Abstract
Purpose
This open-label phase III trial evaluated efficacy and tolerability of linifanib versus sorafenib in patients with advanced hepatocellular carcinoma (HCC) without prior systemic therapy.
Patients and Methods
Patients were randomly assigned in a 1:1 ratio to linifanib 17.5 mg once daily or sorafenib 400 mg twice daily. Patients were stratified by region (Outside Asia, Japan, and rest of Asia), Eastern Cooperative Oncology Group performance score (ECOG PS; 0 or 1), vascular invasion or extrahepatic spread (yes or no), and hepatitis B virus (HBV) infection (yes or no). The primary end point of the study was overall survival (OS). Secondary end points were time to progression (TTP) and objective response rate (ORR) per RECIST v1.1.
Results
We randomly assigned 1,035 patients (median age, 60 years; Asian, 66.6%; ECOG PS 0, 65.2%; HBV, 49.1%; vascular invasion or extrahepatic spread, 70.1%). Median OS was 9.1 months on the linifanib arm (95% CI, 8.1 to 10.2) and 9.8 months on the sorafenib arm (95% CI, 8.3 to 11.0; hazard ratio [HR], 1.046; 95% CI, 0.896 to 1.221). For prespecified stratification subgroups, OS HRs ranged from 0.793 to 1.119 and the 95% CI contained 1.0. Median TTP was 5.4 months on the linifanib arm (95% CI, 4.2 to 5.6) and 4.0 months on the sorafenib arm (95% CI, 2.8 to 4.2; HR, 0.759; 95% CI, 0.643 to 0.895; P = .001). Best response rate was 13.0% on the linifanib arm versus 6.9% on the sorafenib arm. Grade 3/4 adverse events (AEs); serious AEs; and AEs leading to discontinuation, dose interruption, and reduction were more frequent with linifanib (all P < .001).
Conclusion
Linifanib and sorafenib had similar OS in advanced HCC. Predefined superiority and noninferiority OS boundaries were not met for linifanib and the study failed to meet the primary end point. TTP and ORR favored linifanib; safety results favored sorafenib.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third most common cause of cancer deaths worldwide, after lung and stomach cancer.1,2 More than 75% of all HCC cases occur in the Asia-Pacific region and are associated with chronic hepatitis B virus (HBV) infection,3,4 with an increased incidence in the United States and Europe over the last decade. Despite the available treatment options for HCC, the incidence and mortality rates are nearly equal.5 A minority of patients are diagnosed with resectable HCC,6,7 whereas approximately 70% to 85% of HCC patients have locally advanced unresectable or metastatic disease at diagnosis.1,6–8 Up to 70% of patients who undergo potentially curative procedures will have recurrent, advanced-stage disease within 5 years.9,10 Thus, effective systemic therapies are needed for the vast majority of patients with HCC.
HCC is a highly vascularized tumor characterized by overexpression of vascular endothelial growth factor (VEGF).11 Three important proangiogenic factors, VEGF, platelet-derived growth factor (PDGF), and basic fibroblast growth factor, are involved in hepatocarcinogenesis and participate in the neovascularization, invasiveness, and metastatic potential of HCC.11 Elevated VEGF is associated with poor prognosis and survival as well as recurrent disease in HCC.12 VEGF receptor 1 and VEGF receptor 2 are expressed on endothelial cells and provide survival signals to nearby tumor cells.13 PDGF is angiogenic for microvascular sprouting endothelial cells,14 and overexpression has been linked to the increased metastatic potential of HCC.15 Given that VEGF and PDGF expression is correlated with metastatic potential of tumor cells and the degree of microvessel density,15–17 inhibitors of VEGF and PDGF signaling are frequently applied agents for HCC.
Sorafenib is a multitargeted tyrosine kinase inhibitor that blocks the activity of Raf serine/threonine kinase isoforms, VEGFR-2 and -3, PDGFR β, c-KIT, FLT-3, and RET, to inhibit tumor angiogenesis and cell proliferation,18–20 and is currently the worldwide standard treatment for advanced HCC based on data from two large randomized trials, both showing an improvement in overall survival (OS) when compared with placebo.21–23
Linifanib (ABT-869) is a novel ATP-competitive inhibitor of all VEGF and PDGF receptor tyrosine kinases that lacks significant activity against representative cytosolic tyrosine kinases and serine/threonine kinases.24 In an open-label, phase II trial, linifanib demonstrated significant clinical activity as monotherapy in patients with advanced HCC.25 The independently assessed time to progression (TTP; median, 5.4 months) and OS (median, 9.7 months) among the trial population, 89% of whom were of Asian race, compared favorably with the corresponding efficacy outcomes for patients in the phase III sorafenib trial conducted in the Asia-Pacific region.23 On the basis of these results, we compared efficacy and tolerability of linifanib versus sorafenib in patients with advanced or metastatic HCC who had not received prior systemic therapy. Given the lack of established second-line treatment options in HCC, the primary end point of this study was OS. Secondary end points were TTP and objective response rate (ORR).
