Abstract
Understanding the molecular basis of ligand binding to receptors provides insights useful for rational drug design. This work describes development of a new antagonist radioligand of the type 1 cholecystokinin receptor (CCK1R), (2-fluorophenyl)-2,3-dihydro-3-[(3-isoquinolinylcarbonyl)amino]-6-methoxy-2-oxo-l-H-indole-3-propanoate (T-0632), and exploration of the molecular basis of its binding. This radioligand bound specifically with high affinity within an allosteric pocket of CCK1R. T-0632 fully inhibited binding and action of CCK at this receptor, while exhibiting no saturable binding to the closely related type 2 cholecystokinin receptor (CCK2R). Chimeric CCK1R/CCK2R constructs were used to explore the molecular basis of T-0632 binding. Exchanging exonic regions revealed the functional importance of CCK1R exon 3, extending from the bottom of transmembrane segment (TM) 3 to the top of TM5, including portions of the intramembranous pocket as well as the second extracellular loop region (ECL2). However, CCK1R mutants in which each residue facing the pocket was changed to that present in CCK2R had no negative impact on T-0632 binding. Extending the chimeric approach to ECL2 established the importance of its C-terminal region, and site-directed mutagenesis of each nonconserved residue in this region revealed the importance of Ser208 at the top of TM5. A molecular model of T-0632-occupied CCK1R was consistent with these experimental determinants, also identifying Met121 in TM3 and Arg336 in TM6 as important. Although these residues are conserved in CCK2R, mutating them had a distinct impact on the two closely related receptors, suggesting differential orientation. This establishes the molecular basis of binding of a highly selective nonpeptidyl allosteric antagonist of CCK1R, illustrating differences in docking that extend beyond determinants attributable to distinct residues lining the intramembranous pocket in the two receptor subtypes.
Introduction
In recent years, the solution of more than two dozen crystal structures of class A G protein–coupled receptors (GPCRs) has provided high-resolution insights that have added considerably to the power of structure-guided drug development (Shoichet and Kobilka, 2012; Vaidehi et al., 2014). This is particularly true for drugs targeting the helical bundle region that is highly conserved in this group of receptors. An understanding of the varied residues lining the small molecule–binding pocket high in the helical bundle of these receptors has provided a useful starting point for understanding the size and chemical characteristics of potential ligands. It has also become possible to extend these insights computationally, particularly when a series of ligands can be used to guide such refinement (Cawston et al., 2012; Harikumar et al., 2013).
In the current work, we use a small-molecule antagonist ligand of the type 1 cholecystokinin (CCK) receptor (CCK1R), (2-fluorophenyl)-2,3-dihydro-3-[(3-isoquinolinylcarbonyl)amino]-6-methoxy-2-oxo-l-H-indole-3-propanoate (T-0632) (Taniguchi et al., 1996a,b), that is reported to be more selective than other more commonly used benzodiazepine antagonists. It is reported to distinguish the CCK1R from the type 2 CCK receptor (CCK2R) with 23,000-fold selectivity, while the classic benzodiazepine antagonist 3S(−)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4- benzodiazepine-3-yl)-1H-indole-2-carboxamide (L-364,718) exhibits 1500-fold selectivity (Taniguchi et al., 1996a). The molecular basis of the selectivity of benzodiazepine antagonists like L-364,718 and (S)-1-(3-iodophenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)urea (BDZ-1) has recently been carefully evaluated, with specific, nonconserved residues within the intramembranous interhelical pocket identified as key determinants (Cawston et al., 2012). The selectivity for these benzodiazepine ligands can be reversed by substituting these key residues into the other subtype of CCK receptor (Cawston et al., 2012).
We now demonstrate the ability to directly radioiodinate T-0632 to yield a new high-affinity allosteric antagonist radioligand of CCK1R and to use this new reagent to directly explore the molecular determinants of binding. Of particular interest, these determinants also extend to the second extracellular loop region (ECL2), and the insights support substantial differences in conformation of this pocket in the closely related types 1 and 2 CCK receptors. This also helps to explain previous observations using photoaffinity labeling and fluorescence analysis of the orthosteric peptide ligand, CCK, binding with the same high affinity to these two receptors, although with distinct poses while occupying distinct regions of the two receptors (Dong et al., 2005; Harikumar et al., 2005). These data extend our understanding of the basis of small-molecule antagonist binding to CCK1R, including an important role for ligand interactions with Arg336 high in transmembrane segment (TM) 6 and with ECL2.
Materials and Methods
Reagents.
CCK (CCK-26-33) was from Peninsula Laboratories (Belmont, CA). Benzodiazepine CCK1R antagonists L-364,718 and BDZ-1 (Akgun et al., 2009; Cawston et al., 2012) were kindly provided by Dr. R. Freidinger (Merck Laboratories, West Point, PA) and Dr. P.S. Portoghese (University of Minnesota, Minneapolis, MN), respectively. The solid-phase oxidant, N-chlorobenzenesulfonamide (Iodo-Beads), was from Pierce Chemical Co. (Rockford, IL). Fetal Clone II and tissue culture medium were from Gibco (Grand Island, NY). Quest Fluo-8 AM was from AAT Bioquest Inc. (Sunnyvale, CA). All other reagents were of analytical grade.
Preparation of T-0632 and Its Iodination.
The acid form of T-0632 was prepared as a racemic mixture through modifications of the literature procedure (Yamada et al., 1995). In this, synthesis of the key intermediate, 2-fluoro-N-(3-methoxyphenyl)aniline, was accomplished via a Chan-Lam coupling between 3-methoxyphenylboronic acid and 2-fluoroaniline. All intermediates and the final product of the seven-step synthesis were fully characterized by NMR. A form of T-0632 modified by electrophilic iodination of T-0632–methyl ester using iodine monochloride in glacial acetic acid at 90°C was also prepared and characterized. The position of iodination was determined by comparison of the NMR of the product of this reaction with that of T-0632. The analogous radioiodinated form of T-0632 was prepared using 10 μg T-0632 and 1 mCi Na125I with exposure to an Iodo-Bead for 15 seconds. The radioiodinated product was purified by reversed-phase high-performance liquid chromatography on octadecylsilane to yield specific radioactivity of ∼2000 Ci/mmol. This procedure was also used to prepare radioligands [125I]CCK (Cawston et al., 2012) and [125I]BDZ-1 (Cawston et al., 2012).
Receptor Source.
Chinese hamster ovary (CHO) cell lines stably expressing the wild-type (WT) human CCK1R (CHO-CCK1R) and WT human CCK2R (CHO-CCK2R) (Cheng et al., 2003), which were prepared previously, were used for the current study. In addition, CHO cell lines stably expressing chimeric CCK1R/CCK2R constructs in which distinct binding pocket–facing TM residues in CCK1R were changed to those in CCK2R (Harikumar et al., 2013) were used. These included CHO lines expressing CCK1R TM2 [CCK1R(N2.61T)], TM3 [CCK1R(T3.28V,T3.29S)], TM6 [CCK1R(I6.51V,F6.52Y)], and TM7 [CCK1R(L7.39H)] constructs (Table 1) (Harikumar et al., 2013). Additionally, CHO cell lines stably expressing chimeric CCK2R/CCK1R (B/A) constructs in which exonic regions representing larger portions of each receptor were exchanged (B1A2-5, B1-2A3-5, B1-3A4-5, and B1-4A5) (Wu et al., 1997; Potter et al., 2012) were used. Cells were cultured at 37°C in an environment containing 5% CO2 on tissue culture plasticware in Ham’s F-12 medium with 5% Fetal Clone II supplement. Cells were passaged approximately twice a week and lifted mechanically before use. Membranes were prepared using centrifugation over a sucrose gradient, as we have described (Hadac et al., 1996), and were stored at −80°C in Krebs-Ringer-HEPES medium [25 mM HEPES (pH 7.4), 104 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM KH2PO4, and 1.2 mM MgSO4] containing 0.01% soybean trypsin inhibitor and 1 mM phenylmethylsulfonyl fluoride until ready for use.
TABLE 1.
Nomenclature of CCK1R-CCK2R receptor chimeras
| Constructs | Nomenclature | Construct Sequences |
|---|---|---|
| TM site replacement constructs | CCK1R TM2 | CCK1R(N98T) [N2.61T]a |
| CCK1R TM3 | CCK1R(T117V,T118S) [T3.28V,T3.29S]a | |
| CCK1R TM6 | CCK1R(I329V,F330Y) [I6.51V,F6.52Y]a | |
| CCK1R TM7 | CCK1R(L356H) [L7.39H]a | |
| Exonic chimeric constructs | B1A2-5 | CCK2R(Met1-Arg50)-CCK1R(Glu38-Gln428) |
| B1-2A3-5 | CCK2R(Met1-Met134)-CCK1R(Gly122-Gln428) | |
| B1-3A4-5 | CCK2R(Met1-Thr217)-CCK1R(Trp209-Gln428) | |
| B1-4A5 | CCK2R(Met1-Leu269)-CCK1R(Arg251-Gln428) | |
| ECL2 chimeric constructs | CCK1R (ECL2 chimer 1) | CCK1R(Met1-Pro184)-CCK2R(Val198-Gln204)-CCK1R(Cys196-Gln428) |
| CCK1R (ECL2 chimer 2) | CCK1R(Met1-Pro184)-CCK2R(Val198-Arg214)-CCK1R(Gln207-Gln428) | |
| CCK2R (ECL2 chimer 3) | CCK2R(Met1-Pro197)-CCK1R(Phe185-Met195)-CCK2R(Cys205-Gly447) | |
| CCK2R (ECL2 chimer 4) | CCK2R(Met1-Pro197)-CCK1R(Phe185-Gln206)-CCK2R(Gln215-Gly447) |
Residues in brackets represent the nomenclature of Ballesteros and Weinstein (1992). The numbers in parentheses represent the amino acid codons in the WT receptor proteins, which include the signal sequences of each receptor because the specific sites of processing and removal for these receptors have not been established.
