Abstract
Purpose
Insomnia is a distressing and often persisting consequence of cancer. Although cognitive behavioral therapy for insomnia (CBT-I) is the treatment of choice in the general population, the use of CBT-I in patients with cancer is complicated, because it can result in transient but substantial increases in daytime sleepiness. In this study, we evaluated whether CBT-I, in combination with the wakefulness-promoting agent armodafinil (A), results in better insomnia treatment outcomes in cancer survivors than CBT-I alone.
Patients and Methods
We report on a randomized trial of 96 cancer survivors (mean age, 56 years; female, 87.5%; breast cancer, 68%). The primary analyses examined whether ≥ one of the 7-week intervention conditions (ie, CBT-I, A, or both), when compared with a placebo capsule (P) group, produced significantly greater clinical gains. Insomnia was assessed by the Insomnia Severity Index and sleep quality by the Pittsburgh Sleep Quality Inventory. All patients received sleep hygiene instructions.
Results
Analyses controlling for baseline differences showed that both the CBT-I plus A (P = .001) and CBT-I plus P (P = .010) groups had significantly greater reductions in insomnia severity postintervention than the P group, with effect sizes of 1.31 and 1.02, respectively. Similar improvements were seen for sleep quality. Gains on both measures persisted 3 months later. CBT-I plus A was not significantly different from CBT-I plus P (P = .421), and A alone was not significantly different from P alone (P = .584).
Conclusion
CBT-I results in significant and durable improvements in insomnia and sleep quality. A did not significantly improve the efficacy of CBT-I or independently affect insomnia or sleep quality.
INTRODUCTION
Difficulties with initiating and/or maintaining sleep are reported by 30% to 60% of individuals with cancer,1–4 and high rates of insomnia persist well beyond completion of radiation therapy and chemotherapy.5–7 For example, in a study of 300 breast cancer survivors (median time since treatment onset, 48 months), Savard et al8 found that 51% of their participants reported insomnia symptoms, and nearly 20% of this sample met diagnostic criteria for insomnia. These prevalence rates are especially striking, considering most epidemiologic studies report approximately 30% of adults in the general population experience insomnia symptoms, and approximately 10% meet diagnostic criteria for an insomnia disorder.9,10 Insomnia in cancer survivors is particularly troublesome, because it tends to be unremitting,11 and untreated insomnia is associated with diminished quality of life,12 risk for both medical and psychiatric morbidities,13 and costs to the individual and society.14 Given the prevalence of insomnia both during and after cancer treatment and the substantial consequences of untreated insomnia, it stands to reason that insomnia disorder should be a focus for treatment after cancer therapy.
Cognitive behavioral therapy for insomnia (CBT-I) comprises behavioral techniques, cognitive restructuring, and education. CBT-I is based on rectification of the mismatch between sleep ability and sleep opportunity, as implemented with sleep restriction therapy; application and use of operant conditioning paradigms, as accomplished with stimulus control therapy; identification and correction of sleep-interfering behaviors, as carried out with sleep hygiene instructions; optimization of circadian effects on sleep via regularization of the sleep-wake schedule; and use of cognitive restructuring techniques to reduce stress responses that affect sleep.15,16
Although CBT-I is effective in treating insomnia, between 32% and 89% of patients treated with CBT-I do not consistently follow treatment recommendations.17 One reason for this low adherence is the temporary daytime sleepiness and/or worsening of daytime function that accompany the sleep-restriction component of CBT-I.18,19 We wanted to determine whether providing patients with the wakefulness-promoting agent armodafinil (A; Nuvigil; Teva Pharmaceuticals, Petah Tikva, Israel) would result in greater adherence to CBT-I instructions, in addition to our objective of providing additional data on the efficacy of CBT-I in cancer survivors. A previous study of the addition of modafinil (Provigal; Cephalon, Frazer, PA) to a CBT-I regimen showed increased CBT-I adherence.20 In addition, we thought it possible that A alone might produce improvements in insomnia based on the concept that A, like CBT-I, could result in increased activity during, as well as prolongation of, the wake period. These effects, regardless of how they are initiated, are thought to allow for better sleep initiation and consolidation. We hypothesized that the three intervention strategies (ie, CBT-I, A, or both), compared with the placebo (P) -only group, would improve insomnia, with the largest effects occurring with the combined strategy.
PATIENTS AND METHODS
Patients
Cancer survivors with chronic insomnia in two Northeastern cities were recruited from cancer clinics and via letters and advertising between September 2008 and November 2012, when the funded recruitment window ended. Eligibility criteria remained essentially unchanged from December 2008, when the original criterion of having breast cancer was broadened to include having any cancer, and the criterion of receiving chemotherapy was broadened to include radiation therapy. Other eligibility criteria that remained unchanged were that participants must have completed all chemotherapy and/or radiotherapy ≥ 1 month before study start and must demonstrate no measurable disease. Participants were required to discontinue any prescribed or over-the-counter sleep medications for 1 week before beginning the baseline data collection as well as during the 11-week study period. They also had to have self-reported problems with insomnia for at least 3 months and stated that it began or became worse with the onset of cancer or treatment. Patients who had ever received modafinil or A or undergone CBT-I and those who had a history of seizures or severe headaches, substance or alcohol abuse, sleep apnea, or an unstable medical or psychiatric illness were not eligible. The institutional review boards of the University of Rochester and University of Pennsylvania approved the protocol, and patients provided written informed consent.
