Abstract
In pursuit of the goal to understand and eventually reproduce the diverse functions of the brain, a key challenge lies in reverse engineering the peculiar biology-based “technology” that underlies the brain’s remarkable ability to process and store information. The basic building block of the nervous system is the nerve cell, or “neuron,” yet after more than 100 years of neurophysiological study and 60 years of modeling, the information processing functions of individual neurons, and the parameters that allow them to engage in so many different types of computation (sensory, motor, mnemonic, executive, etc.) remain poorly understood. In this paper, we review both historical and recent findings that have led to our current understanding of the analog spatial processing capabilities of dendrites, the major input structures of neurons, with a focus on the principal cell type of the neocortex and hippocampus, the pyramidal neuron (PN). We encapsulate our current understanding of PN dendritic integration in an abstract layered model whose spatially sensitive branch-subunits compute multidimensional sigmoidal functions. Unlike the 1-D sigmoids found in conventional neural network models, multidimensional sigmoids allow the cell to implement a rich spectrum of nonlinear modulation effects directly within their dendritic trees.
Keywords: Contextual modulation, dendrites, dendritic spike, multilayer network, multiplicative interaction, single-neuron model, synaptic integration
I. INTRODUCTION
Whether the goal is a scientific one—to understand the computing functions of neural tissue, or an engineering one—to imitate some aspect of brain function using neuromorphic technology, it is crucial to develop simplified models of individual neurons that concisely describe their computing functions, while abstracting away nonessential biological details. The cost of not having good single-neuron models is potentially high: if in studying a neural circuit, the neuroscientist operates under flawed assumptions regarding the capabilities of the various neuron types that make up the circuit, he/she may fail to design appropriate experiments, encounter difficulties interpreting experimental data, and be steered away from correct conclusions as to how the circuit works. Pitfalls also await the neuroengineer who attempts to build neuromorphic hardware without a solid understanding of the computing capabilities of individual neurons: the large investment of time and money required to move through the hardware development cycle may be wasted if critical capabilities of individual neurons are omitted from the design.
In this paper, we review both the history and recent progress in our understanding of the computing functions of individual neurons, with the following limitations in scope. First, we focus on dendritic integration in the pyramidal neuron (PN), one of a multitude of neuron types in the central nervous system (CNS), but a particularly important one as it is the principal cell of the neocortex and hippocampus. Some of our conclusions will likely apply to other neuron types, while others may not. Second, we focus on spatial integration of synaptic inputs, where multiple synapses are activated in fixed spatial arrangements on one or more dendrites, and time-averaged responses are measured at the soma. Third, we focus on the principles of synaptic integration within a dendritic tree or subtree consisting of a relatively homogeneous set of dendrites emanating from a central node. In a stellate-form cell, this might correspond to the entire dendritic tree. In a PN, subtrees fitting this general description include the basal tree emanating from the soma, and tuft dendrites emanating from the main apical branch point [1]. More global interactions between subtrees are beyond the scope of this paper, but have been discussed elsewhere [2]–[11].
For previous reviews and a broad range of perspectives on the topic of dendritic inte.gration and single neuron computation, see [12]–[23].
II. THE TRADITIONAL VIEW OF THE NEURON
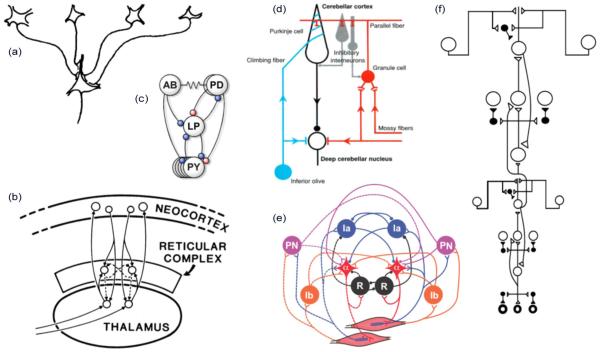
The classical view of dendritic integration in neurons of the CNS is that excitatory and inhibitory synaptic currents collected from across the dendritic arbor are funneled to the soma, where the net current determines the cell’s output firing rate. This view that dendrites exist mainly to increase a neuron’s receptive surface area, but confer no additional processing capabilities [24], [25], is in keeping with the long-running tendency to leave dendrites out of the picture in multineuron models covering virtually every part of the brain and aspect of brain function [26]–[48] (Fig. 1). The notion that dendrites are computationally superfluous has also been reinforced by the introduction of numerous dendrite-less “point neuron” models in the neural computation literature [49]–[54] (see [55] for a commentary on [49]); though it should be emphasized that the use of point neuron models may be entirely appropriate for certain questions and/or levels of analysis.
Fig. 1.
Examples of neural circuit models constructed from “point neurons” containing a single integrative node. (a) Model of a “simple” cell in visual cortex [34]. (b) “Seachlight” model of attention [56]. (c) Subset of the stomatogastric ganglion circuit [36]. (d) Cerebellar circuit [43]. (e) Spinal cord circuit [42]. (f) Circuit in visual cortex [57].
III. CHALLENGES TO THE POINT NEURON HYPOTHESIS
The key assumption in a point neuron model is that the cell has a single integrative node at the soma where all excitatory and inhibitory effects are combined. Though it continues to enjoy broad acceptance, this radically simplified view of the neuron began to face serious challenges already decades ago, both 1) from neurophysiological data indicating that dendrites are capable of generating “active” spike-like responses with varying degrees of locality and based on a variety of ionic mechanisms [1], [6], [11] (see [14] for more references from the pre-1990 literature), [58]–[82]; and 2) from biophysical studies showing that a neuron’s cable properties promote spatially restricted synaptic interactions [61], [65], [83]–[104]. Taken together, these two effects mean that a neuron is capable, in principle, of carrying out multiple local computations simultaneously within its dendritic tree. But what local computations? How many can occur simultaneously, and what are their allowable spatial arrangements in the dendritic arbor? And how are the results of the local computations combined from across the dendritic tree to determine the cell’s overall output?
IV. THERE HAVE BEEN A VARIETY OF IDEAS ABOUT DENDRITIC COMPUTATION
Early theorists proposed that dendrites might engage in a variety of local computations, and they contemplated different ways that the results of those computations might be combined at the soma. Koch et al. introduced the notion of a dendritic “subunit” [88], a subregion within the dendritic tree in which local synaptic computations can take place with relatively little interaction with other subunits. They also showed that the divisive interaction between an excitatory synapse and a shunting or “silent” inhibitory synapse (i.e., whose reversal potential is at or near the cell’s resting potential) could provide a logical and–not operation, where the inhibition acts like a local “veto” signal. They suggested that this operation, repeated in many different subunits across the dendritic tree, could provide a biophysical mechanism for direction selectivity [89], along the lines proposed by Barlow and Levick [105]. Shepherd and Brayton [100] demonstrated that voltage-dependent Na+ channels could implement logical and and or operations between nearby synapses, and proposed that a dendritic tree might act like a hierarchical boolean logic network [12]. Rall and Segev [98] showed that mixtures of active and passive spines could produce complicated and varied nonlinear interactions between inputs to multiple branches of a dendritic tree. Zador et al. [106] showed that a voltage-dependent K+ channels could produce a xor interaction between inputs delivered to two different dendritic sites. Other early models of dendritic integration included the “contextron,” a simplified neuron model in which a set of dedicated modulatory inputs enabled different dendritic subunits at different times [107]; the “sigma-pi” unit, in which the dendrites provided a set of low-order product terms between groups of synapses that were summed at the soma [108]–[110]; and the “clusteron,” a model neuron that captured the fact that dendrites containing N-methyl-D-aspartate (NMDA) and voltage-dependent Na+ channels exhibit the property of “cluster sensitivity,” that is, they respond more strongly to excitatory synapses activated in multiple spatial clusters in comparison to the same total amount of input delivered diffusely [67], [92]–[94], [111].
The idea that a neuron computes a sum of nonlinear terms, where each term represents the interaction between a group of nearby synaptic inputs, is more or less explicit in several of the aforementioned models. This abstraction of dendritic integration has the advantage that it is specific regarding the mathematical operations that are supposed to be taking place in the dendritic tree, but is vague regarding the constraints that exist on the spatial distribution of dendritic sites that can be simultaneously involved in these local nonlinear operations, in relation to the physical branching structure of the neuron. For example, when a distal site is synaptically activated and a local computation takes place, should the result of that computation, as it flows to the cell body, influence or not influence an ongoing computation at a more proximal site? Do the neuron’s computational subunits have soft or hard boundaries, and to what extent are the boundaries warped by branch points, the soma, etc.? Are dendritic subunits organized hierarchically, and if so, is the hierarchy shallow or deep?
V. THE TWO-LAYER MODEL
In search of a model of dendritic integration that retained the same basic sum-of-nonlinear-terms form as several earlier models, but which was more explicit about the relationship between the structure of a PN dendritic tree and the structure of the overall computation, we introduced the “two-layer model” (2LM) (Fig. 2). The 2LM incorporates several morphology-related simplifications. First, it aims to describe the behavior of only a portion of the overall dendritic arbor, specifically, a subtree consisting of a uniform set of thin dendrites emanating from a central node. Second, given that most of the excitatory synapses impinging on PN thin dendrites lie on long, unbranched terminal sections [112]–[114], we assumed that the tree consisted only of unbranched terminal dendrites. Third, given that cable theory tells us that voltage communication within a dendrite is relatively efficient whereas communication between dendrites is relatively poor [88], [97], [104], we assumed communication within a dendrite was perfect and communication between dendrites was negligible.
Fig. 2.
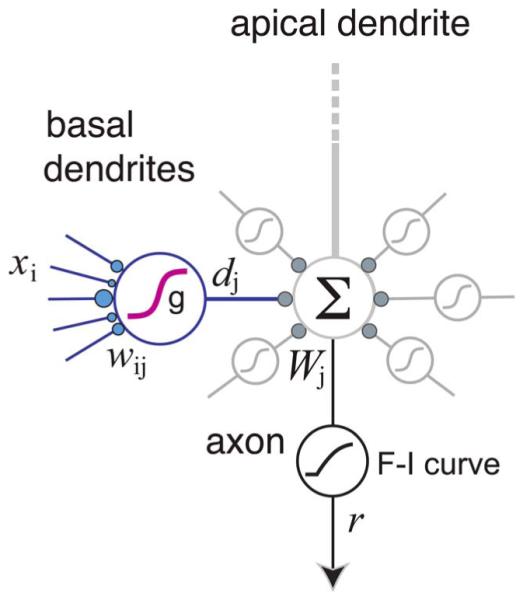
Two-layer model of a pyramidal neuron dendritic subtree. Each dendritic “subunit” computes a weighted sum of its inputs (weights are shown as blue circles), and then applies a nonlinear subunit function g(·) to produce the dendritic output d. These outputs are then summed with weights and fed into the global F–I curve to produce the neuron’s response r. A model of this form was used to predict average spike rates produced by a biophysically detailed compartmental model [96]. Depiction here is intended to suggest the basal subtree; apical oblique and apical tuft dendrites are thought to behave similarly [1], [91], though the model for the cell as a whole also involves nonlinear interactions between subtrees [6], [7], [11], [18] (see Fig. 8).