A series of site mutations replacing one or two residues within ECL2 of CCK1R with an alanine or corresponding CCK2R residue(s) were also prepared. These constructs include M195A, R197A, R197V, F198H/L199R, L200W, N202S, V204R, Q206R, and S208T, representing mutations of nonconserved residues within the C-terminal region of ECL2 of CCK1R. Additionally, the conserved Met121 at the top of TM3 and Trp209 at the top of TM5 were mutated to alanines. All these mutation constructs were prepared using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and using WT human CCK1R in pcDNA3.1 vector as the template, with the products verified by direct DNA sequencing. In addition, chimeric constructs swapping the ECL2 segment of CCK1R(Phe185-Met195) with CCK2R(Val198-Gln204), as well as swapping larger ECL2 portions of CCK1R(Phe185-Gln206) with CCK2R(Val198-Arg214), were prepared (Table 1). These were done by excising sequences of the WT receptor cDNAs and replacing them with the corresponding sequences of the other receptor. Some receptor cDNA fragments of interest were joined together using newly introduced restriction sites. The junctions of these constructs were verified by direct DNA sequencing. Two rat CCK1R ECL2 site-mutant constructs (D203K and M205L) that were prepared previously (Dong et al., 2009) were also used. Receptor constructs were expressed transiently in COS cells after transfection using a modified DEAE-dextran protocol that included 10% dimethylsulfoxide shock and 0.1 mM chloroquine diphosphate treatment (Holtmann et al., 1996). Cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 5% Fetal Clone II, and they were studied 48 hours after transfection. Arg337 in the rat CCK1R, which is analogous to Arg336 in the human CCK1R, was also mutated to Ala for another series of studies. This mutant was stably expressed in the CHO cell line that was established as we have reported (Dong et al., 2008).
Ligand-Binding Assay.
The binding characteristics of T-0632, CCK, BDZ-1, and L-364,718 were determined in standard radioligand competition-binding assays using receptor-bearing membranes. In brief, ∼7.5-μg membranes of WT CHO-CCK1R or CHO-CCK2R or CHO cells expressing chimeric CCK1R/CCK2R TM constructs were incubated at room temperature for 1 hour with a constant amount of radioligand [125I]T-0632 or [125I]CCK (Cawston et al., 2012) or [125I]BDZ-1 (Cawston et al., 2012) (∼10,000 cpm) in 100 μl of Krebs-Ringer-HEPES medium containing 0.2% bovine serum albumin and 0.01% soybean trypsin inhibitor in the absence or presence of increasing concentrations of nonlabeled T-0632, CCK, BDZ-1, or L-364,718. Membrane-bound and -free radioligand were separated by filtration through a Unifilter-96 GF/B filter plate in a FilterMate harvester (PerkinElmer Life Sciences, Waltham, MA), as we have described (Cawston et al., 2012). Radioactivity in the plate was quantified using a TopCount NXT counter (PerkinElmer). A well established cell-binding assay was used to characterize binding of T-0632 and CCK to COS cells expressing chimeric CCK1R/CCK2R constructs exchanging larger portions of exonic regions of each receptor (Table 1), as well CCK1R site-mutant constructs (Dong et al., 2011). Competition-binding data were analyzed using the LIGAND program of Munson and Rodbard (1980), which depends on weighted least-squares curve fitting, and these were plotted using the GraphPad Prism software suite (GraphPad Software, Inc., La Jolla, CA). Data were also fit to the allosteric ternary complex model using Prism software.
Biologic Activity Assays.
The biologic activity of T-0632 was evaluated using the CHO-CCK1R cell line and assays to quantify intracellular calcium and inositol monophosphate-1 (IP1). The calcium assay was performed using Quest Fluo-8 AM on a robotic FlexStation 3 plate reader (Molecular Devices, Sunnyvale, CA) (Harikumar et al., 2013). For this, we evaluated the ability of CCK and T-0632 to stimulate calcium responses and the ability of a fixed concentration of T-0632 (0.1 μM) to modify the CCK dose-response curve, as well as the ability of T-0632 concentrations to inhibit the response to a fixed concentration of CCK (0.1 nM). The IP1 assay developed by Cisbio US Inc. (Bedford, MA) was also used with similar experimental design. All assays were performed in duplicate and repeated at least three times in independent experiments. Concentration-response curves were analyzed and plotted using nonlinear regression analysis in the GraphPad Prism software suite.
Receptor Internalization Studies.
CCK1R internalization studies were performed morphologically using C-terminally fluorescently tagged receptor (CCK1R-YFP), following the procedure we have previously described (Harikumar et al., 2006). The CHO cell line stably expressing the CCK1R-YFP construct (CHO-CCK1R-YFP) has been previously characterized to have normal binding and CCK-stimulated intracellular calcium responses (Harikumar et al., 2006). In brief, CHO-CCK1R-YFP cells grown on glass coverslips were washed twice with ice-cold phosphate-buffered saline (PBS) containing 0.1 mM MgCl2 and 0.08 mM CaCl2 and then incubated with 0.1 μM unlabeled, nonfluorescent CCK or T-0632 for 1.5 hour at 4°C. The coverslips were then washed once in cold PBS, incubated further with prewarmed PBS at 37°C for various time periods (0, 2, 5, 10, 20, and 30 minutes), fixed in 2% paraformaldehyde, washed twice in PBS, and mounted on slides using Vectashield (Vector Laboratories, Burlingame, CA). Labeled cells were examined with a Zeiss Axiovert 200M inverted microscope (Carl Zeiss, Thornwood, NY) equipped for epifluorescence.
Molecular Modeling.
All molecular modeling was conducted using a stochastic global energy optimization procedure in Internal Coordinate Mechanics (Abagyan et al., 1994) with the ICM-Pro package version 3.7-3 (MolSoft LLC, San Diego, CA). This procedure consisted of three iterative steps: 1) random conformational change of a dihedral angle according to the biased-probability Monte Carlo method (Abagyan and Totrov, 1994); 2) local minimization of all free dihedral angles; and 3) acceptance or rejection of the new conformation based on the Metropolis criterion at the simulation temperature, usually at 600 K (Metropolis et al., 1953).
The ligand-guided homology modeling method (Katritch et al., 2010) that we previously used to construct a model of CCK1R in its inactive conformation (Cawston et al., 2012) was used in this work. The inactive structure of this receptor was used as the starting point for the current modeling. A collection of 90 CCK1R antagonist ligands selected from the ChEMBL database (Overington, 2009) was used to refine the ligand-binding pocket of the inactive structure. A distance restraint was used between the ligand and an anchor residue on the receptor to limit the size of the sampling space and to keep the ligand within the pocket during the molecular modeling process. Previously, after attempting to use several different hydrogen bond donor-acceptor pairs, the best models were found to come from use of Asn6.55 as the anchor residue (Cawston et al., 2012). A defined hydrogen bond distance restraint with this residue was used during the simulations, and each ligand was docked to the pocket followed by cycles of stochastic global energy optimization, side-chain sampling, backbone minimization, and loop modeling. This resulted in an ensemble of multiple receptor conformations for T-0632 bound to CCK1R.
Each pocket conformation in the ensemble was then evaluated using a composite score that included the ability to differentiate structurally diverse positives from decoys by docking scores, the percentage of ligands forming hydrogen bonds with the anchor residue (Asn6.55), and the consistency of docking poses based on clustering of these poses as assessed by atomic property fields (Totrov, 2008). The best model was then formally evaluated using plots of receiver operating characteristic curves (Truchon and Bayly, 2007) to evaluate the ability to differentiate a set of 90 known CCK1R antagonists having activities >1 μM seeded into 103 lower-affinity CCK receptor ligands present in the ChEMBL database. The best model achieved an area under the curve value of 93%. These evaluation criteria were harder to achieve than differentiating CCK ligands from random decoys.
Results
T-0632 Radioiodination.
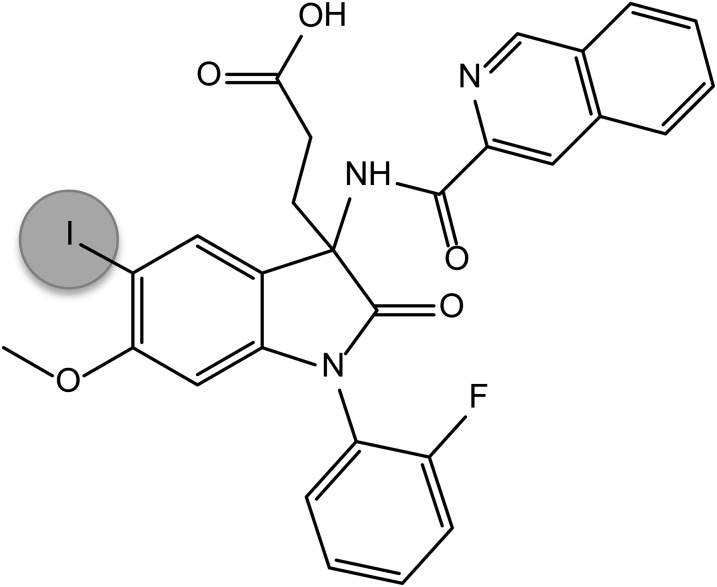
T-0632 was previously reported as a selective CCK1R antagonist (Taniguchi et al., 1996a,b); however, it has not previously been directly radioiodinated or had the molecular basis of its docking characterized. T-0632 was synthesized (Yamada et al., 1995), and its chemical identity was confirmed by NMR. Oxidative radioiodination provided a basis for introduction of an iodine at the 5-position of the indolinone ring system (Fig. 1). The product was purified to homogeneity using reversed-phase high-performance liquid chromatography.
