Abstract
Purpose
National Comprehensive Cancer Network guidelines recommend patients with head and neck cancer (HNC) receive treatment at centers with expertise, but whether provider experience affects survival is unknown.
Patients and Methods
The effect of institutional experience on overall survival (OS) in patients with stage III or IV HNC was investigated within a randomized trial of the Radiation Therapy Oncology Group (RTOG 0129), which compared cisplatin concurrent with standard versus accelerated fractionation radiotherapy. As a surrogate for experience, institutions were classified as historically low- (HLACs) or high-accruing centers (HHACs) based on accrual to 21 RTOG HNC trials (1997 to 2002). The effect of accrual volume on OS was estimated by Cox proportional hazards models.
Results
Median RTOG accrual (1997 to 2002) at HLACs was four versus 65 patients at HHACs. Analysis included 471 patients in RTOG 0129 (2002 to 2005) with known human papillomavirus and smoking status. Patients at HLACs versus HHACs had better performance status (0: 62% v 52%; P = .04) and lower T stage (T4: 26.5% v 35.3%; P = .002) but were otherwise similar. Radiotherapy protocol deviations were higher at HLACs versus HHACs (18% v 6%; P < .001). When compared with HHACs, patients at HLACs had worse OS (5 years: 51.0% v 69.1%; P = .002). Treatment at HLACs was associated with increased death risk of 91% (hazard ratio [HR], 1.91; 95% CI, 1.37 to 2.65) after adjustment for prognostic factors and 72% (HR, 1.72; 95% CI, 1.23 to 2.40) after radiotherapy compliance adjustment.
Conclusion
OS is worse for patients with HNC treated at HLACs versus HHACs to cooperative group trials after accounting for radiotherapy protocol deviations. Institutional experience substantially influences survival in locally advanced HNC.
INTRODUCTION
Overall survival (OS) is improved for patients with cancer who undergo specialized surgical resection at hospitals that perform a large number of procedures (eg, pancreaticoduodenectomy or lung cancer resection).1–6 Individual physician volume may also contribute to in-hospital mortality after cancer resection.7 In a manner analogous to surgery, radiation therapy is a local modality with a high degree of user dependence. Treatment planning and patient care techniques can vary considerably among radiation oncologists. Because of its complexity, radiation therapy treatment planning for head and neck cancer (HNC) in particular has considerable interinstitutional and interphysician variation.8,9
In addition to complex treatment planning, HNC radiotherapy is frequently complicated by acute and chronic toxicities.10 Therefore, robust multidisciplinary coordination of care may be particularly important in HNC. Indeed, current National Comprehensive Cancer Network (NCCN) guidelines recommend that all patients with HNC “need access to the full range of support services and specialists with expertise in the management of patients with HNC for optimal treatment and follow-up.”11 The inference is that suboptimal outcomes may be more likely to occur for patients with HNC when high-volume specialization is not employed.
Therefore, we investigated whether institutional patient accrual volume was associated with OS or progression-free survival (PFS) for patients with HNC enrolled onto a prospective, multicenter, randomized controlled trial conducted by the Radiation Therapy Oncology Group (RTOG; protocol 0129).
PATIENTS AND METHODS
Study Population
RTOG 0129 was a phase III clinical trial conducted from 2002 to 2005 designed to evaluate whether accelerated fractionation (AFX) in comparison with standard fractionation (SFX) radiotherapy could improve OS of patients with HNC treated with concurrent high-dose cisplatin. The experimental design and primary results of the RTOG 0129 trial were published in 201012 and revealed similar 3-year OS in patients receiving AFX and SFX radiotherapy. RTOG 0129 was registered with the National Cancer Institute and approved by the institutional review boards at participating centers. All patients provided written informed consent. This retrospective analysis was not included in the original protocol.
Briefly, eligible patients for RTOG 0129 had untreated, pathologically confirmed, American Joint Committee on Cancer stage III or IV13 squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, or larynx; had Zubrod performance status 0 to 1; were age ≥ 18 years; and had adequate bone marrow, hepatic, and renal function. Patients were randomly assigned to cisplatin concurrent with AFX by concomitant boost radiotherapy (72 Gy delivered in 42 fractions over 6 weeks, inclusive of twice-per-day irradiation for 12 treatment days) or SFX radiotherapy (70 Gy in 35 fractions over 7 weeks). Chemotherapy consisted of intravenous cisplatin 100 mg/m2 of body-surface area on days 1 and 22 for patients assigned to AFX and on days 1, 22, and 43 for patients assigned to SFX.
Prior cigarette smoking in pack-years was obtained at enrollment by interviewer-administered questionnaire. To assess tumor status and late toxicity, follow-up examination and imaging studies were performed every 3 months for 2 years, every 6 months through year 5, and then annually.
Tumor Human Papillomavirus Status
Formalin-fixed, paraffin-embedded tumor specimens were evaluated for human papillomavirus (HPV) type 16 and 12 additional HPV types (types 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68) using the in situ hybridization–catalyzed signal amplification method for biotinylated probes (Dako GenPoint, Carpinteria, CA) as previously described.14
Radiation Therapy Compliance
Radiotherapy plans were reviewed for protocol compliance and categorized as per protocol (PP), acceptable variation (AV), or unacceptable deviation (UD) for total dose delivered, elapsed days, AFX completed, spinal cord dose, and field borders (Appendix Table A1, online only). Field borders were also scored based on adherence to protocol instructions by the principal investigator on primary tumor coverage, coverage of lymphatic space posterior to the spinal cord (right and left), and spinal cord and supraclavicular lymph node coverage.
Statistical Methods
Our principal outcome was the effect of institutional expertise on OS. As a surrogate for institutional expertise, we used institutional accrual volume to 21 HNC clinical trials conducted by the RTOG during the 5-year period (July 30, 1997, to July 29, 2002) immediately before the activation of RTOG 0129. To do so, 3,007 patients enrolled from 303 centers were divided into three approximately equally sized cohorts: high (1,017 patients, 15 centers, ≥ 42 patients per center), middle (1,016 patients, 44 centers, 13 to 41 patients per center), and low (974 patients, 244 centers, one to 12 patients per center). RTOG 0129 outcomes in the middle and low cohorts were similar and combined. The final cohorts were denoted historically low (HLACs) and high accruing centers (HHACs).
