Abstract
Background:
Organophosphorus poisoning (OPP) is a major global public health problem. Pralidoxime has been used in a complimentary role to atropine for the management of OPP. World Health Organization (WHO) recommends use of pralidoxime but studies regarding its role have been inconclusive, ranging from being ineffective to harmful or beneficial.
Materials and Methods:
The present study was undertaken to evaluate the effectiveness of pralidoxime. Eddleston's study was the most compelling factor for our study, as he showed worst outcomes using pralidoxime. Our practice of continuous use of pralidoxime was based on the WHO guidelines and the study by Pawar (2006), which showed better outcome with higher doses of pralidoxime. These conflicting results suggested that a re-evaluation of its use in our clinical practice was indicated.
Results:
There was no difference in mortality rates, hemodynamic parameters and atropine requirements between the AP and A groups. Mean duration of ventilation (3.6 ± 4.6 in AP group vs. 3.6 ± 4.4 in A group) and Intensive Care Unit stay (7.1 ± 5.4 in AP group vs. 6.8 ± 4.7 in A group) was comparable. Serum sodium concentrations showed a correlation with mortality, with lower concentrations associated with better outcomes.
Conclusion:
The study suggests that add-on WHO-recommended pralidoxime therapy does not provide any benefit over atropine monotherapy. Adding pralidoxime does not seem to be beneficial and at the same time does not result in increased mortality rates. Our practice changed after completion of this study, and it has proven to be of significant benefit to patients who had to bear the expense of treatment.
Keywords: Acetyl-cholinesterase, atropine, organophosphorous poisoning, pralidoxime, pseudo-cholinesterase, ventilation
INTRODUCTION
Organophosphorus (OP) compounds, used as insecticides and the agents of chemical warfare, are a major global cause of health problems. Estimates from World Health Organization (WHO) indicate that each year about 1 million accidental and 2 million suicidal poisonings involving OP pesticides occur worldwide.[1] The incidence has steadily increased in the recent past and has reached a level in the developing countries, where it can be called a “social calamity.”
Organophosphorus pesticides are readily available “over the counter” in Kashmir and this easy accessibility coupled with the menace of suicidal poisoning remains the most common modality of poisoning.[2] Since 1990, political unrest has caused great suffering and mental trauma among the residents of Kashmir. The high incidence of Organophosphorus poisoning (OPP) with suicidal intent is but one of many manifestations of the tragic consequences of this unrest.
Organophosphorus agents or their metabolites cause toxicity by inhibiting the function of acetyl-cholinesterase[3] an enzyme responsible for hydrolyzing and inactivating the neurotransmitter acetylcholine. Atropine[4] is an established specific antidote for OPP. WHO-recommends that a second type of antidote called pralidoxime (acetyl-cholinesterase reactivator) be given along with atropine. Although the beneficial effects of atropine are clear, controversy surrounds the role of pralidoxime in treating organophosphate poisoning.
Recent studies regarding use of pralidoxime have shown contrasting outcomes resulting in confusion about the management of OPP patients. Study by Pawar et al.[5] showed decreased morbidity and mortality using high-dose pralidoxime regimen, whereas study by Eddleston et al.[6] concluded that use of pralidoxime was associated with increased mortality.
The present study was designed to critically analyze the role of pralidoxime in patients with moderate to severe poisoning by dividing them into two comparable groups in all aspects using WHO-recommended dose of pralidoxime (30 mg/kg bolus intravenous [I.V] over 20 min followed by 8 mg/kg/h continuous infusion). This dose and continuous infusion protocol is more likely to be associated with steady-state concentrations of pralidoxime in blood.
MATERIALS AND METHODS
This study, “effectiveness of pralidoxime in the treatment of OPP — a randomized, double-blind, placebo-controlled trial” was conducted in the Department of Anesthesiology and Critical Care, Sher-i-Kashmir Institute of Medical Sciences, Soura, Kashmir from July 2010 to August 2012. The institutional review board approved the investigation protocol. 100 adult otherwise healthy patients were included in the study.
