Abstract
Complement dependent cytotoxicity (CDC) significantly contributes to Rituximab (RTX) and Ofatumumab (OFA) efficacies in the treatment of B-cell non-Hodgkin’s lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Human CD59 (hCD59) is a key complement regulatory protein that restricts the formation of the membrane attack complex and thereby inhibits CDC. hCD59 is an important determinant of the sensitivity of NHL and CLL to RTX and OFA treatment. Recently, we developed a specific and potent hCD59 inhibitor, His-tagged ILYd4, which consists of 30 amino acid sequences extending from the N-terminus of ILYd4. Our previously published results indicate that His-tagged ILYd4 can be used as a lead candidate to further develop a potential therapeutic adjuvant for RTX and OFA treatment of RTX-resistant NHL and CLL. However, these studies were conducted using ILYd4 tagged on the N-terminus with 30 additional amino acids (AA) containing 6 X His used for immobilized metal affinity chromatograph. As a further step towards the development of ILYd4-based therapeutics, we investigated the impact of the removal of this extraneous sequence on the anti-hCD59 activity. In this paper, we report the generation and characterization of tag-free ILYd4. We demonstrate that tag-free ILYd4 has over three-fold higher anti-hCD59 activities than the His-tagged ILYd4. The enhanced RTX-mediated CDC effect on B-cell malignant cells comes from tag-free ILYd4’s improved functionality and physical properties including better solubility, reduced tendency to aggregation, and greater thermal stability. Therefore, tag-free ILYd4 is a better candidate for the further development for the clinical application.
Keywords: Rituximab, complement, CD59, intermedilysin, his-tag
INTRODUCTION
Complement dependent cytotoxicity (CDC) significantly contributes to the Rituximab (RTX) and Ofatumumab (OFA) efficacies in cancer therapy [1]. The complement system is the principal part of the innate immune system and plays an important role in host defense [2, 3]. The complement system consists of over 30 soluble and membrane-bound proteins, and is activated by three distinct pathways: the classical, mannose-binding lectin (MBL) and alternative pathways [2, 4]. All three activation pathways converge at the level of C3 to form C5 convertase. C5 convertase then cleaves C5 to form C5a and C5b. The terminal complement activation pathway is initially induced by C5b, followed by the sequential association of C6 to form C5b6, and then C7, C8, and C9. Polymerization of C9 bound to the C5b-8 complex forms a membrane attack complex (MAC), an end-product of complement cascade activation. The MAC creates a lytic pore in the lipid bilayer cell membrane and destroys membrane integrity. This allows the free passage of solutes and water in and out of the cell, which eventually destroys the cell, a process also called as CDC [2-4].
To prevent the potentially harmful effect of complement activation on autologous cells, numerous complement regulatory proteins such as CD46, CD55, and CD59 restrict complement activation at different stages of the various pathways [2, 5]. This complement regulatory system takes advantage of the inherent instability of activation pathway enzymes and reduces the production of activated complement. Among these complement regulators, CD59 is the most important inhibitor for restricting MAC formation [2, 6]. CD59, a glycosylphosphatidylinositol (GPI)-anchored membrane protein, restricts MAC formation by preventing C9 polymerization through binding to both C8 and C9 [7]. Not only does CD59 protect normal cells from deleterious bystander complement attack, but it also confers unwanted protection of cancer cells by limiting the complement activation mediated by a therapeutic antibody (Ab) such as RTX on non-Hodgkin’s lymphoma (NHL) and chronic lymphocytic leukemia (CLL). Numerous findings indicate that CD59 is the most effective complement regulator in protecting these B cell malignancies from RTX-mediated CDC [8, 9]. Dalle et al. have recently found that CD59, but neither CD46 nor CD55, is over-expressed in an in vivo model of RTX-resistant follicular lymphoma-derived tumor cells [10]. In addition, Bannerji et al. reported a significant increase in human CD59 (hCD59) expression in patients who failed to clear CLL cells from peripheral blood after initiation of RTX treatment [11]. Moreover, the sensitivity to CDC effects mediated by OFA on RTX-resistant B-cell malignant cell lines and CLL cells were negatively correlated with the level of CD59 on the cell surface [1]. Thus, up-regulation of hCD59 in NHL and CLL is an important determinant of the sensitivity of these cancer cells to RTX treatment [8, 10, 12]. For these reasons, the development of a molecule capable of abrogating hCD59 function and sensitizing cancer cells to the CDC effect of RTX and OFA is likely to fulfill an urgent unmet clinical need [2, 13]. However, there are problems with the current methods of treatment. The targeted toxicity elicited from anti-hCD59 specific Abs [8, 12, 14], and the poor inhibitory efficacy of C8- or C9-derived peptides limit their therapeutic applications [15].
