Abstract
The yellow fever mosquito, Aedes aegypti, vectors disease-causing agents that adversely affect human health, most notably the viruses causing dengue and yellow fever. The efficacy of current mosquito control programs is challenged by the emergence of insecticide-resistant mosquito populations, suggesting an urgent need for the development of chemical insecticides with new mechanisms of action. One recently identified potential insecticide target is the A. aegypti D1-like dopamine receptor, AaDOP2. The focus of the present study was to evaluate AaDOP2 antagonism both in vitro and in vivo using assay technologies with increased throughput. The in vitro assays revealed AaDOP2 antagonism by four distinct chemical scaffolds from tricyclic antidepressant or antipsychotic chemical classes, and elucidated several structure-activity relationship trends that contributed to enhanced antagonist potency, including lipophilicity, halide substitution on the tricyclic core, and conformational rigidity. Six compounds displayed previously unparalleled potency for in vitro AaDOP2 antagonism, and among these, asenapine, methiothepin, and cis-(Z)-flupenthixol displayed subnanomolar IC50 values and caused rapid toxicity to A. aegypti larvae and/or adults in vivo. Our study revealed a significant correlation between in vitro potency for AaDOP2 antagonism and in vivo toxicity, suggesting viability of AaDOP2 as an insecticidal target. Taken together, this study expanded the repertoire of known AaDOP2 antagonists, enhanced our understanding of AaDOP2 pharmacology, provided further support for rational targeting of AaDOP2, and demonstrated the utility of efficiency-enhancing in vitro and in vivo assay technologies within our genome-to-lead pipeline for the discovery of next-generation insecticides.
Introduction
Mosquitoes transmit pathogens and parasites that cause diseases that adversely affect human health worldwide, including malaria, yellow fever, and dengue. Existing approaches for mosquito control have demonstrated efficacy in reducing incidences of such diseases, but are becoming inadequate due to the emergence of insecticide-resistant mosquito populations (Hemingway and Ranson, 2000; Hemingway, 2014). The need for novel mode-of-action compounds to control mosquitoes is further emphasized by the fact that it has been several decades since a new public health insecticide has been deployed to reduce the spread of vector-borne diseases (Hemingway et al., 2006).
Arthropod G protein–coupled receptors (GPCRs) mediate critical biologic processes (Hauser et al., 2006) and have emerged as potential insecticide targets (Hill et al., 2013). Molecular approaches, including genome sequencing efforts, have identified more than 100 GPCRs within the genomes of several arthropod vector species (Hill et al., 2002; Nene et al., 2007; Arensburger et al., 2010; Kirkness et al., 2010). Among the GPCR superfamily, the biogenic amine receptors are of particular interest because of their crucial roles in insect physiology and behavior (Hauser et al., 2006; Fuchs et al., 2014). For example, the biogenic amine dopamine and its receptors are implicated in a variety of arthropod behaviors, including arousal (Kume et al., 2005), locomotion (Yellman et al., 1997; Draper et al., 2007; Mustard et al., 2010), and olfactory learning (Kim et al., 2007; Riemensperger et al., 2011). It is also notable that dopamine is associated with the salivary function of vectors (Ali, 1997; Sauer et al., 2000; Šimo et al., 2011, 2014), suggesting potential roles for the mediation of pathogen acquisition and transmission during blood feeding. In Aedes aegypti, dopamine is also implicated in sclerotization and ovarian/egg development, as increased dopamine levels were observed in newly emerged adults and following a blood meal (Andersen et al., 2006). The central roles of dopamine systems in fundamental biologic processes offer the dopamine receptors as potential insecticide targets.
A recent study from our invertebrate receptor group supports the pursuit of D1-like dopamine receptors (AaDOP1 and AaDOP2) from the yellow fever mosquito, A. aegypti, as targets for novel mode-of-action insecticides (Meyer et al., 2012). Specifically, AaDOP2 was used as a prototypical target for a “genome-to-lead” approach for the discovery of target-based insecticides, where genomic sequence data were used to drive in vitro functional characterization of recombinant AaDOP receptors in human embryonic kidney (HEK) 293 cells (Meyer et al., 2012). Following pharmacologic characterization, high-throughput screening (HTS)–amenable evaluation of pharmacologically active compounds identified AaDOP2 antagonists that display significant in vivo toxicity to mosquito larvae (Meyer et al., 2012), supporting the validity of targeting AaDOP2 for A. aegypti control.
The present study entailed a robust follow-up pharmacologic analysis of AaDOP2 antagonists identified in a small-molecule screen of the LOPAC1280 library (Meyer et al., 2012). To accomplish this, we developed an HTS-amenable cell-based assay that enabled an in-depth study of AaDOP2 antagonism by tricyclic antidepressants and structurally related compounds. Several of these compounds demonstrated enhanced potency for in vitro AaDOP2 antagonism and greater efficacy for larval death in mosquito bioassays. Importantly, we provided evidence that several AaDOP2 antagonists caused toxicity in adult A. aegypti. Furthermore, we improved upon our previously described genome-to-lead pipeline via implementation of efficiency-enhancing in vivo assay technologies.
Materials and Methods
Cis-(Z)-flupenthixol, clozapine, mianserin, nortriptyline, imipramine, protriptyline, norclomipramine, pirenperone, desipramine, haloperidol, trazodone, fluoxetine, fluvoxamine, buspirone, (+)-butaclamol, amoxapine, amitriptyline, chlorpromazine, doxepin, loratadine, ketotifen, chlorprothixene, loxapine, cyproheptadine, asenapine, diphenhydramine, ritanserin, ketanserin, risperidone, 3-isobutyl-1-methylxanthine, G418, and Dulbecco’s modified Eagle’s medium were purchased from Sigma-Aldrich (St. Louis, MO). Amperozide, methiothepin, clomipramine, SCH-23390 [(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine], LY-310,762 (1-[2-[4-(4-fluorobenzoyl)-1-piperidinyl]ethyl]-1,3-dihydro-3,3-dimethyl-2H-indol-2-one), R59-022 (6-[2-[4-[(4-fluorophenyl)phenylmethylene]-1-piperidinyl]ethyl]-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one), and tomoxetine were purchased from Tocris Bioscience (Ellisville, MO). Benztropine was purchased from Enzo Life Sciences (Farmingdale, NY). The antibiotic-antimycotic 100× solution was purchased from Life Technologies (Grand Island, NY). FetalClone I serum, bovine calf serum, HEPES, and Hanks’ balanced salt solution were purchased from Hyclone (Logan, UT). The homogenous time-resolved fluorescence (HTRF) cAMP kit was purchased from Cisbio Bioassays (Bedford, MA).