Design and Procedures
A computer-generated randomization schedule with a block size of eight, stratified by city and sex, was used to assign participants to one of four groups: CBT-I, A, CBT-I plus A, or neither (ie, factorial design of CBT-I [yes or no] v A [yes or no]; Table 1). The original grant application was approved, with modafinil 100 mg twice per day as the active medication. A switch to A 50 mg twice per day was made at the suggestion of Cephalon, which manufactured both medications and supplied the drug and matching placebo. All patients received written sleep hygiene guidelines (eg, keep bedroom cool and free of light, avoid naps, avoid using alcohol as sleep aid, and so on) at the time of consent. The 7-week CBT-I intervention was provided on an individual basis, followed a published treatment manual,15 and was in accordance with the CBT-I description provided in the Introduction.
Table 1.
47-Day Intervention by Study Arm
| Characteristic | Group One | Group Two | Group Three | Group Four |
|---|---|---|---|---|
| Drug | CBT-I plus placebo | CBT-I plus armodafinil | Placebo | Armodafinil |
| Dosage | Placebo capsule morning and afternoon* | Armodafinil (3 days at 50 mg, 40 days at 100 mg, 4 days at 50 mg)† | Placebo capsule morning and afternoon* | Armodafinil (3 days at 50 mg, 40 days at 100 mg, 4 days at 50 mg)† |
| Therapy | Sleep hygiene guidelines plus 1-hour weekly CBT-I sessions (three in person, four by telephone) | Sleep hygiene guidelines plus 1-hour weekly CBT-I sessions (three in person, four by telephone) | Sleep hygiene guidelines | Sleep hygiene guidelines |
Abbreviation: CBT-I, cognitive behavioral therapy for insomnia.
Placebo matched to armodafinil 50 mg.
On intervention days 1 to 3 and 44 to 47, armodafinil 50 mg was provided in morning, with matching placebo in afternoon; armodafinil 50 mg twice per day was provided on days 4 to 43.
Random assignment was conveyed to a pharmacist, who provided the study coordinator with the appropriate study medications. Patients were told of their random assignment to CBT-I or not after the completion of their 2-week baseline period. All study personnel and patients were blinded to medication (A or P) assignment but not to CBT-I (yes or no) status. Participants had the option of completing measures using paper and pen on scannable forms or using an Internet data portal. Data were electronically transferred to an access database, and data quality was checked by an information analyst.
Treatments
CBT-I was delivered over the course of seven individual sessions occurring once per week. Sessions one, two, and four were conducted in person (duration, 30 to 60 minutes), and sessions three, five, six, and seven (duration, 15 to 30 minutes) were conducted by telephone. Use of telephone sessions in our trial was based on the perspective that fewer in-clinic visits would help with recruitment and retention of participants.
A is a single-isomer formulation of modafinil (R-enantiomer of modafinil). It is indicated for the promotion of wakefulness in several sleep disorders, including narcolepsy, sleep apnea syndrome, and shiftwork disorder. It is available in 50-, 150-, or 250-mg tablets. Clinically, modafinil is thought to exert its effects over a 6- to 8-hour timeframe. A, given its right-shifted pharmacokinetic profile, is thought to be active for a longer period of time. Medication was begun with a 50-mg dose of A in the morning (7 to 9am), along with an afternoon (12 to 2pm) administration of P. After 3 days, active doses of A were provided both in the morning and afternoon (morning, 50 mg; afternoon, 50 mg). Twice-per-day treatment was continued for 40 days, followed by 4 days of 50 mg in the morning and P in the afternoon.
Assessments
Primary outcome.
The Insomnia Severity Index (ISI) is a commonly used seven-item psychometrically validated measure used to rate insomnia, with scores of 0 to 7 indicating absence of insomnia, 8 to 14 indicating subthreshold insomnia symptoms, 15 to 21 indicating moderate insomnia, and 22 to 28 indicating severe insomnia.21–23
Secondary outcome.
The Pittsburgh Sleep Quality Inventory (PSQI) is a commonly used 19-item psychometrically validated measure of sleep quality and disturbances. It has a score range of 0 to 21, with scores ≤ 5 associated with good sleep quality and > 5 associated with poor sleep quality.24
On enrollment, patients provided demographic and clinical information and a brief sleep history. Other assessments were made before (baseline), weekly during the intervention (ISI only), immediately after the intervention (postintervention), and 3 months after the study intervention (follow-up).
