculosis, HIV/AIDS, President’s Emergency Plan for AIDS Relief (PEPFAR), sub-Saharan Africa, collaborative TB/HIV activities
Tuberculosis (TB) is the most common opportunistic illness and a leading cause of death among people living with HIV (PLWH), accounting for about a quarter of HIV-related deaths worldwide.1,2 Among approximately 8.8 million incident cases of TB in 2010, 2.3 million were in Africa, where 39% were co-infected with HIV.2
The US President’s Emergency Plan for AIDS Relief (PEPFAR) has supported a comprehensive package of care in which interventions to address HIV-related TB have received increased funding and support in recent years. Our objectives are to summarize the impact of PEPFAR’s support of collaborative TB/HIV activities and to outline unresolved issues to be addressed by future programming.
Background
PEPFAR’s TB/HIV programming is based on the World Health Organization (WHO) 12-point policy for collaborative TB/HIV activities,3 which are integrated into PEPFAR annual guidance.4 PEPFAR’s resource allocation decisions must meet a “certain impact” criterion; supporting scale-up of TB/HIV activities is emphasized as a funding priority.
From 2005-2011, funding allocated for TB/HIV activities increased from $19 million to $160 million per year. Demonstration projects to boost the programming of collaborative TB/HIV activities were initiated in Ethiopia, Kenya and Rwanda through collaboration between PEPFAR, WHO and national TB and HIV programs in 2005. The main objective of these demonstration projects was to expand HIV testing for TB patients as a gateway for comprehensive care including antiretroviral therapy (ART) and cotrimoxazole preventive therapy (CPT). They were also intended to scale up TB screening and the provision of isoniazid preventive therapy (IPT) for PLWH. These demonstration projects resulted in significantly improved outcomes, particularly for interventions provided for HIV-infected TB patients, which were achieved by addressing policy and programmatic bottlenecks in the three countries. Based on these experiences and also to further enhance the provision of TB interventions among PLWH in more countries, a workshop was co-sponsored by PEPFAR, the Bill and Melinda Gates Foundation and WHO in 2007 to stimulate additional action in PEPFAR-supported countries;5 an additional $50 million was made available to spur on further scale-up. At this workshop, key actions for priority areas were identified. Countries developed draft activity plans based on WHO-recommended activities that formed the basis of subsequent national consultations and consensus building for accelerated programming and funding through PEPFAR as well as other mechanisms such as the Global Fund. This funding was made available via the PEPFAR country operational planning process which is the vehicle through which funding allocations are matched to programmatic priorities.
Primarily through experiences garnered from PEPFAR-supported countries and the involvement of PEPFAR implementing partners, in 2008 WHO branded the essential TB interventions for PLWH as ‘Three Is for HIV/TB’ (Intensified Case Finding, Isoniazid Preventive Therapy, Infection Control).6 Implementation of the ‘Three Is’ in the context of comprehensive collaborative TB/HIV activities has been promoted through integration in PEPFAR-supported care, treatment and laboratory infrastructure ever since. PEPFAR programs are particularly well-placed to catalyze accelerated uptake of the ‘Three Is’ and the requisite coordination of TB and HIV services at the clinic and community levels, utilizing the more than 13,500 HIV care and treatment sites, including 5,200 providing ART.
Successes of PEPFAR
Coordination between TB and HIV Programming
Implementation of collaborative TB/HIV activities requires coordination between TB and HIV programs at all levels.7 The epidemiology of HIV and TB as well as health system factors and challenges specific to individual countries8 must be considered, in order to develop tailored TB and HIV service delivery models.3 PEPFAR implementing partners have provided crucial support to TB/HIV collaboration, and as a result PEPFAR-supported countries in sub-Saharan Africa are among the first to demonstrate that effective coordination and collaboration between TB and HIV programs can result in nationwide coverage of key interventions.9 Setting time-bound targets at national, regional, district, and facility levels in a participatory manner through national TB/HIV coordinating bodies has been instrumental for nationwide scale-up of collaborative TB/HIV activities. Experiences reported by Kenya, Rwanda and Malawi in setting targets for the accelerated implementation of collaborative activities demonstrated their value and helped to mobilize political commitment from the TB and HIV control programs as well as funders. PEPFAR targets for TB/HIV have also helped with accountability. Similarly, creating an environment conducive to the development of appropriate policy, operational guidelines, training manuals and protocols in line with international guidelines has been useful.10 Implementing integrated recording and reporting formats that capture collaborative TB/HIV activities with standardized and harmonized indicators,11 and inclusion of TB components in HIV registers and HIV components in TB registers12 has been crucial to monitoring and evaluating program implementation.
HIV Testing and Counseling of TB Patients and Linkages to HIV Care and Treatment
In the early phase of PEPFAR (2003-2005), there was an urgency to identify PLWH eligible for ART. PEPFAR’s target was to place 2 million persons on ART within the first five-year period. The utility of providing HIV testing in TB clinics was evidenced by seroprevalence surveys among TB patients in southern and east Africa which showed rates of co-infection with HIV between 50-80%.
Initially, incorporating HIV testing of TB patients into routine care was challenging, as the standard of care was voluntary counseling and testing (VCT), which involved one-on-one pre- and post- test counseling that could last an hour or more. Introduction of provider initiated testing and counseling (PITC),13 which promoted an “opt-out” strategy in clinical settings and allowed for abbreviated pre-test counseling, often in a group setting, was an enormous paradigm shift that promised to streamline identification of HIV-infected TB patients so they could receive ART.
PEPFAR support for early implementation of PITC included: assisting with guideline development, modifying recording and reporting systems, procuring test kits, developing linkages to HIV care, and training clinicians. PEPFAR funding supported development of a training-of-trainers implementation package and assignment of Ministry of Health personnel to provide mentorship and supervision of early roll-out initiatives.14 Initially there were concerns that TB clinic staff would not want to assume this extra work. There were also legislative concerns regarding whether untrained healthcare workers could provide the abbreviated counseling of PITC, and whether cadres other than laboratory workers could perform the rapid HIV test.