OS was defined as time from random assignment to death resulting from any cause. Secondary end points were PFS, locoregional failure (LRF), and acute and chronic toxicities. PFS was defined as time from random assignment to local, regional, or distant progression or death resulting from any cause. LRF was defined as time from random assignment to local or regional disease progression; distant metastasis and death unrelated to the index cancer were competing risks. OS and PFS rates were estimated by the Kaplan-Meier method15 and compared by log-rank test.16 LRF rates were estimated by the cumulative incidence method17 and compared by Gray's test.18 Acute toxicity was evaluated weekly during the period of therapy according to the Common Toxicity Criteria for Adverse Events (version 2.0).19 Late toxicities were recorded at each follow-up visit, with attention to soft tissue changes, bony necrosis, changes in central or peripheral nervous system function, and condition of the mucosa.
Analysis was restricted to patients with known HPV status (if oropharynx primary site) and known cigarette pack-years. To evaluate study population bias, pretreatment characteristics and survival outcomes were compared for patients with complete versus missing data. Patient characteristics were compared by Pearson χ2 or Fisher's exact test for categorical variables or Wilcoxon rank sum test18 for ordinal and continuous variables. Cox proportional hazards models were used to evaluate the independent effect of accrual volume after accounting for treatment assignment and known prognostic factors: age, T stage, and N stage, Zubrod performance status, cigarette pack-years, and tumor HPV status. A second model including radiotherapy compliance estimated the percentage of the effect of accrual volume resulting from radiotherapy deviations. Sensitivity analysis was performed by: one, imputing missing values for HPV status (oropharynx only) and pack-years using the Markov chain Monte Carlo algorithm (with 20 imputations) to minimize potential bias from excluding patients from the analysis; two, lowering the HHAC threshold from 42 (top 5% of centers) to 25 patients (top 10% of centers); and three, considering historical accrual as a continuous variable.
RESULTS
Study Population
A total of 743 patients were enrolled, and 721 were eligible for protocol-specified end points (Fig 1). Of these 721, 250 (34.7%) had missing data for tumor HPV status (n = 110; oropharynx only) and/or cigarette smoking pack-years (n = 163). The remaining 471 patients were included. No statistically significant differences in patient or tumor characteristics or survival outcomes were observed for patients with complete versus missing data (Appendix Table A2; Appendix Figs A1 and A2, online only).
Fig 1.
CONSORT diagram. HPV, human papillomavirus; RTOG, Radiation Therapy Oncology Group.
Median age of the analysis population was 56 years, and median number of smoking pack-years was 30. Thirty-eight percent of patients had HPV-positive oropharyngeal cancers, 19% had HPV-negative oropharyngeal cancers, and 44% had nonoropharyngeal primary cancers. Disease was staged as IVA for a majority of patients (77%).
Of the 471 patients, 321 were treated at one of 88 HLACs and 150 at one of 13 HHACs. Median accrual to RTOG 0129 from HLACs was two patients (range, one to 20), and median accrual to RTOG 0129 from HHACs was six patients (range, one to 44). Patient characteristics are listed in Table 1. Patients treated at HLACs and HHACs had similar distributions of treatment assignment, age, cigarette pack-years, tumor HPV status, and N stage. However, patients at HLACs had better performance status (Zubrod of 0: 62% v 52%; P = .04) and lower T stage (T4: 26.5% v 35.3%; P = .002).
Table 1.
Patient and Tumor Characteristics by Accrual Volume
| Characteristic | HHAC (n = 150) |
HLAC (n = 321) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Assigned treatment | .83* | ||||
| SFX plus cisplatin | 75 | 50.0 | 164 | 51.1 | |
| AFX-C plus cisplatin | 75 | 50.0 | 157 | 48.9 | |
| Age, years | .61† | ||||
| Median | 56 | 55 | |||
| Range | 33-82 | 31-79 | |||
| Q1 to Q3 | 50-60 | 50-61 | |||
| Zubrod performance status | .04* | ||||
| 0 | 78 | 52.0 | 199 | 62.0 | |
| 1 | 72 | 48.0 | 122 | 38.0 | |
| Cigarette pack-years | .75† | ||||
| Median | 30.5 | 30 | |||
| Range | 0-152 | 0-137.5 | |||
| Q1 to Q3 | 1.5-50.4 | 6-49 | |||
| Primary site | .43* | ||||
| HPV-positive oropharynx | 62 | 41.3 | 116 | 36.1 | |
| HPV-negative oropharynx | 29 | 19.3 | 59 | 18.4 | |
| Nonoropharynx | 59 | 39.3 | 146 | 45.5 | |
| T stage | .002† | ||||
| T2 | 23 | 15.3 | 94 | 29.3 | |
| T3 | 74 | 49.3 | 142 | 44.2 | |
| T4 | 53 | 35.3 | 85 | 26.5 | |
| N stage | .10† | ||||
| N0 | 34 | 22.7 | 54 | 16.8 | |
| N1 | 26 | 17.3 | 52 | 16.2 | |
| N2a | 11 | 7.3 | 31 | 9.7 | |
| N2b | 41 | 27.3 | 81 | 25.2 | |
| N2c | 27 | 18.0 | 73 | 22.7 | |
| N3 | 11 | 7.3 | 30 | 9.3 | |
| AJCC stage | .28* | ||||
| III | 40 | 26.7 | 71 | 22.1 | |
| IV | 110 | 73.3 | 250 | 77.9 | |
Abbreviations: AFX-C, accelerated fractionation with concomitant boost; AJCC, American Joint Committee on Cancer (ed 5); HHAC, historically high-accruing center; HLAC, historically low-accruing center; HPV, human papillomavirus; Q1, first quartile; Q3, third quartile; SFX, standard fractionation.
Pearson χ2 test.
Wilcoxon rank sum test.
