Abstract
Objective
Although cilostazol is commonly used as an adjunct after peripheral vascular interventions, its efficacy remains uncertain. We assessed the effect of cilostazol on outcomes after peripheral vascular interventions using meta-analytic techniques.
Methods
We searched MEDLINE (1946–2012), Cochrane CENTRAL (1996–2012), and trial registries for studies comparing cilostazol in combination with antiplatelet therapy to antiplatelet therapy alone after peripheral vascular interventions. Treatment effects were reported as pooled risk/hazard ratio (HR) with random-effects models.
Results
Two randomized trials and four retrospective cohorts involving 1522 patients met inclusion criteria. Across studies, mean age ranged from 65 to 76 years, and the majority of patients were male (64%–83%); mean follow-up ranged from 18 to 37 months. Most interventions were in the femoropopliteal segment, and overall, 68% of patients had stents placed. Fooled estimates demonstrated that the addition of cilostazol was associated with decreased restenosis (relative risk [RR], 0.71; 95% confidence interval [CI], 0.60–0.84; P < .001), improved amputation-free survival (HR, 0.63; 95% CI, 0.47–0.85; P = .002), improved limb salvage (HR, 0.42; 95% CI, 0.27–0.66; P < .001), and improved freedom from target lesion revascularization (RR, 1.36; 95% CI, 1.14–1.61; P < .001). There was no significant reduction in mortality among those receiving cilostazol (RR, 0.73; 95% CI, 0.45–1.19; P = .21).
Conclusions
The addition of cilostazol to antiplatelet therapy after peripheral vascular interventions is associated with a reduced risk of restenosis, amputation, and target lesion revascularization in our meta-analysis of six studies. Consideration of cilostazol as a medical adjunct after peripheral vascular interventions is warranted, presuming these findings are broadly generalizable.
During the past 2 decades, endovascular techniques including angioplasty and stent placement have been incorporated into treatment paradigms for patients with peripheral arterial disease.1 Although there are advantages to using minimally invasive techniques for the treatment of peripheral arterial disease, the long-term durability of these interventions remains a significant issue. Late clinical failure in the form of restenosis may compromise clinical outcomes and can necessitate repeated interventions, imparting additional procedural risk and cost.2
Pharmaceutical treatments may decrease postintervention restenosis within treated arterial segments,3,4 potentially resulting in better long-term clinical outcomes and less overall treatment cost by reducing the need for serial interventions. Cilostazol, a phosphodiesterase inhibitor, has pleiotropic effects including inhibition of platelet aggregation, direct arterial vasodilation, and prevention of intimal hyperplasia. Previous reports demonstrated the effectiveness of cilostazol therapy for the prevention of restenosis after percutaneous coronary intervention6,7 and suggested similar benefit after peripheral arterial intervention.8—14 However, no study has summarized the current data demonstrating the effectiveness of cilostazol in preventing restenosis or improving clinical outcomes after peripheral endovascular interventions.
The aim of this study was to systematically review and analyze the effect of cilostazol (in addition to antiplatelet medication) compared with antiplatelet medication alone in preventing restenosis and improving clinical outcomes after endovascular therapy for lower extremity peripheral arterial disease, specifically angioplasty and stent placement.
METHODS
Protocol and study eligibility criteria
We used methodology recommended by the Cochrane Collaboration15 to identify appropriate studies. To report our methods and findings, we used guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.16 We included studies of any design that met the following criteria:
The population studied must be patients undergoing endovascular treatment (angioplasty or stenting) for infrainguinal lower extremity peripheral vascular disease.
The intervention must be cilostazol in the periprocedural setting.
The comparison group may be no cilostazol, an anti- platelet medication, or placebo.
The minimum duration of follow-up had to be 6 months.
The study reported at least one prespecified outcome of interest (restenosis, freedom from amputation, mortality).
We were unable to restrict our analysis to randomized controlled trials (RCTs) because of the small number of studies with this design. Comparison groups included patients taking either no cilostazol or an alternative antiplatelet regimen (tidopidine). We included studies of both angioplasty and stenting, as practice patterns vary and potential benefit is of interest after both procedures. The minimum duration of follow-up was chosen on the basis of the inherent time interval between endovascular treatment and the development of intimal hyperplasia.
Outcome measures
Our primary outcome measure of interest was postintervention restenosis as measured by duplex ultrasonography, computed tomography angiography, or angiography. Restenosis was defined as a peak systolic velocity ratio ≥2.413,14 or >50% vessel diameter reduction10 on follow-up duplex ultrasound examination. Restenosis was reported by three of the studies. Other outcomes of interest included patency, occlusion, freedom from target lesion revascularization, limb salvage, and amputation-free survival. These outcomes were based on the suggested objective performance goals for evaluating the effectiveness of endovascular interventions in the lower extremity.17 In addition to these potential benefits, harms including bleeding complications and adverse drug effects were also considered important outcomes.
In each of the included studies, cilostazol therapy was continued for the duration of follow-up. Compliance with postprocedural cilostazol therapy was specifically detailed in three studies reporting 94%, 100%, and 90% compliance rates.8,10,14 Two of the studies used intention-to-treat analysis to ensure that any bias would underestimate cilostazol benefit.
