Abstract
S-Adenosylmethionine (SAMe), the principal methyl donor that is available as a nutritional supplement, and its metabolite methylthioadenosine (MTA) exert chemopreventive properties against liver and colon cancer in experimental models. Both agents reduced β-catenin expression on immunohistochemistry in a murine colitis-associated colon cancer model. In this study, we examined the molecular mechanisms involved. SAMe or MTA treatment in the colitis-associated cancer model lowered total β-catenin protein levels by 47 and 78%, respectively. In an orthotopic liver cancer model, increasing SAMe levels by overexpressing methionine adenosyltransferase 1A also reduced total β-catenin levels by 68%. In both cases, lower cyclin D1 and c-Myc expression correlated with lower β-catenin levels. In liver (HepG2) and colon (SW480, HCT116) cancer cells with constitutively active β-catenin signaling, SAMe and MTA treatment inhibited β-catenin activity by excluding it from the nuclear compartment. However, in liver (Huh-7) and colon (RKO) cancer cells expressing wild-type Wnt/β-catenin, SAMe and MTA accelerated β-catenin degradation by a glycogen synthase kinase 3-β–dependent mechanism. Both agents lowered protein kinase B activity, but this was not mediated by inhibiting phosphoinositide 3-kinase. Instead, both agents increased the activity of protein phosphatase 2A, which inactivates protein kinase B. The effect of MTA on lowering β-catenin is direct and not mediated by its conversion to SAMe, as blocking this conversion had no influence. In conclusion, SAMe and MTA inhibit Wnt/β-catenin signaling in colon and liver cancer cells regardless of whether this pathway is aberrantly induced, making them ideal candidates for chemoprevention and/or chemotherapy in these cancers.
Introduction
S-Adenosylmethionine (SAMe) is a naturally occurring biomolecule found in our bodies that is synthesized by methionine adenosyltransferase (MAT) isoenzymes (Lu and Mato, 2012). SAMe serves as the primary methyl donor for all trans-methylation reactions and is involved in biosynthesis in which 5′-methylthioadenosine (MTA) is a byproduct (Lu and Mato, 2012). SAMe spontaneously breaks down to MTA extracellularly, and MTA is converted back to SAMe via the methionine salvage pathway intracellularly (Lu and Mato, 2012). Exogenously delivered SAMe and MTA promote apoptosis and attenuate progrowth signals in colon and liver cancer cells (Yang et al., 2004; Chen et al., 2007; Ramani et al., 2008; Li et al., 2009; Lu and Mato, 2012). In HepG2 liver cancer cells, SAMe and MTA can reduce leptin’s mitogenic effect by reducing both extracellular signal-regulated kinase and phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) signaling (Ramani et al., 2008). SAMe and MTA can also inhibit the mitogenic effects of epidermal growth factor, insulin-like growth factor-1, and leptin in colon cancer cells (Chen et al., 2007). In a recent study of azoxymethane/dextran sulfate sodium salt (AOM/DSS)–induced chronic inflammation colon cancer model, we found that SAMe and MTA treatment reduced colonic tumor size and number (Li et al., 2012). SAMe and MTA treatment inhibited the growth-promoting and prosurvival signals of nuclear factor κB and interleukin-6 (Li et al., 2012). We also observed a reduction in active AKT and total β-catenin levels, two proteins that are also involved in growth and proliferation (Li et al., 2012).
β-Catenin is the primary effector molecule in the Wnt signaling pathway (Anastas and Moon, 2013). Although β-catenin is constitutively expressed, the majority of it is degraded. β-catenin is recognized by a destruction complex that consists of Axin, adenomatous polyposis coli (APC), and glycogen synthase kinase 3 (GSK3)β (Anastas and Moon, 2013). GSK3β phosphorylates the N terminus of β-catenin at serines 33 and 37 and threonine 41, which allows it to be recognized by β-transducin repeat-containing E3 ubiquitin protein ligase to target for ubiquitination and subsequently proteasomal degradation (Anastas and Moon, 2013). Wnt signaling plays a major role in growth, survival, and development and in adult tissues containing actively growing cells (Klaus and Birchmeier, 2008; Holland et al., 2013). Some Wnt target genes that promote growth and proliferation include CMYC and CCND1 (cyclin D1) (Clevers, 2006). Different mutations in the Wnt pathway, such as APC, axin, or β-catenin stabilizing mutations, causing aberrant signaling, have been found in many types of cancer, which include colon cancer, hepatocellular carcinoma (HCC), and hepatoblastoma (Giles et al., 2003). The main goals of this study were to determine whether SAMe and MTA can influence the Wnt signaling pathway in colon and liver cancer and define the mechanisms by which SAMe and MTA mediate this effect. Our studies showed that SAMe and MTA can inhibit Wnt signaling by two distinct mechanisms, as follows: one, by reducing the levels of nuclear β-catenin in cancer cells with constitutively active Wnt signaling; and two, by promoting the degradation of β-catenin by increasing the pool of active GSK3β.
Materials and Methods
SAMe in a disulfate p-toluene sulfonate salt was a generous gift from Gnosis SRL (Cairate, Italy). MTA, cycloleucine (1-amino-1-cyclopentanecarboxylic acid), MG132 (Z-Leu-Leu-Leu-al), and LY294002 were obtained from Sigma-Aldrich (St. Louis, MO). The GSK3β inhibitor TDZD-8 was purchased from EMD Millipore (Billerica, MA), and human recombinant Wnt3a was obtained from R&D Systems (Minneapolis, MN). β-Catenin, phospho-β-catenin (s33/s37), phospho-GSK3β (s9), GSK3β, phospho-AKT (t308), phospho-AKT (s473), pan AKT, pan protein phosphatase 2A (PP2A)c, histone H3, and β-tubulin were all purchased from Cell Signaling Technology (Danvers, MA). Phospho-PP2Ac (Y307) and MATα1 antibody was purchased from Abcam (Cambridge, MA). β-Actin antibody was purchased from Sigma-Aldrich. All other reagents used were of analytical grade.
Cell Culture and Treatments.
SW480 (APC mutant), HCT116 (β-catenin–stable mutant), RKO (no mutations), HepG2 (β-catenin–stable N-terminal truncated mutant), and Huh-7 (no mutations, Wnt3 expressed) cells were obtained from the Cell Culture Core at the University of Southern California Research Center for Liver Diseases. All cell lines were tested and authenticated by American Type Culture Collection (Manassas, VA) using short random repeat profiling in August 2014. HepG2 and Huh-7 cells were grown in Dulbecco’s modified Eagle’s medium, SW480 cells in L15, and HCT116 cells in McCoy’s 5A media containing 10% research grade fetal bovine serum (Seradigm, Providence, UT) in a humidified incubator at 37°C with a 5% CO2 atmosphere. In experiments with RKO cells, 4 × 105 cells were plated on six-well plates and grown to 50–55% confluency the following day. Subsequently, cells were treated with 150 ng/ml recombinant Wnt3a concurrently with either 2 mM SAMe (0.5 M stock SAMe dissolved in 1.68 M Tris solution) or 1 mM MTA [0.5 M stock MTA dissolved in dimethylsulfoxide (DMSO)] for 6 hours. For experiments assessing the effect of SAMe and MTA on β-catenin phosphorylation, RKO cells were treated with 20 μM proteasomal inhibitor MG132 for 6 hours concurrently with Wnt3a alone, Wnt3a + SAMe, and Wnt3a + MTA. An antibody to phospho-β-catenin (s33/t37) was used to detect the level of β-catenin phosphorylation after the various treatment combinations. For experiments involving GSK3β inhibition, 20 μM TDZD-8 was added at the same time as the Wnt3a alone, Wnt3a + SAMe, or Wnt3a + MTA treatments for 6 hours. To inhibit the PI3K pathway in RKO cells, 20 μM PI3K inhibitor LY294002 was added alone or concurrently with Wnt3a, SAMe, MTA, Wnt3a + SAMe, or Wnt3a + MTA for 6 hours. Whole-cell lysates were isolated and subjected to Western blotting using antibodies to β-catenin and phospho-AKT (s473) to confirm the effectiveness of LY294002. For Huh-7 cells, 3 × 105 cells were plated on six-well plates the day before treatment with either 2 mM SAMe or 1 mM MTA for 6 hours. All control experiments had 0.2% DMSO final concentration vehicle added to the cells.