Survival and Treatment Efficacy Analyses
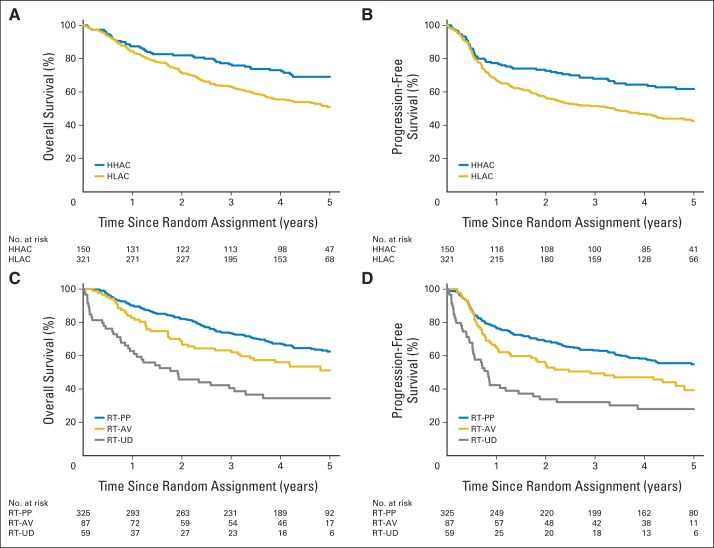
Median follow-up among surviving patients was 4.8 years (range, 1.1 to 6.5). There were 202 deaths among the cohort of 471 patients: 153 among patients treated at HLACs and 49 at HHACs. Patients treated at HLACs had significantly worse OS when compared with patients treated at HHACs (5 years: 51.0%; 95% CI, 45.1 to 56.8 v 69.1%; 95% CI, 61.6 to 76.7; P = .002; hazard ratio [HR], 1.67; 95% CI, 1.21 to 2.31). Patients treated at HLACs also had significantly worse PFS (5 years: 42.7%; 95% CI, 37.0 to 48.4 v 61.8%; 95% CI, 53.8 to 69.7; P < .001; HR, 1.64; 95% CI, 1.22 to 2.20). Kaplan-Meier curves for OS and PFS stratified by institutional accrual are shown in Figures 2A and 2B.
Fig 2.
Kaplan-Meier estimates by (A, B) accrual volume and (C, D) radiotherapy (RT) compliance of (A, C) overall (OS) and (B, D) progression-free survival (PFS). Patients treated at historically low-accruing centers (HLACs) had significantly worse OS (P = .002) and PFS (P < .001) than patients treated at historically high-accruing centers (HHACs). (A) Five-year rates of OS were 51.0% (95% CI, 45.1 to 56.8) in HLAC group and 69.1% (95% CI, 61.6 to 76.7) in HHAC group. (B) Five-year rates of PFS were 42.7% (95% CI, 37.0 to 48.4) in HLAC group and 61.8% (95% CI, 53.8 to 69.7) in HHAC group. Patients with ≥ one RT compliance score of acceptable variation (AV) or with ≥ one RT compliance score of unacceptable deviation (UD) had significantly worse OS (P = .007 and P < .001) and PFS (P = .01 and P < .001) than patients treated per protocol (PP). (C) Five-year rates of OS were 63.0% (95% CI, 57.4 to 68.6) in PP group, 51.1% (95% CI, 40.1 to 62.3) in AV group, and 41.1% (95% CI, 26.6 to 55.6) in UD group. (D) Five-year rates of PFS were 55.6% (95% CI, 50.0 to 61.2) in PP group, 39.3% (95% CI, 28.0 to 50.7) in AV group, and 33.0% (95% CI, 19.2 to 46.8) in UD group.
LRF rates were higher among patients treated at HLACs than HHACs (5 years: 36.4%; 95% CI, 30.9 to 41.9 v 20.8%; 95% CI, 14.1 to 27.5; P < .001). Among PFS events, the first failure event was locoregional in 43.3% of patients at HLACs compared with 33.9% at HHACs, distant in 25.0% and 30.5%, and death without documented progression in 31.7% and 35.6% (P = .43), respectively.
Treatment at an HLAC was associated with a 91% increased risk of death (HR, 1.91; 95% CI, 1.37 to 2.65; P < .001) and an 89% increase in progression or death (PFS: HR, 1.89; 95% CI, 1.39 to 2.56; P < .001) when compared with an HHAC (Table 2), after adjustment for age, T and N stages, performance status, smoking pack-years, tumor HPV status, and treatment assignment. Sensitivity analysis (adjusted for prognostic variables) confirmed the increase in risk of failure for both OS and PFS in patients treated at HLACs in all 721 patients (OS: HR, 1.57; 95% CI, 1.20 to 2.04; P < .001; PFS: HR, 1.56; 95% CI, 1.23 to 1.98; P < .001), when lowering the HHAC threshold to 25 patients (OS: HR, 1.53; 95% CI, 1.14 to 2.04; P = .004; PFS: HR, 1.69; 95% CI, 1.29 to 2.21; P < .001), and when historical accrual volume was considered a continuous variable (for every 10 patients; OS: HR, 0.93; 95% CI, 0.88 to 0.97; P = .001; PFS: HR, 0.92; 95% CI, 0.88 to 0.96; P < .001).
Table 2.
Multivariable Analysis
| End Point | Patients With Complete Data (n = 471) |
All Patients With Data Imputed (n = 721) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| OS | ||||||
| Accrual volume (HLAC v HHAC) | 1.91 | 1.37 to 2.65 | < .001 | 1.57 | 1.20 to 2.04 | < .001 |
| Assigned treatment (SFX v AFX-C) | 0.94 | 0.71 to 1.25 | .69 | 1.07 | 0.85 to 1.35 | .56 |
| Age, years (continuous) | 1.03 | 1.01 to 1.04 | .002 | 1.02 | 1.01 to 1.03 | .002 |
| Zubrod performance status (1 v 0) | 1.53 | 1.16 to 2.04 | .003 | 1.65 | 1.30 to 2.09 | < .001 |
| Cigarette pack-years (continuous) | 1.01 | 1.00 to 1.01 | .01 | 1.01 | 1.00 to 1.01 | .008 |
| T stage (T4 v T2-3) | 2.08 | 1.55 to 2.79 | < .001 | 1.84 | 1.45 to 2.34 | < .001 |
| N stage (N2b-N3 v N0-N2a) | 1.59 | 1.18 to 2.12 | .002 | 1.64 | 1.29 to 2.09 | < .001 |
| HPV status (HPV-negative OP v HPV-positive OP) | 2.34 | 1.52 to 3.61 | < .001 | 2.22 | 1.48 to 3.33 | < .001 |
| HPV status (non-OP v HPV-positive OP) | 2.46 | 1.68 to 3.60 | < .001 | 2.48 | 1.78 to 3.46 | < .001 |
| PFS | ||||||
| Accrual volume (HLAC v HHAC) | 1.89 | 1.39 to 2.56 | < .001 | 1.56 | 1.23 to 1.98 | < .001 |
| Assigned treatment (SFX v AFX-C) | 0.87 | 0.67 to 1.13 | .30 | 0.98 | 0.79 to 1.20 | .83 |
| Age, years (continuous) | 1.02 | 1.01 to 1.04 | .003 | 1.02 | 1.00 to 1.03 | .006 |
| Zubrod performance status (1 v 0) | 1.62 | 1.24 to 2.10 | < .001 | 1.63 | 1.31 to 2.02 | < .001 |
| Cigarette pack-years (continuous) | 1.01 | 1.00 to 1.01 | .001 | 1.01 | 1.00 to 1.01 | .005 |
| T stage (T4 v T2-3) | 1.75 | 1.33 to 2.31 | < .001 | 1.54 | 1.23 to 1.92 | < .001 |
| N stage (N2b-N3 v N0-N2a) | 1.59 | 1.21 to 2.07 | < .001 | 1.55 | 1.25 to 1.93 | < .001 |
| HPV status (HPV-negative OP v HPV-positive OP) | 2.09 | 1.42 to 3.08 | < .001 | 2.05 | 1.46 to 2.87 | < .001 |
| HPV status (non-OP v HPV-positive OP) | 2.06 | 1.46 to 2.89 | < .001 | 2.14 | 1.60 to 2.86 | < .001 |
Abbreviations: AFX-C, accelerated fractionation with concomitant boost; HHAC, historically high-accruing center; HLAC, historically low-accruing center; HPV, human papillomavirus; HR, hazard ratio; OP, oropharynx; OS, overall survival; PFS, progression-free survival; SFX, standard fractionation.