Search methods and study selection
We searched MEDLINE (1946–2012) by the Ovid search engine and Cochrane CENTRAL (1996–2012) for potentially relevant studies in October 2012. We used exploded medical subject headers (MeSH) terms and keywords to generate sets for the following themes: cilostazol, restenosis, stent, peripheral artery disease. The terms restenosis, stent, and peripheral artery disease were searched with the Boolean term or and then intersected with cilostazol with the Boolean term and. We conducted our latest search on October 31, 2012. We considered all types of publications eligible, and no language or other limits were applied.
To identify published and unpublished studies missed during the primary search, we manually searched the reference lists of included articles. To identify unpublished studies, we screened proceedings from the Society for Vascular Surgery Annual Meeting between 2000 and 2012. Two unblinded reviewers independently screened titles and abstracts of the 566 articles from our initial search for eligibility. After obtaining the published text of potentially relevant articles, the same reviewers performed a formal full-text assessment to determine final eligibility. Disagreements about eligibility between reviewers were resolved with a third reviewer by discussion and consensus. All studies that met inclusion criteria were used for data extraction. Articles were screened for overlapping populations to avoid duplicate outcome reporting. We developed a standardized data collection form, tested this form on two studies that met the inclusion criteria, and refined the form accordingly. Two unblinded reviewers independently extracted data from each included study, and a third author checked the resulting data.
Assessment of methodological quality
We formally assessed the methodological quality of each study with two validity scales. For RCTs, we used the Cochrane Collaboration’s risk of bias assessment tool, which contains seven domains that assess for the risk of bias in randomized trials, each of which is categorized as low, high, or unclear risk of bias.18 For the observational studies, we used the Newcastle-Ottawa quality assessment scale, which allocates stars (0–9) for the quality of population selection, comparability, exposure, and outcomes.19
Analysis
We used Revman 5.1 software to analyze and pool each outcome.20 Treatment effects for dichotomous outcomes were assessed through comparison of the hazard ratio (HR) and the relative risk (RR) ratio with the 95% confidence interval (CI) or P value, with each separate outcome assessed at a 99.3% significance level to control type I error across the studies. For the outcome of restenosis, we used raw event rates when necessary to allow formal data pooling. Summary estimates of overall effects were created by incorporating the direction, magnitude, and statistical significance from different studies for like outcomes. Quantitative data summarization was performed on several outcome categories, including patency-related, limb-related, and mortality-related outcome groups. Adjusted outcomes were used when available. Adjusted HRs were reported in cohort studies, whereas RR ratios were reported in RCTs. During analysis, we ranked outcomes by follow-up duration and found no significant trend or association between duration and treatment effect.
In the event that relevant data were not provided in the manuscript, we requested this information from the authors. If these requests were not answered and adequate data for a particular outcome could not be obtained, we excluded the study from the analysis of that outcome.
We assessed variability among the studies by heterogeneity testing when three or more studies reported the same outcome and the results were pooled. We used I2 greater than 50% as a threshold for significant heterogeneity. We assessed publication bias by visually inspecting each study for outcome effect size and sample size as well as by looking for a paucity of small negative studies, which suggests the potential for publication bias.
RESULTS
Patient and study characteristics
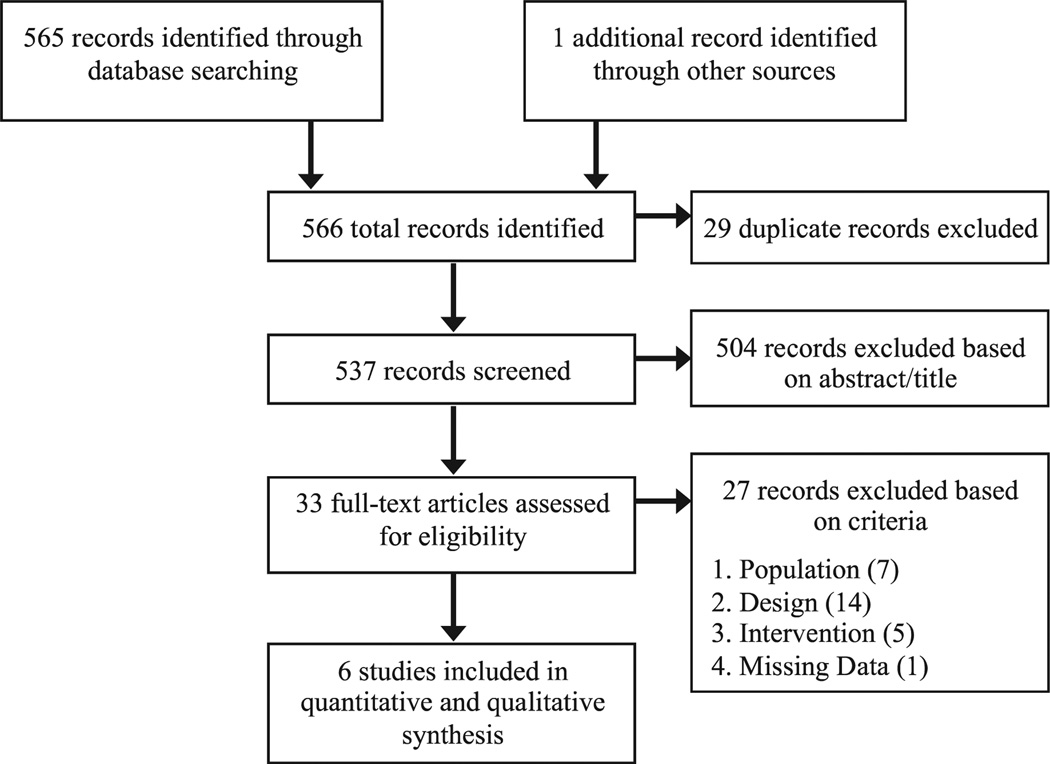
Our initial search strategy identified 537 unique studies that we considered for inclusion. We excluded 504 articles on the basis of screening titles and abstracts and 27 on the basis of full-text review. Ultimately, six studies met all inclusion criteria (Fig 1). The Table displays the baseline characteristics of the two RCTs and four retrospective cohorts that met inclusion criteria. A total of 1522 patients were included in our review. A majority (87%) were from retrospective cohort studies. All studies were conducted in Japan and published between 2008 and 2012. All compared cilostazol with either no cilostazol10,12–4 (n = 4) or an alternative antiplatelet medication8,9 (tidopidine, n = 2), with both groups receiving various cointerventions (aspirin with or without an adjunct antiplatelet medication). Across studies, the mean age ranged from 65 to 76 years, and 64% to 83% were men. The majority of interventions were in the femoropopliteal segment (vs infrapopliteal location), and overall, 68% of patients underwent stent placement. Mean follow-up ranged from 18 to 37 months. The indication for intervention (critical limb ischemia vs claudication) varied across studies. In each study, patient comorbidities were balanced in both treatment groups (in two studies, propensity score matching was used to achieve similar comparison groups). In one report, the entire population was hemodialysis dependent.10
Fig 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Table.
