Abstract
Background
The autogenous vein is the preferred conduit in below-knee vascular reconstructions. However, many argue that prosthetic grafts can perform well in crural bypass with adjunctive antithrombotic therapy. We therefore compared outcomes of below-knee prosthetic versus autologous vein bypass grafts for critical limb ischemia and the use of adjunctive antithrombotic therapy in both settings.
Methods
Utilizing the registry of the Vascular Study Group of New England (2003–2009), we studied 1227 patients who underwent below-knee bypass for critical limb ischemia, 223 of whom received a prosthetic graft to the below-knee popliteal artery (70%) or more distal target (30%). We used propensity matching to identify a patient cohort receiving single-segment saphenous vein yet had remained similar to the prosthetic cohort in terms of characteristics, graft origin/target, and antithrombotic regimen. Main outcome measures were graft patency and major limb amputation within 1 year. Secondary outcomes were bleeding complications (reoperation or transfusion) and mortality. We performed comparisons by conduit type and by antithrombotic therapy.
Results
Patients receiving prosthetic conduit were more likely to be treated with warfarin than those with greater saphenous vein (57% vs. 24%, P < 0.001). After propensity score matching, we found no significant difference in primary graft patency (72% vs. 73%, P = 0.81) or major amputation rates (17% vs. 13%, P = 0.31) between prosthetic and single-segment saphenous vein grafts. In a subanalysis of grafts to tibial versus popliteal targets, we noted equivalent primary patency and amputation rates between prosthetic and venous conduits. Whereas overall 1-year prosthetic graft patency rates varied from 51% (aspirin + clopidogrel) to 78% (aspirin + warfarin), no significant differences were seen in primary patency or major amputation rates by antithrombotic therapy (P = 0.32 and 0.17, respectively). Further, the incidence of bleeding complications and 1-year mortality did not differ by conduit type or antithrombotic regimen in the propensity-matched analysis.
Conclusions
Although limited in size, our study demonstrates that, with appropriate patient selection and antithrombotic therapy, 1-year outcomes for below-knee prosthetic bypass grafting can be comparable to those for greater saphenous vein conduit.
INTRODUCTION
Most vascular surgeons agree that the type of conduit and the site of distal anastomosis are the most relevant predictors of infrainguinal bypass patency.1 Autogenous single-segment vein grafts generally maintain superior patency compared with prosthetic conduits.2,3 Short-segment prosthetic bypasses to above-knee targets can approach the 66–80%4 5-year patency rates obtained with long-segment saphenous vein grafts.5-7 Unfortunately, longer prosthetic grafts do not always perform as well as noted in several retrospective series.8-10
However, many patients with long-segment occlusions lack adequate saphenous vein, either due to inadequate size or because it has been previously harvested, and are thus faced with receiving a less-than-ideal bypass conduit. Accordingly, the adjunctive use of antithrombotic therapy has been repeatedly proposed to improve outcomes in high-risk grafts. For example, the Antiplatelet Trialists Collaboration11 and others12 showed that single antiplatelet therapy (acetylsalicylic acid) was associated with a 43% relative risk reduction of graft occlusion. For prosthetic grafts that cross the knee joint, a recently published randomized trial found that the addition of clopidogrel to aspirin contributed an additional 37% relative risk reduction of graft occlusion.13 Similarly, therapeutic anticoagulation with warfarin may provide a protective effect for prosthetic infrainguinal bypass grafts, especially to infrageniculate targets.14,15
Although antithrombotic adjuncts have shown promise in selected settings, their benefit in real-world practice for patients who undergo lower extremity bypass to below-knee targets remains uncertain. We therefore studied patients prospectively tracked by the Vascular Study Group of New England who underwent infrageniculate bypass with either single-segment saphenous vein or prosthetic conduit for critical limb ischemia and who were simultaneously treated with various antithrombotic regimens. In this manner, we sought to elucidate the real-world use and impact of such adjunctive treatment on patient and graft outcomes at 1 year.
METHODS
Database and Subjects
For this study, we utilized data from the Vascular Study Group of New England (VSGNE).16 The institutional review board at Dartmouth Medical School reviewed and approved this study. Patients underwent surgery by 71 surgeons at 14 academic and community medical centers. Patient-level and operative data were abstracted by trained physicians, nurses, or data-entry personnel before surgery, at hospital discharge from surgery, and at 1-year follow-up. The registry is audited for completeness of procedural inclusion at each center on a semiannual basis.
Constructing the Cohort of Patients Undergoing Below-knee Bypass
We included patients who underwent an open infrainguinal bypass procedure for critical limb ischemia (ischemic rest pain and/or tissue loss) between January 1, 2003 and December 31, 2009. Our analysis was limited to patients whose graft origins were the iliac, femoral, or above-knee popliteal arteries and whose targets were the below-knee popliteal, tibial, or pedal vessels; lower extremity bypass grafts that did not cross the knee joint were excluded. To allow comparison of prosthetic conduit to an “ideal” conduit, we studied only patients who underwent surgery with either a single-segment greater saphenous vein or prosthetic (92% polytetrafluoroethylene and 8% Dacron). We did not include patients who received a vein other than the greater saphenous vein (e.g., arm vein), cryovein, or more than one vein segment. Because multiple bypasses in the same patient do not behave independently, we only analyzed the first lower extremity bypass (LEB) procedure per patient to avoid confounding secondary to within-patient dependence.17 We also excluded patients who lacked sufficient follow-up data (4%). Mean follow-up time for the cohort was 334 days.
Definitions of Antithrombotic Use
The utilization of the following antithrombotic medications is recorded in the VSGNE database: aspirin, warfarin, and clopidogrel. Patients are recorded to be taking these medications preoperatively (within 48 hr of surgery), at hospital discharge from surgery, and at 1-year follow-up. For our analysis, we categorized a subject as taking a given antithrombotic if they were noted to be on the medication either perioperatively or at follow-up. Patients were further categorized by the combination of various antithrombotic therapies recorded for them.