Cisbio HTRF cAMP Dynamic 2 Cell-Based Assay
HEK293 cells stably expressing AaDOP2 (HEK-AaDOP2) or the human D1 dopamine receptor (HEK-hD1) were maintained and cryogenically frozen as previously described (Meyer et al., 2012). To prepare for pharmacologic analysis, cells were thawed and resuspended in assay buffer (Hanks’ balanced salt solution, 20 mM HEPES, 0.1% fatty acid–free bovine serum albumin). To remove cryogenic freezing media, cell suspensions were centrifuged at 500g for 5 minutes, followed by aspiration of the supernatant. Cell pellets were resuspended in assay buffer and seeded into 384-well plates (CulturPlate-384; PerkinElmer, Waltham, MA) at 2000–2500 cells per well and incubated at 37°C and 5% CO2 for 1 hour. Test compounds were added using a 384-well pin tool (V&P Scientific, San Diego, CA). A MultiFlo (BioTek, Winooski, VT) low-volume bulk reagent dispenser was used to dispense 3 μM dopamine (in assay buffer containing 500 μM 3-isobutyl-1-methylxanthine and 0.02% ascorbic acid) to activate AaDOP2. Drug stimulation was carried out at room temperature for 1 hour. Cells were lysed by sequential addition of cAMP-d2 and anti-cAMP cryptate conjugate, both diluted 1:39 in lysis buffer, and were incubated at room temperature for 1 hour. Time-resolved fluorescence resonance energy transfer was measured with a lag time of 100 microseconds and integration time of 300 microseconds using a Synergy4 (BioTek) fluorescence plate reader with a 330/80-nm excitation filter and emission filters of 620/10 and 665/8 nm. Sensitivity parameters were set by reading the cAMP standard curve using the autosensitivity setting. All experimental conditions were read using sensitivity settings obtained for the cAMP standard curve. Cellular cAMP concentrations were estimated in GraphPad Prism (GraphPad Software, La Jolla, CA) by applying the 620/665-nm fluorescence ratio values to a standard curve of known cAMP concentrations.
Cyclic AMP measurements in HEK293 cells stably expressing the human D1 dopamine receptor were performed as described earlier, but 500 nM dopamine was used to stimulate cAMP accumulation.
In Vivo A. aegypti Larval Screen.
Test compounds were evaluated for in vivo toxicity in bioassays against L3 stage A. aegypti larvae in a double-blind manner. In brief, compounds were resuspended in water and added to wells of a 24-well plate (BD Bioscience, San Jose, CA) in duplicate, with each well containing five A. aegypti larvae in 1-ml total volume to achieve a final concentration of 400 μM per well (see Supplemental Fig. 1 for illustrations of the assay format). Plates were incubated at 22°C, and the assay was scored for larval mortality at 24, 48, and 72 hours. Larvae unresponsive to gentle tapping of the plate or touch with a sterile probe were scored as dead.
A. aegypti Adult Concentration-Response Curves.
Test compounds were dissolved in deionized water to a 200 mM stock concentration and serially diluted in Aedes saline (Hayes, 1953) to achieve a dose range of 0.25–20 mM. Four-day-old A. aegypti adult females [average wing length of 3.4 mm, measured as described by Briegel (1990)] were anesthetized on ice, and groups of 20 females were injected with the indicated amounts of test compounds (0.5 μl per mosquito) or Aedes saline alone (control) using a pulled glass capillary needle. Additional uninjected mosquito controls were also included. Mosquitoes were housed in 10-cm diameter × 20-cm height paper coffee cup cages with lace screens (secured with rubber bands) and maintained at 75% humidity with 10% sucrose provided ad libitum via a cotton wick (see Supplemental Fig. 2 for illustrations of injections and mosquito housing). Observations of mortality were made daily for up to 4 days post-treatment. Mosquitoes were scored as “dead” if no movement was observed and confirmed by no response to a gentle touch of the legs with a metal probe. When observed at any time point, moribund adult mosquitoes (i.e., insects incapable of standing, walking, or flying) were scored as dead. At the 24-hour time point, and to a lesser extent at the 48-hour time point, we observed a percentage of the adult mosquito population that was moribund. These mosquitoes did not recover and died by assay endpoint. The moribund phenotype was negligible at 96 hours (less than 1% of the adult population for any replicate dose). LD50 values for test compounds injected into adult mosquitoes were calculated by nonlinear regression using the sigmoidal dose-response equation in the GraphPad Prism software.
Results
In Vitro Evaluation of AaDOP2 Antagonism.
Our previous studies indicated potential value in pursuing AaDOP2 in a target-first approach for developing new insecticides against A. aegypti (Meyer et al., 2012). We also demonstrated the success of utilizing a heterologous cell model, where recombinant AaDOP2 receptors are expressed in HEK293 cells (HEK-AaDOP2) for identification and pharmacologic evaluation of novel AaDOP2 ligands (Meyer et al., 2012). To improve upon our genome-to-lead pipeline for novel insecticide discovery, HEK-AaDOP2 cells were used to develop a cell-based assay that enabled rapid and efficient study of receptor antagonists. The Cisbio HTRF cAMP Dynamic 2 detection methodology was chosen as the assay platform, allowing for the direct detection of cAMP in a 384-well format, and initial experiments were focused on validating cAMP responses to dopamine stimulation using this assay format. As AaDOP2 is a Gαs-coupled D1-like dopamine receptor, stimulation with dopamine results in an enhanced level of cAMP (Meyer et al., 2012). As expected, dopamine treatment displayed a concentration-dependent enhancement of cAMP accumulation with an EC50 of 950 ± 190 nM (n = 5). The EC50 of dopamine was similar to that determined in the previous [3H]cAMP-based quantification method (Meyer et al., 2012). Furthermore, the potency of amitriptyline (the prototypical mosquito-toxic AaDOP2 antagonist) for inhibition of dopamine-stimulated cAMP in the HEK-AaDOP2 cells was similar to that previously reported (Tables 1 and 2) (Meyer et al., 2012; Hill et al., 2013), demonstrating suitability of the HTRF cAMP detection technology for high-throughput cell-based pharmacologic studies of AaDOP2.