Mouse Colon and Liver Tissues.
Colon tissue samples from control AOM/DSS mice and ones treated with SAMe or MTA were isolated, as previously described (Li et al., 2012). The control (empty vector) and MAT1A-overexpressing liver tumor samples used were obtained, as previously described (Yang et al., 2013). Four-month-old Mat1a knockout and age- and gender-matched control livers were obtained, as previously described (Lu et al., 2001). Liver specimens from 3-month-old male C57/B6 mice treated with SAMe (150 mg/kg per day) or MTA (75 mg/kg per day) added to drinking water (6 ml/day based on average intake of mouse per day) for 6 days were obtained as we described (Chen et al., 2007). The control mice were fed 0.2% DMSO via their water supply. Animals were treated humanely, and all procedures were in compliance with our institutions’ guidelines for the use of laboratory animals.
Transient Transfection and Super 8× TOPFlash Reporter Assays.
SW480 (2 × 105), HepG2 (3 × 105), and HCT116 (2 × 105 cells/well) cells were plated on 12-well plates 1 day prior to transfection. Either 0.5 μg Super 8× TOPFlash (Addgene, Cambridge, MA) or 0.5 g Super 8× FOPFlash (Addgene) was cotransfected with 25 ng CMV-pRL Renilla luciferase (Promega, Madison, WI) for 24 hours using jetPRIME transfection reagent (Polyplus Transfection, New York, NY). Either 2 mM SAMe or 1 mM MTA was added 3, 6, or 12 hours prior to the end of the 24-hour transfection period. For experiments involving cycloleucine (MAT inhibitor, to block the conversion of MTA to SAMe), cells were pretreated for 2 hours with 20 mM cycloleucine and followed by cotreatment with MTA for another 3, 6, or 12 hours. After transfection, the cells were lysed and assayed for luciferase activity using Promega’s Dual Luciferase Reporter Assay System. The TOPFlash/FOPFlash reporter activity was normalized to the CMV-pRL activity.
mRNA Isolation of Mouse Colon and Liver Tumor Samples and Quantitative Real-Time Polymerase Chain Reaction.
Total RNA was isolated from colon and liver tumors using the Quick RNA miniprep kit, according to the suggested manufacturer’s protocol (Zymo Research, Irvine, CA). A quantity amounting to 0.5 μg total RNA was used to reverse transcribe cDNA using NxGen M-MuLV Reverse Transcriptase (Lucigen, Middleton, WI) in a 20 μl volume. Two microliters of the newly synthesized cDNA was added to 1× KAPA Probe Fast qPCR Universal mastermix (KAPA Biosystems, Wilmington, MA) and TaqMan mouse probes from Life Technologies (Grand Island, NY) to either c-Myc or Ccnd1. The reactions were run on the LightCycler 480 (Roche, Indianapolis, IN) for quantitative real-time polymerase chain reaction analysis. Expression was normalized to glyceraldehyde 3-phosphate dehydrogenase.
Confocal Analysis of β-Catenin Localization in SW480 Cells.
Confocal analysis of the SW480 cells was done according to the Jackson ImmunoResearch (West Grove, PA) protocol, as previously described (Peng et al., 2013). Briefly, 5 × 105 cells were plated on a glass coverslip in six-well plates and grown overnight. The cells were treated with either SAMe (1 mM to 2 mM) or MTA (0.5 mM to 1 mM) for 12 hours. DMSO (0.2% final concentration) was used as the untreated control. The primary β-catenin antibody was diluted 1:100 for overnight incubation at 4°C. Afterward, a 1:20 diluted fluorescein isothiocyanate-labeled goat anti-rabbit IgG secondary antibody (Jackson ImmunoResearch) was used to label and stain for β-catenin on the fixed SW480 cells. The coverslips with cells were then mounted onto slides with Vectashield mounting medium containing 4′,6′-diamidino-2-phenylindole stain (Vector Laboratiories, Burlington, CA). The samples were visualized by an Eclipse TE300 confocal microscope (Nikon Instruments, Melville, NY).
Protein Isolation and Western Blot Analysis.
Nuclear and cytoplasmic extract isolation for SW480 cells was performed using the NE-PER Nuclear and Cytoplasmic Extraction Reagent kit (Thermo Scientific, Rockford, IL). Protein isolation and Western blotting were done, as previously described (Li et al., 2012). For each sample, 20 μg protein was loaded for Western blot analysis. β-actin was used as a loading control for most Western blots, except for the nuclear and cytoplasmic isolation fractions in which histone H3 and α-tubulin served as loading controls, respectively.
Statistical Analysis.
All data shown represent the mean ± SEM. Statistical analysis was done using analysis of variance, followed by the Fisher’s Student t test for multiple comparisons. All protein quantifications were done by comparing the densitometric values derived from the ratios of the samples to their respective loading controls. Significance was defined by P < 0.05.
Results
SAMe and MTA Reduced β-Catenin Level and Its Target Gene Expression In Vivo.
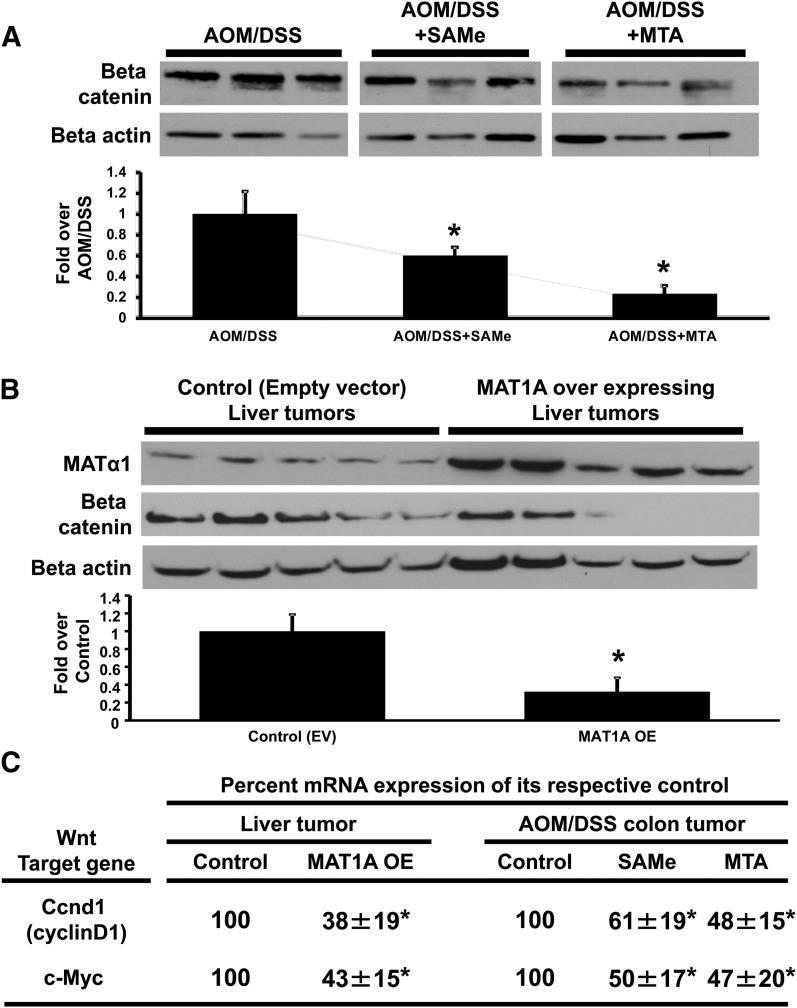
Our previous study showed that SAMe and MTA treatment reduced β-catenin levels on immunohistochemistry in the tumors of an inflammation-induced colon cancer mouse model (Li et al., 2012). To confirm this effect more quantitatively, Western blot analysis was performed and showed that treatment of 100 mg/kg per day SAMe or 75 mg/kg per day MTA reduced total β-catenin levels by 47 and 78%, respectively (Fig. 1A). To determine whether the effect of SAMe on β-catenin also occurs in liver cancer, we used an orthotopic liver cancer mouse model comparing liver tumors derived from injected Hep3B liver cancer cells overexpressing Matα1 protein or an empty vector. Previously, we showed that overexpressing Matα1 in liver cancer cells elevated intracellular SAMe levels by twofold (Li et al., 2010). Figure 1B shows that tumors overexpressing MATα1 have a 68% reduction in total β-catenin as compared with the empty vector control tumors. β-Catenin is the main effector of the Wnt signaling pathway. SAMe- or MTA-treated colon and MATα1-overexpressing liver tumors have lower mRNA levels of two Wnt targets, cyclin D1 and c-Myc, as compared with their respective controls (Fig. 1C). However, SAMe (150 mg/kg per day) and MTA (75 mg/kg per day) treatment of 6 days had no effect on normal liver β-catenin protein expression (Supplemental Fig. 1). This suggests that exogenously delivered SAMe and MTA can affect the Wnt signaling pathway in cancer cells by reducing its main effector β-catenin.
