Abstract
Background:
Schizophrenia is a chronic illness having varied etiology which affects cognition, emotion, perception, and other aspects of behavior. There are data which show possible role of oxidative stress and disturbance in antioxidant mechanisms in various neurological and neuropsychiatric disorders.
Materials and Methods:
Fifty drug-naive schizophrenic patients, who attended psychiatry outpatient department/inpatient department for the 1st time, were selected and compared with 50 age-sex matched healthy controls. The erythrocyte level of malondialdehyde (MDA) - a lipid peroxidation product and marker of oxidative stress, antioxidant enzymes - superoxide dismutase (SOD), glutathione peroxidase (GPX) was estimated. We also correlated the sociodemographic parameters and severity of illness (positive and negative syndrome scale score) with oxidative stress (MDA) and level of antioxidant enzymes (SOD, GPX).
Results:
The level of oxidative stress (MDA) was increased, and the levels of antioxidative enzymes (GPX and SOD) were decreased in schizophrenic patients as compared to normal healthy controls and the difference was statistically significant. No significant relationships of age, sex, educational status, marital status, and PANNS score with oxidative stress (MDA) and antioxidative enzymes (GPX and SOD) level in schizophrenic patients was found; but there was significant relationship of locality with oxidative stress (MDA) and antioxidative enzymes (GPX and SOD) level in schizophrenic patients was found. Urban population have a higher level of MDA, GPX, and SOD than the rural population.
Conclusion:
Our findings put great emphasis on the weak pro/antioxidant defense mechanisms and its role in the pathophysiology of schizophrenia. We can make recommendations of dietary nutritional supplementation and adjunct antioxidants therapy with antipsychotics to treat schizophrenics.
Keywords: Antioxidative enzymes, drug naive, oxidative stress, schizophrenia
INTRODUCTION
Schizophrenia is a clinical syndrome that involves cognition, emotion, perception, and other aspects of behavior. The expression of these manifestations varies across patients and with time, but the effect of the illness is often severe and is usually long-lasting. The disorder usually begins before age 25, persists throughout life, and affects persons of all social classes. Although schizophrenia is discussed as if it is a single disease; it probably comprises a group of disorders with heterogeneous etiologies.[1]
Oxidative stress is an imbalance between oxidants and antioxidants in favor of the oxidants, potentially leading to damage.[2] Antioxidant is “any substance that when present at low concentrations compared with that of an oxidizable substrate, significantly delays or inhibits oxidation of that substrate.”[3]
The potential toxicity of free radicals is counteracted by a number of cytoprotective enzymes and antioxidants that limit the damage. All cells in eukaryotic organisms contain powerful antioxidant enzymes. The three major antioxidant enzymes are superoxide dismutases (SOD), catalases and glutathione (GSH) peroxidases.[4] Although multiple factors can precipitate oxidative stress in cells, the neurotransmitter glutamate is the major effector of this process in the brain, primarily through activation of its ionotropic receptors.[5] The excitatory amino acids and neurotransmitters whose metabolism produces reactive oxygen species, are unique in the brain as sources of oxidative stress. Other sources are generated by the high and constant use of oxygen in the mitochondria to supply the energy needs of these tissues. Free radicals are also produced by cytochrome P450 electron transport and the monoamine oxidase activity of the outer mitochondrial membrane.
Role of oxidative stress has been reported in various neurological diseases such as Alzheimer's disease,[6] Parkinson's disease,[7] Huntington's disease,[8] and cognitive impairment in elderly patients.[9]
There are also evidences suggesting oxidative disturbances in psychiatric disorders such as bipolar mood disorder,[10] schizophrenia,[11] and depression.[12] Previous studies have predominantly examined products of lipid peroxidation and DNA oxidation as markers of oxidative damage. Most data demonstrating oxidative disturbances have examined indirect measures of oxidative status such as peripheral and brain levels of antioxidants, oxidative enzymes, and products. The direct measurement of free radicals is difficult because of their short half-lives and low titers.
