Abstract
Importance
While lower extremity revascularization is effective in preventing amputation, the relationship between spending on vascular care and regional amputation rates remains unclear.
Objective
To test the hypothesis that higher regional spending on vascular care is associated with lower amputation rates in patients with severe peripheral arterial disease (PAD).
Design
Retrospective cohort study.
Setting
United States Medicare patients, 2003-2010
Participants
18,463 patients who underwent major PAD-related amputation.
Exposures
Price-adjusted Medicare spending on revascularization procedures and related vascular care in the year before lower extremity amputation, across hospital referral regions.
Main Outcome Measure(s)
Correlation coefficient between regional spending on vascular care and regional rates of PAD-related amputation.
Results
Among patients ultimately subject to amputation, 64% were admitted to the hospital in the year prior to amputation for revascularization, wound-related care, or both; 36% were admitted only for their amputation. The mean cost of inpatient care in the year before amputation, including the amputation itself, was $22,405, but varied from $11,077 (Bismarck, North Dakota) to $42,613 (Salinas, California) (p<0.001). Patients in high-spending regions were more likely to undergo vascular procedures in crude analyses (12.0 procedures per 10,000 patients in the lowest quintile of spending, 20.4 procedures per 10,000 patients in the highest quintile of spending, p<0.0001), as well as in risk-adjusted analyses (adjusted OR for receiving a vascular procedure in highest quintile of spending = 3.5, 95 % CI 3.2-3.8, p<0.0001). While revascularization was associated with higher spending (R=0.38, p<0.001), higher spending was not associated with lower regional amputation rates (R=0.10, p=0.06). Regions most aggressive in the use of endovascular interventions which most likely to have high spending (R=0.42, p=0.002) and high amputation rates (R=0.40, p=0.004).
Conclusions
Regions that spend the most on vascular care is highest perform the most procedures, especially endovascular interventions, in the year before amputation. However, there is little evidence that higher regional spending is associated with lower amputation rates. This suggests an opportunity to limit costs in vascular care without compromising quality.
Keywords: Cardiovascular sugery, endovascular interventions, cost, peripheral vascular disease, health policy and outcomes research
Introduction
Health care costs attributable to critical limb ischemia, the most severe form of peripheral arterial disease (PAD), have been estimated at nearly 5 billion dollars annually in Medicare patients1, 2. Moreover, with the advent of less invasive endovascular techniques, the use of revascularization procedures for critical limb ischemia (CLI) has increased four-fold since 2003 3. Therefore, many believe that in recent years, vascular care aimed at preventing amputation has become increasingly intensive and expensive4.
However, the costs of revascularization for patients who are at risk for amputation, as well as the costs of amputation procedure itself, remain uncertain. These costs vary significantly according to the type of treatments patients receive. For example, “plain old” balloon angioplasty requires catheters that cost a few hundred dollars each, while newer atherectomy devices, drug-coated balloons, and other endovascular adjuncts can exceed several thousand dollars for each artery treated5. Second, while leg bypass surgery is spared the device-related costs of endovascular interventions, the resultant hospital stay nearly always spans several days, and incurs significant expense6-8. And third, the costs related to the amputation procedure itself remain uncertain, and patients undergoing amputation commonly have post-operative complications and a prolonged hospital stay9, 10. A description of spending patterns for patients at risk for amputation, as well as a delineation of relationships between spending on vascular care and amputation risk, may help to guide physicians and policymakers towards establishing value-based guidelines for the treatment of severe PAD.
Therefore, we characterized Medicare spending related to severe PAD in the year prior to amputation, including costs related to the amputation procedure itself. To ensure we studied vascular care provided to patients with the most severe form of PAD, rather than the discretionary treatment of claudication, we studied care provided to patients in the year prior to major limb amputation as a result of PAD. Using across hospital referral regions as our unit of analysis11, we examined risk-adjusted relationships between spending and amputation risk.
Methods
Databases
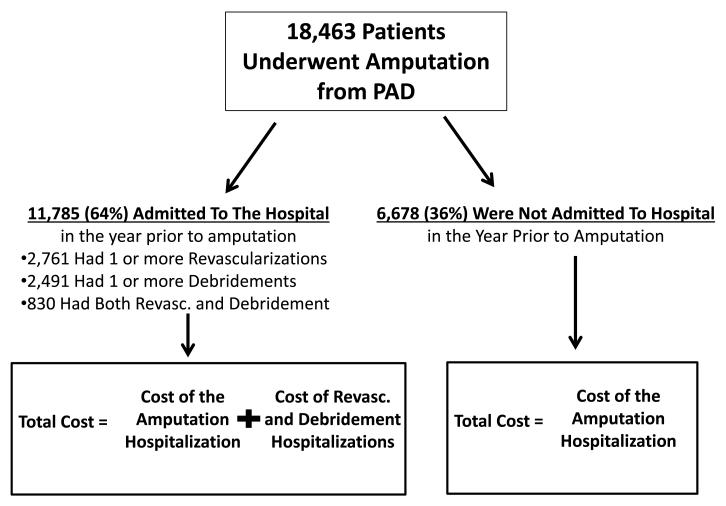
We used Medicare claims (2003-2010) to identify patients with severe PAD, and then examined the costs associated with vascular care in the year prior to amputation. We utilized CPT codes to define both revascularization procedures and leg amputation procedures (above and below-knee only) commonly utilized in the care of patients with severe PAD12. As in prior work, we also ensured that all patients had ICD-9 diagnosis codes for peripheral vascular disease, and underwent major above or below-knee amputation - an indicator of critical limb ischemia3, 13. We recorded the procedure, and age, gender, and race of the beneficiary receiving the procedure. Vital status was determined using the Denominator file, which contains information about eligibility by year for Part B and information about age, gender, and race of eligible beneficiaries (Figure 1).
Figure 1.
Patients, procedures, and hospitalization cost data from the year prior to amputation in our cohort.
We excluded patients under age 66, to allow a one-year “look-back” for comorbidity assessment. Similarly, records with missing values for gender, age, and race strata were also removed from the analysis. We recorded comorbidities including hypertension, diabetes, coronary disease, renal insufficiency, cerebrovascular disease, congestive heart failure, malignancy, measured both individually and in aggregate using the Charlson score. We identified each patient’s zip code of residence and hospital referral region (HRR). as described by the Dartmouth Atlas of Healthcare11. Of the 20,058 patients in our dataset, cost data was unavailable in Medicare claims for 8% (1,595 patients). These patients were excluded from our analysis, but were similar in characteristics to those patients who remained in our cohort.
Studying cost in the year prior to amputation
The severity of PAD can vary significantly, from claudication to limb-threatening ischemia and gangrene. To study a population of patients with similar extent of PAD13, we examined vascular care during the year prior to amputation. By intent, the extent of PAD is inherently similar across patients studied in this manner, as the risk of 1-year limb loss for the entire cohort is 100%4, 14. As reported in prior work, the use of this exposure variable (vascular care in the year prior to amputation) allows us to study care aimed specifically at the treatment of severe PAD, rather than the discretionary treatment of claudication13, 14.
