Abstract
Cancer stem cells (CSCs) have been reported to be critical in the initiation, maintenance, and progression of cancers. The expression of stem cell markers, such as podoplanin (PDPN), CD133, and nestin, may have been correlated with malignant progression. However, the effects of CSCs and stem cell markers on clinical outcomes in cancer patients remain unclear. In this study, we assessed the prognostic roles of glioma CSCs (gCSCs) isolation and stem cell markers in patients with primary glioblastoma (pGBM). A cohort of 39 patients with pGBM was separated into two groups, those positive or negative for gCSCs, and the correlation between gCSC and patient survival was evaluated. We observed significantly different cumulative survival (P = 0.045) when comparing patients positive for gCSCs patients and negative for gCSC. Among the patients positive for gCSCs, we observed no significant differences in survival between those whose gCSCs were each positive or negative for PDPN, CD133, or nestin. This study strongly supports the prognostic value of gCSCs isolation on the survival of patients with pGBM.
1. Introduction
Cancer stem cells (CSCs) are critical in the initiation, maintenance, and progression of tumors and in the development of resistance to therapy [1], although the tumor microenvironment is also important to these processes [2–5]. The accumulation of evidence suggesting a strong association between CSCs and malignancy [6, 7] has led to considerable research on the prognostic role of CSCs in cancer patients [8]. Close associations have been observed between clinical outcomes and the presence of CSC features in various tumors, such as the expression of stem cell markers, genetic features, and the formation of tumor spheres [9–13]. Similar findings have been observed in patients with glioma in that neurosphere formation and the expression of stem cell markers, such as podoplanin (PDPN), CD133, and nestin, were found to be prognostic markers of clinical outcomes [14–16]. In contrast, other studies have found that the presence of CSC features, such as expression of stem cell markers, was not prognostically significant [8, 17, 18], bringing into question the prognostic value of CSC features.
In this study, we assessed the prognostic role of the isolation of glioma CSCs (gCSCs) and expression of stem cell markers (PDPN, CD133, and nestin). These two factors are supposed to be strongly associated with tumor malignancy, in patients with pGBM [15]. We evaluated previously defined populations of gCSCs, with properties that included the ability to form gliomaspheres, to undergo neural differentiation, and to induce tumorigenesis in vivo [6]. In this study, a cohort of 39 patients with pGBM was separated into two groups, those positive or negative for gCSCs, and the correlation between gCSC and patient survival was evaluated. We also assessed the expression of stem cell markers (PDPN, CD133, and nestin) in gCSCs and its relationships with patient survival to address the possible prognostic value of these markers in pGBM.
2. Materials and Methods
2.1. Patient Population
Patients with pGBM treated at two institutions between 2009 and 2013 were included in this study (Table 1); patients with secondary GBM were excluded. All patients were histologically diagnosed by neuropathologists and graded according to the 2007 WHO classification [20]. All patients provided written informed consent, and the study was approved by the Institutional Review Boards of the two institutions (KC10SNSI0466 and 4-2012-0212).
Table 1.
Cohorts of primary glioblastoma patients who were positive or negative for glioma cancer stem cells (gCSCs) [6].
