Abstract
Objective
The goal of this study was to evaluate whether protamine usage after carotid endarterectomy (CEA) increased within the Vascular Study Group of New England (VSGNE) in response to studies indicating that protamine reduces bleeding complications associated with CEA without increasing the risk of stroke.
Methods
We reviewed 10,059 CEAs, excluding concomitant coronary bypass, performed within the VSGNE from January 2003 to July 2012. Protamine use and reoperation for bleeding were evaluated monthly using statistical process control. Twelve centers and 77 surgeons entering the VSGNE between 2003 and 2008 were classified as original participants, and 14 centers and 60 surgeons joining after May 2009 were considered new. Protamine use for surgeons was categorized as rare (<10%), selective (10%-80%), or routine (>80%). Outcome measures were in-hospital reoperation for bleeding, postoperative myocardial infarction (POMI), and stroke or death.
Results
Two significant increases occurred in protamine use for all VSGNE centers over time. From 2003 to 2007, the protamine rate remained stable at 43%. In 2008, protamine usage increased to 52% (P< .01), coincident with new centers joining the VSGNE. Protamine usage then increased to 62% in 2010 (P< .01), shortly after the presentations of the data showing a benefit of protamine. This effect was due to 10 surgeons in the original VSGNE centers who increased their usage of protamine: six surgeons from rare use to selective use and four surgeons to routine use. Reoperation for bleeding was reduced by 0.84% (relative risk reduction, 57.2%) in patients who received protamine (0.6% vs 1.44%; P< .001). There were no differences in POMI (1.1% vs 1.09%) or stroke or death (1.1% vs 1.03%) between protamine treated and untreated patients, respectively. Reoperation for bleeding was decreased for surgeons who used protamine routinely (0.5%; P< .001) compared with selective (1.4%) and rare users (1.5%) of protamine. There were no differences in POMI (0.9%, 1.2%, 1.1%; P = .720) and stroke or death rates (1.0%, 1.2%, 1.0%; P = .656) for rare, selective, and routine users of protamine.
Conclusions
Protamine use increased over time by VSGNE surgeons, most significantly after the presentations of VSGNE-derived data showing the benefit of protamine, and was associated with a decrease in reoperation for bleeding. Improvements in processes of care and outcomes can be achieved in regional quality groups by sharing safety and efficacy data.
The Vascular Study Group of New England (VSGNE) was designed as a regional collaborative to improve vascular health care by collecting and analyzing data for key vascular procedures. Since its inception in 2003, VSGNE quality improvement initiatives have successfully improved the perioperative medical management of vascular patients with increased use of aspirin, β-blockers, and statins.1,2 The collaborative has monitored and improved the regional rate of patching during conventional carotid endarterectomy (CEA), which reduced the restenosis rate at the 1-year follow-up.3
VSGNE data have also provided a basis for substantial clinical research with a quality improvement focus, such as developing a cardiac risk prediction model to improve patient selection.4 Other research studies have used the power of this large database to answer clinical questions such as whether protamine should be administered during CEA.5 This study showed a threefold reduction in reoperation for bleeding, with no increase in potential thrombotic complications, a finding that required a data set of >4000 patients to prove significance.
An unanswered question is whether and how rapidly such a finding might change clinical practice. Results concerning protamine use during CEA were presented by Stone et al5 at a semiannual VSGNE meeting in April 2009, at the Society for Vascular Surgery (SVS) plenary session in June 2009, and were published in the Journal of Vascular Surgery in March 2010. The purpose of our current study was to determine whether this information would increase protamine usage during CEA in a region where the practice pattern was evenly split before these presentations. Secondarily, we sought to confirm the beneficial effects of protamine during CEA in a patient cohort more than twice as large as the original study.