In addition to assuming that each dendrite is a separate subunit, the basic 2LM also assumed that: 1) the effect of the ith synapse onto the jth subunit is fully described by its activation rate xi times its weight wij; 2) a subunit’s input–output (I–O) function is a linear–nonlinear (LN) cascade, first combining its synaptic inputs linearly followed by a stereotyped nonlinear subunit function g(·); 3) the dendritic output “currents” dj are summed at the soma with branch weights Wj; and 4) the total somatic current is fed into the axo-somatic F–I curve to determine the cell’s output firing rate r (Fig. 2).
All of these features of the basic 2LM are captured by
| (1) |
| (2) |
Besides providing a simple model of dendritic integration, this scheme brought three additional advantages. First, the 2LM is isomorphic to a feedforward artificial neural network (ANN), with the dendrites providing a layer of nonlinear computing subunits (also knows as codons, conjunctions, basis functions, hidden units, higher order features, etc.) interposed between the inputs and the output. This isomorphism allows the theory of ANNs, including their learning rules and their various uses as classifiers, function approximators, density estimators, etc., to be brought to bear in the effort to understand how individual neurons contribute to neural circuit function [115]. Second, the idea that a neuron pools the outputs of a set of discrete LN subunits suggests a connection between dendritic form/function and neurophysiological data: the receptive fields (RFs) of sensory neurons have frequently been described as having a two-layer sum-of-subunits structure. Most notably, the receptive fields of V1 “complex cells” are typically modeled as a sum of multiple “simple cell” subunits [34], [116]–[118]. A similar scheme involving the pooling of subunits is thought to account for the increasing spatial invariance of receptive fields along the visual cortical form processing pathway [119]–[123], raising the possibility that dendritic subunits are the physical substrate for a neuron’s functional subunits [83], [85], [88], [91], [124]–[135]. Third, the partitioning of the dendritic tree into discrete integrative units of a specific size facilitates the analysis of memory or processing capacity [130], [136]–[138].
VI. SUPPORT FOR THE TWO-LAYER MODEL
A. Modeling Studies
Given the technical difficulties associated with stimulating real neurons at multiple precisely defined locations and maintaining the health of neurons while recording their responses to large stimulus sets, the first direct support for the 2LM came from computer simulations. In these studies, a biophysically detailed compartmental model was used as a surrogate for a real neuron, and its responses were compared to the predictions of an abstract 2LM over a systematically constructed stimulus set. The logic of this model-to-model comparison strategy is as follows: if the responses of a very complicated, realistic compartmental model, whose evaluation involves numerical integration of thousands of coupled nonlinear differential equations, can be predicted by a model that is so simple that it can be evaluated by hand, then to the extent that the detailed model is a faithful representation of a real neuron, and the stimulus set is a faithful representation of the relevant stimulus space, then the simple model is a useful abstraction of the real neuron’s integrative capabilities. Furthermore, the ways in which the simple model fails to capture the complex model’s behavior can provide valuable clues as to the deficiencies in one or both of the models, or the stimulus set, or the response measure, or all of the above. For example, the detailed model might lack ionic currents crucial for a behavior of interest, or the ion channels that are included might be inappropriately distributed or incorrectly parameterized. The stimulus set used to test the models might be too simple, like testing the performance of a scientific calculator only on problems involving single-digit arithmetic, or functionally inappropriate, like testing the ability to play tennis with a bow and arrow. Even if the task is appropriate, the response measure might be off base, like assessing the quality of a musician’s recorded performance using the video track rather than the sound track. The simple model may also be deficient, either by including invalid simplifications, or by leaving out valid ones. Thus, the model-to-model comparison process is not only a means of testing ideas about single-neuron function and the biophysical basis thereof, but has the added advantage that it produces information useful for improving both the realistic and abstract models.
Several studies have proceeded using this approach. Archie and Mel [83] showed using a compartmental model that a dendritic tree containing four basal dendrites emanating from the soma—a “ball and sticks” morphology—produced firing rates that were more consistent with the predictions of a 2LM with an accelerating subunit function than of a point neuron. Moreover, the model cell captured the response nonlinearities of V1 complex cells whose receptive fields had been previously described by a two-layer “energy” model [117], [139]. However, given the simplified dendritic morphology and stimulus set used in the study, and the lack of a quantitative assessment of prediction performance under different 2LM assumptions, the strength of the conclusions that could be drawn from that study was limited. A more demanding test of the 2LM was carried out using a more biophysically realistic compartmental model of a CA1 pyramidal cell, using a larger, more complex stimulus set [96]. We found the 2LM with a sigmoidal subunit function outperformed the other variants tested, and explained 67% of the firing rate variance that could be attributed to variations in the spatial distribution of synaptic inputs—compared to 11% for a point neuron model.
This test of the 2LM was also lacking, however, in that the simulation experiments did not control for within-branch spatial variations, made no allowance for dendrite-specific subunit nonlinearities, and could not distinguish direct voltage-dependent interactions between dendrites in the first layer from nonlinear interactions occurring in the second layer arising from the cell’s axo-somatic F–I curve. Lacking these controls, it was not possible to decide whether the substantial fraction (33%) of unexplained firing rate variance seen in this study was due to either a fundamental breakdown of the 2LM’s core assumption of subunit independence, versus an inadequate representation of the subunit and somatic nonlinearities. In a recent follow-up study, we found that when the nonlinear dendritic I–O functions are properly characterized in terms of the steady state currents they produce, and the somatic F–I curve is properly taken into account, the two core assumptions of the 2LM—that 1) synaptic integration occurs independently in different subunits, and 2) subunit outputs combine linearly at the soma—are upheld with remarkable accuracy [84].
B. Experimental Studies
A basic prediction of the 2LM is that the responses to two inputs delivered to the same dendritic branch should combine as if the inputs are summed by an LN subunit with a sigmoidal nonlinearity, whereas the responses to two inputs delivered to two different branches should combine linearly at the soma—as long as the cell remains subthreshold for somatic spiking [95], [96], or the somatic F–I curve happens to be linear [84]. These predictions were confirmed in experiments in brain slices [74], with a caveat discussed in Section VII.
The idea that thin terminal dendrites are the main integrative compartments in PNs has also received support from anatomical data: dendritic spine volumes and postsynaptic density sizes measured in EM images were found to decrease steadily along the length of CA1 oblique dendrites [140]. This gradual falloff in synaptic “weight” moving from the branch origin, where the input resistance is low, to the branch tip, where the input resistance is high, suggests that as a group these synapses are “designed” to be uniform contributors to voltage signaling in that specific dendritic compartment, rather than at the soma. (For a related point on the role of spines in equalizing local voltage responses, see [141]). Additional circumstantial support for dendritic subunitization lies in the evidence that dendrites are, or contain, or would benefit from being or containing, synaptic plasticity compartments [92], [106], [127], [128], [130], [136], [138], [142]–[154]; for review, see [22].
VII. PROBLEMS WITH THE 2LM ASSUMPTION THAT DENDRITES ARE LOCATIONLESS
A core assumption of the basic 2LM is that a dendrite sums its inputs independent of their location within the subunit. Notwithstanding its value in linking dendritic trees to ANNs and other equivalent layered computational frameworks, the assumption that a dendrite is a locationless LN unit is a crude approximation from the perspective of cable theory, and has become increasingly untenable as evidence accumulates regarding the location dependence of synaptic integration effects in PN dendrites.
Several lines of evidence suggest that modeling a PN dendrite as a locationless integrative unit is too simple. It has been known for decades that dendritic structures, unlike uniform cables, exhibit highly asymmetric voltage attenuation and summation effects [61], [65], [89], [97], [102], [104], [155], [156]. These asymmetries arise both from the difference in input impedance at the two ends of the dendrite (high at the distal tip, low at the perisomatic end; see [157, Ch. 3]), and from the fact that the “readout” of a dendrite is at the proximal end, since it is only the current reaching the soma that participates in the cell’s second layer of processing. (This statement ignores the effect of direct dendro–dendritic communication, which do occur [158] but are beyond the scope of this paper.)
A striking illustration of the importance of location within a PN dendrite lies in the systematic change in a dendrite’s sigmoidal I–O curve when a single focal excitatory stimulus is applied at different distances from the soma [61], [62], [71] (Fig. 3). When the intensity of dendritic excitation is gradually increased and the response is measured at the soma, the I–O curve typically exhibits these three general features: an initial slow rise at low stimulus intensities, followed by a steep jump in amplitude when the local dendritic spike threshold is crossed, followed by a saturation of the response magnitude at high stimulus intensities [1], [70], [76], [159]. The parameters of the sigmoid vary systematically with dendritic location, however. As the stimulation site moves farther out along the branch, the spike threshold decreases steadily, given that it is inversely related to the input resistance [65], [99], [156]. The amplitude of the sigmoid also declines steadily, given the distance-dependent attenuation of a dendritic spike as it passively propagates from the site of initiation to the soma [61], [65], [71], [159] [Fig. 3(b) and (c)]. Other parameters can influence the I–O curve as well, including the spatial spread of the excitation about its center [61], [67], [92]–[94], [160], and the spine neck resistance [84], [141], [161], both of which alter the sigmoidal nonlinearity. Thus, depending on its center, spread, and other parameters that may depend on location, each excitatory input to a dendrite can choose from a spectrum of different sigmoidal I–O curves—a fact that is irreconcilable with the locationless LN subunit assumption.
Fig. 3.
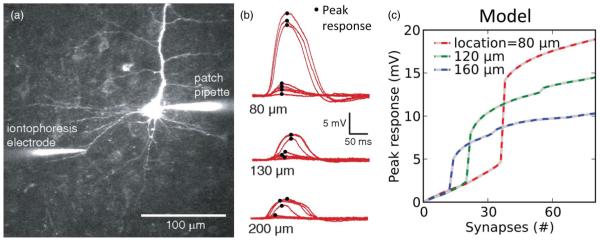
Example of location dependence of dendritic I–O curves. (a)–(b) Data from [71] showing location dependence of NMDA spikes. (a) Illustration of experimental setup: glutamate iontophoresis activates synapses at specific dendritic locations. (b) Voltage traces recorded at the soma for various stimulus sites along the dendritic length. As stimulation intensity is increased, a threshold is crossed and a local “NMDA spike” is generated. (c) Compartmental model from [61] shows a similar location dependence of the threshold and peak somatic voltage response. Curves show peak voltage responses recorded at the soma [similar to the peak of the traces shown in (b)] for stimulus sites at increasing distances from the soma (modified from [61]).
An interesting side effect of the spatial dependence of a dendrite’s sigmoidal I–O curve is that conventional notions of synaptic integration can be turned on their head. For example, an excitatory stimulus of a given intensity can have a larger effect on the soma when it is delivered to a distal site compared to more proximal sites, if the intensity is sufficient to cross the local spike threshold distally but not proximally [compare blue to red and green curves in Fig. 3(c) for 12 synapses]. Or, the somatic response can be largest for a stimulus delivered at mid-dendrite compared to the same stimulus delivered more proximally or distally [compare green to red and blue curves in Fig. 3(c), for 30 synapses].
In summary, even when only a single excitatory input pathway is activated, let alone an arbitrary spatial pattern of excitation, the asymmetric passive cable properties interacting with location-dependent dendritic spike thresholds means that a PN thin dendrite cannot be described as an LN function with a stereotyped sigmoidal nonlinearity. But what is the alternative? In Section VIII, we discuss our recent efforts to capture with greater generality the spatial integration capabilities of a single active dendrite, including both pure excitatory, as well as excitatory–inhibitory spatial interactions.