Overall, PEPFAR-supported scale-up of HIV counseling and testing in TB clinics has been enormously successful. In 19 PEPFAR-supported countries in Africa, between 2003 and 2009, HIV testing in TB patients rose from 4% to 61%, compared to testing in non-PEPFAR supported African countries which rose from 1% to 40%.15 A key component of this successful scale-up was the development of a standard implementation package, which guided countries through the critical steps necessary to roll out this intervention.16 In addition, guidelines for PITC and monitoring and evaluation further enhanced countries’ ability to implement this activity. The model of performing HIV testing in the TB clinic and avoiding referral to a VCT clinic was a vast improvement.17,18 Overall, concerns about TB clinic staff resenting extra duties were not borne out.19 Early adopters of PITC reported successful outcomes. In Kenya, between 2006 and 2009, the proportion of TB patients who underwent HIV testing increased from 60% to 88%.20 Similar findings were seen in Zambia, especially when patients received PITC within TB clinics.21
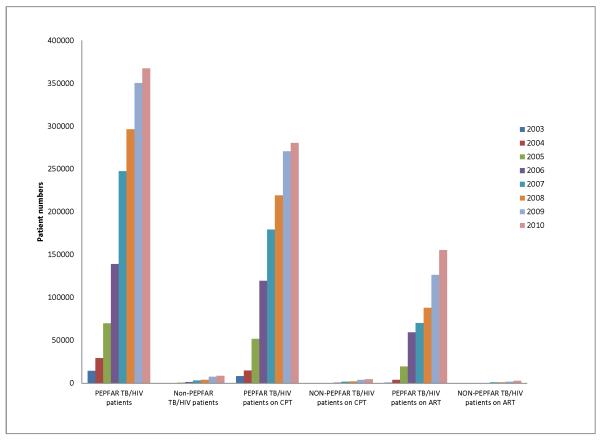
Linking HIV-infected TB patients to HIV care services has also increased dramatically as a result of PEPFAR support. From 2003 to 2009, in 19 PEPFAR-supported countries in Africa, the proportion of estimated HIV-infected TB patients receiving ART increased from 0.1% to 13.1%, compared to an increase from 0% to 2.7% in non-PEPFAR-supported countries.15 Provision of CPT has become routine in TB clinics in many PEPFAR countries once the patients are identified by the system. For example, in 2010 77% of notified HIV positive TB patients were provided CPT in PEPFAR countries as compared to 55% in non-PEPFAR countries. However timely provision of ART to HIV-infected TB patients has been more difficult to operationalize. In 2010, 42% of HIV positive TB patients in PEPFAR countries received ART, as compared to 34% in non-PEPFAR countries.
Intensified TB Case Finding
Early identification of PLWH suspected of having TB, followed by a timely diagnostic workup and prompt initiation of treatment, can improve patient outcomes and reduce transmission in communities and healthcare settings.22,23 TB screening is also essential in evaluating PLWH for IPT eligibility by ensuring exclusion of TB disease to avoid the risk of isoniazid monotherapy.24
PEPFAR implementing partners have supported the roll-out of screening tools for symptoms related to TB for use in HIV care and treatment settings. Initially, efforts to screen PLWH for TB were inconsistent and non-standardized, resulting in missed opportunities to identify TB suspects and cases. More recently, WHO has proposed a standard TB screening tool for PLWH based on a meta-analysis,25 which has resulted in substantial improvements in performing and reporting TB screening among PLWH. In 2010, PEPFAR-supported programs reported 2.9 million PLWH were screened for TB in HIV care or treatment settings.26
An ongoing challenge has been confirming a TB diagnosis among PLWH with a positive symptom screen. With the roll-out of new molecular diagnostics such as GeneXpert MTB/RIF, the impetus for intensified case finding is enhanced as the availability of a rapid diagnostic test represents a quantum leap in patients’ access to early and accurate diagnosis, as well as timely and effective treatment. It will be necessary to support national HIV programs and clinicians to ensure that screening tools are adapted and rapidly implemented as part of HIV care and treatment.
TB Infection Control
When PEPFAR was initiated, TB infection control was largely a neglected issue in resource-limited settings, particularly in Africa. An acute awareness of the need for TB infection control came following an outbreak of extensively drug-resistant TB (XDR-TB) among PLWH in a rural community in KwaZulu-Natal, South Africa, in which 52 of 53 patients died within a median of 16 days: nearly two thirds of these patients were thought to have acquired their infection from a previous hospital admission.27 Furthermore, there was increasing evidence that health care workers in low- and middle-income countries were at substantially higher risk of developing TB than the general population.28 This came at a time when PEPFAR was scaling-up HIV care and treatment programs, bringing PLWH into health care settings that lacked TB infection control practices and where rates of TB were often over 500 per 100,000.29
In 2006, PEPFAR supported the development of interim guidelines on TB Infection Control in the context of HIV 30 and in 2009, worked with WHO to publish revised policy and guidelines on TB Infection Control.31 These updated guidelines emphasized the importance of management and oversight of TB infection control activities, as well as the basic approach of administrative and environmental controls and personal respiratory protection.
PEPFAR immediately emphasized scale-up of TB infection control activities through its technical documents and the country planning process, supporting training of national-level program implementers, engineers, and architects. Support was also provided to develop national TB infection control guidelines32 and to scale-up training of health care workers. PEPFAR funds supported pilot testing of clinic-based infection control initiatives,33 and most recently the development of a TB infection control implementation package for ART clinics and other outpatient settings,34 which focuses on simple behaviors including identifying, separating and fast-tracking coughing patients, covering cough with tissues, sleeve, or mask, and keeping windows open where feasible.