Sample Size
Our target accrual in the original protocol was 226 participants. We assumed a 20% attrition rate and projected the resulting 180 participants (45 per arm) would have 80% power to detect a difference in ISI mean post-pre change scores between arms of 1.7. This assumed a longitudinal analysis using estimation by generalized estimating equation and possible linear splines to represent trajectories in each arm. Because of our ability to randomly assign only 96 patients, and the fact that the original planned analysis would be problematic with this smaller sample size, we changed the primary analysis to analysis of covariance (ANCOVA) using preintervention ISI as the covariate, with appropriate contrasts to evaluate the four planned comparisons (ie, P group compared with other three groups, and CBT-I plus A compared with CBT-I plus P). With four comparisons (ie, significance level, .0125; standard deviation, 4.7; pre-post correlation, 0.58; determined from randomized controlled trial of yoga for sleep quality among cancer survivors25), we calculated that 96 participants would have 80% power to detect a difference in mean change between arms of 4.2, corresponding to an effect size of 0.90.
Statistical Analyses
ANCOVA was used for the postintervention score (average of two postintervention weeks), controlling for the score at the time of consent (preintervention). Using appropriate contrasts, the mean post-pre change was estimated for CBT-I plus A versus P, CBT-I plus P versus P, A versus P, and CBT-I plus A versus CBT-I plus P. P values less than .0125 were considered statistically significant (Bonferroni adjustment).
Analyses were performed by intention to treat, although 23 (24%) of the 96 randomly assigned eligible patients did not provide postintervention data. The missing value patterns (monotone dropout, 75%; sporadic missing data, 25%) were examined through visual inspection and logistic regression of missing data versus treatment arm and demographic characteristics. We also tested whether the rate of missing data depended on the previous ISI value. We found no evidence the data were not missing at random and therefore assumed a missing-at-random mechanism.26 Multiple imputation (MI; SAS PROC MI: 100 complete data sets per arm, Markov chain Monte Carlo single-chain method with 200 burn-in iterations, EM posterior-mode starting values, Jeffrey's prior based on baseline and postintervention ISI, and daytime sequelae portion of ISI—leaving out sleep continuity items that would be affected by CBT-I intervention—for each intermediate time point) was used to evaluate the sensitivity of the results to the missing data. The MI analysis results were similar to the complete case analyses, in which only those patients who provided postintervention data were included. Both sets of results are listed in Table 2; however, for space reasons, we provide only the MI results elsewhere in the report, because they are less likely to be biased because of the missing data.
Table 2.
Comparison of Insomnia and Sleep Quality at Postintervention by Study Conditions
| Comparison | Estimate | SE | 95% CI | P* | Effect Size |
|---|---|---|---|---|---|
| Insomnia† | |||||
| CBT-I plus placebo v placebo | |||||
| Complete case | −5.13 | 1.53 | −8.19 to −2.06 | .001 | −1.06 |
| MI | −4.93 | 1.85 | −8.63 to −1.22 | .010 | −1.02 |
| CBT-I plus armodafinil v placebo | |||||
| Complete case | −6.92 | 1.55 | −10.02 to −3.81 | < .001 | −1.43 |
| MI | −6.36 | 1.84 | −10.02 to −2.69 | .001 | −1.31 |
| Armodafinil v placebo | |||||
| Complete case | 1.57 | 1.60 | −1.64 to 4.77 | .333 | 0.32 |
| MI | 1.04 | 1.89 | −2.74 to 4.82 | .584 | 0.21 |
| CBT-I plus armodafinil v CBT-I plus placebo | |||||
| Complete case | −1.79 | 1.54 | −4.86 to 1.29 | .249 | −0.37 |
| MI | −1.43 | 1.78 | −4.91 to 2.05 | .421 | −0.29 |
| Sleep Quality‡ | |||||
| CBT-I plus placebo v placebo | |||||
| Complete case | −3.48 | 1.09 | −5.66 to −1.30 | .002 | −0.99 |
| MI | −3.22 | 1.17 | −5.57 to −0.88 | .008 | −0.92 |
| CBT-I plus armodafinil v placebo | |||||
| Complete case | −4.12 | 1.15 | −6.42 to −1.82 | .001 | −1.17 |
| MI | −4.03 | 1.21 | −6.45 to −1.61 | .002 | −1.14 |
| Armodafinil v placebo | |||||
| Complete case | 1.33 | 1.15 | −0.97 to 3.63 | .251 | 0.38 |
| MI | 1.40 | 1.23 | −1.05 to 3.86 | .257 | 0.40 |
| CBT-I plus armodafinil v CBT-I plus placebo | |||||
| Complete case | −0.64 | 1.13 | −2.89 to 1.61 | .572 | −0.18 |
| MI | −0.81 | 1.18 | −3.11 to 1.50 | .494 | −0.23 |
NOTE. Analyses are presented as both complete case and MI because of frequent missing data. Estimates and associated statistics refer to differences between groups in mean change from baseline. Effect size is standardized mean difference.
Abbreviations: ANCOVA, two-way analysis of covariance; CBT-I, cognitive behavioral therapy for insomnia; MI, multiple imputation.
P values denote improvements compared with placebo from comparison by ANCOVA, controlling for values at time of consent.
By Insomnia Severity Index.
By Pittsburgh Sleep Quality Index.