VIII. WHEN MULTIPLE INPUTS ARE ACTIVATED, THE SUBUNIT ACTS LIKE A MULTIDIMENSIONAL SIGMOID
In the first experimental test of the 2LM model, we compared summation of two colocalized inputs within the same dendrite to summation of two inputs delivered to different dendrites [74]. In a final experiment in that study in which two inputs to the same branch were increasingly separated, we showed that the within-branch summation rule changed qualitatively as a function of separation distance. Both the data and theory available at the time were insufficient to describe the effect in any generality, however.
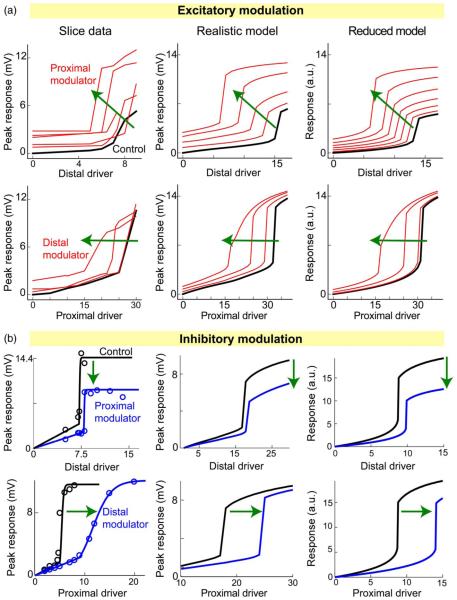
To address this, we carried out a more extensive set of two-input summation experiments both in brain slices and computer models, and in both excitatory–excitatory [61] and excitatory–inhibitory [65] configurations. To facilitate interpretation of the results, in each case, one of the inputs was designated the “driver” input, whose sigmoidal I–O curve was plotted along the abscissa, while the other input, whether more proximal or more distal, and whether excitatory or inhibitory, was designated the “modulator.” The effect of the modulator on the driver’s I–O curve was then analyzed. Fig. 4 shows examples of the data gathered in the slice experiments, and comparable results from two different types of models: 1) a “realistic” model, consisting of a reconstructed PN morphology discretized into 443 compartments, containing multiple types of ion channels and time-varying synaptic conductances, and whose somatic response was calculated through numerical integration of thousands of coupled nonlinear differential equations using the NEURON simulation package [162]; and 2) a “reduced” model, consisting of two compartments with time-invariant synaptic conductances and whose response was the solution of one nonlinear algebraic equation. The effects of the modulator on the driver’s sigmoidal I–O curve were similar in all three “models” (slice, realistic, and reduced), for both excitatory and inhibitory modulation at both proximal and distal sites (Fig. 4). This similarity is important as it tells us that the reduced model, though radically simplified compared to the realistic model, not to mention a real neuron, is nonetheless adequate to describe the time-averaged analog location-dependent interactions of two spatially separated inputs to an active dendrite.
Fig. 4.
Location-dependent two-input synaptic integration effects. (a) Excitatory–excitatory interactions, adapted from [61]. Left column shows peak somatic responses recorded in brain slices as a function of excitatory stimulus intensity (“Driver”), similar to Fig. 3(c). Each red curve was generated for a fixed value of the excitatory modulation. Distal/proximal labels indicate location of the driver or modulator relative to the soma. Middle column shows comparable results in a realistic 443-compartmental model with full membrane and synaptic dynamics. Right column is from a time-invariant two-compartment model. (b) Plots are organized as in (a) but for an inhibitory modulatory input (from [65]).
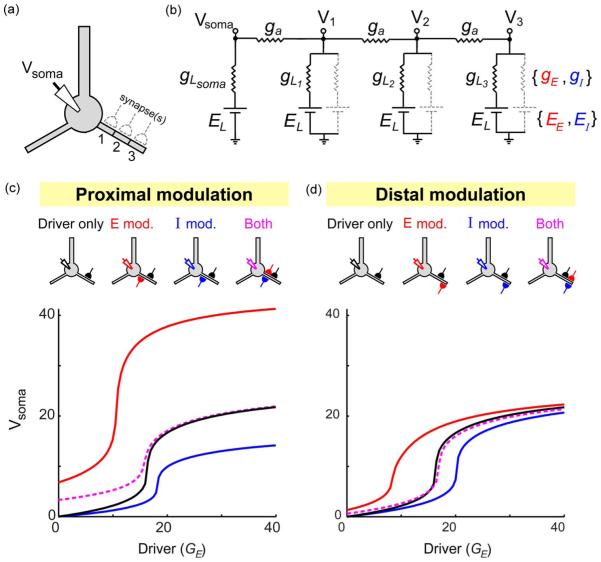
The general rules of two-input summation are summarized in Fig. 5. First, a proximal modulator affects both the threshold and the amplitude of the driver’s I–O curve, lowering the threshold and increasing the amplitude in the case of an excitatory modulator, and raising the threshold while decreasing the amplitude in the case of an inhibitory modulator [Fig. 5(c)]. In contrast, a distal modulator affects only the threshold of the driver’s input-output curve, lowering the threshold (i.e. left-shifting the I–O curve) when the modulator is excitatory, and raising the threshold when the modulator is inhibitory (Fig. 5(d); see also [99], [156]). Finally, as suggested by the first two generalizations, excitatory and inhibitory modulators have similar effects, but in opposite directions. Consistent with this, when excitatory and inhibitory modulators are applied simultaneously at the same location, their effects on the threshold and amplitude roughly cancel [Fig. 5(c) and (d), dashed magenta curves].
Fig. 5.
Excitatory and inhibitory spatial modulation effects are roughly equal but opposite. (a) Schematic shows neuron with three potential sites of synaptic input. (b) Circuit model with three dendritic and one somatic compartments. Parameters were: gL1 = 10, gL2 = 2, gL3 = 1, ga = 6, EL = −70 mV, EE = 0 mV, EI = −70 mV. Excitatory synapses were modeled as voltage-dependent NMDA-type channels, with gE = GE/(1 + exp(−(Vx + 23:7)/12:5)) where Vx is the voltage at the synapse location. Conductances were in arbitrary units. (c) Schematics of neurons/synapses show an excitatory “Driver” input delivered to mid-dendrite (black synapse), coupled with a proximal excitatory (red) or inhibitory (blue) modulator input, or both. I–O curves are shown for each case (see legend for color code). Excitatory and inhibitory modulator conductances were GE = 40 and gI = 18. The somatic voltage was calculated using the method described in [65]. (d) Similar to (c), but for distal modulation (GE = 60 and gI = 24).
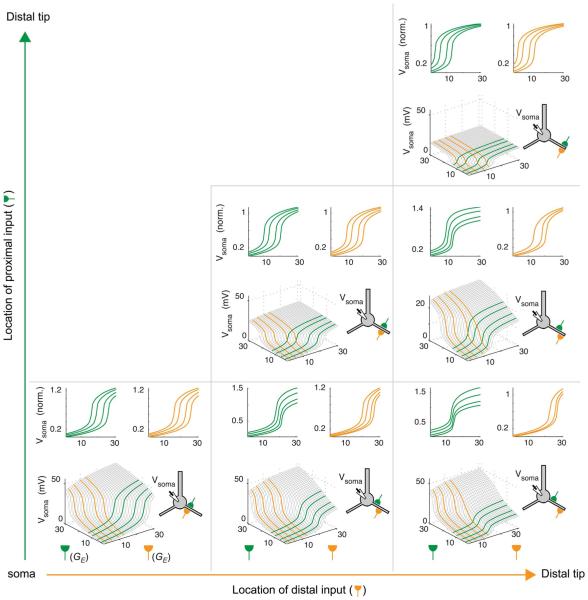
To gain a more comprehensive overview of the space of two-input spatial integration effects, including the effects of both relative and absolute location, we systematically varied the locations of two inputs to a dendrite, and for each input configuration, plotted the somatic response as a 2-D surface plot (Fig. 6). The three subplots on the main diagonal show cases with two colocalized inputs at increasing distances from the soma. The plots are all symmetric 2-D sigmoids (and thus effectively degenerate to 1-D sigmoids as in the basic 2LM), but with lower threshold and amplitude as the distance from the soma increases. This is consistent with the distance-dependent changes in sigmoid parameters seen for a single input to a PN (Fig. 3). In each subplot in Fig. 6, several 1-D slices are collected into vertically normalized “marginal” plots, so that the effects of proximal modulation (green) and distal modulation (orange) can be explicitly viewed. Given the rough interchangeability of excitatory and inhibitory modulation effects (see Fig. 5), the 1-D slice plots can be interpreted as showing either type of modulation, where inhibition pushes the curves to the right, and excitation pushes the curves to the left.
Fig. 6.
Spectrum of two-input location effects. Surface plots show effect of conjoint stimulation by two inputs (schematic of neuron shows locations). Inputs are given in normalized conductance units (similar to Fig. 5). Three panels on the main diagonal show cases with colocalized inputs at different distances from the soma. Green and orange line plots in top row of each panel show color-coded slices from surface plots to highlight the effect one input has on the I–O curve of the other.
In the lower right subplot, which corresponds to the case with maximally separated inputs, the 2-D sigmoid is most asymmetric, clearly showing the distinction between threshold plus amplitude modulation by the proximal input (green) versus pure threshold modulation by the distal input (orange).
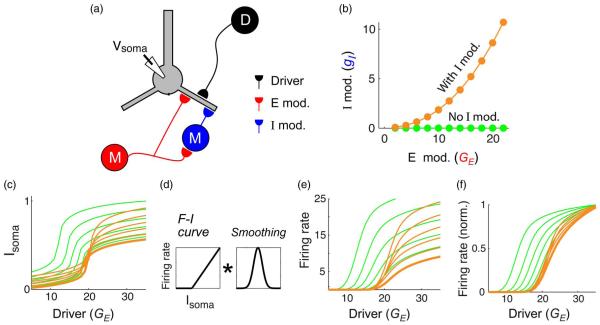
IX. MULTIPLE MODULATION EFFECTS CAN BE COMBINED
The rough cancellation of the excitatory and inhibitory modulators illustrated in Fig. 5 raises the intriguing possibility that multiple modulators may be combined to achieve a rich spectrum of modulation effects. To illustrate this idea, we constructed an example in which a driver input (D) projects to mid-dendrite, while a modulatory input (M) acts both through a monosynaptic excitatory input to a proximal site, and a disynaptic inhibitory input to a distal site via a dendrite-targeting interneuron (I) [Fig. 7(a)]. According to the “rules” of proximal–distal modulation laid out in Figs. 5 and 6, as the modulatory input increases, its effects should be to: 1) lower the threshold and boost the amplitude of the driver’s I–O curve due to the proximal excitatory modulator, while 2) raising the threshold of the driver’s I–O curve without affecting the amplitude due to the distal inhibitory modulator. If the two modulatory effects combine “rationally,” in the sense that the two opposing threshold effects cancel, the net result of the modulation should be a relatively pure multiplicative scaling of the driver’s I–O curve. The orange scatter data in Fig. 7(b) show the slightly superlinear relation between the levels of modulatory excitation and inhibition needed to achieve the multiplicative scaling effect. (This type of gentle superlinear relation could arise from various sources. For example, the interneuron could have an accelerating F–I curve, or the excitatory synapses onto the interneuron could have facilitating short-term synaptic dynamics.) The effect of this compound modulator is shown by the orange curves in Fig. 7(c)–(e), where the lowest orange curve is the driver’s I–O curve with no modulation. For comparison, the green curves show the result where the inhibition is “blocked,” leaving only the excitatory proximal modulation effect [as seen in Fig. 5(c)]. When the set of orange curves is passed through a typical F–I nonlinearity and a temporal/spatial averaging operation to represent the transformation from subthreshold voltage signals to firing rates under in vivo-like conditions, the result is a roughly multiplicative modulation [Fig. 7(e)]. To make the approximate scaling relationship clearer, the I–O curves are also shown vertically normalized [Fig. 7(f)].