Many challenges remain in scaling up TB infection control activities. The need is enormous, and the targeted service settings include over 13,500 PEPFAR-supported facilities providing care and treatment services for PLWH in high TB prevalence settings. TB infection control measures involve vigilance for multiple, simple behaviors and require ongoing monitoring and supervision. Evaluation of burgeoning programs is of critical importance if gains are to be maintained.
Broader Impact of PEPFAR Support of TB/HIV Integration
TB Diagnostics and Health Systems Strengthening
Addressing TB among PLWH required significant investment in health systems. Improving TB diagnosis remains a critical challenge and requires improved laboratory services. Support to TB laboratory services through PEPFAR has included educating and enlarging the workforce; improving equipment, supply chains, and specimen referral networks; addressing biosafety concerns; developing lab information systems; and supporting and evaluating new diagnostic techniques.35,36 More recently, PEPFAR has promoted comprehensive support to developing quality laboratory systems and building professional linkages through support to the first African Society for Laboratory Medicine. Infrastructure improvements have included renovations of health facilities, laboratories, training schools and support for the development of national reference laboratories (NRL), including TB. PEPFAR support for the establishment of a Field Epidemiology and Lab Training Program (FELTP) in some countries has promoted opportunities for capacity building of National TB Program and NRL staff.
Impact of ART Scale-up on TB Control at the Population Level
ART has the potential to contribute substantially to TB control. In cohort studies conducted in high-income and resource-limited settings, ART is associated with 54-92% reduction in TB incidence rates, with a pooled summary effect of 67% (95% CI, 61%-73%).37 Important data from two PEPFAR-supported countries show a positive impact of ART on TB control at the community level. In South Africa, ART scale-up was found to be associated with a reduced community prevalence of HIV-associated TB38 and in Malawi, with a reduction in the TB notification rate in the general population.39
Several factors have undermined the potential for ART to have an even more substantial impact on TB control. Since ART is typically initiated at low CD4+ counts, many patients have a current TB diagnosis when starting ART.40,41 In addition, low ART coverage and poor retention in care mean that the overall distribution of CD4+ counts in populations may not improve sufficiently to have a more substantial TB preventive effect.
PEPFAR’s goal of scaling up ART from 4 to 6 million in the next 24 months is enabling many countries to increase the threshold for initiating ART to a CD4+ count of <350 cells/μl. This initiative along with PEPFAR’s support of programs to increase HIV counseling and testing and improve linkage and retention in HIV care may have considerable impact on TB morbidity and mortality, if used synergistically with IPT, intensified case finding and infection control.42
TB/HIV Integration as a Model for the Larger Global Health Initiative
As programs shift from emergency initiatives to sustainability, lessons learned in the pursuit of TB/HIV collaboration illuminate key principles of the US President’s Global Health Initiative (GHI).43 Collaborative TB/HIV activities are prototypic of GHI’s key concepts of coordination, collaboration, integration, and systems strengthening. Nascent progress demonstrated over the last seven years, particularly from TB program entry points, provides guideposts and best practice examples for integration, bridging programmatic cultures, as well as the value of community engagement. Various models of TB/HIV integration have evolved demonstrating that adaptations must be modified to countries’ contextual milieu.44 It has been documented that integration of TB and HIV services offers real benefits to patients and the health system.9 Programs implementing TB/HIV services also exemplify and enhance the HIV continuum of care model, ensuring that programs link to and between HIV services and other health sector services. Efforts should continue to identify and maximize synergies and benefits across TB and HIV programs as these activities should be the standard of care for co-infected persons.
Ongoing Challenges to be Addressed
Isoniazid Preventive Therapy
IPT has been shown to reduce TB incidence among PLWH45 and has been recommended by WHO as part of a comprehensive package of HIV care.46 Although successful ART substantially reduces TB risk,47 evidence supports an additive protective benefit from concomitant IPT use among individuals on ART.48,49 As such, IPT remains a highly relevant strategy to decrease the burden of TB among PLWH in the context of ART programmatic scale-up.
In many PEPFAR-supported countries, scale-up of IPT has been a challenge. Close to 180,000 PLWH were reported to have received IPT in 2010, representing 12% of the nearly 1.5 million PLWH who were reported to be newly enrolled in HIV care.2 Despite the advocacy of PEPFAR partners, ideological conservatism and lack of consensus on the part of national HIV and TB program managers have hampered efforts to implement this strategy. A commonly cited barrier to IPT implementation is concern about emergence of isoniazid resistance because of inadequate patient adherence and difficulty excluding TB before starting IPT,50-52 despite evidence suggesting that the effect of IPT on isoniazid resistance is likely to be small.53 Other implementation hurdles include the absence of standard TB screening algorithms or operating procedures,51 and the lack of systems to monitor program implementation.50
A notable exception is Ethiopia, where PEPFAR has supported IPT implementation since 2006. In 2010, 6,636 PLWH in Ethiopia received IPT, as compared to 1,983 in 2005. Key strategies employed by PEPFAR partners in Ethiopia included sponsoring a national symposium on management of latent TB infection in PLWH, which led to the development of national guidelines; supporting the development of provider support tools and registers; ensuring availability of isoniazid; and training and mentoring HIV providers.
Following the recent release of WHO guidelines on TB screening and IPT among PLWH,46 IPT implementation has begun in several countries. Support is needed to bring these efforts to scale, to approach the Global Plan’s target of providing IPT to all those attending HIV care services who are eligible for it by 2015.54
Progress in TB Diagnostics
The lack of simple, accurate, low-cost diagnostic tests for TB is a critical weakness in our ability to tackle the HIV-associated TB epidemic, since reliable diagnosis is fundamental for case finding and treatment as well as to implementation of IPT. Diagnoses are often missed or delayed due to the non-specific clinical presentation and high rates of sputum smear-negative, extrapulmonary and disseminated disease in PLWH.55,56 Chest radiography has limited utility and culture-based diagnosis is too slow, costly and technically complex for most resource-limited settings.