TABLE 1.
Evaluation of antidepressant compounds from distinct classes for antagonism of the AaDOP2 receptor
The effect of various concentrations of antidepressant compounds was tested for inhibition of 3 µM dopamine-stimulated cAMP in HEK-AaDOP2 receptor cells. Data represent the mean ± S.E.M. IC50 values for at least three independent experiments.
| Compound | IC50 ± S.E.M. | Chemical Class |
|---|---|---|
| nM | ||
| (+)-Butaclamol | 260 ± 32 | DR antagonist |
| Amitriptyline | 5.1 ± 1.2 | TCA |
| Amoxapine | 20 ± 8.4 | TeCA |
| Atomoxetine | No inhibitiona | NRI |
| Clomipramine | 56 ± 18 | TCA |
| Desipramine | 3300 ± 600 | TCA |
| Doxepin | 20 ± 6.2 | TCA |
| Fluoxetine | No inhibitiona | SSRI |
| Fluvoxamine | No inhibitiona | SSRI |
| Imipramine | 360 ± 34 | TCA |
| Norclomipramine | 670 ± 35 | TCA |
| Nortriptyline | 140 ± 50 | TCA |
| Protriptyline | 600 ± 250 | TCA |
| SCH-23390 | 1300 ± 340 | D1DR antagonist |
| Trazodone | No inhibitiona | SARI |
| Venlafaxine | No inhibitiona | SNRI |
D1DR, selective D1-like dopamine receptor antagonist; DR antagonist, nonselective dopamine receptor antagonist; NRI, norepinephrine reuptake inhibitor; SARI, serotonin antagonist and reuptake inhibitor; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; TeCA, tetracyclic antidepressant.
Less than 10% inhibition at 3 µM compound.
TABLE 2.
Pharmacologic characterization of compounds for antagonist activity against the AaDOP2 receptor
The effect of various concentrations of compounds was tested for inhibition of 3 µM dopamine-stimulated cAMP in HEK-AaDOP2 receptor cells. The data represent the mean ± S.E.M. IC50 values for at least three independent experiments.
| Compound | IC50 ± S.E.M. |
|---|---|
| nM | |
| (+)-Butaclamol | 160 ± 31 |
| Amitriptyline | 7.2 ± 1.2 |
| Amperozide | 570 ± 110 |
| Aripiprazole | 6500 ± 770 |
| Asenapine | 0.30 ± 0.06 |
| Benztropine | 340 ± 41 |
| Chlorpromazine | 17 ± 0.88 |
| Chlorprothixene | 1.2 ± 0.39 |
| Cis-(Z)-flupenthixol | 0.35 ± 0.07 |
| Clozapine | 14 ± 2.9 |
| Cyproheptadine | 6.5 ± 1.9 |
| Diphenhydramine | 7500 ± 2800 |
| Haloperidol | 4300 ± 1000 |
| Ketanserin | 3200 ± 360 |
| Ketotifen | 750 ± 180 |
| Loratadine | 18,000 ± 1800 |
| Loxapine | 5.9 ± 1.4 |
| LY-310,762 | 3000 ± 820 |
| Methiothepin | 0.25 ± 0.05 |
| Mianserin | 130 ± 24 |
| Olanzapine | 11 ± 2.2 |
| Pirenperone | 680 ± 98 |
| R59-022 | 53 ± 13 |
| Risperidone | 150 ± 41 |
| Ritanserin | 500 ± 110 |
Our previous screen of the LOPAC1280 library identified 51 active compounds as AaDOP2 antagonists, including several tricyclic antidepressants (Meyer et al., 2012). Importantly, two tricyclic antidepressant compounds, amitriptyline and doxepin, cause significant mortality of mosquito larvae in whole-organism bioassays, suggesting the potential of tricyclic antidepressants as insecticide lead compounds (Meyer et al., 2012). To gain an understanding of the chemical features that are important for AaDOP2 antagonist activity, pharmacologic evaluation of additional small molecules that are structurally related to the tricyclic leads was carried out in the cell-based assay described earlier. Specifically, nine tricyclic antidepressants and five antidepressant compounds lacking a tricyclic core were studied for their ability to antagonize the cAMP accumulation in response to dopamine treatment (3 μM) in HEK-AaDOP2 cells. All nine tricyclic compounds displayed concentration-dependent antagonist activity against AaDOP2, with IC50 values less than 3 μM, whereas compounds representing other classes of antidepressants displayed less than 10% inhibition at 3 μM (Fig. 1; Table 1).
Fig. 1.
Concentration-response curves for selected AaDOP2 antagonists. Test compounds were evaluated for the ability to inhibit dopamine (3 µM)-stimulated cAMP in HEK-AaDOP2 cells. Data points represent the mean ± S.E.M. for at least three independent experiments.
To identify novel AaDOP2 antagonists with chemical structures distinct from the tricyclic antidepressant ring scaffold, we evaluated concentration-dependent effects of a suite of additional active compounds identified in our previous small-molecule screen (Meyer et al., 2012) together with structurally related compounds, enabling an initial in vitro structure-activity relationship (SAR) analysis. As performed earlier, test compounds were studied for their ability to modulate dopamine-stimulated (3 µM) cAMP accumulation in HEK-AaDOP2 cells (Table 2). Interestingly, six compounds were more potent antagonists than the prototypical AaDOP2 antagonist amitriptyline (Table 2). Furthermore, asenapine, methiothepin, and cis-(Z)-flupenthixol displayed subnanomolar IC50 values for inhibition of dopamine-stimulated cAMP in HEK-AaDOP2 cells (Table 2).
