Abstract
There are ongoing efforts to develop pain therapeutics with novel mechanisms of action that avoid common side effects associated with other analgesics. The anticonvulsant neuropeptide galanin is a potent regulator of neuronal excitability and has a well established role in pain modulation, making it a potential target for novel therapies. Our previous efforts focused on improving blood-brain-barrier penetration and enhancing the metabolic stability of galanin analogs to protect against seizures. More recently, we designed peripherally acting galanin analogs that reduce pain-related behaviors by acting in the periphery and exhibit preferential binding toward galanin receptor (GalR)2 over GalR1. In this study, we report preclinical studies of a monodisperse oligoethylene glycol–containing galanin analog, NAX 409-9 (previously reported as GalR2-dPEG24), in rodent analgesic and safety models. Results obtained with NAX 409-9 in these tests were compared with the representative analgesics gabapentin, ibuprofen, acetylsalicylic acid, acetaminophen, and morphine. In mice that received intraplantar carrageenan, NAX 409-9 increased paw withdrawal latency with an ED50 of 6.6 mg/kg i.p. NAX 409-9 also increased the paw withdrawal threshold to mechanical stimulation following partial sciatic nerve ligation in rats (2 mg/kg). Conversely, NAX 409-9 had no effect in the tail flick or hot plate assays (up to 24 mg/kg). Importantly, NAX 409-9 did not negatively affect gastrointestinal motility (4–20 mg/kg), respiratory rate (40–80 mg/kg), or bleed time (20 mg/kg). These studies illustrate that this nonbrain-penetrating galanin analog reduces pain behaviors in several models and does not produce some of the dose-limiting toxicities associated with other analgesics.
Introduction
At least 1.5% of the general population suffers from chronic pain, with at least 50 million affected in the US alone (Taylor, 2006). Unfortunately, current treatment options often do not provide complete relief of symptoms. In addition, the leading classes of analgesics, including opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), and anticonvulsants, each carry specific and dose-limiting side effects. Given the various potential adverse events, when coupled with inadequate pain relief, patients suffering from chronic pain often experience a significantly diminished quality of life. Therefore, there is a large unmet need for therapeutics with novel mechanisms of action, reduced adverse effects, and diminished abuse potential.
Like many other neuropeptides, galanin is a potent modulator of neuronal excitability. Galanin and its putative receptors involved in nociception—galanin receptor (GalR)1 and GalR2—are expressed at sites of pain mediation, including the dorsal root ganglion (DRG) and the dorsal horn of the spinal cord (Lang et al., 2007). Levels of this neuropeptide increase in the spinal cord, DRG, and sensory neurons in various models of sciatic nerve injury (Wiesenfeld-Hallin et al., 1992a,b; Hökfelt et al., 1994). Similarly, galanin levels increase in the spinal cord following carrageenan-induced inflammation (Ji et al., 1995). Overexpression of galanin attenuates painful stimuli (Holmes et al., 2003; Wiesenfeld-Hallin et al., 2005), whereas antisense galanin oligonucleotides (Ji et al., 1994) or receptor antagonists (Verge et al., 1993) result in increased pain behaviors. Exogenously administered galanin is thought to dampen hyperexcitability in the DRG and spinal cord after nerve injury as a result of its inhibitory action on slow depolarizing ventral root potentials (Yanagisawa et al., 1986; Nussbaumer et al., 1989). Injection into the hypothalamic arcuate nucleus increases withdrawal latencies to noxious thermal and mechanical stimulation in intact rats and rats with carrageenan-induced inflammation (Sun et al., 2003). Similarly, centrally administered galanin increases withdrawal latency in carrageenan-induced hyperalgesia and has been proposed to act both pre- and postsynaptically (Hua et al., 2005a). Mice with a null mutation in galanin show reduced pain behaviors following partial nerve injury (Kerr et al., 2000) and show diminished spinal hyperexcitability in comparison with wild-type mice (Kerr et al., 2001). Furthermore, intrathecally administration of GalR2-preferring peptides induces allodynia in intact rats, whereas intrathecal galanin or GalR1-preferring peptides diminish pain behaviors and GalR2-preferring peptides show no effect in nerve injury models (Liu and Hökfelt, 2000; Liu et al., 2001). Conversely, several galanin ligands, nonselective for GalR1/GalR2, have been shown to be antinociceptive (reviewed in Lang et al., 2007, and Mitsukawa et al., 2008). In addition, a GalR2-preferring unmodified galanin fragment, gal(2–11), reversed nerve injury-induced allodynia (Hulse et al., 2011). The facilitating effects observed with low intrathecal doses contrast with several reports showing galanin or galanin-selective receptor agonists inhibiting pain behaviors. Under conditions of mild or moderate evoked pain, endogenous galanin may mediate pain signaling in naive animals, whereas exogenously administered galanin diminishes pain behaviors (Kerr et al., 2001; Gundlach and Jungnickel, 2006), which may become more apparent in states of chronic pain and/or inflammation. An additional role may also be the downregulation of inflammation through both GalR1 and GalR2. Therefore, galanin may show a differential role in pain, depending on pain state, site of action, and concentration. Despite these apparent discrepancies, galanin remains an important modulator of pain, and galanin agonists represent potentially novel therapeutic compounds for the treatment of pain.
Systemic administration of neuropeptides is largely hindered by poor metabolic stability. We have previously shown enhanced metabolic stability, blood-brain-barrier penetration, and antiseizure activity of modified galanin analogs that contain a critical amount of lipophilicity and cationization to rationally selected domains of the active galanin neuropeptide (Bulaj et al., 2008). Our prototype galanin analog, NAX 505-5, has demonstrated potent antiseizure activity in several animal models (White et al., 2009; Jequier Gygax et al., 2014). We have also recently shown that peptides containing monodisperse oligoethyleneglycol (dPEG) modifications, with minimal penetration into the central nervous system, effectively reduce pain-related behaviors in the formalin and carrageenan models of pain (Zhang et al., 2013). These dPEG modifications applied to neuropeptides like galanin provide potential first-in-class neurotherapeutics for pain. In this work, we describe the further pharmacologic characterization and toxicity screening of a novel dPEG-containing GalR2-preferring analog, NAX 409-9 [referred to previously as Gal-R2-dPEG24 (Zhang et al., 2013)]. These studies illustrate that this nonbrain-penetrating analog reduces pain behaviors in several animal models and does not show some of the dose-limiting toxicities associated with representative analgesics, including reduced gastrointestinal (GI) motility and respiratory depression for opiates and increased bleed time for NSAIDs.
Materials and Methods
Animals.
Male albino CF-1 mice (18–38 g; Charles River, Portage, MI) and Male Sprague-Dawley rats (200–225 g) were used as experimental animals. All animals were allowed free access to food and water, except during testing procedures. Experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Utah and were carried out in accordance with the Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health (Bethesda, MD).
Compounds.