Introduction of the Xpert MTB/RIF assay represents a key breakthrough. This rapid molecular assay can be used close to the point-of-care by operators with little technical expertise, enabling diagnosis of TB and simultaneous assessment of rifampicin resistance to be performed within 2 hours using unprocessed sputum samples or specimens from extrapulmonary sites.57 Testing a single sputum sample detects 98%-100% of smear-positive pulmonary TB and between 57% and 83% of smear-negative disease in adults presenting with suspected TB.57
As a critical cross-cutting health systems strengthening activity, PEPFAR welcomed WHO’s policy statement endorsing the Xpert MTB/RIF assay,58 and is committed to supporting scale-up and appropriate use of this new technology.59 As of December 2011, PEPFAR programs procured or budgeted for over 130 Xpert machines. Additional resources are planned to further accelerate scale-up of this new technology, including procurement of another 100 machines and 300,000 cartridges as well as provision of technical assistance to support their appropriate use. Significantly greater investment will be needed, however, if this test is to be placed at peripheral health facilities so that it is as near point-of-care as is feasible, where patients will benefit from this rapid diagnostic.
Early Initiation of ART for HIV-Infected TB Patients
Without ART, case fatality is considerably higher in HIV-infected TB patients than in non HIV-infected patients. Mortality risk increases as the CD4+ count declines, and case fatality is highest in the first two months of TB treatment.60-63 Key interventions, which include CPT and ART, must therefore occur early in TB treatment.
Three randomized controlled studies have demonstrated that earlier initiation of ART (within 2 to 4 weeks) in HIV-infected TB patients with CD4+ counts <50 cells/μL is associated with reduced mortality, despite an increased risk of immune reconstitution inflammatory syndrome (IRIS).64-66 The 2012 WHO Policy on Collaborative TB/HIV Activities recommends that ART should be started within 2 weeks after the onset of TB treatment in PLWH with a CD4+ count <50 cells/μL and as early as possible in the remaining cases.3
In 2010, only 34% of notified TB cases were HIV tested and of these only 46% started on ART.2 The priority is to ensure increased ART uptake, and, if CD4+ counts are measured, that ART starts within two weeks of TB treatment in those with CD4+ counts <50 cells/μL. Co-location of TB and ART services is the key to the implementation of this strategy. In this regard, PEPFAR has supported a variety of country models for provision of dual services to co-infected patients, including initiation of ART within TB clinics.67 The new PEPFAR support for an “AIDS-free Generation”, which will strive to more rapidly scale up timely HIV treatment, will hopefully reduce HIV-related mortality among TB patients and TB control more broadly.
TB in HIV-Exposed and -Infected Children
Addressing TB among HIV-infected children remains a challenge.68 Underlying immunosuppression and other HIV-related lung diseases complicate the diagnosis of TB, particularly among the youngest children. New diagnostic methods are unlikely to significantly benefit children, as many children do not easily produce sputum, and specimens are frequently culture-negative even where culture is available.69 Point-of-care diagnostic techniques for all children are needed as is an effective vaccine that can safely be administered to HIV-infected children.70 Despite advances, children lag behind in HIV treatment efforts, and prevention of mother-to-child transmission has not yet been fully scaled-up. Opportunities to fully realize linkages across programs include taking full advantage of all routine and HIV service-delivery entry points to screen for TB and HIV in children and ensuring that contact tracing of infectious TB cases occurs. Better routine data gathering efforts of both TB and HIV status in children can direct strategies for program integration and improvement.71
Monitoring and Evaluation
Monitoring and evaluation of collaborative TB/HIV activities has been challenging, as often it requires review of two information systems, i.e. the National TB surveillance system and the ART patient monitoring system, which are rarely linked and often lack functional feedback loops. PEPFAR must continue to support national programs to transition to HIV/TB linked reporting systems that capture collaborative TB/HIV activities with standardized and harmonized indicators, consistent with WHO recommendations.12 Operational research on improving linkages as well as monitoring and evaluation systems between National TB programs and HIV care and treatment services is a critical need to fully achieve the rewards from progress made. PEPFAR’s new focus on operations research, termed implementation science, promises a welcome addition to the programmatic successes.72
Conclusions
TB remains the most significant cause of morbidity and mortality among PLWH in sub-Saharan countries with a high prevalence of both diseases. Working in collaboration with host countries, international partners, and other stakeholders, PEPFAR has supported a comprehensive package of care which has included interventions to address HIV-related TB. As a result, PEPFAR-supported countries have made significant gains in HIV testing and counseling of TB patients and linkage to HIV care and treatment, intensified TB case finding, and TB infection control. Future programming must address accelerated implementation of IPT, increased access to new TB diagnostics, early initiation of ART for HIV-infected TB patients, and strengthening systems to monitor and evaluate program implementation. Efforts should continue to identify and maximize synergies and benefits across TB and HIV programs, as collaborative TB/HIV activities should be the standard of care for individuals affected by both diseases.
PANEL 1. Case Study: Rwanda.
Implementation of WHO TB/HIV policy guidelines
In Rwanda, a TB/HIV collaborative policy was implemented in 2005,73 and a national coordinating body for TB/HIV activities, the TB/HIV Technical Working Group,74 was established in 2006. The working group, whose members include the National TB Division, National HIV/AIDS Division, National Referral Laboratory, district hospitals, health centers, and PEPFAR partners, meets quarterly to monitor and plan implementation of TB/HIV activities. At peripheral level, TB/HIV activities are coordinated through Quality Improvement Teams. One-stop TB/HIV Services have been rolled-out nationwide, whereby TB/HIV patients are treated for both TB and HIV at TB clinics until they complete TB treatment.75 Each TB diagnosis and treatment center receives integrated TB and TB/HIV supervision by central and district level teams on a quarterly basis.