Using the factorial nature of the design, we performed a similar analysis for ISI using drug (yes or no), CBT-I (yes or no), and drug * CBT-I interaction as the factors and baseline as the covariate. In addition, a longitudinal analysis to compare the group trajectories of ISI over the 7-week intervention period is reported in the Appendix and Appendix Fig A1 (online only). We used SAS software (version 9.2; SAS Institute, Cary, NC) SPSS software (version 19; SPSS, Chicago, IL), and R software (version 3; http://www.r-project.org) for analyses as appropriate.
RESULTS
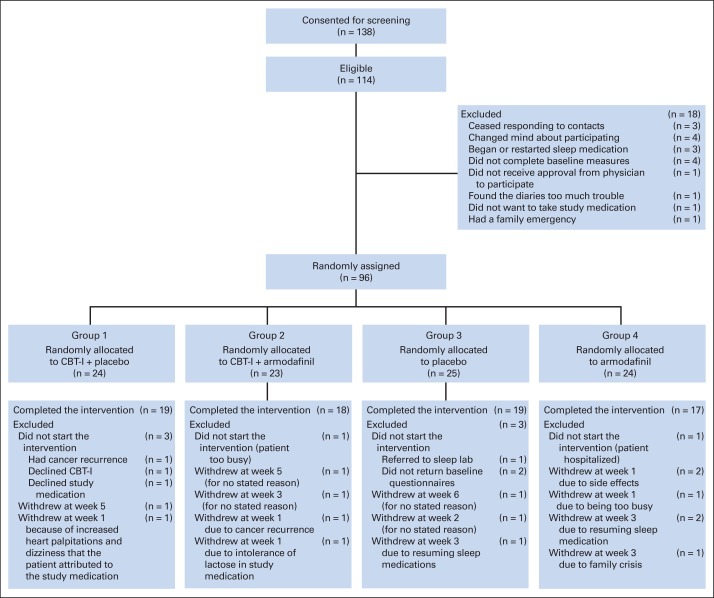
Of the 138 patients who consented to screening, 114 were eligible, and 96 were randomly assigned; 88 patients (eligible, 77%; randomized assigned, 92%) began the intervention, and 73 patients (83% of 88 patients beginning intervention) completed the 7-week intervention (Fig 1). Average compliance with the study medication, as determined by the returned study medication cards, was > 90% for all study arms and did not differ significantly by group. No serious related adverse events were reported. One grade 2 possibly related event (tingling, numbness, and weakness in legs) occurred, as did two probably related grade 2 events (headaches). All three events occurred in patients assigned to CBT-I plus A. Table 3 lists the baseline characteristics by treatment group; there were no important differences between the treatment groups for any baseline characteristics.
Fig 1.
Trial profile. CBT-I, cognitive behavioral therapy for insomnia.
Table 3.
Demographic and Clinical Characteristics of Participants by Study Group
| Characteristic | CBT-I Plus Placebo (n = 24) |
CBT-I Plus Armodafinil (n = 23) |
Placebo (n = 25) |
Armodafinil (n = 24) |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||
| Mean | 59 | 56 | 52 | 57 | ||||
| SD | 9.9 | 10.2 | 11.5 | 7.4 | ||||
| Sex | ||||||||
| Male | 3 | 12 | 1 | 4 | 7 | 28 | 1 | 4 |
| Female | 21 | 87 | 22 | 96 | 18 | 72 | 23 | 96 |
| Ethnicity | ||||||||
| Non-Hispanic | 23 | 96 | 22 | 96 | 24 | 96 | 22 | 92 |
| Unknown | 1 | 4 | 1 | 4 | 1 | 4 | 2 | 8 |
| Race | ||||||||
| White | 23 | 96 | 21 | 91 | 19 | 76 | 23 | 96 |
| African American | 1 | 4 | 2 | 9 | 4 | 16 | 1 | 4 |
| Unknown or other | 2 | 8 | ||||||
| Education | ||||||||
| > High school | 20 | 83 | 19 | 86 | 23 | 92 | 23 | 96 |
| ≤ High school | 4 | 17 | 3 | 14 | 2 | 8 | 1 | 4 |
| Married | 13 | 54 | 16 | 70 | 18 | 72 | 12 | 50 |
| Time from last cancer treatment to intervention, days | ||||||||
| Mean | 1,625 | 1,647 | 654 | 1,363 | ||||
| Minimum | 136 | 48 | 112 | 104 | ||||
| Maximum | 7,071 | 10,034 | 1,957 | 6,115 | ||||
| Type of cancer | ||||||||
| Breast | 16 | 67 | 17 | 74 | 15 | 60 | 17 | 71 |
| Other | 8 | 33 | 6 | 26 | 10 | 40 | 7 | 29 |
| Type of cancer treatment | ||||||||
| Chemotherapy | 17 | 71 | 17 | 74 | 21 | 84 | 22 | 92 |
| Radiotherapy | 19 | 79 | 18 | 78 | 17 | 68 | 17 | 71 |
| Receiving hormone therapy | ||||||||
| Yes | 3 | 12 | 4 | 17 | 5 | 20 | — | — |
| No | 19 | 79 | 19 | 80 | 16 | 64 | 19 | 79 |
| Unknown | 2 | 8 | 4 | 16 | 5 | 21 | ||
| Insomnia at time of consent* | ||||||||
| Mean | 15.2 | 15.1 | 14.7 | 14.7 | ||||
| SD | 4.6 | 5.4 | 5.3 | 4.4 | ||||
Abbreviations: CBT-I, cognitive behavioral therapy for insomnia; SD, standard deviation.
