Abstract
Background:
Procedural discomfort is experienced by patients during the establishment of subarachnoid block even after good preoperative counseling and adequate premedication. To enhance comfort, procedural sedation that would provide good analgesia, faster recovery, and amnesia is necessary.
Materials and Methods:
Patients with American Society of Anesthesiologists Status I and II posted for elective surgeries under subarachnoid block were premedicated with injection midazolam 0.05 mg/kg and preloaded with 10 ml/kg ringer lactate solution. They were randomized into three groups of 30 each. Group K0.3 received ketamine 0.3 mg/kg, Group K0.4 received ketamine 0.4 mg/kg and Group K0.5 received ketamine 0.5 mg/kg intravenously. University of Michigan sedation score, ease of positioning, prick response, verbal response, hallucinations, recall of procedure, and patient satisfaction were evaluated.
Results:
There was statistically significant difference in sedation among the three groups. Increased dose necessitated help of two persons to position the patient, which showed statistically significant difference. Verbal response was seen early in Group K0.3 (4.67 ± 2.84 min). There was no recall of experience of subarachnoid block procedure in any of the groups in spite of back muscle contraction or patient movement. Hence, all patients in all three groups were satisfied and were willing to undergo subarachnoid block, if the situation arises.
Conclusion:
Ketamine in the dose of 0.3 mg/kg provided sufficient sedation for allaying procedural discomfort due to less sedation, less positional difficulty, early verbal response, no hallucinations, no recall of performance of procedure, and good patient satisfaction.
Keywords: Ketamine, subanesthetic dose, subarachnoid block
INTRODUCTION
Regional anesthesia has become more popular than general anesthesia because of its simplicity and less complications.[1] Procedural discomfort is experienced during the establishment of subarachnoid block even after good preoperative counseling and premedication. This could be due to multiple reasons such as cold operating environment, new people, positioning, and obviously the procedure itself.[2] Furthermore, patients would experience pain during the needle insertion in spite of administration of local anesthetic. To overcome these problems and to enhance the patient's comfort, procedural sedation that would provide good analgesia, faster recovery, and amnesia is necessary.[3] We chose ketamine in our study as it is short acting, a potent analgesic in the subanesthetic doses and does not depress respiration.[4,5] Midazolam added to ketamine also provides ante grade amnesia.[6] We compared the efficacy of three subanesthetic doses of ketamine to evaluate its required dose to prevent procedural discomfort with least complications.
MATERIALS AND METHODS
After getting approval from hospital Ethics Committee, a randomized double-blind prospective study was conducted in 90 patients aged between 18 and 60 years of American Society of Anesthesiologists Status I and II posted for elective surgeries under subarachnoid block. Patients with compromised cardiovascular or respiratory function, bleeding diathesis, and pregnancy were excluded from the study. Procedures involving epidural placement were also excluded. After obtaining informed consent, premedication was done with oral diazepam 10 mg in the night and 10 mg on the morning of the surgery. After ensuring 8 h nil per oral status, all patients were premedicated with injection midazolam 0.05 mg/kg and preloaded with 10 ml/kg ringer lactate solution in the preoperative holding area. The patients were shifted to the operation room table, and the baseline hemodynamic parameters were recorded. The patients were randomized to one of the three groups by closed envelope technique. Group K0.3 received ketamine 0.3 mg/kg, Group K0.4 received ketamine 0.4 mg/kg and Group K0.5 received ketamine 0.5 mg/kg intravenously. After opening the envelope, the study drug was prepared, made up to 10 ml and administered by an assistant. The patient identification was written on a slip and put back into an envelope and sealed. The person doing the procedure and monitoring along with the patient is blinded to the dose. The equal volume test solution was given intravenously by an assistant who was not involved in the administration of anesthesia and monitoring. The study parameters were carefully evaluated. University of Michigan sedation score [Table 1] was noted 3 min after giving the drug and the patient was put into the lateral position. Ease of positioning for subarachnoid block (while turning the patient to the lateral position) was assessed by a three-point scale:
Table 1.