Calculating Price-Adjusted Medicare Spending in the Year Prior to Amputation
In this analysis, for a global assessment of the costs of critical limb ischemia, we studied inpatient costs during the year prior to amputation, including costs related to revascularization, wound debridement, as well as management of cellulitis. This encompassed both diagnostic (such as a diagnostic angiogram) and therapeutic invasive vascular procedures. We also studied costs incurred during the amputation procedure itself. Spending was aggregated at the level of the hospital referral region, as defined in the Dartmouth Atlas11.
Costs were then adjusted for regional differences in Medicare payments, adjusted for inflation given the year of the procedure, and reported as “price-adjusted” Medicare spending15. Finally, to specifically consider the impact of revascularization procedures alone, we studied costs specifically associated with revascularization, exclusive of amputation-related care.
Calculating population-based regional major leg amputation rate
Population-based regional amputation rate was calculated across hospital referral regions, using the total number of major amputations as the numerator, and total number of patients in the region (determined from the mid-year census estimate) as the denominator. Toe amputations and forefoot amputations were not considered in this analysis.
Examining relationships between regional spending and rates of amputation
After defining regional spending in the year prior to amputation and calculating population-based regional rates of major amputation, we examined the associations between these two variables. These associations were displayed using scatter plots between the exposure variable and the outcome variable, at the regional level. Correlation coefficients were calculated between the exposure and outcome.
To adjust for differences in patient characteristics across regions, we generated quintiles of spending and population-based amputation rates, and adjusted for differences in comorbidities and Charlson score across quintiles of spending using backwards stepwise logistic regression models. Models adjusted for patient-level comorbidities as outlined in Table 1, and a cut-off of p<0.20 was established for model inclusion. We censored regions where fewer than 11 amputations occurred, in accordance with guidelines regarding preservation of patient confidentiality from the Centers for Medicare and Medicaid Services. All calculations were performed using SAS (Cary, NC) and STATA (College Station, Texas). Institutional Review Board approval was obtained from the Committee for Protection of Human Subjects from Dartmouth Medical School.
Table 1.
Characteristics of Vascular Amputees in Medicare Claims 2007-2009, by quintile of spending in year prior to amp on revasc/hosp. Patient characteristics of those undergoing amputation, by quintile of spend in the year prior to amputation.
| Quintile of spending | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Overall (n=l 8,463) Very Low | Low | Medium | High | Very High | Ratio (Very High to Very Low) |
p value (non- parametric test of trend) |
||
| Mean spending in this quintile ($) | $22,405 | $17,134 | $20,138 | $21,612 | $23,107 | $27,395 | 1.60 | 0.001 |
| Age (mean) | 78.4 | 78.6 | 78.0 | 78.3 | 78.2 | 78.3 | 1.00 | 0.514 |
| Proportion Male | 50.7 | 52% | 52% | 53% | 53% | 53% | 1.03 | 0.836 |
| Proportion African American | 28 | 14% | 18% | 22% | 22% | 20% | 1.45 | 0.001 |
| Proportion with Diabetes | 49.1 | 48% | 46% | 50% | 49% | 49% | 1.03 | 0.007 |
| Proportion with Congestive Heart Failure | 35.8 | 32% | 35% | 36% | 35% | 37% | 1.14 | 0.001 |
| Proportion with Coronary Artery Diseaes | 13.6 | 13% | 12% | 14% | 15% | 15% | 1.18 | 0.001 |
| Proportion with Renal Insufficency | 17.7 | 14% | 17% | 17% | 17% | 19% | 1.29 | 0.001 |
| Charlson Score | 3.5 | 3.0 | 3.3 | 3.5 | 3.5 | 3.6 | 1.18 | 0.001 |
| Proportion Undergoing invasive diagnostic or therapeutic vascular procedure | 46.8 | 39.4 | 46.6 | 46.2 | 48.0 | 54.1 | 1.37 | 0.001 |
| Per Capita Income ($) | $18,867 | $18,707 | $19,226 | $18,721 | $18,822 | $18,857 | 1.01 | 0.001 |
Results
Patient characteristics, revascularization, and hospitalization rates
We identified 18,463 patients who underwent major PAD-related amputation between 2003 and 2010. Overall, patients had a mean age of 78 years, and 51% of patients were male. Patients commonly had a history of diabetes (49%), heart failure (35%), and coronary disease (14%) (Table 1).
Within this cohort, 11,785 (64%) had a hospitalization during the year prior to amputation, while the remaining 36% were not admitted for a PAD-related reason during this same period. Of the 11,785 admitted to the hospital, 2,762 (15%) underwent an inpatient revascularization procedure, and 2,491 (14%) had a debridement procedure performed during a hospital admission in the year prior to amputation.
Overall, amputation-specific, and revascularization-based spending
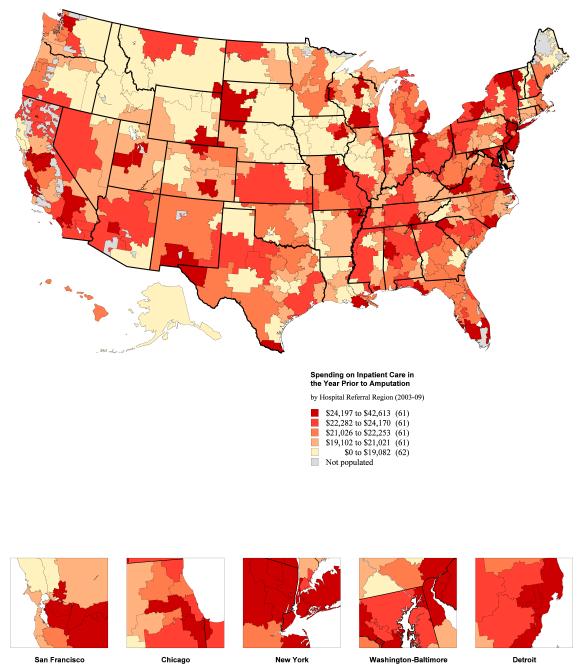
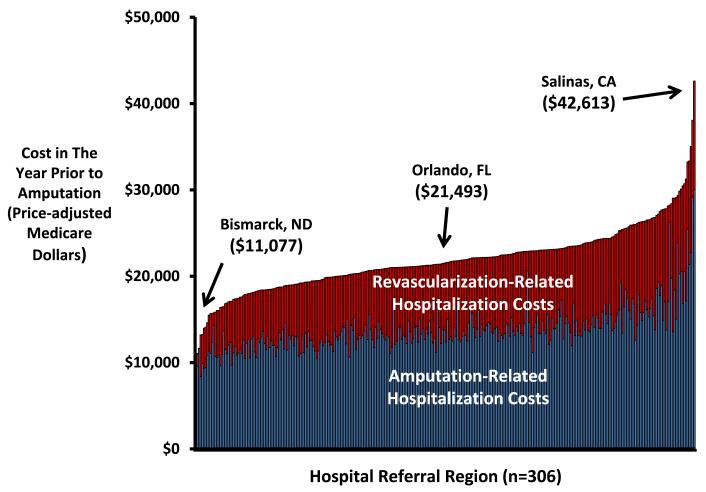
The mean total cost of inpatient vascular care in the year prior to amputation, including the amputation itself, was $22,405 (95% CI $22,145-22,666) per patient. The hospital referral regions with the lowest mean spending on overall inpatient care in the year prior to amputation were Bismarck, North Dakota ($11,077, 95% CI $7,399-14,754), Lebanon, New Hampshire ($13,206, 95% CI $8,870-17,541), and Meridian, Mississippi ($14,120, 95% CI $10,320-17,921). Costs in the year prior to amputation were highest in Paterson, New Jersey ($35,040, 95% CI $23,658-46,421), Ridgewood, New Jersey ($38,070, 95% CI $7,123 - 69,017), and Salinas, California ($42,613, 95% CI $14,041-71,185) (Figure 2).