Fig. 7.
Compound modulation circuit for multiplicative scaling. (a) Schematic of input configuration. (b) Orange scatter data show relation between excitatory modulation strength [red input in (a)] and inhibitory modulation strength [blue input in (a)]. Green data points indicate the case with only excitatory modulation, i.e., with inhibitory modulation “blocked.” (c) Orange curves show subthreshold voltage calculated using the four-compartmental model shown in Fig. 5. The effect of the compound modulator is primarily an increase in amplitude with a slight lowering of the threshold. For comparison, green curves show modulation with inhibition blocked [comparable to the red curve in Fig. 5(c)]. (d) Transformation to firing rate is modeled by applying a threshold-linear F–I curve followed by convolution with a smoothing kernel. (e) Firing rate curves obtained by applying the transformation in (d) to the data in (c). Orange curves resulting from compound modulation show approximate multiplicative scaling. The case without inhibition is shown for comparison (green curves). (f) Height-normalized firing rate curves from panel (e) illustrating the near invariance in orange I–O curves up to a variable scaling factor.
To summarize, a PN’s nonlinear analog spatial processing capabilities provide the local circuit with access to a broad spectrum of modulation effects that could be useful in many different types of computations. In particular, a modulatory pathway (whether contextual, attentional, cross-modal, etc.) would distribute its excitatory contacts nonuniformly along the proximal–distal axis of the PN’s thin dendrites to achieve a desired mixture of threshold lowering versus amplitude boosting, while biasing its projections onto different classes of interneurons to achieve the desired mixture of threshold elevation versus gain suppression [163]. The diversity of computations that can be implemented through this type of compound modulation effect has barely begun to be explored.
X. DISCUSSION
A. Current State of the 2LM
A major theme of this review is that a key assumption of the original 2LM is too simple: thin dendrites emanating from a soma or main trunk are not well described as locationless LN subunits. Rather, based on close agreement between experimental and modeling studies, we have arrived at the following conclusions: 1) given that a single input pathway can produce a spectrum of 1-D sigmoids depending on its location and other parameters, and that two input pathways can produce an even richer spectrum of 2-D branch functions depending on their locations and parameters, and there is evidence that this process continues to higher dimensions [164], it is appropriate to describe a PN thin dendrite as a multidimensional sigmoidal unit; 2) the multidimensional sigmoidal interactions between synapses within a PN dendrite can be reproduced by simple time-invariant circuits, which may open the door to compact analog hardware implementations of dendrites in the future [165]; 3) the distinct effects of distal versus proximal modulation are straightforward to state: distal modulation has a pure threshold-altering effect, whereas proximal modulation has combined threshold and amplitude effects [65]; for related ideas, see [89], [99], [102], [156], and [166]; 4) excitatory and inhibitory modulators have similar effects but in opposite directions; and 5) to the limited extent that the issue has been explored, the effects of multiple modulators combine rationally, in the sense that their effects on threshold and amplitude roughly “add” when they act in the same direction, and roughly “cancel” when they act in opposite directions (Fig. 7).
In contrast to the LN subunit assumption, which is clearly in need of revision, the other key assumption of the 2LM—that thin dendrites act as independent subunits [74], [83], [95], [96]—has received further support in recent years [1], [84], [140], [155]. Thus, our current working model of a PN subtree retains its two-layer structure, but includes an upgraded definition of the subunit function
| (3) |
where E(x) and I(x) are the spatial patterns of excitation and inhibition, respectively, impinging on a dendrite. No analytical expression is currently available for function g(E(x); I(x)) for arbitrary spatial patterns of excitation and inhibition. However, to the extent that the E(x) and I(x) patterns can be captured by a coarse spatial quantization, such as in the single-input, two-input, and three-input cases discussed above, the time-averaged response of the dendrite can be evaluated by solving a circuit like the one shown in Fig. 5 (or using a table lookup). While these multidimensional branch functions arising from spatial interactions are more complicated than the locationless 1-D subunit functions of the original two-layer model, they are far less complicated than a biophysically detailed compartmental model of a dendrite, and are therefore valuable as abstractions of dendritic spatial processing capabilities.
B. Scope and Extensions of the Model
To reiterate the limitations in our scope in this paper, the augmented 2LM that includes within-dendrite spatial effects as shown in Fig. 8 is most appropriate for describing the integrative behavior of a dendritic tree or subtree whose synapses lie predominantly on a uniform set of long, unbranched terminal dendrites emanating from a common central node. This “ball and sticks” structure applies to many types of CNS neurons, however, so that our conclusions may have broad applicability. It is also important to note that the augmented 2LM is a generalization of the basic 2LM model, and so encompasses single-neuron models that do not make use of their within-dendrite spatial processing capabilities [83], [130], [132], [133], [136]–[138]. The simplest way this “degeneracy” could occur is if excitatory inputs project to dendrites in a spatially unbiased fashion. A further limitation has been our focus on time-averaged spatial integration behavior. While spatial integration is clearly important, many interesting forms of dendritic processing will likely involve timing in some critical way [89], [155], [167]–[170]. How best to include timing effects into a model that has no representation of time is not clear, especially if the goal is to retain a model that is simple enough so that it can be evaluated practically by hand.
Fig. 8.
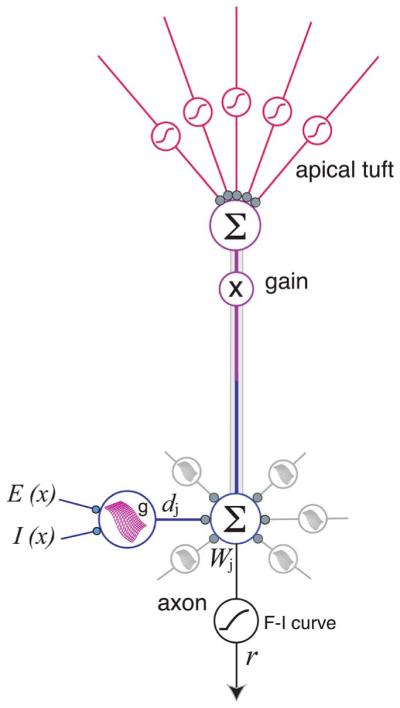
Augmented model of the PN is an elaboration of the 2LM shown in Fig. 2. Dendritic subunits are shown having multidimensional sigmoidal I–O functions that depend on the spatial distributions of excitatory and inhibitory inputs to that dendrite [61], [65]. Output of apical tuft is shown setting the response gain for the whole cell [1], [6], [7], [175]. Apical tuft and oblique dendrites may also show within-branch location effects, but this has not yet been tested.
Even within the realm of spatial processing, a number of additional parameters will likely interact with the location effects we have discussed here, including 1) the NMDA–AMPA ratio, which will influence the maximum achievable slope of the sigmoidal nonlinearity; 2) short-term synaptic dynamics, which can significantly change the integrative “arithmetic” of the cell [21], [171], [172], and 3) ion channel gradients, which may introduce additional location-dependent summation effects even within individual branches [62], [173].
A further elaboration of the two-layer model, in which the core assumption of subunit independence is relaxed, is also likely to be needed to capture the breadth of synaptic integration effects in the CNS. It is obvious that few real dendritic trees conform faithfully to the assumption that all of their dendrites are terminal, unbranched, and emanate directly from a central node, i.e., the morphology that would tend to maximize dendritic independence. Instead, thin dendrites within a typical subtree are electrically coupled to varying degrees, such that branches that are close, particularly sister branches joined at a branch point, show nontrivial levels of subthreshold voltage interaction that leads to a partial breakdown of their functional independence [1], [84], [159]. One interesting possibility is that the sharing of subthreshold voltage signals between nearby subunits is a feature rather than a bug. For example, fully independent subunits might be the appropriate targets for statistically independent channels of information, e.g., see [174], whereas immediately neighboring subunits whose responses are correlated by subthreshold voltage communication may be appropriate targets for partially correlated information channels. More work will be needed to sort out this issue.
XI. CONCLUSION
The past 30 years have brought major progress in our understanding of the principles of synaptic integration in active dendritic trees. The modern view of the neuron, supported by the triad of experimental data, mathematical analysis, and computer modeling, has evolved based on an accumulation of insights from many corners, and has brought us to a point where, though we are still far from having a complete picture of the roles that individual neurons play in the circuits of the brain, it can now be said with confidence that a neuron’s information processing capabilities go well beyond the capacity to integrate and fire. Recent advances in the ability to measure dendritic processing in the intact brain will make it much easier to pin down the computing functions of individual pyramidal neurons and other cells types moving forward, so that our 100+ year wait to learn what a single neuron does may be approaching its end [11], [68], [73], [79], [176], [177].
Acknowledgments
This work was supported in part by the National Institutes of Mental Health under Grant MH065918-01 and the U.S.–Israel Binational Science Foundation under Grant 2009341.
Biographies
Monika P. Jadi received the B.E. degree in electronics and power engineering from the National Institute of Technology, Nagpur, India, in 1995, the M.S. degree in electrical engineering from the University of Missouri, Rolla, MO, USA, in 1997, and the Ph.D. degree in biomedical engineering from the University of Southern California, Los Angeles, CA, USA, in 2010.

From 1995 to 1997, she worked as a Research and Teaching Assistant at the University of Missouri. From 1997 to 2004, she worked as a Senior Staff Engineer at Motorola, contributing to the architecture and development of real-time software for paging and cellular base stations. From 2004 to 2010, she worked as a Research and Teaching Assistant with the Department of Biomedical Engineering, University of Southern California. She is currently a Postdoctoral Fellow in the laboratory of Terrence Sejnowski at the Salk Institute for Biological Sciences, La Jolla, CA, USA. Her broad research interest includes neural computation at various levels in the brain from the synapse, to single neuron to networks of neurons. She has studied the contribution of inhibitory neurons in the brain to single-neuron computation as well as oscillatory dynamics in neural networks.
Dr. Jadi has been a recipient of the Dean’s Fellowship at the University of Missouri, Women in Science and Engineering Fellowship at the University of Southern California, and the National Eye Institute Training Fellowship at the Salk Institute. She has been a member of the Society for Neuroscience since 2004.
Bardia F. Behabadi received the B.S. degree in biomedical engineering from The Johns Hopkins University, Baltimore, MD, USA, in 2002 and the Ph.D. degree in biomedical engineering from the University of Southern California, Los Angeles, CA, USA, in 2011.