PATIENTS AND METHODS
Study Population
Patients ages ≥ 18 years with unresectable or metastatic HCC, Child-Pugh Class A liver function, Eastern Cooperative Oncology Group (ECOG) performance score (PS) of 0 to 1, adequate hepatic (bilirubin ≤ 3.0 mg/dL; ALT/AST ≤ 5× upper limit of normal [ULN]; albumin ≥ 2.8 g/dL; partial thromboplastin time ≤ 1.5× ULN; international normalized ratio < 1.5), hematologic (absolute neutrophil count [ANC] ≥ 1.0 × 109/L; platelets ≥ 50 × 109/L or ≥75 × 109/L with splenomegaly [per physical examination or reported on radiographic imaging]), and renal (creatinine ≤ 1.5× ULN) parameters, no prior systemic treatment for HCC, and a measurable lesion based on RECIST v1.126 were eligible. Eligibility criteria also included no prior local therapy (including liver-directed therapy) within 4 weeks before study drug administration and no radionuclide treatment within 6 months (or five half-lives); no evidence of untreated brain or meningeal metastases; no evidence of proteinuria at baseline; no symptomatic or persistent uncontrolled hypertension (> 140/90 mmHg); and patients could not be receiving therapeutic anticoagulation therapy or antiretroviral therapy for HIV.
Study Design and Treatment
This randomized, open-label phase III study was performed at 186 sites by 207 investigators in 28 countries worldwide. The study was approved by the institutional review board or independent ethics committee of each participating center and complied with the International Conference on Harmonization Good Clinical Practice guidelines and applicable local regulatory requirements. All patients provided written, informed consent.
Patients were randomly assigned in a 1:1 ratio to receive linifanib or sorafenib. Linifanib 17.5 mg was orally self-administered daily with at least 120 mL of water under fasting conditions in the evening. There were no scheduled dosing breaks; dose reductions or drug interruptions owing to study drug-related toxicities were allowed per discretion of the investigator. Sorafenib 400 mg was orally self-administered twice daily per the locally approved product label or applicable Summary of Product Characteristics.
Study visits were conducted weekly during the first 3 weeks and then on day 1 every 3 weeks thereafter (starting with week 4). Patients with controlled disease and with tolerable adverse effects received treatment until disease progression; unacceptable drug related toxicities; or until they required cancer-related surgery, radiation therapy, or alternate antineoplastic agents. If a dose reduction of linifanib was needed, the dose was decreased by 5 mg for the first reduction followed by 2.5 mg for all subsequent reductions. Sorafenib-related toxicities were managed according to the approved product label in that country.
Outcomes and Assessments
OS was defined as the number of days from the day the patient received random assignment to the date of the patient's death from any cause. TTP was defined as the number of days from the date of randomization to the date of earliest disease progression. Progression-free survival (PFS) was defined as the number of days from the date of randomization to the date the patient experienced an event of disease progression or death (all causes of mortality) if disease progression was not reached. ORR was defined as the proportion of patients with complete response (CR) or partial response (PR). TTP, PFS, and ORR were all based on RECIST, v1.1.26 Radiographic tumor assessments were performed at screening, every 6 weeks until week 42, and every 9 weeks thereafter. Treatment emergent adverse events (AEs) were summarized by system organ class and preferred term according to the Medical Dictionary for Regulatory Activities (MedDRA). In addition, AEs of hemorrhage were summarized based on a narrow standardized MedDRA query, excluding clinical laboratory terms. AE severity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). When an investigator determined that a patient should discontinue the study, a final visit was conducted. All patients were to have one follow-up visit approximately 30 days after the last dose of linifanib or sorafenib.
Statistical Methods
In our current study, both noninferiority and superiority hypotheses were tested. This design would allow for superiority assessment only if and after noninferiority was achieved. Because no single prior study of linifanib compared safety outcomes versus sorafinib, noninferiority testing was planned in case safety data favored linifanib. Assuming the true hazard ratio (HR) in favor of the linifanib group is 0.80, a total of 667 deaths would be needed for the study to have 80% power at a one-sided α level of 0.025 to detect a statistically significant treatment effect for the linifanib group using the log-rank test for OS. Two interim analyses, one for futility alone and one for both efficacy and futility, were performed and reviewed by an independent data monitoring committee when approximately 200 deaths (30% of the required number of events) and 333 deaths (50% of the required number of events) were observed, respectively. The Lan-DeMets alpha spending function with an O'Brien-Fleming boundary was to be used to ensure that the one-sided false positive rate would be 0.025 or less for OS.
Using a noninferiority test on the primary efficacy end point of OS with a noninferiority margin of 1.0491, the power of the study to declare noninferiority is tabulated in the Data Supplement for the same range of possible HRs (one interim analysis for futility alone and one interim analysis for both efficacy and futility are assumed when approximately 200 and 333 deaths occur, respectively). The power for HRs of 0.80, 0.82, and 0.85 was 93%, 88%, 80%, and 74%, respectively.