Figure 2.
National map (panel A) and histogram (panel B) demonstrating regional spending on vascular care and hospitalizations in the year prior to amputation, by hospital referral region.
The mean regional spending on revascularization or debridement (exclusive of the amputation) in the year prior to amputation was $8,316 (95% CI $8,150-8,483) per patient. The regions with the lowest mean spending on revascularization or debridement were Muncie, Indiana ($1,277, 95% CI % $60-5,582), Duluth, Minnesota ($3,342 (95% CI $1,141-5,542) and Topeka, Kansas ($4,199, 95% CI $1,445-6,953) Regions with the highest mean spending were St. Paul, Minnesota (14,063, 95% CI $4,698-23,427), Toledo, Ohio ($14,107, 95% CI $9,763-18,450), and Harlingen, Texas ($14,120, 95% CI $10,553-17,686).
The mean spending for the amputation procedure itself was $14,088 (95% CI $13,898-14,278) per patient. The regions with the lowest mean spending on the amputation-related hospitalization were Lebanon, New Hampshire ($8,368, 95% CI $6,076-10,659), Meridian, Mississippi ($9,408, 95% CI $7,333-11,484), and Bismarck, North Dakota ($9,541, 95% CI $6,382-12,700). The hospital referral regions with the highest mean spending on the amputation procedure itself were Paterson, New Jersey ($22,725, 95% CI $12,859-32,590), Rapid City, South Dakota, ($25,448, 95% CI $3,605-47,292), and Salinas, California ($30,039, 95% CI $12,195-47,884).
Variation in the proportion of all costs related to revascularization
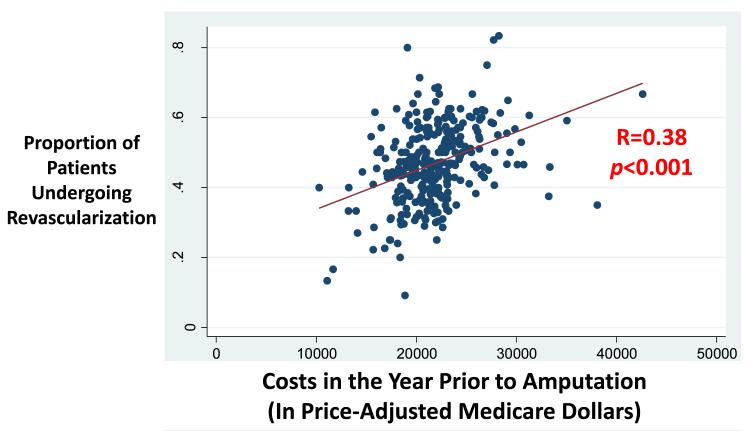
The proportion of all costs related to hospitalizations for revascularization, cellulitis, or debridement represented less than 10% of all costs in many regions, such as Pueblo, Colorado (7%), Grand Junction, Colorado (9%), and Redding, California (10%). However, revascularization and other procedural care represented more than 50% of all costs in Waterloo, Iowa (51%), Burlington, Vermont (52%), and Sun City, Arizona (53%). In 90 of the 307 hospital referral regions, more than 40% of spending in the year prior to amputation was attributable to revascularization, rather than wound care or the amputation procedure itself. There was a positive correlation between the proportion of patients treated with revascularization and the costs incurred in the year prior to amputation (R=0.38, p<0.001) (Figure 3).
Figure 3.
Scatterplot depicting the relationship between regional spending rates and regional revascularization rate in the year prior to amputation.
Differences in patient characteristics, by quintile of spending
We examined differences in patient characteristics between high and low spending regions, across quintiles of spending (Table 1). In regions where spending was highest (mean spending of $27,395), patients undergoing amputation were more likely to be African-American (14% in very slow spending regions, 20% in very high spending regions), and were slightly more likely to have coronary artery disease (13% in very slow spending regions, 15% in very high spending regions). Charlson comorbidity scores were slightly higher in regions where spending was highest (3.0 in very slow spending regions, 3.6 in very high spending regions). As shown in Table 1, while many of these differences were statistically significant given our large sample, clinical differences in patients across quintiles of spending were small.
Use of invasive vascular care, by quintile of spending
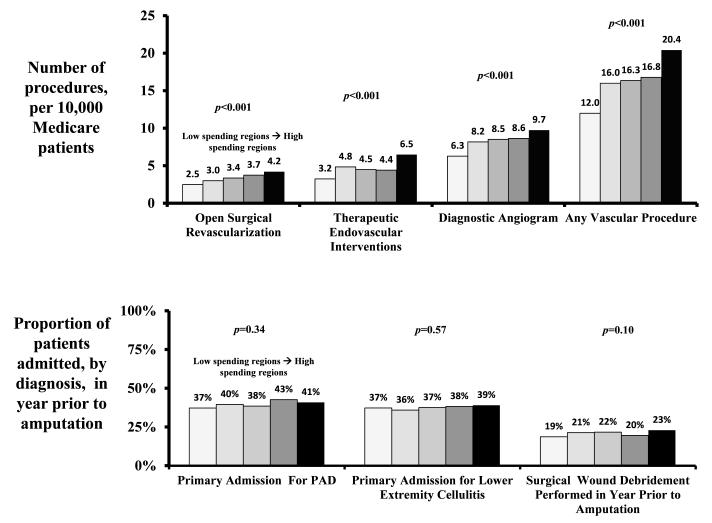
In regions with higher spending, patients were more likely to be treated with invasive vascular care. For example, patients in higher spending regions were more likely to undergo a vascular procedure such as a diagnostic or therapeutic angiogram or open surgical procedure (12.0 procedures per 10,000 patients in the lowest quintile of spending, 20.4 procedures per 10,000 patients in the highest quintile of spending, p<0.0001) (Figure 4).
Figure 4.
Differences in revascularization and non-revascularization care, across quintile of hospital spending in the year prior to amputation.