From 2004 to 2011, he worked as a Research and Teaching Assistant with the Department of Biomedical Engineering, University of Southern California. Since 2012, he has been a Senior Engineer at Qualcomm Research, San Diego, CA, USA. His research interests include neural computation, machine learning, and artificial intelligence.
Alon Poleg-Polsky was born in Magnitogorsk, Russia, in 1980, and immigrated to Haifa, Israel, in 1990. He received the MD and Ph.D. degrees in neuroscience from the Technion—Israel Institute of Technology, Haifa, Israel, in 2007 and 2009, respectively. The main focus of his Ph.D. training, under the supervision of Prof. J. Schiller, was to examine dendritic signal processing in fine dendrites and NMDA spike generation using experimental and modeling approaches.

Between 2008 and 2010, he combined his medical and scientific training as a medical director of a startup company, Nephera, in Haifa, Israel. In 2010, he joined Jeffrey S. Diamond’s lab at the National Institutes of Health (NIH), Bethesda, MD, as a visiting fellow. In his current postdoctoral studies. he is applying his skills in understanding retinal physiology, specifically focusing on direction selectivity circuit.
Dr. Poleg-Polsky is a member of the Society for Neuroscience. He serves as a reviewer for the Journal of Neuroscience and the Journal of Physiology. During his studies, he received numerous awards, including the Wolf prize for graduate students (2005) and the Rothschild Fellowship (2010).
Jackie Schiller was born in 1960. She received the Ph.D. degree in neurobiology from the Hebrew University, Jerusalem, Israel, in 1993.

She was a Postdoctoral Fellow in the Laboratory of Bert Sakmann at the Max-Planck Institute of Medical Sciences between 1993 and 1995. Between 1995 and 1997, she was a Postdoctoral Fellow in the Laboratory of David Clapham at the Mayo. She joined the Technion—Israel Institute of Technology, Haifa, Israel, as a faculty member in 2000 and became a full Professor with the Faculty of Medicine in 2011. She currently heads the Rappaport Neuroscience center at the Technion. Her work focuses on understanding the processing capabilities of principle cortical neurons with special emphasis on the nonlinear computational capabilities of dendrites in cortical pyramidal neurons. She uses advanced electrophysiological, imaging, histology, and modeling tools to decipher the properties of dendrites. She has recently been exploring the role of active dendritic processing in the cortex of awake behaving animals.
Prof. Schiller has been a Principle Investigator on grants from Israel Science Foundation, United States-Israel Binational Science Foundation, German-Israel Foundation for Scientific Research and Development and Minerva Foundation.
Bartlett W. Mel was born in Berkeley, CA, USA, in 1960. He received the B.S. degree in electrical engineering and computer science from the University of California at Berkeley, Berkeley, CA, USA, in 1982 and the Ph.D. degree in computer science from the University of Illinois at Urbana-Champaign, Urbana, IL, USA, in 1989.

He was a Postdoctoral Fellow in the Laboratory of Christof Koch at the California Institute of Technology (Caltech), Pasadena, CA, USA, from 1989 to 1994. He has been on the faculty of the Biomedical Engineering Department, University of Southern California (USC), Los Angeles, CA, USA, since 1994, with joint appointments in Psychology and the Neuroscience Ph.D. Program. His work focuses on understanding the information processing mechanisms of the brain, with intertwined scientific and engineering goals. A major focus has been to study the “arithmetic” underlying synaptic integration in the dendritic trees of CNS neurons using computer models at multiple levels of abstraction, and to explore how the computing capabilities of individual neurons may contribute to the sensory, motor, and memory-related functions of the brain. On the engineering side, he has focused on the development of neurally inspired algorithms in the attempt to reproduce the remarkable functions of the visual cortex, including algorithms for edge detection, contour extraction, feature learning, and viewpoint-invariant object recognition.
Dr. Mel has been a Principal Investigator on grants from the Office of Naval Research (ONR), the Army Research Office (ARO), the Defense Advanced Research Projects Agency (DARPA), the National Science Foundation (NSF), the National Institutes of Health (NIH), and the Israel–U.S. Binational Foundation, and has served on grant review panels for NSF and NIH. He has been the recipient of a Hewlett-Packard/AEA Fellowship, a McDonald-Pew Foundation Fellowship, and an NSF Career Award. In 2010–2011, he served as Program Chair and General Chair of the Computational and Systems Neuroscience (COSYNE) conference.
Footnotes
This paper reviews the computational capabilities that neurons’ highly intricate and nonlinear dendritic trees endow them with.
Contributor Information
MONIKA P. JADI, Computational Neurobiology Laboratory, Salk Institute for Biological Studies, La Jolla, CA 92037 USA
BARDIA F. BEHABADI, Qualcomm Research, San Diego, CA 92121 USA
ALON POLEG-POLSKY, Synaptic Physiology Section, National Institute of Neurobiological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892 USA.
JACKIE SCHILLER, Department of Physiology, Technion Medical School, Haifa 31096, Israel.
BARTLETT W. MEL, Department of Biomedical Engineering and Neuroscience Graduate Program, University of Southern California, Los Angeles, CA 90089 USA
REFERENCES
- [1].Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: A new unifying principle. Science. 2009 Aug.325(5941):756–760. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- [2].Ang CW, Carlson GC, Coulter DA. Hippocampal CA1 circuitry dynamically gates direct cortical inputs preferentially at theta frequencies. J. Neurosci. 2005 Oct.25(42):9567–9580. doi: 10.1523/JNEUROSCI.2992-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dvorak-Carbone H, Schuman EM. Patterned activity in stratum lacunosum moleculare inhibits CA1 pyramidal neuron firing. J. Neurophysiol. 1999 Dec.82(6):3213–3222. doi: 10.1152/jn.1999.82.6.3213. [DOI] [PubMed] [Google Scholar]
- [4].Hasselmo ME, Barkai E. Cholinergic modulation of activity-dependent synaptic plasticity in the piriform cortex and associative memory function in a network biophysical simulation. J. Neurosci. 1995 Oct.15(10):6592–6604. doi: 10.1523/JNEUROSCI.15-10-06592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nature Neurosci. 2005 Dec.8(12):1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- [6].Larkum ME, Zhu JJ, Sakmann B. Dendritic mechanisms underlying the coupling of the dendritic with the axonal action potential initiation zone of adult rat layer 5 pyramidal neurons. J. Physiol. 2001 Jun.533:447–466. doi: 10.1111/j.1469-7793.2001.0447a.x. pt. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Larkum ME, Senn W, Lüscher H-R. Top-down dendritic input increases the gain of layer 5 pyramidal neurons. Cereb. Cortex. 2004 Oct.14(10):1059–1070. doi: 10.1093/cercor/bhh065. [DOI] [PubMed] [Google Scholar]
- [8].Oakley JC, Schwindt PC, Crill WE. Dendritic calcium spikes in layer 5 pyramidal neurons amplify and limit transmission of ligand-gated dendritic current to soma. J. Neurophysiol. 2001 Jul.86(1):514–527. doi: 10.1152/jn.2001.86.1.514. [DOI] [PubMed] [Google Scholar]
- [9].Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002 Apr.416(6882):736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- [10].Takahashi H, Magee JC. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron. 2009 Apr.62(1):102–111. doi: 10.1016/j.neuron.2009.03.007. [DOI] [PubMed] [Google Scholar]
- [11].Xu N, Harnett MT, Williams SR, Huber D, O’Connor DH, Svoboda K, Magee JC. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature. 2012 Dec.492(7428):247–251. doi: 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]
- [12].Shepherd GM, Koch C. Dendritic electrotonus and synaptic integration. In: Shepherd GM, editor. The Synaptic Organization of the Brain. Oxford Univ. Press; Oxford, U.K.: 1990. pp. 439–473. [Google Scholar]
- [13].Segev I. Single neuron models: Oversimple, complex and reduced. Trends Neurosci. 1992 Nov.15(11):414–421. doi: 10.1016/0166-2236(92)90003-q. [DOI] [PubMed] [Google Scholar]
- [14].Mel BW. Information processing in dendritic trees. Neural Comput. 1994 Nov.6(6):1031–1085. [Google Scholar]
- [15].Mel BW. Why have dendrites? A computational perspective. In: Stuart G, Spruston N, Häusser M, editors. Dendrites. 2nd Oxford Univ. Press; New York, NY, USA: 2008. pp. 421–440. [Google Scholar]
- [16].Segev I, London M. Untangling dendrites with quantitative models. Science. 2000 Oct.290(5492):744–750. doi: 10.1126/science.290.5492.744. [DOI] [PubMed] [Google Scholar]
- [17].Magee JC. Dendritic integration of excitatory synaptic input. Nature Rev. Neurosci. 2000 Dec.1(3):181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- [18].Häuser M, Mel BW. Dendrites: Bug or feature? Current Opinion Neurobiol. 2003 Jun.13(3):372–383. doi: 10.1016/s0959-4388(03)00075-8. [DOI] [PubMed] [Google Scholar]
- [19].London M, Häusser M. Dendritic computation. Annu. Rev. Neurosci. 2005;28:503–532. doi: 10.1146/annurev.neuro.28.061604.135703. [DOI] [PubMed] [Google Scholar]
- [20].Spruston N, Stuart GJ, Häusser M. Dendritic integration. In: Stuart G, Spruston N, Häusser M, editors. Dendrites. 2nd Oxford Univ. Press; New York, NY, USA: 2008. pp. 351–400. [Google Scholar]
- [21].Silver RA. Neuronal arithmetic. Nature Rev. Neurosci. 2010 Jun.11(7):474–489. doi: 10.1038/nrn2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Branco T, Häusser M. The single dendritic branch as a fundamental functional unit in the nervous system. Current Opinion Neurobiol. 2010 Aug.20(4):494–502. doi: 10.1016/j.conb.2010.07.009. [DOI] [PubMed] [Google Scholar]
- [23].Major G, Larkum ME, Schiller J. Active properties of neocortical pyramidal neuron dendrites. Annu. Rev. Neurosci. 2013 Jul.36:1–24. doi: 10.1146/annurev-neuro-062111-150343. [DOI] [PubMed] [Google Scholar]
- [24].Cash S, Yuste R. Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron. 1999 Feb.22(2):383–394. doi: 10.1016/s0896-6273(00)81098-3. [DOI] [PubMed] [Google Scholar]
- [25].Yuste R, Urban R. Dendritic spines and linear networks. J. Physiol. 2004 Nov.98(4–6):479–486. doi: 10.1016/j.jphysparis.2005.09.014. [DOI] [PubMed] [Google Scholar]