University of Michigan sedation score

Patient turned on his own,
Patient turned with the help of one person,
Patient turned with the help of more than one person.
The parameters monitored (heart rate, mean arterial blood pressure, oxygen saturation, respiratory rate, and sedation score) were recorded every 3 min for 15 min. With aseptic precautions, subarachnoid block was performed using 25 gauge Quinke's spinal needle without local infiltration. The desired volume of injection bupivacaine 0.5% heavy was injected intrathecally. Response to spinal needle insertion was noted and graded by the 4-point score [Table 2]. Then the patient was placed in the supine position for surgery. Apnea, airway obstruction and involuntary movements if present were noted. Time interval between ketamine injection and response to verbal commands (defined as precisely answering questions about name, date of birth and home address) was noted in minutes. Hallucinations, if occurred were graded (nil, mild, moderate, and severe) and treated with injection midazolam. In the recovery area, when patient was fully awake, patient recall of the subarachnoid block procedure was noted as yes or no. The patient satisfaction was assessed by asking whether he or she would undergo spinal procedure again, if the situation arises.
Table 2.
Response to spinal needle insertion

Demographic profile was expressed as mean ± standard deviation hemodynamic parameters; SpO2, respiratory rate, and verbal response were assessed by one-way ANOVA. Sedation score, ease of positioning, and prick response were compared by nonparametric Mann–Whitney U-test. Hallucinations, recall of procedure and patient satisfaction were assessed by Chi-squire test. P < 0.05 was considered as statistically significant.
RESULTS
The demographic profile was comparable with no statistically significant difference among three groups. Demographic profile is summarized in Table 3.
Table 3.
Demographic data
Mean heart rate and mean blood pressure from baseline to 15 min are depicted in Figure 1. There was no statistically significant difference among the three groups.
Figure 1.
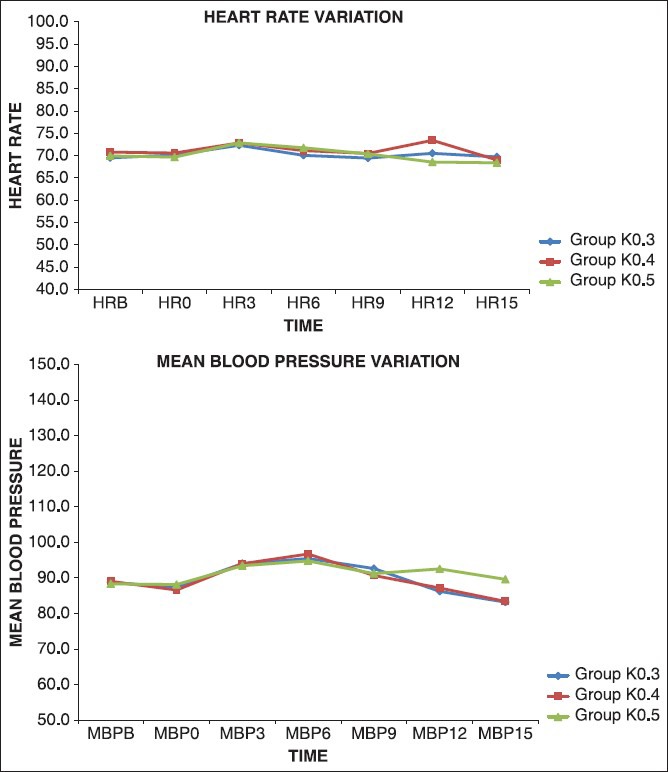
Hemodynamic changes observed with three different doses of ketamine
No statistically significant difference was found in SpO2 and respiratory rate among the three groups. The lowest SpO2 observed in Groups K0.3, 0.4, and 0.5 were 98%, 97%, and 96%, respectively. Respiratory rate ranged from 14 to 16 breaths/min in all three groups. None of the patients in any of the groups had airway obstruction, apnea, or involuntary movements.