Chemical synthesis of NAX 505-5 and NAX 409-9 was previously described (Bulaj et al., 2008; Zhang et al., 2013). Both analogs were synthesized by PolyPeptide Laboratories (San Diego, CA), and delivered as a lyophilized material (acetate salt, purity >95%). Compounds were dissolved in their respective vehicle solvents and administered by intraperitoneal injection. The vehicle solvents for each compound were as follows: saline (0.9% NaCl; for morphine solutions), 0.5% methylcellulose (for gabapentin, acetaminophen, acetylsalicylic acid, and ibuprofen), and 1% Tween 20 in 0.9% NaCl (saline; for NAX 409-9 and NAX 505-5). Testing procedures were conducted at the previously determined time-to-peak effect (TPE) for each compound: morphine (30 minutes), gabapentin (120 minutes), acetaminophen (60 minutes), acetylsalicylic acid (30 minutes), ibuprofen (30 minutes), and NAX 409-9 (60 minutes).
Carrageenan.
A state of localized inflammation was induced in mice by injecting carrageenan (25 μl, 2% in saline, λ-carrageenan; Sigma-Aldrich, St. Louis, MO) subcutaneously into the plantar surface of the right hindpaw. Paw withdrawal responses from thermal stimulation were assessed according to previously described methods (Hargreaves et al., 1988; Dirig et al., 1997; Hua et al., 2005b). Mice were placed in plexiglass chambers on top of a heated glass surface (30°C). Thermal stimulation was applied with a projection bulb below the glass surface. Latency to paw withdrawal was measured from the onset of heat application until a full paw withdrawal occurred. Two measurements were obtained from each paw, with at least 1 minute between assessments, and subsequently averaged to obtain the mean withdrawal latency. Experimental conditions, including animal habituation, glass plate temperature, and projection bulb intensity (35% of maximum), produced baseline withdrawal latencies for noninjected (contralateral) paws and carrageenan-injected (ipsilateral) paws of 6–9 seconds and 2–4 seconds, respectively. Compounds were administered prior to testing, and the TPE for each compound coincided with the peak inflammatory effect of carrageenan (unpublished observations; 3 hours postinjection). Paw withdrawal latencies were obtained at this time point, as described above. For dose-response studies, mice were considered protected if their ipsilateral paw latency was greater than 50% of the maximal effect latency (adapted from Tallarida, 1987). Paw withdrawal latency was calculated using latency values from vehicle-treated animals, with the following formula: [(contralateral latency − ipsilateral latency)/2] + ipsilateral latency. The number of protected/unprotected animals for each dose was then analyzed using Probit to obtain a median effective dose (ED50, with 95% confidence interval) value. For time course studies, withdrawal latencies were obtained 30, 60, and 90 minutes following NAX 409-9 injection (150, 180, and 210 minutes postcarrageenan). Withdrawal latencies were obtained, as described above, and the area under the curve (AUC) was obtained for each paw, in each of the vehicle and NAX-409-9 treatment groups.
Partial Sciatic Nerve Ligation.
The Seltzer partial sciatic nerve ligation (PSNL) model has been described previously (Seltzer et al., 1990). Anesthesia was induced in rats using 4–5% isoflurane, and the depth of anesthesia was maintained using 2–3% isoflurane and monitored by response to tail pinch. The upper thigh was shaved and cleaned with ethanol and betadine. A small incision was made through the skin and underlying muscle of the upper thigh and separated to expose the sciatic nerve. The nerve was separated from the surrounding connective tissue and slightly elevated using fine curved forceps. Approximately one-third to one-half of the nerve was tied off by passing a needle attached to size 8 nylon suture through the nerve. This ligation was performed dorsal to where the sciatic nerve bifurcates and the location of the ligation were similar for each animal. Rats were monitored daily following surgery for infection or untoward postsurgical complications. Approximately 2 weeks following surgery, withdrawal responses to mechanical stimulation by von-Frey fibers were used to obtain withdrawal thresholds before and following drug treatment. Filaments were applied to the mid-paw plantar surface until a slight bend in the fiber occurred or the rat displayed a spontaneous paw withdrawal or flinch. The maximal stimulation applied was 300 g. Individual paw responses to mechanical stimulation were used to quantify mechanical allodynia using a set of calibrated nylon von-Frey monofilaments and the Dixon up-down method (Dixon, 1991; Chaplan et al., 1994). This testing paradigm allowed for determination of the 50% paw withdrawal threshold (PWT). On the day of testing, rats received an initial stimulation, using von-Frey filaments, to obtain a predrug PWT. After compound administration, rats were tested again, to obtain a postdrug PWT. Pre- and postdrug PWTs were compared for statistical analysis and graphical representation, and data are expressed as a percentage of predrug PWT.
Mouse Tail Flick.
Tail flick responses were measured using a radiant beam focused on the tail while using an automated analgesic meter (IITC, Woodland Hills, CA). Mice were gently placed in a restraint chamber, and a light beam (peak intensity of 75 mJ; 50% of maximum intensity) was focused on a point 2–5 mm from the tip of the tail. The light beam sensor was positioned such that the beam automatically shut off when the mouse moved its tail in response to the thermal stimulation or reached a maximum light exposure time of 25 seconds. The trigger temperature (prewarming) was 25°C, and the idle intensity was set at 1% of maximum. The latency to tail flick is the time from the onset of light beam until movement of the tail. Data are presented as mean latencies to tail flick.
Mouse Hot Plate.
Pain reflexes in response to placement on a heated glass surface (55°C) were measured in mice using a Hot Plate Analgesia Meter (IITC). Mice were placed on a heated glass surface (25.4 cm × 25.4 cm) that is surrounded by a clear plexiglass cage (19 cm tall). The latency to respond to the thermal stimulus with either a hindpaw lick, hindpaw flick, or jump (whichever response occurred first) was then recorded. The maximal time spent on the heated surface was 30 seconds. Data are presented as mean hindpaw withdrawal latencies.
GI Motility.
Effects of NAX 409-9 on GI motility were studied in mice, and results obtained with NAX 409-9 were compared with those of morphine. A charcoal bolus (250 μl oral gavage; 10% activated charcoal, 5% gum Arabic, and 85% dH2O by weight) was administered to mice at the TPE following vehicle (1% Tween 20/saline), morphine (2 mg/kg; 30 minutes), or NAX 409-9 (4, 10, and 20 mg/kg; 60 minutes) administration. Mice were sacrificed at 20 minutes following administration of the charcoal bolus, and the GI tract was removed. Connective tissue surrounding the GI tract was detached, and the tissue was placed in a linear arrangement, to allow for measurement of the charcoal bolus. The transit distance of the charcoal bolus was recorded as a percentage of total distance traveled from the pyloric sphincter to the cecum. Data are presented as percentage of total transit distance (entire length of small intestine).
Respiratory Rate.
Following compound administration, mice were placed under light restraint, and a pressure transducer that measured respiratory rate was placed against the thoracic wall of each animal. Data samples were measured using a PowerLab system with Chart software (ADInstruments). After a 1- to 2-minute habituation time, respirations were measured in each animal for approximately 1 minute. The mean respiratory rate for this period was obtained and compared with the total mean respiratory rate for vehicle-treated mice.
Bleed Time.