- [26].Albus JS. A theory of cerebellar function. Math. Biosci. 1971 Feb.10(1–2):25–61. [Google Scholar]
- [27].Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995 Nov.7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- [28].Crick F. Function of the thalamic reticular complex: The searchlight hypothesis. Proc. Nat. Acad. Sci. USA. 1984 Jul.81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fukushima K, Miyake S, Ito T. Neocognitron: A neural network model for a mechanism of visual pattern recognition. IEEE Trans. Syst. Man Cybern. 1983 Sep-Oct;SMC-13(5):826–834. [Google Scholar]
- [30].Grossberg S, Mingolla E, Ross WD. Visual brain and visual perception: How does the cortex do perceptual grouping? Trends Neurosci. 1997 Mar.20(3):106–111. doi: 10.1016/s0166-2236(96)01002-8. [DOI] [PubMed] [Google Scholar]
- [31].Hasselmo ME. Neuronal rebound spiking, resonance frequency and theta cycle skipping may contribute to grid cell firing in medial entorhinal cortex. Philos. Trans. Roy. Soc. Lond. B, Biol. Sci. 2014;369(1635) doi: 10.1098/rstb.2012.0523. DOI: 10.1098/rstb.2012.0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hebb DO. The Organization of Behavior: A Neuropsychological Approach. Wiley; New York, NY, USA: 1949. [Google Scholar]
- [33].Hopfield JJ. Neural networks and physical systems with emergent collective computational abilities. Proc. Nat. Acad. Sci. USA. 1982 Apr.79(8):2554–2558. doi: 10.1073/pnas.79.8.2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J. Physiol. 1962 Jan.160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Itti L, Koch C. Computational modelling of visual attention. Nature Rev. Neurosci. 2001 Mar.2(3):194–203. doi: 10.1038/35058500. [DOI] [PubMed] [Google Scholar]
- [36].Marder E, Eisen JS. Electrically coupled pacemaker neurons respond differently to same physiological inputs and neurotransmitters. J. Neurophysiol. 1984 Jun.51(6):1362–1374. doi: 10.1152/jn.1984.51.6.1362. [DOI] [PubMed] [Google Scholar]
- [37].Marr D. A theory of cerebellar cortex. J. Physiol. 1969 Jun.202(2):437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10(10):408–415. [Google Scholar]
- [39].Miller KD, Keller JB, Stryker MP. Ocular dominance column development: Analysis and simulation. Science. 1989 Aug.245(4918):605–615. doi: 10.1126/science.2762813. [DOI] [PubMed] [Google Scholar]
- [40].Olshausen BA, Field DJ. How close are we to understanding v1? Neural Comput. 2005 Aug.17(8):1665–1699. doi: 10.1162/0899766054026639. [DOI] [PubMed] [Google Scholar]
- [41].Pouget A, Beck JM, Ma WJ, Latham PE. Probabilistic brains: Knowns and unknowns. Nature Neurosci. 2013 Sep.16(9):1170–1178. doi: 10.1038/nn.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Raphael G, Tsianos GA, Loeb GE. Spinal-like regulator facilitates control of a two-degree-of-freedom wrist. J. Neurosci. 2010 Jul.30(28):9431–9444. doi: 10.1523/JNEUROSCI.5537-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Raymond JL, Lisberger SG, Mauk MD. The cerebellum: A neuronal learning machine? Science. 1996 May;272(5265):1126–1131. doi: 10.1126/science.272.5265.1126. [DOI] [PubMed] [Google Scholar]
- [44].Riesenhuber M, Poggio T. Hierarchical models of object recognition in cortex. Nature Neurosci. 1999 Nov.2(11):1019–1025. doi: 10.1038/14819. [DOI] [PubMed] [Google Scholar]
- [45].Stringer SM, Rolls ET. Learning transform invariant object recognition in the visual system with multiple stimuli present during training. Neural Netw. 2008 Sep.21(7):888–903. doi: 10.1016/j.neunet.2007.11.004. [DOI] [PubMed] [Google Scholar]
- [46].Wei Z, Wang X-J, Wang D-H. From distributed resources to limited slots in multiple-item working memory: A spiking network model with normalization. J. Neurosci. 2012 Aug.32(33):11228–11240. doi: 10.1523/JNEUROSCI.0735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Willshaw DJ, Buneman OP, Longuet-Higgins HC. Non-holographic associative memory. Nature. 1969 Jun.222(5197):960–962. doi: 10.1038/222960a0. [DOI] [PubMed] [Google Scholar]
- [48].Zipser D, Andersen RA. A back-propagation programmed network that simulates response properties of a subset of posterior parietal neurons. Nature. 1988 Feb.331(6158):679–684. doi: 10.1038/331679a0. [DOI] [PubMed] [Google Scholar]
- [49].Lapicque L. Recherches quantitatives sur l’excitation électrique des nerfs traitée comme une polarization. J. Physiol. Pathol. Gen. 1907;9:620–635. [Google Scholar]
- [50].McCulloch W, Pitts W. A logical calculus of the ideas immanent in nervous activity. Bull. Math. Biol. 1943 Dec.5(4):115–133. [PubMed] [Google Scholar]
- [51].Rosenblatt F. Principles of Neurodynamics: Perceptrons and the Theory of Brain Mechanisms. Spartan Books; East Lansing, MI, USA: 1962. [Google Scholar]
- [52].Widrow B, Hoff ME. Associative Storage and Retrieval of Digital Information in Networks of Adaptive ‘Neurons’. In: Bernard DEE, Kare DMR, editors. Biological Prototypes and Synthetic Systems. Vol. 1. Springer-Verlag; New York, NY, USA: 1962. p. 160. [Google Scholar]
- [53].Rumelhart DE, McClelland JL. Parallel Distributed Processing: Explorations in the Microstructure of Cognition. Vol. 1. MIT Press; Cambridge, MA, USA: 1986. [DOI] [PubMed] [Google Scholar]
- [54].Izhikevich EM. Simple model of spiking neurons. IEEE Trans. Neural Netw. 2003 Nov.14(6):1569–1572. doi: 10.1109/TNN.2003.820440. [DOI] [PubMed] [Google Scholar]
- [55].Abbott LF. Lapicque’s introduction of the integrate-and-fire model neuron (1907) Brain Res. Bull. 1999 Dec.50(5–6):303–304. doi: 10.1016/s0361-9230(99)00161-6. [DOI] [PubMed] [Google Scholar]
- [56].Crick F. Function of the thalamic reticular complex: The searchlight hypothesis. Proc. Nat. Acad. Sci. USA. 1984 Jul.81(14):4586–4590. doi: 10.1073/pnas.81.14.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Raizada RDS, Grossberg S. Towards a theory of the laminar architecture of cerebral cortex: Computational clues from the visual system. Cereb. Cortex. 2003 Jan.13(1):100–113. doi: 10.1093/cercor/13.1.100. [DOI] [PubMed] [Google Scholar]
- [58].Amitai Y, Friedman A, Connors BW, Gutnick MJ. Regenerative activity in apical dendrites of pyramidal cells in neocortex. Cereb. Cortex. 1993 Feb.3(1):26–38. doi: 10.1093/cercor/3.1.26. [DOI] [PubMed] [Google Scholar]
- [59].Antic SD, Zhou W-L, Moore AR, Short SM, Ikonomu KD. The decade of the dendritic NMDA spike. J. Neurosci. Res. 2010 Nov.88(14):2991–3001. doi: 10.1002/jnr.22444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ariav G, Polsky A, Schiller J. Submillisecond precision of the input-output transformation function mediated by fast sodium dendritic spikes in basal dendrites of CA1 pyramidal neurons. J. Neurosci. 2003;23(21):7750–7758. doi: 10.1523/JNEUROSCI.23-21-07750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Behabadi BF, Polsky A, Jadi M, Schiller J, Mel BW. Location-dependent excitatory synaptic interactions in pyramidal neuron dendrites. PLoS Comput. Biol. 2012;8(7):e1002599. doi: 10.1371/journal.pcbi.1002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Branco T, Häusser M. Synaptic integration gradients in single cortical pyramidal cell dendrites. Neuron. 2011 Mar.69(5):885–892. doi: 10.1016/j.neuron.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Eccles JC, Libet B, Young RR. The behaviour of chromatolysed motoneurones studied by intracellular recording. J. Physiol. 1958 Aug.143(1):11–40. doi: 10.1113/jphysiol.1958.sp006041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Golding NL, Spruston N. Dendritic sodium spikes are variable triggers of axonal action potentials in hippocampal CA1 pyramidal neurons. Neuron. 1998;21(5):1189–1200. doi: 10.1016/s0896-6273(00)80635-2. [DOI] [PubMed] [Google Scholar]
- [65].Jadi M, Polsky A, Schiller J, Mel BW. Location-dependent effects of inhibition on local spiking in pyramidal neuron dendrites. PLoS Comput. Biol. 2012;8(6):e1002550. doi: 10.1371/journal.pcbi.1002550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kandel ER, Spencer WA. Electrophysiological properties of an archicortical neuron. Ann. New York Acad. Sci. 1961 Sep.94:570–603. doi: 10.1111/j.1749-6632.1961.tb35560.x. [DOI] [PubMed] [Google Scholar]
- [67].Katona G, Kaszás A, Turi GF, Hájos N, Tamás G, Vizi ES, Rózsa B. Roller coaster scanning reveals spontaneous triggering of dendritic spikes in CA1 interneurons. Proc. Nat. Acad. Sci. USA. 2011 Feb.108(5):2148–2153. doi: 10.1073/pnas.1009270108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Lavzin M, Rapoport S, Polsky A, Garion L, Schiller J. Nonlinear dendritic processing determines angular tuning of barrel cortex neurons in vivo. Nature. 2012 Oct.490(7420):397–401. doi: 10.1038/nature11451. [DOI] [PubMed] [Google Scholar]
- [69].Llinás R, Sugimori M. Electrophysiological properties of in vivo Purkinje cell dendrites in mammalian cerebellar slices. J. Physiol. 1980 Aug.305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Losonczy A, Magee JC. Integrative properties of radial oblique dendrites in hippocampal CA1 pyramidal neurons. Neuron. 2006;50(2):291–307. doi: 10.1016/j.neuron.2006.03.016. [DOI] [PubMed] [Google Scholar]
- [71].Major G, Polsky A, Denk W, Schiller J, Tank DW. Spatiotemporally graded NMDA spike/plateau potentials in basal dendrites of neocortical pyramidal neurons. J. Neurophysiol. 2008 May;99(5):2584–2601. doi: 10.1152/jn.00011.2008. [DOI] [PubMed] [Google Scholar]
- [72].Milojkovic BA, Zhou W-L, Antic SD. Voltage and calcium transients in basal dendrites of the rat prefrontal cortex. J. Physiol. 2007 Dec.585:447–468. doi: 10.1113/jphysiol.2007.142315. pt. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Palmer LM, Shai AS, Reeve JE, Anderson HL, Paulsen O, Larkum ME. NMDA spikes enhance action potential generation during sensory input. Nature Neurosci. 2014;17:383–390. doi: 10.1038/nn.3646. [DOI] [PubMed] [Google Scholar]
- [74].Polsky A, Mel BW, Schiller J. Computational subunits in thin dendrites of pyramidal cells. Nature Neurosci. 2004;7(6):621–627. doi: 10.1038/nn1253. [DOI] [PubMed] [Google Scholar]
- [75].Poolos NP, Kocsis JD. Dendritic action potentials activated by NMDA receptor-mediated EPSPs in CA1 hippocampal pyramidal cells. Brain Res. 1990 Aug.524(2):342–346. doi: 10.1016/0006-8993(90)90714-m. [DOI] [PubMed] [Google Scholar]