Fig. 1.
SAMe and MTA reduced β-catenin protein levels and Wnt target genes in colon and liver cancer. Western blot analysis of β-catenin levels was done in (A) individual colon tumors of AOM/DSS mice treated with SAMe or MTA and in (B) individual liver tumors derived from Hep3B liver cancer cells overexpressing Matα1 (resulting in elevated SAMe levels) injected into the mouse liver as compared with their respective controls. (C) Quantitative real-time polymerase chain reaction analysis measured Wnt target genes c-Myc and Ccnd1 in the colon and liver cancer mouse models. The mRNA expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase. All data were derived from individual tumors isolated from three to five mice. The graphs below the blots represent the densitometric values expressed as fold of their respective controls from individual tumors of three to five mice. *P < 0.05 versus respective control.
SAMe and MTA Reduced TOPFlash Reporter Activity in Both Colon and Liver Cancer Cell Lines.
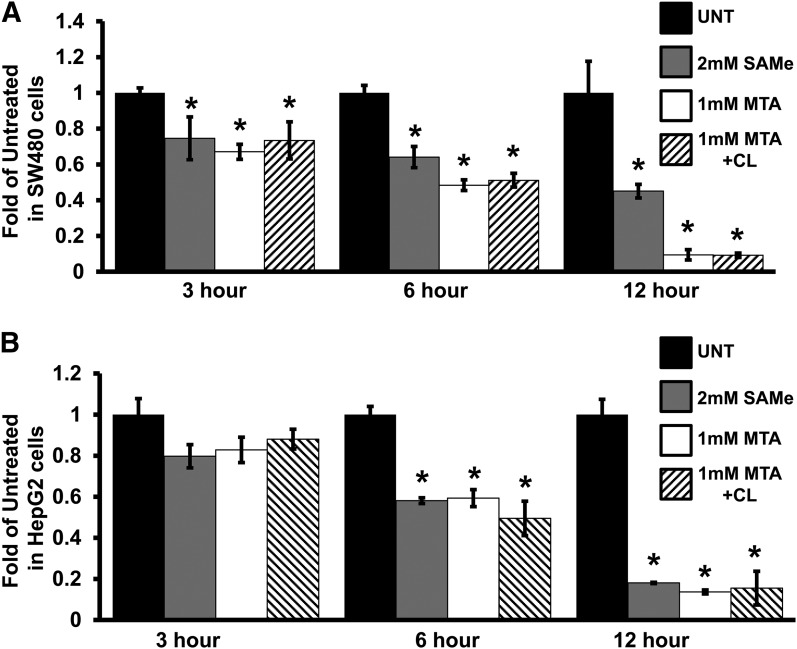
To determine whether SAMe- and MTA-mediated reduction of Wnt target genes is at the transcriptional level, we used a super TOPFlash reporter containing multimerized Wnt response elements transiently transfected in colon cancer (SW480, APC mutant; HCT116 cells, β-catenin–stable mutant) and liver cancer (HepG2, β-catenin–stable N-terminal truncated mutant) cell lines with constitutively aberrant active Wnt signaling (de La Coste et al., 1998; Gayet et al., 2001). In SW480 and HepG2 cells, SAMe and MTA lowered reporter activity in a time-dependent manner (Fig. 2). Similar results were obtained with HCT116 cells (data not shown). No reporter activity in the negative control reporter construct FOPFlash was observed in any of the cell lines (data not shown). MTA can convert back to SAMe via the methionine salvage pathway (Lu and Mato, 2012). To examine whether the effect of MTA on TOPFlash activity was through SAMe, cells were pretreated with 20 mM cycloleucine, a MAT inhibitor that blocks MTA’s conversion to SAMe. Pretreatment with cycloleucine for 2 hours followed by cotreatment with MTA did not alter MTA’s effect on TOPFlash reporter activity in both cell lines (Fig. 2).
Fig. 2.
SAMe/MTA reduced T cell–specific factor/β-catenin signaling in colon and liver cancer cell lines. Super 8× TOPFlash reporter plasmid contains multiple Wnt response elements (T cell–specific factor/β-catenin sites) that test for Wnt responsiveness. SAMe/MTA treatment reduced Wnt activity in a time-dependent manner (3, 6, and 12 hours) in (A) SW480 colon cancer and (B) HepG2 liver cancer cells. The SW480 and HepG2 cells were pretreated for 2 hours with 20 mM cycloleucine (CL), followed by cotreatment with 1 mM MTA for 3, 6, or 12 hours. Graphs represent data from at least three independent experiments, with numbers normalized to CMV-pRL and expressed as a ratio of the TOPFlash activity over that of the empty vector controls. *P < 0.05 versus respective control. UNT, untreated vehicle control (0.2% DMSO final concentration).
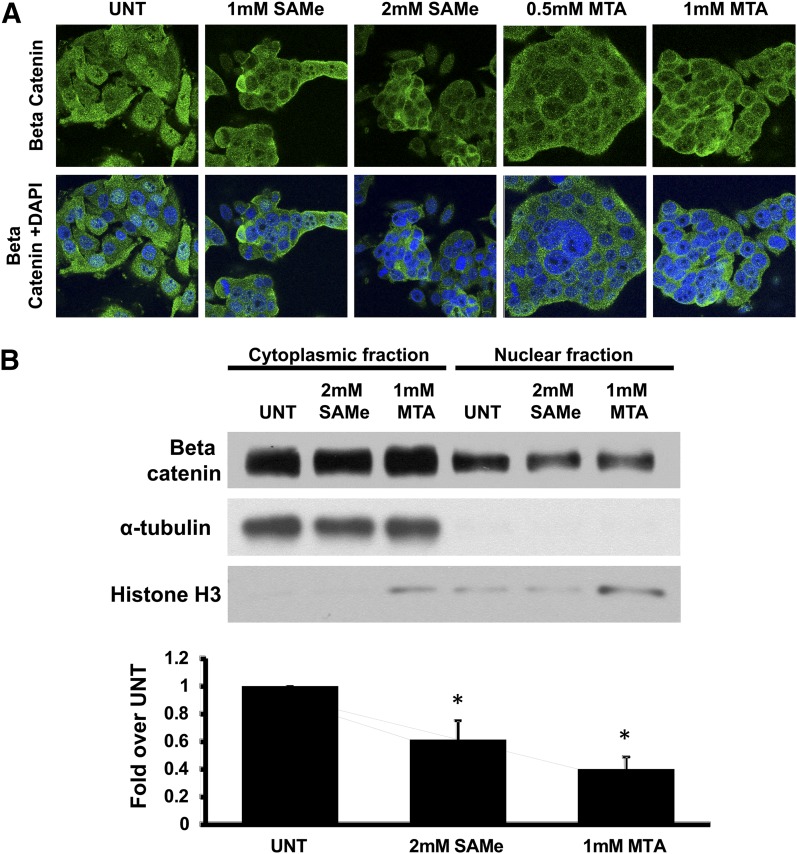
SAMe and MTA Reduced Nuclear β-Catenin Accumulation in SW480 Colon Cancer Cells.