The distribution of OS was estimated for each treatment group using Kaplan-Meier methodology. Estimated median survival time and 95% CI for the estimated median survival time were presented for each treatment group. Both noninferiority and superiority hypotheses were tested for the primary efficacy end point of OS using the Cox proportional hazards model with treatment as a factor, stratified by region (outside Asia, Japan, or rest of Asia), ECOG PS (0 v 1), vascular invasion or extrahepatic spread (yes v no), and hepatitis B virus infection (yes v no). Within each stratum, a permutated-block randomization method was used to generate the patient randomization schedules. Noninferiority for OS was tested first with a margin value of 1.0491. If noninferiority was declared for OS, then superiority was to be tested for OS. The HR and the corresponding CI were estimated using the stratified Cox proportional hazards model. Additional details of the statistical methods are provided in the Data Supplement (online only).
After enrollment was complete, the data were analyzed by the independent data monitoring committee, at which time they recommended stopping the trial based on futility to show superiority (HR for OS, 0.989; 95% CI, 0.821 to 1.192; 455 OS events). The investigators were notified and the study was amended for early closure. To allow time to plan subsequent treatment, the remaining active patients (n = 184) continued on study drug per investigator discretion. The results presented are based on the final analysis as conducted per protocol at 667 OS events. The data cutoff was on May 31, 2012.
RESULTS
Study Conduct, Patients, and Treatment Administration
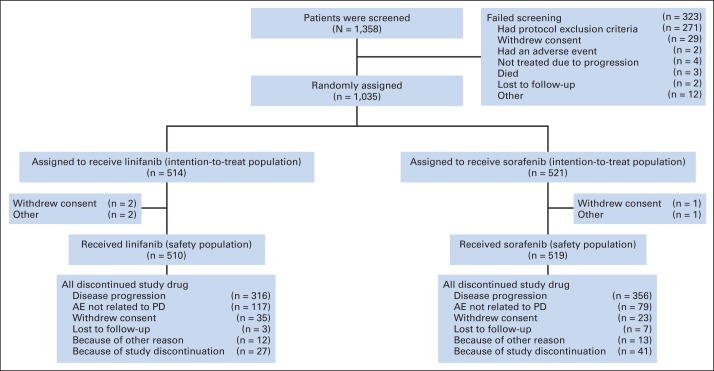
We randomly assigned 1,035 patients to receive linifanib 17.5 mg once daily (n = 514) or sorafenib 400 mg twice per day (n = 521). The efficacy population comprised all 1,035 patients (Fig 1). Six patients did not receive study medication and the remaining 1,029 patients (linifanib, n = 510; sorafenib, n = 519) received at least one dose of study medication and made up the safety analysis population (Fig 1). The treatment arms were well balanced for demographic, disease, prior treatment characteristics and stratification subgroups (Table 1). Overall, most patients were male (84.6%), Asian (66.6%), had HBV infection (53.2%), Child-Pugh Class A liver function (94.4%), ECOG PS 0 (64.4%), and Barcelona Clinic Liver Cancer stage C HCC (82.3%).
Fig 1.
CONSORT diagram. AE, adverse event; PD, progressive disease.
Table 1.
Baseline Patient and Disease Characteristics
| Characteristic | Linifanib (n = 514) |
Sorafenib (n = 521) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 59 | 60 | ||
| Range | 21-84 | 23-87 | ||
| Male sex | 444 | 86.4 | 436 | 83.7 |
| Region | ||||
| Outside Asia | 175 | 34.0 | 171 | 32.8 |
| Japan | 40 | 7.8 | 44 | 8.4 |
| Rest of Asia | 299 | 58.2 | 306 | 58.7 |
| Underlying risk factors for cirrhosis*† | ||||
| HBV | 275 | 53.5 | 276 | 53.0 |
| HCV | 130 | 25.3 | 129 | 24.8 |
| Alcohol cirrhosis | 66 | 12.8 | 63 | 12.1 |
| Hemochromatosis | 5 | 1.0 | 4 | 0.8 |
| Other | 103 | 20.0 | 116 | 22.3 |
| ECOG performance status† | ||||
| 0 | 323 | 62.8 | 344 | 66.2 |
| 1 | 191 | 37.2 | 176 | 33.8 |
| Child-Pugh class‡ | ||||
| A | 484 | 93.2 | 493 | 95.0 |
| B | 30 | 5.8 | 26 | 5.0 |
| BCLC stage | ||||
| B | 81 | 15.8 | 102 | 19.6 |
| C | 433 | 84.2 | 418 | 80.4 |
| Vascular invasion† | ||||
| Yes | 238 | 46.3 | 211 | 40.5 |
| Extrahepatic spread† | ||||
| Yes | 307 | 59.7 | 296 | 56.8 |
| No. of target lesions at baseline | ||||
| 1 | 129 | 24.8 | 111 | 21.4 |
| 2 | 233 | 46.5 | 247 | 47.7 |
| 3 | 70 | 13.7 | 103 | 19.9 |
| ≥ 4 | 75 | 14.6 | 57 | 11.0 |
| Sites of extrahepatic spread | ||||
| Lung | 172 | 33.5 | 152 | 29.2 |
| Lymph node | 142 | 27.6 | 132 | 25.3 |
| Brain | 1 | 0.2 | 0 | 0 |
| Bone | 40 | 7.8 | 52 | 10.0 |
| Peritoneum | 21 | 4.1 | 17 | 3.3 |
| Other | 54 | 10.5 | 52 | 10.0 |
| Prior locoregional therapies | ||||
| Yes | 233 | 45.3 | 241 | 46.3 |
| Prior oncology surgery | 157 | 30.1 | ||
| Yes | 153 | 29.1 | ||
| α-fetoprotein, ng/mL§ | ||||
| Median | 352 | 415 | ||
| > ULN | ||||
| No. of patients | 430 | 448 | ||
| % | 84.8 | 86.3 | ||
| Hepatitis B infection‖ | ||||
| No | 263 | 51.2 | 264 | 50.7 |
| Yes | 251 | 48.8 | 257 | 49.3 |
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; CRF, case report form; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; IVRS, interactive voice response system; ULN, upper limit of normal.