By Insomnia Severity Index.
Mean severity of insomnia during the postintervention period for the four study conditions were as follows: CBT-I plus P, 5.61; CBT-I plus A, 3.35; P, 10.47; and A, 12.17 (Table 2; Fig 2). ANCOVA with MI and controlling for values at time of consent showed that participants in both the CBT-I plus A (P = .001) and CBT-I plus P groups (P = .010) had significantly less insomnia than those in the P group. The A and P groups were not significantly different (P = .584), nor was the CBT-I plus A group significantly different from the CBT-I plus P group (P = .421).
Fig 2.
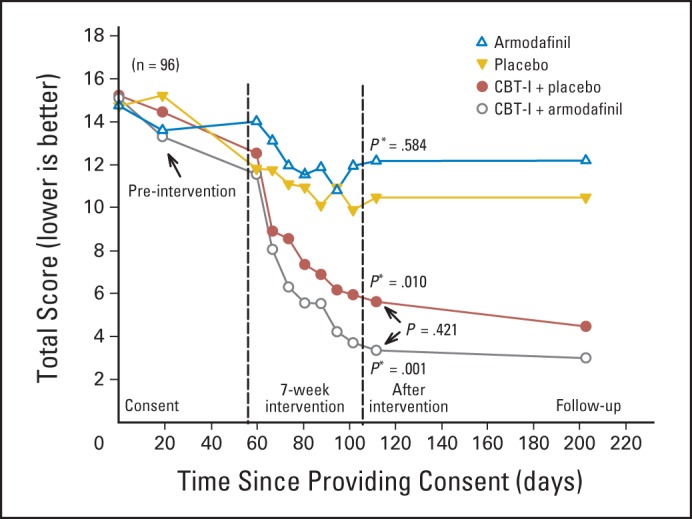
Insomnia Severity Index. Nos. per study arm at beginning of intervention, postintervention, and follow-up were as follows: cognitive behavioral therapy for insomnia (CBT-I) plus placebo, 21, 18, and 16; CBT-I plus armodafinil, 22, 17, and 16; placebo, 22, 17, and 19; and armodafinil, 23, 15, and 14, respectively. (*) P values indicate difference from placebo using analysis of covariance, with multiple imputation and controlling for values at time of consent.
From the factorial analysis, the estimated improvement on the ISI from receiving CBT-I was a significant 7.40 points (P < .001). We note that an 8.5-point reduction on the ISI is associated with moderate improvement,27 and 60% of the CBT-I participants achieved at least this level of relief, compared with only 20% of the non–CBT-I participants. The estimated improvement from receiving A was a nonsignificant 1.43 points (P = .424). The CBT-I by A interaction was also not significant (P = .346), indicating that the effect of CBT-I was independent of treatment with A. The findings from the longitudinal analyses, which are reported in the Appendix (online only), were similar.
We also compared the change from postintervention ISI with follow-up ISI (average of weeks 23 and 24) using a one-way ANOVA. None of the groups showed a statistically significant change from post-treatment to follow-up (P = .505).
Our findings on sleep quality as assessed by the PSQI total score mirrored the findings with the ISI (Table 2; Fig 3). Mean scores using MI on the postintervention PSQI in groups one to four were 4.69, 4.21, 8.32, and 9.46, respectively, indicating good sleep quality (ie, scores ≤ 5) for both the CBT-I plus P and CBT-I plus A groups and poor sleep quality (ie, scores > 5) for both the A and P groups. ANCOVA with MI and controlling for values at time of consent showed that participants in both the CBT-I plus A (P = .002) and CBT-I plus P groups (P = .008) had better sleep quality than those in the P group. The A and P groups were not significantly different (P = .257), nor was the CBT-I plus A group significantly different from the CBT-I plus P group (P = .494).
Fig 3.