We studied patients with a prosthetic bypass who were on aspirin alone (n = 67), aspirin plus clopidogrel (aspirin/clopidogrel, n = 30), aspirin plus warfarin (aspirin/warfarin, n = 93), and aspirin plus clopidogrel plus warfarin (aspirin/clopidogrel/warfarin, n = 33). Comparisons between outcomes were performed across these different combinations of antithrombotic therapy. We found that the following antithrombotic combinations were each found in <5% of the subjects who met our inclusion criteria: clopidogrel alone (n = 7), warfarin alone (n = 12), and clopidogrel plus warfarin (n = 4). Furthermore, only 11 subjects were on no medication. Our study was unlikely to draw any generalizable conclusions from the impact of these drug combinations given the small sample sizes of clopidogrel alone, warfarin alone, or clopidogrel plus warfarin. Therefore, we analyzed only aspirin alone, aspirin/clopidogrel, aspirin/warfarin, and aspirin/clopidogrel/warfarin.
Matching Cohorts
Given the observational nature of our data set, conduit type and antithrombotic regimen were not randomly assigned, but instead decisions regarding conduit type and antithrombotic treatment were made by the treating physician. To control for the nonrandom decision to utilize an autologous or prosthetic bypass conduit, we used propensity matching methods to create similar patient cohorts.18 First, we created a logistic regression model utilizing all available patient demographic and clinical data to predict the likelihood of a patient receiving a prosthetic conduit (see Table AI in Appendix). Each patient was then assigned a score (the propensity score), based on the number of each of the characteristics the patient possessed. Next, we matched patients who received a prosthetic conduit to patients with a greater saphenous vein conduit by stratified propensity score as described by Becker and colleagues.19 This ensured that our two cohorts were matched equally in terms of age, race, gender, medical comorbidity, anticoagulation therapy, indication, and operative details. Chi-squared or t-test comparisons were performed for every variable between the two cohorts to verify even matching.
Definitions of Outcome Measures
Our main outcome measures were primary graft patency and the incidence of major lower extremity amputation. Primary patency was defined as uninterrupted patency of the bypass graft with no procedure or intervention of the conduit itself after implantation.20 Patency was assessed by physical examination and duplex scan. Below-knee and above-knee amputations qualified as major leg amputations. Secondary outcomes assessed were patient survival and bleeding complications. We defined a bleeding complication as either a return to the operating room for bleeding or a perioperative blood transfusion of >2 units of packed red blood cells.
Statistical Analysis
When comparing patient demographics, we applied chi-squared analysis for categorical variables and t-test or analysis of variance (ANOVA) for continuous variables, depending on the number of groups compared. The chi-squared analysis was used to compare bleeding complications. We utilized life-table analysis to compare mortality, the incidence of major amputation, and primary patency at long-term follow-up, to account for the fact that not every patient had a follow-up visit at exactly 1 year. Survival curves were generated using the Kaplan–Meier technique. Log-rank tests were used to determine the level of significance between comparisons. The 95% confidence intervals (CIs) were reported when appropriate, and P < 0.05 was considered significant.
All analyses were performed using Microsoft Excel (Microsoft Corp., Redmond, WA) and Stata (StataCorp, College Station, TX) software.
RESULTS
Characteristics of Patients Undergoing Lower Extremity Bypass
We identified 1227 patients who underwent LEB for critical limb ischemia between 2003 and 2009 and met our inclusion criteria. Ischemic rest pain was noted in 535 (44%) and tissue loss in 692 (56%) patients. Of these, 1004 (82%) received greater saphenous vein (GSV) and 223 (18%) received prosthetic conduit. Patient and operative characteristics for this cohort prior to propensity score matching are shown in Table 1. Subjects who received CSV were more likely to be male (69% vs. 63%, P = 0.01). Furthermore, they had a lower incidence of coronary disease (37% vs. 53%, P < 0.001) and far less often had a previous arterial bypass (24% vs. 49%, P < 0.001). Patients with a prosthetic graft were more often treated with warfarin in addition to aspirin (42% vs. 18%, P < 0.001) or with warfarin in addition to aspirin and clopidogrel (15% vs. 6%, P < 0.001). Conversely, patients with GSV were more commonly treated with aspirin alone (56% vs. 30%, P < 0.001) or with aspirin plus clopidogrel (20% vs. 13%, P = 0.02).
Table I.