Patients with multiple hepatic histories were counted for each type of hepatic history.
Per electronic CRF.
Child-Pugh class was determined from assessments most proximal to the beginning of treatment. Per protocol, patients were Child-Pugh class A before randomization.
Percentages calculated on nonmissing values.
Data from IVRS, which were used for stratification. Other stratification factors from IVRS are shown in Figure 3.
Patients' mean duration of exposure to linifanib and sorafenib was 127.2 days (range, 2 to 775 days) and 127.8 days (range, 3 to 729 days), respectively (Appendix Table A1 [online only]). No statistically significant differences in duration of exposure were observed between treatment groups. Patients' mean daily dose was 13.7 mg for the linifanib arm (standard deviation, 4.42 mg) and 667.1 mg for the sorafenib arm (standard deviation, 164.36 mg). The mean linifanib dose-intensity was 78.2% and the mean sorafenib dose-intensity was 83.4%, which was significantly higher (P < .001). Of patients receiving linifanib or sorafenib, 55.9% and 40.8%, respectively, had a dose reduction. The most common reasons for study drug discontinuation were disease progression (linifanib arm, 68.6%; sorafenib arm 62.0%) and AEs not related to progressive disease (linifanib arm, 15.2%; sorafenib arm, 22.9%; Fig 1).
Efficacy
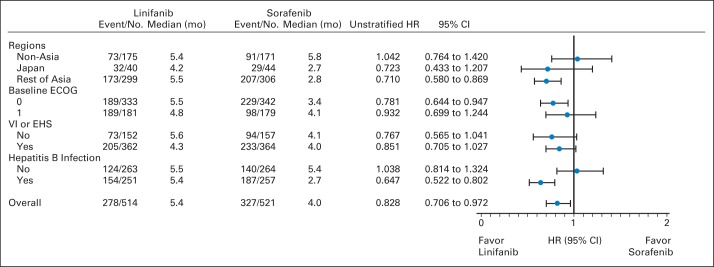
Median OS was 9.1 months (95% CI, 8.1 to 10.2) for patients receiving linifanib and 9.8 months (95% CI, 8.3 to 11.0) for patients receiving sorafenib (Fig 2). Compared with the sorafenib group, the median stratified HR was 1.046 (95% CI, 0.896 to 1.221) for the linifanib group (Fig 2). OS was not superior with linifanib treatment. Furthermore, the HR 95% CI upper limit of 1.221 did not meet the prespecified study definition of linifanib noninferiority (upper limit of HR 95% CI < 1.0491). We conducted analyses of OS by prespecified stratification subgroups (region: outside Asia, Japan, or rest of Asia; baseline ECOG PS: 0 v 1; vascular invasion or extrahepatic spread: yes v no; and HBV infection: yes v no). For all prespecified subgroups, the OS HRs ranged from 0.793 to 1.119 and the 95% CI contained 1.0 (Fig 3). Therefore, OS was similar across all prespecified subgroups. Overall death rates were similar between the linifanib group (66.5%) and sorafenib group (65.3%).
Fig 2.
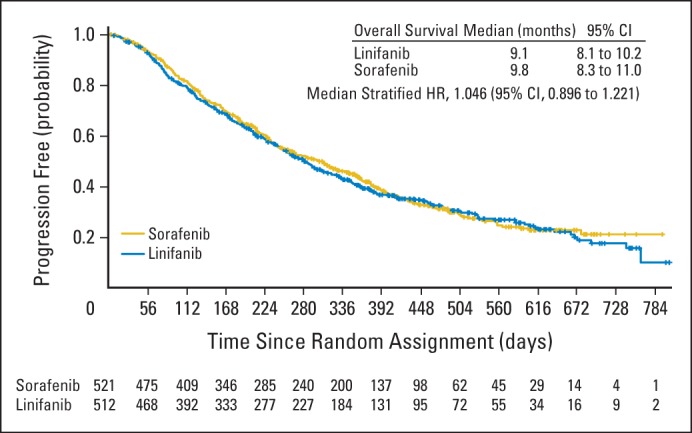
Kaplan-Meier analysis of overall survival with a cutoff point at the 667th patient death. HR, hazard ratio.