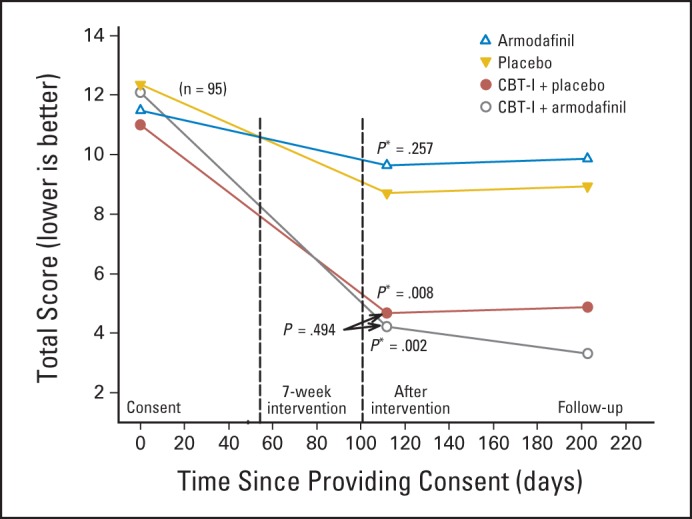
Pittsburgh Sleep Quality Index. Nos. per study arm at beginning of intervention, postintervention, and follow-up were as follows: cognitive behavioral therapy for insomnia (CBT-I) plus placebo, 21, 18, and 15; CBT-I plus armodafinil, 22, 14, and 16; placebo, 22, 17, and 15; and armodafinil, 22, 14, and 14, respectively. (*) P values indicate difference from placebo using analysis of covariance, with multiple imputation and controlling for values at time of consent.
ANCOVA with MI and controlling for values at time of consent to assess the main effects of CBT-I and A and their interaction on the PSQI showed the estimated improvement from receiving CBT-I was a statistically significant 5.43 points (P < .001). The estimated improvement from receiving A was a statistically nonsignificant 0.81 points (P = .496). The CBT-I by A interaction was also not statistically significant (P = .198), indicating that the CBT-I plus A effects are additive. As with the ISI, none of the groups showed a statistically significant change in PSQI from postinteraction to follow-up (P = .881).
DISCUSSION
A 7-week treatment program of CBT-I was effective in treating insomnia in cancer survivors. This finding is in keeping with reports from several recent studies.18,19,28–30 CBT-I produced strong and consistent reductions in insomnia severity from the time of consent to post-treatment, both with and without the addition of A. As assessed with the ISI, patients receiving CBT-I reported rapid improvement, with substantial change occurring by the second week of therapy. Continual improvements were observed through the remainder of the intervention. The decrease in insomnia remained stable 3 months after the conclusion of CBT-I. Improvement in sleep quality as assessed by the PSQI for those receiving CBT-I compared with those who did not mirrored the insomnia findings.
Contrary to our positive findings regarding CBT-I, our hypothesis that the addition of A to CBT-I would improve outcomes in treating insomnia in cancer survivors was not supported. The modest improvements on the ISI and PSQI observed in both the complete-case and MI analyses for the CBT-I plus A group compared with CBT-I plus P group did not reach statistical significance. A alone compared with P also showed no improvement on either the ISI or PSQI for any analyses. These findings parallel the outcomes in a prior study published by our research group.20 In that study, a single dose of modafinil (100 mg every morning), alone or in combination with CBT-I, in 30 patients with insomnia disorder did not directly affect sleep continuity as assessed prospectively and serially with sleep diaries. It was found, however, that CBT-I along with modafinil improved daytime sleepiness. A subsequent report will describe on the impact of A on treatment adherence, daytime sleepiness, and sleep continuity in the current trial.
Although the findings from the this study are consistent with our previous findings, it is possible that the fixed-dosage regimen (morning, 50 mg; afternoon, 50 mg) used in this protocol contributed to the lack of observed effects. Two interpretations are possible. First, the afternoon dosage may not have allowed enough time for the drug to clear before sleep onset, serving to compromise the efficacy of CBT-I by making sleep onset more difficult. Second, and conversely, the afternoon dose may have been insufficient to prolong wakefulness until the prescribed time for bed. It is possible that both explanations are correct and that these outcomes vary by individual and thus can only be addressed by a study that embraces a flexible dosing approach (ie, allows for amount of medication and time of administration to be varied by participant). An additional reason for the failure of this study to support our hypothesis concerning A may have been our low accrual (only 138 of targeted 226 patients). Although low accrual may have reduced our ability to detect an effect for A, the CIs for the CBT-I plus A and CBT-I plus P groups and the consistency of findings across the two drug groups indicate that as used, A is unlikely to have any clinically important effects on sleep quality or insomnia. The failure of A to produce benefits additive to those of CBT-I for the treatment of insomnia should discourage the use of this drug in oncologic practice.
The primary strength of our study was its longitudinal nature, with weekly assessments and 3-month follow-up. These multiple assessment points and positive findings regarding CBT-I in both of the study arms in which it was used allow us to conclude that CBT-I results in robust improvement in insomnia and sleep quality and that cancer survivors with insomnia receive rapid and lasting benefit from it. Considering the prevalence of insomnia in patients with cancer and survivors, the potential for poorer outcomes if insomnia remains untreated,31–34 and the efficacy of CBT-I in treating the disorder, it is desirable that providers and patients obtain increased access to evidence-based nonpharmacologic sleep interventions as an integral part of comprehensive cancer care.
