Abstract
Purpose
Although American Society of Clinical Oncology guidelines discourage the use of tumor marker assessment for routine surveillance in nonmetastatic breast cancer, their use in practice is uncertain. Our objective was to determine use of tumor marker tests such as carcinoembryonic antigen and CA 15-3/CA 27.29 and associated Medicare costs in early-stage breast cancer survivors.
Methods
By using Surveillance, Epidemiology, and End Results-Medicare records for patients diagnosed with early-stage breast cancer between 2001 and 2007, tumor marker usage within 2 years after diagnosis was identified by billing codes. Logistic regression models were used to identify clinical and demographic factors associated with use of tumor markers. To determine impact on costs of care, we used multivariable regression, controlling for other factors known to influence total medical costs.
Results
We identified 39,650 eligible patients. Of these, 16,653 (42%) received at least one tumor marker assessment, averaging 5.7 tests over 2 years, with rates of use per person increasing over time. Factors significantly associated with use included age at diagnosis, diagnosis year, stage at diagnosis, race/ethnicity, geographic region, and urban/rural status. Rates of advanced imaging, but not biopsies, were significantly higher in the assessment group. Medical costs for patients who received at least one test were approximately 29% greater than costs for those who did not, adjusting for other factors.
Conclusion
Breast cancer tumor markers are frequently used among women with early-stage disease and are associated with an increase in both diagnostic procedures and total cost of care. A better understanding of factors driving the use of and the potential benefits and harms of surveillance-based tumor marker testing is needed.
INTRODUCTION
Routine monitoring for breast cancer recurrence with tumor markers (carcinoembryonic antigen [CEA],1,2 CA 15-3,1,2 and CA 27.29) is not recommended for women with locoregional disease who have been treated with curative intent.3,4 These recommendations are based on studies showing that intensive monitoring with laboratory studies and diagnostic imaging modalities does not improve survival compared with physical examination.5–9 Two prospective trials5–8,10 evaluated intensive follow-up versus standard follow-up in patients with early-stage breast cancer in the 1980s, and in another study, 126 patients were retrospectively classified as having intensive or minimal follow-up.9 None of these studies found a significant difference in overall survival. A survey conducted before publication of more recent evidence suggested that a substantial fraction of clinicians ordered breast cancer tumor markers in at least some of their patients with stage I to III breast cancer.11 Recent surveys of oncologists suggest that a substantial minority of oncologists continue to order tumor marker tests.11–13
Today's cost-conscious environment and focus on evidence-based care has heightened calls for ending the use of serial monitoring by using tumor markers. For example, breast cancer tumor markers were highlighted in the American Society of Clinical Oncology's (ASCO's) Cost of Care Task Force and the joint American Board of Internal Medicine/American Society of Clinical Oncology (ASCO) “Choosing Wisely” campaign as an opportunity to reduce cost of care without harming patient outcomes.14 Beyond the costs of the tests themselves, tumor marker assessment may lead to further unnecessary downstream testing as a result of false positives resulting from relatively poor assay specificity.15 However, the actual frequency of use of these tests and additional health care usage associated with testing are unknown.
The objective of this study was to determine usage of tumor marker assessment and associated Medicare costs in early-stage breast cancer among survivors age 65 years or older with locoregional breast cancer. We characterized follow-up testing and total costs of care among those who underwent tumor marker testing compared with those who were not tested, hypothesizing that patients receiving tumor marker tests would have higher rates of follow-up tests and procedures and higher overall costs of care than those who did not. The findings from this study may be useful for policymakers, clinicians, and researchers considering ways to reduce the use of or improve the evidence base for use of tumor markers in women with locoregional breast cancer.
METHODS
Patient Population
The Surveillance, Epidemiology, and End Results (SEER)-Medicare database includes pathologic, staging, health care use, cost, and survival information for more than 94% of Medicare enrollees diagnosed with cancer in the SEER regions.14,16 We evaluated patients included in the SEER-Medicare database from 2001 to 2007. The study population was restricted to patients with breast cancer who were continuously enrolled in Medicare Part A and Part B for a period beginning 12 months before diagnosis through 24 months after diagnosis. We limited our follow-up period to 2 years from diagnosis to minimize the likelihood that the patients in our cohort would have developed recurrent disease during the follow-up period (much of which is metastatic). Patients with breast cancer recurrence in the 2 years after diagnosis were identified by using a validated algorithm developed by Chubak et al17 and were excluded from the study. Recurrence was indicated by any of the following: a second breast cancer record in the SEER registry, a mastectomy claim at least 180 days after primary cancer diagnosis, or a diagnosis code for a secondary non-breast malignant neoplasm occurring at least 180 days after primary cancer diagnosis.