Characteristics of included studies
| Author | Year | Design | No. | Age, years |
Male, % |
Indication, % CLIa |
Lesion location, % femoropoplitealb |
Intervention, % stentc |
Comparisond | Cointerventiond | Mean follow-up, months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soga | 2011 | Retrospective cohort | 618 | 72 ± 11 | 69% | 100% | 35% | 37% | No cilostazol | Aspirin, dopidogrel | 21 |
| Soga | 2012 | Retrospective cohort | 562 | 73 ± 9 | 76% | 26% | 100% | 100% | No cilostazol | Aspirin, clopidogrel | 25 |
| Ikushima | 2011 | Retrospective cohort | 28 | 76 ± 7 | 82% | 36% | 100% | 100% | Tidopidine | Aspirin | 18 |
| Iishi | 2010 | Retrospective cohort | 109 | 65 ± 11 | 64% | 21% | 100% | 65% | No cilostazol | Aspirin | 37 |
| Soga | 2009 | RCT | 78 | 71 ± 8 | 83% | 0% | 100% | 46% | No cilostazol | Aspirin, tidopidine | 24 |
| Iida | 2008 | RCT | 127 | 70 ± 8 | 72% | 25% | 100% | 87% | Tidopidine | Aspirin | 36 |
CLI, Critical limb ischemia; RCT, randomized controlled trial.
Indications included critical limb ischemia vs claudication.
Lesion locations include femoropopliteal and infrapoplitcal.
Interventions include stenting (with or without angioplasry) and angioplasty alone.
Aspirin dose, 81–200 mg/day; ticlopidine dose, 200 mg/day; clopidogrel dose, 75 mg/day.
There was minimal heterogeneity from study to study with a consistent set of findings for patency- and limb-related outcomes. The mortality results of the retrospective cohort studies were heterogeneous (I2 = 63%). Sensitivity analysis demonstrated that removal of studies with a majority of critical limb ischemia patients yielded an acceptably low I1. Thus, the heterogeneity among these studies is likely to be due to the severity of underlying illness associated with patients presenting with critical limb ischemia.
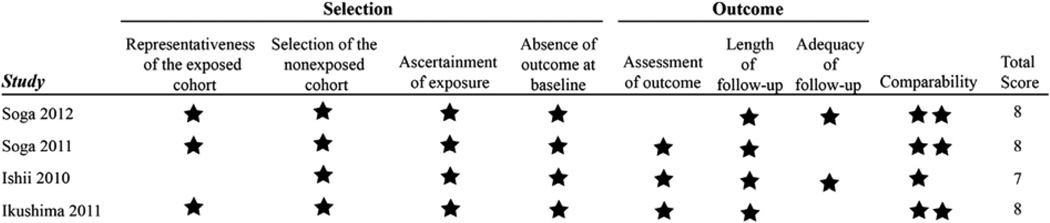
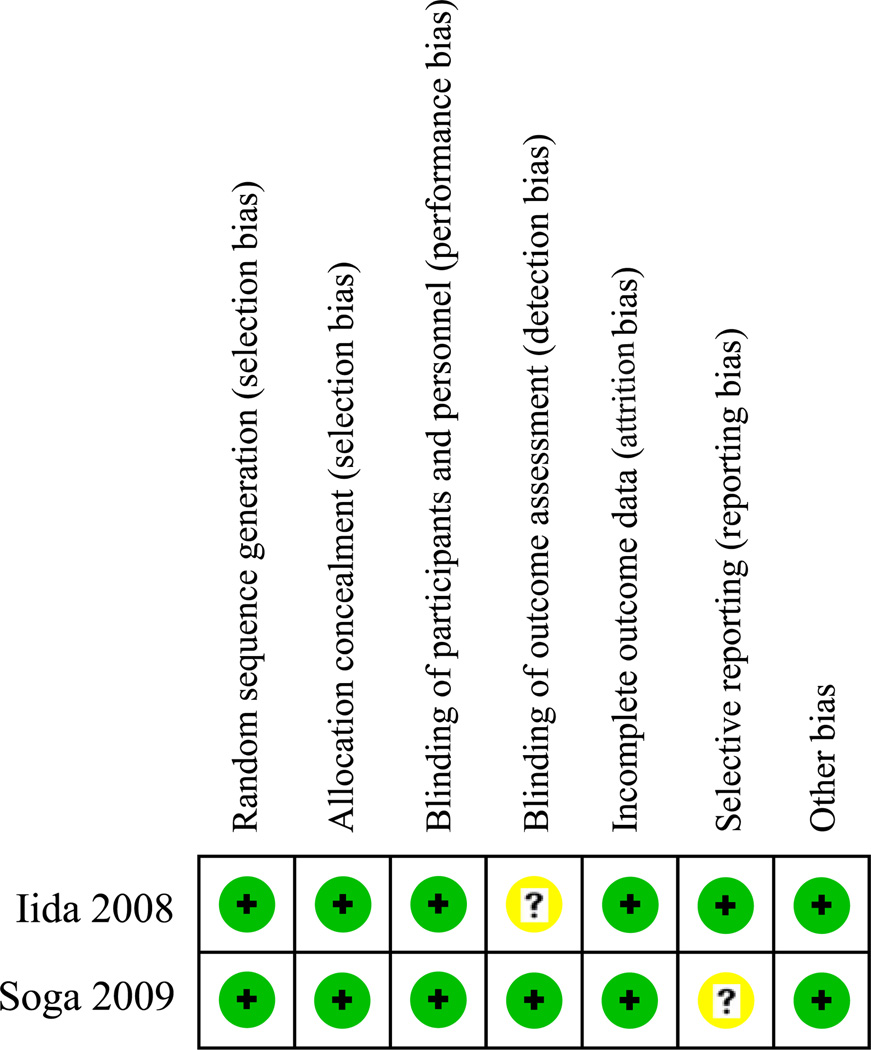