The majority of sporadic and heritable forms of colon cancer have mutations that inactivate the ability of the cell to degrade β-catenin (Giles et al., 2003). The stabilized β-catenin can accumulate in the nucleus to act on Wnt target genes. In SW480 cells, SAMe and MTA did not alter total β-catenin mRNA nor protein levels (data not shown), yet TOPFlash activity fell (Fig. 2A). This prompted us to examine whether SAMe and MTA altered β-catenin subcellular localization. Confocal analysis shows that 12-hour treatment of SAMe or MTA reduced nuclear β-catenin content as compared with the DMSO (0.2% final) control (Fig. 3A). This was confirmed by Western blot analysis of the cytoplasmic and nuclear fractions of SW480 cells (Fig. 3B). Similar results were observed with HepG2 cells (data not shown).
Fig. 3.
SAMe and MTA reduced nuclear β-catenin content in SW480 colon cancer. (A) Representative images of SW480 colon cancer cells (APC mutant) treated for 12 hours with varying doses of SAMe (1 and 2 mM) or MTA (0.5 mM and 1 mM). Confocal analysis was done to visualize the fluorescein isothiocyanate–labeled β-catenin signal (green: top images). The bottom set shows merged images of β-catenin and the 4′,6′-diamidino-2-phenylindole–stained nuclei. Images are representative of three independent experiments and visualized at 600× magnification under oil immersion. (B) Western blot analysis of β-catenin levels in the cytoplasmic and nuclear fraction of SW480 cells after 12 hours of 2 mM SAMe or 1 mM MTA treatment. α-Tubulin and histone H3 served as cytoplasmic and nuclear markers, respectively. The graph below the blots represents the densitometric values expressed as fold of their respective controls from three independent experiments. *P < 0.05 versus respective control.
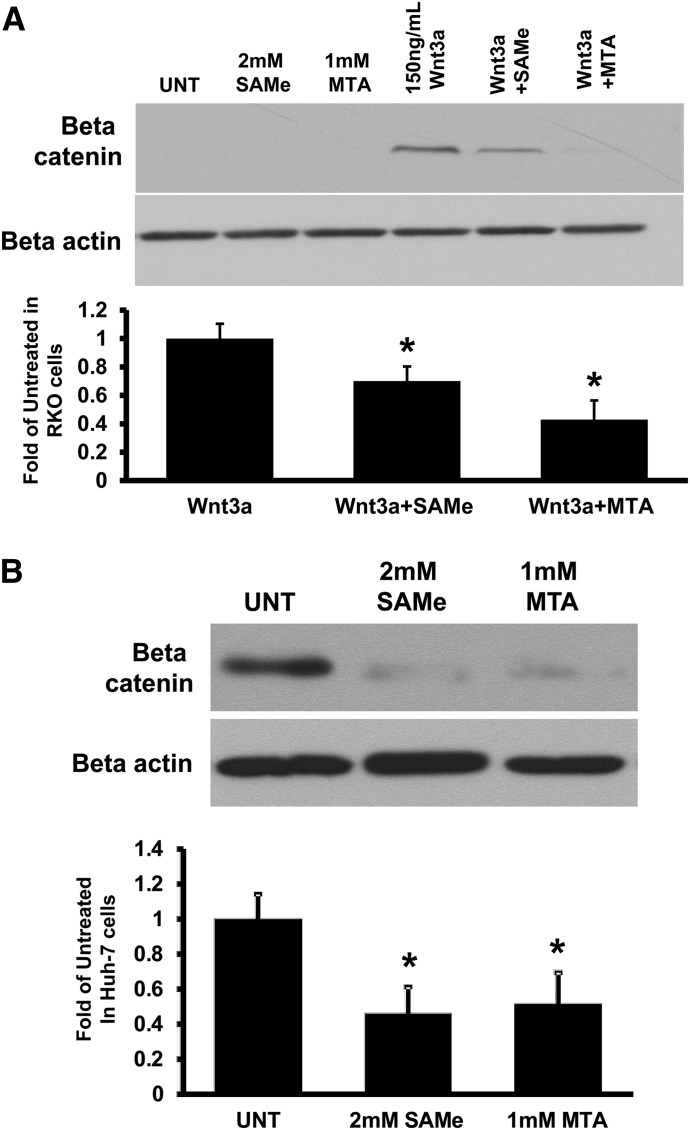
SAMe and MTA Reduced β-Catenin Levels in Colon and Liver Cancer Cell Lines Containing No Mutations in β-Catenin Destruction Complex.
Because SAMe- or MTA-treated AOM/DSS colon cancer tumors and the MATα1-overexpressing liver tumors have lower total β-catenin levels (Fig. 1), we suspect these agents can also inhibit Wnt/β-catenin signaling by another mechanism in cells with intact Wnt signaling. To investigate this possibility, we treated RKO colon cancer cells (basal Wnt activity, β-catenin protein not detectable due to rapid degradation) with recombinant Wnt3a to inhibit β-catenin degradation. Cotreatment of SAMe or MTA with Wnt3a for 6 hours lowered β-catenin protein levels by 30 and 57%, respectively, as compared with Wnt3a alone (Fig. 4A). β-Catenin mRNA levels were not affected by SAMe or MTA treatment, suggesting that the mechanism lies at the protein level (data not shown). To demonstrate that this effect was also true for liver cancer cells, we treated Huh-7 cells, which have no mutations in the Wnt pathway, but endogenously express Wnt3 so that β-catenin protein is detectable at baseline (Wei et al., 2011), with SAMe or MTA for 6 hours. Similar to RKO cells, SAMe and MTA treatment lowered β-catenin protein levels in Huh-7 cells (Fig. 4B).
Fig. 4.
SAMe and MTA lowered Wnt-mediated activation of β-catenin in RKO colon cancer and Huh-7 liver cancer cells with normal intact Wnt pathway. (A) RKO cells cotreated with 150 ng/ml human recombinant Wnt3a to active Wnt signaling pathway were treated with 2 mM SAMe or 1 mM MTA for 6 hours. (B) Huh-7 cells (stabilized β-catenin due to the cells expressing endogenous Wnt3) were treated with either 2 mM SAMe or 1 mM MTA for 6 hours. Western blot analysis was done to examine β-catenin levels. The graphs below the blots represent the densitometric values expressed as fold of their respective controls from at least three independent experiments. All values were normalized to β-actin. *P < 0.05 versus respective control. UNT, untreated vehicle control (0.2% DMSO final concentration).
β-Catenin Degradation Mediated by SAMe and MTA Requires Active GSK3β.
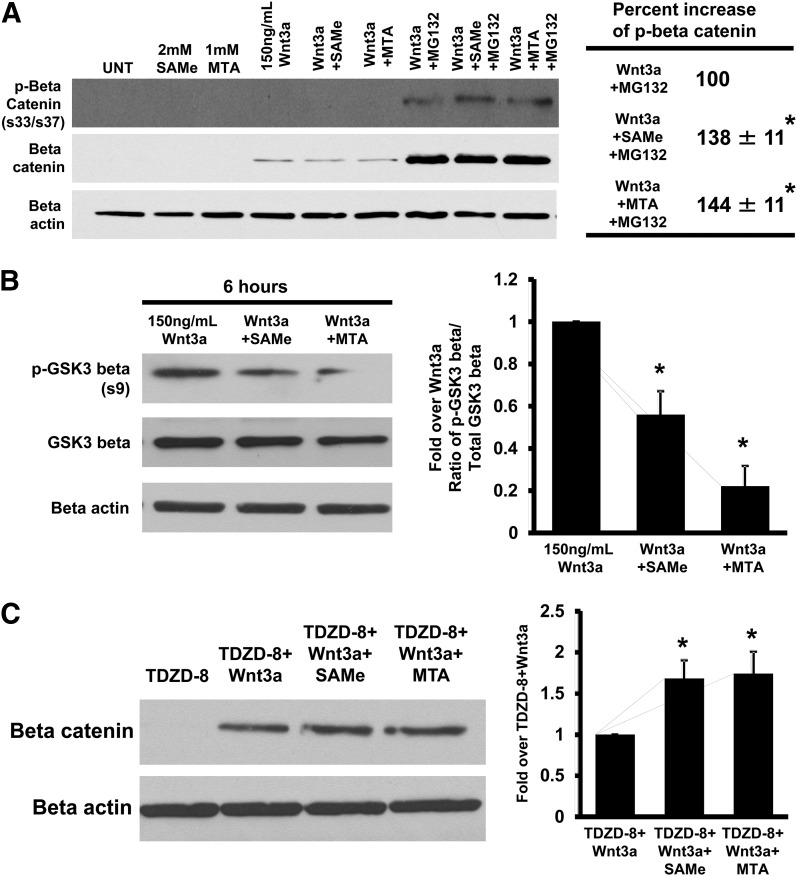
The phosphorylation of β-catenin at the N terminus is required for its recognition by the proteasomal complex to promote its degradation (Clevers, 2006). We examined whether the lowering of β-catenin levels by SAMe and MTA was the result of accelerated degradation. Prior to Wnt3a ± SAMe/MTA treatment, RKO cells were pretreated with the proteasomal inhibitor MG132, which enables the detection of the phosphorylated form of β-catenin (at serines 33 and 37 and threonine 41) by Western blot analysis. In the presence of MG132, SAMe and MTA elevated phosphorylated β-catenin by about 40% (Fig. 5A). The phosphorylation of these sites is mediated by GSK3β (Anastas and Moon, 2013). During Wnt signaling, GSK3β is normally inactivated in order for β-catenin stabilization to happen, and one way this occurs is via GSK3β phosphorylation at serine 9 (Carnero, 2010). We next examined whether SAMe and MTA treatment increased the level of active GSK3β. Figure 5B shows that SAMe and MTA were able to reduce the inactivated form of GSK3β (phospho-GSK3 at serine 9) by 44 and 78%, respectively. Furthermore, inhibition of GSK3β altogether by the small molecule inhibitor TDZD-8 completely abolished the ability of SAMe and MTA to reduce β-catenin levels in RKO cells (Fig. 5C).