Recently, we developed a specific and potent hCD59 inhibitor His-tagged ILYd4 [16], and demonstrated that it enhances CDC in vitro and in vivo, thereby sensitizing RTX resistant lymphoma cells and primary CLL to RTX [16-18]. Furthermore, His-tagged ILYd4 also enhances CDC effects mediated by OFA on malignant B-cells, RTX-resistant cell lines, and primary CLL cells [1]. By defining PK/PD profiles of His-tagged ILYd4 in mice, we showed that by itself His-tagged ILYd4 does not adversely mediate in vivo hemolysis of hCD59-expressing erythrocytes [17]. Moreover, His-tagged ILYd4 alone does not trigger lysis or ADCC effect in cells ex vivo and in vivo [1, 16-18]. Our previous results demonstrated that the sensitivity to CDC effects mediated by OFA or RTX on RTX-resistant malignant B-cell lines and CLL cells negatively correlated with the level of CD59 on the cell surface [1]. These results rationalize the use of ILYd4 as a potential therapeutic adjuvant for RTX and OFA treatment of RTX-resistant NHL and CLL [1, 17].
Although we have conducted extensive in vitro and in vivo proof of concept studies and developed suits of assays for further ILYd4 optimization, there are still some questions to be addressed before ILYd4 becomes the therapeutic drug for clinical application. For example, it remains to be seen whether potential side effects other than hemolysis emerge upon reaching the maximum tolerated dose (MTD) in mice. To this end, we need to improve the solubility of His-tagged ILYd4, which does not exceed 1mg/ml in PBS buffer. Our His-tagged ILYd4 construct has a 6xHis sequence linked to the N-terminus of the ILYd4 through a 24 AA sequence that includes an Xpress™ epitope and enterokinase cleavage recognition sequence [1, 16, 17]. It is conceivable that these additional AAs used for the purification of ILYd4 may affect the activities of the native ILYd4 through changing the physical properties and functionality of ILYd4. Indeed, an affinity tag such as His has been reported to negatively affect the biological activities of the target proteins, resulting in diminished or altered biological activity [19-21]. Therefore, our next step towards the development of ILYd4-based therapeutics is to determine how this extraneous 30 AAs sequence influences the ILYd4 activity. Here, we report the generation and characterization of tag-free ILYd4 and demonstrate that tag-free ILYd4 has over three-fold higher anti-hCD59 activities than His-tagged ILYd4 to enhance RTX-mediated CDC effect on malignant B-cells through improving ILYd4’s functionality and physical properties including solubility, monomeric character, and metabolic stability.
METHODS AND MATERIALS
1) Original and RTX Resistant B-cell Malignancy Cell Lines, and Cell Culture
The human B-cell lymphoma cell lines ARH-77 and RL were purchased from and authenticated by the ATCC (Manassas, VA), and passaged less than 50 times. RTX-resistant cell lines RamosR51.2 were generated according to previously published method [14, 17]. Those resistant cell lines that survived complement attack induced by RTX at concentrations of 51.2 μg/ml in the presence of 10% (v/v) normal human serum or NHS (Valley Biomedical, Winchester, VA) as a source of complement, were named as RamosR51.2. To further ensure the drug resistance of RamosR51.2 cells, we further treated them for 4 times with the RTX (72.6 μg/ml) and complement (10% NHS) before performing the following CDC experiment.
2) Preparation of His-tagged and Tag-free ILYd4
The His-tagged ILYd4 were purified using the immobilized metal affinity chromatography (IMAC) (Novagen, San Diego, CA) according to our previously published procedure [16, 22]. Removal of endotoxin from the recombinant protein ILY and ILYd4 was accomplished by Detoxi-Gel column (Thermo Scientific, Waltham, MA) to achieve endotoxin levels that were lower than 0.1 EU/ml as determined by Recombinant Factor C Endotoxin Detection System (Lonza, Walkersville, MD). His-tagged ILYd4 was dialyzed against PBS buffer containing 10% glycerol, 9 mM MES, pH 6.4, and stored at −80°C for further use.
Tag-free ILYd4 was prepared through the His-tagged form by removal of the extraneous sequence with Enterokinase (Sydlabs, BP000034-GD8) and re-chromatography on IMAC column (Fig. 1A). Briefly, the His-tagged ILYd4 was diluted with 20mM Tris-HCl, 50mM NaCl pH 7.5 to keep a concentration of approx 0.2 mg/mL. The enterokinase was added to the solution to achieve His-tagged ILYd4 to enzyme ratio of 1: 100 (w/w). The digestion mixture was incubated for overnight at room temperature. After the digestion, the 30AA His-tag containing fragment was removed by IMAC and the tag-free ILYd4 was collected by further dialysis against PBS buffer containing 10% glycerol, 9 mM MES, pH 6.4, analyzed for integrity and the purity, and stored at −80°C for further use.