Patients were excluded from the analysis if they had any of the following: unknown month of diagnosis; breast cancer that was not the first primary cancer diagnosis; diagnosis made at autopsy, by death certificate, or in same calendar month as death; SEER-recorded stage IV (metastatic disease), stage 0 (in situ disease), or unknown stage at diagnosis; those who died within 24 months after diagnosis; and those younger than age 65 years who qualified for Medicare on the basis of disability and/or end-stage renal disease. We also excluded patients enrolled in health maintenance organizations (HMOs), because claims data to track tumor marker or other health care use were unavailable. Patients with no claims of any type in the 2-year period after diagnosis, indicative of missing claims data rather than a true lack of use, were also excluded.
Identification of Use of Tumor Markers and Other Diagnostic Tests
Tabulation of tumor marker testing began at diagnosis. CEA testing (Healthcare Common Procedure Coding System [HCPCS] code 82378) was identified uniquely; however, CA 15-3 and CA 27.29 testing could not be separated because one code is used for both tests (HCPCS code 86300). To avoid duplicate counting of tumor markers tests, a 1-week exclusion window was created around each patient test in which additional claims for tests were not counted on the basis of discussions with clinical specialists (N.L.H. and J.R.G.).
Use of ultrasound, computed tomography, magnetic resonance imaging, positron emission tomography, bone scan, biopsy procedures, and chemotherapy was determined by using Medicare claims during the period from 3 months to 24 months after diagnosis (the first 2 months after diagnosis were excluded to minimize capture of tests performed for initial evaluation of the extent of disease). Claims indicating imaging procedures and chemotherapy drugs and their administration were identified by using relevant HCPCS, Revenue Center, and International Classification of Diseases, 9th edition, Clinical Modification (ICD-9-CM) Volume 3 procedure codes; claims indicating biopsy procedures were identified by using HCPCS, ICD-9-CM Volume 3 codes, and diagnostic related group codes.
Analysis of Factors Influencing Testing Decisions
We developed a logistic regression model to determine associations between tumor characteristics and demographic factors at diagnosis and receipt of one or more tumor marker tests. The dependent variable was receipt of at least one tumor marker test in the 2 years after cancer diagnosis. Independent variables included age, diagnosis year, race/ethnicity, SEER tumor stage at diagnosis, estrogen receptor status, progesterone receptor status, SEER registry (ie, geographic location), urban/rural status, and noncancer comorbidity score based on claims data in the 12 months before diagnosis.16,18 Tests of statistical significance were two-sided, and all analyses were conducted by using SAS 9.3 (SAS Institute, Cary, NC).
Analysis of Cost Data
Total cost of care was measured as reimbursements for claims filed between months 3 and 24 after the breast cancer diagnosis date. A linear regression model was used to estimate the effect of tumor marker testing on total cost of care. Total cost was approximately log-normally distributed; therefore, log-transformed cost of care was analyzed as a continuous response variable. A categorical variable indicating whether a patient underwent at least one tumor marker assessment within 2 years after diagnosis was included in the model, as well as the variables that were used in the logistic regression model. All statistical tests were two-sided. Parameter estimates from the regression model were exponentiated to display results as percent increases or decreases in total cost of care. SEs and 95% CIs were constructed by using the delta method.19 A second regression model was limited to patients with stage I disease only, as part of a sensitivity analysis. Institutional review board approval was obtained by Fred Hutchinson Cancer Research Center in October 2013.
RESULTS
Participant Demographics
We identified 218,697 women diagnosed with locoregional breast cancer from 2001 to 2007. After applying initial exclusion criteria, there were 53,059 patients remaining. An additional 13,409 patients were identified as having locally recurrent or metastatic disease by using the recurrence algorithm, resulting in a total of 39,650 patients eligible for analysis (Fig 1).
Fig 1.