METHODS
Patients and database
We reviewed 10,059 CEAs performed in 9260 patients in the VSGNE from January 2003 to July 13, 2012. During the study period, 787 patients had two CEAs and six patients had three CEAs. Among the study patients, carotid disease was asymptomatic in 6034 (60%) and symptomatic in 4025 (40%). The analysis did not include 235 patients who had a concomitant coronary artery bypass procedure. From 2003 to 2012, there were 26 centers and 137 surgeons in the VSGNE. Of these, 12 centers and 77 surgeons were categorized as “original” because they joined the VSGNE before 2008, and 14 centers and 60 surgeons were categorized as “new” because they joined the VSGNE after 2008.
Study end points
Protamine administration intrao-peratively or after CEA was recorded in the registry. Four patients were excluded from analysis due to lack of protamine data. Surgeons were categorized into three groups according to their practice pattern of protamine use: (1) “rare” if protamine was used in <10% of CEAs; (2) “selective” if protamine was used in 10% to 80% of CEAs; or (3) “routine” if protamine was used in >80% of the patients. Surgeons who performed <10 CEAs were excluded from analysis of protamine use practice pattern. The registry captures the use of heparin and protamine but does not record dosages. Heparin activity, as measured by partial thromboplastin time or activated clotted time, is not recorded in the registry.
The primary end point was the rate of protamine use during CEA. Secondary end points included reoperation for bleeding after CEA, postoperative myocardial infarction (POMI), and the combined outcome of any stroke or death during the same hospitalization. POMI was defined as a troponin elevation according to individual center criteria, electrocardiogram with new Q waves, new ST or T-wave changes, or documentation by clinical criteria or echocardiogram or other imaging modality. Stroke severity was defined as major if it resulted in blindness or disability or caused nonindependent living and as minor if symptoms were nondisabling. Bleeding after CEA was captured as reoperation for bleeding. The registry does not record re-exploration for bleeding after skin closure but before leaving the operating room or more minor neck hematomas that did not require reoperation. The VSGNE also records reoperation for a neurologic event, which was not examined in this study. Use of drains was not included in the analysis because these data were only recorded in the VSGNE after 2010.
Intervention
The benefits of protamine were previously presented at the VSGNE semiannual meeting in April 2009 and the Vascular Annual Meeting in June 2009 and later reported by Stone et al in the Journal of Vascular Surgery publication in March 2010.5 However, no formal VSGNE quality improvement interventions were undertaken to increase the use of protamine, such as team meetings or plan, do, study, act cycles. Further, feedback on protamine use was not provided to individual surgeons or centers during the time period included in this study.
Statistical analysis: statistical process control
The protamine usage rate was evaluated monthly in a statistical process control (SPC) chart. A P chart was used because the total number of procedures per month varied from 46 to 167 over time, as more centers joined the VSGNE. SPC charts distinguish special cause variation from random variation.6
If a process is “stable,” then the protamine usage rate will vary between the upper and lower control limits, which are set at 3 sigma (~3 standard deviations from the mean). Any increase or decrease in the rate of protamine usage within this range is considered random variation. If a significant change occurs in the system, then the SPC chart indicates special cause variation. Although there are various definitions for determining a special cause variation, we used the commonly accepted criterion of 8 or more consecutive points above the central line. A shift in the data to this degree has a probability of less than 0.01. The central line was established and fixed using the first 2 years of data collection (number of procedures using protamine divided by total number of procedures). SPC charts were used to evaluate protamine usage rate for the all centers combined and the original centers alone. Separate SPC charts were constructed for surgeons with rare and selective protamine use. Reoperation for bleeding was also measured with a P chart.
For each of the surgeons’ protamine usage categories, the χ2 test was used to determine whether there was a difference in protamine usage between original and new centers for procedures done in 2010 or later. The χ2 test was also used to determine whether the three outcome variables (reoperation for bleeding, POMI, and stroke or death) were different if protamine was used. A P value of ≤ .05 was considered statistically significant. Statistical analysis was performed using STATA 12.0 software (Stata-Corp LP, College Station, Tex).