Patient Population Analysis
Sociodemographic characteristics and medical comorbidities were compared for patients at HLACs versus HHACs to evaluate the possible contribution of these factors to observed survival differences (Table 3). HLACs had a higher proportion of uninsured patients (either self-pay or no means of payment) than HHACs (12.3% v 4.3%; P = .009), but no other differences were noted. Patients at HLACs and HHACs were also similar with regard to history of cardiovascular, respiratory, hepatic, renal, thromboembolic, hormonal, neurologic, and infectious illnesses.
Table 3.
Socioeconomic and Comorbidity Status by Accrual Volume
| Factor | HHAC (n = 150) |
HLAC (n = 321) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Highest educational level completed | .11* | ||||
| Grade 1 to 8 | 1 | 0.7 | 22 | 6.9 | |
| Grade 9 to 11 | 22 | 14.7 | 46 | 14.3 | |
| High school graduate or GED | 54 | 36.0 | 123 | 38.3 | |
| Vocational school | 10 | 6.7 | 20 | 6.2 | |
| Associate's degree or some college | 17 | 11.3 | 46 | 14.3 | |
| Bachelor's degree | 18 | 12.0 | 30 | 9.3 | |
| Advanced degree | 9 | 6.0 | 14 | 4.4 | |
| Other | 2 | 1.3 | 6 | 1.9 | |
| Unknown/prefer not to answer | 17 | 11.3 | 14 | 4.4 | |
| Insurance status | .009† | ||||
| Other | 30 | 20.0 | 44 | 13.7 | |
| Private insurance | 78 | 52.0 | 135 | 42.1 | |
| Medicare | 12 | 8.0 | 21 | 6.5 | |
| Medicare and private insurance | 5 | 3.3 | 8 | 2.5 | |
| Medicaid | 4 | 2.7 | 25 | 7.8 | |
| Medicaid and Medicare | 1 | 0.7 | 3 | 0.9 | |
| Military or VA | 2 | 1.3 | 35 | 10.9 | |
| Self-pay | 1 | 0.7 | 11 | 3.4 | |
| No means of payment | 5 | 3.3 | 27 | 8.4 | |
| Unknown | 12 | 8.0 | 12 | 3.7 | |
| History of heart problems | .75‡ | ||||
| No | 135 | 90.0 | 284 | 88.5 | |
| Yes | 15 | 10.0 | 37 | 11.5 | |
| History of lung problems | .36‡ | ||||
| No | 136 | 90.7 | 299 | 93.1 | |
| Yes | 14 | 9.3 | 22 | 6.9 | |
| History of high blood pressure | .83‡ | ||||
| No | 106 | 70.7 | 222 | 69.2 | |
| Yes | 44 | 29.3 | 99 | 30.8 | |
| History of bleeding problems | .45‡ | ||||
| No | 149 | 99.3 | 314 | 97.8 | |
| Yes | 1 | 0.7 | 7 | 2.2 | |
| History of circulation problems | .56‡ | ||||
| No | 138 | 92.0 | 301 | 93.8 | |
| Yes | 12 | 8.0 | 20 | 6.2 | |
| History of liver problems | .60‡ | ||||
| No | 146 | 97.3 | 308 | 96.0 | |
| Yes | 4 | 2.7 | 13 | 4.0 | |
| History of diabetes or sugar in urine | .13‡ | ||||
| No | 135 | 90.0 | 302 | 94.1 | |
| Yes | 15 | 10.0 | 19 | 5.9 | |
| History of kidney or urine problems | 1.00‡ | ||||
| No | 147 | 98.0 | 314 | 97.8 | |
| Yes | 3 | 2.0 | 7 | 2.2 | |
| History of stroke | .77‡ | ||||
| No | 145 | 96.7 | 312 | 97.2 | |
| Yes | 5 | 3.3 | 9 | 2.8 | |
| History of thyroid problems | .24‡ | ||||
| No | 148 | 98.7 | 310 | 96.6 | |
| Yes | 2 | 1.3 | 11 | 3.4 | |
| History of seizure | .41‡ | ||||
| No | 147 | 98.0 | 309 | 96.3 | |
| Yes | 3 | 2.0 | 12 | 3.7 | |
| History of HIV/AIDS | .32‡ | ||||
| No | 149 | 99.3 | 321 | 100.0 | |
| Yes | 1 | 0.7 | 0 | 0.0 | |
| History of frequent infections | 1.00‡ | ||||
| No | 149 | 99.3 | 318 | 99.1 | |
| Yes | 1 | 0.7 | 3 | 0.9 | |
| History of psychological problems | 1.00‡ | ||||
| No | 144 | 96.0 | 308 | 96.0 | |
| Yes | 6 | 4.0 | 13 | 4.0 | |
| History of other illness | .08‡ | ||||
| No | 142 | 94.7 | 287 | 89.4 | |
| Yes | 8 | 5.3 | 34 | 10.6 | |
Abbreviations: GED, General Educational Development; HHAC, historically high-accruing center; HLAC, historically low-accruing center; VA, Veterans Affairs.