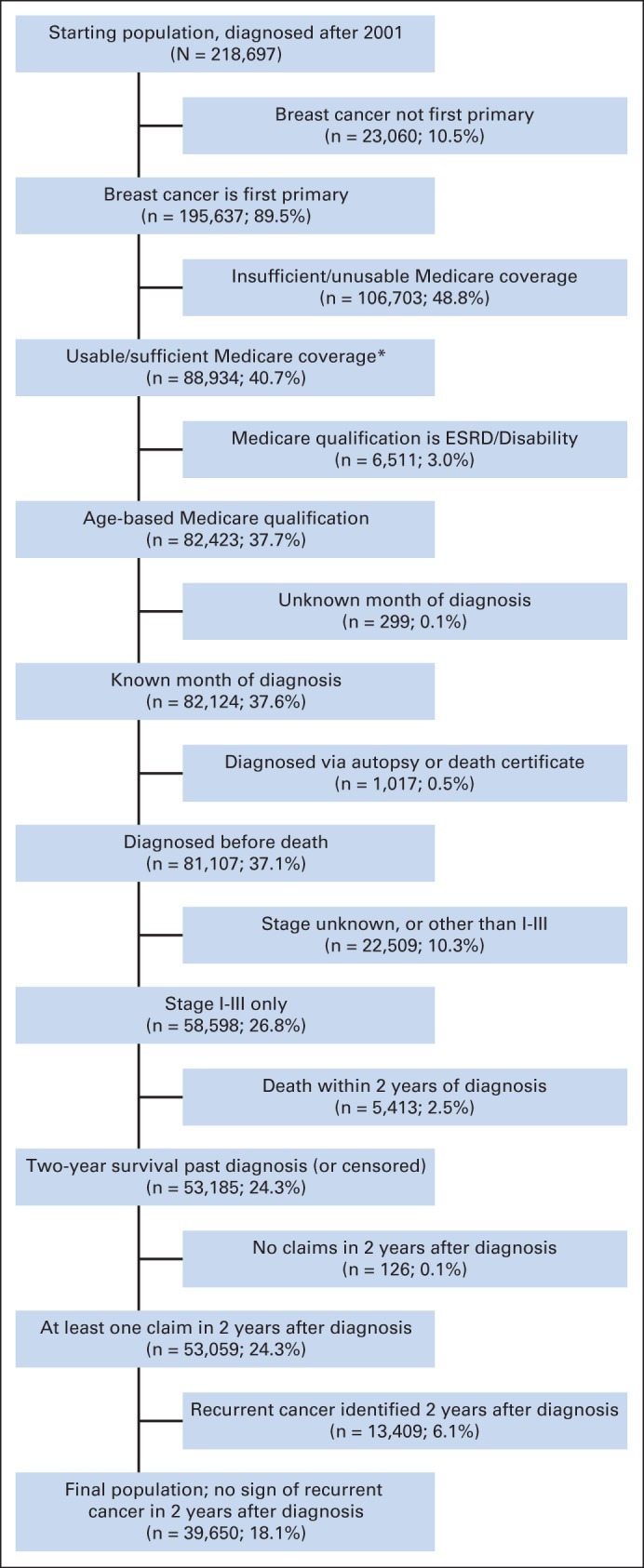
CONSORT diagram. (*) Covered in Medicare Parts A and B 12 months before diagnosis through 24 months after diagnosis (and not enrolled in a health maintenance organization). If patients died earlier than 24 months after diagnosis, they were required to be enrolled only until death. ESRD, end-stage renal disease.
Tumor Marker Usage and Receipt of Other Procedures
Among the 39,650 eligible patients, 16,653 (42%) received at least one tumor marker test within 2 years of diagnosis. Patients who were tested received, on average, 2.3 CEA tests and 3.4 CA 15-3/CA 27.29 tests. Table 1 provides demographic characteristics of the eligible patient population by use of tumor marker tests. Those who underwent tumor marker testing were younger than those who did not (age 74.8 years v age 76.1 years, respectively; P < .01). Patients of Hispanic origin were the most likely to receive testing, whereas American Indian/Alaskan native patients were least likely. Patients with stage I disease were less likely to receive testing than those with stage II or III disease. The use of tumor marker testing increased steadily over time, with approximately 38% of patients receiving testing in 2001 versus 46% of patients in 2007.
Table 1.
Demographic and Clinical Characteristics of Patients Receiving or Not Receiving Tumor Marker Testing
| Characteristic | Test |
No Test |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Mean age at diagnosis, years | 74.8 | 76.1 | ||
| Race/ethnicity* | ||||
| White | 14,238 | 41.5 | 20,034 | 58.5 |
| African American | 842 | 39.6 | 1,283 | 60.4 |
| American Indian/Alaskan native | 28 | 34.6 | 53 | 65.4 |
| Asian/Pacific Islander | 658 | 45.5 | 789 | 54.5 |
| Hispanic | 818 | 52.5 | 739 | 47.5 |
| Other/unknown | 69 | 41.1 | 99 | 58.9 |
| Tumor stage* | ||||
| 1 | 9,776 | 38.6 | 15,538 | 61.4 |
| 2 | 5,841 | 47.0 | 6,599 | 53.0 |
| 3 | 1,036 | 54.6 | 860 | 45.4 |
| Diagnosis year* | ||||
| 2001 | 2,086 | 38.0 | 3,398 | 62.0 |
| 2002 | 2,216 | 39.4 | 3,406 | 60.6 |
| 2003 | 2,196 | 39.6 | 3,349 | 60.4 |
| 2004 | 2,347 | 41.8 | 3,270 | 58.2 |
| 2005 | 2,458 | 43.3 | 3,213 | 56.7 |
| 2006 | 2,601 | 45.4 | 3,123 | 54.6 |
| 2007 | 2,749 | 45.9 | 3,238 | 54.1 |
Percent calculated within each demographic category.