Adjustment for age, sex, race, diabetes, cardiac, and renal disease across quintiles of spending on vascular care accentuated these differences. Overall, patients living in regions in the highest quintile of spending were more than three times as likely to undergo a vascular procedure when compared to patients in regions in the lowest quintile of spending (adjusted OR for receiving a vascular procedure = 3.5, 95 % CI 3.2-3.8, p<0.0001) (Table 2). Similar trends were seen in both crude and adjusted analyses when individually examining open surgical revascularizations, therapeutic endovascular interventions, and diagnostic angiograms (crude rates are shown in Figure 4, and adjusted odds ratios demonstrated in Table 2). We also found that regions where spending on vascular procedures was high also had high spending on the amputation procedure itself (R=0.82, p<0.001)
Table 2.
Multivariable models demonstrating the likelihood of a patient undergoing vascular procedures, across quintiles of spending, as well as the likelihood of being in the highest quintile of amputation rate.
| Characteristic | Likelihood of Undergoing Any Vascular Procedure (Diagnostic/Therapeutic) |
Likelihood of UndergoingAny Endovascular Procedure |
Likelihood of UndergoingAny Open Surgical Procedure |
Likelihood of Being in Highest Quintile of Amputation Rate |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Odds Ratio | 95% Conf. Interval | p Value | Odds Ratio | 95% Conf. Interval | p Value | Odds Ratio | 95% Conf. Interval | p Value | Odds Ratio | 95% Conf. Interval | p Value | |||||
|
|
||||||||||||||||
| Quintile of Spending on Vascular Care | ||||||||||||||||
| Very Low Spending | referent | referent | referent | referent | ||||||||||||
| Low Spending | 1.6 | 1.4 | 1.7 | O.001 | 1.6 | 1.4 | 1.7 | <0.001 | 1.2 | 1.0 | 1.4 | 0.011 | 1.0 | 0.9 | 1.2 | 0.54 |
| Medium Spending | 1.5 | 1.4 | 1.7 | O.001 | 1.5 | 1.4 | 1.7 | <0.001 | 1.2 | 1.0 | 1.4 | 0.024 | 1.0 | 0.9 | 1.2 | 0.554 |
| High Spending | 2.4 | 2.2 | 2.6 | O.001 | 2.3 | 2.1 | 2.5 | <0.001 | 2.4 | 2.1 | 2.7 | <0.001 | 1.0 | 0.9 | 1.1 | 0.857 |
| Very High Spending | 3.5 | 3.1 | 3.8 | O.001 | 3.2 | 2.9 | 3.5 | <0.001 | 4.0 | 3.5 | 4.5 | <0.001 | 0.9 | 0.8 | 1.1 | 0.383 |
| Age | ||||||||||||||||
| Age 65-69 | referent | referent | referent | referent | ||||||||||||
| Age 70-74 | 1.0 | 1.0 | 1.1 | 0.407 | 1.0 | 1.0 | 1.1 | 0.34 | 0.9 | 0.8 | 1.0 | 0.217 | 1.0 | 1.0 | 1.1 | 0.73 |
| Age 75-79 | 1.0 | 0.9 | 1.1 | 0.497 | 1.0 | 0.9 | 1.1 | 0.966 | 1.0 | 0.9 | 1.1 | 0.422 | 1.0 | 0.9 | 1.1 | 0.913 |
| Age 80-85 | 0.8 | 0.7 | 0.9 | O.001 | 0.8 | 0.7 | 0.9 | <0.001 | 0.7 | 0.6 | 0.8 | <0.001 | 0.9 | 0.7 | 0.9 | <0.001 |
| Age>85 | 0.5 | 0.5 | 0.6 | O.001 | 0.6 | 0.5 | 0.6 | <0.001 | 0.5 | 0.4 | 0.5 | <0.001 | 0.9 | 0.5 | 0.6 | <0.001 |
| Diabetes | 1.1 | 1.0 | 1.1 | 0.053 | 1.1 | 1.0 | 1.2 | 0.005 | 1.2 | 1.1 | 1.3 | 0.001 | 0.8 | 0.8 | 0.9 | <0.001 |
| Coronary artery disease | 1.3 | 1.1 | 1.4 | O.001 | 1.2 | 1.1 | 1.3 | <0.001 | 1.3 | 1.2 | 1.5 | <0.001 | 1.0 | 0.9 | 1.1 | 0.912 |
| Congestive heart failure | 0.9 | 0.9 | 1.0 | 0.008 | 0.9 | 0.9 | 1.0 | 0.034 | 0.8 | 0.7 | 0.9 | <0.001 | 1.0 | 0.9 | 1.1 | 0.586 |
| Renal Insufficiency | 1.3 | 1.2 | 1.5 | <0.001 | 1.4 | 1.3 | 1.5 | <0.001 | 0.8 | 0.7 | 0.9 | <0.001 | 1.0 | 0.9 | 1.1 | 0.913 |
| African American Race | 0.8 | 0.7 | 0.8 | <0.001 | 0.8 | 0.7 | 0.8 | <0.001 | 0.8 | 0.8 | 0.9 | <0.001 | 0.3 | 0.3 | 0.3 | <0.001 |
Correlation between spending and amputation rate, by region
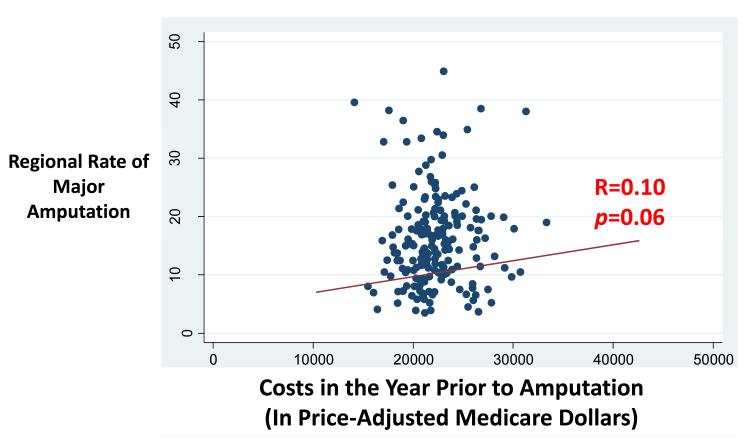
Despite the direct correlation between procedural care and overall spending, we did not find a direct relationship between overall spending in the year prior to amputation and regional amputation rate (R=0.10, p=0.06) (Figure 5). Even in risk adjusted comparisons, there was no significant relationship between the likelihood of being in the highest quintile of amputation rate and overall spending rate (adjusted OR 0.95, 95 %CI 0.9-1.1, p=0.383) (Table 2).
Figure 5.
Scatterplot depicting the relationship between regional spending rates and regional amputation rates.
Across practice patterns, the regions that were most aggressive in the use of endovascular interventions (in the highest 20th percentile) were likely to have high spending (R=0.42, p=0.002) as well as high amputation rates (R=0.40, p=0.004). Conversely, regions that were not aggressive in the use of endovascular interventions (in the lowest 20th percentile) were not likely to be in the highest quintile of amputation rate (R=0.10, p=0.36).