Fig. 1.
T-0632 structure. Shown is the predicted chemical structure of the iodinated form of T-0632 used in this project.
T-0632 Binding Characterization.
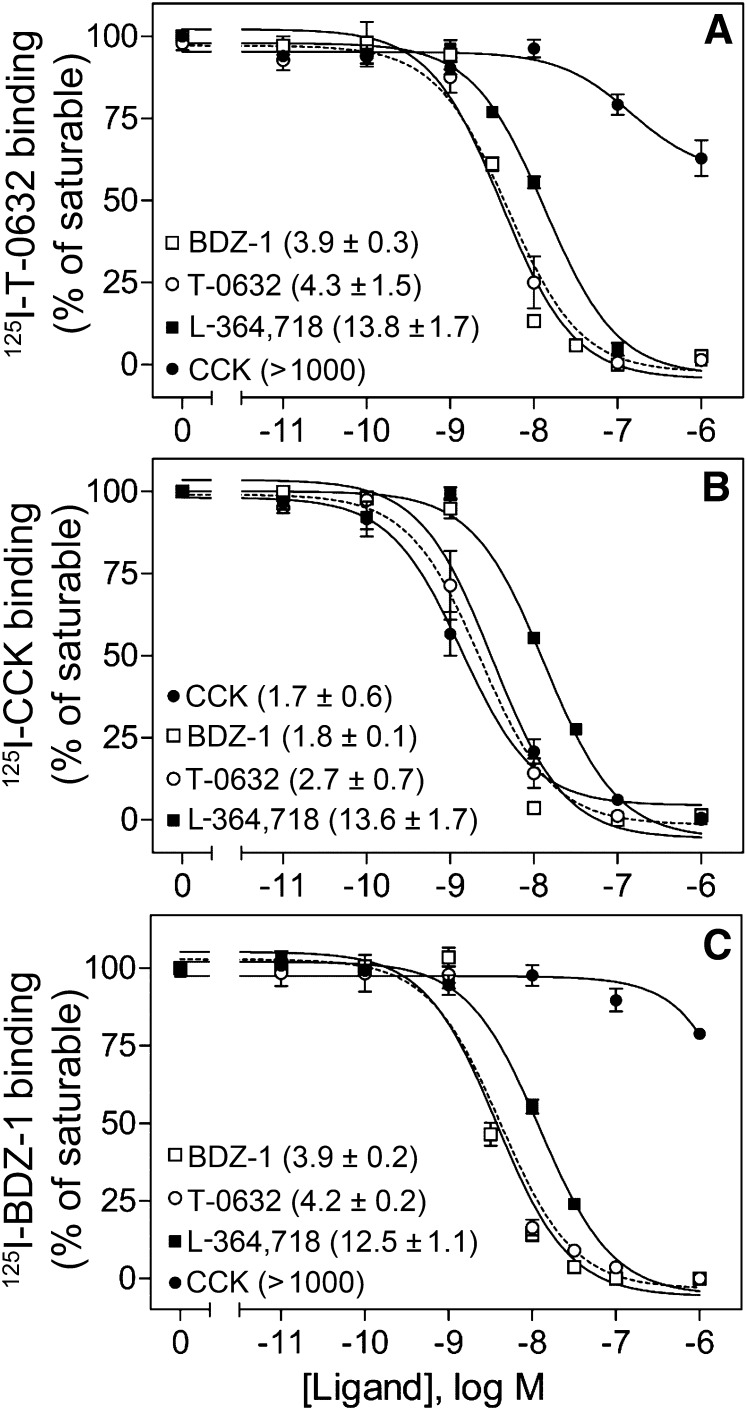
[125I]T-0632 bound saturably and with high affinity to CCK1R. As shown in the top panel of Fig. 2, T-0632 fully competed for [125I]T-0632 binding to this receptor. Analysis of the homologous competition-binding data revealed an IC50 value of 4.3 ± 1.5 nM. Of note, well characterized small-molecule benzodiazepine ligands known to bind to the intramembranous allosteric pocket within CCK1R, L-364,718 (Gao et al., 2008) and BDZ-1 (Cawston et al., 2012), also fully inhibited the saturable binding of [125I]T-0632, while the natural peptide ligand, CCK, inhibited only 37% of saturable binding at concentrations as high as 1 μM. As seen in the other panels in Fig. 2, similar competition binding was performed using [125I]CCK and [125I]BDZ-1 radioligands. Of note, the pattern of competition observed for [125I]T-0632 was most similar to that of [125I]BDZ-1. Whereas CCK did not fully inhibit [125I]T-0632 binding (inhibited 23% of saturable binding), T-0632 did fully inhibit the saturable binding of [125I]CCK, similar to other well characterized allosteric ligands of CCK1R, L-364,718 and BDZ-1 (Hadac et al., 2006; Gao et al., 2008).
Fig. 2.
Binding characterization of T-0632, CCK, and benzodiazepine ligands. Shown are competition-binding curves for T-0632, CCK, BDZ-1, and L-364,718 to compete for binding of radioligand [125I]T-0632 (A), [125I]CCK (B), and [125I]BDZ-1 (C) to membranes from CHO cells stably expressing the type 1 CCK receptor (CHO-CCK1R). Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Data are expressed as means ± S.E.M. of duplicate determinations from three independent experiments. Shown also in parentheses are the IC50 values ± S.E.M. in nanomolar units for each of the curves.
The allosteric ternary complex model was used to analyze equilibrium dissociation constants (Kb) of the orthosteric agonist radioligand, [125I]CCK, and of the allosteric antagonist radioligand, [125I]T-0632, as well as their ternary complex modulator constant, α. Analysis of the data with the [125I]CCK radioligand yielded a log Kb (molar) for CCK of −8.85 ± 0.15, with a log Kb for T-0632 of −8.65 ± 0.11 and a value for log α of less than −100 (α ≈ 0). Analysis of the data with the [125I]T-0632 radioligand yielded a log Kb for T-0632 of −8.30 ± 0.12, with a log Kb for CCK of −6.82 ± 0.10 and a value for log α of −0.23 ± 0.06 (α ≈ 0.60 ± 0.08).
Antagonist Action of T-0632.
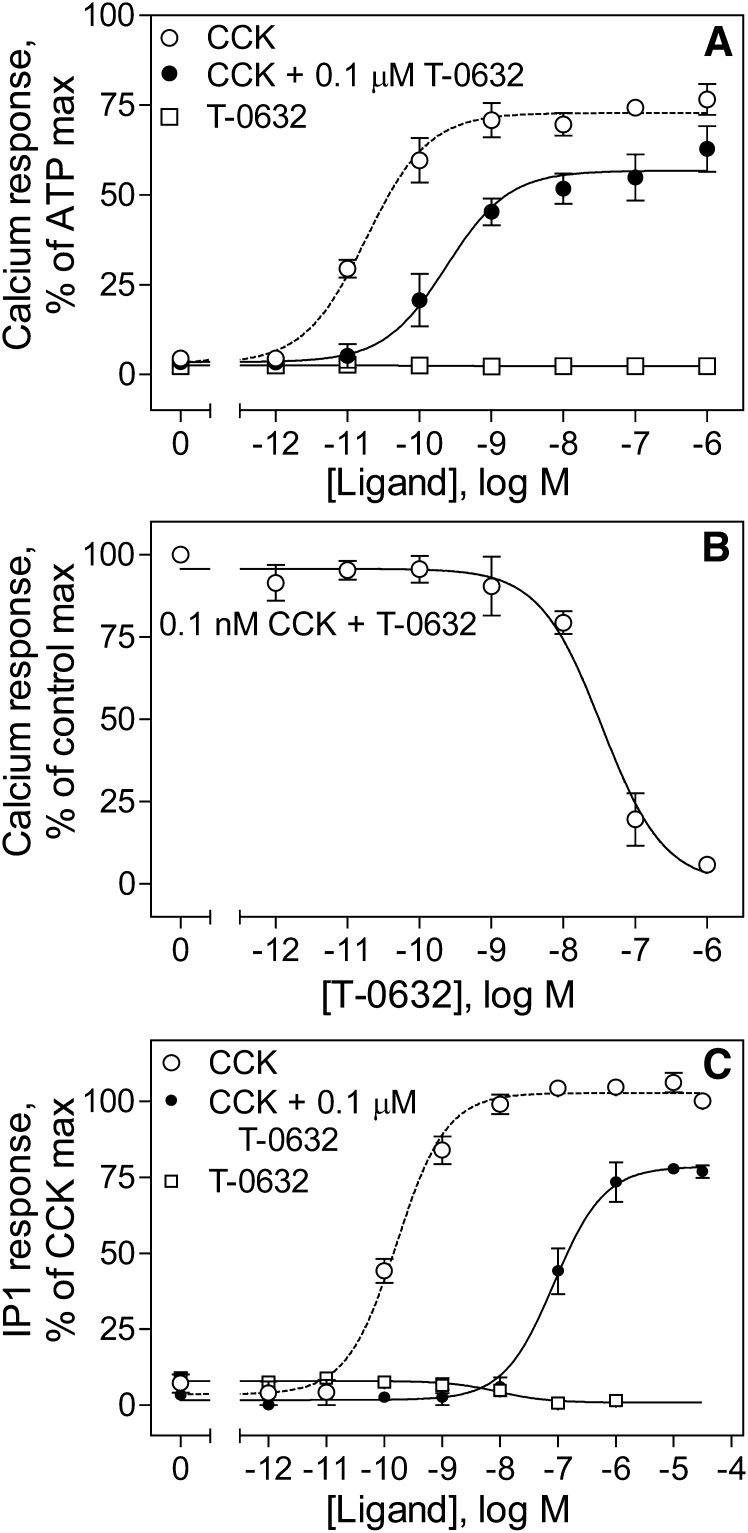
The previously reported antagonist action of T-0632 (Taniguchi et al., 1996a,b) was confirmed by the data illustrated in Fig. 3. These data show the absence of intrinsic agonist activity of T-0632 to stimulate intracellular calcium or IP1 responses in CCK1R-bearing cells. In the IP1 assay, there appears to be inverse agonist activity of T-0632. Additionally, these data show the ability of T-0632 to inhibit CCK-stimulated responses, with right shifts in the calcium and IP1 CCK dose-response curves. Of note, these data also show the previously unrecognized effect of reducing the maximal biologic effect of CCK in both of these assays. This is consistent with negative allosteric modulation of efficacy.