A commonly used method of measuring lipid peroxidation is the performance of thiobarbituric acid reactive substances (TBARS) assays. TBARS are low-molecular-weight substances, consisting malondialdehyde (MDA), which are formed from the decomposition of unstable lipid peroxidation products and react with thiobarbituric acid (TBA) to form fluorescent adducts.[13] Deficiency of glutathione, the major intracellular antioxidant, in its reduced form (GSH), has been observed and suggested to be of pathophysiological significance in schizophrenia as early as 1934,[14] although differences did not reach statistical significance in that study. Significant GSH deficiency has subsequently been reported.[15] Most of the previous studies are done in patients who are on antipsychotics. There are reports that antipsychotics increases generation of free radical within the brain. Hence, the present study tries to examine the oxidative stress by measuring the level of MDA and level of antioxidant enzymes: SOD and glutathione peroxidase (GPX) in drug-naive schizophrenics patients in India. In this study, the correlation between sociodemographic characteristics and PANNS score with the level of MDA, SOD, GPX was also seen.
MATERIALS AND METHODS
The present study was conducted at the Department of Psychiatry, Jawaharlal Nehru Medical College and Hospital, Aligarh Muslim University, Aligarh. Fifty drug-naive patients of schizophrenia who attended psychiatry outpatient department (OPD) or were admitted in the psychiatry ward for the 1st time between July 2010 and September 2011 were selected. Informed consent was taken from each patient/caregivers and normal controls subjects. The study was approved by the Board of Studies of the Department of Psychiatry and Ethical Committee of Faculty of Medicine.
All the subjects were initially evaluated in detail on a semi-structured proforma which included sociodemographic characteristics, clinical history, physical and mental status examination. Diagnosis of schizophrenia was made according to International Classification Of Diseases-10 (ICD-10)[16] diagnostic criteria, independently by two psychiatrists. Then they were assessed using positive and negative syndrome scale (PANSS)[17] for severity of disease.
Inclusion criteria
All cases of schizophrenia that fulfilled ICD-10 diagnostic criteria of schizophrenia
All cases of schizophrenia that were drug-naïve and attended psychiatry OPD/inpatient department for the 1st time
The patients/caregivers who gave informed consent for participation in the study.
Exclusion criteria
Any history of physical and psychiatric illness
History of substance abuse.
Whole blood samples were obtained by venipuncture from patients and controls. These were collected in heparinized tubes. Obtained samples were then centrifuged, and plasma was carefully removed. The prepared red blood cells (RBCs) hemolysate were then used to estimate the following:
MDA by spectrophotometric measuring of TBA reactivity[18]
SOD was estimated using spectrophotometrical method based on auto-oxidation of pyrogallol[19]
Glutathione peroxide (GSX-Px) activity was measured according to the method of Moin.[20]
Subjects were then compared with 50 normal healthy individuals who were age and sex matched and belonging to the general population.
Statistical analysis
All Statistical analyses were done using SPSS software version 17 statistical package for window (Chicago Inc.). Continuous variables were expressed as mean ± standard deviation (Gaussian distribution), range and qualitative data were expressed as a percentage. Depending on normality distribution, unpaired t-test for independent samples and ANOVA was used for comparing continuous variables between two groups. Chi-square test was used to compare qualitative data. All P values were two-tailed and values of P < 0.05 were considered statistically significant. All confidence interval were calculated at 95% level.
OBSERVATION AND RESULTS
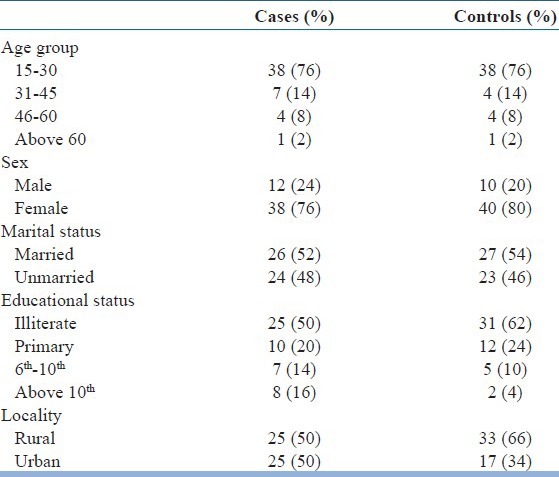
The sociodemographic profiles of the cases were given below in the Table 1. The mean age of the patients was 29.54 ± 11.90 years, mostly female (76%), illiterate (50%) having equal proportions from rural (50%) and urban (50%) background. Majority of the schizophrenics are of the paranoid type (94%), and remaining are catatonic (2%), simple (2%) and the hebephrenic type (2%).
Table 1.
Sociodemographic profile
The comparison of oxidative stress and antioxidants enzyme level in schizophrenic patients with normal healthy controls.