Figs 2 and 3 outline the assessment of methodological quality for each study. Overall, methodological quality was good. Important limitations include the retrospective nature of the four observational studies with potential for selection bias and related confounding. Two of the retrospective cohorts used propensity score matching to mitigate this risk10,13; the other two used adjustment techniques in their regression analyses.9,12 In addition, there was variation in the cointerventions occurring in the cilostazol and comparison groups within the studies. The RCTs had low risk of bias in most of the Cochrane risk-of-bias tool domains, but the open-label design precluded blinding of the study participants and is a potential source of performance bias.
Fig 2.
Newcastle-Ottawa quality assessment scale.
Fig 3.
Cochrane risk-of-bias tool.
Main findings
To organize and facilitate the merging of outcomes reported across the studies, outcomes were divided into three broad categories: patency-related outcomes (including restenosis, patency, and occlusion), limb-related outcomes (including limb salvage, amputation-free survival, and freedom from target lesion revascularization), and mortality-related outcomes. Outcomes from both RCTs and retrospective cohort studies were collectively considered. The follow-up interval associated with these outcomes ranged from 2 to 6 years.
Patency-related outcomes
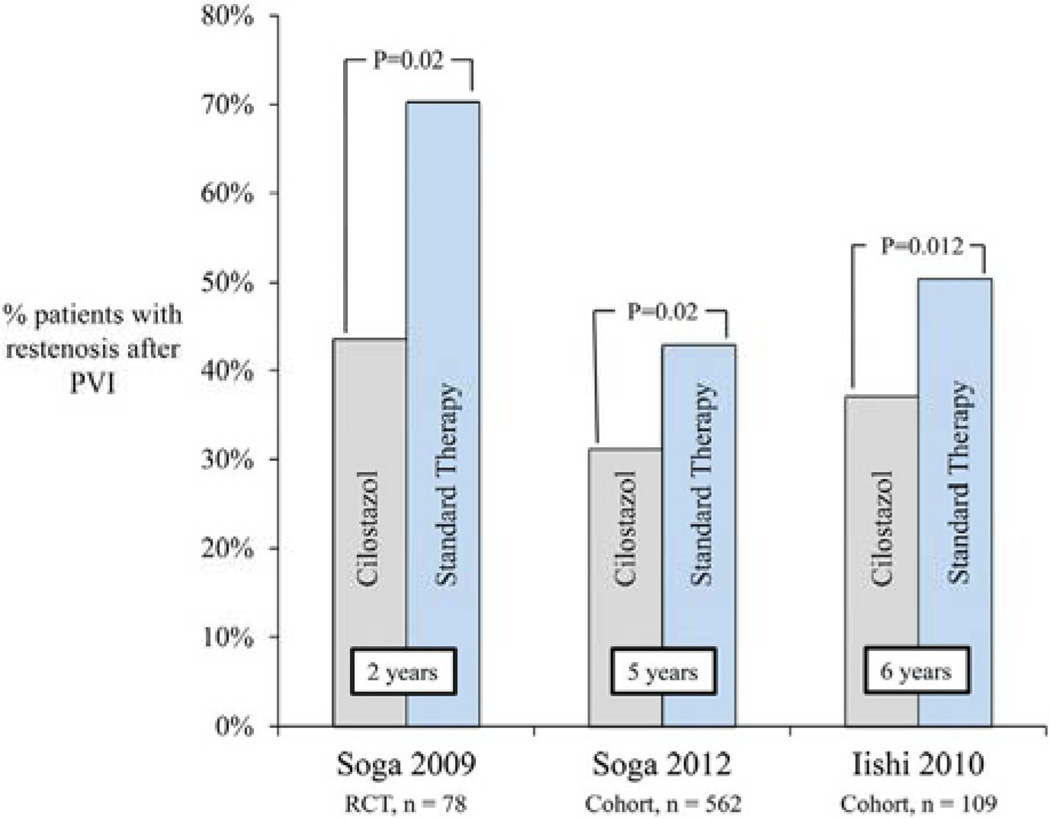
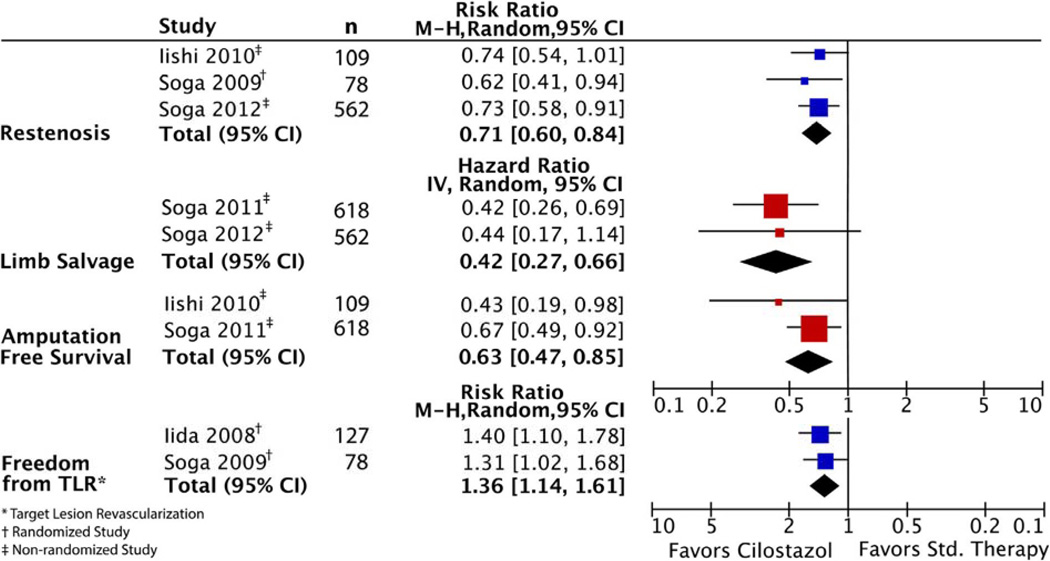
Two studies comparing cilostazol with ticlopidine reported patency; both favored cilostazol (75% vs 30% at 2 years; P = .007, and 73% vs 51% at 3 years; P = .01, respectively).8,9 Restenosis was reported across three studies (Fig 4). In one RCT, cilostazol was associated with a decreased risk of restenosis compared with no cilostazol at 2 years (RR, 0.62; 95% CI, 0.41–0.94; P = .02).14 Similar findings were shown in the two retrospective cohort studies. The first study reported an adjusted HR for restenosis of 0.60 at 5 years (95% CI, 0.43–0.84; P = .003), with a calculated risk ratio of 0.73 (95% CI, 0.58–091; P = .02).13 The second study demonstrated a decreased risk of restenosis at 6 years with an HR of 0.51 (95% CI, 0.27–0.84; P = .008), with a calculated risk ratio of 0.74 (95% CI, 0.54–1.01; P = .05).11 When combined by meta-analytic techniques, the summary estimate demonstrated lower rates of restenosis in patients taking cilostazol (RR, 0.71; 95% CI, 0.60–0.84; P < .001; Fig 5).