| Patients | gCSC candidates | Age (years) | Sex | Podoplanin | CD133 | Nestin |
|---|---|---|---|---|---|---|
| gCSC positive | gCSC0315 | 57 | F | − | + | + |
| gCSC0426 | 44 | F | − | + | + | |
| gCSC0520 | 72 | F | − | + | + | |
| gCSC0713 | 55 | F | − | − | − | |
| gCSC0504 | 39 | M | + | + | + | |
| gCSC1120 | 48 | F | + | + | + | |
| gCSC0503 | 64 | M | + | + | + | |
| gCSC0114 | 53 | M | − | + | + | |
| gCSC0213 | 51 | F | − | + | + | |
| gCSC0228 | 68 | M | + | + | + | |
| gCSC0308 | 61 | M | + | + | + | |
| gCSC0924 | 11 | M | + | + | + | |
| gCSC0510 | 49 | F | + | + | + | |
| gCSC0627 | 61 | M | + | + | + | |
| gCSC0520 | 34 | M | + | + | + | |
|
| ||||||
| gCSC negative | gCSC0406 | 38 | F | |||
| gCSC08241 | 71 | F | ||||
| gCSC1005 | 59 | F | ||||
| gCSC1124 | 63 | F | ||||
| gCSC0226 | 28 | M | ||||
| gCSC0309 | 59 | M | ||||
| gCSC0803 | 60 | F | ||||
| gCSC08242 | 65 | F | ||||
| gCSC0620 | 66 | F | ||||
| gCSC0928 | 46 | M | ||||
| gCSC1108 | 82 | M | ||||
| gCSC0219 | 60 | M | ||||
| gCSC0528 | 66 | F | ||||
| gCSC0610 | 49 | M | ||||
| gCSC0702 | 63 | M | ||||
| gCSC0709 | 24 | M | ||||
| gCSC0816 | 57 | M | ||||
| gCSC0822 | 62 | F | ||||
| gCSC1118 | 57 | F | ||||
| gCSC1218 | 44 | M | ||||
| gCSC0102 | 68 | M | ||||
| gCSC0106 | 57 | F | ||||
| gCSC0529 | 60 | F | ||||
| gCSC1102 | 57 | M | ||||
2.2. Treatments
All patients received combined therapy, consisting of surgery, followed by concurrent chemotherapy and radiotherapy and adjuvant chemotherapy (Table 2) [19]. The aim of surgery was gross total tumor resection, defined as macroscopic removal of 100% of the tumor mass. Patients not suitable for total resection underwent subtotal resection, defined as removal of <100% but ≥90% of the macroscopic tumor mass, or partial resection, defined as removal of <90% of the macroscopic tumor [21]. The extent of tumor resection was estimated by the neurosurgeons and confirmed by postoperative review of magnetic resonance imaging (MRI) scans. All patients received postoperative adjuvant radiotherapy with concomitant and adjuvant temozolomide (TMZ), as described previously [19]. Recurrent tumors were treated with salvage temozolomide (200 mg/m2) [22] in 16 patients and radiotherapy in one patient and not treated in six patients.
Table 2.
Demographic characteristics of the pGBM patient.
| Characteristics | gCSCs positive | gCSCs negative | P value* |
|---|---|---|---|
| (N = 15) | (N = 24) | ||
| Age (years) | 0.188 | ||
| Median | 53 ± 15 | 60 ± 13 | |
| Range | 11–72 | 28–82 | |
| Sex (number [%]) | 0.653 | ||
| Male | 8 (53%) | 11 (46%) | |
| Female | 7 (47%) | 13 (54%) | |
| Mean follow-up (months) | 13.5 ± 8.4 | 19.9 ± 12.4 | 0.225 |
| Pathological diagnosis | Primary glioblastoma | Primary glioblastoma | |
| Treatment | Surgery + Stupp's regimen [19] | Surgery + Stupp's regimen [19] | |
| Extent of surgery (number [%]) | |||
| Total resection | 10 (67%) | 14 (58%) | 0.636 |
| Subtotal resection | 4 (27%) | 8 (33%) | |
| Partial resection | 1 (7%) | 2 (8%) | |
| Recurrences (number [%]) | 11 (73%) | 12 (50%) | 0.192 |
| Treatment of recurrences (number [%]) | |||
| Radiation therapy | 1 (9%) | 0 (0%) | |
| Adjuvant temozolomide | 6 (55%) | 10 (83%) | |
| None | 4 (36%) | 2 (17%) | |
| 1p 19q codeletion by FISH | 1 (7%) | 0 (0%) | 0.385 |
| MGMT methylation by PCR | 7 (47%) | 11 (45%) | 1.000 |
pGBM: primary glioblastoma; gCSCs: glioma cancer stem cells; MGMT: O-6-methylguanine-DNA methyltransferase. *By Mann-Whitney test for continuous variables and by Fisher's exact test for categorical variables.
2.3. Isolation of gCSCs
Tumor specimens had been collected in the operating room from glioblastoma patients undergoing surgery, followed by isolation of gCSCs within 1 hour using a previously described mechanical dissociation method [6]. Only cells that showed the ability to form gliomaspheres, undergo neural differentiation, and induce in vivo tumorigenesis, as described in our previous report [6], had been defined as gCSCs. The isolated gCSC preparations had been assayed for the expression of PDPN, CD133, and nestin by immunocytochemistry (Table 1) [6]. These selection procedures and immunocytochemical analyses had been performed using protocols described in our previous report [6]. The survival outcomes of the patients with confirmed pGBM were followed up.