In 2009, HIV counseling and testing of TB patients was expanded to include TB suspects.76 In 2010 more than 95% of TB suspects received HIV counseling and testing; over 95% of HIV-infected TB patients received CPT, and over 65% received ART.77,78
TB screening is offered to PLWH at enrollment in care and during follow-up visits. In July 2011, IPT was introduced in three pilot sites. Currently, more than 3,300 adults have initiated IPT.78 Scale-up to ten district hospitals is planned by the end of 2012, and to all remaining hospitals by the end of 2013. TB infection control measures have been implemented at 77% of TB diagnosis and treatment centers.78
Linkage of PEPFAR TB/HIV Activities with Ministry of Health
PEPFAR TB/HIV activities are closely linked with those of the Ministry of Health. Ministry of Health and PEFAR implementers worked together to develop the TB and TB/HIV National Strategic Plan, and its monitoring and evaluation plan. During the annual COP process, PEPFAR implementers consider which national strategic plan activities are covered by other funding sources, to avoid duplication of funded activities.
Integration of TB/HIV Monitoring Data
Within health facilities, monitoring and evaluation data related to collaborative TB/HIV activities are primarily captured through integrated paper-based tools (laboratory register, TB cases register and TB treatment sheets), that are also used for other TB data. During the reporting phase, aggregated TB and TB/HIV data are reported in the same paper and electronic formats, and are later sent to district and national level for reporting and archiving.
All TB/HIV indicators are monitored through the above described system. Only one indicator related to TB screening among PLWH is reported by the Ministry of Health HIV/AIDS Division, however for integration, these data are shared and discussed during meetings of the TB/HIV technical working group.
PANEL 2. Case Study: Kenya.
Implementation of 2004 WHO TB/HIV policy guidelines
In Kenya, HIV testing for TB patients as an entry point to collaborative TB/HIV activities was introduced in the third quarter of 2005. Over the next several years, interventions have increased to cover all of the collaborative activities, due to the availability of policy documents and increased resources for the TB and HIV programs.
The TB program put into place a robust monitoring and evaluation system that included revised data capture tools at the service delivery points to reflect the new policy guidelines. A strong team and committees were formed to steer the implementation, and progress was monitored using the new TB/HIV indicators.
Initially stigma was a major challenge to TB/HIV integration, both amongst patients and with health care workers. Special initiatives were put in place to ensure that patients accepted HIV testing in TB clinics and health care workers were comfortable with testing for HIV.
Between 2005 and 2010, HIV testing of TB patients increased from 32% to 91%, while provision of CPT for HIV-infected TB patients increased from 81% to 99%. Availability of CPT at the TB clinic has enabled its rapid uptake over the years, as staff became more comfortable with implementation of this service following training and mentorship.
ART uptake by HIV-infected TB patients, however, has been challenging, although there are signs of improvement following adoption of three models of TB/HIV integration in 2009, based on available infrastructure, human resources and policies. In 2010, 48% of HIV-infected TB patients received ART, up from 26% in 2005. The most successful model is the fully integrated clinic where both TB and HIV services are available, followed by partial integration where the patient is referred to a different building within the setting; the least successful is one in which patients must be referred to another facility to access care.
Lessons learned
Development of policy documents prior to initiating the strategy is critical, as they can serve as advocacy tools and provide guidance when challenges arise. Preparation of comprehensive plans at the national level is also critical prior to rolling out initiatives at the districts. Involvement of patient groups raises awareness and increases acceptability of integrating TB/HIV services. Finally, enabling TB nurses to provide ART in TB clinics was key to successful scale-up of ART for HIV-infected TB patients.
Figure 1. Coverage of cotrimoxazole and antiretroviral therapy among TB/HIV patients identified in PEPFAR and non-PEPFAR funded countries in the WHO African region, 2003-2010.
PEPFAR countries reported in African Region (WHO): Angola, Botswana, Cote d’Ivoire, Democratic Republic of the Congo, Ethiopia, Ghana, Kenya, Lesotho, Malawi, Mozambique, Namibia, Nigeria, South Africa, Swaziland, Tanzania, Uganda, Zambia, Zimbabwe. Non-PEPFAR countries reported: Burkina Faso, Burundi, Central African Republic, Congo, Equatorial Guinea, Gambia, Guinea, Guinea-Bissau, Liberia, Madagascar, Mali, Mauritania, Mauritius, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone. (Source: WHO)
Table 1.