Between 2001 and 2007, the number of CEA tests per patient remained relatively constant, whereas the number of CA 15-3/CA 27.29 tests ordered increased from 2.6 per person to 3.9 per person (data not shown). Patients who underwent tumor marker testing were more likely than those who did not to receive chemotherapy (23.8% v 10.9%), to undergo advanced imaging (62.2% v 47.3%), or to undergo a biopsy (8.0% v 6.9%; Table 2). Among those who did undergo additional procedures, patients who received tumor marker testing had more imaging procedures (2.5 v 2.0; P < .001) but not more biopsy procedures (1.09 v 1.11; P > .05).
Table 2.
Receipt of Other Procedures From 3 to 24 Months After Diagnosis, According to Receiving or Not Receiving Tumor Marker Testing
| Procedure | Test* |
No Test* |
Pdifference | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Imaging | |||||
| No | 6,291 | 37.8 | 12,114 | 52.7 | < .001 (χ2) |
| Yes | 10,362 | 62.2 | 10,883 | 47.3 | |
| No. of procedures | |||||
| Overall† | 2.5 | 2.0 | < .001 (t test) | ||
| CT | 1.1 | 0.9 | |||
| Ultrasound | 0.6 | 0.6 | |||
| MRI | 0.2 | 0.1 | |||
| PET | 0.07 | 0.03 | |||
| PET/CT | 0.1 | 0.05 | |||
| Bone scan | 0.5 | 0.3 | |||
| Chemotherapy | |||||
| No | 12,685 | 76.2 | 20,491 | 89.1 | < .001 (χ2) |
| Yes | 3,968 | 23.8 | 2,506 | 10.9 | |
| No. of treatments | 9.3 | 7.5 | < .001 (t test) | ||
| Biopsy | |||||
| No | 15,315 | 92.0 | 21,414 | 93.1 | < .001 (χ2) |
| Yes | 1,338 | 8.0 | 1,583 | 6.9 | |
| No. of procedures | 1.09 | 1.11 | > .05 (t test) | ||
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography.
Percent calculated from 16,653 patients with tumor marker testing and 22,997 patients without.
Includes CT, ultrasound, MRI, PET scan, PET/CT scan, and bone scan.
Factors Associated With Tumor Marker Use
Several factors were independently associated with tumor marker usage (Table 3). Older women and African Americans were less likely to undergo tumor marker testing. Women diagnosed in the later years of the study and with more advanced SEER stage at diagnosis were more likely to have tumor marker testing. Usage also varied widely on the basis of SEER geographical regions; relative to San Francisco (the reference region), patients diagnosed in Connecticut, Seattle, Atlanta, Los Angeles, Greater California, Kentucky, and New Jersey had a higher likelihood of undergoing testing, whereas patients diagnosed in Iowa, San Jose, and rural Georgia had a lower likelihood of undergoing tumor marker testing. Relative to large metropolitan areas (> 1M population), patients in smaller metropolitan, large urban, or small urban areas were less likely to receive testing, whereas patients in rural areas were more likely to receive tumor marker testing.
Table 3.
Factors Associated With Receiving at Least One Tumor Marker Test in 2 Years After Diagnosis
| Factor | OR | 95% CI |
|---|---|---|
| Age* | ||
| Mean age at diagnosis | 0.96† | 0.96 to 0.97 |
| Year* | ||
| Diagnosis year | 1.05† | 1.04 to 1.07 |
| Comorbidity* | ||
| Klabunde Index‡ | 0.95 | 0.90 to 1.01 |
| Race/ethnicity (v white) | ||
| African American | 0.84† | 0.76 to 0.93 |
| American Indian/Alaskan native | 0.81 | 0.49 to 1.33 |
| Asian/Pacific Islander | 0.99 | 0.86 to 1.13 |
| Hispanic | 1.01 | 0.90 to 1.14 |
| Other/unknown | 0.87 | 0.62 to 1.23 |
| Tumor stage (v stage 1) | ||
| 2 | 1.48† | 1.41 to 1.55 |
| 3 | 2.19† | 1.97 to 2.43 |
| ER status (v negative) | ||
| Positive | 0.98 | 0.90 to 1.07 |
| Borderline | 1.33 | 0.77 to 2.27 |
| Unknown | 0.82 | 0.59 to 1.13 |
| PR status (v negative) | ||
| Positive | 0.92§ | 0.86 to 0.98 |
| Borderline | 0.93 | 0.73 to 1.19 |
| Unknown | 1.07 | 0.78 to 1.47 |
| Geographic region (v San Francisco) | ||
| Connecticut | 2.14† | 1.85 to 2.47 |
| Detroit | 1.00 | 0.86 to 1.15 |
| Hawaii | 1.07 | 0.84 to 1.37 |
| Iowa | 0.36† | 0.30 to 0.43 |
| New Mexico | 1.12 | 0.92 to 1.36 |
| Seattle | 1.75† | 1.52 to 2.02 |
| Utah | 0.91 | 0.75 to 1.09 |
| Atlanta | 1.36† | 1.15 to 1.61 |
| San Jose | 0.56† | 0.46 to 0.69 |
| Los Angeles | 7.72† | 6.67 to 8.92 |
| Rural Georgia | 0.28† | 0.15 to 0.56 |
| Greater California | 2.55† | 2.24 to 2.89 |
| Kentucky | 1.17§ | 1.01 to 1.35 |
| Louisiana | 0.90 | 0.77 to 1.05 |
| New Jersey | 3.49† | 3.07 to 3.96 |
| Urban/rural status (v large metropolitan area with 1M+ population) | ||
| Metropolitan (250,000+) | 0.88† | 0.83 to 0.94 |
| Large urban (20,000+) | 0.80† | 0.73 to 0.89 |
| Small urban (2,500+) | 0.75† | 0.67 to 0.84 |
| Rural (< 2,500) | 1.30§ | 1.06 to 1.58 |
Abbreviations: ER, estrogen receptor; OR, odds ratio; PR, progesterone receptor.