To study whether NAX 409-9 had any effect on bleed time, in comparison with NSAIDs ibuprofen and acetylsalicylic acid, drugs were administered subchronically. NAX 409-9 (20 mg/kg), ibuprofen (75 mg/kg), and acetylsalicylic acid (100 mg/kg) were administered twice daily for 3 days prior to testing. At testing, mice were restrained in a plexiglass chamber during the measurement of bleed time. Methods for determining bleed time have been described previously (Braun et al., 2009). A 3-mm segment of the tail was removed using surgical scissors, and tail bleeding was monitored by gently blotting drops of blood leaving the tail with filter paper every 20 seconds. Care was taken to ensure that the filter paper did not make direct contact with the wound. Blotting continued for up to 20 minutes after tail clip occurred. When no blood appeared on the filter paper after blotting, the total bleed time (tail clip − bleeding cessation) was noted for each animal.
Data Analysis.
Data are presented as means ± S.E.M. Individual groups were compared by t test or, for multiple comparisons, one- or two-way analysis of variance, followed by Newman-Keuls or Bonferroni a posteriori tests, respectively.
Results
Figure 1 shows the peptide sequence derived from the active galanin fragment, gal(1–16), and dPEG modification used in NAX 409-9. This modified neuropeptide is a peripherally acting, GalR2-preferring galanin analog containing a C-terminal dPEG modification. We previously described the synthesis, galanin receptor binding, and effects obtained from pharmacologic screening of this compound (Zhang et al., 2013) in several assays.
Fig. 1.
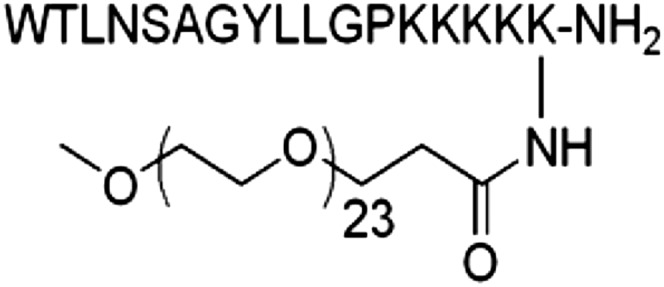
Structure of NAX 409-9. The peptide was derived from the native galanin fragment, gal(1–16), with subsequent C-terminal dPEG modification. The chemical structure, synthesis, galanin receptor binding, and pharmacologic screening of this compound have been previously described (Zhang et al., 2013).
Analgesic Pharmacology
NAX 409-9 Modifies Carrageenan-Induced Thermal Hyperalgesia.
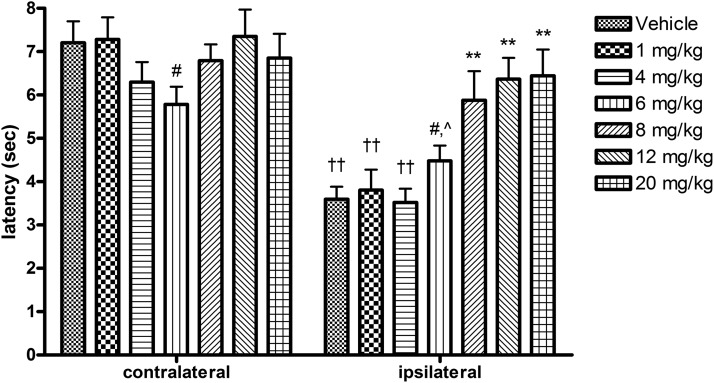
Carrageenan-induced inflammation was verified using a digital caliper to assess paw edema (thickness), measured across the dorso-ventral aspect of each hindpaw after withdrawal latency testing (data not shown). Figure 2 shows contralateral and ipsilateral latencies following thermal stimulation in vehicle- and NAX 409-9–treated mice. There were no differences in contralateral paw latencies between treatment groups, with the exception of 6 mg/kg, which was noted to be significantly lower than 12 mg/kg. However, latencies to paw withdrawal in ipsilateral paws in NAX 409-9–treated mice showed a dose-dependent increase in latency above that observed in vehicle-treated mice (vehicle, 3.5 ± 0.3 seconds; 1 mg/kg, 3.8 ± 0.5 seconds; 4 mg/kg, 3.5 ± 0.3 seconds; 6 mg/kg, 4.5 ± 0.4 seconds; 8 mg/kg, 5.9 ± 0.7 seconds; 12 mg/kg, 6.4 ± 0.5 seconds; and 20 mg/kg, 6.4 ± 0.6 seconds). At doses of 8 mg/kg and greater, ipsilateral latencies in NAX 409-9–treated mice were significantly elevated above the latencies observed in vehicle-treated mice. Maximal efficacy was observed at 20 mg/kg.
Fig. 2.
Dose-response study of NAX 409-9 in the carrageenan model of thermal hyperalgesia. Male CF-1 mice received intraplantar carrageenan (25 μl, 2% solution in saline) in the right (ipsilateral) hindpaw. Three hours later, both left (contralateral) and right hindpaws were subjected to thermal stimulation (focused light beam beneath glass platform). The paw withdrawal (latency) is shown for each paw in each group. One hour prior to latency determination, mice received NAX 409-9 (intraperitoneal injection) at 1 (N = 8), 4 (N = 12), 6 (N = 12), 8 (N = 12), 12 (N = 18), or 20 mg/kg (N = 8), or vehicle (1% Tween 20 in saline, N = 19). NAX 409-9 produced a dose-dependent increase in latency to paw withdrawal in the ipsilateral paw, starting at doses above 6 mg/kg. **P < 0.01 compared with vehicle, 1 mg/kg, and 4 mg/kg ipsilateral latencies. ††P < 0.01 compared with contralateral latencies in the same treatment group. #P < 0.05 compared with 12 mg/kg on the same side paw. ^P < 0.05 compared with 20 mg/kg on the same side paw.
To compare antinociceptive effects of NAX 409-9 with those of commonly used analgesics, representative compounds from different classes of drugs were tested in the carrageenan assay and ED50 values were then determined (see Table 1). Dose ranges for each compound tested were 1–20 mg/kg (NAX 409-9), 3–60 mg/kg (gabapentin), 25–150 mg/kg (ibuprofen), 25–200 mg/kg (acetylsalicylic acid), and 0.05–4 mg/kg (morphine).
TABLE 1.
ED50 [95% confidence interval (CI)] values for NAX 409-9 and representative analgesic compounds in the carrageenan model of thermal hyperalgesia
| Compound | ED50a | 95% CIa |
|---|---|---|
| mg/kg | ||
| NAX 409-9 | 6.6 | 5.6–7.8 |
| Gabapentin | 28.6 | 23.8–34.2 |
| Ibuprofen | 10.5 | n.d.b |
| Acetylsalicylic acid | 49.3 | 29.8–81.4 |
| Morphine | 2.2 | 0.3–15.2 |
n.d., not determined.
ED50 and CI values were calculated by identifying the number of animals in each treatment group protected (above 50% effect threshold, determined in vehicle-treated animals).
The CI for ibuprofen included all doses administered during testing.