Wilcoxon rank sum test; other and unknown were excluded.
Fisher's exact test of self-pay and no means of payment versus all others; unknown was excluded.
Fisher's exact test.
Toxicity and Protocol Compliance Analysis
Incidences of grade ≥ 3 acute toxicity (any), acute mucositis, and late toxicity were similar between the groups (Table 4). Incidence of late mucositis was higher at HHACs but low in both groups (HHACs, 6.8%; HLACs, 2.9%; P = .08).
Table 4.
Toxicity and Treatment Compliance by Accrual Volume
| Toxicity/Compliance | HHAC |
HLAC |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Acute grade 3 to 5 toxicity | 118 of 150 | 78.7 | 255 of 321 | 79.4 | .85* |
| Acute grade 3 to 5 mucositis | 57 of 150 | 38.0 | 106 of 321 | 33.0 | .29* |
| Late grade 3 to 5 toxicity† | 42 of 146 | 28.8 | 106 of 310 | 34.2 | .25* |
| Late grade 3 to 5 mucositis† | 10 of 146 | 6.8 | 9 of 310 | 2.9 | .05* |
| No. of cisplatin cycles delivered | .91‡ | ||||
| 0 | 0 | 0.0 | 2 | 0.6 | |
| 1 | 10 | 6.7 | 29 | 9.0 | |
| 2 | 91 | 60.7 | 176 | 54.8 | |
| 3 | > 49 | 32.7 | 114 | 35.5 | |
| Cisplatin dose delivered, mg/m2 | .99‡ | ||||
| Mean | 221.4 | 219.6 | |||
| SD | 57.1 | 64.0 | |||
| Median | 200.0 | 200.0 | |||
| Range | 100.0-301.8 | 0.0-308.6 | |||
| Q1 to Q3 | 200.0-300.0 | 198.9-299.1 | |||
| Radiation total dose, Gy | .32‡ | ||||
| Median | 70.79 | 71.5 | |||
| Range | 44.1-74.85 | 0-76.02 | |||
| Q1 to Q3 | 70-72 | 70-72 | |||
| Radiation total fractions | .71‡ | ||||
| Median | 35 | 35 | |||
| Range | 25-42 | 0-51 | |||
| Q1 to Q3 | 35-42 | 35-42 | |||
| Radiation therapy elapsed days | .004‡ | ||||
| Median | 47 | 49 | |||
| Range | 30-71 | 0-102 | |||
| Q1 to Q3 | 43-50 | 44-52 | |||
Abbreviations: HHAC, historically high-accruing center; HLAC, historically low-accruing center; Q, quartile; SD, standard deviation.
Pearson χ2 test.
Late period defined as > 90 days after start of radiation therapy.
Wilcoxon rank sum test.
Patients treated at HLACs and HHACs received similar cisplatin doses and cycles and radiation doses and numbers of fractions, but duration of therapy was longer at HLACs (range, 0 to 120 v 30 to 71 days; first to third quartile, 44 to 52 v 43 to 50; median, 49 v 47 days; P = .004; Table 4). The overall radiotherapy plan score at HLACs was more likely than at HHACs to deviate from protocol (inclusive of AV and UD; 18.1% v 6%; P < .001). In general, there were more cases of protocol variation (considered within acceptable range) in the HLAC group for total dose, field border, fractionation, and elapsed days. At least one component of the treatment plan or delivery was scored as UD more often at HLACs versus HHACs (11% v 5%; P = .04). Common causes of UD included an excess of elapsed days of treatment (HLAC v HHAC, 3%; n = 10 v 0.7%; n = 1) and field borders not PP (HLAC v HHAC, 8%; n = 26 v 5%; n = 7).
An analysis of the effect of treatment compliance on outcome revealed OS and PFS to be significantly lower among patients with AV or UD when compared with patients treated PP (Figs 2C and 2D). Effect of historical accrual volume did not differ by fractionation arm. Therefore, we evaluated whether the differences in OS and PFS for patients treated at HLACs versus HHACs could be explained by radiotherapy compliance.
Accrual volume remained independently associated with OS and PFS in multivariable analysis after consideration of treatment compliance effect (OS: HR, 1.72; 95% CI, 1.23 to 2.40; PFS: HR, 1.73; 95% CI, 1.28 to 2.36). UD (but not AD) from radiotherapy protocol independently increased the risk of death (OS: HR, 2.56; 95% CI, 1.75 to 3.74) and progression or death (PFS: HR, 2.31; 95% CI, 1.62 to 3.30) when compared with PP radiation therapy. By comparing the HR for accrual volume before and after the addition of radiotherapy compliance with the multivariable model, we estimated that only 21% and 18% of the effect of accrual volume on OS and PFS, respectively, resulted from radiotherapy protocol noncompliance.
DISCUSSION
In a secondary analysis of RTOG 0129, we observed significantly worse OS and PFS among patients with HNC treated at institutions with historically low- as compared with historically high-volume accrual to RTOG trials. Risk of death or progression was 90% greater for patients at HLACs. There were higher locoregional recurrence rates at HLACs compared with HHACs. Deviations from protocol therapy were more common at HLACs than HHACs and independently increased risk of death but did not entirely explain the survival benefit from HHAC treatment. These findings suggest that experienced providers likely execute superior treatment plans and may better support patients through treatment.
Prior publications have associated patient volume with HNC survival outcome. Population-based data from the National Cancer Data Base demonstrated that treatment at high-volume research facilities was associated with higher 90-day, 1-year, and 4-year survival for patients with locally advanced laryngeal cancer.20 Furthermore, an analysis of HNC outcomes in a SEER-Medicare database demonstrated that patients treated at high-volume hospitals had a trend toward better survival compared with patients treated at low-volume hospitals, even though they were not more likely to receive NCCN guideline therapy.21 This growing body of evidence suggests patients with HNC who are treated at high-volume centers have better outcomes.
Our results are also supported by a prior report from the Trans Tasman Radiation Oncology Group (TROG 02.02), in which major radiation plan deficiencies in HNC treatment were strongly associated with institutional enrollment volume. Major deficiencies were reported for 5.4% of patients at sites contributing ≥ 20 patients versus 29.8% of patients at sites contributing < five patients.22 Furthermore, patients treated with major radiation plan deficiencies had an absolute OS reduction of 20% (50% v 70%; P < .001) and locoregional control reduction of 24% (54% v 78%; P < .001) at 2 years. The RTOG 0129 protocol included several predefined quality measures for radiotherapy (eg, total dose delivered, elapsed days, hyperfractionation completed, spinal cord dose, and boost field borders) but did not include minimal dose to the gross and planning target volumes, which were analyzed in the TROG 02.02 study. Therefore, we were unable to use identical metrics. These or other unmeasured quality indices may account for some of the outcome differences between HLACs and HHACs not explained by the available compliance measures in RTOG 0129.