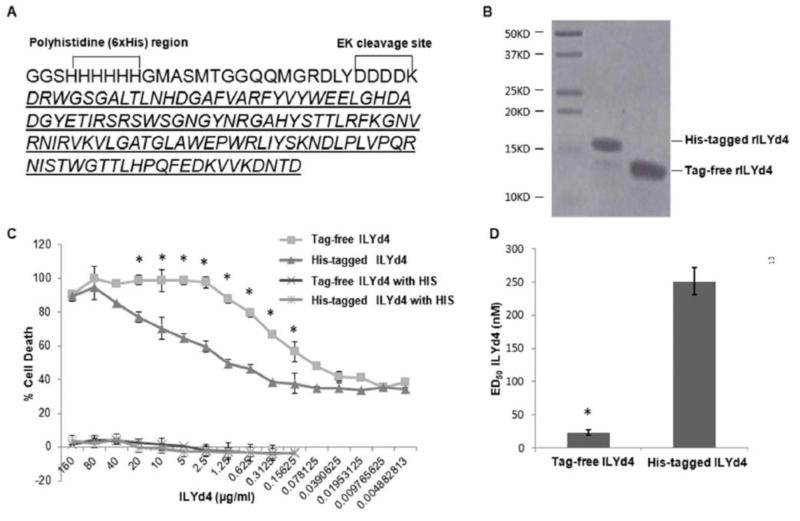
Fig. (1). Purification of tag-free ILYd4 and characterization of its functionality.
(A) AA sequence of His-tagged ILYd4. Italicized letters indicate the Tag-free ILYd4 sequence. EK: enterokinase. (B) The purity of both His-tagged ILYd4 (6μg) and tag-free ILYd4 (8μg) analyzed by 15% SDS-PAGE gel. (C) Complement-mediated assays on human erythrocytes. The human erythrocytes were sensitized with 1% (w/v) anti-human erythrocyte Ab with 5% (v/v) NHS in the presence of the serial dilutions of His-tagged ILYd4 and tag-free ILYd4. Five % (v/v) heat-inactivated human serum (HIS) was used as a negative control. (D) The mean ED50 of tag-free ILYd4 for human erythrocytes was ten-fold lower than that of His-tagged ILYd4. Results are mean ± SD of three different experiments. *: P < 0.01 v.s. His-tagged ILYd4.
The purity of both His-tagged ILYd4 and tag-free ILYd4 was analyzed in reversed phase-HPLC (RP-HPLC, Agilent HP1100 HPLC system) with a C8 column (Agilent ZORBAX 300SB-C8, 2.1*100mm, 3.5μm). Mobile phase A was 0.1%TFA in milli-Q water, and mobile Phase B was 0.085%TFA in acetonitrile. Samples were eluted with a linear gradient of 30–100% (v/v) mobile Phase B, at a flow rate of 0.25 ml/min at 25°C for at least 15 minutes.
3) Complement-mediated Assays on Human Erythrocytes
Sensitivity of 1% (v/v) human erythrocytes to human complement-mediated lysis in the presence of serial dilutions of ILYd4 was assessed by the anti-human erythrocyte Ab-sensitized erythrocyte method, as described by Hu et al [6, 22]. We used NHS as a source of complement to test the activity of His-tagged and tag-free ILYd4. In both cases, and heat-inactivated human serum (HIS) was used as a negative control. Cells were incubated at 37 °C for half an hour. The amount of hemoglobin released from lysed erythrocytes was determined by the absorbance of the supernatant at 414 nm, and the percent lysis was calculated as follows: {(test OD414 - blank OD414) / (total lysis OD414 - blank OD414)} ×100. The total lysis sample was obtained by adding pure water to the erythrocyte pellet.