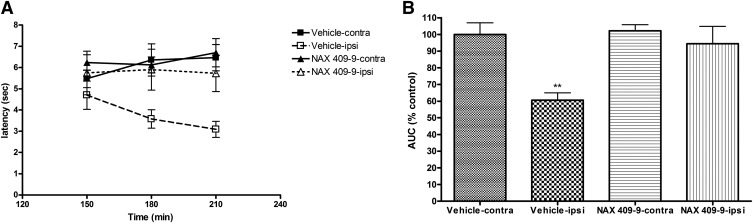
To study the duration of antinociceptive effect following NAX 409-9 treatment, animals were tested 150, 180, and 210 minutes after administration of either vehicle (1% Tween 20/saline) or NAX 409-9 (12 mg/kg) (Fig. 3A). Carrageenan reduced withdrawal latency (in ipsilateral paws) starting at 150 minutes in vehicle-treated mice (4.7 ± 0.7 seconds) and reached a maximal hyperalgesic effect at 180–210 minutes (3.6 ± 0.4 and 3.1 ± 0.4 seconds for 180 minutes and 210 minutes, respectively). By comparison, the ipsilateral paw latency in NAX 409-9–treated mice was unchanged across this time course (5.7 ± 1.0, 5.9 ± 1.0, and 5.7 ± 0.9 seconds for 150, 180, and 210 minutes, respectively). AUC values for these data are shown in Fig. 3B. Although the overall AUC was diminished in ipsilateral paws of vehicle-treated mice (expressed as a percentage of vehicle-contralateral latency AUC; 61 ± 4%; P < 0.01), NAX 409-9 ipsilateral latencies were comparable to control (vehicle-contralateral AUC; 100 ± 7%).
Fig. 3.
Time course of effect of NAX 409-9 (12 mg/kg) in the carrageenan model of thermal hyperalgesia. Mice were evaluated for thermal response latencies at 150, 180, and 210 minutes following carrageenan administration (corresponding to 30, 60, and 90 minutes following administration of NAX 409-9, 12 mg/kg) or vehicle (1% Tween 20/saline). NAX 409-9 was administered such that the time-to-peak effect for the compound (1 hour) corresponded with maximal hyperalgesia following carrageenan administration (approximately 3 hours). (A) Shows the time course of effect of carrageenan administration (administered at time = 0 minute) and treatment with either vehicle (squares) or NAX 409-9 (triangles). Responses in both left (contralateral; filled squares, vehicle; filled triangles, NAX 409-9) and carrageenan-injected (right, ipsilateral; open squares, vehicle; open triangles, NAX 409-9) paws are shown across the time of testing. (B) Shows the area under the curve for each paw (ipsilateral and contralateral) in each group (vehicle or NAX 409-9), normalized to the contralateral paw in vehicle-treated animals (% control). Carrageenan-treated paws show reduced withdrawal latency, with a maximal effect at 210 minutes. NAX 409-9 reversed this reduction in paw withdrawal latency in ipsilateral paws. N = 8 for each group. **P < 0.01 compared with all other groups.
NAX 409-9 Antinociceptive Effect in the PSNL Model.
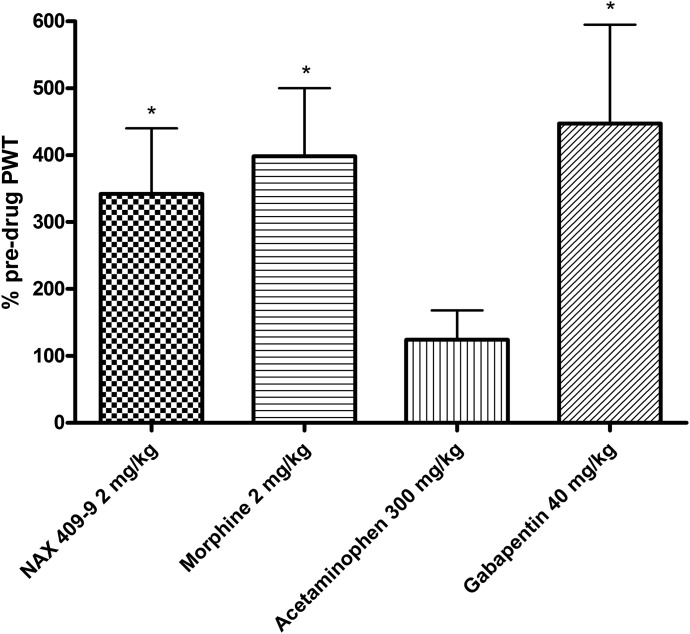
NAX 409-9 and other analgesics were tested in the rat PSNL model. On the day of testing, rats received an initial stimulation (predrug PWT) prior to drug administration. This was then followed by compound administration and retesting (i.e., postdrug PWT). Data are presented as a percentage of predrug PWT. As illustrated in Fig. 4, NAX 409-9 significantly reversed the mechanical allodynia (342 ± 98%) associated with PSNL. By comparison, the representative analgesics morphine (2 mg/kg; 398 ± 102%) and gabapentin (40 mg/kg; 447 ± 148%) also significantly increased PWT, whereas the PWT following acetaminophen administration (124 ± 44%) was not significantly different from vehicle-treated control animals.
Fig. 4.
Antinociceptive activity of NAX 409-9 and representative analgesic compounds in the Seltzer PSNL model of neuropathic pain in rats (Seltzer et al., 1990). The sciatic nerve in the right hind leg of each rat was ligated 2 weeks prior to testing. Rats were injected (intraperitoneally) with the various compounds, at the doses listed above, and tested at various time points (1 hour, NAX 409-9, acetaminophen; 2 hours, morphine; or 4 hours, gabapentin), based on previous time course studies (data not shown). Data are presented as a percentage of predrug PWT. NAX 409-9 (2 mg/kg) increased the PWT in a manner comparable to that of morphine (2 mg/kg) and gabapentin (40 mg/kg), whereas acetaminophen was ineffective at 300 mg/kg. N = 8 for each group. *P < 0.05 compared with predrug PWT values.
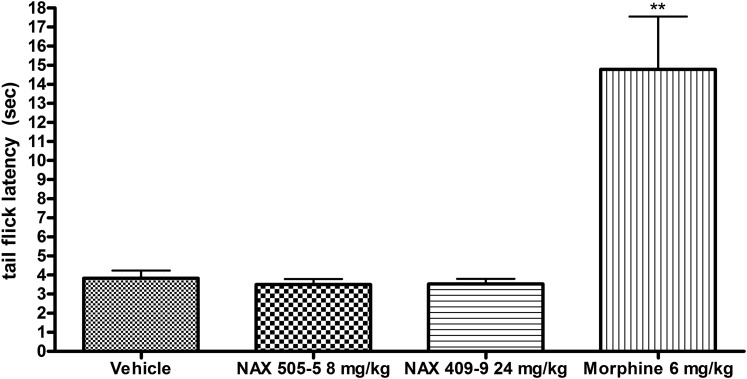
Effect of NAX 409-9 on Mouse Tail Flick Latency.
NAX 409-9 was evaluated for antinociceptive efficacy in a model of acute pain, the tail flick assay. In this model, tail flick responses to an acute thermal stimulus appear to be mediated by a central reflex mechanism. Therefore, NAX 409-9 was tested in comparison with the centrally acting (GalR1-preferring) galanin analog, NAX 505-5. We have previously described the anticonvulsant efficacy of NAX 505-5 (Bulaj et al., 2008; White et al., 2009). Figure 5 shows that neither NAX 505-5 (8 mg/kg) nor NAX 409-9 (24 mg/kg) produced significantly different tail flick latencies (3.5 ± 0.3 seconds for each analog), in comparison with vehicle (3.8 ± 0.4 seconds). In contrast, morphine (6 mg/kg) significantly elevated tail flick latency (14.8 ± 2.9 seconds; P < 0.01 compared with vehicle).