Fig. 3.
Inhibition by T-0632 of the CCK-stimulated biologic responses in CHO-CCK1R cells. (A) Intracellular calcium responses in CHO-CCK1R cells incubated with increasing concentrations of T-0632, CCK, or CCK in the presence of a constant amount of T-0632 (0.1 μM). (B) Inhibition of intracellular calcium responses to 0.1 nM CCK in CHO-CCK1R cells incubated with increasing concentrations of T-0632. (C) IP1 responses in CHO-CCK1R cells incubated with increasing concentrations of T-0632, CCK, or CCK in the presence of a constant amount of T-0632 (0.1 μM). Data points represent means ± S.E.M. of data from three independent experiments performed in duplicate, normalized relative to the responses stimulated by 0.1 mM ATP (A), by 0.1 nM CCK in the absence of T-0632 (B), and by 10 μM CCK (C).
Effects of T-0632 on CCK1R Internalization.
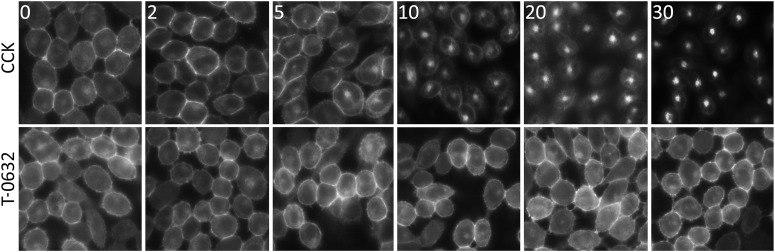
Figure 4 shows the effects of CCK and T-0632 on internalization of the CCK1R-YFP expressed on the CHO-CCK1R-YFP cell line. CCK stimulated internalization of CCK1R-YFP in a time-dependent manner, while T-0632 occupation had no effect on CCK1R-YFP internalization.
Fig. 4.
Effects of T-0632 on CCK1R internalization. Shown are time courses of internalization of the CCK1R-YFP in CHO cells induced by CCK (top row) and T-0632 (bottom row). The numbers on the left top of each panel indicate the time in minutes for ligand incubation with the cells. Immunofluorescent images are representative of three independent experiments.
Elucidation of the Molecular Basis of Binding of T-0632.
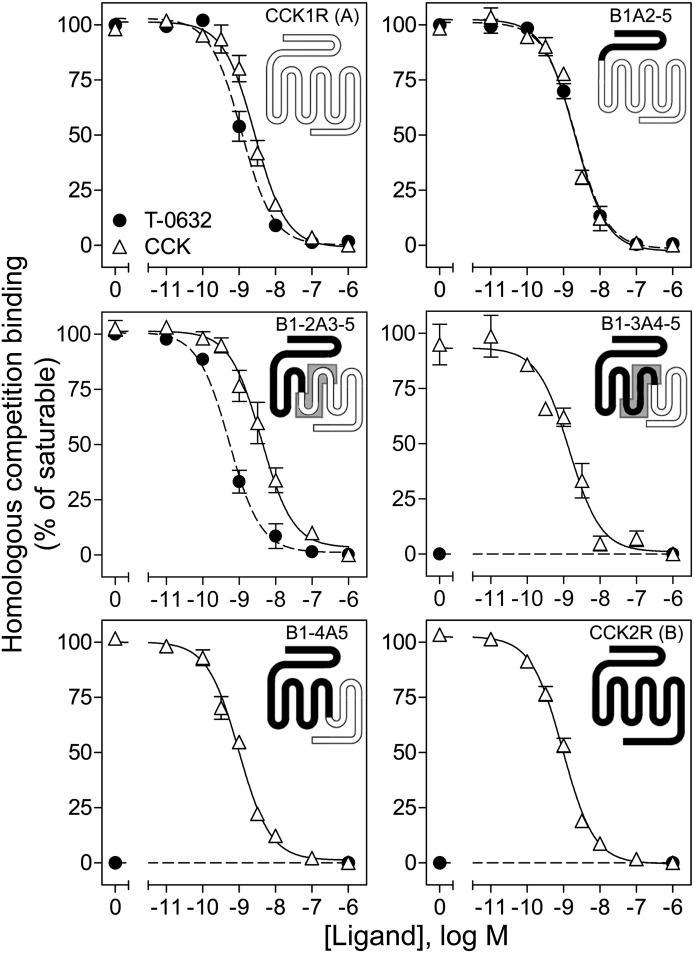
We were able to take advantage of the inability of T-0632 to bind to or to activate the closely structurally related GPCR CCK2R, by using chimeric CCK2R/CCK1R receptors to explore the molecular basis of its binding to CCK1R (Fig. 5). The region of docking was first approximated by exchanging exonic regions of CCK1R and CCK2R (Table 1) (Wu et al., 1997; Potter et al., 2012). Figure 5 shows homologous competition curves for binding of T-0632 and CCK to COS cells expressing each of these constructs. Constructs B1A2-5 and B1-2A3-5, which included exon 3 of CCK1R, bound T-0632 normally, while constructs B1-3A4-5 and B1-4A5, which did not include the exon 3 region of CCK1R, exhibited no T-0632 binding. This clearly localized key determinants for binding to exon 3, which encodes the region of CCK1R, from the bottom of TM3 to the top of TM5, including ECL2.
Fig. 5.
Identification of selectivity determinants for T-0632 binding using chimeric CCK1R/CCK2R exonic constructs. Shown are homologous competition curves for binding of T-0632 and CCK to COS cells expressing each of these chimeric CCK1R/CCK2R exonic constructs. Region of interest is shaded in gray (see text). Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Data are expressed as means ± S.E.M. of duplicate determinations from three independent experiments.
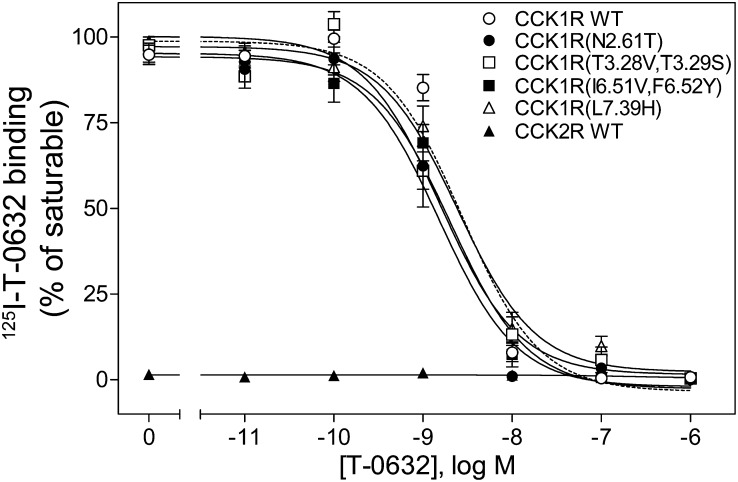
This region is known to contribute to the previously characterized benzodiazepine-binding pocket (Cawston et al., 2012). Because T-0632 binds selectively to CCK1R and not to CCK2R, it was logical to extend this analysis by studying the residues lining this pocket that are distinct in CCK1R and CCK2R. These residues have been exchanged in a series of chimeric CCK1R/CCK2R constructs that were previously used in the characterization of the benzodiazepine ligands of this receptor (Cawston et al., 2012; Harikumar et al., 2013). We studied the constructs in which those CCK1R residues facing this pocket that are distinct in CCK1R and CCK2R were changed to those in CCK2R: CCK1R(N2.61T), CCK1R(T3.28V,T3.29S), CCK1R(I6.51V,F6.52Y), and CCK1R(L7.39H) (Table 1) (Cawston et al., 2012; Harikumar et al., 2013). Figure 6 shows that none of these constructs had their T-0632 binding significantly affected by these modifications, suggesting that the key selectivity determinants likely reside elsewhere in the receptor.
Fig. 6.