Fig 4.
Restenosis after peripheral vascular intervention (PVT). RCT, Randomized controlled trial.
Fig 5.
Forest plot of main outcomes. CI, Confidence interval.
Vessel occlusion was reported in two studies. The rate of in-stent occlusion in one of the RCTs was 5.1% in the cilostazol group vs 16.2% in the group not receiving cilostazol at 2 years, but this was not statistically significant (P = .12).14 However, in the cohort study reporting occlusion, cilostazol was associated with reduced in-stent occlusion at 5 years with an adjusted HR of 0.55 (95% CI, 0.31–0.97; P = .04).13
Limb-related outcomes
Limb-related outcomes reported in two or more studies included freedom from target lesion revascularization, amputation-free survival, and limb salvage.
Freedom from target lesion revascularization was improved in the cilostazol groups with RR 1.40 (95% CI, 1.10–1.78; P = .04) and RR 1.31 (95% CI, 1.02–1.68; P < .05) at 2 and 3 years, respectively.8,14 Amputation-free survival was improved in the cilostazol group in both studies reporting this outcome with HR 0.67 (95% CI, 0.49–0.92; P = .01) and HR 0.43 (95% CI, 0.19–0.98; P < .05) at 5 and 6 years, respectively.10,12 Finally, limb salvage at 5 years in patients with critical limb ischemia was reported in two cohort studies. In the first study, cilostazol was associated with improved limb salvage (adjusted HR, 0.42; 95% CI, 0.26–0.69; P < .001).12 In the second study, there was a trend toward improved limb salvage, but this was not statistically significant (adjusted HR, 0.44; 95% CI, 0.17–1.14; P = .09).13 The summary estimates significantly favor cilostazol for each set of limb-related outcomes (freedom from target lesion revascularization, amputation-free survival, and limb salvage). When combined by meta-analytic techniques, the summary estimate for freedom from target lesion revascularization is RR 1.36 (95% CI, 1.14–1.61; P < .001); for amputation-free survival, HR 0.63 (95% CI, 0.47–0.85; P = .002); and for limb salvage, HR 0.42 (95% CI, 0.27–0.66; P < .001; Fig 5).