The level of oxidative stress (MDA) was increased and the level of antioxidative enzymes (GPX and SOD) was decreased in schizophrenic patients as compared to normal healthy controls. The mean level and standard deviation of the cases were MDA (48.9 ± 8.2 nmol/ml Hb), GPX (30.3 ± 7.6 U/mg Hb), SOD (21.4 ± 7.5 U/mg Hb) and controls were MDA (10.7 ± 5.4 nmol/ml Hb), GPX (66.2 ± 7.7 U/mg Hb), SOD (52.4 ± 7.9 U/mg Hb). The mean difference between cases and controls was significant (<0.05) [Table 2 and Figure 1].
Table 2.
Comparison of oxidative stress and antioxidants enzyme level in schizophrenic patients with normal healthy controls
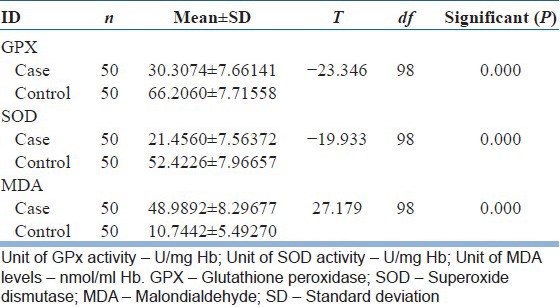
Figure 1.
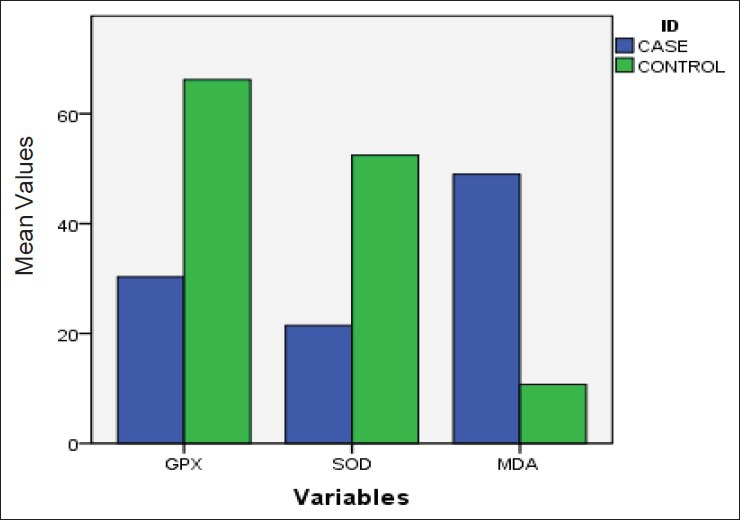
Comparison of oxidative stress and antioxidants enzyme level in schizophrenic patients with normal healthy controls
There was no significant relationship of age, sex, educational status, marital status, and severity of illness (PANSS score) with oxidative stress (MDA) and antioxidative enzymes (GPX and SOD) level in schizophrenic patients was found, but there was significant relationship of locality with oxidative stress (MDA) and antioxidative enzymes (GPX and SOD) level in schizophrenic patients was found. Urban population have a higher level of MDA, GPX, and SOD than the rural population.
DISCUSSION
The brain and nervous system possess high potential for the initiation of free radical reactions because of extensive aerobic metabolism which can cause more damage in the brain and nervous system relative to other tissues due to insufficient antioxidative protection.[21] Dopamine and norepinephrine are associated with the production of free radicals and increased catecholamines metabolism in certain conditions also increases the free radical burden.[22] Similarly, trauma and ischemia of the brain generate free radicals which are particularly related to reperfusion or reoxygenation of the tissue.[23] The brain contains both enzymatic and nonenzymatic antioxidants against free radical damage. The enzymatic antioxidants include SOD, glutathione peroxidase (GSH-Px), catalase, glutathione reductase, and glucose-6-phosphate dehydrogenase.[24] Lipid peroxidation and antioxidative defense mechanisms in RBCs to a certain extent reflect the state of the cell membranes of different tissues including brain tissue.[25] We investigated pro/antioxidant status in RBCs of schizophrenic patients with both positive and negative symptoms.