Fig. 5.
The inhibitory effect of SAMe and MTA on β-catenin requires GSK3β. (A) RKO cells were pretreated for 2 hours with 20 μM proteasome inhibitor MG132. After pretreatment, the cells were treated with 150 ng/ml Wnt3a with or without 2 mM SAMe or 1 mM MTA for 6 hours. Western blot analysis was done with antibodies to total and phosphorylated β-catenin (s33/37). The table to the right represents the ratio of phosphorylated to total β-catenin percent, normalized to β-actin. (B) RKO cells were cotreated with 150 ng/ml Wnt3a and 2 mM SAMe or 1 mM MTA for 6 hours to look at GSK3β serine 9 phosphorylation status. The graph to the right represents the fold ratio of phosphorylated to total GSK3β, normalized to β-actin. (C) RKO cells were treated with 20 μM TDZD-8 concurrently with 150 ng/ml Wnt3a and 2 mM SAMe or 1 mM MTA for 6 hours. Western blot of β-catenin was done to assess the effect of TDZD-8 on SAMe- and MTA-mediated downregulation of β-catenin. The graph to the right represents the densitometric values expressed as fold of their respective controls. All data are derived from three independent experiments. *P < 0.05 versus respective control. UNT, untreated vehicle control (0.2% DMSO).
AKT and PP2A Are Involved in SAMe and MTA’s Effect on GSK3β Phosphorylation Status.
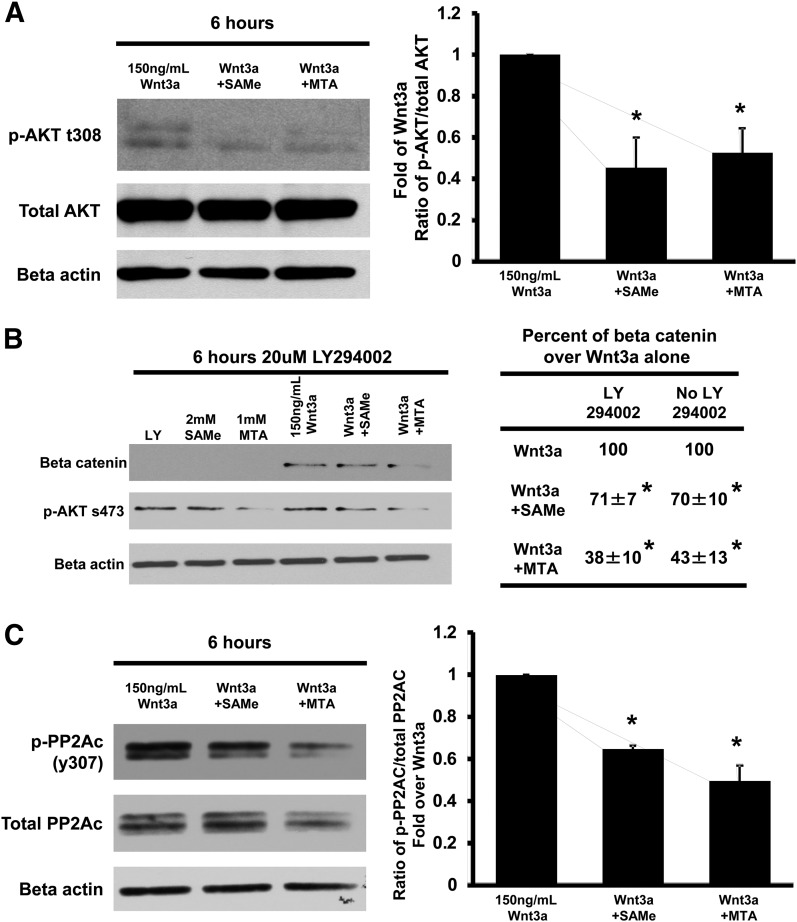
SAMe and MTA treatment inhibited AKT activity in inflammation-induced colon cancer (Li et al., 2012), and AKT is one of the kinases that phosphorylate GSK3β serine 9 (Mishra, 2010). To assess whether this might be the mechanism, we examined the phosphorylation status of AKT and confirmed that SAMe and MTA treatment reduced phosphorylated AKT (Fig. 6A). We next examined whether the effect of SAMe and MTA was mediated via PI3K, the upstream regulator of AKT. Blocking PI3K with LY294002 did not prevent SAMe- or MTA-mediated reduction in β-catenin in the presence of Wnt3a (Fig. 6B). Because PI3K was not involved, we examined whether SAMe and MTA might have increased the activity of PP2A, which is known to dephosphorylate and inactivate AKT (Kuo et al., 2008). Phosphorylation of PP2A catalytic subunit at tyrosine 307 inactivates PP2A (Janssens and Goris, 2001), and Fig. 6C shows that SAMe and MTA treatment reduced the level of phospho-PP2Ac at tyrosine 307, which is consistent with higher PP2A activity.
Fig. 6.
SAMe and MTA reduced AKT and PP2Ac phosphorylation in RKO cells. (A) Western blot analysis of phosphorylated AKT at threonine 308 in RKO cells treated with 150 ng/ml Wnt3a and 2 mM SAMe or 1 mM MTA for 6 hours. The graph to the right represents the fold ratio of phosphorylated to total AKT, normalized to β-actin. (B) RKO cells were cotreated with 20 μM LY294002 with or without 150 mg/ml Wnt3a and 2 mM SAMe or 1 mM MTA for 6 hours. Western blot was done with antibodies to β-catenin and phosphorylated AKT at serine 473. The table to the right compares the densitometric values expressed as fold of their respective controls. (C) Western blot was done to phosphorylated PP2Ac at tyrosine 307 and total PP2Ac on RKO cells treated with 150 ng/ml Wnt3a and 2 mM SAMe or 1 mM MTA for 6 hours. The graph to the right represents the ratio of phosphorylated to total PP2Ac, normalized to β-actin. All experiments were done from three independent experiments. *P < 0.05 versus respective control.
β-Catenin Expression Is Increased When SAMe Level Is Reduced.