RESULTS
Protamine use
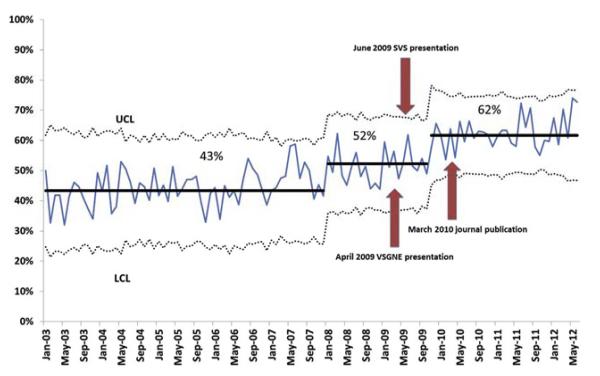
Over the entire study from 2003 to 2012, protamine was used during 53% of CEAs. As shown in Fig 1, protamine use remained stable from 2003 through 2007 at 43%. Beginning in January 2008, there was a significant increase in protamine use to 52% (P < .01). This was due to new centers and surgeons joining the VSGNE with a higher than average protamine usage rate. By November 2009, ~6 months after two VSGNE presentations reporting the benefit of protamine to reduce reoperation for bleeding, the rate of protamine use showed a special cause increase to 62% (P < .01) and remained elevated through the end of the study. To account for the possible confounding effects of new centers entering the VSGNE, we performed a separate P chart analysis of only the original centers and surgeons. The results were similar, with protamine use increasing to 50% in early 2008 and to 63% starting after April 2010 following the presentations and journal article (Supplementary Fig, online only). We compared cohorts from 2003 to 2008 and from 2008 to 2012 and found similar patient and operative characteristics, except for a higher rate of patients aged >80 years, lower rate of symptomatic carotid disease, and fewer eversion CEAs in the latter group (Supplementary Table, online only). The rate of patch angioplasty, which has been associated with a decrease in reoperation for bleeding, and use of perioperative antiplatelet agents was similar.
Fig 1.
Statistical process control (SPC) chart shows protamine use during carotid endarterectomy (CEA) for all surgeons participating in the Vascular Study Group of New England (VSGNE) from January 2003 to July 2012. LCL, Lower control limit; SVS, Society for Vascular Surgeons; UCL, upper control limit.
Rare, selective, and routine protamine use by surgeons at the original VSGNE centers
During the entire study duration, 77 surgeons from the 12 original VSGNE centers performed 8372 CEAs (83%); of these, 14 performed <10 procedures and were not categorized into protamine usage groups. Twenty-five surgeons were “routine” protamine users and performed 44% of the procedures. Their protamine usage rate was ≥94% throughout the study. Nineteen surgeons were “selective” protamine users and performed 25% of the procedures. Their protamine usage rate was 15% until 2009, when it increased to >50% through 2012. Nineteen surgeons were “rare” protamine users and performed 31% of the procedures. Their protamine usage rate was stable at 4% from until 2009, but it increased significantly to 10% by August 2010, after VSGNE presentations and publication by Stone et al.5 By analysis of individual surgeon practice, 10 of the original 77 surgeons in the VSGNE significantly increased their use of protamine. Of these, six surgeons went from rare to selective use and four went from rare or selective use to routine use.
For the 10 surgeons (improvers), protamine usage increased from 7% to 49% and their rate of reoperation for bleeding was reduced from 1.8% to 1% (Fig 2). This difference was not statistically significant, likely due to a small sample size with a power of .31 at α = .05. From 2003 to 2008, the improvers’ reoperative rate was not significantly different from the surgeons (n = 10) who rarely used protamine (1.8% vs 1.6%; Fisher exact test P=.115). This is an expected finding because both groups rarely used protamine during this time frame.
Fig 2.