Mortality-related, outcomes
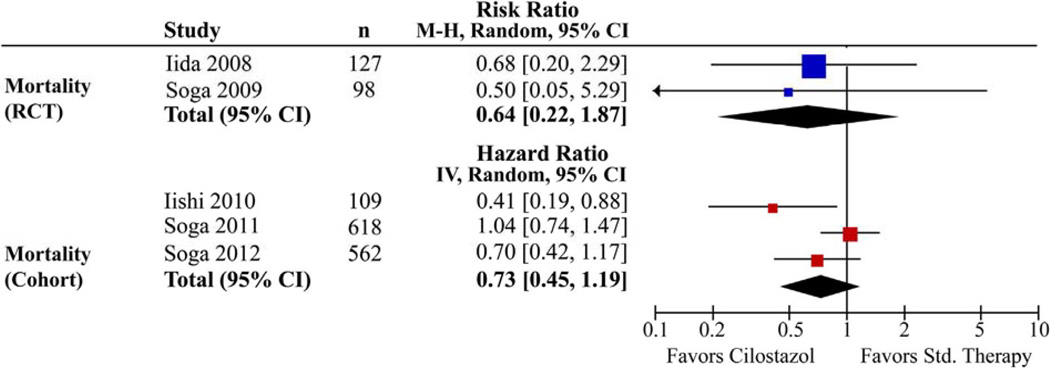
Mortality-related outcomes were reported in five studies. In both RCTs, cilostazol therapy had no significant effect on mortality at 2 years (RR, 0.50; 95% CI, 0.05–5.29; P = .6) and 4 years (RR, 0.68; 95% CI, 0.20–2.29; P = .53).8,14 The retrospective cohort studies demonstrated similar results, with no difference in all-cause mortality at 5 years (HR, 0.7; 95% CI, 0.42–1.17; P = .2)13 and no difference in overall survival at 5 years (HR, 1.04; 95% CI, 0.74–1.47; P = .84)12 between the cilostazol and comparison groups. One study, however, documented improvement in overall survival among the patients in the cilostazol group (HR, 0.41; 95% CI, 0.19–0.88; P = .02).10 The summary estimates calculated for mortality-related outcomes showed no significant difference between the cilostazol and comparison groups in both the RCTs (RR, 0.64; 95% CI, 0.22–1.87; P = .4) and cohort studies (HR, 0.73; 95% CI, 0.45–1.19; P = .2) (Fig 6).
Fig 6.
Forest plot of mortality outcomes. CI, Confidence interval; RCT, randomized controlled trial.
Regarding adverse drug effects, one RCT found no difference in bleeding complications between the cilostazol and ticlopidine groups.8 Adverse drug effects reported in three of the studies included palpitations (7%), headache (4%), and peripheral edema (2%).8,10,14
DISCUSSION
Main findings
Improving the durability of endovascular interventions is important, as these procedures are common and treatment failure can have severe consequences. In the current review, we attempt to systematically evaluate the available evidence on the effectiveness of cilostazol to improve durability and to prevent adverse clinical outcomes after peripheral endovascular interventions.
Across studies, patients treated with cilostazol after peripheral intervention consistently experienced improved patency- and limb-related outcomes. Whereas patency and mortality are important, limb-related outcomes may best capture outcomes most important to patients after peripheral vascular interventions. In the groups receiving cilostazol, there were improvements in limb salvage, freedom from target lesion revascularization, and amputation-free survival across multiple studies. Overall, there was no significant improvement in mortality-related outcomes (death, cardiovascular death, overall survival) across studies, with the exception of one retrospective study reporting increased survival in patients receiving cilostazol.
The findings of improved patency- and limb-related outcomes were consistent across studies despite variation in indication, intervention, and comparison. Associations between cilostazol and improved freedom from target lesion revascularization were established by RCT; associations between cilostazol and improved limb salvage and amputation-free survival were established from retrospective cohort data. Whereas the consistency of these findings provides important information about the effectiveness of cilostazol, all studies were performed in a similar setting (Japan), where the pharmaceutical company that markets cilostazol is located. Given the inherent differences in population demographics and exposures between Japan and other settings, there may be inadequate evidence to generalize findings from one population to heterogeneous populations worldwide.