Identification of selectivity determinants for T-0632 binding using CCK1R/CCK2R TM site-mutant constructs. Shown are homologous competition curves for binding of T-0632 to membranes prepared from CHO cells expressing CCK1R/CCK2R TM constructs in which those CCK1R residues facing this pocket that are distinct in CCK1R and CCK2R were changed to those in CCK2R (Cawston et al., 2012; Harikumar et al., 2013). Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Data are expressed as means ± S.E.M. of duplicate determinations from three independent experiments.
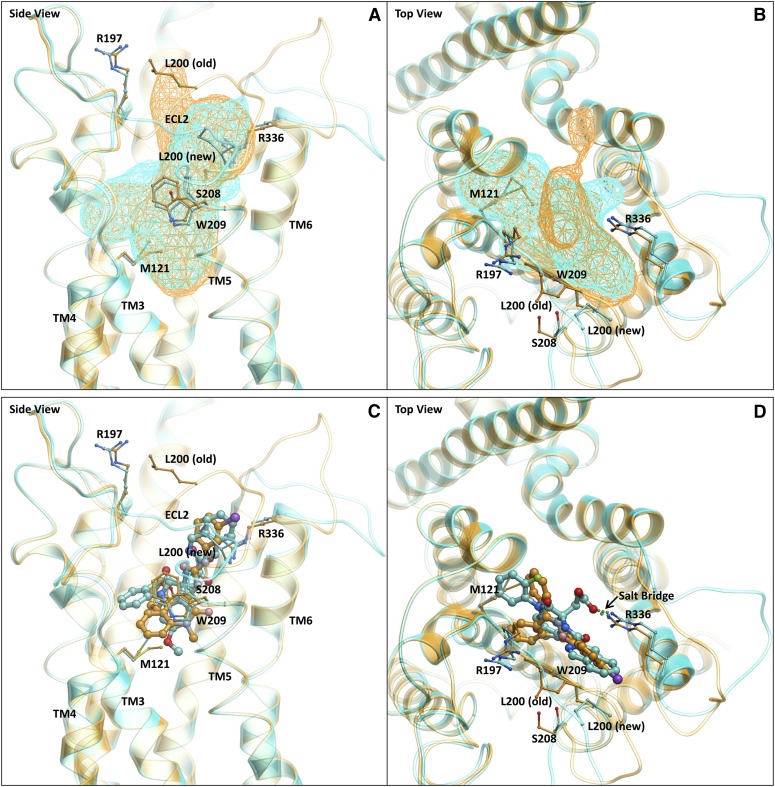
We attempted to dock T-0632 into our previous molecular model of the benzodiazepine antagonist–occupied CCK1R (Cawston et al., 2012) (Fig. 7). This demonstrated effective docking within the pocket, but also revealed key potential interactions with ECL2, which was also implicated in the exon exchange approach. Of note, the length of ECL2 is distinct in CCK1R and CCK2R, with this region four residues shorter in CCK2R (Fig. 8). This deletion is on the N-terminal side of the conserved cysteine residue that is known to be involved in the conserved disulfide bond linking the top of TM3 and ECL2 (Ding et al., 2003). The working molecular model supported an interaction in the C-terminal side of this cysteine, but it was important to study the entire loop. Following refinement of the model, we found that ECL2 moved out of the pocket between TM4 and TM5, causing a change in the volume and shape of the allosteric pocket compared with the previous model (Cawston et al., 2012) (Fig. 7A).
Fig. 7.
Molecular model of T-0632 bound to CCK1R. In all images, the new model described in this work (Supplemental Fig. 1) is represented in cyan ribbon and stick and the old model (Supplemental Fig. 2) (Cawston et al., 2012) is displayed in gold ribbon and stick. The viewpoints are from the TM “side” between TM4 and TM5 (A and C) and from the “top” through the N-terminal extracellular surface (B and D). (A) and (B) illustrate the comparison of the allosteric binding pockets of the old (gold wire mesh) and new (cyan wire mesh) models. A change in volume and shape was observed due to the movement of ECL2. (C) and (D) illustrate the comparison of the binding poses of benzodiazepine (gold stick) and T-0632 (cyan stick) in the old (gold ribbon) and new (cyan) models, respectively. A salt bridge was shown to be present between the carboxylate of T-0632 and Arg336 of CCK1R.
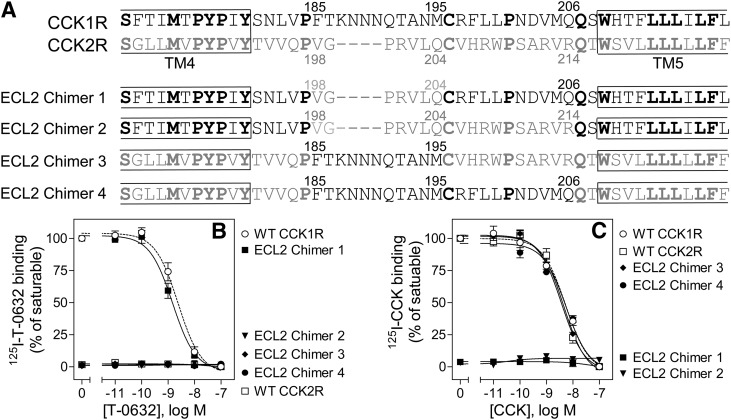
Fig. 8.
Binding characterization of CCK1R/CCK2R ECL2 chimeric constructs. Shown are an illustration of the CCK1R/CCK2R ECL2 chimeric constructs used (A) and homologous competition curves for binding of T-0632 (B) and CCK (C) to COS cells expressing these constructs. Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Data are expressed as means ± S.E.M. of duplicate determinations from three independent experiments.
The importance of ECL2 was subsequently studied by mutagenesis. We arbitrarily divided this loop into three segments: the distal region, from the conserved Cys205 (in CCK2R) to TM5; the middle region, spanning the conserved Pro197 to Cys205, that includes the segment of variable length between CCK1R and CCK2R; and the region proximal to Pro197. Substitution of the shorter middle ECL2 segment from CCK2R into CCK1R (ECL2 chimer 1) did not alter T-0632 affinity, although [125I]CCK binding was lost (Fig. 8). Likewise, insertion of the middle ECL2 segment from CCK1R into CCK2R (chimer 3) did not engender [125I]T-0632 binding, indicating that this segment does not contribute to T-0632 binding and specificity. The additional substitution of the distal ECL2 segment of CCK2R into CCK1R (chimer 2) abolished [125I]T-0632 binding; however, the extended substitution of the middle and distal ECL2 segments of CCK1R into CCK2R did not engender binding (chimer 4). This indicates that, while critical, the role of the distal region of ECL2 was contextual on the tertiary structure of CCK1R. A role of the distal ECL2 segment in T-0632 binding was consistent with our working model for docking of T-0632 (Fig. 7).
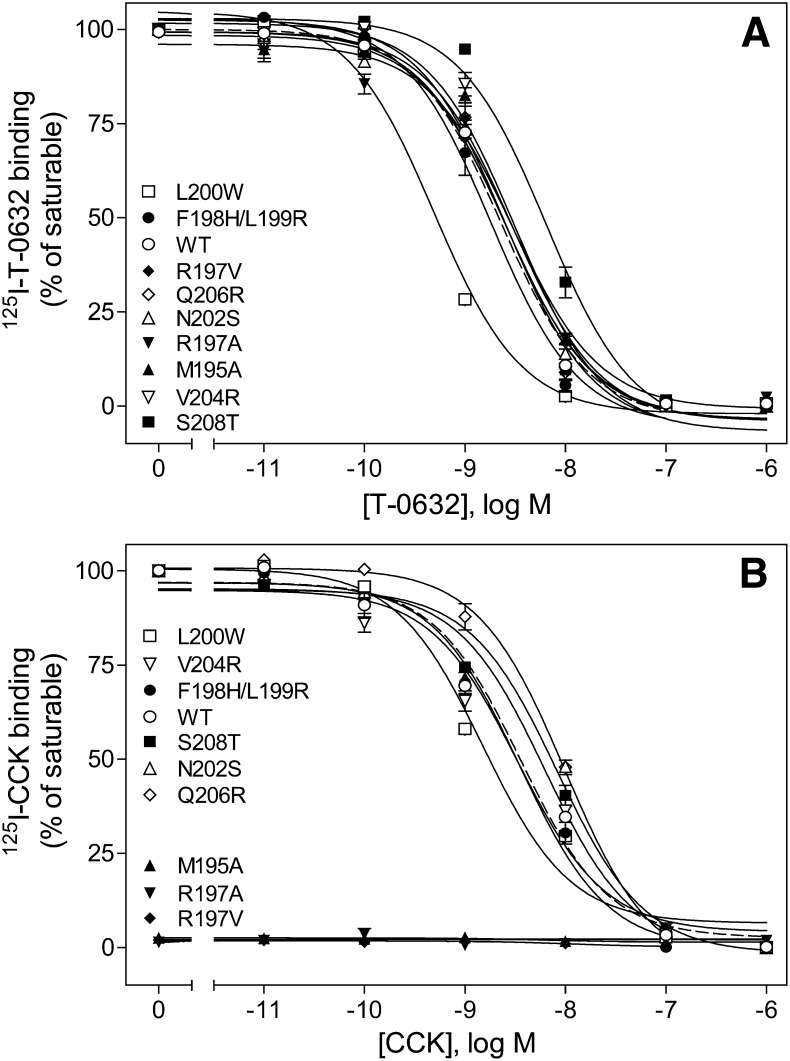
We next mutated each of the residues in this region of CCK1R (Fig. 9; Table 2). There was minimal or no loss of T-0632 binding for mutation of each of these residues normally present in the ECL2 region of CCK1R to the equivalent residues in CCK2R. Even the Arg197 residue, which has major importance for CCK binding and action, had no effect on T-0632 binding. The model predicted that Arg197 would be directed away from the allosteric pocket, thereby explaining the lack of effect on T-0632 binding. Of note, a residue just outside this region, Ser208, was found to be important, with the S208A mutant found to bind T-0632 with affinity ∼3-fold lower than that of WT CCK1R, while CCK bound normally to this construct. Also of interest, replacement of Leu200 with a larger hydrophobic residue, Trp, significantly enhanced T-0632 binding affinity. Indeed, in our model this residue is adjacent to the hydrophobic isoquinoline moiety within T-0632, and the Trp substitution was predicted to provide improved docking of T-0632.