Minimal sedation was observed in 22 patients in Group K0.3, whereas only seven of them in Group K0.4 and three of them in the Group K0.5 had minimal sedation [Figure 2]. There was statistically significant difference in the degree of sedation when intergroup comparison was made. With regard to the ease of positioning, 5 patients in Group K0.3 and 2 patients in Group K0.4 turned on their own, while none turned on their own in Group K0.5. 22 patients in Group K0.5 had to be positioned with the help of two persons [Figure 2]. Ease of positioning was significantly different between the three groups. When the response to prick was assessed, Groups K0.4 and K0.5 did not show any gross patient movement, but in 3 patients of Group K0.3, this observation was made. Prick response scores were significantly high in Group K0.3 when compared with Group K0.4 [Figure 2].
Figure 2.
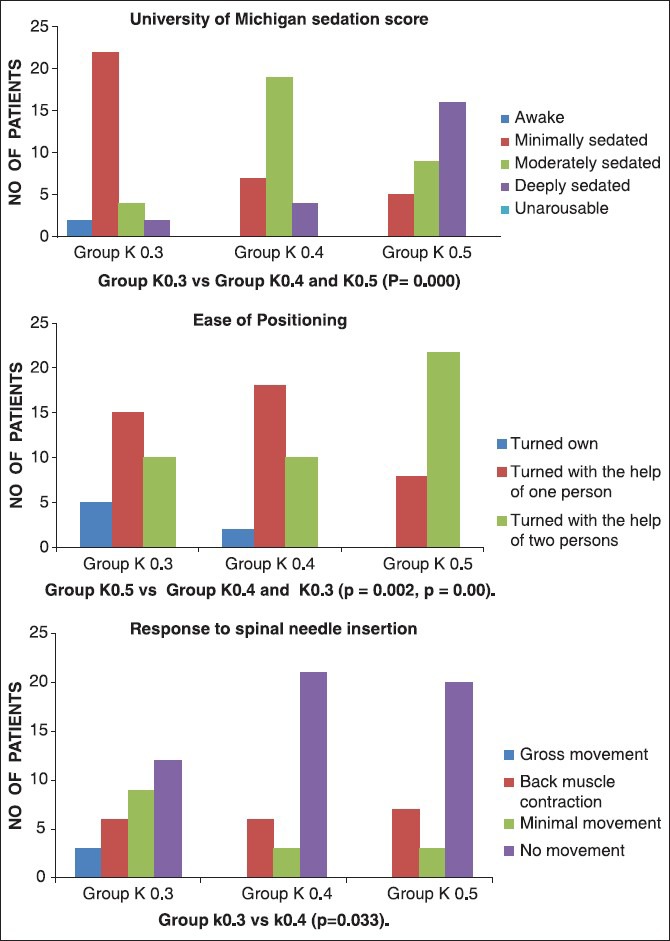
Ease of positioning, prick response, University of Michigan sedation score observed among three groups
Early verbal response was seen in Group K0.3 (4.67 ± 2.84 min) when compared with Group K0.4 (6.53 ± 2.47) and Group K0.5 (6.93 ± 2.24), which was statistically significant. Only 1 patient showed mild hallucination in Group K0.5. None of the patients in all three groups could recall spinal needle prick. All patients in all three groups were satisfied with the spinal procedure and were willing to undergo it, if the situation arises.