In general, the included studies showed improvement in patency-related outcomes and minimal impact on mortality-related outcomes with cilostazol therapy. These results are similar to those of several large meta-analyses reported in the cardiology literature regarding cilostazol after percutaneous coronary intervention, documenting decreased restenosis and improved freedom from revascularization in this population.6,7 Two studies of cilostazol after peripheral intervention narrowly missed meeting inclusion criteria (because of inclusion of iliac interventions and inadequate length of follow-up) but drew similar conclusions about the benefit of cilostazol.11,21
Whereas these results support the consideration of cilostazol as a medical adjunct after peripheral intervention, there are several potential barriers to promoting cilostazol use in appropriate candidates in a real-world clinical environment. Cost may be a significant issue for some patients, as the purchase price for cilostazol ranges from approximately $4 to $5 per day.22 Previous reports have established the relative safety of cilostazol and an acceptable low risk of serious side effects and no increase in bleeding events over placebo,23,24 but polypharmacy and compliance are concerns in this patient population, as medication regimens become increasingly complex. Further, across the studies, harms of cilostazol were not consistently addressed.
Limitations
The six studies that met inclusion criteria differed in several important ways. The indication for intervention varied from 100% critical limb ischemia to 100% claudication, with several studies reporting a mixed population. Whereas measures like amputation-free survival are important in patients with critical limb ischemia, the event rate is low in claudicants, and this outcome may not be accurately reflected in this population. Mortality was a rare outcome, leading to wide confidence intervals and thus low precision in summary estimates. This meta-analysis may be underpowered to detect associations between cilostazol and mortality. Within the studies, outcomes were not stratified by presenting symptoms (claudication vs critical limb ischemia) or treated segment (femoropopliteal vs infrapopliteal), limiting our ability to study these patient subgroups separately.
The type of intervention also varied between studies: the majority was femoropopliteal stent placement, but balloon angioplasty of femoropopliteal and tibial arteries was included in several studies. The studies also varied in their comparisons and cointerventions. Four studies compared cilostazol with no cilostazol, whereas two studies compared cilostazol with ticlopidine, an antiplatelet medication. Aspirin was an important cointervention provided in both arms of each study, and depending on surgeon preference, dopidogrel was given as a cointervention to both groups in some studies. Despite these differences in comparison groups and cointerventions, the studies had similar outcomes: improvement in patency and freedom from target lesion revascularization with no significant difference in mortality.
In general, the quality of the evidence was good with low risk of bias across most RCT domains and high-quality scores for the retrospective cohort studies. However, the RCTs were open label, and sample sizes were relatively small (n = 80 and n = 127). Lack of blinding within the RCT and cohort studies is a significant potential source of bias. There may be patient characteristics that influenced the providers’ decision to add cilostazol, potentially introducing unmeasured selection bias. Overall, the consistency of the results across studies allowed us to integrate outcomes, enhancing the internal validity of the review. Adjusted results were used whenever possible to minimize the potential for confounding.
One important consideration is the potential for publication bias; all reviewed studies demonstrated significant benefit in one or more of the outcomes measured. The assessment of publication bias is difficult, as few studies met our inclusion criteria. To minimize this bias, our search strategy included abstracts, trial registries, and meeting proceedings in an attempt to minimize publication bias and to find all relevant studies, and no studies or abstracts reported lack of a positive finding (or any negative finding) between cilostazol and outcome.
Other factors related to the introduction of bias included variability in the measuring and reporting of outcomes, necessitating qualitative analysis with limited formal statistical pooling of data. Several authors were contacted for additional data about unreported outcomes without success; thus, there were often only two or three studies reporting a given outcome. Further, our strict inclusion criteria precluded the use of some potentially relevant data. One study included iliac artery interventions11; although the main results were similar to those of other studies included in our review, iliac interventions have better patency-related outcomes, and this study was not included. A second study on cilostazol administration after tibial angioplasty had 3-month follow-up21; we thought it inappropriate to include this as the time interval in which to observe restenosis may be inadequate.
CONCLUSIONS
On the basis of available evidence, adding cilostazol to antiplatelet therapy after endovascular interventions for lower extremity peripheral arterial disease is associated with improved patency- and limb-related outcomes, resulting in decreased restenosis as well as improved limb salvage, amputation-free survival, and freedom from target lesion revascularization. Consideration of cilostazol as a medical adjunct in appropriate candidates is warranted to potentially improve limb-related outcomes and durability after lower extremity angioplasty and stent placement for peripheral arterial disease. All of the studies reviewed on cilostazol after peripheral interventions were performed in a similar setting in Japan, and the majority of these studies were retrospective in nature. Further RCTs on heterogeneous populations are needed to investigate the generalizability of these findings.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
Presented at the Twenty-seventh Annual Meeting of the Eastern Vascular Society, White Sulphur Springs, WVa, September 21, 2013.