Characteristics of patients who underwent below-knee bypass
| Variable | Unmatched cohort
|
Matched cohort
|
||||
|---|---|---|---|---|---|---|
| GSV (n = 1004) | Prosthetic (n = 223) | P | GSV (n = 204) | Prosthetic (n = 204) | P | |
| Patient characteristics | ||||||
| Male gender | 692 (69) | 141 (63) | 0.01 | 151 (74) | 131 (64) | 0.07 |
| White race | 984 (98) | 221 (99) | 0.45 | 199 (98) | 202 (99) | 0.53 |
| Living at home | 957 (95) | 205 (93) | 0.06 | 193 (95) | 189 (94) | 0.6 |
| Independently ambulatory | 734 (73) | 153 (69) | 0.43 | 136 (67) | 139 (68) | 0.26 |
| Age (yr) | 69.4 ± 11.9 | 71.6 ± 11 | 0.14 | 71.4 ± 11.5 | 70.9 ± 11.1 | 0.64 |
| 40–49 | 51 (5) | 9 (4) | 0.51 | 6 (3) | 9 (4) | 0.43 |
| 50–59 | 162 (16) | 29 (13) | 0.24 | 24 (12) | 28 (14) | 0.55 |
| 60–69 | 258 (26) | 47 (21) | 0.15 | 54 (26) | 45 (22) | 0.3 |
| 70–79 | 302 (30) | 81 (36) | 0.07 | 59 (29) | 73 (36) | 0.14 |
| 80–89 | 203 (20) | 51 (23) | 0.38 | 51 (25) | 43 (21) | 0.35 |
| 90–100 | 21 (2) | 6 (3) | 0.58 | 8 (4) | 6 (3) | 0.59 |
| Smoking (prior or current) | 813 (81) | 183 (83) | 0.56 | 179 (88) | 167 (83) | 0.12 |
| Hypertension | 875 (87) | 198 (89) | 0.73 | 176 (86) | 181 (89) | 0.45 |
| Chronic obstructive pulmonary disease | 287 (29) | 66 (30) | 0.76 | 74 (36) | 58 (28) | 0.1 |
| Diabetes | 553 (55) | 121 (54) | 0.82 | 106 (52) | 113 (55) | 0.49 |
| Coronary disease | 370 (37) | 118 (53) | <0.001 | 105 (51) | 108 (53) | 0.77 |
| Dialysis | 90 (9) | 17 (8) | 0.74 | 20 (10) | 15 (7) | 0.62 |
| Congestive heart failure | 201 (20) | 50 (22) | 0.43 | 49 (24) | 44 (22) | 0.56 |
| Previous arterial bypass | 238 (24) | 110 (49) | <0.001 | 94 (46) | 99 (49) | 0.62 |
| Previous angioplasty/stent | 208 (21) | 65 (29) | 0.006 | 50 (25) | 60 (29) | 0.27 |
| Previous amputation | 48 (5) | 10 (5) | 0.85 | 10 (5) | 9 (4) | 0.81 |
| Operative characteristics | ||||||
| Graft side—right | 527 (53) | 106 (48) | 0.18 | 100 (49) | 97 (48) | 0.77 |
| Graft origin | ||||||
| External iliac | 4 (0.4) | 10 (4.5) | <0.001 | 2 (1) | 9 (4) | 0.07 |
| Common femoral | 638 (64) | 184 (83) | <0.001 | 159 (78) | 166 (81) | 0.39 |
| Profunda femoris | 33 (3.3) | 4 (1.8) | 0.24 | 7 (3) | 4 (2) | 0.36 |
| Superficial femoral | 272 (27) | 21 (9.4) | <0.001 | 29 (14) | 21 (10) | 0.23 |
| Above-knee popliteal | 57 (5.7) | 4 (1.8) | 0.02 | 7 (3) | 4 (2) | 0.36 |
| Graft recipient | ||||||
| Below-knee popliteal | 448 (45) | 155 (70) | <0.001 | 144 (71) | 139 (68) | 0.59 |
| Tibioperoneal trunk | 55 (5.5) | 16 (7.2) | 0.33 | 15 (7) | 13 (6) | 0.7 |
| Anterior tibial | 128 (13) | 14 (6.3) | 0.006 | 14 (7) | 14 (7) | 1 |
| Posterior tibial | 159 (16) | 18 (8.1) | 0.003 | 15 (7) | 18 (9) | 0.59 |
| Peroneal | 92 (9.2) | 15 (6.7) | 0.24 | 11 (5) | 15 (7) | 0.42 |
| Dorsalis pedis | 60 (6) | 2 (0.9) | 0.002 | 2 (1) | 2 (1) | 1 |
| Posterior tibial– ankle | 51 (5) | 3 (1.4) | 0.01 | 2 (1) | 3 (1) | 0.65 |
| Antithrombotic group | ||||||
| Aspirin | 567 (56) | 67 (30) | <0.001 | 71 (35) | 65 (32) | 0.53 |
| Aspirin/clopidogrel | 205 (20) | 30 (13) | 0.02 | 29 (14) | 30 (15) | 0.89 |
| Aspirin/warfarin | 176 (18) | 93 (42) | <0.001 | 78 (38) | 79 (39) | 0.92 |
| Aspirin/clopidogrel/warfarin | 56 (5.6) | 33 (15) | <0.001 | 26 (13) | 30 (15) | 0.57 |
Data expressed as number (%) or mean ± SD.
Characteristics of the Matched Cohort
Utilizing the stratified propensity score, we were able to match 204 patients who received GSV with 204 patients who had the same likelihood of receiving a saphenous vein conduit, but actually had prosthetic conduit placement. Table 1 depicts the matched cohort to be primarily white, elderly males. Of these, the majority smoked and had hypertension whereas about half also had coronary disease or diabetes.
In the matched cohort of bypass patients, baseline operative and patient characteristics were comparable (Table 1). Specifically, similar numbers of patients with GSV compared with prosthetic had coronary artery disease (51% vs. 53%, respectively, P = 0.77) and previous arterial bypass (46% vs. 49%, respectively, P = 0.62). In addition, the below-knee popliteal artery was the distal target in 71% of GSV patients and in 68% of prosthetic patients (P = 0.59), with the proportion of tibial and pedal targets also comparable between groups (Table 1). Last, 35% of GSV patients were on aspirin alone compared with 32% of prosthetic patients (P = 0.53) and 38% vs. 39%, respectively, were on aspirin plus warfarin (P = 0.92).
Outcomes by Type of Conduit
A comparison of outcome measures between the group of patients receiving GSV and the group receiving a prosthetic conduit is shown in Figure 1 and Table 2. Primary graft patency at 1 year was maintained in 70% of GSV patients and in 72% of prosthetic patients (P = 0.9). Within the first year after bypass, major limb amputation occurred in 11% of patients with GSV and 16% of patients with a prosthetic (P = 0.07). Whereas those with GSV had a slightly higher 1-year survival rate than those with a prosthetic (88% vs. 79%, P = 0.01), about 14% of patients in each group incurred bleeding complications in the perioperative period (P = 0.98; Table 2).
Fig. 1.