DISCUSSION
Since the discovery of ketamine by Domino et al.,[7] research had been done to determine its various clinical uses. Some used ketamine to provide preemptive analgesia as it prevented or reversed the already established central sensitization.[8,9] Ketamine also produced local anesthesia and had been compared with lignocaine and prilocaine.[10] Subanesthetic doses had been extensively used in postoperative pain relief.[11] Apart from its use for extradural analgesia,[12] it also provided neuroprotective action. It had been used for septic,[13] hemorrhagic shock, and status asthmaticus.[14]
Ketamine had been popularized for procedural sedation since many years. Green and Johnson published a comprehensive review of ketamine sedation[15] and a case series of 108 episodes of pediatric sedation in an emergency department.[16] It had an excellent safety profile with low incidence of side-effects. Sedation can be described as a continuous process ranging from minimal sedation extending to general anesthesia.[17] The specialty of ketamine sedation was that it does not follow the continuous process of gradually increasing sedation with cardiorespiratory depression, toward a state of general anesthesia.[18] Sedation increased patient comfort during regional anesthesia and increased the patient's acceptance.[19] Ketamine produced dose-dependent sedation with statistically significant difference among three groups. Our findings were similar to the review done by Green and Johnson.[15]
Subarachnoid block procedure, though well-explained to the well-premedicated patients preoperatively, exposure to the new operation room environment and its people, positioning for spinal procedure and the fear of pain during spinal needle insertion result in procedural discomfort. Procedural sedation can overcome these problems. Various drugs had been used to produce sedation for neuraxial block such as midazolam, propofol, remifentanil, sevoflurane, and ketamine either alone or in combination.[20,21,22,23,24]
Ketamine, in subanesthetic doses produced less hemodynamic disturbances.[25] Concurrent midazolam successfully suppressed these minimal responses and provided cardiovascular stability.[26] Our study had similar observations with all the three doses due to low dose ketamine used in conjunct with midazolam. Mean blood pressure increased by 6 min with all the three doses and came to near baseline value by 15 min. This rise in blood pressure helped in avoiding hypotension due to sympathetic blockade, which was an interesting and useful finding. This observation was similar to when propofol and ketamine were used for induction of general anesthesia where the cardio stimulant effects of ketamine, even in subanesthetic doses, balanced the cardio depressant effects of propofol.[27] Heart rate increased minimally, which returned to baseline by 15 min. The maximum heart rate in all the three groups was 73.43 ± 9.47.
We looked at the positional difficulty due to sedation. Few patients in Group K0.3 (n = 5) and Group K0.4 (n = 2) turned on their own, while none turned on their own in Group K0.5. More patients in Group K0.5 (n = 22) required the help of two persons due to increased sedation. Our study confirmed that increased dose necessitated help of two persons to position the patient. Otherwise, with doses of 0.3 or 0.4 mg/kg, positioning can be satisfactorily achieved with the help of one person to make the patient comfortable.
We looked at the required ketamine dose to prevent response to spinal needle prick. Though gross patient movement was not observed in Groups K0.4 and K0.5, 3 patients in Group K0.3 had this presentation. Our study showed that there was no patient movement in most of the patients in all the three groups. This could be due to the intense analgesia provided by ketamine.[28]
Respiratory rate and SpO2 changes were minimal and were not statistically significant. This could be attributed to the subanesthetic dose used.[28] Ketamine preserved the integrity of laryngeal and pharyngeal reflexes with minimal effect on central respiratory drive.[29] The incidence of upper airway obstruction and snoring was not recorded. None of the patients in any of the groups had airway obstruction, apnea or involuntary movements.
Verbal response was seen early in Group K0.3 (4.67 ± 2.84 min). This could be due to the low dose used.[28] This quick recovery allowed the patients to cooperate during the procedure, while remaining sedated.
Mild hallucinations were seen only in one person who received 0.5 mg/kg ketamine and was not observed in the other groups at all. This low incidence could be due to concurrent use of midazolam[26] and subanesthetic dose of ketamine used.[30]
We also looked further, whether patients who moved due to spinal needle prick remembered the event after surgery. There was no recall of spinal procedure in any of the groups in spite of back muscle contraction or movement. This could be the reason for good patient satisfaction in all the three groups as they were willing to undergo spinal procedure, if the situation arises. Recall of noxious spinal needle insertion[31] was not present due to intense analgesia provided by ketamine and ante grade amnesia[32] provided by midazolam.