Patients with clinical evidence of OPP (bronchorrhoea, increased salivation, miosis or fasciculations) or report by relatives of poison ingestion were included in the study. Patients with Bardin Grade 2 (hypotension and deteriorating level of consciousness) and Bardin Grade 3 (Grade 2 and stupor, abnormal chest X-ray) were shifted from emergency department to Intensive Care Unit (ICU) for further management. Pralidoxime was administered only in ICU. Patients with history of alleged OP intake, age >14 years, and clinical signs and symptoms of OPP were included in the study. Exclusion criteria were age (<14 or >60 years), known pregnancy, pralidoxime administration before ICU admission, carbamate poisoning, any chronic illnesses, ≥12 h interval from time of poisoning to initiation of treatment. The randomization was done as per computer generated random number table.
All patients were managed by OPP protocol on presentation to the hospital that included decontamination and resuscitation for airway, breathing and circulation. Decontamination, resuscitation and atropine were started in the emergency department immediately and continued in ICU. Atropine was given as I.V bolus of 2-3 mg and dose doubled every 5 min until atropinisation was achieved. Thereafter, atropine was continued as an infusion at the rate of 20-80 mcg/kg/min and titrated to the target end points.
Baseline characteristics including age, sex, pseudo-cholinesterase levels, and severity of poisoning, type of compound ingested were recorded. Pseudo-cholinesterase levels were measured at the time of admission, after 24, 72 and 120 h, and on the day of discharge or last level measured before death.
All the patients received atropine 2-3 mg I/V stat on arrival. The dose was doubled every 5 min till atropinisation. Atropine was given as a continuous infusion at the rate of 20-80 mcg/min titrated to target end points for atropine therapy (heart rate >100 bpm, dry mouth, clear lungs on auscultation, normalization of bowel sounds, mid dilated pupils). Pralidoxime was given to the study subjects in the treatment group (AP) as per WHO-recommendations (30 mg/kg loading dose over 30 min followed by 8 mg/kg/h continuous infusion for a maximum of 7 days) presuming that this would be the maximum period of active acetyl-cholinesterase inhibition in most patients, or until clinical improvement that is, resolution of muscle fasciculations and weakness, or until atropine was no longer required indicating the presence of sufficient cholinesterase levels at the synapses. Placebo group (A) received I.V saline. Atropine was withdrawn slowly over a period of 3-5 days.
Patients were assessed for cholinergic signs, atropine requirements, muscle power and the level of consciousness by Glasgow Coma Scale on regular basis. Outcome parameters analyzed were mortality, need for intubation, need for ventilation and duration of ventilation, duration of stay in ICU, need for tracheostomy, incidence of Intermediate Syndrome, infections and other complications.
RESULTS
The two groups were comparable with respect to age, weight and sex distribution. The mean age for AP and A groups was 29.1 ± 10.9 years and 28.2 ± 9.9 years respectively (P > 0.05).
There were no significant differences in the OP compounds ingested between the two groups (P > 0.05). The number of patients who consumed dimethyl and diethyl compounds in AP group was 35 (70%) and 9 (18%) whereas 33 patients (66%) consumed dimethyl and 10 (20%) patients ingested diethyl agent in A group. Nature of the compound could not be known in 13 (13%) of patients [Table 1]. Heart rate, systolic blood pressure and diastolic blood pressure (mean ± standard deviation [SD]) at different time intervals did not differ significantly between the two groups (P > 0.05).
Table 1.
Demographics
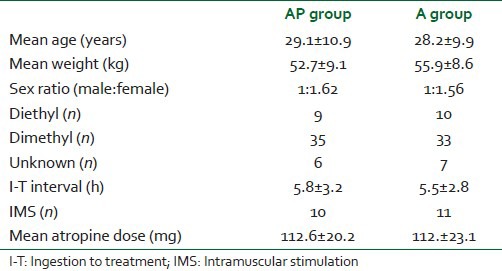
In A group, pseudo-cholinesterase levels (mean ± SD) on admission were 1476.9 ± 431.2 for survivors and 916.9 ± 311.7 for nonsurvivors (P > 0.05). The pseudo-cholinesterase levels (mean ± SD) at discharge or death in this group were 4516.2 ± 976.9 and 3170 ± 1503.2 respectively (P < 0.05). In AP group, pseudo-cholinesterase levels (mean ± SD) on admission was 1318.6 ± 481.1 for survivors and 915.1 ± 544.8 for patients who died (P < 0.05), whereas the levels (mean ± SD) at discharge were 4456.4 ± 563.9 in survivors and 1463.8 ± 576.4 among nonsurvivors respectively [Table 2].