Analyzed by using the natural log transformation and adjusted for age, diagnosis year, Klabunde index, race, tumor stage, tumor receptor status, geographic region, and urban/rural status.
P < .001, Wald χ2.
Klabunde Index value missing for 848 participants.
P < .05, Wald χ2.
Cost Evaluation
The total cost of care for women who received tumor marker testing was 29.4% higher than the cost for those who did not, after accounting for differences in patient demographics and clinical factors (P < .001; Table 4). When the analysis was restricted to patients with stage I disease, the total cost of care for those who underwent testing was approximately 24.3% higher compared with total cost of care for those who did not undergo testing (P < .001). Younger age, later diagnosis year, higher comorbidity index, and higher tumor stage were each associated with higher mean costs of care, independent of tumor marker testing (Table 4). Estimated total cost of care also varied significantly by race/ethnicity and SEER geographic region. Tumor marker testing increased the overall cost of care by 34.7% in months 3 to 12 after diagnosis and by 28.4% in months 13 to 24 after diagnosis (Table 5). By using parameter estimates from the model, the average cost during months 3 to 12 after diagnosis for women who received no testing was $12,468 compared with $16,794 for women who received tumor marker testing; during months 13 to 24, average costs were $7,675 with no testing and approximately $9,855 with testing.
Table 4.
Results From Regression Analyses of Cost of Care 3 to 24 Months After Diagnosis, With or Without Receiving at Least One Tumor Marker Test During the 2 Years After Diagnosis
| Factor | % Increase (+) or Decrease (−) in Total Cost of Care* | 95% CI |
|---|---|---|
| Receipt of at least one tumor marker test† | ||
| No (reference) | ||
| Yes | +29.4‡ | +26.6 to +32.2 |
| Age (years) | ||
| Mean age at diagnosis§ | −1.7‡ | −1.8 to −1.5 |
| Year | ||
| Diagnosis year§ | +5.4‡ | +4.9 to +5.9 |
| Comorbidity | ||
| Klabunde Index§ | +55.7‡ | +51.7 to +59.7 |
| Race (v white) | ||
| African American | −0.1 | −3.1 to +6.2 |
| American Indian/Alaskan native | −13.9 | −33.4 to +5.5 |
| Asian/Pacific Islander | −23.1‡ | −27.8 to −18.3 |
| Hispanic | −6.9‖ | −11.8 to −2.0 |
| Other/unknown | −23.7‖ | −35.5 to −12.0 |
| Tumor stage (v stage 1) | ||
| 2 | +18.1‡ | +15.5 to 20.7 |
| 3 | +64.3‡ | +56.3 to +72.3 |
| Estrogen receptor status (v negative) | ||
| Positive | −18.4‡ | −21.5 to −15.2 |
| Borderline | −4.8 | −28.8 to +19.1 |
| Unknown | −9.3 | −22.7 to +4.2 |
| Progesterone receptor status (v negative) | ||
| Positive | −2.9‖ | −5.7 to −0.0 |
| Borderline | +1.6 | −9.8 to +13.1 |
| Unknown | −17.9‖ | −29.8 to −6.1 |
| Geographic region (v San Francisco) | ||
| Connecticut | −2.4 | −8.9 to +4.1 |
| Detroit | +15.8‡ | +8.2 to +23.5 |
| Hawaii | −12.9‖ | −22.5 to −3.2 |
| Iowa | −28.6‡ | −33.7 to −23.6 |
| New Mexico | −17.5‡ | −24.9 to −10.3 |
| Seattle | −12.1‡ | −17.9 to −6.2 |
| Utah | −27.0‡ | −33.1 to −20.9 |
| Atlanta | −11.9‖ | −18.7 to −5.2 |
| San Jose | +3.6 | −4.9 to +12.0 |
| Los Angeles | +1.4 | −5.1 to +7.9 |
| Rural Georgia | −10.7 | −29.3 to +7.8 |
| Greater California | −7.3‖ | −12.7 to −1.9 |
| Kentucky | −27.8‡ | −32.7 to −23.0 |
| Louisiana | −15.4‡ | −21.2 to −9.6 |
| New Jersey | −4.7 | −10.3 to +0.8 |
| Urban/rural status (v large metropolitan area with 1M+ population) | ||
| Metropolitan (250,000+) | −2.0 | −4.8 to +0.8 |
| Large urban (20,000+) | −3.5 | −8.0 to +0.9 |
| Small urban (2,500+) | −7.1‖ | −11.5 to −2.7 |
| Rural (< 2,500) | −8.2‖ | −16.1 to −0.3 |
Analyzed using the natural log transformation (70 participants not included because of no costs).