2.4. Statistical Analysis
The primary study outcome was overall survival (OS), measured from the date of surgery confirming the diagnosis of pGBM to the date of the last follow-up visit or death. Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank (Mantel-Cox) test. Patients' demographic characteristics were compared using the Mann-Whitney U test for continuous variables and by Fisher's exact test for categorical variables. All statistical analyses were performed using SPSS version 18.0KO software (SPSS Korea, Seoul, Korea), with P < 0.05 considered statistically significant.
3. Results
3.1. Patients
Of the 39 pGBM patients treated at our institution from April 2009 to December 2013, fifteen were categorized as positive and twenty-four as negative for gCSCs (Table 1). These patients included 19 males and 20 females, ranging in age from 11 to 82 years. In 15 of the 39 pGBM patients, gCSCs were isolated. Immunocytochemical analysis had showed that [6], of the 15 patients positive for gCSCs, nine had PDPN+, fourteen had CD133+, and fourteen had nestin+ gCSCs (Table 1).
3.2. Patient Survival
The demographic and clinical characteristics of the 39 pGBM patients are shown in Table 2. The mean survival time of all 39 pGBM patients was 725 days. The mean survival time of the twenty-four gCSC negative patients (855 days) was much longer than that of the 15 gCSC positive patients (520 days). There were no statistically significant differences in age (P = 0.188), gender (P = 0.653), mean follow-up (P = 0.225), extent of surgery (P = 0.636), recurrence rate (P = 0.192), 1p 19q codeletion (P = 0.385), and O-6-methylguanine-DNA methyltransferase (MGMT) methylation (P = 1.000) between the gCSC positive and gCSC negative groups of patients. Kaplan-Meier curves displaying the proportion of OS are demonstrated in Figure 1, showing statistically significant difference in cumulative survival (P = 0.045) between the two groups.
Figure 1.
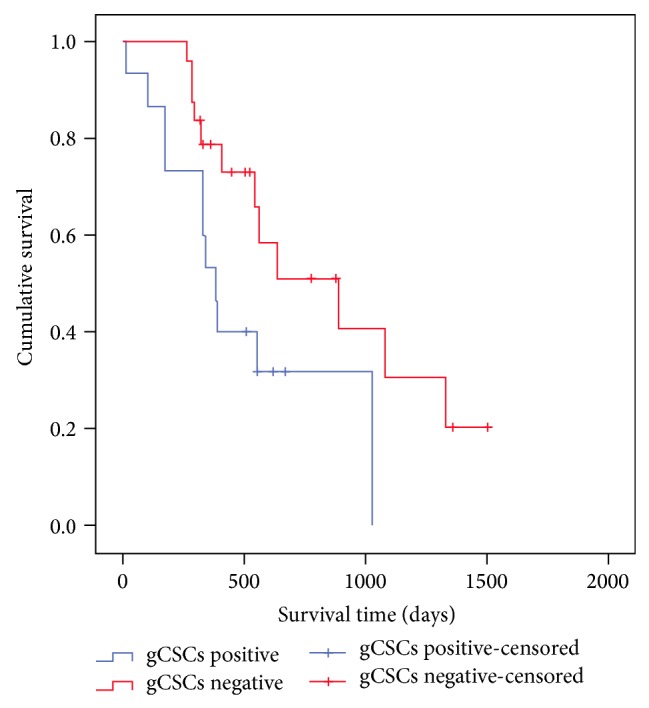
Kaplan-Meier survival curves of pGBM patients positive and negative for gCSCs (P = 0.045 as calculated by the log-rank test).
Among the patients positive for gCSCs, we observed no significant differences in survival between those whose gCSCs were positive (nine patients) or negative (six patients) for PDPN expression (P = 0.619), positive (14 patients) or negative (one patient) for CD133 (P = 0.079), and positive (14 patients) or negative (one patient) for nestin (P = 0.079).