Fig. 9.
Binding characterization of ECL2 site mutants. Shown are homologous competition curves for binding of T-0632 (A) and CCK (B) to COS cells expressing ECL2 site-mutant constructs. Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Data are expressed as means ± S.E.M. of duplicate determinations from three independent experiments.
TABLE 2.
Analysis of homologous competition-binding curves for CCK1R constructs
Values represent means ± S.E.M. of data from a minimum of three independent assays performed in duplicate. Two-tailed t tests were performed to determine the significance of differences.
| Constructs | T-0632 Binding | CCK Binding | ||
|---|---|---|---|---|
| Ki | Bmax | Ki | Bmax | |
| nM | (× 103/cell) | nM | (× 103/cell) | |
| WT | 2.3 ± 0.6 | 191 ± 55 | 3.6 ± 0.6 | 140 ± 36 |
| M121A | 42.4 ± 7.6* | 125 ± 40 | 13.2 ± 3.1 | 146 ± 39 |
| M195A | 3.0 ± 0.4 | 150 ± 46 | N.D. | N.D. |
| R197A | 3.0 ± 0.5 | 50 ± 21 | N.D. | N.D. |
| R197V | 2.5 ± 0.6 | 100 ± 35 | N.D. | N.D. |
| F198H/L199R | 1.8 ± 0.4 | 65 ± 17 | 3.5 ± 0.6 | 110 ± 34 |
| L200W | 0.4 ± 0.04* | 63 ± 20 | 1.5 ± 0.6 | 174 ± 40 |
| N202S | 2.6 ± 0.5 | 111 ± 39 | 8.3 ± 0.4* | 45 ± 15 |
| D203Ka | 0.9 ± 0.3 | 74 ± 13 | 3.1 ± 0.3 | 138 ± 10 |
| V204R | 3.2 ± 0.7 | 103 ± 44 | 3.3 ± 1.1 | 150 ± 39 |
| M205La | 1.4 ± 0.5 | 112 ± 21 | 2.1 ± 0.2 | 117 ± 30 |
| Q206R | 2.6 ± 0.6 | 146 ± 33 | 9.9 ± 0.9* | 145 ± 47 |
| S208T | 6.4 ± 1.2* | 170 ± 47 | 6.2 ± 2.0 | 145 ± 32 |
| W209A | N.D. | N.D. | N.D. | N.D. |
| R336Aa | N.D. | N.D. | N.D. | N.D. |
N.D., not detectable.
Represents data in rat CCK1R construct.
P < 0.05 vs. WT receptor.
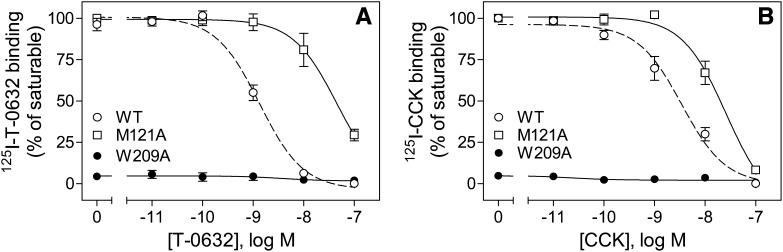
Our refined molecular model also predicted possible interactions and important determinants of T-0632 binding within this pocket that are conserved in both CCK1R and CCK2R. We next studied those residues using alanine-replacement mutants (Figs. 10 and 11; Table 2). The W209A mutant was not tolerated, eliminating both CCK and T-0632 binding, likely due to misfolding and/or trafficking. However, the M121A mutant had a much more profound negative impact on T-0632 binding than on CCK binding. Thus, while conserved in sequence between CCK1R and CCK2R, this residue must be presented quite differently in the conformation of these two CCK receptor subtypes.
Fig. 10.
Binding characterization of conserved TM site mutants. Shown are homologous competition curves for binding of T-0632 (A) and CCK (B) to COS cells expressing site-mutant constructs M121A and W209A. Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Data are expressed as means ± S.E.M. of duplicate determinations from three independent experiments.
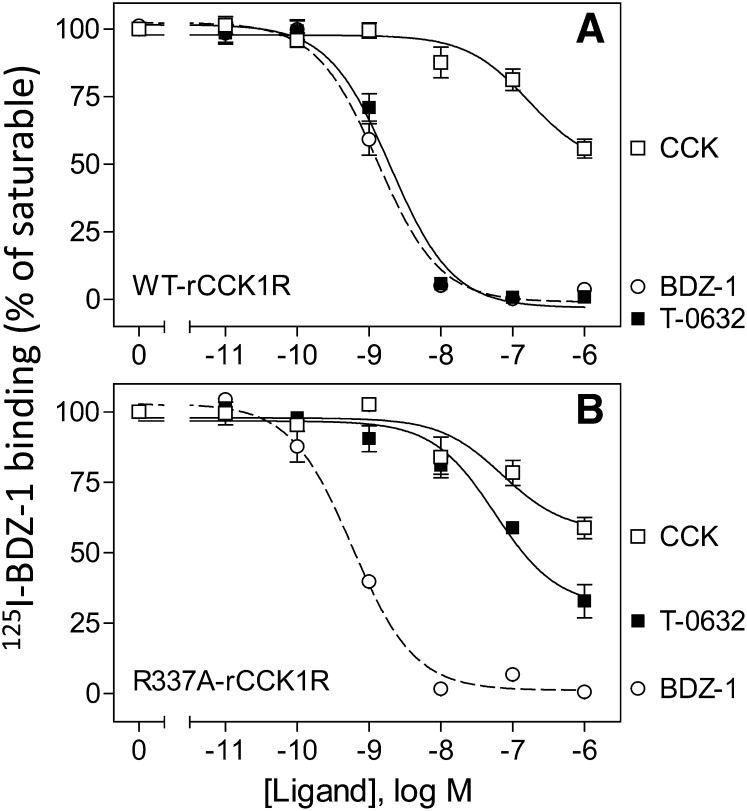
Fig. 11.
Binding characterization of TM6 mutant. Shown are competition curves for CCK, T-0632, and BDZ-1 to compete for binding radioligand, [125I]BDZ-1, to membranes from CHO cells expressing WT rat CCK1R (A) and the R337A mutant construct (B). Values represent percentages of maximal saturable binding that were observed in the absence of competitor. Data are expressed as means ± S.E.M. of duplicate determinations from three independent experiments.
Another particularly interesting residue that is also conserved in CCK1R and CCK2R was Arg336 at the top of TM6. The working molecular model suggested the possibility of formation of a salt bridge between a carboxylate group present in T-0632 that is absent in the benzodiazepine ligands and receptor residue Arg336. We previously prepared and studied the analogous residue in the rat CCK1R (Arg337 in the rat CCK1R), based on a series of studies showing its importance for CCK peptide binding (Gigoux et al., 1999a; Gouldson et al., 2000; Martin-Martinez et al., 2005). Indeed, this mutation in rat CCK1R, R337A, markedly reduced CCK binding (Fig. 11) while having no effect on the binding of BDZ-1, a classic benzodiazepine ligand. In contrast, the binding of T-0632 was markedly reduced (80-fold shift in affinity) in this mutant, supporting the model prediction.
The best explanation for these data is that there are structural differences in the helical bundle conformation of CCK1R and CCK2R. The chimeric receptor approach is highly dependent on conservation of structure, with focused differences determining distinct functional properties. This set of data strongly supports differences in conformation of these two closely related receptors. This is also consistent with the distinct modes of docking the same CCK peptides to both receptors that has been proposed previously (Dawson et al., 2002; Miller and Gao, 2008).
Discussion
GPCRs are remarkable for their ability to change conformation, providing the basis for binding highly diverse ligands that approach from their extracellular surfaces and that result in changes in the conformation of the receptor cytosolic face that facilitate coupling with G proteins and initiation of other effector pathways. This ability to change shapes also results in a broad energy landscape that includes a variety of conformations compatible with binding distinct ligands with different patterns of biologic effects.
In the current work, we examine the molecular basis of binding of a unique, highly selective, nonpeptidyl small-molecule antagonist ligand to the CCK1R, T-0632 (Taniguchi et al., 1996a,b). This was facilitated by the development of a high-specific-radioactivity and high-affinity radioiodinated form of T-0632. Most radioligands used to study CCK receptor binding have been analogs of the natural peptide ligand, CCK (Powers et al., 1988). A benzodiazepine radioligand was also recently reported (Akgun et al., 2009), providing the first well defined allosteric radioligand for this receptor that provides an ability to screen for other small-molecule allosteric ligands independent of effects on the binding of the orthosteric peptide radioligand. This broadens the capability for identifying unique ligands in competition-binding assays. Based on the properties now reported, this new T-0632 radioligand should have similar capabilities, as well as the advantage of being much more selective for CCK1R over CCK2R.