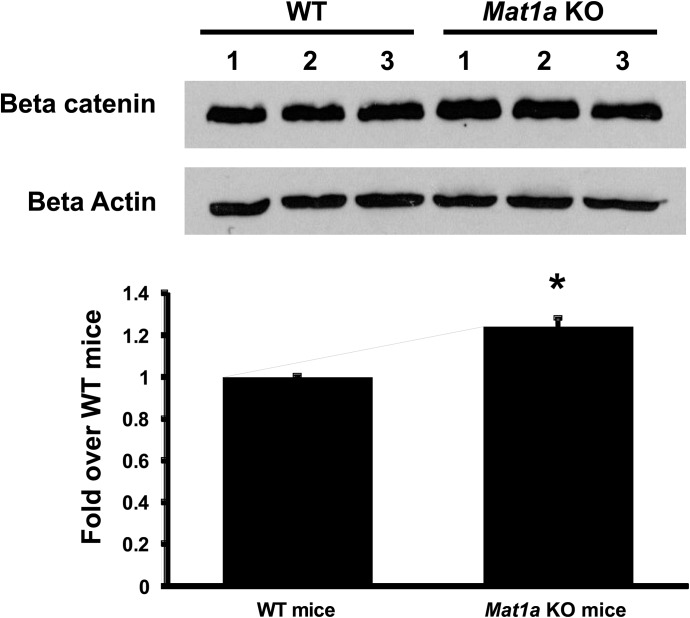
Mat1a knockout mice have reduced hepatic SAMe levels (Lu et al., 2001). Figure 7 shows β-catenin protein levels are 20% higher in 4-month-old Mat1a knockout mice livers as compared with age- and gender-matched wild-type mice.
Fig. 7.
In vivo reduction of SAMe levels in mouse livers elevated β-catenin protein levels. Western blot analysis of β-catenin expression comparing mouse livers isolated from 4-month-old Mat1a knockout (KO) male mice to age/gender-matched wild-type (WT) mice. The graph below the blots represents the fold difference of β-catenin protein levels over its appropriate age/gender-matched wild-type mouse. These experiments were from three different 4-month-old Mat1a KO and WT male livers. *P < 0.05 versus respective control.
Discussion
Wnt signaling, with its action mediated by β-catenin, is known to be aberrantly active in many different cancers, as it promotes proliferation and prosurvival signals for the cancer cells (Anastas and Moon, 2013). Over 85% of all colon cancers have mutations in either APC, axin, or β-catenin (stabilizing), whereas 30% of HCC have Wnt signaling mutations (Giles et al., 2003). There is growing evidence that aberrant Wnt signaling is influential in many cancer types, such as liver, lung, and breast, despite lower mutation rates (Howe and Brown, 2004; Mazieres et al., 2005; Bengochea et al., 2008). It has been reported that up to 95% of liver tumors have aberrant Wnt signaling (Bengochea et al., 2008). We showed that SAMe and MTA, naturally occurring biomolecules, can reduce tumor load in the chronic inflammation-induced colon cancer model and reduced total β-catenin levels on immunohistochemistry (Li et al., 2012). This prompted us to examine whether SAMe and MTA can influence Wnt signaling.
In this study, we examined whether SAMe and MTA can affect Wnt signaling by using the colonic tumors from the AOM/DSS mouse model and hepatic tumors from Hep3B liver cancer cells that overexpress Matα1 (resulting in elevated SAMe levels) in an orthotopic HCC mouse model. β-Catenin levels and two Wnt target genes, c-Myc and Ccnd1, commonly upregulated in both cancers, were reduced in both the treated colon and liver mouse tumors (Fig. 1). One previous study showed a reduction of β-catenin levels in normal primary rat hepatocytes cultured for 24 hours with 4 mM SAMe; however, the mechanism was not fully elucidated (Yamaji et al., 2011). In addition, hepatocytes rapidly dedifferentiate in culture, so that by 24 hours, MAT1A expression is reduced to 15% of baseline and SAMe level to 50% (Tomasi et al., 2009). Thus, the effect of SAMe in this rapidly dedifferentiating cultured cell model may not represent the response of normal liver. Indeed, pharmacologic doses of SAMe or MTA treatment of 6 days had no effect on β-catenin protein expression in normal liver (Supplemental Fig. 1). These results suggest pharmacologic SAMe and MTA inhibit β-catenin signaling only in de-differentiated cells.
SAMe and MTA treatment inhibited β-catenin activity as they reduced TOPFlash reporter activity in both SW480 and HepG2 cells (Fig. 2). Both agents had no influence on β-catenin expression as these cells contain mutations that prevent β-catenin degradation. This prompted us to examine whether SAMe and MTA treatment altered the subcellular localization of β-catenin. Indeed, we observed that both compounds reduced the nuclear content of β-catenin (Fig. 3). The mechanism by which β-catenin translocates to the nucleus is not entirely clear, as several models have been proposed. In gliomas, β-catenin required FoxM1 for nuclear translocation (Zhang et al., 2011). FoxM1 is overexpressed in a variety of cancers, including colon and liver (Pilarsky et al., 2004). In NIH-3T3 cells, β-catenin nuclear translocation was mediated by the following components of the nuclear pore complex: nucleoporins Nup62, Nup153, and RanBP2/Nup358 (Sharma et al., 2012). Others showed that β-catenin phosphorylation at serine 552 by AKT caused a translocation from the adherens junctions to the cytosolic and nuclear compartments (Fang et al., 2007) and enhanced its binding to 14-3-3 ζ, a nucleocytoplasmic transporter protein, to elevate TOPFlash activity (Brunet et al., 2002). However, AKT can phosphorylate Chibby and 14-3-3, forming a tripartite complex, and facilitate nuclear export of β-catenin (Li et al., 2008). Li et al. (2008) suggests that the opposing activities of AKT may be due to the subcellular fractions of AKT being exposed to different targets. Whether SAMe and MTA affect any of these processes that promote β-catenin nuclear transport dynamics is a subject of future investigation.
In SAMe- or MTA-treated AOM/DSS colon tumors and orthotopic liver cancers overexpressing MATα1 (Fig. 1), total β-catenin levels are reduced, suggesting these molecules can inhibit Wnt/β-catenin by another mechanism in cells with wild-type Wnt signaling (such as RKO and Huh-7 cells). Treatment with the proteasomal inhibitor MG132 in RKO cells showed that SAMe and MTA promoted β-catenin degradation by increasing phosphorylation of serines 33 and 37 (Fig. 5A). Because GSK3β is responsible for β-catenin at these sites, we examined whether SAMe and MTA affected GSK3β activity and found that both agents raised the active pool of GSK3β (lower phospho-GSK3β S9; Fig. 5B). This is in agreement with Yamaji et al. (2011); however, our results conflicted when we tested whether SAMe and MTA’s inhibitory effect on β-catenin required GSK3β. Using the GSK3β-specific inhibitor TDZD-8, the ability of SAMe and MTA to lower β-catenin levels was abolished (Fig. 5C), but Yamaji et al. (2011), using either Kenpaullone or PHZ1123, found no influence. Differences in cell types may attribute the different effects exerted by SAMe. Also, the primary hepatocytes were cultured longer (48 hours versus 6 hours) and at a dose of SAMe double that of our study.
GSK3β serine 9 phosphorylation (leading to its inactivation) is mediated by multiple upstream kinases that include protein kinase A, AKT, protein kinase C, p90 ribosomal S6 kinase/MAPK-activating protein, and p70 ribosomal S6 kinase (Mishra 2010). We focused on AKT because our previous work showed SAMe and MTA inhibited AKT activation in AOM/DSS colon tumors (Li et al., 2012). We confirmed this in RKO cells (Fig. 6A). Reduced AKT activity can be the result of either reduced PI3K (its upstream kinase) or increased PP2A activity (Kuo et al., 2008). Treatment of RKO cells with the PI3K inhibitor LY294002 did not affect SAMe- and MTA-mediated reduction in β-catenin level (Fig. 6B), ruling out involvement of PI3K. PP2A activity is modulated by methylation and phosphorylation, with the former activating and the latter attenuating its activity (Leulliot et al., 2004). SAMe and MTA treatment reduced PP2Ac phosphorylation, thus increasing the levels of active PP2A (Fig. 6C). This is consistent with Yamaji et al. (2011), who observed that SAMe activated PP2A. Although the effect of SAMe may be via PP2A methylation, this is not the mechanism for MTA, as it was still effective even when its conversion to SAMe was blocked with cycloleucine. In contrast to SAMe, MTA is not a methyl donor and can inhibit protein methylation (Lu and Mato, 2012). How MTA increases PP2A activity is not clear at present and will require further investigation. Many mitogens are known to influence β-catenin activity and growth in part via AKT and PP2A (Desbois-Mouthon et al., 2001; Lee et al., 2010; Paul et al., 2013; Zhai et al., 2013). Our results suggest that one key mechanism for SAMe and MTA to block the effect of mitogens is at the level of AKT and PP2A.