Fig 3.
Analysis of overall survival by prespecified stratification subgroups, per interactive voice response system for stratification factors. ECOG, Eastern Cooperative Oncology Group; EHS, extrahepatic spread; HR, hazard ratio; mo, months; VI, vascular invasion.
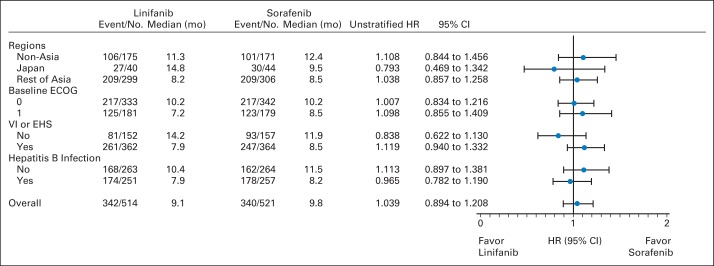
Median TTP was 5.4 months for patients receiving linifanib (95% CI, 4.2 to 5.6) and 4.0 months for patients receiving sorafenib (95% CI, 2.8 to 4.2; Fig 4). Compared with the sorafenib group, the stratified HR was 0.759 for the linifanib group (95% CI, 0.643 to 0.895), which was statistically significant (P = .001). The TTP advantage for linifanib was maintained until approximately 400 days of treatment; however, once the number of at-risk patients was reduced to fewer than 30 in each arm, the estimated probability of progression favored the sorafenib arm for the remaining patients. Median PFS was 4.2 months for patients receiving linifanib (95% CI, 4.1 to 5.4) and 2.9 months for patients receiving sorafenib (95% CI, 2.8 to 4.0). Compared with the sorafenib arm, the stratified HR was 0.813 for the linifanib arm (95% CI, 0.697 to 0.948), which was statistically significant (P = .008). For TTP in prespecified subgroups, the upper limit of the 95% CI of the HR for linifanib versus sorafenib was less than 1.0 in patients in Asia (excluding Japan) who had a baseline ECOG PS of 0 and HBV infection (Appendix Fig A1 [online only]). Therefore, TTP seemed to be significantly more favorable with linifanib than with sorafenib in these three prespecified subgroups; TTP was similar with either treatment in the other subgroups.
Fig 4.
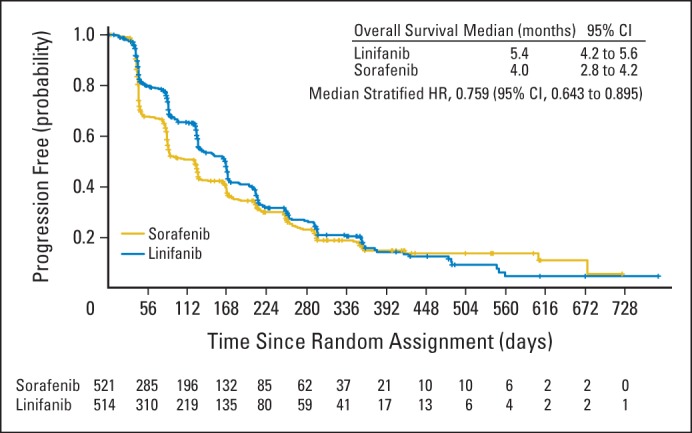
Kaplan-Meier analysis of time to progression. HR, hazard ratio.
A total of 67 patients receiving linifanib (13.0%) and 36 patients receiving sorafenib (6.9%) had a CR or PR per RECIST, v1.1 (Table 2). A total of 52 patients receiving linifanib (10.1%) and 32 patients receiving sorafenib (6.1%) had a confirmed ORR (CR or PR), which was significantly different between treatment groups (P = .018) (Table 2).
Table 2.
Secondary End Points: TTP or ORR
| End Point | Linifanib 17.5 mg Once Daily (n = 514) | Sorafenib 400 mg Twice per Day (n = 521) |
|---|---|---|
| TTP | ||
| Median | 5.4 | 4.0 |
| 95% CI | 4.2 to 5.6 | 2.8 to 4.2 |
| Disease progression events | ||
| No. of patients | 278 | 327 |
| % | 54.1 | 62.8 |
| HR | 0.759 | |
| 95% CI* | 0.643 to 0.895 | |
| P† | .001 | |
| Best response rate, %‡ | 13.0 | 6.9 |
Abbreviations: HR, hazard ratio; ORR, objective response rate; TTP, time to progression.
Stratified Cox proportional hazards model for comparison with sorafenib group.
Stratified log-rank test (two sided).
Confirmed response rates were 10.1% and 6.1%, respectively.