- [76].Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404(6775):285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- [77].Schiller J, Schiller Y, Stuart G, Sakmann B. Calcium action potentials restricted to distal apical dendrites of rat neocortical pyramidal neurons. J. Physiol. 1997;505:605–616. doi: 10.1111/j.1469-7793.1997.605ba.x. pt. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Schwindt PC, Crill WE. Local and propagated dendritic action potentials evoked by glutamate iontophoresis on rat neocortical pyramidal neurons. J. Neurophysiol. 1997 May;77(5):2466–2483. doi: 10.1152/jn.1997.77.5.2466. [DOI] [PubMed] [Google Scholar]
- [79].Smith SL, Smith IT, Branco T, Häusser M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature. 2013 Nov.503(7474):115–120. doi: 10.1038/nature12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994 Jan.367(6458):69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- [81].Wei DS, Mei YA, Bagal A, Kao JP, Thompson SM, Tang CM. Compartmentalized and binary behavior of terminal dendrites in hippocampal pyramidal neurons. Science. 2001 Sep.293(5538):2272–2275. doi: 10.1126/science.1061198. [DOI] [PubMed] [Google Scholar]
- [82].Wong RK, Prince DA, Basbaum AI. Intradendritic recordings from hippocampal neurons. Proc. Nat. Acad. Sci. USA. 1979 Feb.76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Archie KA, Mel BW. A model for intradendritic computation of binocular disparity. Nature Neurosci. 2000 Jan.3(1):54–63. doi: 10.1038/71125. [DOI] [PubMed] [Google Scholar]
- [84].Behabadi BF, Mel BW. Mechanisms underlying subunit independence in pyramidal neuron dendrites. Proc. Nat. Acad. Sci. USA. 2013 Dec.111(1):498–503. doi: 10.1073/pnas.1217645111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Borg-Graham LJ, Grzywacz NM. A model of the directional selectivity circuit in retina: Transformations by neurons singly and in concert. In: McKenna TM, Davis JL, Zornetzer SF, editors. Single Neuron Computation. Academic; New York, NY, USA: 1992. pp. 347–375. [Google Scholar]
- [86].Borst A, Euler T. Seeing things in motion: Models, circuits, mechanisms. Neuron. 2011 Sep.71(6):974–994. doi: 10.1016/j.neuron.2011.08.031. [DOI] [PubMed] [Google Scholar]
- [87].Hao J, Wang X, Dan Y, Poo M, Zhang X. An arithmetic rule for spatial summation of excitatory and inhibitory inputs in pyramidal neurons. Proc. Nat. Acad. Sci. USA. 2009 Dec.106(51):21 906–21 911. doi: 10.1073/pnas.0912022106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Koch C, Poggio T, Torre V. Retinal ganglion cells: A functional interpretation of dendritic morphology. Philos. Trans. Roy. Soc. Lond. B, Biol. Sci. 1982 Jul.298(1090):227–263. doi: 10.1098/rstb.1982.0084. [DOI] [PubMed] [Google Scholar]
- [89].Koch C, Poggio T, Torre V. Nonlinear interactions in a dendritic tree: Localization, timing, role in information processing. Proc. Nat. Acad. Sci. USA. 1983 May;80(9):2799–2802. doi: 10.1073/pnas.80.9.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nature Neurosci. 2004 Apr.7(4):373–379. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- [91].Mel BW, Ruderman DL, Archie KA. Translation-invariant orientation tuning in visual ‘complex’ cells could derive from intradendritic computations. J. Neurosci. 1998;18(11):4325–4334. doi: 10.1523/JNEUROSCI.18-11-04325.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Mel BW. The clusteron: Toward a simple abstraction for a complex neuron. In: Moody J, Hanson S, Lippmann R, editors. Advances in Neural Information Processing Systems. Vol. 4. Morgan Kaufmann; San Mateo, CA: 1992. pp. 35–42. [Google Scholar]
- [93].Mel BW. Synaptic integration in an excitable dendritic tree. J. Neurophysiol. 1993;70(3):1086–1101. doi: 10.1152/jn.1993.70.3.1086. [DOI] [PubMed] [Google Scholar]
- [94].Mel BW. NMDA-based pattern discrimination in a modeled cortical neuron. Neural Comput. 1992;4(4):502–517. [Google Scholar]
- [95].Poirazi P, Brannon T, Mel BW. Arithmetic of subthreshold synaptic summation in a model CA1 pyramidal cell. Neuron. 2003 Mar.37(6):977–987. doi: 10.1016/s0896-6273(03)00148-x. [DOI] [PubMed] [Google Scholar]
- [96].Poirazi P, Brannon T, Mel BW. Pyramidal neuron as two-layer neural network. Neuron. 2003 Mar.37(6):989–999. doi: 10.1016/s0896-6273(03)00149-1. [DOI] [PubMed] [Google Scholar]
- [97].Rall W, Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys. J. 1973 Jul.13(7):648–687. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Rall W, Segev I. Functional possibilities for synapses on dendrites and on dendritic spines. In: Edelman GM, Gall WE, Cowan WM, editors. Synaptic Function. Wiley; New York, NY, USA: 1987. pp. 605–636. [Google Scholar]
- [99].Rhodes P. The properties and implications of NMDA spikes in neocortical pyramidal cells. J. Neurosci. 2006 Jun.26(25):6704–6715. doi: 10.1523/JNEUROSCI.3791-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shepherd GM, Brayton RK. Logic operations are properties of computer-simulated interactions between excitable dendritic spines. Neuroscience. 1987 Apr.21(1):151–165. doi: 10.1016/0306-4522(87)90329-0. [DOI] [PubMed] [Google Scholar]
- [101].Tuckwell HC. Introduction to Theoretical Neurobiology. 1–2. Cambridge University Press; Cambridge, U.K.: 1988. [Google Scholar]
- [102].Vu ET, Krasne FB. Evidence for a computational distinction between proximal and distal neuronal inhibition. Science. 1992 Mar.255(5052):1710–1712. doi: 10.1126/science.1553559. [DOI] [PubMed] [Google Scholar]
- [103].Woolf TB, Shepherd GM, Greer CA. Local information processing in dendritic trees: Subsets of spines in granule cells of the mammalian olfactory bulb. J. Neurosci. 1991 Jun.11(6):1837–1854. doi: 10.1523/JNEUROSCI.11-06-01837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zador AM, Agmon-Snir H, Segev I. The morphoelectrotonic transform: A graphical approach to dendritic function. J. Neurosci. 1995;15(3):1669–1682. doi: 10.1523/JNEUROSCI.15-03-01669.1995. pt. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Barlow HB, Levick WR. The mechanism of directionally selective units in rabbit’s retina. J. Physiol. 1965 Jun.178(3):477–504. doi: 10.1113/jphysiol.1965.sp007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zador AM, Claiborne BJ, Brown TH. Nonlinear pattern separation in single hippocampal neurons with active dendritic membrane. In: Moody J, Hanson S, Lippmann R, editors. Advances in Neural Information Processing Systems 4. Vol. 4. MIT Press; Cambridge, MA, USA: 1991. pp. 51–58. [Google Scholar]
- [107].Mel BW. A connectionist learning model for 3-D mental rotation, zoom, pan; Proc. 8th Annu. Conf. Cogn. Sci. Soc..1986. pp. 562–571. [Google Scholar]
- [108].Mel BW. MURPHY: A robot that learns by doing; Proc. Neural Inf. Process. Syst. Conf..1987. pp. 544–553. [Google Scholar]
- [109].Mel BW. A connectionist model may shed light on neural mechanisms for visually guided reaching. J. Cogn. Neurosci. 1991;3(3):273–292. doi: 10.1162/jocn.1991.3.3.273. [DOI] [PubMed] [Google Scholar]
- [110].Durbin R, Rumelhart DE. Product units: A computationally powerful and biologically plausible extension to backpropagation networks. Neural Comput. 1989 Mar.1(1):133–142. [Google Scholar]
- [111].Makara JK, Magee JC. Variable dendritic integration in hippocampal CA3 pyramidal neurons. Neuron. 2013 Dec.80(6):1438–1450. doi: 10.1016/j.neuron.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Beaulieu C, Colonnier M. A laminar analysis of the number of round-asymmetrical and flatsymmetrical synapses on spines, dendritic trunks, cell bodies in area 17 of the cat. J. Comput. Neurol. 1985 Jan.231(2):180–189. doi: 10.1002/cne.902310206. [DOI] [PubMed] [Google Scholar]
- [113].Megias M, Emri Z, Freund TF, Gulyas AI. Total number and distribution of inhibitory and excitatory synapses on hippocampal CA1 pyramidal cells. Neuroscience. 2001;102(3):527–540. doi: 10.1016/s0306-4522(00)00496-6. [DOI] [PubMed] [Google Scholar]
- [114].Elston GN, DeFelipe J. Spine distribution in cortical pyramidal cells: A common organizational principle across species. Progr. Brain Res. 2002;136:109–133. doi: 10.1016/s0079-6123(02)36012-6. [DOI] [PubMed] [Google Scholar]
- [115].Bishop CM. Neural Networks for Pattern Recognition. Oxford Univ. Press; New York, NY, USA: 1995. [Google Scholar]
- [116].Heeger DJ. Normalization of cell responses in cat striate cortex. Vis. Neurosci. 1992 Aug.9(2):181–197. doi: 10.1017/s0952523800009640. [DOI] [PubMed] [Google Scholar]
- [117].Ohzawa I, DeAngelis GC, Freeman RD. Encoding of binocular disparity by complex cells in the cat’s visual cortex. J. Neurophysiol. 1997 Jun.77(6):2879–2909. doi: 10.1152/jn.1997.77.6.2879. [DOI] [PubMed] [Google Scholar]
- [118].Touryan J, Felsen G, Dan Y. Spatial structure of complex cell receptive fields measured with natural images. Neuron. 2005 Mar.45(5):781–791. doi: 10.1016/j.neuron.2005.01.029. [DOI] [PubMed] [Google Scholar]
- [119].Fukushima K. Neocognitron: A self organizing neural network model for a mechanism of pattern recognition unaffected by shift in position. Biol. Cybern. 1980;36(4):193–202. doi: 10.1007/BF00344251. [DOI] [PubMed] [Google Scholar]
- [120].Oram MW, Perrett DI, Hietanen JK. Directional tuning of motion-sensitive cells in the anterior superior temporal polysensory area of the macaque. Exp. Brain Res. 1993;97(2):274–294. doi: 10.1007/BF00228696. [DOI] [PubMed] [Google Scholar]
- [121].Mel BW. SEEMORE: Combining color, shape, texture histogramming in a neurally inspired approach to visual object recognition. Neural Comput. 1997 May;9(4):777–804. doi: 10.1162/neco.1997.9.4.777. [DOI] [PubMed] [Google Scholar]
- [122].Cadieu C, Kouh M, Pasupathy A, Connor CE, Riesenhuber M, Poggio T. A model of V4 shape selectivity and invariance. J. Neurophysiol. 2007 Sep.98(3):1733–1750. doi: 10.1152/jn.01265.2006. [DOI] [PubMed] [Google Scholar]
- [123].Rust NC, Dicarlo JJ. Selectivity and tolerance (‘invariance’) both increase as visual information propagates from cortical area V4 to IT. J. Neurosci. 2010 Sep.30(39):12978–12995. doi: 10.1523/JNEUROSCI.0179-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Bollmann JH, Engert F. Subcellular topography of visually driven dendritic activity in the vertebrate visual system. Neuron. 2009 Mar.61(6):895–905. doi: 10.1016/j.neuron.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002 Aug.418(6900):845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- [126].Hubel DH. Exploration of the primary visual cortex, 1955–78. Nature. 1982 Oct.299(5883):515–524. doi: 10.1038/299515a0. [DOI] [PubMed] [Google Scholar]