4) CDC Assays
Cell viability was determined by propidium iodide (PI) staining as described [8, 17]. Briefly, 105 cells were treated with RTX together with serial dilutions of ILYd4 in the presence of NHS as a source of complement for 2 hours at 37°C. Since ARH-77 and RL cell lines were more resistant to RTX-mediated CDC effects [18, 23, 24] than RamosR51.2 cell lines [24], we used in the CDC assay 20 μg/ml or 10 μg/ml of RTX for treating ARH-77, and RL, or RamosR51.2 cell lines, respectively with 20% (v/v) of NHS as a source of complement. After washing with 1% (w/v) BSA/PBS, the cells (in 100 μl) were incubated with 10 μl PI (50 μg/ml) at room temperature for 5 min and immediately analyzed on the FACScan. The PI negative population was regarded as live cells. Percentage of cell death was calculated using the following formula: (%) = 100 × {1 − (live cells in treated sample / live cells in untreated control)}. ED50, defined as the effective dose requires for achieving 50% cell death, was calculated based on the percentage of CDC-mediated cell deaths in the presence of the serial dilutions of ILYd4. As such, ED50 presents the efficacy of each protein to different incubation conditions.
5) Characterization of the pH Dependent Solubility of His-tagged and Tag-free ILYd4
His-tagged ILYd4 and tag-free protein were dialyzed against three buffers: (1) 20 mM MES, 150 mM, pH 5.0; (2) PBS, pH 7.4; and (3) 10 mM Tris, 150 mM NaCl, pH 9.0. After dialysis, OD600 of solutions was taken to measure the turbidity. The protein solutions were centrifuged at 10000 rpm for 10min. Measurement of the supernatant at OD280 provided the protein concentration. The pellet was resuspended in buffer (20mM PB, pH 6.0). Both the quantities of the proteins in the supernatant or pellet were also analyzed by SDS-PAGE analysis.
6) Characterization of the Aggregation of His-tagged and Tag-free ILYd4
Ten % of the blue Native-PAGE gel (Invitrogen, Native PAGE Novex Bis–Tris Gel system) was used to characterize the aggregation status of the protein.
7) Thermal Stability Studies
We used complement-mediated haemolytic assay as described above to compare the thermostability of the tag-free with His-tagged ILYd4 following storage of 0.6 mg/ml solution in PBS buffer (pH: 7.4) at 25°C or 40°C over different time periods (1, 3, 7 and 14 days). Considering that complement-mediated haemolysis of human erythrocytes also depends on the degree of sensitization of human erythrocyte with anti-human erythrocyte Abs and the level of complement activity in NHS used in different experiments, we used in each experiment the tag-free ILYd4 or His-tagged ILYd4 that were stored at −80°C as an internal control to measure the complement-mediated hemolysis of Ab-sensitized human erythrocytes. 10% (v/v) NHS was used as the source of complement for the experiments. Also, we used 10% blue native gel and 15% SDS-PAGE and RP-HPLC with a C8 column to characterize the physical property and aggregation status of both proteins at each time point.
8) Statistical Analysis
The statistical significance of the differences between the group means was determined using one-way ANOVA to compare variance. Results were expressed as the mean ± SD of data obtained from three separate experiments. A P value <0.05 was considered significant.
RESULTS
1) Tag-free ILYd4 has ten-fold higher CDC effect on Ab-sensitized Human Erythrocytes than His-tagged ILYd4
To test the functionality of tag-free ILYd4, we cleaved the His-tag from the His-tagged ILYd4 (Fig. 1A). To determine the purity of purified tag-free ILYd4 and His-tagged ILYd4, we used RP-HLPC and SDS-PAGE to quantitatively analyze the proteins. We obtained over 99% purity of tag-free and His-tagged ILYd4 respectively (Fig. 1B and Supplemental Fig. 1). To compare the functionality of tag-free with His-tagged ILYd4, we used the complement assay with human erythrocytes from a healthy individual. The human erythrocytes were sensitized with anti-human erythrocyte Abs and lysed by the NHS from the same health individual, which was used as a source of complement. Titration with ascending concentration of either tag-free ILYd4, or His-tagged ILYd4 revealed that the former rendered anti-human Ab sensitized erythrocytes significantly more sensitive to complement-mediated hemolysis than the His-tagged ILYd4 (Fig. 1C). The nature of complement-mediated hemolytic effect was confirmed by demonstrating that the HIS did not mediate any hemolysis (Fig. 1C). It is important to note that ILYd4 itself did not mediate any hemolysis if the complement is inactivated. This result is consistent with our previous findings that ILYd4 itself is not toxic to erythrocytes [1, 16-18]. The ED50 of tag-free ILYd4 was approximately ten-fold lower than that of His-tagged ILYd4 (Fig. 1D). It has been previously reported that domain 4 of ILY binds to hCD59 through amino acids 42-58 that participate in binding of hCD59 to C8 and C9 [25, 26]. We and others further demonstrated that ILYd4 is a potent and specific anti-hCD59 inhibitor [1, 16, 17, 27]. Taken together, these results indicate that tag-free ILYd4 has ten-fold higher anti-hCD59 activities than His-tagged ILYd4.