Safety
The overall safety summary and specific grade 3/4 AEs occurring in more than 3% of patients in either treatment arm are listed in Table 3. Grade 3/4 AEs; serious AEs; and AEs leading to discontinuation, dose interruption, or reduction were more frequent on the linifanib arm versus the sorafenib arm (all P < .001). Of the patients receiving sorafenib, 75.0% experienced a grade 3 AE or higher; of the patients receiving linifanib 85.3% did so. Grade 3/4 AEs that were observed more frequently on the linifanib arm than the sorafenib arm (P < .05) were hypertension (20.8% v 10.6%), fatigue (9.6% v 4.8%), hepatic encephalopathy (7.3% v 3.3%), asthenia (7.1% v 2.1%), ascites (6.1% v 3.3%), thrombocytopenia (5.3% v 2.1%), hypokalemia (4.7% v 2.3%), vomiting (4.3% v 0.8%), and hypoglycemia (3.1% v 0.8%). The only grade 3/4 AE observed more frequently on the sorafenib arm than the linifanib arm (P < .05) was increased ALT (4.8% v 2.2%). On the linifanib arm, 52.4% of patients experienced a serious AE and 36.3% of patients experienced an AE that led to discontinuation of study drug. On the sorafenib arm, 38.5% of patients experienced a serious AE and 25.4% of patients experienced an AE that led to discontinuation of study drug. An AE of hemorrhage (all-grade bleeding event) was experienced by 27.3% of patients receiving linifanib and 17.7% of patients receiving sorafenib, the most common of which were epistaxis and gingival bleeding.
Table 3.
Safety Summary
| Adverse Event | Linifanib 17.5 mg Once Daily (n = 510) |
Sorafenib 400 mg Twice per Day (n = 519) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Any AE | 508 | 99.6 | 511 | 98.5 |
| Any AE that could be related to SD | 483 | 94.7 | 481 | 92.7 |
| Any AE grade ≥ 3 | 435 | 85.3* | 389 | 75.0 |
| Any serious AE | 267 | 52.4* | 200 | 38.5 |
| Any AE leading to SD discontinuation | 185 | 36.3* | 132 | 25.4 |
| Any AE leading to SD interruption | 389 | 76.3* | 261 | 50.3 |
| Any AE leading to SD reduction | 231 | 45.3* | 162 | 31.2 |
| Any fatal AEs | 83 | 16.3 | 73 | 14.1 |
| Deaths | 351 | 68.8 | 338 | 67.1 |
| Grade 3 or 4 AEs occurring in > 3% of patients in either arm | ||||
| Hypertension | 106 | 20.8* | 45 | 10.6 |
| Palmar-plantar erythrodysesthesia syndrome | 70 | 13.7 | 77 | 14.8 |
| AST increased | 62 | 12.2 | 65 | 12.5 |
| Diarrhea | 61 | 12.0 | 48 | 9.2 |
| Fatigue | 49 | 9.6† | 25 | 4.8 |
| Hepatic encephalopathy | 37 | 7.3† | 17 | 3.3 |
| Asthenia | 36 | 7.1* | 11 | 2.1 |
| Hyperbilirubinemia | 32 | 6.3 | 21 | 4.0 |
| Ascites | 31 | 6.1† | 17 | 3.3 |
| Thrombocytopenia | 27 | 5.3† | 11 | 2.1 |
| Hypokalemia | 24 | 4.7† | 12 | 2.3 |
| Blood bilirubin increased | 23 | 4.5 | 18 | 3.5 |
| Abdominal pain | 23 | 4.5 | 14 | 2.7 |
| Decreased appetite | 22 | 4.3 | 13 | 2.5 |
| Vomiting | 22 | 4.3* | 4 | 0.8 |
| Neutropenia | 20 | 3.9 | 12 | 2.3 |
| Hyponatremia | 19 | 3.7 | 17 | 3.3 |
| Leukopenia | 18 | 3.5 | 12 | 2.3 |
| Platelet count decreased | 17 | 3.3 | 10 | 1.9 |
| Hypoglycemia | 16 | 3.1† | 4 | 0.8 |
| Anemia | 15 | 2.9 | 28 | 5.4 |
| ALT increased | 11 | 2.2 | 25 | 4.8† |
Abbreviations: AE, adverse event; SD, study drug.
P < .001 for comparison between the sorafenib and linifanib groups.
P < .05 for comparison between the sorafenib and linifanib groups.
DISCUSSION
Our phase III trial, comparing the efficacy of oral linifanib to that of sorafenib, failed to meet the primary end point: OS was not significantly different between the two treatments. Median OS was 9.1 months for linifanib (95% CI, 8.1 to 10.2) and 9.8 months for sorafenib (95% CI, 8.3 to 11.0). OS was similar across all prespecified subgroup analyses (region: outside Asia, Japan, or rest of Asia; baseline ECOG PS: 0 v 1; vascular invasion or extrahepatic spread: yes v no; and HBV infection: yes v no).