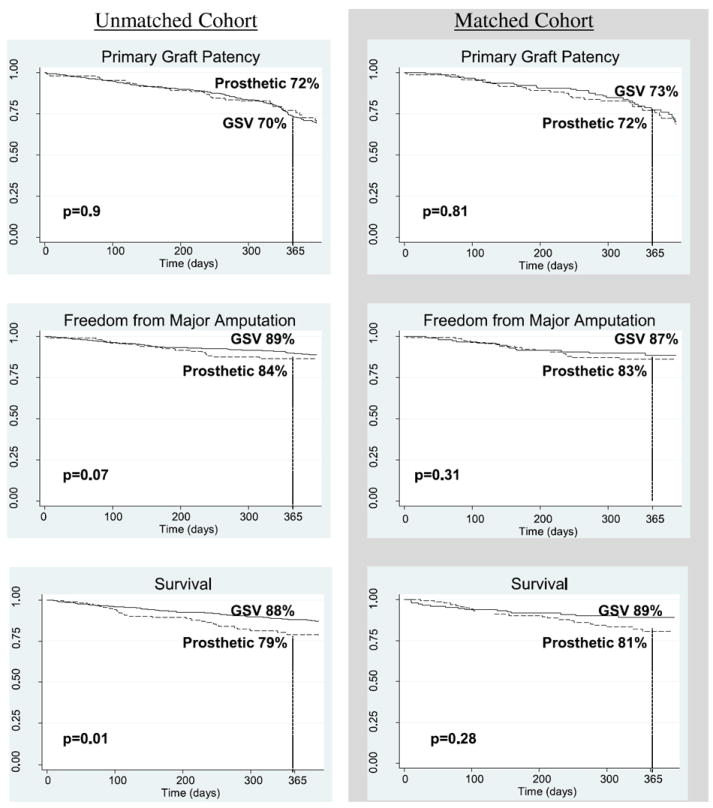
Comparison of outcomes between conduit types: Kaplan–Meier curves depicting the comparison of outcomes between greater saphenous vein (GSV) and prosthetic below-knee bypass conduits.
Table II.
Outcomes by antithrombotic therapy: Comparison of outcomes between greater saphenous vein (GSV) and prosthetic below-knee bypass conduits, broken down by the various combinations of antithrombotic therapy
| Outcome measure | Unmatched cohort
|
Matched cohort
|
||||
|---|---|---|---|---|---|---|
| GSV [events/number at risk (%)] | Prosthetic [events/number at risk (%)] | P | GSV [events/number at risk (%)] | Prosthetic [events/number at risk (%)] | P | |
| Bleeding complicationsa | 139/1004 (14) | 31/223 (14) | 0.98 | 26/204 (13) | 29/204 (14) | 0.66 |
| Aspirin | 72/567 (13) | 8/67 (12) | 0.86 | 10/71 (14) | 7/65 (11) | 0.56 |
| Aspirin/clopidogrel | 27/205 (13) | 4/30 (13) | 0.98 | 2/29 (7) | 4/30 (13) | 0.41 |
| Aspirin/warfarin | 30/176 (17) | 14/93 (15) | 0.67 | 9/78 (12) | 13/79 (16) | 0.38 |
| Aspirin/clopidogrel/warfarin | 10/56 (18) | 5/33 (15) | 0.74 | 5/26 (19) | 5/30 (17) | 0.8 |
| Primary patency at 1 yearb | 455/650 (70) | 91/127 (72) | 0.9 | 97/133 (73) | 83/116 (72) | 0.81 |
| Aspirin | 261/356 (73) | 29/39 (74) | 0.26 | 30/43 (70) | 28/38 (74) | 0.6 |
| Aspirin/clopidogrel | 84/135 (62) | 6/11 (55) | 0.85 | 13/20 (65) | 6/11 (55) | 0.66 |
| Aspirin/warfarin | 83/114 (73) | 42/53 (79) | 0.24 | 39/50 (78) | 35/44 (80) | 0.52 |
| Aspirin/clopidogrel/warfarin | 27/45 (59) | 14/24 (60) | 0.85 | 15/20 (75) | 14/23 (59) | 0.65 |
| Major amputation rate at 1 yearb | 74/658 (11) | 21/134 (16) | 0.07 | 18/133 (13) | 20/120 (17) | 0.31 |
| Aspirin | 35/359 (10) | 11/42 (26) | <0.001 | 7/43 (16) | 10/41 (24) | 0.24 |
| Aspirin/clopidogrel | 11/140 (8) | 0/12 (0) | 0.4 | 1/20 (5) | 0/12 (0) | 0.47 |
| Aspirin/warfarin | 17/114 (15) | 5/51 (9) | 0.24 | 7/50 (14) | 5/42 (9) | 0.44 |
| Aspirin/clopidogrel/warfarin | 11/45 (25) | 5/26 (21) | 0.77 | 3/20 (14) | 5/25 (20) | 0.14 |
| Survival at 1 yearb | 648/735 (88) | 124/157 (79) | 0.01 | 135/152 (89) | 116/143 (81) | 0.28 |
| Aspirin | 358/398 (90) | 40/49 (81) | 0.17 | 45/50 (89) | 40/47 (85) | 0.88 |
| Aspirin/clopidogrel | 137/157 (87) | 9/14 (64) | 0.3 | 21/22 (95) | 9/14 (64) | 0.19 |
| Aspirin/warfarin | 108/132 (82) | 52/67 (78) | 0.5 | 48/58 (83) | 45/56 (80) | 0.77 |
| Aspirin/clopidogrel/warfarin | 45/48 (94) | 23/27 (87) | 0.18 | 21/22 (95) | 22/26 (85) | 0.37 |
Bleeding complications = reoperation for bleeding and/or transfusion of >2 units of packed red blood cells.
Life-table analysis at 1 year after procedure.
The results among the matched cohort were similar. As depicted in Figure 1, overall primary graft patency rates were comparable for propensity-matched patients with a venous conduit and prosthetic conduit (73% and 72%, respectively, P = 0.81). Major amputation rates were 13 % among patients with a venous bypass graft and 17% among those with a prosthetic graft (P = 0.31). There was no difference in 1-year survival between propensity-matched subjects with GSV (89%) and prosthetic conduit subjects (81%, P = 0.28). Within the matched cohort, bleeding complications were encountered in 26 (13%) patients who received GSV and in 29 (14%) patients who received a prosthetic conduit, also a nonsignificant difference (P = 0.66).