Our current work showed exogenous treatment of liver and colon cancer cells with SAMe or MTA reduced β-catenin signaling regardless of whether they harbor mutations in the Wnt signaling pathway. Raising endogenous SAMe level via overexpressing MATα1 in liver cancer cells also reduced β-catenin signaling. In all of these models, we showed previously that these treatments inhibit tumor growth, invasion, and metastasis, but increase apoptosis (Yang et al., 2004, 2013; Li et al., 2010, 2012). β-catenin signaling is well known to enhance growth (Clevers, 2006), but the effect of GSK3β on apoptosis is conflicting (Jacobs et al., 2012). Although SAMe and MTA have multiple actions (Lu and Mato, 2012), inhibiting Wnt/β-catenin signaling may also contribute to the overall tumor-suppressive effects.
Although raising SAMe levels in cancer cells inhibits β-catenin signaling, lowering SAMe level results in the opposite, namely higher β-catenin signaling, as demonstrated by Mat1a knockout livers (Fig. 7). Supporting this, cyclin D1 and c-Myc expression is higher in Mat1a knockout livers (Chen et al., 2004; Tomasi et al., 2009). These results show that, in hepatocytes, β-catenin signaling is regulated by SAMe level.
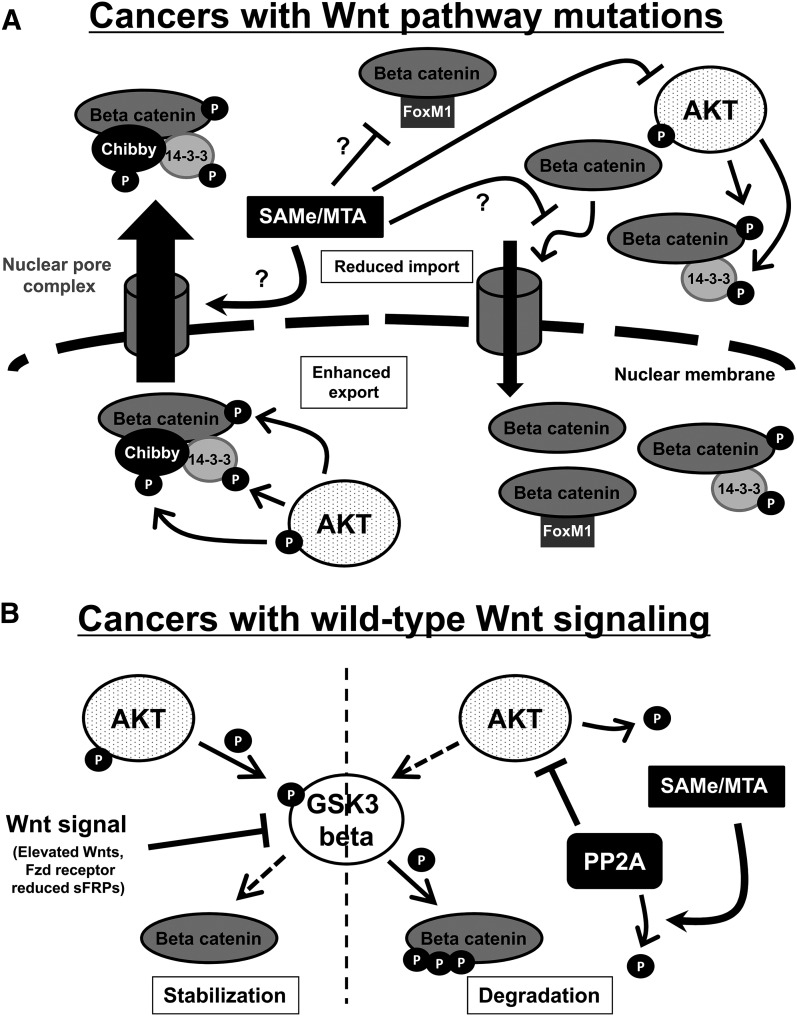
In 2012, there were approximately 8.2 million cancer related deaths worldwide; the majority were caused by cancers of the lung, liver, stomach, colon, and breast (Ferlay, 2013). Aberrant Wnt signaling is common in all of these cancers (Anastas and Moon, 2013). SAMe and MTA can inhibit β-catenin, the main effector of Wnt signaling, as well as other oncogenic mechanisms such as AKT to promote apoptosis and attenuate growth progression in liver and colon cancers (Lu and Mato, 2012). More importantly, these compounds are anti-apoptotic in normal hepatocytes (Ansorena et al., 2002; Yang et al., 2004) and have no toxic effects toward normal colon epithelial cells (Li et al., 2012). Because SAMe and MTA are naturally occurring compounds found in our bodies, it makes them the ideal candidates for preventing and/or treating cancers with aberrant Wnt signaling. Figure 8 summarizes the key findings from the current study.
Fig. 8.
SAMe and MTA inhibit β-catenin signaling in liver and colon cancer cells by multiple mechanisms. (A) SAMe and MTA reduce the nuclear accumulation of β-catenin in colon or liver cancers containing mutations to the Wnt pathway. SAMe and MTA may attenuate one or more proposed mechanisms of β-catenin nuclear import and/or enhance β-catenin export. For nuclear import, SAMe and MTA may reduce the interaction of β-catenin to proteins that may aid its translocation to the nucleus, such as FoxM1, which contains a nuclear localization signal. Because SAMe and MTA can reduce AKT activity, it may reduce the phosphorylation of β-catenin at serine 552 and the nucleocytoplasmic transporter 14-3-3 to limit its nuclear import. SAMe and MTA may also potentially prevent β-catenin nuclear transport by disrupting its interaction with proteins of the nuclear pore complex. In addition, SAMe and MTA may enhance the export of β-catenin by enhancing Chibby and/or 14-3-3 to translocate to the nucleus, where, when phosphorylated by nuclear AKT, interact with β-catenin, leading to nuclear export of β-catenin/Chibby/14-3-3 complex. Nuclear AKT may be immune to the effects of exogenously added SAMe and MTA. These hypothetical schemes remain to be examined. (B) SAMe and MTA reduce β-catenin protein levels in colon and liver cancer cells with intact wild-type Wnt signaling. SAMe and MTA enhance GSK3β-mediated degradation of β-catenin by elevating the activity of PP2A (indicated by lower levels of phospho-PP2Ac at tyrosine 307, the inactive form). More active PP2A can dephosphorylate AKT (threonine 308), reduce its activity, and thus reduce AKT-mediated inactivation of GSK3β at serine 9. The dashed arrows represent the lack of kinase activity toward its target substrate.
Supplementary Material
Abbreviations
- AKT
protein kinase B
- AOM
azoxymethane
- APC
adenomatous polyposis coli
- DMSO
dimethylsulfoxide
- DSS
dextran sulfate sodium salt
- HCC
hepatocellular carcinoma
- MAT
methionine adenosyltransferase
- MTA
methylthioadenosine
- SAMe
S-adenosylmethionine
Authorship Contributions
Participated in research design: Li, Mato, Lu.
Conducted experiments: Li, Peng, Yang, Kurniawidjaja, Panthaki, Zheng.
Performed data analysis: Li, Peng, Yang.
Wrote or contributed to the writing of the manuscript: Li, Lu.