The identified increase in lipid peroxidation product (MDA) in RBC, which was consistent with the findings reported by Altuntas et al. and Herken et al.[15,26] A lot of studies reported increased level of MDA in plasma[11,27,28,29,30] and unchanged level of MDA was also reported by Ranjekar et al.[32] but it has smaller (n - 31) sample size.[31] Study by Dadheech et al. from India also reported increased level of MDA.[31]
The antioxidative enzymes (GPX and SOD) are decreased in our study which correlate with the findings reported by Ranjekar et al., Li et al., Ben Othmen et al.[30,32,33] and Dadheech et al. from India.[31] However, some studies also reported increased and unchanged levels.[10,11,26]
Decreased RBCs GSH-Px activity in schizophrenia could be explained by several factors. It is possible that it was a result of its oxidative inactivation[34] or because of its kinetic properties. The affinity of selenium GSH-Px for glutathione is low,[35] so GSH-Px is not saturated with glutathione even at high concentrations of this substrate. Decreased glutathione content, found in other studies[15] also supports this hypothesis. However in a recent, small sample size study in first episode drug-naive schizophrenic reports decreased glutathione level and increased GSH-Px level.[36] Though we have not differentiated between acute and chronic cases, the obtained decrease in this enzyme activity could be a consequence of exhausted adaptive response to a long-lasting oxidative stress during chronicity of this disease. In a recent study, statistically significant difference between the deficit schizophrenia and nondeficit schizophrenia in terms of oxidative stress and serum total antioxidant level has been reported implicating as both having a different disease entity.[37]
Red blood cells have been extensively studied as a susceptible target for oxidative damage, since they are long-lived cells and very rich in Fe2+ -containing molecules, primarily Hb that generate oxygen radicals.[38,39]
Overall, the data suggest that the changes in susceptibility of RBCs lipids to peroxidation observed in schizophrenics may be explained in part by change in levels of saturated and unsaturated fatty acids in RBC membranes. It can be assumed that decreased SOD activity and decreased GSH-Px activity might result in accumulation of H2O2 and other hydroperoxides in erythrocytes of schizophrenic. It could be responsible for further production of free radicals in Fenton reaction[40] in nervous tissue and amplification of oxidation of susceptible molecules, which could amplify the damage of neurons and lead to their death. Despite important physiological roles of NO, excessive formation or inadequate degradation of this compound has been suggested an important factor in the genesis of neurological disorders.[41] Furthermore, NO may cause impairment in mitochondrial permeability,[42] which affect ATP synthesis and organelle's ability to sequester excess cellular Ca2+,[43] both of which could contribute to neuronal death and alter the thought processing in schizophrenia. Marked oxidative stress in schizophrenia, increased level of intracellular Ca2+, and reactive oxygen species can be potent activators of (mitogen-activated protein) kinases[44] and associated activation of transcription factor NF-κB.[45] This factor regulates the expression of genes coding cell adhesion molecule proteins, nitric oxide synthase, proinflammatory cytokines, all of which play diverse roles in neuronal development, signal transduction, synaptic stabilization, neurogenesis, learning, and memory.
Our findings support the hypothesis of disturbances of oxidative stress and antioxidant mechanisms in schizophrenia. Further, antioxidant effects of established antipsychotic agents provide indirect evidence for oxidative pathophysiological mechanisms in schizophrenia. Abnormalities in levels of antioxidants and oxidative products have been reported to reverse over the course of treatment with atypical antipsychotics, coinciding with symptomatic improvement.[46,47] A recent meta-analysis report oxidative stress abnormalities might be independent of antipsychotics in first episode psychosis, it also says total antioxidant status, RBC catalase, and plasma nitrite might be state markers for acute exacerbations of psychosis and RBC SOD might be trait markers; however, more longitudinal studies are needed to prove this.[48]
We can make recommendations of possible use of antioxidants as an adjunct therapy with antipsychotics. Few trials are done with Vitamin C and E, Gingko biloba extract and N-acetylcysteine.[49,50,51,52]
Our study also evaluates the relation of sociodemographic parameters and severity of illness measured by PANSS score on oxidative stress and antioxidative enzymes.
We found, there was no significant association of age, sex, educational status, marital status, and PANNS score with oxidative stress (MDA) and antioxidative enzymes (GPX and SOD) in the schizophrenic patients except incidentally significantly higher values of all the three parameters (MDA, GPX, SOD) was seen in urban population than rural one. Which can be due to modern lifestyles, different dietary habits and increased psychological stress in urban population? However, we cannot draw any inference because level of all the three parameters (MDA, GPX, and SOD) was increased.