4. Discussion
We observed statistically significant difference in OS between gCSC positive and gCSC negative patients with pGBM. As CSCs may influence tumor growth and resistance to treatment [1], gCSCs are supposed to have a prognostic role in predicting the clinical outcome in pGBM patients. CSC features are reported to have been associated with poorer prognosis in various types of cancers [9–13]. Similar findings were reported in patients with a glioma in that the formation of neurosphere correlated with poorer clinical outcomes [14, 23, 24]. Our findings of the statistical analyses present reliable evidence that the isolation of gCSC is an independent prognostic factor for the clinical outcome of patients with pGBM.
To identify further candidate prognostic markers in patients with pGBM, we analyzed the relationship between the presence of PDPN+, CD133+, and nestin+ gCSCs and survival in pGBM patients. Some studies have reported that PDPN, CD133, and nestin expression are prognostic in glioma patients. Ernst et al. [25] and Mishima et al. [15] reported that PDPN expression was prognostic in patients with astrocytomas. Expression of CD133 [26] and nestin [27] was associated with poorer outcomes in many cancers, including brain cancers. In this series, however, there were no statistically significant differences in overall survivals between PDPN+, CD133+, and nestin+ gCSCs and PDPN−, CD133−, and nestin− gCSCs. The mean survival times of PDPN-expressing gCSCs positive and negative groups were similar, 400 days and 408 days, respectively, and there was no significant difference in OS between two groups (P = 0.619). CD133 and nestin were expressed in most of the patients positive for gCSCs and there was only one case of CD133− and nestin− gCSC, so it was impossible to compare the two groups statistically. These findings indicate that the presence of PDPN-, CD133-, and nestin-expressing gCSCs was not prognostic indicators for survival in pGBM patient. This conclusion is supported by data from other studies, which reported that expression of stem cell markers did not have prognostic significance in glioma patients [8, 18, 28].
In this study, only pGBM patients were included for adjusting the grade of glioma. We previously reported that the rates of existence of gCSC increase proportionally as the WHO grades of glioma rise [6]. In the study including samples from various grades of gliomas, the poorer prognosis correlated with the isolation of gCSC should be affected by the grade of glioma because of the close correlation of the gCSC isolation rates and the grade of glioma. Because of the trend of step-by-step increase of gCSC isolation rate according to the WHO grades of gliomas, the stem cell markers should be expressed more in the patients with higher grade gliomas. We showed that most of the gCSCs expressed the stem cell markers, such as CD133 and nestin, in this study.
There are some limitations in this study. The in vitro assay for the isolation of gCSC takes length duration and requires the technical expertise to perform it. So, it may not be suitable for predicting the prognosis of the pGBM patients with short survival periods. We investigated the expression of stemness surface antigens only in the neurospheres and not in the parent tumors. Regarding the duration and technical needs of this in vitro assay, investigation of the expression of stemness surface antigens in the parent tumors may be more proper for evaluation of the prognosis. These suggest the need for additional studies assessing the associations between the stemness surface antigens in the parent tumor and clinical outcomes in pGBM patients.
5. Conclusion
In conclusion, we showed that the presence of gCSCs alone was significantly prognostic of OS in patient with pGBM. Although we found that the presence of PDPN+, CD133+, and nestin+ gCSCs was not prognostic of OS, our findings suggest that this issue warrants further investigation. We are currently performing a continued study in a larger numbers of patients to further address the detailed role of gCSCs in pGBM patients.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2006427) and by a Grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1509). It was also supported by the National Research Foundation and Ministry of Education, Science and Technology, Korean Government, through its National Nuclear Technology Program (2012M2B2B1055639).
Conflict of Interests
The authors declare no conflict of interests.
Authors’ Contribution
Byung Ho Kong, Ju Hyung Moon, and Yong-Min Huh contributed equally to this work as co-first authors.