Discussion
In this descriptive analysis, we demonstrate that costs of inpatient care in the year prior to amputation for patients with critical limb ischemia are over $20,000 per patient for inpatient care alone. Further, these costs vary more than two-fold across hospital referral regions in the United States. Much of this variation is driven by differences in the use of revascularization treatments, rather than differences in patient characteristics or costs related to amputation itself. Moreover, there is little evidence to suggest that higher spending on invasive vascular care, especially endovascular care, in the year prior to amputation is associated with lower regional rates of amputation.
Accurate measurement of the true cost of critical limb ischemia is difficult2, 16, 17. The chronic nature and broad spectrum of PAD, coupled with the far-reaching effects of limb loss on functional status and the ability to live independently, make it challenging to determine its financial impact. Prior studies have examined the inpatient costs of vascular care (Table 3). For example, the REACH registry investigators studied the two-year costs of 25,763 patients systemic atherosclerosis18, 19. For patients who underwent revascularization or amputation, costs easily averaged over $10,000 per patient during the two years following enrollment in the registry - a cost much higher than those patients with mild coronary disease. European patients enrolled in the REACH registry also demonstrated high costs when they required revascularization or amputation, but the magnitude of these costs was much less than in the United States20. Finally, investigators from Minnesota extrapolated costs from statewide data to provide national cost estimates for the impact of hospitalization for PAD on cost. Therefore, while our study is limited to only patients in Medicare, it provides cost estimates that are consistent with other estimates, and provide national-level detail for each individual region of the United States.
Table 3.
Studies reporting cost estimates for inpatient care for patients with severe peripheral arterial disease. Studies examining inpatient costs in patients at risk for amputation from severe PAD.
| Study Author | Patinets (n) | Cost Estimate in Year Prior to Amputation | Comment | |
|---|---|---|---|---|
| REACH (Europe) REACH (US) Minnesota state-based registry | Smolderens et al Mahoney et al Peacock et al | 10,287 2,396 20 per 100,000 | $4,136 $10,430 $32,129 | Two year costs in Europe, calculated from Euros Two year costs in the US, among patients with severe disease Population-based estimates from Minnesota |
In our study, some regions spent less than $13,000, on average, in the year prior to amputation, while other regions spent $30,000 or more in the year prior to amputation. Using these “natural experiments”, we found little evidence to suggest that most expensive strategies are associated with better outcomes. While we acknowledge the well-known weaknesses of administrative claims21, 22, patients were roughly similar in many important demographic and comorbidity variables across strata of spending, and adjustment for any statistical differences had little impact on our findings. Therefore, it appears unlikely that these large differences in spending can be explained simply by differences in patient characteristics.
This suggests that an important opportunity exists, given the right kind of evidence, to save money while still providing high quality care for patients with severe PAD. While much of this necessary evidence will come from clinical trials23, 24, examination of the cost and effectiveness of these treatments in “real-world” settings will be important as well9, 25, 26. National registries, such as the Society for Vascular Surgery’s Vascular Quality Initiative (VQI)27, the National Surgical Quality Improvement Program (NSQIP)28, and the National Cardiovascular Data Registry29, will need to incorporate the right endpoints. This means measuring both efficacy and cost to determine which strategies are effective in limiting amputation risk in patients with critical limb ischemia, in the most cost-effective manner.
Prior work by our group14 and others30, 31 suggested that more vascular care – as measured by any type of diagnostic or therapeutic vascular procedure in the year prior to amputation - is related to lower risks of amputation14. Are these findings discordant with those reported herein? We believe not. Dramatic differences in cost can exist related to the manner in which patients with critical limb ischemia are treated. For example, patients treated with simple “plain-old balloon angioplasty” or a single surgical revascularization will have a much lower costs when compared to patients receiving multiple rounds of atherectomy, drug -eluting balloons, or other more expensive endovascular adjuncts32-35. Our current analyses found that the highest spending rates -and the highest amputation rates - occurred in regions where multiple endovascular interventions were used commonly.
Different interpretations of the spending patterns described in our study are plausible as well. For example, one might argue that a region could provide high-quality preventive and invasive vascular care, and thereby prevent many patients from ever requiring amputation. Within a region like this, overall spending on vascular care would be high, and amputation rates would be low. However, we found few regions where spending and the overall intensity (measured by the number of procedures13) of vascular care was high, and amputation rates were low. In fact, only 3 of 307 regions – (Fort Lauderdale and Fort Myers, Florida, and Madison, Wisconsin) fit this description. Therefore, while plausible, this alternative explanation does not appear to represent an alternative explanation for our findings.
Our study has several important limitations. First, as our work considered only inpatient costs, and does not directly capture outpatient care that may be provided in wound care centers, outpatient angiography suites, and ambulatory imaging centers36-38. Even though the significant burden of comorbidities carried by patients with critical limb ischemia most commonly necessitates hospital-based care, vascular care is increasingly provided in outpatient settings39, 40. Our future work will consider not only hospital-based care, but also care that is provided in ambulatory environments.
Second, our observational dataset derives from administrative claims, and cannot provide patient-level clinical detail as to the extent of peripheral arterial disease, or surgical-level specifics at the time of revascularization. However, our cohort was purposefully designed to consider only those patients with the most severe peripheral arterial disease, such that all patients studied had a limb-loss rate of 100%, an algorithm reflected in our prior publications4,13, 14. Third, as sidedness is not indicated on Medicare claims, we cannot be sure that revascularization procedures and amputations all occurred on the same limb. However, prior work by our group suggested that differential sides occur in fewer than 10% of procedures41. Fourth, our study examined price-adjusted spending among Medicare beneficiaries. Therefore, our results may not be readily generalizable to younger patients insured by non-federal payers. However, Medicare patients comprise more than 80% of patients at risk for amputation, and price-adjusted Medicare spending represents a well-proven measure to examine utilization on the national scale42. Therefore, we find little evidence to suggest that our findings are not readily applicable to most patients at risk for limb amputation.
In conclusion, Medicare spending on patients with severe PAD varies more than twofold across the United States, and regions where spending is highest perform the most revascularization procedures in the year prior to amputation. And while our prior work suggests that access to revascularization is a key component in preventing amputation, our current analysis offer little evidence to suggest that more expensive vascular care offers a marginal advantage over less expensive vascular interventions14. In the current era of accountable care organizations, where quality and cost must be equally considered43-45, saving money and preventing amputation appear to be two achievable and complementary goals in the care of patients with peripheral arterial disease.
Acknowledgements
The authors wish to thank Jonathan S. Skinner, PhD, for his critiques and insights during the preparation of this work.
Funding/Support: Dr. Goodney was supported by a K-08 Career Development Award from the NHLBI (1K08HL05676-01), and an American Vascular Association / American College of Surgeons Supplemental Funding Award.