Pharmacologic selectivity for the targeted insect over humans and other animals is a critical attribute of potential insecticides. To address this concern, several of the most potent AaDOP2 antagonists were evaluated for antagonist activity in HEK293 cells stably expressing the human D1 dopamine receptor (HEK-hD1 cells) and compared with the hD1 antagonist, SCH-23390. Each compound inhibited 500 nM dopamine-stimulated cAMP in the HEK-hD1 cells and displayed IC50 values between 19 and 13,000 nM (Table 3). However, in contrast to the hD1-selective antagonist SCH-23390, all of these compounds were more potent antagonists of AaDOP2 than hD1, suggesting potential species-selective pharmacologic profiles for these compounds.
TABLE 3.
Assessment of compound potency for human D1 receptor antagonism
The effect of various concentrations of compounds was tested for inhibition of 500 nM dopamine-stimulated cAMP in HEK-hD1 cells. The data represent the mean ± S.E.M. IC50 values for four independent experiments.
| Compound | IC50 ± S.E.M. | Relative Fold Selectivity (AaDOP2/hD1) |
|---|---|---|
| nM | ||
| Amitriptyline | 1100 ± 110 | 170 |
| Amperozide | 13000 ± 680 | 23 |
| Asenapine | 150 ± 11 | 500 |
| Chlorpromazine | 750 ± 80 | 44 |
| Chlorprothixene | 49 ± 8.5 | 41 |
| Cis-(Z)-flupenthixol | 19 ± 1.7 | 54 |
| Cyproheptadine | 1400 ± 160 | 220 |
| Doxepin | 2500 ± 240 | 130 |
| Loxapine | 300 ± 31 | 51 |
| Methiothepin | 83 ± 9.0 | 330 |
| SCH-23390 | 1.2 ± 0.20 | 0.0009 |
In Vivo Toxicity of AaDOP2 Antagonists: Effects on A. aegypti Larvae.
An important second step in our insecticide discovery effort was the evaluation of the in vivo activity of compounds identified and characterized in the cell-based in vitro studies. We developed an A. aegypti larval screen that can be performed in a 24-well plate format, allowing rapid assessment of in vivo toxicity for compounds identified as potent antagonists in the in vitro studies. This assay was designed to also enable evaluation of speed-to-kill and support prioritization of compounds for further study. Twenty-five compounds were tested using this approach (Table 4), and 10 compounds [asenapine, chlorpromazine, benztropine, methiothepin, cis-(Z)-flupenthixol, chlorprothixene, loxapine, mianserin, amperozide, and clomipramine) caused 70–100% larval mortality within 24 hours post-treatment. These compounds were faster-acting and caused greater mortality of mosquito larvae at the 24-hour treatment time point than our previously identified lead compound for insecticide development, amitriptyline. Notably, asenapine, chlorpromazine, and amperozide caused greater than 70% mortality of the mosquito population within 30 minutes, and cis-(Z)-flupenthixol, chlorprothixene, mianserin, loxapine, and methiothepin caused greater than 70% mortality within 3 hours (data not shown). We also identified five compounds with moderate mosquito toxicity (i.e., 40–70% mortality at 24 hours postexposure) and nine compounds with limited or no toxicity to mosquito larvae (i.e., 0–40% mortality at 24 hours) (Table 4). The in vivo larval mortality data show a significant correlation with in vitro potency values for antagonism of dopamine-stimulated cAMP in HEK-AaDOP2 cells (r = −0.770, n = 25, P > 0.0001; Fig. 2), providing an important line of evidence that AaDOP2 antagonism is linked to larval toxicity.
TABLE 4.
In vivo toxicity of test compounds to A. aegypti larvae
Data represent the mean ± S.E.M. of three independent experiments.
| Compound | Larval Mortality |
||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| % | |||
| Amitriptyline | 63 ± 20 | 87 ± 7 | 93 ± 3 |
| Amperozide | 93 ± 7 | 93 ± 7 | 93 ± 7 |
| Asenapine | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Benztropine | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Chlorpromazine | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Chlorprothixene | 87 ± 9 | 93 ± 7 | 100 ± 0 |
| Cis-(Z)-flupenthixol | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Clomipramine | 70 ± 21 | 93 ± 3 | 93 ± 3 |
| Desipramine | 30 ± 25 | 40 ± 30 | 43 ± 28 |
| Diphenhydramine | 63 ± 12 | 77 ± 9 | 83 ± 9 |
| Fluoxetine | 43 ± 30 | 53 ± 24 | 53 ± 24 |
| Fluvoxamine | 27 ± 22 | 33 ± 28 | 43 ± 24 |
| Imipramine | 53 ± 26 | 63 ± 20 | 80 ± 12 |
| Ketanserin | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Loxapine | 97 ± 3 | 100 ± 0 | 100 ± 0 |
| LY-310,762 | 0 ± 0 | 3 ± 3 | 3 ± 3 |
| Methiothepin | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| Mianserin | 97 ± 3 | 97 ± 3 | 97 ± 3 |
| Norclomipramine | 40 ± 31 | 63 ± 19 | 70 ± 15 |
| Nortriptyline | 43 ± 28 | 63 ± 19 | 73 ± 15 |
| Pirenzepine | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Protriptyline | 37 ± 12 | 43 ± 13 | 53 ± 23 |
| SCH-23390 | 3 ± 3 | 23 ± 12 | 47 ± 23 |
| Tomoxetine | 20 ± 15 | 30 ± 20 | 30 ± 20 |
| Venlafaxine | 3 ± 3 | 7 ± 7 | 13 ± 9 |
| Control (water only) | 0 ± 0 | 1 ± 1 | 3 ± 1 |
Fig. 2.
Correlation analysis of test compounds evaluated for in vitro potency (IC50 values in HEK-AaDOP2 cells) and in vivo toxicity (percent mortality of A. aegypti L3-stage larvae following 24-hour treatment). The in vitro potency values for compounds that provided less than 10% inhibition of dopamine-stimulated cAMP in HEK-AaDOP2 cells were set to 20 µM.
In Vivo Toxicity of AaDOP2 Antagonists: Effects on Adult A. aegypti.