During the 2 years after diagnosis, excluding the 2 months immediately after diagnosis.
P < .001, two-sided t test.
Analyzed as a continuous variable.
P < .05, two-sided t test.
Table 5.
Results From Regression Analyses of Cost of Care, With or Without Receiving at Least One Tumor Marker Test During the 2 Years (3 to 12 months and 13 to 24 months) After Diagnosis
| Cost Time Period | % Increase (+) in Total Cost of Care* | 95% CI |
|---|---|---|
| Months 3 to 12 after diagnosis | ||
| Receipt of tumor marker test† | ||
| No (reference) | ||
| Yes | +34.7‡ | +31.4 to +38.0 |
| Months 13 to 24 after diagnosis | ||
| Receipt of tumor marker test† | ||
| No (reference) | ||
| Yes | +28.4‡ | +24.9 to +31.9 |
Analyzed by using the natural log transformation.
At any time during the 2 years after diagnosis.
P < .001, two-sided t test.
Because higher stage of disease is associated with increased ordering of tumor markers, and because chemotherapy is more likely to be administered to those with higher-stage disease, we separately analyzed the costs of care in years 1 and 2 after diagnosis. Costs during year 2 remained higher in the group assessed with tumor markers, although chemotherapy would likely have been completed in year 1, suggesting that the increased cost is not solely a result of chemotherapy administration (Table 5).
DISCUSSION
Despite longstanding recommendations against routine use of tumor markers for surveillance of patients with early-stage breast cancer, our analysis of SEER-Medicare records for women age 65 or older finds that testing persists and—at least for the tumor marker MUC1 antigens CA 15-3 and CA 27.29—is increasing over time. Those for whom tumor markers were ordered had significantly and substantially higher total costs of care over 2 years of observation compared with those who did not. The observed 29% higher costs in the tumor marker group appears to be driven in part by greater rates of imaging (but not biopsy). If our findings are generalizable to all women diagnosed with locoregional breast cancer, as seems likely, given our observation of a trend toward increasing rates of use in younger ages, breast cancer care that includes tumor marker testing represents a large financial cost to the health care system.
Our findings may have important implications for practice policy. The American Board of Internal Medicine, in conjunction with ASCO, highlighted use of breast cancer tumor markers in its “Choosing Wisely” campaign.14 The development of the list for oncology was led by ASCO's Cost of Cancer Care Task Force, a multidisciplinary group of oncologists committed to addressing the underlying issues contributing to the rising cost of cancer care. The apparent widespread acceptance of tumor marker testing among locoregional cancer survivors suggests that efforts to curb their use will be challenging. It is also important to note that the association between tumor marker use and higher costs should not be interpreted casually. It is possible, for example, that clinicians who order tumor marker tests may be more aggressive in their use of testing (and perhaps treatment) than those who do not. Efforts that focus on reduction in tumor marker tests alone may be missing a larger issue of clinician beliefs regarding the value of testing in general. Recently, Han et al20 found that 72% of oncologists hold beliefs consistent with the overuse of tumor marker tests. Factors associated with these beliefs are older age, international medical graduate status, lower self-efficacy (confidence in knowledge), and greater perceptions of ambiguity (conflicting expert recommendations) regarding survivorship care. Our data also show wide variation in use among geographic regions in the United States. Interventions aimed at curbing overuse of breast cancer tumor markers may need to address specific beliefs about the efficacy of tumor markers and other tests in the setting of studies that some may feel have not resolved the question of their benefit in this population. Outreach in areas with the highest rates of use would provide the greatest potential for impact.