All patients treated in RTOG 0129 received three-dimensional conformal radiotherapy. Because modern intensity-modulated radiation therapy (IMRT) requires a higher level of expertise than three-dimensional conformal radiotherapy, our analysis may have underestimated the impact of provider expertise in the IMRT era. IMRT allows for a substantial reduction in parotid dose and therefore subjective and objective improvements in xerostomia without loss of efficacy.23–25 However, IMRT substantially increases the complexity of contouring and treatment planning. In fact, target delineation is often nonuniform; Hong et al26 observed major differences in delineated clinical target volumes from a predefined gross tumor volume, even among recognized experts in HNC. Furthermore, the first RTOG study to evaluate the feasibility of IMRT for early-stage oropharyngeal carcinoma (RTOG 0022) observed higher treatment failure rates among patients treated with major dosimetric protocol deviations.27 Given that target delineation deviations were observed more frequently at HLACs than HHACs with three-dimensional conformal therapy, the increased complexity of target delineation associated with IMRT may exacerbate outcome differences. However, RTOG currently collects more dose-volume histogram data on individual plans, a factor that may reduce variation in treatment planning.
In our analysis, measured deviations from protocol therapy did not entirely explain differences in OS and PFS by accrual volume. Only approximately 20% of the effect of accrual volume on OS and PFS could be explained by poor compliance with protocol-specified radiotherapy. HHACs were frequently synonymous with academic tertiary care centers, including members of NCCN and National Cancer Institute–designated cancer centers. A myriad of additional institution-specific factors that may contribute to outcomes were not assessed, including presence of tumor board, number of colleagues, years of practice, presence of a residency training program, and ancillary support services such as speech and swallowing therapists, dietetics and nutritional support, and specialized nursing—all of which may be more robust at HHACs compared with HLACs. Such support services may limit treatment interruptions through advanced management of toxicities. Indeed, a slight but significant increase in treatment duration was observed at HLACs versus HHACs. However, the reported acute toxicities did not differ.
We cannot entirely exclude a possible contribution of referral bias leading to differences in patient populations treated at HLACs versus HHACs and hence differences in outcomes. However, we note that the higher numbers of individuals with poor performance status and T4 tumors at HHACs versus HLACs would bias toward poorer survival. In contrast, patients treated at HLACs were more likely to be uninsured, and socioeconomic status28–31 and uninsured status32 has been associated with less favorable outcomes in HNC. However, insurance status had no effect on PFS or OS (data not shown). We observed no differences in the prevalence of comorbidities or deaths resulting from unknown causes at HLACs versus HHACs. A limitation to our analysis is that accrual to RTOG clinical trials may not be an entirely accurate measure of overall treatment volume at some centers, because of competing institutional protocols and/or treatment volume off protocol.
Nevertheless, our comparative effectiveness research data provide direction toward improvements in research and treatment for patients with HNC. First, cancer centers and training programs should prioritize specialization, particularly in HNC management. Second, clinical trialists should consider the possible contribution of accrual volume on outcome through stratification or other means. Third, clinicians and patient advocates should take steps to improve access of patients with HNC to oncologists who specialize in HNC and who treat patients at high volume centers. Additional alternatives to mitigate this disparity in outcomes include: increased access to and use of contouring atlases to reduce differences in target delineation and normal tissue contouring, validation and implementation of autocontouring software, and continuing medical education focused on target delineation and treatment planning in HNC.
Supplementary Material
Acknowledgment
Presented in part at the 48th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 1-5, 2012.
M.L.G. and Q.Z. had full access to all data in this study and take full responsibility for integrity of data and accuracy of data analysis; design and conduct of study; collection, management, analysis, and interpretation of data; preparation, review, or approval of manuscript; and decision to submit manuscript for publication.
Glossary Terms
- accelerated fractionation:
radiation dose fractionation schedule with an effective rate of dose accumulation exceeding the traditional 10 Gy delivered in five fractions per week.
- comparative effectiveness research:
the generation and synthesis of evidence that compares the benefits and harms of alternative methods to prevent, diagnose, treat, and monitor a clinical condition or to improve the delivery of care. The purpose of comparative effectiveness research is to assist consumers, clinicians, purchasers, and policy makers to make informed decisions that will improve health care at the individual and population levels.
- conformal radiation therapy:
an irradiation technique developed to limit the highest radiation dose to volumes at risk for tumors while sparing surrounding normal tissues. Treatment planning is based on three-dimensional reconstructions of individual patient anatomy.
- multivariate proportional hazards model:
a general method in medical statistics used to analyze the influence of several (patient-specific) covariates on time-to-event end points. No assumption is made concerning the form of the underlying time-to-event curve. The only assumption made is that the effect of the covariates on the hazard rate in the study population is multiplicative and does not change over time.
- planning target volume (PTV):
volume encompassing the clinical target volume that is introduced for radiation treatment planning and evaluation to ensure that the prescribed absorbed dose will actually be delivered to all parts of the clinical target volume with a clinically acceptable probability. It takes into account uncertainties and variations in set-up, positioning, and target motion.
Appendix
Table A1.
Radiation Therapy Scoring Criteria
| Parameter | PP | AV | UD |
|---|---|---|---|
| Total dose | ≤ 4% variation | > 4% to 9% variation | > 9% variation |
| Elapsed days | |||
| SFX | 46-52 | 53-60 | > 60 |
| AFX-C | 39-49 | 50-57 | > 57 |
| Hyperfractionation | ≤ 2 days missed | 3-5 days missed | > 5 days missed |
| Spinal cord dose, Gy | < 47 | 47-50 | > 50 |
Abbreviations: AFX-C, accelerated fractionation with concomitant boost; AV, acceptable variation; PP, per protocol; SFX, standard fractionation; UD, unacceptable deviation.
Table A2.