Toxicity to adult female A. aegypti is considered an important property of any lead molecule because only adult female mosquitoes are responsible for the transmission of disease-causing agents. Therefore, we developed an adult A. aegypti assay to evaluate the effects of AaDOP2 antagonists following introduction to the insect hemocoel via microinjection. Four of the most potent in vitro and/or most efficacious compounds in the larval bioassay were assessed for toxicity (LD50) and speed-to-kill in adult mosquito bioassays (Fig. 3; Table 5). All compounds tested caused dose-dependent toxicity to adult A. aegypti and were capable of providing 100% mortality at all time points, whereas <6% mortality was observed for the saline-injected and uninjected controls throughout the 96-hour experiments. Cis-(Z)-flupenthixol was the most potent compound, having an LD50 of 1.26 nmol/mosquito following 24-hour exposure (Fig. 3). Chlorpromazine and cis-(Z)-flupenthixol became more potent over the course of the 4-day assay, as LD50 values decreased by ∼2- to 3-fold from the 24- to 96-hour time points for these compounds. In contrast, the LD50 values for amitriptyline and amperozide remained relatively stable over the same treatment duration, suggesting that these compounds reach their maximum potency earlier than chlorpromazine and cis-(Z)-flupenthixol (Table 5).
Fig. 3.
Concentration-response curves of adult A. aegypti female mortality 24 hours after injection with AaDOP2 antagonists. Each data point represents the mean ± S.E.M. for three independent experiments. No mortality was observed in saline-injected or uninjected controls at the 24-hour time point.
TABLE 5.
Toxicity of injected AaDOP2 antagonists to 4-day-old adult female A. aegypti
LD50 values (nanomole per mosquito) were calculated from dead and moribund mosquitoes, and represent the mean ± S.E.M. of three independent experiments. The average percent mortality was less than 6% for both injected and uninjected controls throughout the experiment.
| Compound | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|
| Amitriptyline | 3.78 ± 0.02 | 3.39 ± 0.02 | 3.09 ± 0.02 | 3.06 ± 0.02 |
| Amperozide | 2.19 ± 0.03 | 1.98 ± 0.02 | 1.92 ± 0.07 | 1.92 ± 0.03 |
| Chlorpromazine | 2.34 ± 0.02 | 1.97 ± 0.02 | 1.30 ± 0.03 | 1.27 ± 0.02 |
| Cis-(Z)-flupenthixol | 1.26 ± 0.01 | 0.67 ± 0.02 | 0.46 ± 0.03 | 0.42 ± 0.02 |
Discussion
The active ingredients of the major existing neurotoxic classes of insecticides target acetylcholinesterases (organophosphates and carbamates), GABA receptors (organochlorines), and sodium channels (pyrethroids) within insect nervous systems (Hemingway and Ranson, 2000). Continued efficacy of modern commercial insecticides is threatened by the development of insect populations that are resistant to these chemicals (Hemingway and Ranson, 2000), emphasizing the urgency of developing insecticides with new modes of action. Our recent study identified the dopamine receptors of A. aegypti as potential targets for yellow fever mosquito control (Meyer et al., 2012). Specifically, larval toxicity was observed for two tricyclic antidepressant compounds (amitriptyline and doxepin) that display AaDOP2 antagonism (Meyer et al., 2012).
To better understand the chemical basis of A. aegypti toxicity observed in vivo, compounds with activity profiles similar to amitriptyline and doxepin at human targets (i.e., GPCRs and/or biogenic amine transporters) were evaluated for in vitro AaDOP2 modulation and in vivo efficacy in larval bioassays. Several known GPCR-targeting ligands, including tricyclic antidepressants and antipsychotics, demonstrated potent AaDOP2 antagonism and insecticide activity. However, compounds from other antidepressant classes (e.g., selective serotonin reuptake inhibitors and selective norepinephrine reuptake inhibitors) were largely inactive, suggesting GPCR modulation, rather than biogenic amine transporters, as a contributing mechanism for the observed larval toxicity. Further validating AaDOP2 as a viable insecticide target, our data revealed a significant correlation between the in vitro potency of AaDOP2 antagonists and the toxicity of these compounds to mosquito larvae in vivo (Fig. 2). However, it should be noted that benztropine and amperozide, which caused rapid and high larval mortality (Table 4), had somewhat moderate in vitro potency at AaDOP2 (IC50 values of 340 ± 41 and 570 ± 110 nM, respectively). Amperozide and benztropine interact with several different mammalian GPCR families (U'Prichard et al., 1977; Kanba and Richelson, 1984; Bolden et al., 1992; Arnt and Skarsfeldt, 1998), suggesting the possibility that modulation of additional A. aegypti GPCRs could contribute to the in vivo toxicity of these compounds. Alternatively, such differences between the in vitro potency and the magnitude of in vivo toxicity for a given compound may reflect complex factors that impact in vivo insecticidal activity, including differences in the physicochemical properties of compounds that affect absorption through the insect cuticle, dissemination through insect tissues to the target site, and detoxification by insect gut and hemolymph enzymes. Nonetheless, the correlation between the in vitro potencies for AaDOP2 antagonism and larval toxicities suggests that optimizing compounds for potency and selectivity in vitro may be an efficient way to identify and prioritize new lead compounds.