T-0632 was previously proposed as a competitive antagonist of CCK, based on its ability to compete for CCK radioligand binding (Taniguchi et al., 1996a). While we have also observed T-0632 inhibition of CCK-stimulated biologic activity, we now demonstrate that CCK does not fully inhibit T-0632 radioligand binding, consistent with an allosteric mode of docking of this compound. Interestingly, we did not observe the reciprocal effect when CCK was used as the radioligand and T-0632 was used as the unlabeled inhibitor; in that situation, complete inhibition was observed. This is most likely due the fact that the radioligands label different receptor states in the membrane preparations. Specifically, T-0632 would be expected to prefer the inactive receptor state (R), whereas CCK, as an agonist, would preferentially label the active, G protein–coupled state (RG); the complete inhibition of CCK binding by T-0632 in the latter instance thus reflects negative modulation of both CCK-binding affinity and the ability of CCK to promote receptor–G protein coupling. Functionally, an allosteric mode of action that was consistent with reduced CCK signaling efficacy was further supported by the biologic activity studies, in which we observed not only right shifts in the CCK dose-response curves for stimulating intracellular calcium and IP1 in the presence of T-0632, but also a reduction in the maximal biologic responses to CCK in the presence of T-0632 in these assays.
Quantitative analysis of the data generated using the two radioligands—the orthosteric agonist radioligand, [125I]CCK, and the allosteric antagonist radioligand, [125I]T-0632—provided further noteworthy insights. The log Kb of CCK from the allosteric fit for CCK inhibiting binding of radiolabeled [125I]T-0632 (6.82 ± 0.10) was significantly different from the log Kb of CCK inhibiting [125I]CCK binding (−8.85 ± 0.15). This is consistent with the former interaction monitoring events happening on the R state of the receptor (low agonist affinity and low negative cooperativity), while the second interaction is monitoring events happening on the RG state of the receptor (high agonist affinity and competition with CCK, yet high negative cooperativity with T-0632). Of particular interest, the affinity of T-0632 does not appear to be different between these two states (log Kb for T-0632 determined using the CCK radioligand, −8.65 ± 0.11; and determined using the T-0632 radioligand, −8.30 ± 0.12), suggesting that the allosteric binding pocket is less sensitive to receptor conformation than the orthosteric site, while the cooperativity between these sites is very sensitive to receptor conformation.
This work also extends our understanding of the nature of small-molecule antagonist binding to CCK1R. The current molecular model is shown to be distinct from the one that we previously reported that was based largely on benzodiazepine ligands (Cawston et al., 2012). Our current understanding can be further generalized using T-0632 that includes a carboxylate group that was absent in the previously studied benzodiazepines. The current model predicts that this group is present with a salt bridge to a residue high in TM6, Arg336. This is supported by experimental data showing marked negative impact of replacing the Arg336 of CCK1R on T-0632 binding while having no effect on benzodiazepine ligand binding.
Another major difference is the role played by the distal (C-terminal) region of ECL2 for T-0632 docking. Indeed, this region of class A GPCRs has been suggested to be quite important for function, although the themes for various family members have been quite distinct. A range of conformations have been described in various receptors, with rhodopsin having ECL2 function as a lid to constrain retinal (Palczewski et al., 2000) while ECL2 is present as an extended sheet in some members of this family that bind peptide ligands (Zhang et al., 2012; Hulme, 2013). The movement of ECL2 has been suggested as being important in the activation of family A GPCRs, helping to propagate the conformational changes that are believed to occur in TM5 (Ahuja et al., 2009; Hulme, 2013). Crystal structures have also clearly supported key roles of ECL2 in the docking of small-molecule ligands to class A GPCRs (Dore et al., 2011; Kruse et al., 2013). In these papers, ZM241385 is clearly shown to interact with this region of ECL2 in the adenosine A2A receptor (Dore et al., 2011) and small allosteric modulators have key interactions with ECL2 in the muscarinic acetylcholine receptor (Dore et al., 2011; Kruse et al., 2013). This region of ECL2 has been studied in CCK receptors. CCK1R residues, including Arg187 (Dong et al., 2007), Met195 (Gigoux et al., 1998), and Arg197 (Gigoux et al., 1999b; Ding et al., 2002; Arlander et al., 2004), are important for CCK binding and signaling. In CCK2R, ECL2 residue His207 is important for binding and signaling of both gastrin and CCK (Silvente-Poirot and Wank, 1996; Silvente-Poirot et al., 1998); however, mutation of the analogous residue in CCK1R (Phe198) had no effect on the binding of either CCK or T-0632 in the current studies.
Given the high-affinity binding of T-0632 to CCK1R and absence of binding to CCK2R, it was unexpected to find substantial negative impact on T-0632 binding by mutating residues that were conserved in these two subtypes of CCK receptors. Alanine replacements for Met121 and Arg336 in CCK1R had marked negative effects on T-0632 binding. This suggests that these residues, while conserved in sequence, likely assume different positions in the folded receptor. This provides further support for the interpretation that conformations of the pockets within these closely related GPCRs are distinct.
This work also adds a new, very useful, and highly selective antagonist radioligand that binds directly to the helical bundle region of CCK1R, increasing the tools useful for drug discovery at this physiologically important GPCR.
Supplementary Material
Acknowledgments
The authors thank M. L. Augustine and A. S. Ball for their excellent technical assistance. The authors also thank other Wellesley students who contributed to the syntheses of T-0632 analogs: A. Kanyer, L. J. Kim, J. Kwon, and E. A. McLoughlin.
Abbreviations
- BDZ-1
(S)-1-(3-iodophenyl)-3-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)urea
- CCK
cholecystokinin
- CHO
Chinese hamster ovary
- ECL2
second extracellular loop region
- GPCR
G protein–coupled receptor
- L-364,718
3S(−)-N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4- benzodiazepine-3-yl)-1H-indole-2-carboxamide
- PBS
phosphate-buffered saline
- T-0632
(2-fluorophenyl)-2,3-dihydro-3-[(3-isoquinolinylcarbonyl)amino]-6-methoxy-2-oxo-l-H-indole-3-propanoate
- TM
transmembrane segment
- WT
wild type
Authorship Contributions
Participated in research design: Dong, Haines, Miller.
Conducted experiments: Dong, Vattelana, Lam, Orry.
Performed data analysis: Dong, Lam, Orry, Abagyan, Christopoulos, Sexton, Miller.
Wrote or contributed to the writing of the manuscript: Dong, Lam, Orry, Abagyan, Christopoulos, Sexton, Haines, Miller.
Footnotes
This work was supported by the National Institutes of Health [Grant DK032878] and by the Mayo Clinic. A.C. and P.M.S. are Principal Research Fellows of the National Health and Medical Research Council of Australia.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Abagyan R, Totrov M. (1994) Biased probability Monte Carlo conformational searches and electrostatic calculations for peptides and proteins. J Mol Biol 235:983–1002. [DOI] [PubMed] [Google Scholar]
- Abagyan R, Totrov M, Kuznetsov D. (1994) ICM: a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem 15:488–506. [Google Scholar]
- Ahuja S, Hornak V, Yan EC, Syrett N, Goncalves JA, Hirshfeld A, Ziliox M, Sakmar TP, Sheves M, Reeves PJ, et al. (2009) Helix movement is coupled to displacement of the second extracellular loop in rhodopsin activation. Nat Struct Mol Biol 16:168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgün E, Körner M, Gao F, Harikumar KG, Waser B, Reubi JC, Portoghese PS, Miller LJ. (2009) Synthesis and in vitro characterization of radioiodinatable benzodiazepines selective for type 1 and type 2 cholecystokinin receptors. J Med Chem 52:2138–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlander SJ, Dong M, Ding XQ, Pinon DI, Miller LJ. (2004) Key differences in molecular complexes of the cholecystokinin receptor with structurally related peptide agonist, partial agonist, and antagonist. Mol Pharmacol 66:545–552. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. (1992) Analysis and refinement of criteria for predicting the structure and relative orientations of transmembranal helical domains. Biophys J 62:107–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston EE, Lam PC, Harikumar KG, Dong M, Ball AM, Augustine ML, Akgün E, Portoghese PS, Orry A, Abagyan R, et al. (2012) Molecular basis for binding and subtype selectivity of 1,4-benzodiazepine antagonist ligands of the cholecystokinin receptor. J Biol Chem 287:18618–18635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZJ, Harikumar KG, Holicky EL, Miller LJ. (2003) Heterodimerization of type A and B cholecystokinin receptors enhance signaling and promote cell growth. J Biol Chem 278:52972–52979. [DOI] [PubMed] [Google Scholar]
- Dawson ES, Henne RM, Miller LJ, Lybrand TP. (2002) Moleular models for cholecystokinin-A receptor. Pharmacol Toxicol 91:290–296. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Dolu V, Hadac EM, Schuetz M, Miller LJ. (2003) Disulfide bond structure and accessibility of cysteines in the ectodomain of the cholecystokinin receptor: specific mono-reactive receptor constructs examine charge-sensitivity of loop regions. Receptors Channels 9:83–91. [PubMed] [Google Scholar]