In this study, all the participant patients with schizophrenia had never taken any treatment earlier. Thus, this study supports the hypothesis that, there is a disturbance in antioxidant enzyme system in schizophrenia due to the increased oxidative stress, and this further intensifies with the aging process and chronic stage of the illness. A stressful life and polluted environment also make a contribution in deteriorating the condition. This also emphasizes the importance of nutrient antioxidant supplementation to support the entire antioxidant defense system.
Limitations of the present study
Other parameters which influence oxidative stress and antioxidant enzymes level were not included in the study like dietary habits, smoking habits, lifestyles, etc
All the selected cases were of an acute nature which might influence the oxidative stress and antioxidant enzymes level. Comparisons were needed to be done with chronic cases
Measurement of oxidative stress and antioxidant enzymes level were done before the treatment was started, no subsequent measurement was done to verify the changes in these parameters with the treatment or improvement in the disease process.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Benjamin JS, Irwin KH, Virginia AS. Synopsis of Psychiatry. 10th ed. Lippincott Williams and Wilkins, Wolters Kluwer; 1997. Schizophrenia; p. 467. Indian edi. [Google Scholar]
- 2.Sies H. Oxidative stress. Introductory remarks. In: Sies H, editor. Oxidative Stress. London: Academic Press; 1985. [Google Scholar]
- 3.Halliwell B, Gutreridge JM. 2nd ed. Oxford, UK: Clarendon Press; 1989. Free Radicals in Biology and Medicine. [Google Scholar]
- 4.Soboll S, Gründel S, Harris J, Kolb-Bachofen V, Ketterer B, Sies H. The content of glutathione and glutathione S-transferases and the glutathione peroxidase activity in rat liver nuclei determined by a non-aqueous technique of cell fractionation. Biochem J. 1995;311:889–94. doi: 10.1042/bj3110889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dumuis A, Sebben M, Haynes L, Pin JP, Bockaert J. NMDA receptors activate the arachidonic acid cascade system in striatal neurons. Nature. 1988;336:68–70. doi: 10.1038/336068a0. [DOI] [PubMed] [Google Scholar]
- 6.Frautschy SA, Baird A, Cole GM. Effects of injected Alzheimer beta-amyloid cores in rat brain. Proc Natl Acad Sci U S A. 1991;88:8362–6. doi: 10.1073/pnas.88.19.8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson's disease: Evidence supporting it. Ann Neurol. 1992;32:804–12. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 8.Beal MF. Mitochondria free radicals and neurodegeneration. Curr Opin Neurobiol. 1996;6:661–6. doi: 10.1016/s0959-4388(96)80100-0. [DOI] [PubMed] [Google Scholar]
- 9.Breteler MM, van Amerongen NM, van Swieten JC, Claus JJ, Grobbee DE, van Gijn J, et al. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging. The Rotterdam Study. Stroke. 1994;25:1109–15. doi: 10.1161/01.str.25.6.1109. [DOI] [PubMed] [Google Scholar]
- 10.Abdalla DS, Monteiro HP, Oliveira JA, Bechara EJ. Activities of superoxide dismutase and glutathione peroxidase in schizophrenic and manic-depressive patients. Clin Chem. 1986;32:805–7. [PubMed] [Google Scholar]
- 11.Kuloglu M, Ustundag B, Atmaca M, Canatan H, Tezcan AE, Cinkilinc N. Lipid peroxidation and antioxidant enzyme levels in patients with schizophrenia and bipolar disorder. Cell Biochem Funct. 2002;20:171–5. doi: 10.1002/cbf.940. [DOI] [PubMed] [Google Scholar]
- 12.Bilici M, Efe H, Köroglu MA, Uydu HA, Bekaroglu M, Deger O. Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments. J Affect Disord. 2001;64:43–51. doi: 10.1016/s0165-0327(00)00199-3. [DOI] [PubMed] [Google Scholar]