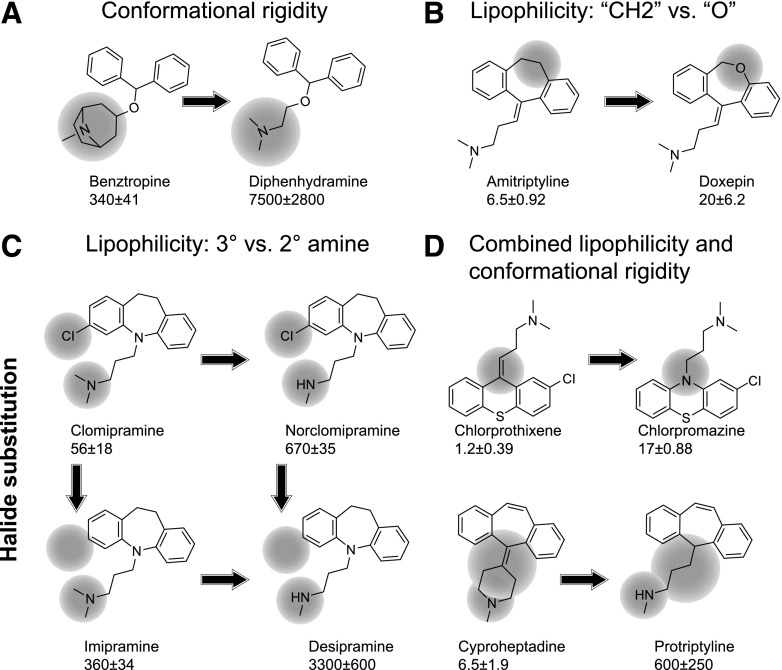
The in vitro evaluation of the chosen compounds for modulation of AaDOP2 provided preliminary insight into the relationship between chemical structure and the potency of AaDOP2 antagonism. One SAR trend suggests that conformational rigidity contributes to the potency of AaDOP2 antagonists. For example, compounds with 6- or 7-membered central rings were generally the most potent AaDOP2 antagonists (Supplemental Fig. 3; Tables 1 and 2). However, the moderate potency of R59-022, risperidone, benztropine, and amperozide (IC50 values ranging from 53 to 570 nM) indicates that the central ring is not essential for antagonist activity. Benztropine was ∼22-fold more potent than diphenhydramine, suggesting that conformational control of the amine moiety also contributes to the potency of compounds with no central ring (Fig. 4A). Another SAR trend suggested that greater lipophilicity may enhance AaDOP2 antagonist potency, as was observed by the ∼3-fold greater potency of amitriptyline over doxepin (Fig. 4B). Furthermore, ligands with tertiary amines (clomipramine, imipramine, amitriptyline, and loxapine) were ∼5- to 100-fold more potent than the secondary amine analogs of these compounds (norclomipramine, desipramine, nortiptyline, and amoxapine, respectively), demonstrating the influence of amine state on AaDOP2 antagonist potency (Fig. 4C; Supplemental Fig. 3). Also, clomipramine and norclomipramine displayed ∼5- to 6-fold more potent IC50 values than imipramine and desipramine, respectively, suggesting that halide substituents of aromatic rings within the tricyclic core can increase the potency of the identified antagonists (Fig. 4C). Enhancements in AaDOP2 antagonist potency were also apparent when considering combinations of chemical properties, such as lipophilicity and halide substitution (Fig. 4C, clomipramine versus desipramine) or lipophilicity and conformational rigidity (Fig. 4D). The chemical scaffolds identified earlier and their key structural features that contribute to AaDOP2 antagonism may be used to guide further lead optimization studies.
Fig. 4.
(A–D) Structure-activity relationship trends for AaDOP2 receptor antagonists. Compound names and in vitro IC50 values (nanomolar) for AaDOP2 antagonism were included.
The in vitro and in vivo data presented here support the hypothesis that targeting GPCR-mediated processes is a viable strategy for identifying insecticidal compounds. However, a major challenge associated with this approach is the development of ligands that are selective for disruption of biologic activity in A. aegypti but not in humans or other higher eukaryotes. To date, all reports indicate that compounds that display both AaDOP2 antagonism and in vivo efficacy are also known to have biologic activity in humans. Our studies identified compounds that are highly selective for targeting AaDOP2 receptors over the human D1 dopamine receptor (Table 3), but antipsychotics and tricyclic antidepressants potently interact with other families of mammalian GPCRs, including histamine, serotonin, adrenergic, and muscarinic receptors (Cusack et al., 1994; von Coburg et al., 2009). The development of ligands that selectively target biologic activity in A. aegypti over humans and other animals could potentially be addressed by using cell-based in vitro assays to screen against panels of human GPCRs. Also, virtual or in silico screening methods are emerging as promising approaches for the study of GPCR modulators (Shoichet and Kobilka, 2012). Such computational methodologies for lead optimization of antipsychotics and tricyclic antidepressants are strengthened by recently reported human GPCR crystal structures from histamine (Shimamura et al., 2011), serotonin (Wang et al., 2013), dopamine (Chien et al., 2010), adrenergic (Rasmussen et al., 2007; Warne et al., 2008), and muscarinic (Haga et al., 2012; Kruse et al., 2012) receptor families. The combination of these in vitro and in silico approaches is expected to provide insight into the molecular determinants of selectivity for AaDOP2 versus human GPCRs, and may ultimately produce mosquito GPCR-selective small molecules.
Pharmacologic selectivity considerations are multifold, as ligand selectivity for AaDOP2 receptors over nontarget insects (e.g., honeybees), in addition to selectivity over human GPCRs, is also paramount. Pharmacologic screening panels can be assembled for invertebrate targets to better understand ligand pharmacology at these receptors. For example, cross-species comparative pharmacologic studies of invertebrate dopamine receptor modulation can be expanded to include GPCRs from nontarget insects. Furthermore, upon genome mining and cloning of additional biogenic amine receptors (in addition to AaDOP1 and AaDOP2), AaDOP2 antagonists can be screened for modulation of other A. aegypti GPCRs, including muscarinic acetylcholine, serotonin, and octopamine/tyramine receptors (Nene et al., 2007). These pharmacologic efforts are expected to provide a deeper understanding of small-molecule modulation of invertebrate GPCRs, and may ultimately allow for target-based pesticide discovery efforts related to other pest arthropods.