Appendix
1. CPT and ICD-9 codes used to delineate the revascularization and amputation procedures in our cohort.
Appendix 1.
CPT codes
| OPEN | |
|---|---|
| Inflow: | Outflow: |
| 35521 Bypass graft, with vein;axillary-femoral | 35302 Thromboendarterectomy, including patch graft, if performed;superficial femoral artery |
| 35351 Thromboendarterectomy, including patch graft, if performed;iliac | 35303 Thromboendarterectomy, including patch graft, if performed;popliteal artery |
| 35355 Thromboendarterectomy, including patch graft, if performed;iliofemoral | 35304 Thromboendarterectomy, including patch graft, if performed;tibioperoneal trunk artery |
| 35361 Thromboendarterectomy, including patch graft, if performed;combined aortoiliac | 35305 Thromboendarterectomy, including patch graft, if performed;tibial or peroneal artery, |
| 35363 Thromboendarterectomy, including patch graft, if performed;combined aortoiliofemoral | 35306 Thromboendarterectomy, including patch graft, if performed;each additional tibial |
| 35537: aortoiliac bypass | 35371 Thromboendarterectomy, including patch graft, if performed;common femoral |
| 35538 Bypass graft, with veln;aortobi-iliac | 35372 Thromboendarterectomy, including patch graft, if performed;deep (profunda) femoral |
| 35539 Bypass graft, with vein;aortofemoral | 35533 Bypass graft, with vein;axillary-femoral-femoral |
| 35540 Bypass graft, with vein;aortobifemoral | 35556 Bypass graft, with vein;femoral-popliteal |
| 35541 Bypass graft, with vein | 35558 Bypass graft vein;femoral-femoral |
| 35546 Aortofemoral bypass with vein. | 35566 Bypass graft, with vein;femoral-anterior tibial, posterior tibial, peroneal artery |
| 35539: Aortofemoral graft with vein.For aortofemoral graft with vein | 35571 Bypass graft, with vein;popliteal-tibial, -peroneal artery or other distal vessels |
| 35548 Bypass graft, with vein;aortoiliofemoral, unilateral | 35583 In-situ vein bypass; femoral-popliteal |
| 35549 Bypass graft, with vein;aortoiliofemoral, bilateral | 35585 In-situ vein bypass; femoral-anterior tibial, posterior tibial, or peroneal artery |
| 35551 Bypass graft, with vein;aortofemoral-popliteal | 35587 In-situ vein bypass;popliteal-tibial, peroneal |
| 35563 Bypass graft, with vein;ilioiliac | 35656 Bypass graft, with other than vein;femoral-popliteal |
| 35565 Bypass graft, with vein;iliofemoral | 35666 Bypass graft, with other than vein;femoral-anterior tibial, posterior tibial, or personal artery |
| 35621 Bypass graft, with other than vein;axillary-femoral | 35671 Bypass graft, with other than vein;popliteal-tibial or -peroneal artery |
| 35623 Bypass graft, with other than vein;axillary-popliteal or -tibial | 35681 Bypass graft, composite, prostetic and vein |
| 35637 Bypass graft, with other than vein;aortoiliac | 35682 Bypass graft;autogenous composite, two segments of veins from two locations |
| 35638 Bypass graft, with other than vein;aortobi-iliac | 35683 Bypass graft;autogenous composite, three or more segments of vein |
| 35646 Bypass graft, with other than vein;aortobifemoral | 35879 Revision, lower extremity arterial bypass, without thrombectomy, open; with vein patch angioplasty |
| 35647 Bypass graft, with other than vein;aortofemoral | 35881 Revision, lower extremity arterial bypass, without thrombectomy, open;with segmental vein interposition |
| 35651 Bypass graft, with other than vein;aortofemoral-popliteal | 35883 Revision, femoral anastomosis of synthetic arterial bypass graft in groin, open; with nonautogenous patch graft |
| 35654 Bypass graft, with other than vein;axillary-femoral-femoral | 35884 Revision, femoral anastomosis of synthetic arterial bypass graft in groin, open;with autogenous vein patch graft |
| 35661 Bypass graft, with other than vein;femoral-femoral | |
| 35663 Bypass graft, with other than vein;ilioiliac | |
| 35665 Bypass graft, with other than vein;iliofemoral | |
| ENDO: | |
| Inflow: | Outflow: |
| 35452 Transluminal balloon angioplasty, open;aortic | 35456 Transluminal balloon angioplasty, open;femoral-popliteal |
| 35454 Transluminal balloon angioplasty, open;iliac | 35459 Transluminal balloon angioplasty, open;tibioperoneal trunk and branches |
| 35472 Transluminal balloon angioplasty, percutaneous;aortic | 35470 Transluminal balloon angioplasty, percutaneous;tibioperoneal trunk or branches, each vessel |
| 35473 Transluminal balloon angioplasty, percutaneous;iliac | 35474 Transluminal balloon angioplasty, percutaneous;femoral-popliteal |
| 35481 Transluminal peripheral atherectomy, open;aortic | 35483 Transluminal peripheral atherectomy, open;femoral-popliteal |
| 35482 Transluminal peripheral atherectomy, open;iliac | 35485 Transluminal peripheral atherectomy, open;tibioperoneal trunk and branches |
| 35491 Transluminal peripheral atherectomy, percutaneous;aortic | 35493 Transluminal peripheral atherectomy, percutaneous;femoral-popliteal |
| 35492 Transluminal peripheral atherectomy, percutaneous;iliac | 35495 Transluminal peripheral atherectomy, percutaneous;tibioperoneal trunk and branches |
| 37205 Transcatheter placement of an intravascular stent(s), (except coronary, carotid, and vertebral vessel), percutaneous; initial vessel | |
| 37206 Transcatheter placement of an intravascular stent(s), (except coronary, carotid, and vertebral vessel), percutaneous;each additional vessel | |
| 37207 Transcatheter placement of an intravascular stent(s), (non-coronary vessel), open; initial vessel | |
| 37208 Transcatheter placement of an intravascular stent(s), (non-coronary vessel), open;each additional vessel | |
| Diagnostic Only Endovascular Procedures: | |
| 36200 | introduction of catheter, aorta |
| 36245 | selective catheter placement, arterial system, each first-order, lower- extremity |
| 36246 | selective catheter placement, arterial system, second-order, lower- extremity |
| 36247 | selective catheter placement, arterial system, third-order, lower- extremity |
| 36248 | selective catheter placement, arterial system, beyond third order, lower- extremity |
| CPT Code | Outcome Measure: Amputation |
| 27590 | Amputation, thigh, through femur, any level; |
| 27591 | Amputation, thigh, through femur, any level; immediate fitting technique including first cast |
| 27592 | Amputation, thigh, through femur, any level; open, circular (guillotine) |
| 27880 | Amputation, leg, through tibia and fibula; |
| 27881 | Amputation, leg, through tibia and fibula; with immediate fitting technique |
| 27882 | Amputation, leg, through tibia and fibula; open, circular (guillotine) |
| 28805 | Amputation, foot; transmetatarsal |
Footnotes
Conflict of Interest Disclosures: The authors report no conflicts of interest pertinent to this manuscript.