Past studies have found more modest use of breast cancer tumor markers among women with local and regional stage breast cancer. In a substudy of a Cancer and Leukemia Group B trial among 8,541 women with stage II breast cancer treated with adjuvant chemotherapy, 37% of patients reported having tumor markers assessed at least once in the previous year during routine follow-up, and 11% to 18% reported undergoing imaging studies (computed tomography and bone scans).21 Similar findings were reported in a SEER-Medicare data analysis of patients diagnosed in the 1990s, with even higher rates reported for patients who were observed by a medical oncologist.11 A survey in 2007 asked 900 US oncologists how they would monitor a patient with stage IIIA breast cancer after completion of chemotherapy.12 Twenty-two percent of respondents stated they would order the breast cancer tumor marker CA 15-3 at every follow-up visit, and 16% reported that they would check chest radiographs every 6 months.
The large geographical variation we found in use of tumor markers is similar to geographical variation that has been found in SEER-Medicare studies evaluating other technologies. Observed variation in region-specific use of tests and procedures has been attributed to regional differences in patient characteristics, demand for services, and area-wide practice styles that influence individual physician's ordering behavior.22–24 It is unclear why tumor marker testing is more common in rural areas. Possible reasons include differences in physician characteristics in rural versus urban settings that predict propensity to order tests (eg, site and type of medical training), reduced access to other forms of surveillance, such as mammography, that compel clinicians to substitute tumor marker testing, or variation in rural versus urban patient preferences for testing.
Our work has several limitations. The SEER-Medicare database includes patients age 65 years or older, and thus our study is representative of women who are enrolled in fee-for-service type Medicare plans. Tumor marker testing for women enrolled in Medicare Advantage Plans may differ from our findings. It is possible that tumor markers are used more frequently in younger breast cancer survivors, given their longer expected life span and the potential impact of recurrence on the life span of these individuals. It is also likely that tumor marker testing would be increased in younger patients, given the higher incidence of more aggressive disease in that population. Our findings could therefore underestimate the financial impact of tumor marker testing. We were not able to distinguish between markers and imaging tests ordered in response to patient symptoms versus surveillance testing in asymptomatic patients. In addition, although we observed higher rates of advanced imaging tests in the tumor marker group, we were unable to determine whether the bulk of higher expenditures in the tumor marker cohort was strictly a result of tumor marker usage driving downstream health expenditures or whether unobserved clinical factors, perhaps related to the practice style of the clinicians, were driving spending for these patients. To limit the inadvertent inclusion of patients with concerning symptoms or locally recurrent or metastatic disease, we limited our observation window to the initial 2 years after diagnosis and omitted patients with diagnosis and/or procedure codes associated with recurrent and/or metastatic disease. A small proportion of such patients still may have been included in our data set. Approximately 49% of women were removed from our initial database because of gaps in Medicare coverage or enrollment in a Medicare HMO. This may influence the representativeness of the final sample, because those excluded for incomplete insurance coverage or HMO enrollment may have different (and likely lower) rates of tumor marker testing. Finally, we were not able to validate the billing records for tumor marker testing in Medicare data. Although it is inappropriate to expect all physicians to always follow guidelines precisely for every patient, our findings suggest that a substantial number of physicians are practicing outside the guidelines.
In summary, we identified a high frequency of tumor marker testing within the SEER-Medicare population during the first 2 years of surveillance after completion of primary therapy for breast cancer, despite ASCO recommendations against routine testing with breast cancer tumor markers and a lack of evidence that early detection of recurrent disease has an impact on breast cancer outcomes. Furthermore, testing was associated with increased health care use and cost in the 2-year period after diagnosis. Further research is necessary to determine the motivations behind tumor marker use and if use of these markers leads to improved survival or quality of life.