Missing Data Analysis
| Factor | Complete Data (n = 471) |
Missing HPV Status* and/or Cigarette Pack-Years (n = 250) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Assigned treatment | .62† | ||||
| SFX plus cisplatin | 239 | 50.7 | 122 | 48.8 | |
| AFX-C plus cisplatin | 232 | 49.3 | 128 | 51.2 | |
| Age, years | .31‡ | ||||
| Median | 56 | 56 | |||
| Range | 31-82 | 26-82 | |||
| Q1 to Q3 | 50-61 | 49-63 | |||
| Zubrod performance status | .47† | ||||
| 0 | 277 | 58.8 | 140 | 56.0 | |
| 1 | 194 | 41.2 | 110 | 44.0 | |
| T stage | .08‡ | ||||
| T2 | 117 | 24.8 | 51 | 20.4 | |
| T3 | 216 | 45.9 | 112 | 44.8 | |
| T4 | 138 | 29.3 | 87 | 34.8 | |
| N stage | .28‡ | ||||
| N0 | 88 | 18.7 | 48 | 19.2 | |
| N1 | 78 | 16.6 | 29 | 11.6 | |
| N2a | 42 | 8.9 | 18 | 7.2 | |
| N2b | 122 | 25.9 | 67 | 26.8 | |
| N2c | 100 | 21.2 | 73 | 29.2 | |
| N3 | 41 | 8.7 | 15 | 6.0 | |
| AJCC stage | .14† | ||||
| III | 111 | 23.6 | 47 | 18.8 | |
| IV | 360 | 76.4 | 203 | 81.2 | |
| Accrual volume | .34† | ||||
| HHAC | 150 | 31.8 | 71 | 28.4 | |
| HLAC | 321 | 68.2 | 179 | 71.6 | |
| OS | .76§ | ||||
| 5-year estimate, % | 56.9 | 57.9 | |||
| 95% CI | 52.2 to 61.6 | 51.3 to 64.4 | |||
| HR | Reference | 0.96 | |||
| 95% CI | 0.76 to 1.22 | ||||
| PFS | .96§ | ||||
| 5-year estimate, % | 48.8 | 47.1 | |||
| 95% CI | 44.1 to 53.5 | 40.4 to 53.8 | |||
| HR | Reference | 0.99 | |||
| 95% CI | 0.80 to 1.23 | ||||
Abbreviations: AFX-C, accelerated fractionation with concomitant boost; AJCC, American Joint Committee on Cancer; HHAC, historically high-accruing center; HLAC, historically low-accruing center; HPV, human papillomavirus; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; Q, quartile; SFX, standard fractionation.
Oropharynx only.
Pearson χ2 test.
Wilcoxon rank sum test.
Log-rank test.
Fig A1.
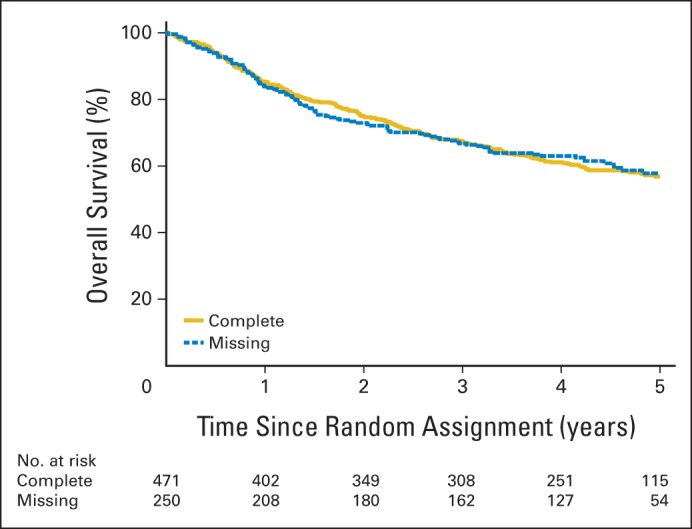
Kaplan-Meier estimates of overall survival for patients with complete data versus missing data.
Fig A2.
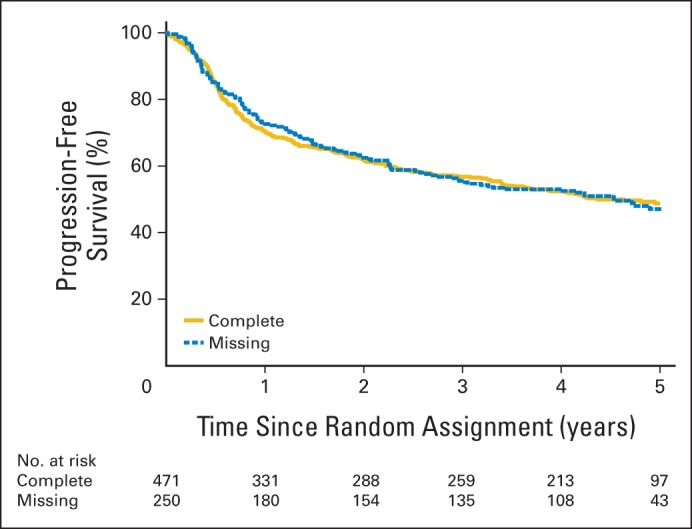
Kaplan-Meier estimates of progression-free survival for patients with complete data versus missing data.