Table 2.
Pseudo-cholinesterase levels (IU/L) on admission and discharge/death in two groups
The incidence of intermediate syndrome was comparable with no statistically significant difference between two groups (P > 0.05). There was no statistically significant difference in total atropine dose requirement between the two groups (P > 0.05).
Ventilatory support was required in 60 patients. 31 out of 50 patients (62%) required ventilatory support in Group AP whereas 29 out of 50 patients (58%) required ventilatory support in the Group A (P > 0.05). Mean duration of ventilator days was comparable between two groups.
The two groups did not differ significantly with respect to complications (P > 0.05). Pneumonia was the most frequent complication, observed in 34% of patients in AP group and 26% in A group. The other complications observed in decreasing frequency were cardiac dysrhythmias (22% vs. 18%), noncardiogenic pulmonary edema (8% vs. 4%) and urinary tract infection (6% vs. 12%) in AP and A groups respectively.
36% of patients presented with hypernatremia (serum sodium >145) on admission. Higher admission serum sodium levels were associated with worst outcomes [Figure 1]. Mortality rate was 55.5% in patients who presented with hypernatremia (>145) compared to 10.9% in patients who presented with normal serum sodium levels (>145). The correlation between hypernatremia and mortality was statistically significant (P < 0.05).
Figure 1.
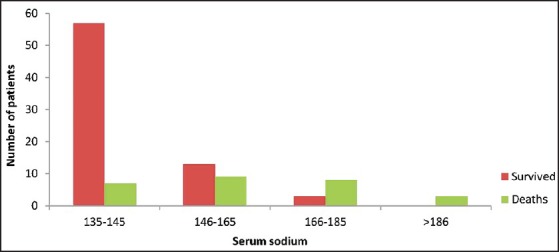
Serum sodium levels among survivors and nonsurvivors
Duration of ventilation (mean ± SD) was 3.5 ± 4.6 days in AP group and 3.6 ± 4.4 days in A group [Figure 2]. The groups showed no significant difference with P > 0.05 (0.8663) using one-way analysis of variance (ANOVA). Mean duration of ICU stay (in days) between the two groups failed to show any statistically significant difference (P > 0.05) with 7.1 ± 5.4 days in group AP compared to 6.8 ± 4.7 days in group A [Table 3].
Figure 2.
Days on ventilator versus mortality
Table 3.
Comparison of clinical outcomes between two groups
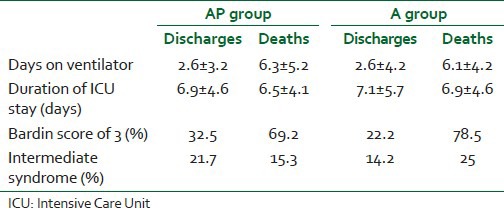
Ingestion to treatment interval (I-T) in two groups were comparable [Table 3] and showed no statistical difference with P > 0.05 using one-way ANOVA (5.8 ± 3.2 h for AP group and 5.5 ± 2.8 h in A group).
Case fatality was slightly higher in A Group (28%) compared to those in AP group (26%) with overall mortality rate of 27%. A one-way ANOVA showed no statistical difference between the two groups.
Relation of secondary outcomes to mortality
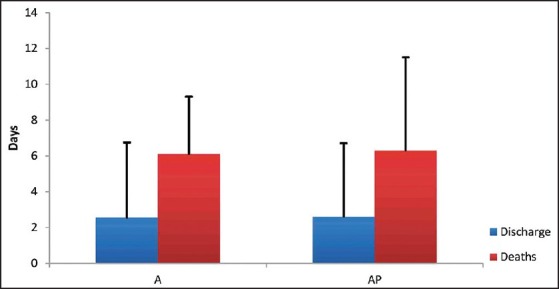
Mortality was seen only in ventilated patients with 42% mortality in AP group and 48.2% in A group. Discharged patients spend lesser days on ventilator compared to patients who died as shown in Table 3 (P < 0.05). Duration of ICU stay was comparable between survivors and nonsurvivors in both groups [Figure 3].