Key Research Questions of Importance to Integration of TB and HIV Programs in High HIV-TB prevalence settings
| Collaborative TB/HIV Activities | Research question | Expected programmatic impact |
|---|---|---|
|
| ||
|
A. Establish the mechanisms for collaboration
| ||
| A.1. Coordinating bodies for TB/HIV activities A.2. Surveillance of HIV prevalence in TB patients A.3. Joint TB/HIV planning A.4. Monitoring and evaluation |
1. What are the best models of delivery of integrated HIV and TB services which may also include maternal and child health and chronic non-communicable diseases like diabetes? | 1. Increased uptake of HIV testing and care for TB patients, and better TB case finding in pre-ART and ART clinics |
|
| ||
|
B. Decrease the burden of TB in PLHIV
| ||
| B.1. Establish intensified TB case-finding (ICF) B.2. Introduce isoniazid preventive therapy (IPT) B.3. Ensure TB infection control (IC) B.4. Better coverage and earlier use of ART |
1. Does a strategy of empirical TB treatment reduce early mortality in PLWH with low CD4+ counts who are about to start ART compared with a strategy of ICF using established or new TB diagnostic tests? | 1. Reduced early mortality in PLWH starting ART |
| 2. Does universal HIV testing and early initiation of ART reduce individual and community risk of TB? | 2. Reduced TB incidence in PLWH and high HIV-prevalence communities | |
| 3. What is the optimum frequency of repeat ICF after baseline screening in pre-ART and ART clinics? | 3. Evidence to guide baseline and serial ICF leading to increased TB diagnosis | |
| 4. What is the most cost-effective TB diagnostic screening algorithm for ICF in pre-ART and ART clinics using smear microscopy, Xpert MTB/RIF and urine LAM? | 4. Evidence to guide the strategic use of current TB diagnostic tests leading to increased TB diagnosis | |
| 5. In health facility and congregate settings, how should TB infection control be routinely monitored, recorded and reported? | 5. Better implementation of TB infection control practices | |
| 6. Is long-term IPT in PLWH before or after the start of ART acceptable to patients and effective in reducing risk of TB without generating drug resistance? | 6. Increased use of IPT which is effective and safe in reducing the risk of TB | |
|
| ||
| C. Decrease the burden of HIV in TB patients | ||
| C.1. Provide HIV testing and counselling C.2. Introduce HIV prevention methods C.3. Introduce CPT C.4. Ensure HIV/AIDS care and support C.5. Introduce ART |
1. Does HIV testing of TB suspects and referral of HIV-positive patients to structured HIV care lead to a better prognosis in this group? | 1. Reduced mortality in TB suspects and improved likelihood of TB being diagnosed within structured HIV care |
| 2. What is the most effective and safest dose of rifabutin to use with protease inhibitors in second-line ART? | 2. Improved treatment of TB in PLWH who have failed first-line ART | |
| 3. Can mobile phone technology improve adherence to care and treatment for HIV-infected TB patients? | 3. Improved TB treatment success and better retention in HIV care and ART for co-infected patients | |
TB = tuberculosis; CPT = cotrimoxazole preventive therapy; ART = antiretroviral therapy; PLWH = people living with HIV/AIDS; ICF = intensified TB case finding; IPT = isoniazid preventive therapy; IC = infection control; LAM = lipoarabinomannan
Acknowledgements
We thank Annabel Baddeley of WHO for her support.
SDL is funded by the Wellcome Trust, London, UK. AAH receives grant support through the Centers for Disease Control and Prevention, including PEPFAR funds.
Sources of Funding/Conflicts: Various authors have professional relationships PEPFAR (either as employees of PEPFAR-supported US Government agencies or as grantees/contractors) as outlined in the Copyright Transfer Agreement Forms.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the United States government.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Getahun H, Gunneberg C, Granich R, Nunn P. HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis. 2010;50(Suppl 3):S201–S207. doi: 10.1086/651492. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization . Global tuberculosis control: WHO report 2011. Geneva: 2011. [Google Scholar]
- 3.World Health Organization . WHO Policy on collaborative TB/HIV activites: guidelines for national programmes and other stakeholders. Geneva: 2012. [PubMed] [Google Scholar]
- 4.Technical considerations provided by PEPFAR technical working groups for FY 2012 COPS and ROPS. http://www.pepfar.gov/documents/organization/169737.pdf.
- 5.Meeting report. Accelerating the implementation of collaborative HIV/Tb activities in selected sub-Saharan African countries. Mar 6-7, 2007.
- 6.World Health Organization . WHO Three I’s Meeting Report. Intensified Case Finding (ICF), Isoniazid Preventive Therapy (IPT) and TB Infection Control (IC) for people living with HIV. Geneva: 2008. [Google Scholar]
- 7.Havlir DV, Getahun H, Sanne I, Nunn P. Opportunities and Challenges for HIV Care in Overlapping HIV and TB Epidemics. JAMA: The Journal of the American Medical Association. 2008;300:423–430. doi: 10.1001/jama.300.4.423. [DOI] [PubMed] [Google Scholar]
- 8.Howard AA, El-Sadr WM. Integration of tuberculosis and HIV services in sub-Saharan Africa: lessons learned. Clin Infect Dis. 2010;50(Suppl 3):S238–S244. doi: 10.1086/651497. [DOI] [PubMed] [Google Scholar]
- 9.Uyei J, Coetzee D, Macinko J, Guttmacher S. Integrated delivery of HIV and tuberculosis services in sub-Saharan Africa: a systematic review. Lancet Infect Dis. 2011;11:855–867. doi: 10.1016/S1473-3099(11)70145-1. [DOI] [PubMed] [Google Scholar]
- 10.Getahun H, Scano F, Nunn P. Implementation of collaborative tuberculosis/HIV activities: policy and programmatic issues. In: Schaaf HS, Zumla AI, editors. Tuberculosis: A Comprehensive Clinical Reference. Saunders/Elsevier; Philadelphia: 2009. [Google Scholar]
- 11.World Health Organization . A guide to monitoring and evaluation for collaborative TB/HIV activities. WHO, UNAIDS and OGAC; Geneva, Switzerland: 2009. ( WHO/HTM/TB/2009.414, WHO/HTM/HIV 09.01 ). [Google Scholar]
- 12.World Health Organization . Three interlinked patient monitoring systems for HIV care/ART, MCH/PMTCT and TB/HIV: Standardized minimum data set and illustrative tools. WHO; Geneva: 2009. [Google Scholar]