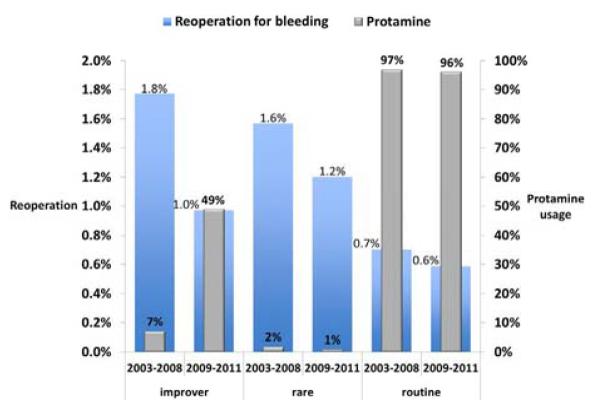
Reoperation for bleeding among the 10 improvers compared to surgeons with rare and routine protamine use.
From 2003 to 2008, the improvers’ rate of reoperation for bleeding was significantly higher than the 17 surgeons who used protamine routinely (1.8% vs 0.7%; P = .012). From 2009 to 2011, the improvers’ reoperative rate was reduced to 1%, which was not statistically significantly different (P = .08). Notably, these surgeons’ protamine usage (49%) did not reach the level of surgeons who routinely used protamine (96%).
Secondary outcome measures
Of patients who did not receive protamine during CEA, 1.4% required reoperation for bleeding compared with only 0.6% of patients who received protamine (P < .001). In contrast, protamine use had no effect on the rate of POMI (1.1% with, 1.1% without protamine; P > .99) or stroke or death (1.1% with, 1.0% without protamine; P = .740). There were no differences in stroke or death rates in patients with symptomatic carotid disease based on protamine use (1.3% rare, selective 1.4%, routine 1.5%; P = .412).
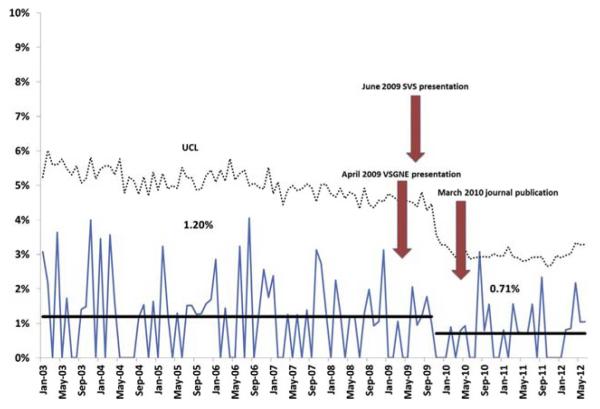
Surgeons using protamine routinely had the lowest rate of reoperation for bleeding, at 0.5% (Table). There was no difference in the rate of POMI or stroke or death between rare, selective, and routine users of protamine. Using SPC, we identified a decrease in the rate of reoperation for bleeding after CEA from 1.2% to 0.7% beginning in the fourth quarter of 2009 (Fig 3), after the presentation by Stone et al at VSGNE and SVS Vascular Annual Meeting.5
Table.
Postoperative complications stratified by surgeon protamine use
|
Reoperation for bleeding (P < .001a) |
POMI (P = .720) |
Stroke or death (P = .656) |
||||
|---|---|---|---|---|---|---|
|
No
|
Yes
|
No
|
Yes
|
No
|
Yes
|
|
| Protamine use categories | No. (%) | No. (%) [95% CI] | No. (%) | No. (%) [95% CI] | No. (%) | No. (%) [95% CI] |
| Rare(<10%) | 2706 (98.5) | 40 (1.5) [29-54] | 2718 (99.1) | 26 (0.9) [17-38] | 2719(99) | 27 (1.0) [18-39] |
| Selective (10% to 80%) | 2554 (98.6) | 35 (1.4) [24-49] | 2559 (98.8) | 30 (1.2) [20-43] | 2558 (98.8) | 31 (1.2) [21-44] |
| Routine (>80%) | 4545 (99.5) | 24 (0.5) [15-36] | 4517 (98.9) | 51 (1.1) [38-67] | 4524 (99) | 45 (1.0) [32-60] |
CI, Confidence interval; POMI, postoperative myocardial infarction.