AUTHOR CONTRIBUTIONS
Conception and design: CW, DS, DW, RP, PG
Analysis and interpretation: CW, SG, RL
Data collection: CW, SG, RL
Writing the article: CW, SG, RL, PG
Critical revision of the article: CW, DS, DW, RP, PG
Final approval of the article: CW, DS, DW, RP, PG
Statistical analysis: CW, SG
Obtained funding: Not applicable
Overall responsibility: PG
REFERENCES
- 1.Schillings M, Sabeti S, Loewe C, Dick P, Amighi J, Mlekusch W, et al. Balloon angioplasty versus implantation of nitinol stems in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 2.Mitra AK, Agrawal DK. In stent restenosis: bane of the stent era. J Clin Pathol. 2006;59:232–239. doi: 10.1136/jcp.2005.025742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorffler-Melly J, Koopman MM, Prins MH, Buller HR. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev. 2005;1:CD002071. doi: 10.1002/14651858.CD002071.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Robertson L, Ghouri MA, Kovacs F. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD002071.pub3. CD002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dindyal S, Kyriakides C. A review of cilostazol, a phosphodiesterase inhibitor, and its role in preventing both coronary and peripheral arterial restenosis following endovascular therapy. Recent Pat Cardiovasc Drug Discov. 2009;4:6–14. doi: 10.2174/157489009787260025. [DOI] [PubMed] [Google Scholar]
- 6.Biondi-Zoccai GG, Lotrionte M, Anselmino M, Moretti C, Agostoni P, Testa L, et al. Systematic review and meta-analysis of randomized clinical trials appraising the impact of cilostazol after percutaneous coronary intervention. Am Heart J. 2008;155:1081–1089. doi: 10.1016/j.ahj.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 7.Jang JS, Jin HY, Seo JS, Yang TH, Kim DK, Kim DS, et al. A meta-analysis of randomized controlled trials appraising the efficacy and safety of cilostazol after coronary artery stent implantation. Cardiology. 2012;122:133–143. doi: 10.1159/000339238. [DOI] [PubMed] [Google Scholar]
- 8.Iida O, Nanto S, Uematsu M, Morozumi T, Kitakaze M, Nagata S. Cilostazol reduces restenosis after endovascular therapy in patients with femoropopliteal lesions. J Vase Surg. 2008;48:144–149. doi: 10.1016/j.jvs.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 9.Ikushima I, Yonenaga K, Iwakiri H, Nagoshi H, Kumagai H, Yamashita Y. A better effect of cilostazol for reducing in-stent restenosis after femoropopliteal artery stent placement in comparison with ticlopidine. Med Devices (Auckl) 2011;4:83–89. doi: 10.2147/MDER.S21629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii H, Kumada Y, Toriyama T, Aoyama T, Takahashi H, Tanaka M, et al. Effects of oral cilostazol 100 mg BID on long-term patency after percutaneous transluminal angioplasty in patients with femoropopliteal disease undergoing hemodialysis: a retrospective chart review in Japanese patients. Clin Ther. 2010;32:24–33. doi: 10.1016/j.clinthera.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 11.Ishii H, Kumada Y, Toriyama T, Aoyama T, Takahashi H, Yamada S, et al. Cilostazol improves long-term patency after percutaneous transluminal angioplasty in hemodialysis patients with peripheral artery disease. Clin J Am Soc Nephrol. 2008;3:1034–1040. doi: 10.2215/CJN.05761207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soga Y, Iida O, Hirano K, Suzuki K, Kawasaki D, Miyashita Y, et al. Impact of cilostazol after endovascular treatment for infrainguinal disease in patients with critical limb ischemia. J Vase Surg. 2011;54:1659–1667. doi: 10.1016/j.jvs.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Soga Y, Iida O, Hirano K, Suzuki K, Yokoi H, Nobuyoshi M. Restenosis after stent implantation for superficial femoral artery disease in patients treated with cilostazol. Catheter Cardiovasc Interv. 2012;79:541–548. doi: 10.1002/ccd.23304. [DOI] [PubMed] [Google Scholar]
- 14.Soga Y, Yokoi H, Kawasaki T, Nakashima H, Tsurugida M, Hikichi Y, et al. Efficacy of cilostazol after endovascular therapy for femoropopliteal artery disease in patients with intermittent claudication. J Am Coll Cardiol. 2009;53:48–53. doi: 10.1016/j.jacc.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Cochrane handbook for systematic reviews of interventions. The Cochrane Collaboration. 2011 Available at: http://www.cochrane-handbook.org.
- 16.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 17.Conte MS, Geraghty PJ, Bradbury AW, Hevelone ND, Lipsitz SR, Moneta GL, et al. Suggested objective performance goals and clinical trial design for evaluating catheter-based treatment of critical limb ischemia. J Vase Surg. 2009;50:1462–1473. e1–e3. doi: 10.1016/j.jvs.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al., editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses; 3rd Symposium on Systematic Reviews: Beyond the Basics; 2000. [Google Scholar]
- 20.Review Manager (RevMan) 5.1. Copenhagen: The Nordic Cochrane Centre: Cochrane Collaboration. 2011 [Google Scholar]
- 21.Soga Y, Iida O, Kawasaki D, Hirano K, Yamaoka T, Suzuki K. Impart of cilostazol on angiographic restenosis after balloon angioplasty for infrapopliteal artery disease in patients with critical limb ischemia. Eur J Vase Endovasc Surg. 2012;44:577–581. doi: 10.1016/j.ejvs.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Dindyal S, Kyriakides C. Regarding “Impact of cilostazol after endovascular treatment for infrainguinal disease in patients with critical limb ischemia”. J Vase Surg. 2012;55:891. doi: 10.1016/j.jvs.2011.10.133. author reply: 892-2. [DOI] [PubMed] [Google Scholar]
- 23.Pratt CM. Analysis of the cilostazol safety database. Am J Cardiol. 2001;87:28D–33D. doi: 10.1016/s0002-9149(01)01719-2. [DOI] [PubMed] [Google Scholar]
- 24.Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-term Effects) J Vase Surg [serial on the Internet] 2008;47:330–336. doi: 10.1016/j.jvs.2007.10.009. Available at: http://onlinelibrary.wiley.eom/o/cochrane/clcentral/articles/993/CN-00628993/frame.html. [DOI] [PubMed] [Google Scholar]