References
- 1.Sulman E., Aldape K., Colman H. Brain tumor stem cells. Current Problems in Cancer. 2008;32(3):124–142. doi: 10.1016/j.currproblcancer.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Kim Y. G., Jeon S., Sin G.-Y., et al. Existence of glioma stroma mesenchymal stemlike cells in Korean glioma specimens. Child's Nervous System. 2013;29(4):549–563. doi: 10.1007/s00381-012-1988-1. [DOI] [PubMed] [Google Scholar]
- 3.Kong B. H., Shin H.-D., Kim S.-H., Mok H.-S., Shim J.-K., Lee J.-H., Shin H.-J., Huh Y.-M., Kim E.-H., Park E.-K., Chang J. H., Kim D.-S., Hong Y.-K., Kim S. H., Lee S.-J., Kang S.-G. Increased in vivo angiogenic effect of glioma stromal mesenchymal stem-like cells on glioma cancer stem cells from patients with glioblastoma. International Journal of Oncology. 2013;42(5):1754–1762. doi: 10.3892/ijo.2013.1856. [DOI] [PubMed] [Google Scholar]
- 4.Kwak J., Shin H. J., Kim S. H., et al. Isolation of tumor spheres and mesenchymal stem-like cells from a single primitive neuroectodermal tumor specimen. Child's Nervous System. 2013;29(12):2229–2239. doi: 10.1007/s00381-013-2201-x. [DOI] [PubMed] [Google Scholar]
- 5.Lim H.-Y., Kim K. M., Kim B. K., Shim J.-K., Lee J.-H., Huh Y.-M., Kim S.-H., Kim E.-H., Park E.-K., Shim K.-W., Chang J. H., Kim D.-S., Kim S. H., Hong Y.-K., Lee S.-J., Kang S.-G. Isolation of mesenchymal stem-like cells in meningioma specimens. International Journal of Oncology. 2013;43(4):1260–1268. doi: 10.3892/ijo.2013.2053. [DOI] [PubMed] [Google Scholar]
- 6.Kong B. H., Park N.-R., Shim J.-K., Kim B.-K., Shin H.-J., Lee J.-H., Huh Y.-M., Lee S.-J., Kim S.-H., Kim E.-H., Park E.-K., Chang J. H., Kim D.-S., Kim S. H., Hong Y.-K., Kang S.-G., Lang F. F. Isolation of glioma cancer stem cells in relation to histological grades in glioma specimens. Child's Nervous System. 2013;29(2):217–229. doi: 10.1007/s00381-012-1964-9. [DOI] [PubMed] [Google Scholar]
- 7.Shin G.-Y., Shim J.-K., Lee J.-H., Shin H.-J., Lee S.-J., Huh Y.-M., Kim E.-H., Park E.-K., Kim S.-H., Chang J. H., Kim D.-S., Hong Y.-K., Kim S. H., Kang S.-G., Lang F. F. Changes in the biological characteristics of glioma cancer stem cells after serial in vivo subtransplantation. Child's Nervous System. 2013;29(1):55–64. doi: 10.1007/s00381-012-1963-x. [DOI] [PubMed] [Google Scholar]
- 8.Kim K.-J., Lee K.-H., Kim H.-S., Moon K.-S., Jung T.-Y., Jung S., Lee M.-C. The presence of stem cell marker-expressing cells is not prognostically significant in glioblastomas. Neuropathology. 2011;31(5):494–502. doi: 10.1111/j.1440-1789.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 9.Pirozzi G., Tirino V., Camerlingo R., La Rocca A., Martucci N., Scognamiglio G., Franco R., Cantile M., Normanno N., Rocco G. Prognostic value of cancer stem cells, epithelial-mesenchymal transition and circulating tumor cells in lung cancer. Oncology Reports. 2013;29(5):1763–1768. doi: 10.3892/or.2013.2294. [DOI] [PubMed] [Google Scholar]
- 10.Wang X., Ren H., Zhao T., et al. Stem cell factor is a novel independent prognostic biomarker for hepatocellular carcinoma after curative resection. Carcinogenesis. 2014;35(10):2283–2290. doi: 10.1093/carcin/bgu162. [DOI] [PubMed] [Google Scholar]
- 11.Kok M., Koornstra R. H., Margarido T. C., Fles R., Armstrong N. J., Linn S. C., Van't Veer L. J., Weigelt B. Mammosphere-derived gene set predicts outcome in patients with ER-positive breast cancer. The Journal of Pathology. 2009;218(3):316–326. doi: 10.1002/path.2544. [DOI] [PubMed] [Google Scholar]
- 12.Kim H. S., Yoo S. Y., Kim K. T., Park J. T., Kim H. J., Kim J. C. Expression of the stem cell markers CD133 and nestin in pancreatic ductal adenocarcinoma and clinical relevance. International Journal of Clinical and Experimental Pathology. 2012;5(8):754–761. [PMC free article] [PubMed] [Google Scholar]