Fig. 5.
Effect of NAX 409-9 in an acute assay of antinociceptive activity, the tail flick assay. Following compound administration, mice were placed under restraint for assessment of latency to tail flick following thermal stimulation (focused light). Mice were treated with either vehicle (1% Tween 20/saline), NAX 505-5 (8 mg/kg), NAX 409-9 (24 mg/kg), or morphine (6 mg/kg). Neither the GalR1-preferring, centrally active analog NAX 505-5 nor NAX 409-9 was effective in increasing tail flick latency, in comparison with morphine. N = 4–8 per group. **P < 0.01 compared with vehicle.
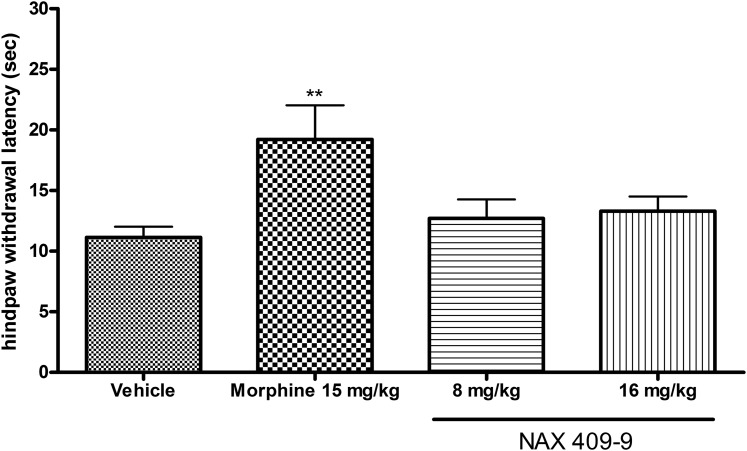
Effect of NAX 409-9 on Hindpaw Withdrawal Latency in the Hot Plate Assay.
NAX 409-9 was tested in the hot plate assay at 8 and 16 mg/kg, and results were compared with the opiate analgesic morphine (15 mg/kg). In comparison with vehicle-treated mice, morphine significantly elevated hindpaw withdrawal latency (19.2 ± 2.8 seconds; P < 0.01), whereas NAX 409-9 had no effect on withdrawal latency (12.7 ± 1.6, 13.3 ± 1.2 seconds, for 8 and 16 mg/kg, respectively) (see Fig. 6).
Fig. 6.
Effect of NAX 409-9 in the hot plate test for evaluation of acute antinociceptive activity. Following compound administration, mice were placed on a heated plate (55°C) within a plexiglass container. Mice were treated with either NAX 409-9, at 8 and 16 mg/kg, or morphine (15 mg/kg). Whereas morphine increased latency to hindpaw withdrawal, NAX 409-9 had no effect at the doses tested. N = 8–14 per group. **P < 0.01 compared with vehicle.
Toxicity Studies
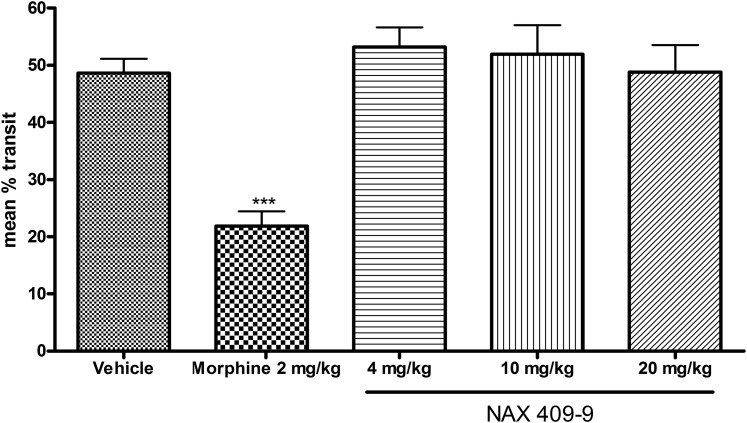
Effects of NAX 409-9 on GI Motility in Mice.
When compared with vehicle-treated mice, a therapeutic dose of morphine (2 mg/kg) significantly reduced charcoal transit in the GI tract of mice (22.6 ± 0.6% of total transit distance). In comparison, NAX 409-9 at 4, 10, or 20 mg/kg had no effect on charcoal transit (respectively, 53.2 ± 8.3, 51.9 ± 12.5, and 48.8 ± 11.6% of total transit distance) (see Fig. 7).
Fig. 7.
Effects of NAX 409-9 and morphine on gastrointestinal motility in mice. Following administration of a charcoal bolus (250 μl oral gavage; 10% activated charcoal, 5% gum Arabic, 85% dH2O by weight) and vehicle (1% Tween 20/saline), morphine (2 mg/kg), or NAX 409-9 (4, 10, or 20 mg/kg) administration, mice were sacrificed and intestinal tracts were removed. Total movement of the charcoal bolus was measured and expressed as a percentage of total intestinal transit distance. Morphine reduced overall intestinal transit distance (***P < 0.001, compared with all other groups), whereas NAX 409-9 at doses up to 20 mg/kg had no effect. Group sizes were N = 6 (NAX 409-9 and morphine) and N = 17 (vehicle).
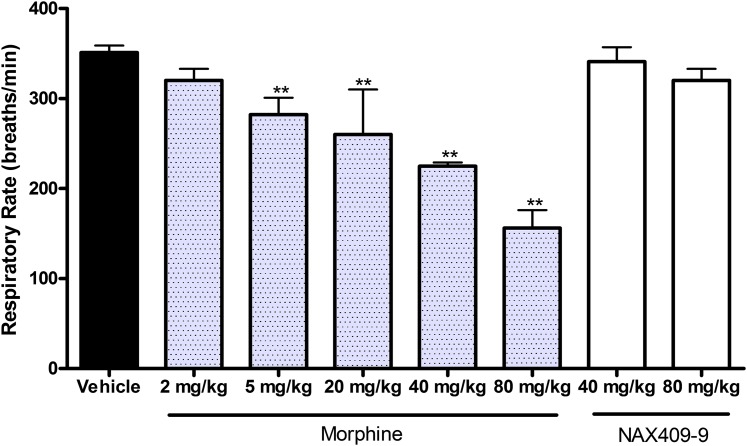
Effects of NAX 409-9 on Respiratory Rate in Mice.
The effect of morphine and NAX 409-9 on respiratory rate was evaluated in mice. In comparison with vehicle-treated mice (351 ± 8 breaths/min), morphine at doses greater than 5 mg/kg significantly decreased respiratory rate (282 ± 19; 260 ± 50; 225 ± 4; 156 ± 20 breaths/min for 5, 20, 40, and 80 mg/kg morphine; P < 0.01, compared with vehicle). At comparable doses of NAX 409-9 (40 and 80 mg/kg), no effects on respiratory rate were observed (341 ± 16 and 320 ± 13 breaths/min, respectively; see Fig. 8).
Fig. 8.