To date, sorafenib is the only approved systemic drug therapy for patients with advanced HCC. Based on the increasing knowledge of the large number of molecular pathways involved in HCC, numerous targets specific and/or broad spectrum tyrosine kinase inhibitors have been developed and tested in first- and second-line therapy. So far, the results have been, at best, somewhat disappointing. The phase III trial of sunitinib versus sorafenib was terminated early owing to safety and lack of efficacy (median OS, 7.9 v 10.2 months; HR, 1.30).27 The phase III trial of brivanib versus sorafenib also did not meet its primary end point of noninferiority in OS.28 In addition, brivanib also failed to improve OS when compared with placebo in second-line HCC treatment.29 Adding other tyrosine kinase inhibitors to sorafenib also failed, so far, to improve clinical outcomes; an example of this were the results of a study that explored adding erlotinib to sorafenib (median OS, 9.5 v 8.5 months; HR, 0.929).30 Whether tyrosine kinase inhibitors targeting the MET signal transduction pathway will improve outcome for patients with advanced HCC is currently being investigated in second-line treatment studies.31
The secondary objectives of our study were to assess the TTP and ORR of linifanib compared with sorafenib. Patients receiving linifanib had a significantly longer TTP than patients receiving sorafenib (P = .001). ORR was also significantly higher on linifanib compared with sorafenib (P = .018). The response rates according to RECIST v1.1 for linifanib (13.0%) and sorafenib (6.9%) each compare favorably to previous phase III trials of sorafenib in advanced HCC patients.21,23 Unfortunately, the improvements in TTP, PFS, and ORR did not translate to improvements in OS, a finding that has been reported with another antiangiogenic agent in the treatment of HCC.29 TTP was chosen as a key secondary end point to determine the effect of linifanib on tumor progression. The link between tumor progression and survival in HCC may be complicated because of the competing risk of death as a result of liver dysfunction.29
A greater portion of patients receiving linifanib than those receiving sorafenib experienced AEs that were grade ≥ 3; serious; or led to the reduction, interruption, or discontinuation of the study drug. The most frequent grade 3/4 AEs were hypertension and palmar-plantar erythrodysesthesia syndrome. The AEs reported in our study are similar to those seen in other studies of linifanib and with other agents in the VEGF/PDGF receptor tyrosine kinase inhibitor class.23,27,29
In summary, linifanib and sorafenib resulted in similar OS in advanced HCC. Predefined superiority and noninferiority OS boundaries were not met for linifanib, and the study failed to meet the primary end point. TTP and ORR favored linifanib whereas safety results favored sorafenib.
Supplementary Material
Acknowledgment
We thank the trial participants and site personnel who made this study possible. Qin Qin and Keith J. Gaddie provided data analysis support and editorial assistance, respectively. Both are employees of AbbVie.
Appendix
Table A1.
Summary of Drug Exposure
| Drug Exposure | Linifanib (n = 514) |
Sorafenib (n = 521) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Duration of study drug, days | ||||
| Mean | 127.2 | 127.8 | ||
| Median | 87 | 84 | ||
| Range | 2-775 | 3-729 | ||
| Duration, interval days | ||||
| > 0-21 | 77 | 15.1 | 54 | 10.4 |
| > 21-42 | 62 | 12.2 | 93 | 17.9 |
| > 42-63 | 55 | 10.8 | 83 | 16.0 |
| > 63-84 | 47 | 9.2 | 37 | 7.1 |
| > 84-105 | 48 | 9.4 | 42 | 8.1 |
| > 106 | 221 | 43.3 | 210 | 40.5 |
| Average daily dose, mg | ||||
| Mean | 13.7 | 667.1 | ||
| Median | 13.8 | 765.9 | ||
| Range | 3.2-70 | 200-800 | ||
| Dose-intensity, % | ||||
| Mean | 78.2 | 83.4 | ||
| Median | 78.8 | 95.7 | ||
| Range | 18.6-400 | 25-100 | ||
Fig A1.
Analysis of time to progression by prespecified stratification subgroups, per interactive voice response system for stratification factors. ECOG, Eastern Cooperative Oncology Group; EHS, extrahepatic spread; HR, hazard ratio; mo, months; VI, vascular invasion.
Footnotes
Supported by AbbVie.