- [127].Iannella NL, Launey T, Tanaka S. Spike timing-dependent plasticity as the origin of the formation of clustered synaptic efficacy engrams. Front. Comput. Neurosci. 2010;4 doi: 10.3389/fncom.2010.00021. DOI: 10.3389/fncom.2010. 00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Iannella N, Tanaka S. Synaptic efficacy cluster formation across the dendrite via STDP. Neurosci. Lett. 2006 Jul.403(1–2):24–29. doi: 10.1016/j.neulet.2006.03.079. [DOI] [PubMed] [Google Scholar]
- [129].Lee D, Lin B-J, Lee AK. Hippocampal place fields emerge upon single-cell manipulation of excitability during behavior. Science. 2012 Aug.337(6096):849–853. doi: 10.1126/science.1221489. [DOI] [PubMed] [Google Scholar]
- [130].Legenstein R, Maass W. Branch-specific plasticity enables self-organization of nonlinear computation in single neurons. J. Neurosci. 2011 Jul.31(30):10787–10802. doi: 10.1523/JNEUROSCI.5684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Majaj NJ, Carandini M, Movshon JA. Motion integration by neurons in macaque MT is local, not global. J. Neurosci. 2007 Jan.27(2):366–370. doi: 10.1523/JNEUROSCI.3183-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Migliore M, Novara G, Tegolo D. Single neuron binding properties and the magical number 7. Hippocampus. 2008;18(11):1122–1130. doi: 10.1002/hipo.20480. [DOI] [PubMed] [Google Scholar]
- [133].Morita K. Possible role of dendritic compartmentalization in the spatial working memory circuit. J. Neurosci. 2008 Jul.28(30):7699–7724. doi: 10.1523/JNEUROSCI.0059-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Sivyer B, Williams SR. Direction selectivity is computed by active dendritic integration in retinal ganglion cells. Nature Neurosci. 2013;16:1848–1856. doi: 10.1038/nn.3565. [DOI] [PubMed] [Google Scholar]
- [135].Hawkins J, Ahmad S, Dubinsky D. Hierarchical Temporal Memory Including HTM Cortical Learning Algorithms. Numenta Inc.; 2011. [Google Scholar]
- [136].Poirazi P, Mel BW. Impact of active dendrites and structural plasticity on the memory capacity of neural tissue. Neuron. 2001 Mar.29(3):779–796. doi: 10.1016/s0896-6273(01)00252-5. [DOI] [PubMed] [Google Scholar]
- [137].Goldman MS, Levine JH, Major G, Tank DW, Seung HS. Robust persistent neural activity in a model integrator with multiple hysteretic dendrites per neuron. Cereb. Cortex. 2003 Nov.13(11):1185–1195. doi: 10.1093/cercor/bhg095. [DOI] [PubMed] [Google Scholar]
- [138].Wu XE, Mel BW. Capacity-enhancing synaptic learning rules in a medial temporal lobe online learning model. Neuron. 2009 Apr.62(1):31–41. doi: 10.1016/j.neuron.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Adelson EH, Bergen JR. Spatiotemporal energy models for the perception of motion. J. Opt. Soc. Amer. A. 1985 Feb.2(2):284–299. doi: 10.1364/josaa.2.000284. [DOI] [PubMed] [Google Scholar]
- [140].Katz Y, Menon V, Nicholson DA, Geinisman Y, Kath WL, Spruston N. Synapse distribution suggests a two-stage model of dendritic integration in CA1 pyramidal neurons. Neuron. 2009 Jul.63(2):171–177. doi: 10.1016/j.neuron.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Gulledge AT, Carnevale NT, Stuart GJ. Electrical advantages of dendritic spines. PloS One. 2012;7(4):e36007. doi: 10.1371/journal.pone.0036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Roo M. De, Klauser P, Muller D. LTP promotes a selective long-term stabilization and clustering of dendritic spines. PLoS Biol. 2008 Sep.6(9):e219. doi: 10.1371/journal.pbio.0060219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012 Mar.483(7387):92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Gordon U, Polsky A, Schiller J. Plasticity compartments in basal dendrites of neocortical pyramidal neurons. J. Neurosci. 2006;26(49):12717–12726. doi: 10.1523/JNEUROSCI.3502-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Govindarajan A, Israely I, Huang S-Y, Tonegawa S. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011 Jan.69(1):132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Harvey CD, Svoboda K. Locally dynamic synaptic learning rules in pyramidal neuron dendrites. Nature. 2007;450(7173):1195–1200. doi: 10.1038/nature06416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Kleindienst T, Winnubst J, Roth-Alpermann C, Bonhoeffer T, Lohmann C. Activity-dependent clustering of functional synaptic inputs on developing hippocampal dendrites. Neuron. 2011 Dec.72(6):1012–1024. doi: 10.1016/j.neuron.2011.10.015. [DOI] [PubMed] [Google Scholar]
- [148].Larkum ME, Nevian T. Synaptic clustering by dendritic signalling mechanisms. Current Opinion Neurobiol. 2008 Jun.18(3):321–331. doi: 10.1016/j.conb.2008.08.013. [DOI] [PubMed] [Google Scholar]
- [149].Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008 Mar.452(7186):436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- [150].Makara JK, Losonczy A, Wen Q, Magee JC. Experience-dependent compartmentalized dendritic plasticity in rat hippocampal CA1 pyramidal neurons. Nature Neurosci. 2009 Dec.12(12):1485–1487. doi: 10.1038/nn.2428. [DOI] [PubMed] [Google Scholar]
- [151].Makino H, Malinow R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron. 2011 Dec.72(6):1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Sajikumar S, Frey JU. Resetting of ‘synaptic tags’ is time- and activity-dependent in rat hippocampal CA1 in vivo. Neuroscience. 2004;129(2):503–507. doi: 10.1016/j.neuroscience.2004.08.014. [DOI] [PubMed] [Google Scholar]
- [153].Takahashi N, Kitamura K, Matsuo N, Mayford M, Kano M, Matsuki N, Ikegaya Y. Locally synchronized synaptic inputs. Science. 2012 Jan.335(6066):353–356. doi: 10.1126/science.1210362. [DOI] [PubMed] [Google Scholar]
- [154].McBride TJ, Rodriguez-Contreras A, Trinh A, Bailey R, Debello WM. Learning drives differential clustering of axodendritic contacts in the barn owl auditory system. J. Neurosci. Off. J. Soc. Neurosci. 2008 Jul.28(27):6960–6973. doi: 10.1523/JNEUROSCI.1352-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Branco T, Clark BA, Häusser M. Dendritic discrimination of temporal input sequences in cortical neurons. Science. 2010 Sep.329(5999):1671–1675. doi: 10.1126/science.1189664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Gidon A, Segev I. Principles governing the operation of synaptic inhibition in dendrites. Neuron. 2012 Jul.75(2):330–341. doi: 10.1016/j.neuron.2012.05.015. [DOI] [PubMed] [Google Scholar]
- [157].Koch C. Biophysics of Computation: Information Processing in Single Neurons. Oxford Univ. Press; Oxford, U.K.: 1998. [Google Scholar]
- [158].Shepherd GM. The Synaptic Organization of the Brain. Oxford Univ. Press; Oxford, U.K.: 2004. [Google Scholar]
- [159].Milojkovic BA, Radojicic MS, Antic SD. A strict correlation between dendritic and somatic plateau depolarizations in the rat prefrontal cortex pyramidal neurons. J. Neurosci. 2005 Apr.25(15):3940–3951. doi: 10.1523/JNEUROSCI.5314-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Polsky A, Mel BW, Schiller J. Encoding and decoding bursts by NMDA spikes in basal dendrites of layer 5 pyramidal neurons. J. Neurosci. 2009 Sep.29(38):11891–11903. doi: 10.1523/JNEUROSCI.5250-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Harnett MT, Makara JK, Spruston N, Kath WL, Magee JC. Synaptic amplification by dendritic spines enhances input cooperativity. Nature. 2012 Nov.491(7425):599–602. doi: 10.1038/nature11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Carnevale NT, Hines ML. The NEURON Book. Cambridge Univ. Press; Cambridge, U.K.: 2006. [Google Scholar]
- [163].Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: The logic of connections between molecularly distinct interneurons. Nature Neurosci. 2013;16(8):1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Jin L, Behabadi BF, Mel BW. Dimensionality of dendritic computation; Proc. Neurosci. Meeting Planner; New Orleans, LA, USA. 2012. [Google Scholar]
- [165].Hasler J, Marr B. Finding a roadmap to achieve large neuromorphic hardware systems. Front. Neurosci. 2013;7 doi: 10.3389/fnins.2013.00118. DOI: 10.3389/fnins.2013.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Pouille F, Watkinson O, Scanziani M, Trevelyan AJ. The contribution of synaptic location to inhibitory gain control in pyramidal cells. Physiol. Rep. 2013 Oct.1(5):e00067. doi: 10.1002/phy2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Rall W. Theoretical significance of dendritic trees for neuronal input-output relations. In: Reiss RF, editor. Neural Theory and Modeling. Stanford Univ. Press; Stanford, CA, USA: 1964. pp. 73–97. [Google Scholar]
- [168].Softky W. Sub-millisecond coincidence detection in active dendritic trees. Neuroscience. 1994 Jan.58(1):13–41. doi: 10.1016/0306-4522(94)90154-6. [DOI] [PubMed] [Google Scholar]
- [169].Magee JC. Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J. Neurosci. 1998 Oct.18(19):7613–7624. doi: 10.1523/JNEUROSCI.18-19-07613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Wei Y. A novel neuronal mechanism for temporal order discrimination. Univ. Southern California; Los Angeles, CA, USA: 2011. Doctor of Philosophy thesis, Neuroscience. [Google Scholar]
- [171].Abbott LF, Varela JA, Sen K, Nelson SB. Synaptic depression and cortical gain control. Science. 1997 Jan.275(5297):220–224. doi: 10.1126/science.275.5297.221. [DOI] [PubMed] [Google Scholar]
- [172].Volman V, Levine H, Ben-Jacob E, Sejnowski TJ. Locally balanced dendritic integration by short-term synaptic plasticity and active dendritic conductances. J. Neurophysiol. 2009 Dec.102(6):3234–3250. doi: 10.1152/jn.00260.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Narayanan R, Johnston D. Functional maps within a single neuron. J. Neurophysiol. 2012 Nov.108(9):2343–2351. doi: 10.1152/jn.00530.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Zhou C, Mel BW. Cue combination and color edge detection in natural scenes. J. Vis. 2008;8(4):4–25. doi: 10.1167/8.4.4. [DOI] [PubMed] [Google Scholar]
- [175].Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999 Mar.398(6725):338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- [176].Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 2013 Jul.499(7458):295–300. doi: 10.1038/nature12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Jia H, Rochefort NL, Chen X, Konnerth A. Dendritic organization of sensory input to cortical neurons in vivo. Nature. 2010 Apr.464(7293):1307–1312. doi: 10.1038/nature08947. [DOI] [PubMed] [Google Scholar]