- 13.Bao B., Ali S., Ahmad A., et al. Differentially expressed miRNAs in cancer-stem-like cells: markers for tumor cell aggressiveness of pancreatic cancer. Stem Cells and Development. 2014;23(16):1947–1958. doi: 10.1089/scd.2013.0551. [DOI] [PubMed] [Google Scholar]
- 14.Laks D. R., Masterman-Smith M., Visnyei K., et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells. 2009;27(4):980–987. doi: 10.1002/stem.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mishima K., Kato Y., Kaneko M. K., Nishikawa R., Hirose T., Matsutani M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathologica. 2006;111(5):483–488. doi: 10.1007/s00401-006-0063-y. [DOI] [PubMed] [Google Scholar]
- 16.Dahlrot R. H., Hermansen S. K., Hansen S., Kristensen B. W. What is the clinical value of cancer stem cell markers in gliomas? International Journal of Clinical and Experimental Pathology. 2013;6(3):334–348. [PMC free article] [PubMed] [Google Scholar]
- 17.Schoppmann S. F., Berghoff A. S., Jesch B., Zacherl J., Nirtl N., Jomrich G., Maroske F., Streubel B., Mesteri I., Birner P. Expression of podoplanin is a rare event in sporadic gastrointestinal stromal tumors and does not influence prognosis. Future Oncology. 2012;8(7):859–866. doi: 10.2217/fon.12.71. [DOI] [PubMed] [Google Scholar]
- 18.Christensen K., Schrøder H. D., Kristensen B. W. CD133 identifies perivascular niches in grade II-IV astrocytomas. Journal of Neuro-Oncology. 2008;90(2):157–170. doi: 10.1007/s11060-008-9648-8. [DOI] [PubMed] [Google Scholar]
- 19.Stupp R., Mason W. P., van den Bent M. J., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 20.Louis D. N., Ohgaki H., Wiestler O. D., et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathologica. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramm C. M., Wagner S., van Gool S., Schmid H., Sträter R., Gnekow A., Rutkowski S., Wolff J. E. A. Improved survival after gross total resection of malignant gliomas in pediatric patients from the HIT-GBM studies. Anticancer Research. 2006;26(5B):3773–3779. [PubMed] [Google Scholar]
- 22.Hart M. G., Garside R., Rogers G., Stein K., Grant R. Temozolomide for high grade glioma. The Cochrane Database of Systematic Reviews. 2013;4 doi: 10.1002/14651858.CD007415.pub2.CD007415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panosyan E. H., Laks D. R., Masterman-Smith M., Mottahedeh J., Yong W. H., Cloughesy T. F., Lazareff J. A., Mischel P. S., Moore T. B., Kornblum H. I. Clinical outcome in pediatric glial and embryonal brain tumors correlates with in vitro multi-passageable neurosphere formation. Pediatric Blood and Cancer. 2010;55(4):644–651. doi: 10.1002/pbc.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beier D., Wischhusen J., Dietmaier W., Hau P., Proescholdt M., Brawanski A., Bogdahn U., Beier C. P. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathology. 2008;18(3):370–377. doi: 10.1111/j.1750-3639.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst A., Hofmann S., Ahmadi R., et al. Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clinical Cancer Research. 2009;15(21):6541–6550. doi: 10.1158/1078-0432.CCR-09-0695. [DOI] [PubMed] [Google Scholar]
- 26.Pallini R., Ricci-Vitiani L., Banna G. L., Signore M., Lombardi D., Todaro M., Stassi G., Martini M., Maira G., Larocca L. M., De Maria R. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clinical Cancer Research. 2008;14(24):8205–8212. doi: 10.1158/1078-0432.CCR-08-0644. [DOI] [PubMed] [Google Scholar]
- 27.Strojnik T., Røsland G. V., Sakariassen P. O., Kavalar R., Lah T. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surgical Neurology. 2007;68(2):133–143. doi: 10.1016/j.surneu.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 28.Chinnaiyan P., Wang M., Rojiani A. M., et al. The prognostic value of nestin expression in newly diagnosed glioblastoma: report from the radiation therapy oncology group. Radiation Oncology. 2008;3(1, article 32) doi: 10.1186/1748-717X-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]