Figure 3.
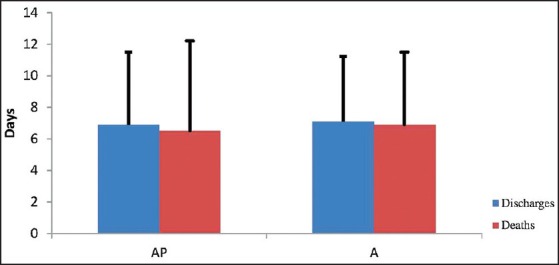
Intensive Care Unit stay — discharges versus deaths
Igher Bardin scores were observed among nonsurvivors (69.2% and 78.5%) in AP and A groups respectively [Table 3].
DISCUSSION
Organophosphates rank foremost in the list of agents that cause acute poisoning in the developing countries. The management of acute organophosphate poisoning depends on its severity. In mild cases, removing the patient from the area of exposure and low dose of atropine may suffice. However, in severe cases, resuscitation, artificial ventilation and high-doses of antidotes become necessary.
Since its discovery in 1956, pralidoxime has been used in the management of OPP in addition to atropine. While efficacy of atropine is clinically proven, clinical experience with pralidoxime has been controversial. It was in the early nineties when researchers like Duval et al.,[7] conducted clinical trials raising questions regarding the efficacy of pralidoxime in management of OPP. All these studies revealed that add-on pralidoxime therapy either did not offer any added benefit or was associated with worsening of the outcome when compared to treatment with atropine alone.
The present study shows that administration of the WHO-recommended regimen of pralidoxime to patients with symptomatic OPP does not offer any benefit. The primary outcome that is, mortality rate in patients treated with pralidoxime was neither increased nor showed any decrease compared to patients who were treated with atropine only (28% vs. 26%).
Eddleston et al.[6] studied the effectiveness of pralidoxime (2 g loading dose over 20 min, followed by infusion of 0.5 g/h for up to 7 days) in patients with OPP and concluded that WHO-recommended dose of pralidoxime is most likely to be ineffective, and may be harmful. The present study used pralidoxime dose (30 mg/kg loading dose over 30 min followed by 8 mg/kg/h continuous infusion for maximum of 7 days) almost similar to the doses used in Eddleston study, however, the mortality rates in treatment and placebo groups were same unlike Eddleston who showed increased mortality in treatment group (24.8% vs. 15.8%). Pawar et al.[5] used pralidoxime regimens similar to that recommended by the WHO and found that high-dose infusion of pralidoxime significantly reduced mortality compared with standard bolus regimen.
Systematic review of oximes by Buckley et al.[8] identified two RCTs (182 people) of pralidoxime with different comparisons and outcomes. Neither of the RCTs found any benefit of pralidoxime. In the first RCT, Johnson et al.[9] found that larger doses of pralidoxime were associated with increased mortality. In another RCT, Cherian et al.[10] found out that infusion dose of pralidoxime was associated with increased mortality compared to placebo. Meta-analysis by Peter et al.[11] found limited evidence about the effect of oximes on mortality or need for ventilation. Limited evidence of worse outcomes for patients treated with oximes was found by Rahimi et al.[12] Meta-analysis by Blain[13] showed weak evidence of any benefit or harm between placebo or no oximes or different regimens versus each other as far as the need for ventilation. Blain also found weak evidence whether oximes are more effective than placebo or no oximes at reducing mortality in people with acute OPP.