2) Tag-free ILYd4 has Three- to Four-fold Higher RTX-mediated CDC effect on B-cell malignancy Cells than His-tagged ILYd4
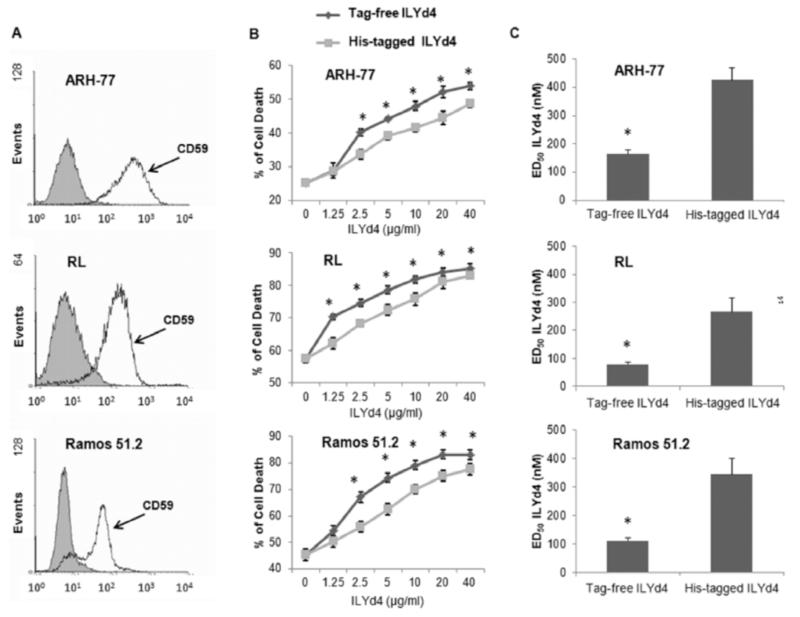
We further investigated whether tag-free like His-tagged ILYd4 enhances RTX-mediated CDC effect on B-cell malignancies such as primary lymphoma cells RL and ARH-77 as well as RTX-resistant Ramos cell line RamosR51.2 [1, 16, 17]. The selection of these cell lines is based on the previous finding that they are very resistant to RTX-mediated CDC due to the high expression of hCD59 on their surface (Fig. 2A) [1]. We found that tag-free ILYd4 also significantly enhanced RTX-mediated CDC on RL, ARH-77 and Ramos51.2 (Fig. 2B). The ED50 of tag-free ILYd4 for RL, ARH-77 and Ramos51.2 were three to four-fold lower than that of His-tagged ILYd4 (Fig. 2C). These results suggest that tag-free ILYd4 enhances several-fold higher RTX-mediated CDC activity to kill B-cell malignancies through the inhibition of hCD59 function than His-tagged ILYd4. This lower enhancement effect of tag-free ILYd4 on B-cell malignancy cells as compared to the enhancement of their lytic activity on human anti-erythrocyte Ab sensitized erythrocytes can be attributed to the fact that the nucleated cells but not erythrocytes possess several protective mechanisms against the cytolytic effect of the MAC, including anti-apoptotic genes, and endocytosis/shedding of MAC [2, 28]. Taken together, these results indicate that as compared to His-tagged ILYd4, the tag-free ILYd4 may be a better clinical candidate for enhancing RTX activity in the treatment of RTX-resistant B-cell malignancies.
Fig. (2). Tag-free ILYd4 has higher RTX-mediated CDC effect on B-cell malignancy cells than His-tagged ILYd4.
(A) The expression of CD59 on the cell surface of ARH-77 RL, and RTX-resistant cell lines RamosR51.2. Grey and white curves represent the staining with IgG plus FITC conjugated secondary Ab and with anti-hCD59 Ab plus FITC conjugated secondary Ab, respectively. (B) CDC effect on B-cell malignancy cells in the presence of the serial dilutions of the tag-free and His-tagged ILYd4. (C) ED50s of tag-free and His-tagged ILYd4 for ARH-77 RL, and Ramos51.2. Result are mean ± SD of three independent experiments. *P<0.01 v.s. His-tagged ILYd4.