Presented at the Quality, Cost, and Outcomes Research (QCOR), Conference of the American Heart Association, Wednesday, May 9th, 2012, Atlanta, Georgia.
References
- 1.Peacock JM, Keo HH, Yu X, Oldeberg N, Duval S, Henry TD, Jaff MR, Baumgartner I, Hirsch AT. Abstract 5788: The incidence and health economic burden of critical limb ischemia and ischemic amputation in minnesota: 2005-2007. Circulation. 2009;120:S1148. [Google Scholar]
- 2.Peacock JM, Keo HH, Duval S, Baumgartner I, Oldenburg NC, Jaff MR, Henry TD, Yu X, Hirsch AT. The incidence and health economic burden of ischemic amputation in minnesota, 2005-2008. Prev Chronic Dis. 2011;8:A141. [PMC free article] [PubMed] [Google Scholar]
- 3.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. Journal of Vascular Surgery. 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Quanstrum KH, Henke P, Dimick JB, Birkmeyer JD. Racial disparities in endof-limb care among medicare amputees. Journal of Vascular Surgery. doi: 10.1016/j.jvs.2011.02.035. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Http://www.Ptca.Org/press_rel/20050414_2pr_boston.Html.
- 6.Cronenwett JL, Likosky DS, Russell MT, Eldrup-Jorgensen J, Stanley AC, Nolan BW. A regional registry for quality assurance and improvement: The vascular study group of northern new england (vsgnne) J Vasc Surg. 2007;46:1093–1101. doi: 10.1016/j.jvs.2007.08.012. discussion 1101-1092. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen LL, Lipsitz SR, Bandyk DF, Clowes AW, Moneta GL, Belkin M, Conte MS. Resource utilization in the treatment of critical limb ischemia: The effect of tissue loss, comorbidities, and graft-related events. Journal of Vascular Surgery. 2006;44:971–975. doi: 10.1016/j.jvs.2006.07.035. discussion 975-976. [DOI] [PubMed] [Google Scholar]
- 8.Siracuse JJ, Giles KA, Pomposelli FB, Hamdan AD, Wyers MC, Chaikof EL, Nedeau AE, Schermerhorn ML. Results for primary bypass versus primary angioplasty/stent for intermittent claudication due to superficial femoral artery occlusive disease. J Vasc Surg. 2012;55:1001–1007. doi: 10.1016/j.jvs.2011.10.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodney PP, Nolan BW, Schanzer A, Eldrup-Jorgensen J, Stanley AC, Stone DH, Likosky DS, Cronenwett JL. Factors associated with death 1 year after lower extremity bypass in northern new england. J Vasc Surg. 2010;51:71–78. doi: 10.1016/j.jvs.2009.07.123. [DOI] [PubMed] [Google Scholar]
- 10.Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, Ruckley CV, Raab GM. Bypass versus angioplasty in severe ischaemia of the leg (basil) trial: A description of the severity and extent of disease using the bollinger angiogram scoring method and the transatlantic inter-society consensus ii classification. J Vasc Surg. 2010;51:32S–42S. doi: 10.1016/j.jvs.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 11.Dartmouth atlas of healthcare. 2007 Oct 1st; www.dartmouthatlas.org.
- 12.Cpt schedule. 2012 Jun 1st; www.ama-assn.org/resources/doc/cpt/cpt-ruc-calendar.pdf. www.ama-assn.org/resources/doc/cpt/cpt-ruc-calendar.pdf.
- 13.Goodney PP, Travis LL, Nallamothu BK, Holman K, Suckow B, Henke PK, Lucas FL, Goodman DC, Birkmeyer JD, Fisher ES. Variation in the use of lower extremity vascular procedures for critical limb ischemia. Circ Cardiovasc Qual Outcomes. 2012;5:94–102. doi: 10.1161/CIRCOUTCOMES.111.962233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodney PP, Holman K, Henke PK, Travis LL, Dimick JB, Stukel TA, Fisher ES, Birkmeyer JD. Regional intensity of vascular care and lower extremity amputation rates. J Vasc Surg. 2012 doi: 10.1016/j.jvs.2012.11.068. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb DJ, Zhou W, Song Y, Andrews KG, Skinner JS, Sutherland JM. Prices don’t drive regional medicare spending variations. Health Aff (Millwood) 2010;29:537–543. doi: 10.1377/hlthaff.2009.0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durham CA, Mohr MC, Parker FM, Bogey WM, Powell CS, Stoner MC. The impact of socioeconomic factors on outcome and hospital costs associated with femoropopliteal revascularization. J Vasc Surg. 2010;52:600–606. doi: 10.1016/j.jvs.2010.04.011. discussion 606-607. [DOI] [PubMed] [Google Scholar]
- 17.Panayiotopoulos YP, Tyrrell MR, Owen SE, Reidy JF, Taylor PR. Outcome and cost analysis after femorocrural and femoropedal grafting for critical limb ischaemia. Br J Surg. 1997;84:207–212. [PubMed] [Google Scholar]
- 18.Mahoney EM, Wang K, Keo HH, Duval S, Smolderen KG, Cohen DJ, Steg G, Bhatt DL, Hirsch AT. Vascular hospitalization rates and costs in patients with peripheral artery disease in the united states. Circ Cardiovasc Qual Outcomes. 2010;3:642–651. doi: 10.1161/CIRCOUTCOMES.109.930735. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney EM, Wang K, Cohen DJ, Hirsch AT, Alberts MJ, Eagle K, Mosse F, Jackson JD, Steg PG, Bhatt DL. One-year costs in patients with a history of or at risk for atherothrombosis in the united states. Circ Cardiovasc Qual Outcomes. 2008;1:38–45. doi: 10.1161/CIRCOUTCOMES.108.775247. [DOI] [PubMed] [Google Scholar]
- 20.Smolderen KG, Wang K, de Pouvourville G, Bruggenjurgen B, Rother J, Zeymer U, Parhofer KG, Steg PG, Bhatt DL, Magnuson EA. Two-year vascular hospitalisation rates and associated costs in patients at risk of atherothrombosis in france and germany: Highest burden for peripheral arterial disease. Eur J Vasc Endovasc Surg. 2012;43:198–207. doi: 10.1016/j.ejvs.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell JB, Bubolz T, Paul JE, Pashos CL, Escarce JJ, Muhlbaier LH, Wiesman JM, Young WW, Epstein RS, Javitt JC. Using medicare claims for outcomes research. Med Care. 1994;32:JS38–51. [PubMed] [Google Scholar]
- 22.Tseng C-L, Rajan M, Miller DR, Hawley G, Crystal S, Xie M, Tiwari A, Safford M, Pogach L. Use of administrative data to risk adjust amputation rates in a national cohort of medicare-enrolled veterans with diabetes. Medical Care. 2005;43:88–92. [PubMed] [Google Scholar]
- 23.Conte MS, Bandyk DF, Clowes AW, Moneta GL, Seely L, Lorenz TJ, Namini H, Hamdan AD, Roddy SP, Belkin M, Berceli SA, DeMasi RJ, Samson RH, Berman SS. Results of prevent iii: A multicenter, randomized trial of edifoligide for the prevention of vein graft failure in lower extremity bypass surgery. J Vasc Surg. 2006;43:742–751. doi: 10.1016/j.jvs.2005.12.058. discussion 751. [DOI] [PubMed] [Google Scholar]
- 24.Adam DJ, Beard JD, Cleveland T, Bell J, Bradbury AW, Forbes JF, Fowkes FGR, Gillepsie I, Ruckley CV, Raab G, Storkey H. participants Bt. Bypass versus angioplasty in severe ischaemia of the leg (basil): Multicentre, randomised controlled trial.[see comment] Lancet. 2005;366:1925–1934. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 25.Goodney PP, Nolan BW, Schanzer A, Eldrup-Jorgensen J, Bertges DJ, Stanley AC, Stone DH, Walsh DB, Powell RJ, Likosky DS, Cronenwett JL. Factors associated with amputation or graft occlusion one year after lower extremity bypass in northern new england. Ann Vasc Surg. 2010;24:57–68. doi: 10.1016/j.avsg.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 26.Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. doi: 10.1016/j.jvs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 27.The society for vascular surgery’s vascular quality initiative. The Vascular Quality Initiative. www.vascularqualityinitiative.org.