Outcomes by Level of Distal Anastomosis
We analyzed primary outcomes based on whether patients had their distal bypass anastomosis at the below-knee popliteal artery or at a tibial/pedal vessel (Fig. 2). Within the unmatched cohort, those who had a venous conduit to the below-knee popliteal artery maintained 78% primary graft patency at 1 year and had a 1-year major amputation rate of 8%. Patients with a prosthetic graft to the same target level had a primary patency rate of 74% (P = 0.24) and a 15% rate of major amputation (P = 0.02). For subjects who had their graft to the tibial/pedal vessels, the 1-year primary patency rates were lower than those for the below-knee popliteal artery (GSV 62%, prosthetic 66%), but there was no significant difference by conduit type (P = 0.52). Furthermore, the incidence of amputation in patients with grafts targeting the tibial/pedal arteries in the unmatched cohort did not differ by venous or prosthetic conduit (14% vs. 19%, respectively, P = 0.33).
Fig. 2.
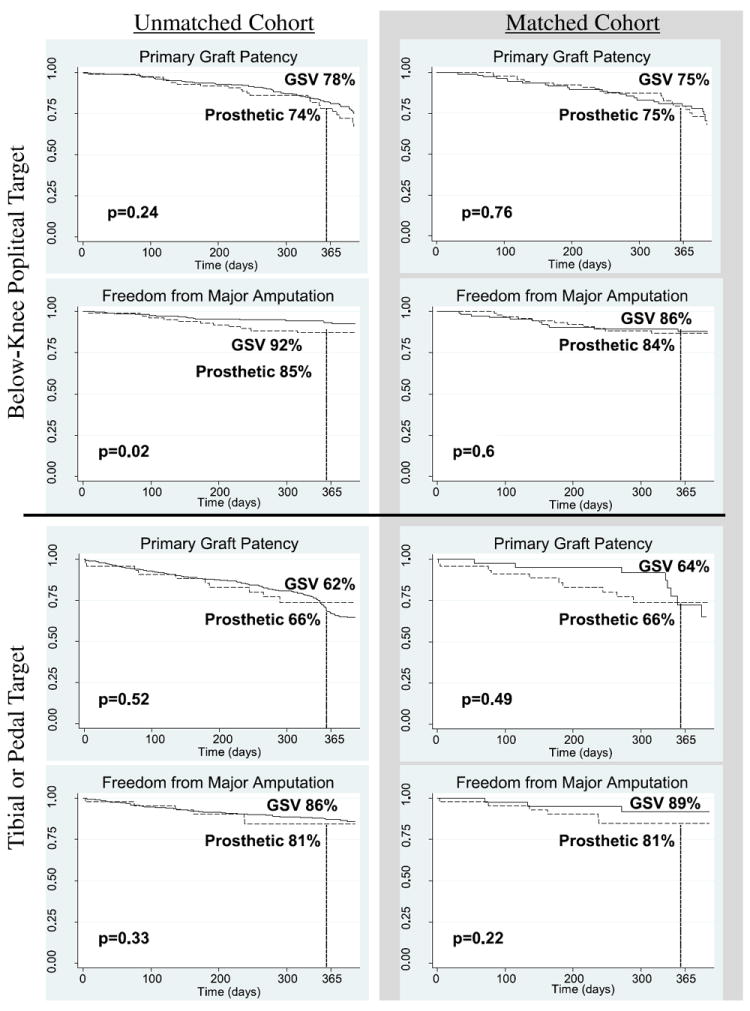
Comparison of outcomes by level of distal anastomosis: Kaplan–Meier curves depicting the comparison of primary outcome measures between greater saphenous vein (GSV) and prosthetic conduits, broken down by distal anastomotic level of bypass grafts.
As shown in Figure 2, primary outcomes by distal anastomosis did not vary significantly within the matched cohort. One-year primary graft patency rates for bypass to the below-knee popliteal artery were 75% for GSV and prosthetic conduits (P = 0.76). The rate of major amputation among patients with that distal anastomotic level was 14% for venous and 16% for prosthetic conduit (P = 0.6). When compared with patients in the matched cohort with grafts to the tibial or pedal vessels, the primary patency rates were 64% for GSV and 66% for prosthetic (P = 0.49), whereas the incidence of amputation within 1 year was 11% for GSV and 19% for prosthetic bypass patients (P = 0.22).
Outcomes by Antithrombotic Regimen
For both the unmatched and matched cohorts, when analyzed by subgroups of similar antithrombotic regimens (aspirin, aspirin/clopidogrel, aspirin/warfarin, and aspirin/clopidogrel/warfarin), we saw no difference in the number of bleeding complications between patients who had a venous or prosthetic bypass graft (Table 2). In addition, we did not find any significant differences in 1-year primary graft patency or patient survival between GSV and prosthetic conduit patients in the unmatched or matched cohorts when we subanalyzed them by type of antithrombotic therapy (Table 2). In the unmatched cohort, we noted a higher amputation rate in the subgroup of patients who were only on aspirin and received a prosthetic versus venous conduit (26% vs. 10%, respectively, P < 0.001). This difference, however, was absent in the matched cohort (prosthetic 24% vs. GSV 16%, P = 0.24). Further, there were no differences in amputation rates within the remaining antithrombotic subgroups (Table 2).
Within the matched cohort, the amount of bleeding complications did not vary by medication usage among patients with a GSV graft (aspirin 13%, aspirin/clopidogrel 13%, aspirin/warfarin 17%, and aspirin/clopidogrel/warfarin 18%, P = 0.86) or for patients with a prosthetic graft (aspirin 12%, aspirin/clopidogrel 13%, aspirin/warfarin 15%, and aspirin/clopidogrel/warfarin 15%, P = 0.49). However, the range of primary graft patency rates in the matched cohort varied based on the type of antithrombotic regimen subjects were administered. Among patients with GSV, those who were on all three antithrombotic medications had the lowest primary patency (59%), followed closely by patients on aspirin/clopidogrel (62%). Of the patients on aspirin alone, 73% achieved primary patency as did 73% of those on aspirin/warfarin. This difference in patency rates among vein conduits was statistically significant (P = 0.01). However, the primary patency rates of prosthetic grafts did not vary significantly by antithrombotic therapy (aspirin 74%, aspirin/clopidogrel 55%, aspirin/warfarin 79%, and aspirin/clopidogrel/warfarin 60%, P = 0.32).