To conclude, ketamine in the dose of 0.3 mg/kg provided sufficient sedation for allaying procedural discomfort since it caused less sedation, less positional difficulty, early verbal response, no hallucinations, no recall of spinal procedure and good patient satisfaction. The only disadvantage observed was the response to spinal needle prick seen in few cases though they were not appreciated. Ketamine in subanesthetic dose may increase apprehensive patients to undergo surgery under subarachnoid block.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Wu CL, Fleisher LA. Outcomes research in regional anesthesia and analgesia. Anesth Analg. 2000;91:1232–42. doi: 10.1097/00000539-200011000-00035. [DOI] [PubMed] [Google Scholar]
- 2.Liu SS, McDonald SB. Current issues in spinal anesthesia. Anesthesiology. 2001;94:888–906. doi: 10.1097/00000542-200105000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Austin TR. Low dose ketamine and diazepam during spinal analgesia. Anesthesia. 1980;35:391–2. doi: 10.1111/j.1365-2044.1980.tb05130.x. [DOI] [PubMed] [Google Scholar]
- 4.Sadove MS, Shulman M, Hatano S, Fevold N. Analgesic effects of ketamine administered in subdissociative doses. Anesth Analg. 1971;50:452–7. [PubMed] [Google Scholar]
- 5.White PF, Way WL, Trevor AJ. Ketamine & Its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–36. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Gjessing J, Tomlin PJ. Intravenous sedation and regional analgesia. Anesthesia. 1977;32:63–9. doi: 10.1111/j.1365-2044.1977.tb11561.x. [DOI] [PubMed] [Google Scholar]
- 7.Domino EF, Chodoff P, Corssen G. Pharmacologic effects of ci-581, a new dissociative anesthetic, in man. Clin Pharmacol Ther. 1965;6:279–91. doi: 10.1002/cpt196563279. [DOI] [PubMed] [Google Scholar]
- 8.Katz J. Pre-emptive analgesia: Evidence, current status and future directions. Eur J Anaesthesiol Suppl. 1995;10:8–13. [PubMed] [Google Scholar]
- 9.Dahl JB, Kehlet H. The value of pre-emptive analgesia in the treatment of postoperative pain. Br J Anaesth. 1993;70:434–9. doi: 10.1093/bja/70.4.434. [DOI] [PubMed] [Google Scholar]
- 10.Dowdy EG, Kaya K, Gocho Y. Some pharmacologic similarities of ketamine, lidocaine, and procaine. Anesth Analg. 1973;52:839–42. [PubMed] [Google Scholar]
- 11.Dich-Nielsen JO, Svendsen LB, Berthelsen P. Intramuscular low-dose ketamine versus pethidine for postoperative pain treatment after thoracic surgery. Acta Anaesthesiol Scand. 1992;36:583–7. doi: 10.1111/j.1399-6576.1992.tb03523.x. [DOI] [PubMed] [Google Scholar]
- 12.Naguib M, Adu-Gyamfi Y, Absood GH, Farag H, Gyasi HK. Epidural ketamine for postoperative analgesia. Can Anaesth Soc J. 1986;33:16–21. doi: 10.1007/BF03010903. [DOI] [PubMed] [Google Scholar]
- 13.Yli-Hankala A, Kirvelä M, Randell T, Lindgren L. Ketamine Anesthesia in a patient with septic shock. Acta Anaesthesiol Scand. 1992;36:483–5. doi: 10.1111/j.1399-6576.1992.tb03502.x. [DOI] [PubMed] [Google Scholar]
- 14.Heshmati F, Zeinali MB, Noroozinia H, Abbacivash R, Mahoori A. Use of ketamine in severe status asthmaticus in intensive care unit. Iran J Allergy Asthma Immunol. 2003;2:175–80. [PubMed] [Google Scholar]
- 15.Green SM, Johnson NE. Ketamine sedation for pediatric procedures: Part 2, Review and implications. Ann Emerg Med. 1990;19:1033–46. doi: 10.1016/s0196-0644(05)82569-7. [DOI] [PubMed] [Google Scholar]
- 16.Green SM, Nakamura R, Johnson NE. Ketamine sedation for pediatric procedures: Part 1, A prospective series. Ann Emerg Med. 1990;19:1024–32. doi: 10.1016/s0196-0644(05)82568-5. [DOI] [PubMed] [Google Scholar]