χ2
Fig 3.
Statistical process control (SPC) chart shows reoperation for bleeding after carotid endarterectomy (CEA) for all surgeons participating in the Vascular Study Group of New England (VSGNE) from January 2003 to July 2012. SVS, Society for Vascular Surgeons; UCL, upper control limit.
DISCUSSION
This study reports an increase in the use of protamine during CEA coinciding with presentations and a publication of VSGNE-derived data documenting the safety and benefits of heparin reversal. Stone et al5 reviewed 4587 CEAs within the VSGNE and found no differences in stroke rates (0.78% vs 1.15%; P = .2), myocardial infarction (1.1% vs 0.91%; P = .51), or death (0.23% vs 0.32%; P = .57), with a decrease in reoperation for bleeding (0.64% vs 1.66%; P = .001) in treated and untreated patients, respectively.5 This study extends those findings, showing an absolute risk reduction of 0.84% and relative risk reduction of 57.2% in reoperation for bleeding in a large cohort more than double the original population.
On the basis of the prior study, we examined surgeon practice patterns and outcomes. We used an SPC chart to examine protamine use over time. The temporal association of the increase in protamine use with the Stone et al5 data strongly suggests a direct link between the reporting of shared data and changes in individual surgeon practice patterns. To further validate this conclusion, we were able to contact nine of 10 surgeons who increased their protamine usage to ask them what influenced their change in protamine usage. Attempts at a more formal survey of all surgeons were invalid due to a low response rate. All nine surgeons responded that this was due to the presentations or the journal article, or both. One quotation from this electronic survey was, “The change was related to the paper out of the VSGNE that showed benefit in decreased bleeding, return to OR, LOS, etc, without any significant increased risk associated with use.”
Unlike our prior quality improvement projects to increase perioperative medication usage, we did not identify bleeding after CEA as a specific quality improvement project and did not recommend a threshold for protamine use. A formal quality improvement team was not formed, and tests of change were not conducted. Instead, the simple reporting of the benefit and safety of protamine appears responsible for a measurable practice change. This rapidity of this change was remarkable.
Research has shown that rapid translation of clinical research findings into current practice is difficult.7-9 The method by which clinicians translate research into practice has been described as a process of awareness to acceptance to adoption.9 Traditionally, scientific meetings and journals have focused on continuing medical education to promote awareness and acceptance, with little attention to adoption. Numerous strategies have been espoused to improve the adoption of clinical research. These include the dissemination of practice guidelines by opinion leaders or professional societies, “coproduction” of knowledge, auditing with performance feedback, computerized decision support, and academic detailing.7,8,10,11 Coproduction of research has been described as a partnership between academic and clinical staff to design research studies immediately relevant to practice.10 Academic detailing refers to personalized education often occurring directly in the practice setting. Because change in practice is complex, the most successful initiatives combine multiple types of interventions to affect change.
The coproduction of knowledge strategy is inherent within the VSGNE. Our working theory is that surgeons who contribute to the VSGNE feel “ownership” of the data and are thus more likely to respond to the data with practice change. The VSGNE also uses academic detailing through direct contact among surgeons in the region. Our hypothesis is supported by the collaborative work of the Northern New England Cardiovascular Disease Study Group, which reduced mortality after coronary artery bypass grafting by providing feedback from outcomes data, training in continuous quality improvement techniques, and site visits. Postoperative mortality decreased by 24%, demonstrating that such efforts may have a direct effect on practice patterns and can improve patient outcomes.12 Our findings should encourage regional quality groups to use SPC methodology in real time and share these results with their members.