- 13.World Health Organization Department of HIV/AIDS. Joint United Nations Programme on HIV/AIDS . Guidance on provider-initiated HIV testing and counseling in health facilities. WHO, UNAIDS; Geneva: 2007. [Google Scholar]
- 14.Bock NN, Nadol P, Rogers M, et al. Provider-initiated HIV testing and counseling in TB clinical settings: tools for program implementation. Int J Tuberc Lung Dis. 2008;12:S69–S72. [PubMed] [Google Scholar]
- 15.Gunneberg C, Sculier D, Reid A, et al. Comparison of progress in provision of HIV testing and ART for TB patients--African region, the rest of the world and PEPFAR supported countries. Presented at: 6th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Rome. 2011; Abstract TUPE485. [Google Scholar]
- 16.Coggin WL, Ryan CA, Holmes CB. Role of the US President’s Emergency Plan for AIDS Relief in responding to tuberculosis and HIV coinfection. Clin Infect Dis. 2010;50(Suppl 3):S255–S259. doi: 10.1086/651499. [DOI] [PubMed] [Google Scholar]
- 17.Van Rie A, Sabue M, Jarrett N, et al. Counseling and testing TB patients for HIV: evaluation of three implementation models in Kinshasa, Congo. Int J Tuberc Lung Dis. 2008;12:S73–S78. [PubMed] [Google Scholar]
- 18.Pevzner ES, Vandebriel G, Lowrance DW, et al. Evaluation of the rapid scale-up of collaborative TB/HIV facilities in Rwanda, 2005-2009. BMC Public Health. 2011;11 doi: 10.1186/1471-2458-11-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corneli A, Jarrett NM, Sabue M, et al. Patient and provider perspectives on implementation models of HIV counseling and testing for patients with TB. Int J Tuberc Lung Dis. 2008;12:S79–S84. [PubMed] [Google Scholar]
- 20.Sitieni J, Kipruto H, Nganga, et al. HIV testing and treatment among tuberculosis patients--Kenya, 2006-2009. MMWR. 2010;59:1514–1517. [PubMed] [Google Scholar]
- 21.Mwinga A, Mwananyambe N, Kaneme C, et al. Provider-initiated HIV testing and counseling of TB patients--Livingstone District, Zambia, September 2004-December 2006. MMWR. 2008;57:285–289. [PubMed] [Google Scholar]
- 22.Currie CS, Williams BG, Cheng RC, Dye C. Tuberculosis epidemics driven by HIV: is prevention better than cure? AIDS. 2003;17:2501–2508. doi: 10.1097/01.aids.0000096903.73209.ac. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . Guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings. WHO; Geneva: 1999. ( WHO/CDS/TB/99 269 ). [Google Scholar]
- 24.World Health Organization Preventive therapy against tuberculosis in people living with HIV. Policy Statement. Weekly Epidemiological Record. 1999;74:385–400. [Google Scholar]
- 25.Getahun H, Kittikraisak AW, Heilig CM, Corbett EL, Ayles H, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8:e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PEPFAR Annual Progress Report. 2010.
- 27.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 28.Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low and midle-income countries: a systematic review. PLoS Med. 2006;3:e494. doi: 10.1371/journal.pmed.0030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization . Global tuberculosis control: surveillance, planning, financing. WHO Report 2006. WHO; Geneva: 2006. [Google Scholar]
- 30.World Health Organization. Centers for Disease Control and Prevention . Tuberculosis infection control in the era of expanding HIV care and treatment: an addendum to WHO guidelines for the prevention of tuberculosis in health care facilities in resource-limited settings. CDC; Atlanta: 2006. [Google Scholar]
- 31.World Health Organization . WHO policy on TB infection control in health-care facilities, congregate settngs and households. Geneva: 2009. ( WHO/HTM/TB/2009.419 ). [PubMed] [Google Scholar]
- 32.Republic of Ghana Ministry of Health. Standard operating procedures for TB and airborne infection prevention and control in Ghana. 2010.
- 33.Verkuijl S, Maharaj S, Jagwer G, Flam R, Howard AA. TB infection control in resource-limited settings in the era of expanding HIV care and treatment: lessons learnt from the Eastern Cape, South Africa [abstract 492). Programs and Abstracts of the 4th South African AIDS Conference; Durban, South Africa. 2009. [Google Scholar]
- 34.Nakashima A, Lipke V, Emerson C, et al. Implementing TB Infection Control in outpatient settings: a training and implmentation package. Centers for DIsease Control and Prevention and Zambian Ministry of Health; 2012. Accessed at http://wwwdev.cdc.gov/globalaids/Resources/pmtct-care/tuberculosis-infection-control.html. [Google Scholar]
- 35.Parsons L, Somoskovi A, Gutierrez C, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev. 2011;24:314–350. doi: 10.1128/CMR.00059-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nkengasong J, Nsuguba P, Nwanyanwu O, et al. Laboratory systems and services are critical in global health: time to end the neglect? Am J Clin Pathol. 2012;134:373. doi: 10.1309/AJCPMPSINQ9BRMU6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis. 2010;10:489–498. doi: 10.1016/S1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 38.Middelkoop K, Bekker LG, Myer L, et al. Antiretroviral Program Associated with Reduction in Untreated Prevalent Tuberculosis in a South African Township. Am J Respir Crit Care Med. 2010;182:1080–5. doi: 10.1164/rccm.201004-0598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zachariah R, Bemelmans M, Akesson A, et al. Reduced tuberculosis notification associated with scaling up antiretroviral treatment in rural Malawi. Int J Tuberc Lung Dis. 2011;15:933–937. doi: 10.5588/ijtld.10.0666. [DOI] [PubMed] [Google Scholar]
- 40.Lawn SD, Fraenzel A, Kranzer K, Caldwell J, Bekker LG, Wood R. Provider-initiated HIV testing increases access of patients with HIV-associated tuberculosis to antiretroviral treatment. S Afr Med J. 2011;101:258–262. doi: 10.7196/samj.4392. [DOI] [PubMed] [Google Scholar]
- 41.Boulle A, Van CG, Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 42.Lawn SD, Harries AD, Williams BG, et al. Antiretroviral therapy and the control of HIV-associated tuberculosis. Will ART do it? Int J Tuberc Lung Dis. 2011;15:571–581. doi: 10.5588/ijtld.10.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.The United States Government Global Health Initiative Strategy. 2012 http://www.pepfar.gov/documents/organization/136504.pdf.