- 13.Fukunaga K, Yoshida M, Nakazono N. A simple, rapid, highly sensitive and reproducible quantification method for plasma malondialdehyde by high-performance liquid chromatography. Biomed Chromatogr. 1998;12:300–3. doi: 10.1002/(SICI)1099-0801(199809/10)12:5<300::AID-BMC751>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Looney JM, Childs HM. The lactic acid and glutathione content of the blood of schizophrenic patients. J Clin Invest. 1934;13:963–8. doi: 10.1172/JCI100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altuntas I, Aksoy H, Coskun I, Cayköylü A, Akçay F. Erythrocyte superoxide dismutase and glutathione peroxidase activities, and malondialdehyde and reduced glutathione levels in schizophrenic patients. Clin Chem Lab Med. 2000;38:1277–81. doi: 10.1515/CCLM.2000.201. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Geneva: World Health Organization; 1992. The ICD-10 Classification of Mental and Behavioral Disorders: Clinical Description and Diagnostic Guidelines. [Google Scholar]
- 17.Stanley RK, Abraham F, Lewis AO. The positive and negative syndrome scale (panss) for schizophrenia. Schizophr Bull. 1987;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 18.Andreeva II, Kotemjakin AA, Kishkun AA. A modified thiobarbituric acid test for measuring lipid peroxidation products. Lab Delo. 1988;1:41–3. [PubMed] [Google Scholar]
- 19.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 20.Moin VM. A simple specific method for assays of red cells glutathione peroxidase activity. Lab Delo. 1986;12:724–727. [PubMed] [Google Scholar]
- 21.Halliwell B. Oxidants and the central nervous system: Some fundamental questions. Is oxidant damage relevant to Parkinson's disease, Alzheimer's disease, traumatic injury or stroke? Acta Neurol Scand Suppl. 1989;126:23–33. doi: 10.1111/j.1600-0404.1989.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 22.Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinines. Mol Pharmacol. 1978;14:633–43. [PubMed] [Google Scholar]
- 23.McCord JM. Oxygen-derived radicals and reperfusion injury. Ann Intern Med. 1987;107:526–45. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 24.Singh R, Pathak DN. Lipid peroxidation and glutathione peroxidase, glutathione reductase, superoxide dismutase, catalase and glucose-6-phosphate dehydrogenase activities in FeCl3-induced epileptogenic foci in rat brain. Epilepsia. 1990;31:15–26. doi: 10.1111/j.1528-1157.1990.tb05354.x. [DOI] [PubMed] [Google Scholar]
- 25.Vilkov GA, Kiroi RI, Stepnina EG, Smirnova OB, Kovalenko VA, Trapezontseva RA. Lipid peroxidation and microviscosity of erythrocyte membranes in patients with schizophrenia. Zh Nevropatol Psikhiatr Im S S Korsakova. 1991;91:45–7. [PubMed] [Google Scholar]
- 26.Herken H, Uz E, Ozyurt H, Sögüt S, Virit O, Akyol O. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol Psychiatry. 2001;6:66–73. doi: 10.1038/sj.mp.4000789. [DOI] [PubMed] [Google Scholar]
- 27.Akyol O, Herken H, Uz E, Fadillioglu E, Unal S, Sögüt S, et al. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients. The possible role of oxidant/antioxidant imbalance. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:995–1005. doi: 10.1016/s0278-5846(02)00220-8. [DOI] [PubMed] [Google Scholar]
- 28.Mahadik SP, Mukherjee S, Scheffer R, Correnti EE, Mahadik JS. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol Psychiatry. 1998;43:674–9. doi: 10.1016/s0006-3223(97)00282-5. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XY, Tan YL, Cao LY, Wu GY, Xu Q, Shen Y, et al. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr Res. 2006;81:291–300. doi: 10.1016/j.schres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Ben Othmen L, Mechri A, Fendri C, Bost M, Chazot G, Gaha L, et al. Altered antioxidant defense system in clinically stable patients with schizophrenia and their unaffected siblings. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:155–9. doi: 10.1016/j.pnpbp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Dadheech G, Mishra S, Gautam S, Sharma P. Evaluation of antioxidant deficit in schizophrenia. Indian J Psychiatry. 2008;50:16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, et al. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–22. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- 33.Li HC, Chen QZ, Ma Y, Zhou JF. Imbalanced free radicals and antioxidant defense systems in schizophrenia: A comparative study. J Zhejiang Univ Sci B. 2006;7:981–6. doi: 10.1631/jzus.2006.B0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awasthi YC, Beutler E, Srivastava SK. Purification and properties of human erythrocyte glutathione peroxidase. J Biol Chem. 1975;250:5144–9. [PubMed] [Google Scholar]