From a scientific standpoint, our current study reinforces the initial VSGNE conclusions by Stone et al5 in a study population of now >10,000 CEAs. This conclusive demonstration of a benefit of protamine without thrombotic complication is important, because several early retrospective studies and small, randomized trials reported an association between protamine use and an increase in postoperative stroke.13-15 Other literature, including a report from the General Anaesthetic vs Local Anaesthetic for Carotid Surgery (GALA) Trial, has shown conflicting evidence, with reduction in hematoma when using protamine but no differences in reoperation rates.16-17
The rate for returning to the operating room for bleeding in our study was 1.4% for those who did not receive protamine. For patients who received protamine, the reoperation for bleeding was significantly reduced to 0.6%, with the lowest bleeding rates (0.4%) attributed to surgeons who routinely used protamine. The need for reoperation in even a small percentage of our patients is not without consequences. Stone et al5 found that these patients have a higher incidence of clinically significant POMI, stroke, and in-hospital mortality. We estimate that the number needed to treat is 111 patients to prevent one reoperation for bleeding.
The National Hospital Discharge Survey documented 140,000 CEAs were performed in 2009.18 National data on protamine use during CEA are not available. Survey data from the SVS have estimated protamine use at between 48% and 54%.19,20 Extrapolating from these data, ~64,400 to 72,800 patients did not receive protamine during CEA. Administration of protamine to the remaining patients could potentially prevent 540 to 610 reoperations for bleeding each year and the adverse outcomes associated with reoperation. Data on protamine use from the Vascular Quality Initiative will help clarify these hypothetical calculations. Although we did not consider cost, at an expense of $0.56-$0.71/mg, protamine is likely very cost effective.
Few nationally endorsed quality indicators exist for vascular surgery. Metrics from the Surgical Care Improvement Project focus mainly on prophylactic antibiotic use.21 Because infection is a rare complication of CEA, other metrics are required to better assess the quality of care. Mortality and volume are under consideration as quality measures by the Agency for Healthcare Research and Quality.22 Although use of patch angioplasty was recommended based on grade 1/level A evidence in the SVS practice guidelines for the management of extracranial carotid disease, patching is not a quality measure for conventional CEA.23 Protamine use was not addressed in this SVS document nor by the European Society for Vascular Surgery guidelines.24 We believe that protamine reversal of heparin during CEA is also a useful quality measure.
This study has some limitations. Although the VSGNE captures an extensive number of data points, other cofounding variables not collected by the registry could have affected the rate of reoperation for bleeding. We recognize that other factors could contribute to the increase in use of protamine in the VSGNE, such as discussions within a practice group that reveal variation in practice. However, our query of the surgeons who increased protamine usage confirmed that the Stone el al presentations or the journal article, or both, prompted their change, despite previous controversy in the literature about this subject.
Fewer symptomatic patients underwent CEA in the 2008 to 2012 cohort, but it is unlikely this masked any deleterious effect of protamine, given the nearly identical rates of stroke and death between protamine treatment groups. We analyzed the effect of protamine on significant postoperative hemorrhage, POMI, and stroke or death, but did not have data on other adverse reactions such as anaphylaxis, pulmonary hypertension, and systemic hypotension. However, we know that such complications were not severe enough to influence mortality. We also could not account for the dosing of heparin or protamine used by surgeons. However, such dosing likely varies widely, which provides even more support for the beneficial effect of protamine.
CONCLUSIONS
Protamine use increased over time by VSGNE surgeons due to the presentations of VSGNE-derived data showing the benefit of protamine. The increase in protamine use was associated with a decrease in reoperation for bleeding after CEA, without an increase in the incidence of stroke or POMI. This demonstrates that improvements in a process of care leading to improved outcomes can be achieved in regional quality groups by sharing safety and efficacy data.