- 17.American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–17. doi: 10.1097/00000542-200204000-00031. [DOI] [PubMed] [Google Scholar]
- 18.Howes MC. Ketamine for paediatric sedation/analgesia in the emergency department. Emerg Med J. 2004;21:275–80. doi: 10.1136/emj.2003.005769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CL, Naqibuddin M, Fleisher LA. Measurement of patient satisfaction as an outcome of regional anesthesia and analgesia: A systematic review. Reg Anesth Pain Med. 2001;26:196–208. doi: 10.1053/rapm.2001.22257. [DOI] [PubMed] [Google Scholar]
- 20.Mackenzie N, Grant IS. Propofol for intravenous sedation. Anesthesia. 1987;42:3–6. doi: 10.1111/j.1365-2044.1987.tb02936.x. [DOI] [PubMed] [Google Scholar]
- 21.Mingus ML, Monk TG, Gold MI, Jenkins W, Roland C. Remifentanil versus propofol as adjuncts to regional anesthesia. Remifentanil 3010 Study Group. J Clin Anesth. 1998;10:46–53. doi: 10.1016/s0952-8180(97)00220-1. [DOI] [PubMed] [Google Scholar]
- 22.Ibrahim AE, Ghoneim MM, Kharasch ED, Epstein RH, Groudine SB, Ebert TJ, et al. Speed of recovery and side-effect profile of sevoflurane sedation compared with midazolam. Anesthesiology. 2001;94:87–94. doi: 10.1097/00000542-200101000-00018. [DOI] [PubMed] [Google Scholar]
- 23.Singh R, Batra YK, Bharti N, Panda NB. Comparison of propofol versus propofol-ketamine combination for sedation during spinal anesthesia in children: Randomized clinical trial of efficacy and safety. Paediatr Anaesth. 2010;20:439–44. doi: 10.1111/j.1460-9592.2010.03286.x. [DOI] [PubMed] [Google Scholar]
- 24.Yeh FC, Hsu CS, So EC, Chan YF, Chen JY, Shieh JP. Low dose ketamine and midazolam as supplements for spinal anesthesia. Acta Anaesthesiol Sin. 1999;37:15–9. [PubMed] [Google Scholar]
- 25.Mion G, Villevieille T. Ketamine pharmacology: An update (pharmacodynamics and molecular aspects, recent findings) CNS Neurosci Ther. 2013;19:370–80. doi: 10.1111/cns.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartwright PD, Pingel SM. Midazolam and diazepam in ketamine Anesthesia. Anesthesia. 1984;39:439–42. doi: 10.1111/j.1365-2044.1984.tb07312.x. [DOI] [PubMed] [Google Scholar]
- 27.Hui TW, Short TG, Hong W, Suen T, Gin T, Plummer J. Additive interactions between propofol and ketamine when used for anesthesia induction in female patients. Anesthesiology. 1995;82:641–8. doi: 10.1097/00000542-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Slogoff S, Allen GW, Wessels JV, Cheney DH. Clinical experience with subanesthetic ketamine. Anesth Analg. 1974;53:354–8. [PubMed] [Google Scholar]
- 29.Frizelle HP, Duranteau J, Samii K. A comparison of propofol with a propofol-ketamine combination for sedation during spinal anesthesia. Anesth Analg. 1997;84:1318–22. doi: 10.1097/00000539-199706000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Knox JW, Bovill JG, Clarke RS, Dundee JW. Clinical studies of induction agents. XXXVI: Ketamine. Br J Anaesth. 1970;42:875–85. doi: 10.1093/bja/42.10.875. [DOI] [PubMed] [Google Scholar]
- 31.Sharma SK, Gajraj NM, Sidawi JE, Lowe K. EMLA cream effectively reduces the pain of spinal needle insertion. Reg Anesth. 1996;21:561–4. [PubMed] [Google Scholar]
- 32.Twersky RS, Hartung J, Berger BJ, McClain J, Beaton C. Midazolam enhances anterograde but not retrograde amnesia in pediatric patients. Anesthesiology. 1993;78:51–5. doi: 10.1097/00000542-199301000-00009. [DOI] [PubMed] [Google Scholar]