- 28.Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB, Chong V, Fabri PJ, Gibbs JO, Grover F, Hammermeister K, Irvin G, 3rd, McDonald G, Passaro E, Jr., Phillips L, Scamman F, Spencer J, Stremple JF. The department of veterans affairs’ nsqip: The first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National va surgical quality improvement program. Annals of Surgery. 1998;228:491–507. doi: 10.1097/00000658-199810000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw RE, Anderson HV, Brindis RG, Krone RJ, Klein LW, McKay CR, Block PC, Shaw LJ, Hewitt K, Weintraub WS. Development of a risk adjustment mortality model using the american college of cardiology-national cardiovascular data registry (acc-ncdr) experience: 1998-2000. J Am Coll Cardiol. 2002;39:1104–1112. doi: 10.1016/s0735-1097(02)01731-x. [DOI] [PubMed] [Google Scholar]
- 30.Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in medicare patients. J Vasc Surg. 2011;54:420–426. 426, e421. doi: 10.1016/j.jvs.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho V, Wirthlin D, Yun H, Allison J. Physician supply, treatment, and amputation rates for peripheral arterial disease. J Vasc Surg. 2005;42:81–87. doi: 10.1016/j.jvs.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Lumsden AB, Davies MG, Peden EK. Medical and endovascular management of critical limb ischemia. J Endovasc Ther. 2009;16:II31–62. doi: 10.1583/08-2657.1. [DOI] [PubMed] [Google Scholar]
- 33.Bosiers M, Deloose K, Verbist J, Peeters P. Update management below knee intervention. Minerva Cardioangiol. 2009;57:117–129. [PubMed] [Google Scholar]
- 34.Rosales OR, Mathewkutty S, Gnaim C. Drug eluting stents for below the knee lesions in patients with critical limb ischemia : Long-term follow-up. Catheter Cardiovasc Interv. 2008;72:112–115. doi: 10.1002/ccd.21557. [DOI] [PubMed] [Google Scholar]
- 35.Rogers JH, Laird JR. Overview of new technologies for lower extremity revascularization. Circulation. 2007;116:2072–2085. doi: 10.1161/CIRCULATIONAHA.107.715433. [DOI] [PubMed] [Google Scholar]
- 36.Maurel B, Paumier A, Jacobi D, Bleuet F, Martinez R, Lermusiaux P. Ambulatory percutaneous angioplasty in patients with claudication. Ann Vasc Surg. 2011;25:191–196. doi: 10.1016/j.avsg.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 37.Egorova NN, Guillerme S, Gelijns A, Morrissey N, Dayal R, McKinsey JF, Nowygrod R. An analysis of the outcomes of a decade of experience with lower extremity revascularization including limb salvage, lengths of stay, and safety. J Vasc Surg. 2010;51:878–885. 885, e871. doi: 10.1016/j.jvs.2009.10.102. [DOI] [PubMed] [Google Scholar]
- 38.Duijm LE, van der Rijt RH, Cuypers PW, Tielbeek AV, Receveur KJ, Douwes-Draaijer P, Buth J. Outpatient treatment of arterial inflow stenoses of dysfunctional hemodialysis access fistulas by retrograde venous access puncture and catheterization. J Vasc Surg. 2008;47:591–598. doi: 10.1016/j.jvs.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Samson RH. Setting up an outpatient imaging center: Adding computed tomographic angiography, magnetic resonance angiography and an outpatient angiography suite to surgeon-run vascular laboratories. Perspect Vasc Surg Endovasc Ther. 2008;20:333–337. doi: 10.1177/1531003508325056. [DOI] [PubMed] [Google Scholar]
- 40.Burns BJ, Phillips AJ, Fox A, Boardman P, Phillips-Hughes J. The timing and frequency of complications after peripheral percutaneous transluminal angioplasty and iliac stenting: Is a change from inpatient to outpatient therapy feasible? Cardiovasc Intervent Radiol. 2000;23:452–456. doi: 10.1007/s002700010103. [DOI] [PubMed] [Google Scholar]
- 41.Tarry WC, Walsh DB, Birkmeyer NJ, Fillinger MF, Zwolak RM, Cronenwett JL. Fate of the contralateral leg after infrainguinal bypass. J Vasc Surg. 1998;27:1039–1047. doi: 10.1016/s0741-5214(98)70007-2. discussion 1047-1038. [DOI] [PubMed] [Google Scholar]
- 42.Bubolz T, Emerson C, Skinner J. State spending on dual eligibles under age 65 shows variations, evidence of cost shifting from medicaid to medicare. Health Aff (Millwood) 2012;31:939–947. doi: 10.1377/hlthaff.2011.0921. [DOI] [PubMed] [Google Scholar]
- 43.Goodney PP, Fisher ES, Cambria RP. Roles for specialty societies and vascular surgeons in accountable care organizations. J Vasc Surg. 2012;55:875–882. doi: 10.1016/j.jvs.2011.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fisher ES, McClellan MB, Bertko J, Lieberman SM, Lee JJ, Lewis JL, Skinner JS. Fostering accountable health care: Moving forward in medicare. Health Aff (Millwood) 2009;28:w219–231. doi: 10.1377/hlthaff.28.2.w219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher ES, Staiger DO, Bynum JP, Gottlieb DJ. Creating accountable care organizations: The extended hospital medical staff. Health Aff (Millwood) 2007;26:w44–57. doi: 10.1377/hlthaff.26.1.w44. [DOI] [PMC free article] [PubMed] [Google Scholar]