For both GSV and prosthetic patients in the matched cohort, there were no differences in the rates of major amputation between the different antithrombotic subgroups. Although GSV patients who were prescribed aspirin/clopidogrel had a much lower incidence of amputation (5%) than their counterparts (aspirin 16%, aspirin/warfarin 14%, and aspirin/clopidogrel/warfarin 14%), this difference was not significant (P = 0.67). The variation in major amputation rates among prosthetic patients in the matched cohort was also quite variable (aspirin 24%, aspirin/clopidogrel 0%, aspirin/warfarin 9%, and aspirin/clopidogrel/warfarin 20%), yet it also did not reach significance (P = 0.17), likely due to the small number of events in both groups (GSV 18, prosthetic 20). Patients in the matched cohort with venous conduit had little difference in survival according to type of antithrombotic medication: aspirin alone 89%, aspirin/clopidogrel 95%, aspirin/warfarin 83%, and aspirin/clopidogrel/warfarin 95% (P = 0.65). There was slightly more variation, although statistically insignificant, in 1-year survival by antithrombotic therapy among prosthetic graft subjects (aspirin 85%, aspirin/clopidogrel 64%, aspirin/warfarin 80%, and aspirin/clopidogrel/warfarin 85%, P = 0.12).
DISCUSSION
Since the autogenous saphenous vein was first described as a bypass conduit for femoral arterial disease in 1949,21 it has remained the conduit of choice, especially for infrageniculate bypass targets. However, prior reports have demonstrated that an acceptable autologous saphenous vein remains unavailable for as many as 20% of bypass patients.2 Within the VSGNE, a comparable 19% of below-knee bypass patients did not receive a single-segment autologous conduit. These patients often receive a prosthetic bypass conduit while the effect of this “second-line” conduit choice, especially in the era of adjunctive antithrombotic treatments, remains uncertain. Therefore, in our study, we sought to compare 1-year outcomes of infrageniculate bypass patients with critical limb ischemia who received a prosthetic conduit with those who received a single-segment saphenous vein. Surprisingly, we discovered little difference in primary graft patency, or bleeding complications within the first year of surgery and an arguably marginal clinical and statistical benefit in terms of limb salvage and survival for those patients who received a saphenous vein bypass.
Given that several series have demonstrated superior graft patency, reintervention, and limb salvage rates for venous versus prosthetic conduit in across-knee bypass surgery,22-24 why did our study of real-world practice not demonstrate any substantial difference in 1-year outcomes between these conduit choices for infrageniculate bypass? We believe that several possible explanations exist. First, and simplest, it may have been too early to note a difference. Our quality improvement initiative currently evaluates patients at 1-year follow-up, which limits our ability to assess more long-term outcomes in graft patency and limb salvage. It his highly likely that, after 1 year, patency rates for autologous conduit will be favorable to prosthetic conduit. Our future efforts to expand the duration of follow-up recording in the VSGNE will help address this limitation.
Aside from the fact that graft- and limb-related outcomes may not diverge between conduit choices until well beyond the 1-year mark, what other explanations for our findings may exist? It could be that the similarities in outcome by conduit are due to uncontrolled differences in patient characteristics between groups. We found that those patients who had a prosthetic bypass also tended to have distal reconstruction using more proximal crural vessels. In addition, prosthesis patients were more commonly on warfarin. The combination of these factors could potentially increase graft patency rates for the prosthesis group, resulting in the lack of difference in outcomes by conduit type.
In an attempt to compare outcomes across conduit type in a “level playing field, ” we used propensity matching to establish cohorts that were similar in characteristics. However, our results in the matched patient cohort failed to demonstrate significant differences in outcomes within the first year after surgery. Therefore, it seems unlikely that our null finding is fully explained by inequalities in patient characteristics within our data set. It is important to note, however, that, although our database records antithrombotic regimen utilization and our analytic methods adjusted for variation thereof, it remains plausible that our ability to fully account for differences by continued antithrombotic use and adherence is incomplete. Comparisons between antithrombotic regimens, as well as bypass conduit types, would ideally be conducted in the setting of randomized trials.
For example, the CASPAR trial demonstrated that, in a controlled environment, the addition of a second antiplatelet agent (clopidogrel plus aspirin) provides a greater protective effect for infrageniculate prosthetic bypass grafts when compared with aspirin alone.13 Such findings would suggest that the use of clopidogrel is beneficial in patients with high-risk bypass grafts. However, in our observational study, we found that patients who received a prosthetic conduit were less often on clopidogrel plus aspirin than those who received a native venous conduit.
We noted other differences in practice patterns with regard to type of antithrombotic agents that surgeons selected for prosthetic versus autologous conduits. In our cohort, patients with prosthetic bypass grafts were more likely to be on warfarin in addition to aspirin. These decisions were likely driven by earlier work supporting the use of warfarin to extend patency in prosthetic crural bypass. For example, Brumberg and colleagues reported that therapeutic warfarin use in low-flow below-knee prosthetic grafts is associated with significantly improved patency rates.15 Further, a single-center randomization of patients with infrainguinal prosthetic bypass grafts mirrored these findings. Sarac and colleagues detailed an improvement in 3-year primary graft patency by 50% in patients who received warfarin in addition to base-fine aspirin.14
However, although some studies supported the use of warfarin to preserve graft patency, others have refuted this assumption. A large multicenter study in Europe (Dutch Bypass Oral Anticoagulants or Aspirin Study) demonstrated that oral anticoagulants were not associated with improved graft patency for prosthetic femoropopliteal or femorocrural grafts.25 Further, in a study from UCLA, a 20-year review of infrageniculate bypass surgery with prosthetic conduit showed no association between warfarin use and prosthetic bypass patency.26
Despite these mixed findings, our analysis has shown that surgeons in the New England region tend to use antithrombotic therapy more commonly to treat prosthetic conduit patients than those with autogenous saphenous vein. Although no specific societal recommendations exist in this regard,27 our findings show that reasonable outcomes may be achieved for less-than-optimal lower extremity bypass grafts for a duration of at least 1 year.