Effects of NAX 409-9 and morphine on respiratory rate in mice. Following compound (morphine, 2, 5, 20, 40, 80 mg/kg; NAX 409-9, 40, 80 mg/kg) or vehicle administration, mice were placed under light restraint and respiratory rate was determined using a pressure transducer placed adjacent to the abdominal wall. Respiratory rates were compared with the group mean for vehicle-treated mice (N = 19). Morphine at doses >5 mg/kg decreased respiratory rate (**P < 0.01), in comparison with vehicle, whereas NAX 409-9 did not decrease respiratory rate. Group sizes for morphine and NAX 409-9 were N = 4–8.
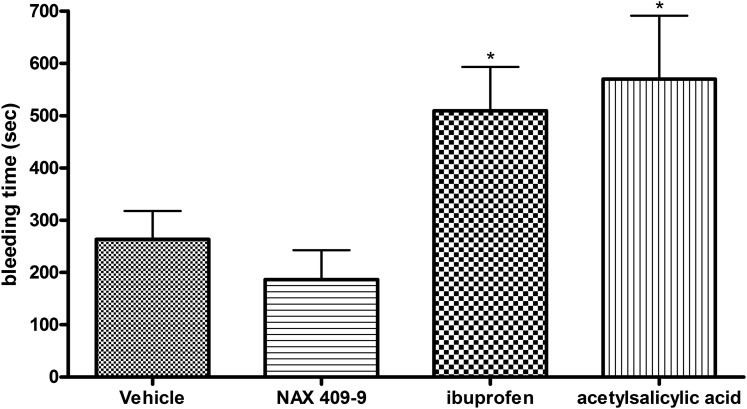
Effect of NAX 409-9 on Bleed Time in Mice.
The effect of NAX 409-9 on bleed time was tested after multiple doses, and results were compared with those obtained for prototype NSAIDs (ibuprofen, acetylsalicylic acid). Following subchronic administration (twice daily for 3 days), NAX 409-9 (20 mg/kg) did not significantly elevate bleed time (187 ± 56 seconds) relative to vehicle (264 ± 54 seconds). In contrast, ibuprofen (75 mg/kg; 509 ± 84 seconds; P < 0.05) and acetylsalicylic acid (100 mg/kg; 570 ± 121 seconds; P < 0.05) increased bleed time following a tail clip in mice (Fig. 9).
Fig. 9.
Effect of NAX 409-9 and NSAIDS (ibuprofen, acetylsalicylic acid) on bleeding time in mice. Mice were administered vehicle (1% Tween 20/saline), NAX 409-9 (20 mg/kg), ibuprofen (75 mg/kg), or acetylsalicylic acid (100 mg/kg) twice daily (intraperitoneal injection) for 3 days. After the final dose on the third day, at the time-to-peak effect for each compound, mice received a tail clip (2–3 mm from tip of tail). Drops of blood were blotted onto filter paper, every 15–20 seconds, without touching the wound, for up to 20 minutes. The total bleed time (se) following tail clip for each compound is shown. Ibuprofen and acetylsalicylic acid significantly increased bleed time (*P < 0.05 compared with vehicle), whereas NAX 409-9 had no effect. N = 14–21 per group.
Discussion
In summary, the results reported in this work show that a novel, peripherally restricted galanin analog has broad analgesic efficacy and avoids common side effects seen with NSAIDs and the opiate morphine. Previously, we reported on the antinociceptive efficacy of this compound in both the formalin and carrageenan models (Zhang et al., 2013). Results in this work extend the previous report by showing the time course of antinociceptive activity in the carrageenan model and the potency of this compound, relative to other analgesics. This report further extends the analgesic profile of this GalR2-preferring agonist to several other pain and toxicity models, and the present data support a role for galanin in mediating inflammation and as a potential therapeutic target for inflammatory pain.
NAX 409-9 and representative analgesics were tested in the carrageenan model of thermal hyperalgesia. This model has been used previously to characterize novel analgesic compounds (Porreca et al., 2006; Yaksh et al., 2006) as well as galanin (Sun et al., 2004; Xiong et al., 2005). Carrageenan has also been shown to induce changes in galanin expression in the DRG, dorsal horn, and the skin (Ji et al., 1995). It is therefore likely that galanin plays a role in response to peripheral inflammation. Injection of galanin into the arcuate nucleus increases withdrawal latencies to noxious thermal and mechanical stimulation in intact rats and rats with carrageenan-induced inflammation (Sun et al., 2003), and centrally administered galanin increases withdrawal latency after carrageenan (Xiong et al., 2005). Similarly, NAX 409-9 has been shown to reduce responses in the formalin model of pain as well as carrageenan (Zhang et al., 2013). Galanin has also been shown to reduce inflammation in an experimental model of intestinal colitis (Talero et al., 2006). In this study, NAX 409-9 dose-dependently increased withdrawal latency in the inflamed paw with a maximal efficacy of 20 mg/kg. Activity of this peripherally acting galanin agonist in the carrageenan model is in agreement with previous reports suggesting a role for galanin in inflammatory pain. Furthermore, NAX 409-9 compares well with representative analgesics morphine, gabapentin, ibuprofen, and acetylsalicylic acid, suggesting potential clinical utility of galanin agonists as novel therapies for pain and inflammation. Whereas there was a significant decrease noted in the contralateral paw latency of the 6 mg/kg treatment group, when compared with 12 mg/kg, this latency was not different than contralateral vehicle latencies and was not observed in any other treatment group.
Galanin has previously been shown to increase dramatically in the spinal cord, DRG, and sensory neurons following sciatic nerve injury (Wiesenfeld-Hallin et al., 1992a,b; Hökfelt et al., 1994). To determine whether peripherally acting GalR2-preferring analogs confer analgesia following nerve injury, we studied the effects of NAX 409-9 in the PSNL model. NAX 409-9 was able to increase PWT in a comparable manner to that of gabapentin and morphine. Acetaminophen, however, did not increase PWT in this model. It is interesting that the peripherally acting galanin analog NAX 409-9 showed comparable activity to the centrally acting analgesics gabapentin and morphine. NAX 409-9 is most likely acting within sensory neurons or DRG, rather than in the spinal cord, and efficacy in the PSNL model suggests an antinociceptive role for GalR2 following nerve injury. These results are similar to those of Hulse et al. (2011), which showed that a GalR2/GalR3-preferring agonist [gal(2–11)] administered peripherally into the receptive field (subcutaneousadministration) was able to elevate mechanical thresholds. From these studies, the authors concluded that peripheral galanin contributes to increased C-fiber thresholds and decreases in spontaneous or evoked pain. In agreement with these observations, activity of NAX 409-9 in the PSNL assay suggests that administrations of a peripherally acting GalR2 agonist can decrease pain-related behaviors following nerve injury.