Supplementary Material
Appendix
We also performed a longitudinal analysis to compare the group trajectories of Insomnia Severity Index (ISI) total scores over the 7-week intervention period and the 2 weeks after the intervention. Figure 2 shows the trajectories. To accommodate the curvature in the profiles, particularly for the cognitive behavioral therapy for insomnia (CBT-I) groups, we fit a linear mixed model with natural cubic smoothing splines (2 knots)—S(Week)—to represent changes over time. (Cubic smoothing splines are piecewise cubic polynomials that can adapt to wide variety of shapes, with minimal sensitivity to random errors.) The fixed effects in the model were S(Week), CBT-I (yes or no), drug (yes or no), and all second- and third-order interactions. Participant-specific slope and intercept were the random effects, with residual error independent of the random effects. Restricted maximum likelihood estimation was used, and inferences were performed using the Kenward-Roger procedure (Kenward MG, Roger JH: Biometrics 53:983-997, 1997).
There was no statistically significant effect of armodafinil on the CBT-I trajectory [P = .338; H0: S(Week) * CBT * drug interaction = 0]. Hence, the shape of the CBT-I trajectory did not significantly change with the addition of armodafinil. The CBT-I effect itself was highly significant [P < .001; H0: S(Week) * CBT interaction = 0]. The drug effect was not significant [P = .7269; H0: S(Week) * drug interaction = 0].
Fig A1.
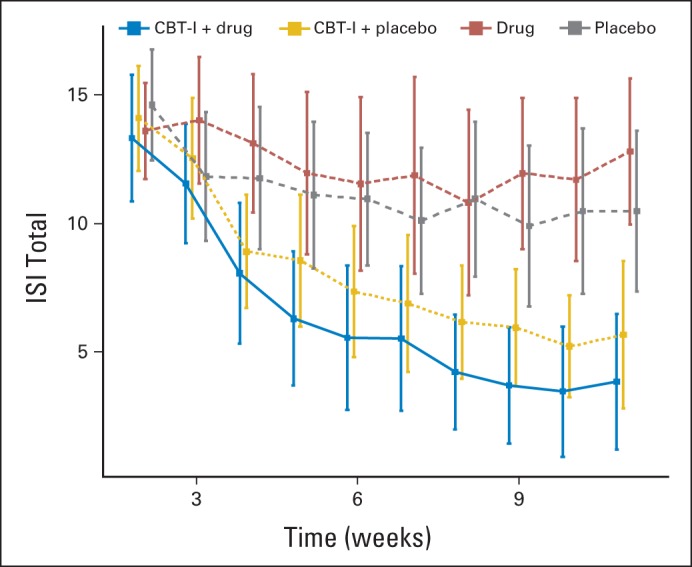
Mean Insomnia Severity Index (ISI) score: total versus week number (with 95% CIs).
Footnotes
Listen to the podcast by Dr Rodin at www.jco.org/podcasts
Supported by National Cancer Institute Grants No. 5 R01 CA126968 and 2R25CA102618-01A1 and by Teva Pharmaceuticals, which provided study medication.
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
Clinical trial information: NCT01091974.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Joseph A. Roscoe, Michael L. Perlis, Michelle Shayne, Josée Savard, Gary R. Morrow
Collection and assembly of data: Joseph A. Roscoe, Charles E. Heckler, Michael L. Perlis, Michelle Shayne, Nina P. Daniels
Data analysis and interpretation: Joseph A. Roscoe, Sheila N. Garland, Charles E. Heckler, Michael L. Perlis, Anita R. Peoples
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Placebo-Controlled Trial of Cognitive Behavioral Therapy and Armodafinil for Insomnia After Cancer Treatment
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Joseph A. Roscoe
No relationship to disclose
Sheila N. Garland
No relationship to disclose
Charles E. Heckler
No relationship to disclose
Michael L. Perlis
Consulting or Advisory Role: InsomniSolv
Research Funding: Teva Pharmaceuticals
Patents, Royalties, Other Intellectual Property: Book royalties from Springer-Verlag; DVD royalties from Flaming Spade Productions
Expert Testimony: Cantor Colburn
Anita R. Peoples
No relationship to disclose
Michelle Shayne
No relationship to disclose
Josée Savard
No relationship to disclose
Nina P. Daniels
No relationship to disclose
Gary R. Morrow
No relationship to disclose
REFERENCES
- 1.Savard J, Villa J, Ivers H, et al. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. J Clin Oncol. 2009;27:5233–5239. doi: 10.1200/JCO.2008.21.6333. [DOI] [PubMed] [Google Scholar]
- 2.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10:419–429. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthews EE, Berger AM, Schmiege SJ, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: A randomized, controlled trial. Oncol Nurs Forum. 2014;41:241–253. doi: 10.1188/14.ONF.41-03AP. [DOI] [PubMed] [Google Scholar]
- 4.Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center–Community Clinical Oncology Program. J Clin Oncol. 2010;28:292–298. doi: 10.1200/JCO.2009.22.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–162. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotronoulas G, Wengström Y, Kearney N. A critical review of women's sleep-wake patterns in the context of neo-/adjuvant chemotherapy for early-stage breast cancer. Breast. 2012;21:128–141. doi: 10.1016/j.breast.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Savard J, Ivers H, Villa J, et al. Natural course of insomnia comorbid with cancer: An 18-month longitudinal study. J Clin Oncol. 2011;29:3580–3586. doi: 10.1200/JCO.2010.33.2247. [DOI] [PubMed] [Google Scholar]
- 8.Savard J, Simard S, Blanchet J, et al. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24:583–590. doi: 10.1093/sleep/24.5.583. [DOI] [PubMed] [Google Scholar]