3) Tag-free ILYd4 is More Soluble than His-tagged ILYd4 Under a Wide Range of pH Conditions
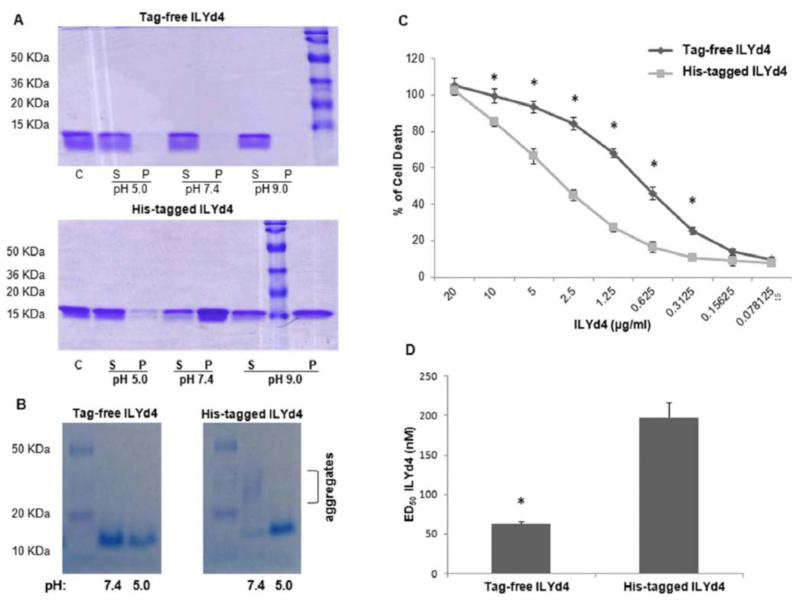
Previously, we noticed that at 1 mg/ml His-tagged ILYd4 in PBS buffer is insoluble and starts to precipitate. Conversely, under the identical condition, tag-free ILYd4 is completely soluble. These findings prompted us to characterize the physical properties of both proteins. To this end, both proteins were dialyzed against the different pH buffers (pH: 5, 7.4 and 9.0) followed by OD600 reading, a routine method for the measuring turbidity in solution, which is indicative of precipitation. Under all pH conditions there was only little precipitation for tag-free ILYd4. In contrast, His-tagged ILYd4 was soluble only at pH 5.0 (Data not shown). Moreover, centrifugation of the dialyzed solutions generated supernatant and pellet fractions that were subjected to SDS-PAGE analysis. Almost complete dissolution of tag-free ILYd4 was observed in all three pH buffers with negligible presence in the pellet (P) (Fig. 3A). In contrast, His-tagged ILYd4 did not dissolve well in all three pH buffers displaying a slightly better solubility in pH 5.0 than in pH 7.4 and 9.0 buffers as evident from the highly intense presence in the P fraction (Fig. 3A). These results indicate that tag-free ILYd4 has better solubility than His-tagged ILYd4. In addition, we run a native gel to investigate the aggregation state of both proteins. Evidently, aggregation was observed for His-tagged in pH 7.4 buffer but was not present in any of the three buffers for tag-free ILYd4 (Fig. 3B). Taken together, these findings suggest that tag-free ILYd4 does not aggregate and is more soluble than the His-tagged ILYd4. These observations may explain the higher anti-hCD59 activity in pH 7.4, the physiological pH, of tag-free ILYd4 than the less soluble and more aggregating His-tagged ILYd4.
Fig. (3). Tag-free ILYd4 has better physical properties and anti-hCD59 activity than His-tagged ILYd4.
(A) Solubility of His-tagged ILYd4 and tag-free ILYd4 in three different pH buffers (pH 5.0, 7.4, and 9.0). We used 15% SDS-PAGE to analyze the fractions - solution (S) and pellet (P) (total 5μg) obtained after centrifugation of the protein dialyzed against three different pH conditions. (B) Native gel (10%) analysis of the aggregation state of the tag-free and His-tagged ILYd4 (5μg) dialyzed against pH 5.0 and 7.4 buffer. (C) Anti-hCD59 activity of the-tag free and His-tagged ILYd4 dialyzed against pH 5.0 buffer by complement-mediated assays on human erythrocytes with 10% (v/v) NHS as a source of complement. (D) ED50 of tag-free ILYd4 for human erythrocytes was three-fold lower than that of His-tagged ILYd4 in pH 5.0 buffer. Results are mean ± SD of three independent experiments. *: P < 0.01 v.s. His-tagged ILYd4.
4) N-terminus Tag of ILYd4 Affects its Functionality
Because an affinity tag such as His may negatively affect the target protein resulting in losing the activity [19], we evaluated the impact of the 30 AAs tag on the complement-mediated hemolysis by carrying out titrations with both His-tagged and tag-free ILYd4 in pH 5.0 buffer, in which both proteins display good solubility (Fig. 3B, right lanes). We observed that tag-free ILYd4 rendered Ab sensitized human erythrocytes significantly more sensitive to complement-mediated hemolysis than His-tagged one (Fig. 3C). The ED50 of the tag-free ILYd4 was approximately three-fold lower than that His-tagged ILYd4 (Fig. 3D). These results indicate that under conditions that support good solubility of both the non-tagged and tagged forms of ILYd4, the tag-free form is more potent than the His-tagged ILYd4.