By contrast, NAX 409-9 did not produce analgesia in models of acute pain (tail flick and hot plate) at doses that were highly effective in the carrageenan model in mice (8–20 mg/kg). Responses in the tail flick assay are mediated via a spinal reflex and therefore not likely to be affected by peripherally acting compounds. However, as the centrally acting GalR1-preferring analog NAX 505-5 was also not highly effective in this model, it is possible that galanin or GalR1 may not produce significant analgesia in response to acute painful states. Moreover, lack of efficacy in hot plate also suggests that galanin may play a less significant role in acute pain. Efficacy in carrageenan and formalin (Zhang et al., 2013), by contrast, suggests that NAX 409-9 is more effective in models of chronic pain or inflammation. It is also noteworthy that the mechanism employed in hot plate and tail flick (thermal stimulation) is different than the chemically mediated hyperalgesia following formalin or the sciatic injury used in the PSNL model. Therefore, it is also likely that the responses observed may be related to the modality of pain used in each animal model. Further studies in similar models of chronic pain, including chronic administration, will further explore the role of GalR2 and inflammation and assess whether this novel analgesic agent can reduce inflammation and/or diminish painful behaviors.
One of the main problems associated with the use of analgesics are dose-limiting side effects. Opioids can reduce GI motility and produce respiratory depression (reviewed in Swegle and Logemann, 2006, and Benyamin et al., 2008), and NSAIDs can increase bleeding risk (reviewed in Schoenfeld et al., 1999, and Laine et al., 2006). Novel analgesic therapies should possess potent activity and avoid common side effects associated with current analgesic therapies. To date, little is known about the potential toxicities of systemic administration of galanin or galanin analogs. To address the question of whether NAX 409-9 negatively affects respiratory rate, GI motility, and bleeding time, we tested NAX 409-9 in various toxicity assays. The effect of NAX 409-9 on GI motility and respiratory rate was compared with that of morphine, whereas the effect of NAX 409-9 on bleed time was compared with that of the NSAIDs ibuprofen and acetylsalicylic acid. Doses of NAX 409-9 used in these studies were in the therapeutic range (i.e., active in the carrageenan assay) or supratherapeutic range (above 20–30 mg/kg). Although morphine showed pronounced activity in reducing GI motility, NAX 409-9 showed no effect at 4–20 mg/kg. Lack of any effect on GI motility by NAX 409-9 suggests that, at therapeutically relevant doses, galanin analogs are unlikely to possess the untoward GI effects of morphine. Morphine also significantly reduced respiratory rate, whereas NAX 409-9 had no effect, up to 80 mg/kg. Not surprisingly, as the central activity of morphine can reduce respiratory activity, this peripherally acting galanin agonist is unlikely to affect respiration by the same mechanism. Lack of effect of NAX 409-9 in reducing respiratory rate suggests a wide therapeutic index for this novel analgesic. Finally, in comparison with NSAIDs, NAX 409-9 was tested in the bleed time assay. NSAIDs, following subchronic administration, significantly increased bleed time, whereas NAX 409-9 had no effect. Therefore, this test shows that NAX 409-9 does not likely possess the same toxicities associated with NSAIDs, which can cause excessive bleeding, particularly bleeding in the GI tract.
As this work describes the potential utility of peripherally acting galanin agonists in the therapeutic treatment of chronic pain and inflammation, future studies should be directed at other models of chronic pain and inflammation that could add to the analgesic profile of NAX 409-9. In addition, future studies should also include evaluation of chronic administration of this peptide in pain models.
In summary, NAX 409-9 represents a novel first-in-class therapeutic with a broad-spectrum analgesic profile that compares favorably to the opiate morphine and the NSAIDs ibuprofen and acetylsalicylic acid. In contrast to morphine and the NSAIDs, NAX 409-9 possesses a more favorable therapeutic index as it is less likely to produce a decrease in respiratory rate or GI motility, and is less likely to increase bleeding tendency. These results certainly support further evaluation of NAX 409-9 and other galanin-based therapeutics for the treatment of pain.
Abbreviations
- AUC
area under the curve
- dPEG
monodisperse oligoethylene glycol
- DRG
dorsal root ganglion
- GalR
galanin receptor
- GI
gastrointestinal
- NSAID
nonsteroidal anti-inflammatory drug
- PSNL
partial sciatic nerve ligation
- PWT
paw withdrawal threshold
- TPE
time-to-peak effect
Authorship Contributions
Participated in research design: Metcalf, Klein, Smith, Bulaj, White.
Conducted experiments: Metcalf, Klein, McDougle, Smith.
Contributed new reagents or analytic tools: Bulaj, McDougle, Zhang.
Performed data analysis: Metcalf, Klein, Smith.
Wrote or contributed to the writing of the manuscript: Metcalf, Klein, McDougle, Zhang, Smith, Bulaj, White.
Footnotes
This work was supported in part by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R21-NS059669 to G.B. and H.S.W.].
G.B. and H.S.W. are scientific cofounders of Neuroadjuvants, Inc.
Some of the data presented in this work has been previously presented as follows: Metcalf CS, Klein BD, McDougle DR, Bulaj G, and White HS (2012) Antinociceptive effects of novel GalR2-specific analogs. Experimental Biology 2012; 2012 Apr 21–25; San Diego, CA. American Society for Pharmacology and Experimental Therapeutics; and Metcalf CS, Klein BD, McDougle DR, Zhang L, Smith MD, Bulaj G, and White HS (2013) Development of peripherally-acting, GalR2-preferring galanin analogs for the treatment of pain. Galanin SFN Pre-Meeting 2013; 2013 Nov 8–9; San Diego, CA.