Here we report significant advancements and modifications to our genome-to-lead insecticide discovery pipeline (Meyer et al., 2012). Incorporation of an in vitro HTRF assay enabled efficient in vitro pharmacologic evaluation and SAR profiling, while offering several advantages over the previously used CRE-mediated luciferase reporter assay for HTS. Direct measurement of cAMP eliminates false positives associated with CRE-luciferase reporter assays that include cAMP/protein kinase A–independent modulation of CRE-mediated transcription (George et al., 1997; Hill et al., 2001) or direct modulation of luciferase (Thorne et al., 2010). Furthermore, the HTRF screening platform was robust enough to support future HTS of small molecules for AaDOP2 antagonist activity in a 384-well format in singlet (i.e., Z′ > 0.5; J. Conley, C. Hill, and V. Watts, unpublished observations), enabling sufficient throughput to carry out the in vitro pharmacologic profiling proposed earlier. The HTRF screening platform also provides flexibility, as it can be used to detect modulation of additional downstream GPCR signaling pathways, including extracellular signal-regulated kinase 1/2 and Ca2+/inositol trisphosphate (Degorce et al., 2009). Other improvements to our established insecticide discovery pipeline for small-molecule modulators of AaDOP2 (Meyer et al., 2012) include the enhanced-throughput larval mosquito bioassay (Table 4) to rapidly assess larval toxicity and the utilization of an injection assay to evaluate toxicity in adult A. aegypti.
In addition to the antipsychotic and tricyclic antidepressant lead optimization and GPCR profiling studies suggested earlier, HTS of diverse small-molecule libraries for the identification of AaDOP2 modulators with novel chemical scaffolds may also be a fruitful endeavor. Especially enticing is the possibility of screening for allosteric modulators of AaDOP2 receptors, as drug discovery campaigns targeting multiple human GPCRs have identified allosteric modulators with unmatched specificity and selectivity (Conn et al., 2009; Wootten et al., 2013). Allosteric modulators are attractive because the orthosteric sites (i.e., the sites of endogenous ligand binding) are largely conserved between the human D1 and AaDOP2, but our published studies suggest that there are opportunities to exploit allosteric sites in the intracellular and extracellular loops where sequence similarity between species is reduced (Meyer et al., 2012; Hill et al., 2013). Our emerging understanding of the chemical basis of AaDOP2 receptor antagonism, together with advancements in assay throughput, suggest that the diverse molecular approaches described earlier can be combined to expedite the discovery of novel ligands that selectively modulate GPCRs of target insects.
The present study describes the in vitro pharmacologic characterization and in vivo efficacy of several AaDOP2 antagonists and demonstrates improvements upon our “genome-to-lead” pipeline (Meyer et al., 2012). Specifically, we report the characterization of compounds with unparalleled in vitro potency for AaDOP2 inhibition and improved efficacy for A. aegypti larval toxicity, and demonstrate toxicity of these compounds to adult mosquitoes. Collectively, our findings provided a major advancement in the development of invertebrate GPCR-targeting technology for novel mode-of-action insecticides.
Supplementary Material
Acknowledgments
The authors thank Dr. Eric Barker (Purdue University) and Dr. David Nichols (Purdue University) for generously providing venlafaxine and aripiprazole, respectively. The authors also thank Isabelle Verona Brust for assistance with figure preparation.
Abbreviations
- GPCR
G protein–coupled receptor
- HEK
human embryonic kidney
- HEK-AaDOP2
HEK293 cells stably expressing AaDOP2
- HEK-hD1
HEK293 cells stably expressing the human D1 dopamine receptor
- HTRF
homogenous time-resolved fluorescence
- HTS
high-throughput screening
- LY-310,762
1-[2-[4-(4-fluorobenzoyl)-1-piperidinyl]ethyl]-1,3-dihydro-3,3-dimethyl-2H-indol-2-one
- R59-022
6-[2-[4-[(4-fluorophenyl)phenylmethylene]-1-piperidinyl]ethyl]-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
- SAR
structure-activity relationship
- SCH-23390
(R)-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine
Authorship Contributions
Participated in research design: Conley, Hill, Meyer, Nuss, Watts.
Conducted experiments: Conley, Doyle, Meyer, Nuss.
Contributed new reagents or analytic tools: Hill, Watts.
Performed data analysis: Conley, Doyle, Hill, Meyer, Nuss, Savinov, Watts.
Wrote or contributed to the writing of the manuscript: Conley, Hill, Meyer, Nuss, Savinov, Watts.
Footnotes
This work was supported by a US Department of Defense, Deployed War Fighter Project award [Grant W911QY]; and a Purdue Research Foundation Trask Innovation award (to C.A.H. and V.J.W.). Additional support was supplied by the Indiana Clinical and Translational Sciences Institute funded, in part, by the National Institutes of Health National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award [Grant UL1-TR001108].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Ali D. (1997) The aminergic and peptidergic innervation of insect salivary glands. J Exp Biol 200:1941–1949. [DOI] [PubMed] [Google Scholar]
- Andersen JP, Schwartz A, Gramsbergen JB, Loeschcke V. (2006) Dopamine levels in the mosquito Aedes aegypti during adult development, following blood feeding and in response to heat stress. J Insect Physiol 52:1163–1170. [DOI] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, et al. (2010) Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330:86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. (1998) Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology 18:63–101. [DOI] [PubMed] [Google Scholar]
- Bolden C, Cusack B, Richelson E. (1992) Antagonism by antimuscarinic and neuroleptic compounds at the five cloned human muscarinic cholinergic receptors expressed in Chinese hamster ovary cells. J Pharmacol Exp Ther 260:576–580. [PubMed] [Google Scholar]
- Briegel H. (1990) Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol 36:165–172. [Google Scholar]
- Chien EYT, Liu W, Zhao Q, Katritch V, Han GW, Hanson MA, Shi L, Newman AH, Javitch JA, Cherezov V, et al. (2010) Structure of the human dopamine D3 receptor in complex with a D2/D3 selective antagonist. Science 330:1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. (2009) Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 8:41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack B, Nelson A, Richelson E. (1994) Binding of antidepressants to human brain receptors: focus on newer generation compounds. Psychopharmacology (Berl) 114:559–565. [DOI] [PubMed] [Google Scholar]