Effectiveness of pralidoxime is limited to clinical trials only. There is some evidence from animal studies that oximes (pralidoxime) affect and reverse the OP binding to acetyl-cholinesterase. However, there are species differences in acetyl-cholinesterase. The studies used single doses of pralidoxime given at the same time with dose of OP insecticide without atropine treatment or supportive care.[14]
Pseudo-cholinesterase levels correlated with the severity of poisoning, with levels <30% of normal in patients with Bardin Class 3 poisoning, as was also reported by Weissmann-Brenner et al.[15] However, pseudo-cholinesterase levels lacked any prognostic significance. There was progressive increase in pseudo-cholinesterase levels among survivors in AP group; however, the increase in pseudo-cholinesterase levels among nonsurvivors in AP group showed minimal change from baseline. Eddleston et al.[6] studied the RBC choline-esterase activity and stated that reactivation of red cell acetyl-cholinesterase may not be essential for survival. However in present study the reactivation (increase in plasma pseudo-cholinesterase levels) showed positive correlation with survival with about three-fold increase from baseline as against minimal increase in patients of AP group who did not survive.
Atropine requirement in milligrams was not significantly different in two groups. Atropine dose correlated with the severity of poisoning, with severely poisoned patients requiring higher doses for initial atropinisation as well as higher infusion rates to maintain adequate atropinisation. Similar observations were seen by Cherian et al.[10]
Hemodynamic profile showed no difference between the two groups. Duration of ICU stay was comparable between two groups with the longest stay of 34 days. Protracted ICU stay was associated with intermediate syndrome, pneumonia and hemodynamic instability. Tracheostomy was required in two patients in A group. These results are congruent with those of Rahimi et al.[12] Higher incidence of intermediate syndrome has been reported by Samuel et al.[16] (61%) compared to our study (20%).
Hypernatremia (serum Na >145) was present in about 36% of patients on admission in our study. This increased serum sodium on admission was associated with worsening of the outcomes irrespective of allocation to either of the study arms. A five-fold increase in mortality was associated with hypernatremia (P < 0.05). Although the cause of hypernatremia in our patients could not be elucidated, yet the alteration in the membrane permeability due to excessive acetylcholine at the synapses could be cited as one possible mechanism. Lyzhnikov et al.[17] believed that excessive acetylcholine due to acute poisoning with phosphorus–organic pesticides changes the membrane permeability towards increasing intracellular potassium and extracellular sodium. Further studies are needed to validate hypernatremia as predictive of poor outcome in these patients.
Various complications reported in our study occurred with equal frequency in both the study arms. Pneumonia and dysrhythmias were the most frequent complications. The factors responsible for arrhythmias were hypoxia, electrolyte disturbances and acidosis as was reported by Karki et al.[18] Case reports have suggested that pralidoxime causes dysrhythmias or respiratory arrest[11,19] but the OP insecticides may also induce these effects. Other complications observed included noncardiogenic pulmonary edema and urinary tract infection. Similar complications were reported by Athar et al.[20] in OP poisoned patients.
CONCLUSION
Add-on WHO-recommended pralidoxime therapy does not seem to provide any benefit over atropine monotherapy and at the same time does not result in increased mortality rates. There is the need for a better antidote. Serum sodium on admission can be of prognostic value and this needs further evaluation. WHO guidelines may need to be reviewed, but with small size of patients in our study, a bigger study will be helpful.
Our practice changed after completion of this study, and it has proven to be of significant benefit to patients who had to bear the expense of treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Joshi S, Biswas B, Malla G. Management of organophosphorus poisoning. Indian J Pharmacol. 2006;41:69–70. [Google Scholar]