5) Tag-free ILYd4 has Better Thermostability than His-tagged ILYd4
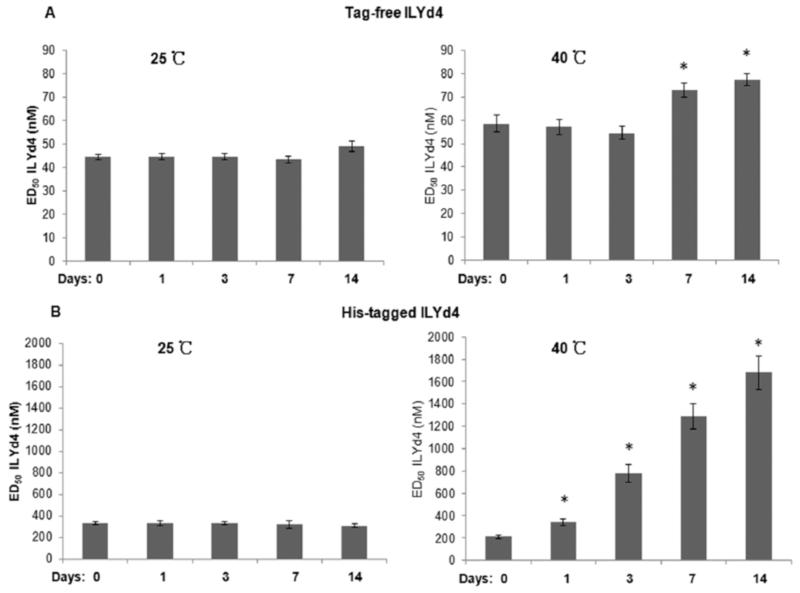
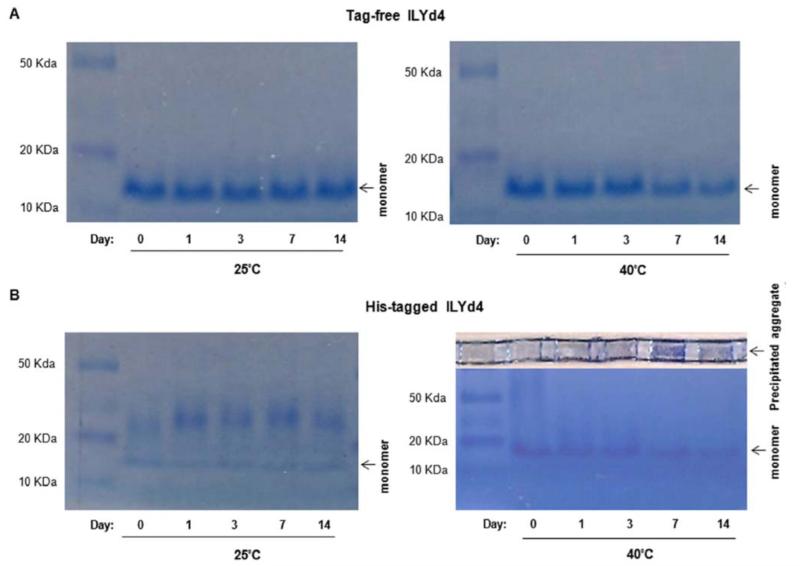
Achieving satisfactory stability is very critical in protein therapeutic development [29]. In general, it is very critical for formulated protein drug products to have long shelf life (usually over 2 years), through which they retain their original structural integrity and full biological activity. Therefore, characterization of the thermostability of a drug candidate is a critical step for the design of the drug formulation strategies that will secure the delivery of a stable drug to the patients [29]. As such, we studied the thermostability of tag-free and His-tagged ILYd4 by monitoring their activities in complement-mediated haemolytic assay following storage in PBS buffer at 25°C or 40°C over different time periods (1, 3, 7 and 14 days). We observed that tag-free ILYd4 stored in PBS buffer at 25°C for periods of up to 7 days maintained its original anti-hCD59 activity. There is a slight loss of activity following storage for 14 days at 25°C (Fig. 4A, and Supplement Fig. 2). After 7 days at 40°C, the tag-free ILYd4 showed a small decline in anti-hCD59 activity (Fig. 4A, and Supplement Fig. 3). In contrast, storage of His-tagged ILYd4 for 14 days at 25°C did not affect its anti-hCD59 activity (Fig. 4B, and Supplement Fig. 4), while at 40°C it progressively lost its activity starting from the first day (Fig. 4B, and Supplement Fig. 5). Taken together, these results indicate that tag-free ILYd4 is more stable in 40°C than His-tagged ILYd4.