Should recommendations for aggressive antithrombotic regimens in the setting of prosthetic bypass grafts be generalized to patients outside of our region? Certainly, treatment with aspirin around lower extremity bypass surgery has been endorsed by treatment guidelines11 and has been well adopted in our region, as 85% of patients were treated with aspirin and >95% of patients were on some type of antiplatelet agent. Further, our study has examined the effect of conduit type and antithrombotic regimen in a large sample of patients who were treated in both community and academic settings. Nevertheless, broader endorsement of regimens for multiple antithrombotic agents would require stronger evidence than our observational data could provide. Future work addressing the interaction between conduit types and adjunctive antithrombotic regimens, in a controlled setting, will be needed to reach such conclusions. Although our study is limited by its retrospective nature, it is nonetheless thought-provoking with regard to short-term use of prosthetic conduit for selective patients who may lack adequate autogenous conduit.
Our study has other limitations. First, we did not include the use of cryopreserved vein, upper extremity vein, spliced or composite vein grafts, or prosthetic grafts with a venous cuff, as we aimed to make uniform comparisons in our analysis. Whereas the addition of a venous interposition cuff to a prosthetic graft may provide a minor benefit,28-30 we were limited in drawing significant statistical conclusions from the use of these and the aforementioned bypass conduit choices. Many investigators, however, have shown promising results with such alternative conduit choices31-33 and further studies focusing on their effectiveness are in progress within our region. Second, the patient cohort studied was predominantly Caucasian. Given that race, especially African American, can negatively impact infrageniculate bypass graft patency and limb salvage rates,34 future work will need to encompass this factor in evaluating high-risk bypass grafts. Third, as previously outlined, our database evaluated patients at 1-year follow-up, which limits us in assessing more long-term outcomes in patency and limb salvage. Fourth, our analysis of bleeding complications was limited to short-term occurrences. We cannot comment on long-term adverse effects that are often associated with chronic anticoagulation, such as hematomas or cerebral bleeds, as our registry does not capture these. Last, we used a broad definition for use of antithrombotic regimens, as our data set records these at perioperative and long-term follow-up time-points only. We cannot surmise from our database if subjects were on antithrombotic therapy primarily for their bypass graft and/or for other underlying medical reasons, such as atrial fibrillation. Limitations such as these would be most appropriately addressed using controlled study designs.
In conclusion, our investigation has shown that, within a 1-year period, prosthetic infrapopliteal bypass grafts can perform competitively with single-segment saphenous vein grafts given appropriate patient selection and antithrombotic therapy. Physician practice patterns appear in line with current recommendations for antiplatelet therapy in peripheral vascular disease patients and further suggest a tendency to employ aggressive antithrombotic therapy for high-risk lower extremity bypass grafts. Further controlled trials, especially those investigating novel antithrombotic therapies, are necessary to better delineate the use of protective adjuncts with prosthetic bypass conduits. Nonetheless, given the statistically different limb salvage rates, single-segment CSV, when available, remains the preferred conduit.
APPENDIX
Table AI.
Multivariate regression model depicting the variables associated with receiving a prosthetic bypass conduit
| Variable | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Antithrombotic therapy | |||
| Aspirin only | 0.22 | 0.15–0.32 | <0.001 |
| Aspirin/clopidogrel/warfarin | 1.8 1.1 | 2.8 0.02 | 1.8 1.1 |
| History of coronary disease | 1.7 | 1.2–2.3 | 0.002 |
| Previous lower extremity bypass | 2.9 | 2.1–4 | <0.001 |
| Age 70–79 yr | 1.5 | 1.1–2.2 | 0.02 |
| Age 80–89 yr | 1.7 | 1.1–2.6 | 0.02 |
| Age ≥90 yr | 3 | 1.1–8.2 | 0.03 |
| Graft origin at superficial femoral artery | 2.6 | 1.7–4.1 | <0.001 |
| Graft target | |||
| Below-knee popliteal artery | 3 | 2.1–4.3 | <0.001 |
| Tibioperoneal trunk | 2.9 | 1.5–5.7 | 0.002 |
Footnotes
Presented at the 40th Annual Symposium of the Society for Clinical Vascular Surgery, Las Vegas, NV, March 14–17, 2012.