Supplementary Material
Supplementary Fig (online only). Statistical process control (SPC) chart shows protamine use during carotid endarterectomy (CEA) by surgeons from original participating centers from January 2003 to July 2012. LCL, Lower control limit; SVS, Society for Vascular Surgeons; UCL, upper control limit; VSGNE, Vascular Study Group of New England.
Supplementary Table (online only). Patient and operative characteristics over time
Footnotes
Author conflict of interest: none.
Presented at the Thirty-ninth Annual Meeting of the New England Society for Vascular Surgery, Boston, Mass, September 21-23, 2012.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
AUTHOR CONTRIBUTIONS Conception and design: DB, BP, AS
Analysis and interpretation: DB, PB, KH, PG, JC, DS
Data collection: DB, RP, KH, PB
Writing the article: DB, RP, JC
Critical revision of the article: DB, RP, JC, KH, JC, DS
Final approval of the article: DB, BP, JC, KH, PG, DS
Statistical analysis: KH, PB
Obtained funding: Not applicable
Overall responsibility: DB
REFERENCES
- 1.Cronenwett J, Likosky D, Russell M, Eldrup-Jorgensen J, Stanley A, Nolan B. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. discussion: 1101-2. [DOI] [PubMed] [Google Scholar]
- 2.Goodney PP, Eldrup-Jorgensen J, Nolan BW, Bertges DJ, Likosky DS, Cronenwett JL. The Vascular Study Group of New England: a regional quality improvement effort to increase beta blocker administration before vascular surgery. J Vasc Surg. 2011;53:1316–28. doi: 10.1016/j.jvs.2010.10.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodney PP, Nolan BW, Eldrup-Jorgensen J, Likosky DS, Cronenwett JL. Vascular Study Group of Northern New England. Restenosis after carotid endarterectomy in a multicenter regional registry. J Vasc Surg. 2010;52:897–904. 905.e1–2. doi: 10.1016/j.jvs.2010.05.005. discussion: 904-5. [DOI] [PubMed] [Google Scholar]
- 4.Bertges DJ, Goodney PP, Zhao Y, Schanzer A, Nolan BW, Likosky DS, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg. 2010;52:674–83. doi: 10.1016/j.jvs.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 5.Stone DH, Nolan BW, Schanzer A, Goodney PP, Cambria RA, Likosky DS, et al. Vascular Study Group of Northern New England: protamine reduces bleeding complications associated with carotid endarterectomy without increasing the risk of stroke. J Vasc Surg. 2010;51:559–64. 564.e1. doi: 10.1016/j.jvs.2009.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan CD, Griffen DL. Advanced statistics: applying statistical process control techniques to emergency medicine: a primer for providers. Acad Emerg Med. 2003;10:883–90. doi: 10.1111/j.1553-2712.2003.tb00634.x. [DOI] [PubMed] [Google Scholar]
- 7.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362:1225–30. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 8.Davis DA, Taylor-Vaisey A. Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ. 1997;157:408–16. [PMC free article] [PubMed] [Google Scholar]
- 9.Green LA, Seifert CM. Translation of research into practice: why we can’t “just do it.”. J Am Board Fam Pract. 2005;18:541–5. doi: 10.3122/jabfm.18.6.541. [DOI] [PubMed] [Google Scholar]
- 10.Rowley E, Morriss R, Currie G, Schneider J. Research into practice: Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for Nottinghamshire, Derbyshire, Lincolnshire (NDL) Implement Sci. 2012;7:40. doi: 10.1186/1748-5908-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rowley E. Protocol for a qualitative study exploring the roles of ‘Diffusion Fellows’ in bridging the research to practice gap in the Nottinghamshire, Derbyshire and Lincolnshire Collaboration for Leadership in Applied Health Research and Care (CLAHRC-NDL) BMJ Open. 2012;2:e000604. doi: 10.1136/bmjopen-2011-000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor GT, Plume SP, Olmstread EM, Mortin JR, Maloney CT, Nugent WC, et al. A regional intervention to improve the hospital mortality associated with coronary artery bypass graft surgery. JAMA. 1996;275:841–6. [PubMed] [Google Scholar]