- 9.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 10.Mellinger GD, Balter MB, Uhlenhuth EH, et al. Insomnia and its treatment: Prevalence and correlates. Arch Gen Psychiatry. 1985;42:225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 11.Morin CM, Bélanger L, Leblanc M, et al. The natural history of insomnia: A population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447–453. doi: 10.1001/archinternmed.2008.610. [DOI] [PubMed] [Google Scholar]
- 12.Lis CG, Gupta D, Grutsch JF. The relationship between insomnia and patient satisfaction with quality of life in cancer. Support Care Cancer. 2008;16:261–266. doi: 10.1007/s00520-007-0314-z. [DOI] [PubMed] [Google Scholar]
- 13.Matteson-Rusby SE, Pigeon WR, Gehrman P, et al. Why treat insomnia? Prim Care Companion J Clin Psychiatry. 2010;12:PCC.08r00743. doi: 10.4088/PCC.08r00743bro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30:263–273. doi: 10.1093/sleep/30.3.263. [DOI] [PubMed] [Google Scholar]
- 15.Perlis ML, Jungquist C, Smith MT, et al. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. New York, NY: Springer; 2005. [Google Scholar]
- 16.Sharma MP, Andrade C. Behavioral interventions for insomnia: Theory and practice. Indian J Psychiatry. 2012;54:359–366. doi: 10.4103/0019-5545.104825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews EE, Arnedt JT, McCarthy MS, et al. Adherence to cognitive behavioral therapy for insomnia: A systematic review. Sleep Med Rev. 2013;17:453–464. doi: 10.1016/j.smrv.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiorentino L, McQuaid JR, Liu L, et al. Individual cognitive behavioral therapy for insomnia in breast cancer survivors: A randomized controlled crossover pilot study. Nat Sci Sleep. 2010;2:1–8. doi: 10.2147/NSS.S8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garland SN, Carlson LE, Stephens AJ, et al. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: A randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32:449–457. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
- 20.Perlis ML, Smith MT, Orff H, et al. The effects of modafinil and cognitive behavior therapy on sleep continuity in patients with primary insomnia. Sleep. 2004;27:715–725. doi: 10.1093/sleep/27.4.715. [DOI] [PubMed] [Google Scholar]
- 21.Morin CM. Insomnia: Psychological Assessment and Management. New York, NY: Guilford Press; 1993. [Google Scholar]
- 22.Morin CM, Beaulieu-Bonneau S, LeBlanc M, et al. Self-help treatment for insomnia: A randomized controlled trial. Sleep. 2005;28:1319–1327. doi: 10.1093/sleep/28.10.1319. [DOI] [PubMed] [Google Scholar]
- 23.Savard MH, Savard J, Simard S, et al. Empirical validation of the Insomnia Severity Index in cancer patients. Psychooncology. 2005;14:429–441. doi: 10.1002/pon.860. [DOI] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Mustian KM, Sprod LK, Janelsins M, et al. Multicenter, randomized controlled trial of yoga for sleep quality among cancer survivors. J Clin Oncol. 2013;31:3233–3241. doi: 10.1200/JCO.2012.43.7707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Little RJA, Rubin DB. Statistical Analysis with Missing Data. Hoboken, NJ: John Wiley and Sons; 2002. [Google Scholar]
- 27.Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Epstein DR, Dirksen SR. Randomized trial of a cognitive-behavioral intervention for insomnia in breast cancer survivors. Oncol Nurs Forum. 2007;34:E51–E59. doi: 10.1188/07.ONF.E51-E59. [DOI] [PubMed] [Google Scholar]
- 29.Espie CA, Fleming L, Cassidy J, et al. Randomized controlled clinical effectiveness trial of cognitive behavior therapy compared with treatment as usual for persistent insomnia in patients with cancer. J Clin Oncol. 2008;26:4651–4658. doi: 10.1200/JCO.2007.13.9006. [DOI] [PubMed] [Google Scholar]
- 30.Savard J, Simard S, Ivers H, et al. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. J Clin Oncol. 2005;23:6097–6106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
- 31.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–235. [PubMed] [Google Scholar]
- 32.Lee KA, Ward TM. Critical components of a sleep assessment for clinical practice settings. Issues Ment Health Nurs. 2005;26:739–750. doi: 10.1080/01612840591008320. [DOI] [PubMed] [Google Scholar]
- 33.Leger D, Guilleminault C, Bader G, et al. Medical and socio-professional impact of insomnia. Sleep. 2002;25:625–629. [PubMed] [Google Scholar]
- 34.Caplette-Gingras A, Savard J, Savard MH, et al. Is insomnia associated with cognitive impairments in breast cancer patients? Behav Sleep Med. 2013;11:239–257. doi: 10.1080/15402002.2012.672940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.