Footnotes
See accompanying editorial on page 136
Supported by Grant No. UC2 CA148570 from the National Cancer Institute, National Institutes of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Julie R. Gralow, Genentech (C), Novartis (C), Roche (C); David L. Veenstra, Genentech (C) Stock Ownership: None Honoraria: None Research Funding: N. Lynn Henry, AstraZeneca, BioMarin Pharmaceuticals, Eli Lilly; Julie R. Gralow, Genentech, Novartis, Amgen, Roche Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Scott D. Ramsey, N. Lynn Henry, William Barlow, David L. Veenstra
Collection and assembly of data: Scott D. Ramsey, David Mummy, Rahber Thariani
Data analysis and interpretation: Scott D. Ramsey, N. Lynn Henry, Julie R. Gralow, Dana K. Mirick, William Barlow, Ruth Etzioni, David Mummy, Rahber Thariani
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Molina R, Jo J, Filella X, et al. C-erbB-2, CEA and CA 15.3 serum levels in the early diagnosis of recurrence of breast cancer patients. Anticancer Res. 1999;19:2551–2555. [PubMed] [Google Scholar]
- 2.Molina R, Jo J, Filella X, et al. c-erbB-2 oncoprotein, CEA, and CA 15.3 in patients with breast cancer: Prognostic value. Breast Cancer Res Treat. 1998;51:109–119. doi: 10.1023/a:1005734429304. [DOI] [PubMed] [Google Scholar]
- 3.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 4.Bast RC, Jr, Ravdin P, Hayes DF, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–1878. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [PubMed] [Google Scholar]
- 5.Rosselli Del Turco M, Palli D, Cariddi A, et al. Intensive diagnostic follow-up after treatment of primary breast cancer: A randomized trial—National Research Council Project on Breast Cancer follow-up. JAMA. 1994;271:1593–1597. doi: 10.1001/jama.271.20.1593. [DOI] [PubMed] [Google Scholar]
- 6.Palli D, Russo A, Saieva C, et al. Intensive vs clinical follow-up after treatment of primary breast cancer: 10-year update of a randomized trial—National Research Council Project on Breast Cancer Follow-up. JAMA. 1999;281:1586. doi: 10.1001/jama.281.17.1586. [DOI] [PubMed] [Google Scholar]
- 7.Liberati A. The GIVIO trial on the impact of follow-up care on survival and quality of life in breast cancer patients: Interdisciplinary Group for Cancer Care Evaluation. Ann Oncol. 1995;6:41–46. doi: 10.1093/annonc/6.suppl_2.s41. [DOI] [PubMed] [Google Scholar]
- 8.Impact of follow-up testing on survival and health-related quality of life in breast cancer patients: A multicenter randomized controlled trial—The GIVIO Investigators. JAMA. 1994;271:1587–1592. doi: 10.1001/jama.1994.03510440047031. [No authors listed] [DOI] [PubMed] [Google Scholar]
- 9.Joseph E, Hyacinthe M, Lyman GH, et al. Evaluation of an intensive strategy for follow-up and surveillance of primary breast cancer. Ann Surg Oncol. 1998;5:522–528. doi: 10.1007/BF02303645. [DOI] [PubMed] [Google Scholar]
- 10. Reference deleted.
- 11.Keating NL, Landrum MB, Guadagnoli E, et al. Surveillance testing among survivors of early-stage breast cancer. J Clin Oncol. 2007;25:1074–1081. doi: 10.1200/JCO.2006.08.6876. [DOI] [PubMed] [Google Scholar]
- 12.Foster JA, Abdolrasulnia M, Doroodchi H, et al. Practice patterns and guideline adherence of medical oncologists in managing patients with early breast cancer. J Natl Compr Canc Netw. 2009;7:697–706. doi: 10.6004/jnccn.2009.0049. [DOI] [PubMed] [Google Scholar]
- 13.Hahn EE, Hays RD, Kahn KL, et al. Use of imaging and biomarker tests for posttreatment care of early-stage breast cancer survivors. Cancer. 2013;119:4316–4324. doi: 10.1002/cncr.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: The top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 15.Chan DW, Beveridge RA, Muss H, et al. Use of Truquant BR radioimmunoassay for early detection of breast cancer recurrence in patients with stage II and stage III disease. J Clin Oncol. 1997;15:2322–2328. doi: 10.1200/JCO.1997.15.6.2322. [DOI] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.Chubak J, Yu O, Pocobelli G, et al. Administrative data algorithms to identify second breast cancer events following early-stage invasive breast cancer. J Natl Cancer Inst. 2012;104:931–940. doi: 10.1093/jnci/djs233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Oehlert GW. A note on the delta method. Am Statistician. 1992;46:27–29. [Google Scholar]
- 20.Han PK, Klabunde CN, Noone AM, et al. Physicians' beliefs about breast cancer surveillance testing are consistent with test overuse. Med Care. 2013;51:315–323. doi: 10.1097/MLR.0b013e31827da908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensley ML, Dowell J, Herndon JE, 2nd, et al. Economic outcomes of breast cancer survivorship: CALGB study 79804. Breast Cancer Res Treat. 2005;91:153–161. doi: 10.1007/s10549-004-6497-9. [DOI] [PubMed] [Google Scholar]
- 22.Quek RG, Master VA, Ward KC, et al. Determinants of the combined use of external beam radiotherapy and brachytherapy for low-risk, clinically localized prostate cancer. Cancer. 2013;119:3619–3628. doi: 10.1002/cncr.28258. [DOI] [PubMed] [Google Scholar]
- 23.Hershman DL, Buono D, McBride RB, et al. Influence of private practice setting and physician characteristics on the use of breast cancer adjuvant chemotherapy for elderly women. Cancer. 2009;115:3848–3857. doi: 10.1002/cncr.24448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandelblatt JS, Berg CD, Meropol NJ, et al. Measuring and predicting surgeons' practice styles for breast cancer treatment in older women. Med Care. 2001;39:228–242. doi: 10.1097/00005650-200103000-00004. [DOI] [PubMed] [Google Scholar]