- Ding XQ, Pinon DI, Furse KE, Lybrand TP, Miller LJ. (2002) Refinement of the conformation of a critical region of charge-charge interaction between cholecystokinin and its receptor. Mol Pharmacol 61:1041–1052. [DOI] [PubMed] [Google Scholar]
- Dong M, Ding XQ, Thomas SE, Gao F, Lam PC, Abagyan R, Miller LJ. (2007) Role of lysine187 within the second extracellular loop of the type A cholecystokinin receptor in agonist-induced activation. Use of complementary charge-reversal mutagenesis to define a functionally important interdomain interaction. Biochemistry 46:4522–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Gao F, Pinon DI, Miller LJ. (2008) Insights into the structural basis of endogenous agonist activation of family B G protein-coupled receptors. Mol Endocrinol 22:1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Lam PC, Pinon DI, Abagyan R, Miller LJ. (2009) Elucidation of the molecular basis of cholecystokinin peptide docking to its receptor using site-specific intrinsic photoaffinity labeling and molecular modeling. Biochemistry 48:5303–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Lam PC, Pinon DI, Hosohata K, Orry A, Sexton PM, Abagyan R, Miller LJ. (2011) Molecular basis of secretin docking to its intact receptor using multiple photolabile probes distributed throughout the pharmacophore. J Biol Chem 286:23888–23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Liu G, Pinon DI, Miller LJ. (2005) Differential docking of high-affinity peptide ligands to type A and B cholecystokinin receptors demonstrated by photoaffinity labeling. Biochemistry 44:6693–6700. [DOI] [PubMed] [Google Scholar]
- Doré AS, Robertson N, Errey JC, Ng I, Hollenstein K, Tehan B, Hurrell E, Bennett K, Congreve M, Magnani F, et al. (2011) Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19:1283–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Sexton PM, Christopoulos A, Miller LJ. (2008) Benzodiazepine ligands can act as allosteric modulators of the Type 1 cholecystokinin receptor. Bioorg Med Chem Lett 18:4401–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigoux V, Escrieut C, Fehrentz JA, Poirot S, Maigret B, Moroder L, Gully D, Martinez J, Vaysse N, Fourmy D. (1999a) Arginine 336 and asparagine 333 of the human cholecystokinin-A receptor binding site interact with the penultimate aspartic acid and the C-terminal amide of cholecystokinin. J Biol Chem 274:20457–20464. [DOI] [PubMed] [Google Scholar]
- Gigoux V, Escrieut C, Silvente-Poirot S, Maigret B, Gouilleux L, Fehrentz JA, Gully D, Moroder L, Vaysse N, Fourmy D. (1998) Met-195 of the cholecystokinin-A receptor interacts with the sulfated tyrosine of cholecystokinin and is crucial for receptor transition to high affinity state. J Biol Chem 273:14380–14386. [DOI] [PubMed] [Google Scholar]
- Gigoux V, Maigret B, Escrieut C, Silvente-Poirot S, Bouisson M, Fehrentz JA, Moroder L, Gully D, Martinez J, Vaysse N, et al. (1999b) Arginine 197 of the cholecystokinin-A receptor binding site interacts with the sulfate of the peptide agonist cholecystokinin. Protein Sci 8:2347–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouldson P, Legoux P, Carillon C, Dumont X, Le Fur G, Ferrara P, Shire D. (2000) Essential role of extracellular charged residues of the human CCK(1) receptor for interactions with SR 146131, SR 27897 and CCK-8S. Eur J Pharmacol 389:115–124. [DOI] [PubMed] [Google Scholar]
- Hadac EM, Dawson ES, Darrow JW, Sugg EE, Lybrand TP, Miller LJ. (2006) Novel benzodiazepine photoaffinity probe stereoselectively labels a site deep within the membrane-spanning domain of the cholecystokinin receptor. J Med Chem 49:850–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadac EM, Ghanekar DV, Holicky EL, Pinon DI, Dougherty RW, Miller LJ. (1996) Relationship between native and recombinant cholecystokinin receptors: role of differential glycosylation. Pancreas 13:130–139. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Cawston EE, Lam PC, Patil A, Orry A, Henke BR, Abagyan R, Christopoulos A, Sexton PM, Miller LJ. (2013) Molecular basis for benzodiazepine agonist action at the type 1 cholecystokinin receptor. J Biol Chem 288:21082–21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Clain J, Pinon DI, Dong M, Miller LJ. (2005) Distinct molecular mechanisms for agonist peptide binding to types A and B cholecystokinin receptors demonstrated using fluorescence spectroscopy. J Biol Chem 280:1044–1050. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Dong M, Cheng Z, Pinon DI, Lybrand TP, Miller LJ. (2006) Transmembrane segment peptides can disrupt cholecystokinin receptor oligomerization without affecting receptor function. Biochemistry 45:14706–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann MH, Hadac EM, Ulrich CD, Miller LJ. (1996) Molecular basis and species specificity of high affinity binding of vasoactive intestinal polypeptide by the rat secretin receptor. J Pharmacol Exp Ther 279:555–560. [PubMed] [Google Scholar]
- Hulme EC. (2013) GPCR activation: a mutagenic spotlight on crystal structures. Trends Pharmacol Sci 34:67–84. [DOI] [PubMed] [Google Scholar]
- Katritch V, Rueda M, Lam PC, Yeager M, Abagyan R. (2010) GPCR 3D homology models for ligand screening: lessons learned from blind predictions of adenosine A2a receptor complex. Proteins 78:197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Ring AM, Manglik A, Hu J, Hu K, Eitel K, Hübner H, Pardon E, Valant C, Sexton PM, et al. (2013) Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature 504:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Martínez M, Marty A, Jourdan M, Escrieut C, Archer E, González-Muñiz R, García-López MT, Maigret B, Herranz R, Fourmy D. (2005) Combination of molecular modeling, site-directed mutagenesis, and SAR studies to delineate the binding site of pyridopyrimidine antagonists on the human CCK1 receptor. J Med Chem 48:4842–4850. [DOI] [PubMed] [Google Scholar]
- Metropolis N, Rosenbluth AW, Rosenbluth MN, Teller AH, Teller E. (1953) Equation of state calculations by fast computing machines. J Chem Phys 21:1087–1092. [Google Scholar]
- Miller LJ, Gao F. (2008) Structural basis of cholecystokinin receptor binding and regulation. Pharmacol Ther 119:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson PJ, Rodbard D. (1980) Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem 107:220–239. [DOI] [PubMed] [Google Scholar]
- Overington J. (2009) ChEMBL. An interview with John Overington, team leader, chemogenomics at the European Bioinformatics Institute Outstation of the European Molecular Biology Laboratory (EMBL-EBI). Interview by Wendy A. Warr. J Comput Aided Mol Des 23:195–198. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. (2000) Crystal structure of rhodopsin: a G protein-coupled receptor. Science 289:739–745. [DOI] [PubMed] [Google Scholar]
- Potter RM, Harikumar KG, Wu SV, Miller LJ. (2012) Differential sensitivity of types 1 and 2 cholecystokinin receptors to membrane cholesterol. J Lipid Res 53:137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SP, Pinon DI, Miller LJ. (1988) Use of N,O-bis-Fmoc-D-Tyr-ONSu for introduction of an oxidative iodination site into cholecystokinin family peptides. Int J Pept Protein Res 31:429–434. [DOI] [PubMed] [Google Scholar]
- Shoichet BK, Kobilka BK. (2012) Structure-based drug screening for G-protein-coupled receptors. Trends Pharmacol Sci 33:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvente-Poirot S, Escrieut C, Wank SA. (1998) Role of the extracellular domains of the cholecystokinin receptor in agonist binding. Mol Pharmacol 54:364–371. [DOI] [PubMed] [Google Scholar]
- Silvente-Poirot S, Wank SA. (1996) A segment of five amino acids in the second extracellular loop of the cholecystokinin-B receptor is essential for selectivity of the peptide agonist gastrin. J Biol Chem 271:14698–14706. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Yazaki N, Endo T, Nagasaki M. (1996a) Pharmacological profile of T-0632, a novel potent and selective CCKA receptor antagonist, in vitro. Eur J Pharmacol 304:147–154. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Yazaki N, Yomota E, Shikano T, Endo T, Nagasaki M. (1996b) Pharmacological profile of T-0632, a novel potent and selective CCKA receptor antagonist, in vivo. Eur J Pharmacol 312:227–233. [DOI] [PubMed] [Google Scholar]
- Totrov M. (2008) Atomic property fields: generalized 3D pharmacophoric potential for automated ligand superposition, pharmacophore elucidation and 3D QSAR. Chem Biol Drug Des 71:15–27. [DOI] [PubMed] [Google Scholar]
- Truchon JF, Bayly CI. (2007) Evaluating virtual screening methods: good and bad metrics for the “early recognition” problem. J Chem Inf Model 47:488–508. [DOI] [PubMed] [Google Scholar]
- Vaidehi N, Bhattacharya S, Larsen AB. (2014) Structure and dynamics of G-protein coupled receptors. Adv Exp Med Biol 796:37–54. [DOI] [PubMed] [Google Scholar]
- Wu V, Yang M, McRoberts JA, Ren J, Seensalu R, Zeng N, Dagrag M, Birnbaumer M, Walsh JH. (1997) First intracellular loop of the human cholecystokinin-A receptor is essential for cyclic AMP signaling in transfected HEK-293 cells. J Biol Chem 272:9037–9042. [DOI] [PubMed] [Google Scholar]
- Yamada K, Hikota M, Shikano T and Nagasaki M (1995) inventors, Tanabe Seiyaku Co., Ltd., assignee. 2-Oxoindoline derivatives as cholecystokinin receptor antagonists. International patent WO 9514668 A1 19950601. 1995 June 1.
- Zhang C, Srinivasan Y, Arlow DH, Fung JJ, Palmer D, Zheng Y, Green HF, Pandey A, Dror RO, Shaw DE, et al. (2012) High-resolution crystal structure of human protease-activated receptor 1. Nature 492:387–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.