- Degorce F, Card A, Soh S, Trinquet E, Knapik GP, Xie B. (2009) HTRF: A technology tailored for drug discovery - a review of theoretical aspects and recent applications. Curr Chem Genomics 3:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper I, Kurshan PT, McBride E, Jackson FR, Kopin AS. (2007) Locomotor activity is regulated by D2-like receptors in Drosophila: an anatomic and functional analysis. Dev Neurobiol 67:378–393. [DOI] [PubMed] [Google Scholar]
- Fuchs S, Rende E, Crisanti A, Nolan T. (2014) Disruption of aminergic signalling reveals novel compounds with distinct inhibitory effects on mosquito reproduction, locomotor function and survival. Sci Rep 4:5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SE, Bungay PJ, Naylor LH. (1997) Evaluation of a CRE-directed luciferase reporter gene assay as an alternative to measuring cAMP accumulation. J Biomol Screen 2:235–240. [Google Scholar]
- Haga K, Kruse AC, Asada H, Yurugi-Kobayashi T, Shiroishi M, Zhang C, Weis WI, Okada T, Kobilka BK, Haga T, et al. (2012) Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature 482:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser F, Cazzamali G, Williamson M, Blenau W, Grimmelikhuijzen CJP. (2006) A review of neurohormone GPCRs present in the fruitfly Drosophila melanogaster and the honey bee Apis mellifera. Prog Neurobiol 80:1–19. [DOI] [PubMed] [Google Scholar]
- Hayes RO. (1953) Determination of a physiological saline solution for Aedes aegypti (L). J Econ Entomol 46:624–627. [Google Scholar]
- Hemingway J. (2014) The role of vector control in stopping the transmission of malaria: threats and opportunities. Philos Trans R Soc Lond B Biol Sci 369:20130431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J, Beaty BJ, Rowland M, Scott TW, Sharp BL. (2006) The Innovative Vector Control Consortium: improved control of mosquito-borne diseases. Trends Parasitol 22:308–312. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Ranson H. (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391. [DOI] [PubMed] [Google Scholar]
- Hill SJ, Baker JG, Rees S. (2001) Reporter-gene systems for the study of G-protein-coupled receptors. Curr Opin Pharmacol 1:526–532. [DOI] [PubMed] [Google Scholar]
- Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. (2002) G protein-coupled receptors in Anopheles gambiae. Science 298:176–178. [DOI] [PubMed] [Google Scholar]
- Hill CA, Meyer JM, Ejendal KFK, Echeverry DF, Lang EG, Avramova LV, Conley JM, Watts VJ. (2013) Re-invigorating the insecticide discovery pipeline for vector control: GPCRs as targets for the identification of next gen insecticides. Pestic Biochem Physiol 106:141–148. [Google Scholar]
- Kanba S, Richelson E. (1984) Histamine H1 receptors in human brain labelled with [3H]doxepin. Brain Res 304:1–7. [DOI] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. (2007) D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci 27:7640–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkness EF, Haas BJ, Sun W, Braig HR, Perotti MA, Clark JM, Lee SH, Robertson HM, Kennedy RC, Elhaik E, et al. (2010) Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc Natl Acad Sci USA 107:12168–12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, et al. (2012) Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482:552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K, Kume S, Park SK, Hirsh J, Jackson FR. (2005) Dopamine is a regulator of arousal in the fruit fly. J Neurosci 25:7377–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JM, Ejendal KFK, Avramova LV, Garland-Kuntz EE, Giraldo-Calderón GI, Brust TF, Watts VJ, Hill CA. (2012) A “genome-to-lead” approach for insecticide discovery: pharmacological characterization and screening of Aedes aegypti D(1)-like dopamine receptors. PLoS Negl Trop Dis 6:e1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustard JA, Pham PM, Smith BH. (2010) Modulation of motor behavior by dopamine and the D1-like dopamine receptor AmDOP2 in the honey bee. J Insect Physiol 56:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi Z, Megy K, Grabherr M, et al. (2007) Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316:1718–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SGF, Choi H-J, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VRP, Sanishvili R, Fischetti RF, et al. (2007) Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 450:383–387. [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Isabel G, Coulom H, Neuser K, Seugnet L, Kume K, Iché-Torres M, Cassar M, Strauss R, Preat T, et al. (2011) Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proc Natl Acad Sci USA 108:834–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JR, Essenberg RC, Bowman AS. (2000) Salivary glands in ixodid ticks: control and mechanism of secretion. J Insect Physiol 46:1069–1078. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Shiroishi M, Weyand S, Tsujimoto H, Winter G, Katritch V, Abagyan R, Cherezov V, Liu W, Han GW, et al. (2011) Structure of the human histamine H1 receptor complex with doxepin. Nature 475:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoichet BK, Kobilka BK. (2012) Structure-based drug screening for G-protein-coupled receptors. Trends Pharmacol Sci 33:268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L, Koči J, Kim D, Park Y. (2014) Invertebrate specific D1-like dopamine receptor in control of salivary glands in the black-legged tick Ixodes scapularis. J Comp Neurol 522:2038–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimo L, Koči J, Žitňan D, Park Y. (2011) Evidence for D1 dopamine receptor activation by a paracrine signal of dopamine in tick salivary glands. PLoS ONE 6:e16158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne N, Auld DS, Inglese J. (2010) Apparent activity in high-throughput screening: origins of compound-dependent assay interference. Curr Opin Chem Biol 14:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U’Prichard DC, Greenberg DA, Snyder SH. (1977) Binding characteristics of a radiolabeled agonist and antagonist at central nervous system alpha noradrenergic receptors. Mol Pharmacol 13:454–473. [PubMed] [Google Scholar]
- von Coburg Y, Kottke T, Weizel L, Ligneau X, Stark H. (2009) Potential utility of histamine H3 receptor antagonist pharmacophore in antipsychotics. Bioorg Med Chem Lett 19:538–542. [DOI] [PubMed] [Google Scholar]
- Wang C, Jiang Y, Ma J, Wu H, Wacker D, Katritch V, Han GW, Liu W, Huang X-P, Vardy E, et al. (2013) Structural basis for molecular recognition at serotonin receptors. Science 340:610–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne T, Serrano-Vega MJ, Baker JG, Moukhametzianov R, Edwards PC, Henderson R, Leslie AGW, Tate CG, Schertler GFX. (2008) Structure of a beta1-adrenergic G-protein-coupled receptor. Nature 454:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D, Christopoulos A, Sexton PM. (2013) Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov 12:630–644. [DOI] [PubMed] [Google Scholar]
- Yellman C, Tao H, He B, Hirsh J. (1997) Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. Proc Natl Acad Sci USA 94:4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.