References
- Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. (2008) Opioid complications and side effects. Pain Physician 11:S105–S120. [PubMed] [Google Scholar]
- Braun A, Varga-Szabo D, Kleinschnitz C, Pleines I, Bender M, Austinat M, Bösl M, Stoll G, Nieswandt B. (2009) Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood 113:2056–2063. [DOI] [PubMed] [Google Scholar]
- Bulaj G, Green BR, Lee HK, Robertson CR, White K, Zhang L, Sochanska M, Flynn SP, Scholl EA, Pruess TH, et al. (2008) Design, synthesis, and characterization of high-affinity, systemically-active galanin analogues with potent anticonvulsant activities. J Med Chem 51:8038–8047. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. [DOI] [PubMed] [Google Scholar]
- Dirig DM, Salami A, Rathbun ML, Ozaki GT, Yaksh TL. (1997) Characterization of variables defining hindpaw withdrawal latency evoked by radiant thermal stimuli. J Neurosci Methods 76:183–191. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. (1991) Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev 15:47–50. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Jungnickel SR. (2006) Galanin and GALP systems in brain – molecular pharmacology, anatomy, and putative roles in physiology and pathology, in Handbook of Biologically Active Peptides (Kastin AJ. ed) pp 753–761, Academic Press, Waltham, MA. [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Zhang X, Wiesenfeld-Hallin Z. (1994) Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci 17:22–30. [DOI] [PubMed] [Google Scholar]
- Holmes FE, Bacon A, Pope RJ, Vanderplank PA, Kerr NC, Sukumaran M, Pachnis V, Wynick D. (2003) Transgenic overexpression of galanin in the dorsal root ganglia modulates pain-related behavior. Proc Natl Acad Sci USA 100:6180–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua XY, Salgado KF, Gu G, Fitzsimmons B, Kondo I, Bartfai T, Yaksh TL. (2005a) Mechanisms of antinociception of spinal galanin: how does galanin inhibit spinal sensitization? Neuropeptides 39:211–216. [DOI] [PubMed] [Google Scholar]
- Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. (2005b) Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur J Neurosci 22:2431–2440. [DOI] [PubMed] [Google Scholar]
- Hulse RP, Wynick D, Donaldson LF. (2011) Activation of the galanin receptor 2 in the periphery reverses nerve injury-induced allodynia. Mol Pain 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jequier Gygax M, Klein BD, White HS, Kim M, Galanopoulou AS. (2014) Efficacy and tolerability of the galanin analog NAX 5055 in the multiple-hit rat model of symptomatic infantile spasms. Epilepsy Res 108:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang Q, Bedecs K, Arvidsson J, Zhang X, Xu XJ, Wiesenfeld-Hallin Z, Bartfai T, Hökfelt T. (1994) Galanin antisense oligonucleotides reduce galanin levels in dorsal root ganglia and induce autotomy in rats after axotomy. Proc Natl Acad Sci USA 91:12540–12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Zhang X, Zhang Q, Dagerlind A, Nilsson S, Wiesenfeld-Hallin Z, Hökfelt T. (1995) Central and peripheral expression of galanin in response to inflammation. Neuroscience 68:563–576. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Cafferty WB, Gupta YK, Bacon A, Wynick D, McMahon SB, Thompson SW. (2000) Galanin knockout mice reveal nociceptive deficits following peripheral nerve injury. Eur J Neurosci 12:793–802. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Gupta Y, Pope R, Thompson SW, Wynick D, McMahon SB. (2001) Endogenous galanin potentiates spinal nociceptive processing following inflammation. Pain 93:267–277. [DOI] [PubMed] [Google Scholar]
- Laine L, Smith R, Min K, Chen C, Dubois RW. (2006) Systematic review: the lower gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Aliment Pharmacol Ther 24:751–767. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Kofler B. (2007) The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharmacol Ther 115:177–207. [DOI] [PubMed] [Google Scholar]
- Liu HX, Brumovsky P, Schmidt R, Brown W, Payza K, Hodzic L, Pou C, Godbout C, Hökfelt T. (2001) Receptor subtype-specific pronociceptive and analgesic actions of galanin in the spinal cord: selective actions via GalR1 and GalR2 receptors. Proc Natl Acad Sci USA 98:9960–9964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Hökfelt T. (2000) Effect of intrathecal galanin and its putative antagonist M35 on pain behavior in a neuropathic pain model. Brain Res 886:67–72. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T. (2008) Galanin, galanin receptors and drug targets. Cell Mol Life Sci 65:1796–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussbaumer JC, Yanagisawa M, Otsuka M. (1989) Pharmacological properties of a C-fibre response evoked by saphenous nerve stimulation in an isolated spinal cord-nerve preparation of the newborn rat. Br J Pharmacol 98:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Vanderah TW, Guo W, Barth M, Dodey P, Peyrou V, Luccarini JM, Junien JL, Pruneau D. (2006) Antinociceptive pharmacology of N-[[4-(4,5-dihydro-1H-imidazol-2-yl)phenyl]methyl]-2-[2-[[(4-methoxy-2,6-dimethylphenyl) sulfonyl]methylamino]ethoxy]-N-methylacetamide, fumarate (LF22-0542), a novel nonpeptidic bradykinin B1 receptor antagonist. J Pharmacol Exp Ther 318:195–205. [DOI] [PubMed] [Google Scholar]
- Schoenfeld P, Kimmey MB, Scheiman J, Bjorkman D, Laine L. (1999) Review article: nonsteroidal anti-inflammatory drug-associated gastrointestinal complications—guidelines for prevention and treatment. Aliment Pharmacol Ther 13:1273–1285. [DOI] [PubMed] [Google Scholar]
- Seltzer Z, Dubner R, Shir Y. (1990) A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain 43:205–218. [DOI] [PubMed] [Google Scholar]
- Sun YG, Gu XL, Lundeberg T, Yu LC. (2003) An antinociceptive role of galanin in the arcuate nucleus of hypothalamus in intact rats and rats with inflammation. Pain 106:143–150. [DOI] [PubMed] [Google Scholar]
- Sun YG, Li J, Yang BN, Yu LC. (2004) Antinociceptive effects of galanin in the rat tuberomammillary nucleus and the plasticity of galanin receptor 1 during hyperalgesia. J Neurosci Res 77:718–722. [DOI] [PubMed] [Google Scholar]
- Swegle JM, Logemann C. (2006) Management of common opioid-induced adverse effects. Am Fam Physician 74:1347–1354. [PubMed] [Google Scholar]
- Talero E, Sánchez-Fidalgo S, Ramón Calvo J, Motilva V. (2006) Galanin in the trinitrobenzene sulfonic acid rat model of experimental colitis. Int Immunopharmacol 6:1404–1412. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. (1987) Manual of Pharmacological Calculations with Computer Programs, Springer-Verlag, New York. [Google Scholar]
- Taylor RS. (2006) Epidemiology of refractory neuropathic pain. Pain Pract 6:22–26. [DOI] [PubMed] [Google Scholar]
- Verge VM, Xu XJ, Langel U, Hökfelt T, Wiesenfeld-Hallin Z, Bartfai T. (1993) Evidence for endogenous inhibition of autotomy by galanin in the rat after sciatic nerve section: demonstrated by chronic intrathecal infusion of a high affinity galanin receptor antagonist. Neurosci Lett 149:193–197. [DOI] [PubMed] [Google Scholar]
- White HS, Scholl EA, Klein BD, Flynn SP, Pruess TH, Green BR, Zhang L, Bulaj G. (2009) Developing novel antiepileptic drugs: characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics 6:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Bartfai T, Hökfelt T. (1992a) Galanin in sensory neurons in the spinal cord. Front Neuroendocrinol 13:319–343. [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Crawley JN, Hökfelt T. (2005) Galanin and spinal nociceptive mechanisms: recent results from transgenic and knock-out models. Neuropeptides 39:207–210. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ, Langel U, Bedecs K, Hökfelt T, Bartfai T. (1992b) Galanin-mediated control of pain: enhanced role after nerve injury. Proc Natl Acad Sci USA 89:3334–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Gao L, Sapra A, Yu LC. (2005) Antinociceptive role of galanin in the spinal cord of rats with inflammation, an involvement of opioid systems. Regul Pept 132:85–90. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Kokotos G, Svensson CI, Stephens D, Kokotos CG, Fitzsimmons B, Hadjipavlou-Litina D, Hua XY, Dennis EA. (2006) Systemic and intrathecal effects of a novel series of phospholipase A2 inhibitors on hyperalgesia and spinal prostaglandin E2 release. J Pharmacol Exp Ther 316:466–475. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Yagi N, Otsuka M, Yanaihara C, Yanaihara N. (1986) Inhibitory effects of galanin on the isolated spinal cord of the newborn rat. Neurosci Lett 70:278–282. [DOI] [PubMed] [Google Scholar]
- Zhang L, Klein BD, Metcalf CS, Smith MD, McDougle DR, Lee HK, White HS, Bulaj G. (2013) Incorporation of monodisperse oligoethyleneglycol amino acids into anticonvulsant analogues of galanin and neuropeptide y provides peripherally acting analgesics. Mol Pharm 10:574–585. [DOI] [PubMed] [Google Scholar]