Footnotes
See accompanying editorial on page 138; listen to the podcast by Dr Eisbruch at www.jco.org/podcasts
Support information appears at the end of this article.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Clinical trial information: NCT00047008.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Evan J. Wuthrick, Qiang Zhang, Mitchell Machtay, Nancy E. Read, Jonathan Harris
Provision of study materials or patients: Phuc Felix Nguyen-Tan
Collection and assembly of data: Evan J. Wuthrick, David I. Rosenthal, Phuc Felix Nguyen-Tan, André Fortin, Craig L. Silverman, Adam Raben, Harold E. Kim, Eric Horwitz, Nancy E. Read, Jonathan Harris, Maura L. Gillison
Data analysis and interpretation: Evan J. Wuthrick, Qiang Zhang, Mitchell Machtay, David I. Rosenthal, Phuc Felix Nguyen-Tan, Nancy E. Read, Jonathan Harris, Qian Wu, Quynh-Thu Le, Maura L. Gillison
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported by Radiation Therapy Oncology Group Grant No. U10 CA21661 and Community Clinical Oncology Program Grant No. U10 CA37422 from the National Cancer Institute.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Institutional Clinical Trial Accrual Volume and Survival of Patients With Head and Neck Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Evan J. Wuthrick
Honoraria: Bayer
Consulting or Advisory Role: Bayer
Research Funding: Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: cGMP as protector for chemotherapy and radiation
Travel, Accommodations, Expenses: Bayer
Qiang Zhang
No relationship to disclose
Mitchell Machtay
Honoraria: Bristol-Myers Squibb, ImClone Systems, Eli Lilly
David I. Rosenthal
No relationship to disclose
Phuc Felix Nguyen-Tan
No relationship to disclose
André Fortin
No relationship to disclose
Craig L. Silverman
No relationship to disclose
Adam Raben
No relationship to disclose
Harold E. Kim
No relationship to disclose
Eric Horwitz
No relationship to disclose
Nancy E. Read
No relationship to disclose
Jonathan Harris
No relationship to disclose
Qian Wu
Employment: Biostat Solution
Consulting or Advisory Role: Biostat Solution
Quynh-Thu Le
Research Funding: Varian, Amgen
Maura L. Gillison
Honoraria: Merck Serono
Consulting or Advisory Role: GlaxoSmithKline, Bristol-Myers Squibb, Pfizer
Travel, Accommodations, Expenses: Merck Serono
REFERENCES
- 1.Luchtenborg M, Riaz SP, Coupland VH, et al. High procedure volume is strongly associated with improved survival after lung cancer surgery. J Clin Oncol. 2013;31:3141–3146. doi: 10.1200/JCO.2013.49.0219. [DOI] [PubMed] [Google Scholar]
- 2.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Talamonti MS, Sener SF, et al. Effect of hospital volume on margin status after pancreaticoduodenectomy for cancer. J Am Coll Surg. 2008;207:510–519. doi: 10.1016/j.jamcollsurg.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Finlayson SR, Tosteson AN, et al. Effect of hospital volume on in-hospital mortality with pancreaticoduodenectomy. Surgery. 1999;125:250–256. [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 6.Birkmeyer JD, Warshaw AL, Finlayson SR, et al. Relationship between hospital volume and late survival after pancreaticoduodenectomy. Surgery. 1999;126:178–183. [PubMed] [Google Scholar]
- 7.Chen YK, Lin HC. Association between urologists' caseload volume and in-hospital mortality for transurethral resection of prostate: A nationwide population-based study. Urology. 2008;72:329–335. doi: 10.1016/j.urology.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Eisbruch A, Gregoire V. Balancing risk and reward in target delineation for highly conformal radiotherapy in head and neck cancer. Semin Radiat Oncol. 2009;19:43–52. doi: 10.1016/j.semradonc.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grégoire V, Daisne JF, Geets X, et al. Selection and delineation of target volumes in head and neck tumors: Beyond ICRU definition. Rays. 2003;28:217–224. [PubMed] [Google Scholar]
- 10.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN Guidelines: Head and Neck Cancers. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [PubMed]
- 12.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaming I, Cooper J, Henson D, et al. AJCC Cancer Staging Manual (ed 5) Philadephia, PA: Lippincott-Raven; 1997. [Google Scholar]
- 14.Huang CC, Qiu JT, Kashima ML, et al. Generation of type-specific probes for the detection of single-copy human papillomavirus by a novel in situ hybridization method. Mod Pathol. 1998;11:971–977. [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 16.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 17.Kalbfleisch J, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: Wiley; 1980. [Google Scholar]
- 18.Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- 19.National Cancer Institute. Cancer Therapy Evaluation Program Common Toxicity Criteria, version 2.0. http://prevention.cancer.gov/files/clinical-trials/common-toxicity-criteria.pdf.
- 20.Chen AY, Fedewa S, Pavluck A, et al. Improved survival is associated with treatment at high-volume teaching facilities for patients with advanced stage laryngeal cancer. Cancer. 2010;116:4744–4752. doi: 10.1002/cncr.25364. [DOI] [PubMed] [Google Scholar]
- 21.Sharma A, Schwartz SM, Méndez E. Hospital volume is associated with survival but not multimodality therapy in Medicare patients with advanced head and neck cancer. Cancer. 2013;119:1845–1852. doi: 10.1002/cncr.27976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters LJ, O'Sullivan B, Giralt J, et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of advanced head and neck cancer: Results from TROG 02.02. J Clin Oncol. 2010;28:2996–3001. doi: 10.1200/JCO.2009.27.4498. [DOI] [PubMed] [Google Scholar]
- 23.Kam MK, Leung SF, Zee B, et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J Clin Oncol. 2007;25:4873–4879. doi: 10.1200/JCO.2007.11.5501. [DOI] [PubMed] [Google Scholar]
- 24.Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–136. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pow EH, Kwong DL, McMillan AS, et al. Xerostomia and quality of life after intensity-modulated radiotherapy vs. conventional radiotherapy for early-stage nasopharyngeal carcinoma: Initial report on a randomized controlled clinical trial. Int J Radiat Oncol Biol Phys. 2006;66:981–991. doi: 10.1016/j.ijrobp.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Hong TS, Tomé WA, Harari PM. Heterogeneity in head and neck IMRT target design and clinical practice. Radiother Oncol. 2012;103:92–98. doi: 10.1016/j.radonc.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisbruch A, Harris J, Garden AS, et al. Multi-institutional trial of accelerated hypofractionated intensity-modulated radiation therapy for early-stage oropharyngeal cancer (RTOG 00-22) Int J Radiat Oncol Biol Phys. 2010;76:1333–1338. doi: 10.1016/j.ijrobp.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong YK, Tsai WC, Lin JC, et al. Socio-demographic factors in the prognosis of oral cancer patients. Oral Oncol. 2006;42:893–906. doi: 10.1016/j.oraloncology.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Groome PA, Schulze KM, Keller S, et al. Explaining socioeconomic status effects in laryngeal cancer. Clin Oncol (R Coll Radiol) 2006;18:283–292. doi: 10.1016/j.clon.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Chu KP, Shema S, Wu S, et al. Head and neck cancer-specific survival based on socioeconomic status in Asians and Pacific Islanders. Cancer. 2011;117:1935–1945. doi: 10.1002/cncr.25723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konski A, Berkey BA, Kian Ang K, et al. Effect of education level on outcome of patients treated on Radiation Therapy Oncology Group Protocol 90-03. Cancer. 2003;98:1497–1503. doi: 10.1002/cncr.11661. [DOI] [PubMed] [Google Scholar]
- 32.Kwok J, Langevin SM, Argiris A, et al. The impact of health insurance status on the survival of patients with head and neck cancer. Cancer. 2010;116:476–485. doi: 10.1002/cncr.24774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.