- 44.Smart T. TB and HIV in Practice. HIV & AIDS Treatment in Practice. 2011 Sep 07;(Issue 181) 2011. [Google Scholar]
- 45.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD000171.pub3. CD000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization . Guidelines for intensifed tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva: 2010. [Google Scholar]
- 47.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis. 2010;10:489–498. doi: 10.1016/S1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 48.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golub JE, Pronyk P, Mohapi L, et al. Isoniazid preventive therapy, HAART, and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23:631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Date AA, Vitoria M, Granich R, Banda M, Fox MY, Gilks C. Implementation of co trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV. Bull World Health Organ. 2010;88:253–259. doi: 10.2471/BLT.09.066522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Getahun H, Granich R, Sculier D, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS. 2010;24:S57–S65. doi: 10.1097/01.aids.0000391023.03037.1f. [DOI] [PubMed] [Google Scholar]
- 52.Lester R, Hamilton R, Charalambous S, et al. Barriers to implementation of isoniazid preventive therapy in HIV clinics: a qualitative study. AIDS. 2010;24:S45–S48. doi: 10.1097/01.aids.0000391021.18284.12. [DOI] [PubMed] [Google Scholar]
- 53.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12:744–751. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stop TB Parternship. The Global Plan to Stop TB 2011-2015. World Health Organization; Geneva: 2011. [Google Scholar]
- 55.Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis. 2011;204(Suppl 4):S1159–S1167. doi: 10.1093/infdis/jir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reid MJ, Shah NS. Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis. 2009;9:173–184. doi: 10.1016/S1473-3099(09)70043-X. [DOI] [PubMed] [Google Scholar]
- 57.Lawn SD, Nicol MP. Xpert(R) MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6:1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization . Rapid implementation of the Xpert MTB/RIF diagnostic test. Geneva: 2011. [Google Scholar]
- 59.US Global Health Programs welcome World Health Organization endorsement of rapid test for tuberculosis. Joint statement by the U S President’s Emergency Plan for AIDS Relief (PEFAR), U S Agency for International Development and U S Department of Health and Human Services. 2010.
- 60.Ackah AN, Coulibaly D, Digbeu H, et al. Response to treatment, mortality, and CD4 lymphocyte counts in HIV-infected persons with tuberculosis in Abidjan, Cote d’Ivoire. Lancet. 1995;345:610. doi: 10.1016/s0140-6736(95)90519-7. [DOI] [PubMed] [Google Scholar]
- 61.Perriens JH, St Louis ME, Mukadi YB, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N Engl J Med. 1995;332:779–784. doi: 10.1056/NEJM199503233321204. [DOI] [PubMed] [Google Scholar]
- 62.Diul MY, Maher D, Harries AD. Tuberculosis case fatality rates in HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 63.Harries AD, Hargreaves NJ, Gausi F, Kwanjana JH, Salaniponi FM. High early death rate in tuberculosis patients in Malawi. Int J Tuberc Lung Dis. 2001;5:1000–1005. [PubMed] [Google Scholar]
- 64.Blanc FX, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Havlir DV, Kendall MA, Ive P, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abdool Karim SS, Naidoo K, Grobler A, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lowrance DW, Makombe S, Harries A, et al. Lower early mortality rates among patients receiving antiretroviral treatment at clinics offering cotrimoxazole prophylaxis in Malawi. J Acquir Immune Def Syndr. 2007;46:56–61. [PubMed] [Google Scholar]
- 68.Marais B, Rabie H, Cotton M. TB and HIV in children--advances in prevention and management. Paed Respir Rev. 2011;12:39–45. doi: 10.1016/j.prrv.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 69.Nicol M, Zar H. New specimens and laboratory diagnostics for childhood TB: progress and prospects. Paed Respir Rev. 2011;12:16–21. doi: 10.1016/j.prrv.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawkridge T, Mahomed H. Prospects for a new, safer and more effective TB vaccine. Paed Respir Rev. 2011;12:46–51. doi: 10.1016/j.prrv.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 71.du Cros P, Bern-Thomas N, Gale M, et al. Counting children: comparing reporting for paediatric HIV and tuberculosis. Bull World Health Organ. 2011;89:855. doi: 10.2471/BLT.11.098582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Padian NS, Holmes CV, McCoy SI, et al. Implementation science for the US President’s Emergency Plan for AIDS Relief (PEPFAR) J Acquir Immune Def Syndr. 2011;56:199–203. doi: 10.1097/QAI.0b013e31820bb448. [DOI] [PubMed] [Google Scholar]
- 73.Programme-National-Integre-de-Lutte-contre-la-Lepre-et-la-Tuberculose . Rapport annuel d’activites 2005. Kigali: 2005. [Google Scholar]
- 74.Programme-National-Integre-de-Lutte-contre-la-Lepre-et-la-Tuberculose . Rapport annuel d’activites 2006. Kigali: 2006. [Google Scholar]
- 75.Tuberculosis Unit-TRACPlus . Handbook of Tuberculosis and TB/HIV in Rwanda. Kigali: 2009. [Google Scholar]
- 76.Tuberculosis Unit-TRACPlus . Report d’activites pour l’annee 2009. Kigali: 2009. [Google Scholar]
- 77.Tuberculosis Unit-TRACPlus. Tuberculosis and Respiratory Communicable Diseases Division/IHDPC/RBC/MOH Annual Report (January-December 2010) 2011;37 [Google Scholar]
- 78.Tuberculosis and Respiratory Communicable Diseases Division/IHDPC/RBC/MOH . Quarterly report (October-December 2011) Vol. 36. Kigali: 2011. [Google Scholar]