Clinical trial information: NCT01009593.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Jiang Qian, AbbVie (C); Mark D. McKee, AbbVie (C); Justin L. Ricker, AbbVie (C); Dawn M. Carlson, AbbVie (C) Consultant or Advisory Role: Masatoshi Kudo, Kowa (C); Yoon-Koo Kang, Bayer (C), Novartis (C); Pei-Jer Chen, Bayer (C), Medigene (C), Traditional Chinese Medicine (C), Bristol-Myers Squibb (C), Roche (C), Janssen Pharmaceuticals (C) Stock Ownership: Jiang Qian, AbbVie; Mark D. McKee, AbbVie; Justin L. Ricker, AbbVie; Dawn M. Carlson, AbbVie Honoraria: Masatoshi Kudo, Bayer, Daiichi Sankyo, Merck Sharpe & Dohme, Chugai Pharmaceutical; Pei-Jer Chen, Bayer, Bristol-Myers Squibb, Roche, Merck, Gilead, Janssen Pharmaceuticals Research Funding: Masatoshi Kudo, Bayer; Yoon-Koo Kang, Bayer, Novartis; Pei-Jer Chen, Bristol-Myers Squibb, Roche, Janssen Pharmaceuticals Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jiang Qian, Justin L. Ricker, Dawn M. Carlson
Provision of study materials or patients: Calin Cainap, Hongming Pan, Ying Cheng, Yoon-Koo Kang, Vera Gorbunova
Collection and assembly of data: Shukui Qin, Wen-Tsung Huang, Ik Joo Chung, Hongming Pan, Ying Cheng, Masatoshi Kudo, Yoon-Koo Kang, Pei-Jer Chen, Han-Chong Toh, Vera Gorbunova, Ferry A.L.M. Eskens, Mark D. McKee, Justin L. Ricker, Dawn M. Carlson, Saied El-Nowiem
Data analysis and interpretation: Calin Cainap, Hongming Pan, Yoon-Koo Kang, Pei-Jer Chen, Jiang Qian, Mark D. McKee, Justin L. Ricker, Dawn M. Carlson, Saied El-Nowiem
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Cancer. http://www.who.int/mediacentre/factsheets/fs297/en/
- 3.Kirk GD, Lesi OA, Mendy M, et al. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004;39:211–219. doi: 10.1002/hep.20027. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn KA, Tsao L, Hsing AW, et al. International trends and patterns of primary liver cancer. Int J Cancer. 2001;94:290–296. doi: 10.1002/ijc.1456. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Global Cancer Facts and Figures 2012. http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf.
- 6.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Di Bisceglie AM, Bruix J, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol. 2008;48(suppl 1):S20–S37. doi: 10.1016/j.jhep.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Miura H, Miyazaki T, Kuroda M, et al. Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol. 1997;27:854–861. doi: 10.1016/s0168-8278(97)80323-6. [DOI] [PubMed] [Google Scholar]
- 12.Poon RT, Ho JW, Tong CS, et al. Prognostic significance of serum vascular endothelial growth factor and endostatin in patients with hepatocellular carcinoma. Br J Surg. 2004;91:1354–1360. doi: 10.1002/bjs.4594. [DOI] [PubMed] [Google Scholar]
- 13.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 14.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49:507–521. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T, Sun HC, Xu Y, et al. Overexpression of platelet-derived growth factor receptor alpha in endothelial cells of hepatocellular carcinoma associated with high metastatic potential. Clin Cancer Res. 2005;11:8557–8563. doi: 10.1158/1078-0432.CCR-05-0944. [DOI] [PubMed] [Google Scholar]
- 16.Tugues S, Fernandez-Varo G, Muñoz-Luque J, et al. Antiangiogenic treatment with sunitinib ameliorates inflammatory infiltrate, fibrosis, and portal pressure in cirrhotic rats. Hepatology. 2007;46:1919–1926. doi: 10.1002/hep.21921. [DOI] [PubMed] [Google Scholar]
- 17.Wada H, Nagano H, Yamamoto H, et al. Expression pattern of angiogenic factors and prognosis after hepatic resection in hepatocellular carcinoma: Importance of angiopoietin-2 and hypoxia-induced factor-1 alpha. Liver Int. 2006;26:414–423. doi: 10.1111/j.1478-3231.2006.01243.x. [DOI] [PubMed] [Google Scholar]
- 18.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst. 2006;98:326–334. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: A multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–844. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 20.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 21.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: Subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 23.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 24.Zhou J, Goh BC, Albert DH, et al. ABT-869, a promising multi-targeted tyrosine kinase inhibitor: From bench to bedside. J Hematol Oncol. 2009;2:33. doi: 10.1186/1756-8722-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toh HC, Chen PJ, Carr BI, et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer. 2013;119:380–387. doi: 10.1002/cncr.27758. [DOI] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Cheng AL, Kang YK, Lin DY, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J Clin Oncol. 2013;31:4067–4075. doi: 10.1200/JCO.2012.45.8372. [DOI] [PubMed] [Google Scholar]
- 28.Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31:3517–3524. doi: 10.1200/JCO.2012.48.4410. [DOI] [PubMed] [Google Scholar]
- 29.Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: Results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509–3516. doi: 10.1200/JCO.2012.47.3009. [DOI] [PubMed] [Google Scholar]
- 30.Zhu AX, Rosmorduc O, Evans J, et al. A phase III, randomized, double-blind, placebo-controlled trial of sorafenib plus erlotinib in patients with hepatocellular carcinoma (HCC). 37th European Society for Medical Oncology Congress; September 28-October 2, 2012; Vienna, Austria. (abstr 917) [Google Scholar]
- 31.Santoro A, Rimassa L, Borbath I, et al. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: A randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55–63. doi: 10.1016/S1470-2045(12)70490-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.