- 35.Mukerjee S, Mahadik SP, Scheffer R, Correnti EE, Kelker H. Impaired antioxidative defense at the onset of psychosis. Schizophr Res. 1996;19:19–26. doi: 10.1016/0920-9964(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 36.Raffa M, Atig F, Mhalla A, Kerkeni A, Mechri A. Decreased glutathione levels and impaired antioxidant enzyme activities in drug-naive first-episode schizophrenic patients. BMC Psychiatry. 2011;11:124. doi: 10.1186/1471-244X-11-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albayrak Y, Ünsal C, Beyazyüz M, Ünal A, Kuloğlu M. Reduced total antioxidant level and increased oxidative stress in patients with deficit schizophrenia – A preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:144–9. doi: 10.1016/j.pnpbp.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Fung LW, Zhang Y. A method to evaluate the antioxidant system for radicals in erythrocyte membranes. Free Radical Biol Med. 1990;9:289–98. doi: 10.1016/0891-5849(90)90003-2. [DOI] [PubMed] [Google Scholar]
- 39.Glen AI, Glen EM, Horrobin DF, Vaddadi KS, Spellman M, Morse-Fisher N, et al. A red cell membrane abnormality in a subgroup of schizophrenic patients: Evidence for two diseases. Schizophr Res. 1994;12:53–61. doi: 10.1016/0920-9964(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 40.Murphy ME, Sies H. Reversible conversion of nitroxyl anion to nitric oxide by superoxide dismutase. Proc Natl Acad Sci U S A. 1991;88:10860–4. doi: 10.1073/pnas.88.23.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heales SJ, Barker JE, Stewart VC, Brand MP, Hargreaves IP, Foppa P, et al. Nitric oxide, energy metabolism and neurological disease. Biochem Soc Trans. 1997;25:939–43. doi: 10.1042/bst0250939. [DOI] [PubMed] [Google Scholar]
- 42.Packer MA, Scarlett JL, Martin SW, Murphy MP. Induction of the mitochondrial permeability transition by peroxynitrite. Biochem Soc Trans. 1997;25:909–14. doi: 10.1042/bst0250909. [DOI] [PubMed] [Google Scholar]
- 43.Strunecka A, Ripova D. What can the investigation of phosphoinositide signalling system in platelets of schizophrenic patients tell us? Prostaglandins Leukot Essent Fatty Acids. 1999;61:1–5. doi: 10.1054/plef.1999.0063. [DOI] [PubMed] [Google Scholar]
- 44.Kyosseva SV, Elbein AD, Griffin WS, Mrak RE, Lyon M, Karson CN. Mitogen-activated protein kinases in schizophrenia. Biol Psychiatry. 1999;46:689–96. doi: 10.1016/s0006-3223(99)00104-3. [DOI] [PubMed] [Google Scholar]
- 45.Mattson MP, Camandola S. NF-kB in neuronal plasticity and neurodegenerative disorders. J Clin Invest. 2001;107:247–54. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dakhale G, Khanzode S, Khanzode S, Saoji A, Khobragade L, Turankar A. Oxidative damage and schizophrenia: The potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–9. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- 47.Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY, Shen YC. The effect of risperidone treatment on superoxide dismutase in schizophrenia. J Clin Psychopharmacol. 2003;23:128–31. doi: 10.1097/00004714-200304000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Flatow J, Buckley P, Miller BJ. Meta-analysis of oxidative stress in schizophrenia. Biol Psychiatry. 2013;74:400–9. doi: 10.1016/j.biopsych.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dakhale GN, Khanzode SD, Khanzode SS, Saoji A. Supplementation of vitamin C with atypical antipsychotics reduces oxidative stress and improves the outcome of schizophrenia. Psychopharmacology (Berl) 2005;182:494–8. doi: 10.1007/s00213-005-0117-1. [DOI] [PubMed] [Google Scholar]
- 50.Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr Res. 2003a;62:195–204. doi: 10.1016/s0920-9964(02)00284-0. [DOI] [PubMed] [Google Scholar]
- 51.Zhang XY, Zhou DF, Zhang PY, Wu GY, Su JM, Cao LY. A double-blind, placebo-controlled trial of extract of Ginkgo biloba added to haloperidol in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2001;62:878–83. doi: 10.4088/jcp.v62n1107. [DOI] [PubMed] [Google Scholar]
- 52.Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, Boulat O, et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology. 2008;33:2187–99. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]