References
- 1.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic) Circulation. 2006;113:e463–5. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 2.Archie JP., Jr Femoropopliteal bypass with either adequate ipsilateral reversed saphenous vein or obligatory polytetrafluoroethylene. Ann Vasc Surg. 1994;8:475–84. doi: 10.1007/BF02133068. [DOI] [PubMed] [Google Scholar]
- 3.Nicoloff AD, Taylor LM, Jr, McLafferty RB, Moneta GL, Porter JM. Patient recovery after infrainguinal bypass grafting for limb salvage. J Vasc Surg. 1998;27:256–63. doi: 10.1016/s0741-5214(98)70356-8. [DOI] [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl. 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Green RM, Abbott WM, Matsumoto T, et al. Prosthetic above-knee femoropopliteal bypass grafting: five-year results of a randomized trial. J Vasc Surg. 2000;31:417–25. [PubMed] [Google Scholar]
- 6.AbuRahma AF, Robinson PA, Holt SM. Prospective controlled study of polytetrafluoroethylene versus saphenous vein in claudicant patients with bilateral above knee femoropopliteal bypasses. Surgery. 1999;126:594–601. [PubMed] [Google Scholar]
- 7.Klinkert P, Post PN, Breslau PJ, van Bockel JH. Saphenous vein versus PTFE for above-knee femoropopliteal bypass. a review of the literature. Eur J Vasc Endovasc Surg. 2004;27:357–62. doi: 10.1016/j.ejvs.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Hunink MG, Wong JB, Donaldson MC, Meyerovitz MF, Harrington DP. Patency results of percutaneous and surgical revascularization for femoropopliteal arterial disease. Med Decis Making. 1994;14:71–81. doi: 10.1177/0272989X9401400109. [DOI] [PubMed] [Google Scholar]
- 9.Schweiger H, Klein P, Lang W. Tibial bypass grafting for limb salvage with ringed polytetrafluoroethylene prostheses: results of primary and secondary procedures. J Vasc Surg. 1993;18:867–74. [PubMed] [Google Scholar]
- 10.Albers M, Battistella VM, Romiti M, Rodrigues AA, Pereira CA. Meta-analysis of polytetrafluoroethylene bypass grafts to infrapopliteal arteries. J Vasc Surg. 2003;37:1263–9. doi: 10.1016/s0741-5214(02)75332-9. [DOI] [PubMed] [Google Scholar]
- 11.Collaborative overview of randomised trials of antiplatelet therapy—II: Maintenance of vascular graft or arterial patency by antiplatelet therapy. BMJ. 1994;308:159–68. [PMC free article] [PubMed] [Google Scholar]
- 12.Dorffler-Melly J, Buller HR, Koopman MM, Prins MH. Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD000536. CD000536. [DOI] [PubMed] [Google Scholar]
- 13.Belch JJ, Dormandy J, Biasi GM, et al. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg. 2010;52:825–33. doi: 10.1016/j.jvs.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Sarac TP, Huber TS, Back MR, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg. 1998;28:446–57. doi: 10.1016/s0741-5214(98)70130-2. [DOI] [PubMed] [Google Scholar]
- 15.Brumberg RS, Back MR, Armstrong PA, et al. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure. J Vasc Surg. 2007;46:1160–6. doi: 10.1016/j.jvs.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: the Vascular Study Group of Northern New England (VSGNNE) J Vasc Surg. 2007;46:1093–101. doi: 10.1016/j.jvs.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Marubini B, Braga M, Leite ML, Petroccione A, Pirotta N. “Within patient”-dependent outcomes in graft occlusion after coronary artery bypass. SINBA Group. Control Clin Trials. 1993;14:296–307. doi: 10.1016/0197-2456(93)90227-5. [DOI] [PubMed] [Google Scholar]
- 18.Rosenbaum P, Rubin D. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 19.Becker S, Ichino A. Estimation of average treatment effects based on propensity scores. Stata J. 2002;2:358–77. [Google Scholar]
- 20.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 21.Kunlin J. Le traitement de l’arterite obliterante par la greffe veineuse. Arch Mal Coeur. 1949;43:371. [Google Scholar]
- 22.Faries PL, Logerfo FW, Arora S, et al. A comparative study of alternative conduits for lower extremity revascularization: all-autogenous conduit versus prosthetic grafts. J Vasc Surg. 2000;32:1080–90. doi: 10.1067/mva.2000.111279. [DOI] [PubMed] [Google Scholar]
- 23.Taylor LM, Jr, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990;11:193–205. doi: 10.1067/mva.1990.17235. [DOI] [PubMed] [Google Scholar]
- 24.Belkin M, Conte MS, Donaldson MC, Mannick JA, Whittemore AD. Preferred strategies for secondary infrainguinal bypass: lessons learned from 300 consecutive reoperations. J Vasc Surg. 1995;21:282–93. doi: 10.1016/s0741-5214(95)70269-5. [DOI] [PubMed] [Google Scholar]
- 25.Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin Study): a randomised trial. Lancet. 2000;355:346–51. [PubMed] [Google Scholar]
- 26.Kashyap VS, Ahn SS, Quinones-Baldrich WJ, et al. Infrapopliteal-lower extremity revascularization with prosthetic conduit: a 20-year experience. Vasc Endovasc Surg. 2002;36:255–62. doi: 10.1177/153857440203600402. [DOI] [PubMed] [Google Scholar]
- 27.Moawad J, Gagne P. Adjuncts to improve patency of infrainguinal prosthetic bypass grafts. Vasc Endovasc Surg. 2003;37:381–6. doi: 10.1177/153857440303700601. [DOI] [PubMed] [Google Scholar]
- 28.Oderich GS, Panneton JM, Yagubyan M, et al. Comparison of precuffed and vein-cuffed expanded polytetrafluoroethylene grafts for infragenicular arterial reconstructions: a case-matched study. Ann Vasc Surg. 2005;19:49–55. doi: 10.1007/s10016-004-0152-0. [DOI] [PubMed] [Google Scholar]
- 29.Kreienberg PB, Darling RC, 3rd, Chang BB, et al. Early results of a prospective randomized trial of spliced vein versus polytetrafluoroethylene graft with a distal vein cuff for limb-threatening ischemia. J Vasc Surg. 2002;35:299–306. doi: 10.1067/mva.2002.121208. [DOI] [PubMed] [Google Scholar]
- 30.Pappas PJ, Hobson RW, 2nd, Meyers MG, et al. Patency of infrainguinal polytetrafluoroethylene bypass grafts with distal interposition vein cuffs. Cardiovasc Surg. 1998;6:19–26. doi: 10.1016/s0967-2109(97)00093-8. [DOI] [PubMed] [Google Scholar]
- 31.Arvela E, Soderstrom M, Alback A, Aho PS, Venermo M, Lepantalo M. Arm vein conduit vs prosthetic graft in infrainguinal revascularization for critical leg ischemia. J Vasc Surg. 2010;52:616–23. doi: 10.1016/j.jvs.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Hughes K, Domenig CM, Hamdan AD, et al. Bypass to plantar and tarsal arteries: an acceptable approach to limb salvage. J Vasc Surg. 2004;40:1149–57. doi: 10.1016/j.jvs.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 33.Alexander J, Gutierrez C, Katz S. Non-greater saphenous vein grafting for infrageniculate bypass. Am Surg. 2002;68:611–4. [PubMed] [Google Scholar]
- 34.Rowe VL, Kumar SR, Glass H, Hood DB, Weaver FA. Race independently impacts outcome of infrapopliteal bypass for symptomatic arterial insufficiency. Vasc Endovasc Surg. 2007;41:397–401. doi: 10.1177/1538574407303679. [DOI] [PubMed] [Google Scholar]