- 13.Mauney MC, Buchanan SA, Lawrence WA, Bishop A, Sinclair K, Daniel TM, et al. Stroke rate is markedly reduced after carotid endarterectomy by avoidance of protamine. J Vasc Surg. 1995;2:264–9. doi: 10.1016/s0741-5214(95)70140-0. discussion: 269-70. [DOI] [PubMed] [Google Scholar]
- 14.Fearn SJ, Parry AD, Picton AJ, Mortimer AJ, McCollum CN. Should heparin be reversed after carotid endarterectomy? A randomized prospective trial. Eur J Vasc Endovasc Surg. 1997;13:394–7. doi: 10.1016/s1078-5884(97)80082-2. [DOI] [PubMed] [Google Scholar]
- 15.Levison JA, Faust GR, Halpern VJ, Theodoris A, Nathan I, Kline RG, et al. Relationship of protamine dosing with postoperative complications of carotid endarterectomy. Ann Vasc Surg. 1999;13:67–72. doi: 10.1007/s100169900222. [DOI] [PubMed] [Google Scholar]
- 16.Treiman RL, Cossman DV, Foran RF, Levin PM, Cohen JL, Wagner WH. The influence of neutralizing heparin after carotid endarterectomy on postoperative stroke and wound hematoma. Vasc Surg. 1990;12:440–5. discussion: 445-6. [PubMed] [Google Scholar]
- 17.Dellagrammaticas D, Lewis SC, Gough MJ. GALA Trial Collaborators. Is heparin reversal with protamine after carotid endarterectomy dangerous? Eur J Vasc Endovasc Surg. 2008;36:41–4. doi: 10.1016/j.ejvs.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention [Accessed March 10, 2013];National hospital discharge survey. 2009 Available at: http://www.cdc.gov/nchs/nhds.htm.
- 19.Quiroz EM, Milner R. A national survey of the use of anti-platelet medications and protamine during carotid endarterectomy and carotid artery stenting: what is the standard? J Vasc Surg. 2011;53:557–8. [Google Scholar]
- 20.Wakefield TW, Lindbald B, Stanley TJ, Nichol BJ, Stanley JC, Bergqvist D, et al. Heparin and protamine use in peripheral vascular surgery: a comparison between surgeons of the Society for Vascular Surgery and the European Society for Vascular Surgery. Eur J Vasc Surg. 1994;8:193–8. doi: 10.1016/s0950-821x(05)80459-1. [DOI] [PubMed] [Google Scholar]
- 21.The Joint Commission [Accessed March 10, 2013];Surgical care improvement project. Available at: http://www.jointcommission.org/surgical_care_improvement_project/
- 22.Agency for Healthcare Research and Quality [Accessed March 10, 2013];National Quality Measures Clearinghouse. Available at: http://www.qualitymeasures.ahrq.gov/browse/in-progress.aspx.
- 23.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease: executive summary. J Vasc Surg. 2011;54:832–6. doi: 10.1016/j.jvs.2011.07.004. [DOI] [PubMed] [Google Scholar]; J Vasc Surg. 2012;55:894. Erratum in: [Google Scholar]
- 24.Liapis CD, Bell PR, Mikhailidis D, Sivenius J, Nicolaides A, Fernandes e Fernandes J, et al. ESVS Guidelines Collaborators. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009;37(4 Suppl):1–19. doi: 10.1016/j.ejvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig (online only). Statistical process control (SPC) chart shows protamine use during carotid endarterectomy (CEA) by surgeons from original participating centers from January 2003 to July 2012. LCL, Lower control limit; SVS, Society for Vascular Surgeons; UCL, upper control limit; VSGNE, Vascular Study Group of New England.
Supplementary Table (online only). Patient and operative characteristics over time