- 2.Malik GM, Mubarik M, Romshoo GJ. Organophosphorus poisoning in the Kashmir Valley, 1994 to 1997. N Engl J Med. 1998;338:1078. doi: 10.1056/NEJM199804093381520. [DOI] [PubMed] [Google Scholar]
- 3.Chugh SN, Agarwal N, Dabla S. Comparative evaluation of “atropine alone” and atropine with pralidoxime in the management of organophosphorus poisoning. J Indian Acad Clin Med. 2006;6:33–7. [Google Scholar]
- 4.du Toit PW, Müller FO, van Tonder WM, Ungerer MJ. Experience with the intensive care management of organophosphate insecticide poisoning. S Afr Med J. 1981;60:227–9. [PubMed] [Google Scholar]
- 5.Pawar KS, Bhoite RR, Pillay CP, Chavan SC, Malshikare DS, Garad SG. Continuous pralidoxime infusion versus repeated bolus injection to treat organophosphorus pesticide poisoning: A randomised controlled trial. Lancet. 2006;368:2136–41. doi: 10.1016/S0140-6736(06)69862-0. [DOI] [PubMed] [Google Scholar]
- 6.Eddleston M, Eyer P, Worek F, Juszczak E, Alder N, Mohamed F, et al. Pralidoxime in acute organophosphorus insecticide poisoning: A randomised controlled trial. PLoS Med. 2009;6:e1000104. doi: 10.1371/journal.pmed.1000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duval G, Rakouer JM, Tillant D, Auffray JC, Nigond J, Deluvallee G. Acute poisoning by insecticides with anticholinesterase activity. Evaluation of the efficacy of a cholinesterase reactivator, pralidoxime. J Toxicol Clin Exp. 1991;11:51–8. [PubMed] [Google Scholar]
- 8.Buckley NA, Eddleston M, Szinicz L. The Cochrane Library. No. 3. Chichester, UK: John Wiley & Sons, Ltd.; 2010. Search date; 2003. Oximes for acute organophosphate pesticide poisoning. [Google Scholar]
- 9.Johnson S, Peter JV, Thomas K, Jeyaseelan L, Cherian AM. Evaluation of two treatment regimens of pralidoxime (1 gm single bolus dose vs 12 gm infusion) in the management of organophosphorus poisoning. J Assoc Physicians India. 1996;44:529–31. [PubMed] [Google Scholar]
- 10.Cherian AM, Peter JV, Samuel J. Effectiveness of P2AM (PAM-pralidoxime) in the treatment of organophosphorus poisoning. A randomized, double-blind, placebo-controlled trial. J Assoc Physicians India. 1997;45:22–4. [Google Scholar]
- 11.Peter JV, Moran JL, Graham P. Oxime therapy and outcomes in human organophosphate poisoning: an evaluation using meta-analytic techniques. Crit Care Med. 2006;34:502–10. doi: 10.1097/01.ccm.0000198325.46538.ad. [DOI] [PubMed] [Google Scholar]
- 12.Rahimi R, Nikfar S, Abdollahi M. Increased morbidity and mortality in acute human organophosphate-poisoned patients treated by oximes: a meta-analysis of clinical trials. Hum Exp Toxicol. 2006;25:157–62. doi: 10.1191/0960327106ht602oa. [DOI] [PubMed] [Google Scholar]
- 13.Blain PG. Organophosphorus poisoning (acute) Clin Evid (Online) 2011 [PMC free article] [PubMed] [Google Scholar]
- 14.Worek F, Reiter G, Eyer P, Szinicz L. Reactivation kinetics of acetylcholinesterase from different species inhibited by highly toxic organophosphates. Arch Toxicol. 2002;76:523–9. doi: 10.1007/s00204-002-0375-1. [DOI] [PubMed] [Google Scholar]
- 15.Weissmann-Brenner A, Friedman LM, David A, Vidan A, Hourvitz A. Organophosphate poisoning: a multihospital survey. Isr Med Assoc J. 2002;4:573–6. [PubMed] [Google Scholar]
- 16.Samuel J, Thomas K, Jeyaseelan L, Peter JV, Cherian AM. Incidence of intermediate syndrome in organophosphorous poisoning. J Assoc Physicians India. 1995;43:321–3. [PubMed] [Google Scholar]
- 17.Lyzhnikov EA, Savina AS, Shepelev VM. Pathogenesis of disorders of cardiac rhythm and conductivity in acute organophasphate insecticide poisoning. Kardiologiia. 1975;15:126–9. [PubMed] [Google Scholar]
- 18.Karki P, Ansari JA, Bhandary S, Koirala S. Cardiac and electrocardiographical manifestations of acute organophosphate poisoning. Singapore Med J. 2004;45:385–9. [PubMed] [Google Scholar]
- 19.Scott RJ. Repeated asystole following PAM in organophosphate self-poisoning. Anaesth Intensive Care. 1986;14:458–60. doi: 10.1177/0310057X8601400424. [DOI] [PubMed] [Google Scholar]
- 20.Athar A, Ara J, Khan EA. Acute organophosphate insecticide poisoning. J Surg Pak. 2008;13:71–4. [Google Scholar]