Footnotes
This work was supported by the National Institutes of Health National Center for Complementary and Alternative Medicine [Grants R01-AT001576 and R01-AT004896 to S.C.L. and J.M.M.]; Plan Nacional of I+D SAF 2011-29851; and Departamento de Educación del Gobierno Vasco (J.M.M.). HepG2, Huh7, RKO, HCT116, and SW480 cells were provided by the Cell Separation and Culture Core, and confocal microscopy was done at the Imaging Core of the University of Southern California Research Center for Liver Diseases supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant P30-DK48522].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Anastas JN, Moon RT. (2013) WNT signalling pathways as therapeutic targets in cancer. Nat Rev Cancer 13:11–26. [DOI] [PubMed] [Google Scholar]
- Ansorena E, García-Trevijano ER, Martínez-Chantar ML, Huang ZZ, Chen L, Mato JM, Iraburu M, Lu SC, Avila MA. (2002) S-adenosylmethionine and methylthioadenosine are antiapoptotic in cultured rat hepatocytes but proapoptotic in human hepatoma cells. Hepatology 35:274–280. [DOI] [PubMed] [Google Scholar]
- Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, et al. (2008) Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer 99:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Kanai F, Stehn J, Xu J, Sarbassova D, Frangioni JV, Dalal SN, DeCaprio JA, Greenberg ME, Yaffe MB. (2002) 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J Cell Biol 156:817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A. (2010) The PKB/AKT pathway in cancer. Curr Pharm Des 16:34–44. [DOI] [PubMed] [Google Scholar]
- Chen H, Xia M, Lin M, Yang H, Kuhlenkamp J, Li T, Sodir NM, Chen YH, Josef-Lenz H, Laird PW, et al. (2007) Role of methionine adenosyltransferase 2A and S-adenosylmethionine in mitogen-induced growth of human colon cancer cells. Gastroenterology 133:207–218. [DOI] [PubMed] [Google Scholar]
- Chen L, Zeng Y, Yang H, Lee TD, French SW, Corrales FJ, García-Trevijano ER, Avila MA, Mato JM, Lu SC. (2004) Impaired liver regeneration in mice lacking methionine adenosyltransferase 1A. FASEB J 18:914–916 . [DOI] [PubMed] [Google Scholar]
- Clevers H. (2006) Wnt/beta-catenin signaling in development and disease. Cell 127:469–480. [DOI] [PubMed] [Google Scholar]
- de La Coste A, Romagnolo B, Billuart P, Renard CA, Buendia MA, Soubrane O, Fabre M, Chelly J, Beldjord C, Kahn A, et al. (1998) Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci USA 95:8847–8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois-Mouthon C, Cadoret A, Blivet-Van Eggelpoël MJ, Bertrand F, Cherqui G, Perret C, Capeau J. (2001) Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation. Oncogene 20:252–259. [DOI] [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. (2007) Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem 282:11221–11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay JSI, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, and Bray F (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. International Agency for Research on Cancer: Available from: http://globocan.iarc.fr, accessed on 22nd of July 2014.
- Gayet J, Zhou XP, Duval A, Rolland S, Hoang JM, Cottu P, Hamelin R. (2001) Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines. Oncogene 20:5025–5032. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. (2003) Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 1653:1–24. [DOI] [PubMed] [Google Scholar]
- Holland JD, Klaus A, Garratt AN, Birchmeier W. (2013) Wnt signaling in stem and cancer stem cells. Curr Opin Cell Biol 25:254–264. [DOI] [PubMed] [Google Scholar]
- Howe LR, Brown AM. (2004) Wnt signaling and breast cancer. Cancer Biol Ther 3:36–41. [DOI] [PubMed] [Google Scholar]
- Jacobs KM, Bhave SR, Ferraro DJ, Jaboin JJ, Hallahan DE, Thotala D. (2012) GSK-3β: a bifunctional role in cell death pathways. Int J Cell Biol 2012:930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Goris J. (2001) Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353:417–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaus A, Birchmeier W. (2008) Wnt signalling and its impact on development and cancer. Nat Rev Cancer 8:387–398. [DOI] [PubMed] [Google Scholar]
- Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. (2008) Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem 283:1882–1892. [DOI] [PubMed] [Google Scholar]
- Lee CH, Hung HW, Hung PH, Shieh YS. (2010) Epidermal growth factor receptor regulates beta-catenin location, stability, and transcriptional activity in oral cancer. Mol Cancer 9:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulliot N, Quevillon-Cheruel S, Sorel I, Li de La Sierra-Gallay I, Collinet B, Graille M, Blondeau K, Bettache N, Poupon A, Janin J, et al. (2004) Structure of protein phosphatase methyltransferase 1 (PPM1), a leucine carboxyl methyltransferase involved in the regulation of protein phosphatase 2A activity. J Biol Chem 279:8351–8358. [DOI] [PubMed] [Google Scholar]
- Li FQ, Mofunanya A, Harris K, Takemaru K. (2008) Chibby cooperates with 14-3-3 to regulate beta-catenin subcellular distribution and signaling activity. J Cell Biol 181:1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ramani K, Sun Z, Zee C, Grant EG, Yang H, Xia M, Oh P, Ko K, Mato JM, et al. (2010) Forced expression of methionine adenosyltransferase 1A in human hepatoma cells suppresses in vivo tumorigenicity in mice. Am J Pathol 176:2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TW, Yang H, Peng H, Xia M, Mato JM, Lu SC. (2012) Effects of S-adenosylmethionine and methylthioadenosine on inflammation-induced colon cancer in mice. Carcinogenesis 33:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TW, Zhang Q, Oh P, Xia M, Chen H, Bemanian S, Lastra N, Circ M, Moyer MP, Mato JM, et al. (2009) S-Adenosylmethionine and methylthioadenosine inhibit cellular FLICE inhibitory protein expression and induce apoptosis in colon cancer cells. Mol Pharmacol 76:192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. (2001) Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci USA 98:5560–5565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu SC, Mato JM. (2012) S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev 92:1515–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazieres J, He B, You L, Xu Z, Jablons DM. (2005) Wnt signaling in lung cancer. Cancer Lett 222:1–10. [DOI] [PubMed] [Google Scholar]
- Mishra R. (2010) Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer 9:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul I, Bhattacharya S, Chatterjee A, Ghosh MK. (2013) Current understanding on EGFR and Wnt/β-catenin signaling in glioma and their possible crosstalk. Genes Cancer 4:427–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Dara L, Li TW, Zheng Y, Yang H, Tomasi ML, Tomasi I, Giordano P, Mato JM, Lu SC. (2013) MAT2B-GIT1 interplay activates MEK1/ERK 1 and 2 to induce growth in human liver and colon cancer. Hepatology 57:2299–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarsky C, Wenzig M, Specht T, Saeger HD, Grützmann R. (2004) Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia 6:744–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani K, Yang H, Xia M, Ara AI, Mato JM, Lu SC. (2008) Leptin’s mitogenic effect in human liver cancer cells requires induction of both methionine adenosyltransferase 2A and 2beta. Hepatology 47:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M, Jamieson C, Johnson M, Molloy MP, Henderson BR. (2012) Specific armadillo repeat sequences facilitate β-catenin nuclear transport in live cells via direct binding to nucleoporins Nup62, Nup153, and RanBP2/Nup358. J Biol Chem 287:819–831. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Tomasi ML, Iglesias-Ara A, Yang H, Ramani K, Feo F, Pascale MR, Martínez-Chantar ML, Mato JM, Lu SC. (2009) S-adenosylmethionine regulates apurinic/apyrimidinic endonuclease 1 stability: implication in hepatocarcinogenesis. Gastroenterology 136:1025–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Chua MS, Grepper S, So SK. (2011) Soluble Frizzled-7 receptor inhibits Wnt signaling and sensitizes hepatocellular carcinoma cells towards doxorubicin. Mol Cancer 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji S, Droggiti A, Lu SC, Martinez-Chantar ML, Warner A, Varela-Rey M. (2011) S-Adenosylmethionine regulates connexins sub-types expressed by hepatocytes. Eur J Cell Biol 90:312–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Cho ME, Li TW, Peng H, Ko KS, Mato JM, Lu SC. (2013) MicroRNAs regulate methionine adenosyltransferase 1A expression in hepatocellular carcinoma. J Clin Invest 123:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Sadda MR, Li M, Zeng Y, Chen L, Bae W, Ou X, Runnegar MT, Mato JM, Lu SC. (2004) S-adenosylmethionine and its metabolite induce apoptosis in HepG2 cells: role of protein phosphatase 1 and Bcl-x(S). Hepatology 40:221–231. [DOI] [PubMed] [Google Scholar]
- Zhai X, Yan K, Fan J, Niu M, Zhou Q, Zhou Y, Chen H, Zhou Y. (2013) The β-catenin pathway contributes to the effects of leptin on SREBP-1c expression in rat hepatic stellate cells and liver fibrosis. Br J Pharmacol 169:197–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, Huang H, Xue J, Liu M, Wang Y, et al. (2011) FoxM1 promotes β-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell 20:427–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.