Fig. (4). Tag-free ILYd4 has better thermostability than His-tagged ILYd4.
(A) The activities of tag-free ILYd4 after storing in PBS buffer in either 25°C or 40°C for different time periods. (B) The activities of His-tagged ILYd4 after storing in PBS buffer in either 25°C or 40°C over different time periods. Results are mean ± SD of three independent experiments. *P<0.01 v.s. day 0.
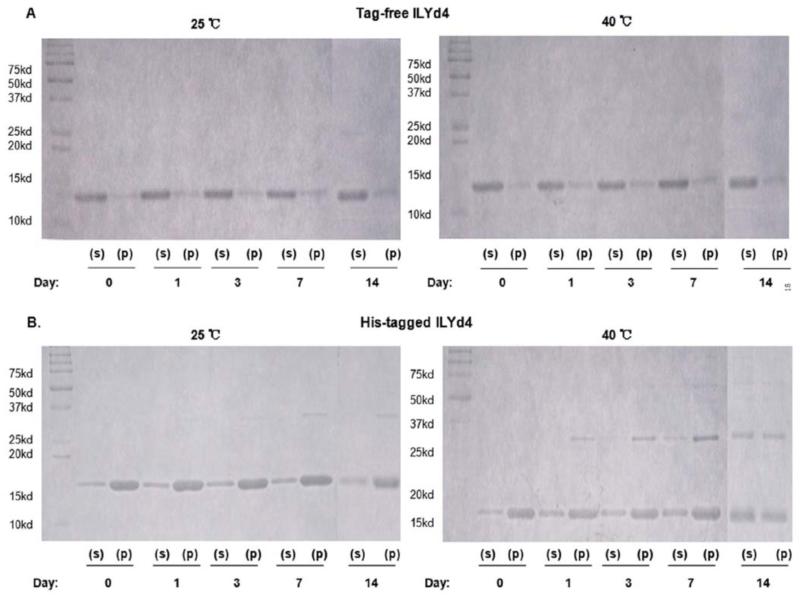
We further explored the underlying mechanism by which the high temperature accelerates the loss of His-tagged ILYd4 activity. Monitoring on native gel revealed the absence of any noticeable aggregation in the tag-free ILYd4 solutions kept for up to 14 days at either 25°C or 40°C (Fig. 5A). In contract, at 25°C, His-tagged ILYd4 forms an aggregate already on day 1 that increases in size over storage time (Fig. 5B). Interestingly, at 25°C there is no change in the amount of monomeric His-tagged ILYd4 throughout the study period. This observation suggest that the fact that there were not changes for anti-hCD59 activity of the His-tagged ILYd4 over storage time at 25°C (Fig. 4B, left panel) originates from the stable steady-state monomeric component present in the solution. At 40°C, the aggregates of the His-tagged ILYd4 precipitate on top of the gel and their amount increases progressively over time (Fig. 5B, right panel). Consistently, we observe time-dependent reduction in the amount of the monomeric His-tagged ILYd4 component in the mixture. This reduction is responsible for the gradual loss in anti-hCD59 activity of the His-tagged ILYd4 over storage time at 40°C (Fig. 4B, right panel). These results were further confirmed by monitoring the stored solutions by C8 RP-HPLC analysis (Supplemental Fig. 6). We observed a time-dependent increase in the area under the curve (AUC) and in the retention time of the peak of His-tagged ILYd4, which was not the case for the tag-free ILYd4. The time-dependent shift in the retention time of the peak of His-tagged ILYd4 is characteristic of its progressive aggregation. These observations are in agreement with the results obtained from the analysis of the stored solutions by the native gel (Fig. 5). Interestingly, SDS-PAGE analysis of the soluble (S) and pellet (P) fractions obtained from the tag-free and the His-tagged ILYd4 solutions stored at 25°C or 40°C for 14 days show a time-dependent increase in the amount of dimer at 25°C and of both the dimer and tetramer initially in the P fraction and later also in S fraction at 40°C of His-tagged ILYd4 but not in the tag-free ILYd4 (Fig. 6). Taken together, time- and temperature-dependent aggregation of the His-tagged ILYd4 explains the extensive loss of its anti-hCD59 activity during storage at 40°C.
Fig. (5). Formation of aggregate occurred in His-tagged but not in tag-free ILYd4 is accelerated by high temperature.
After storing both protein in PBS buffer under 25°C or 40°C over different period of times, we used 10% native gel to determine their aggregation state. (A) The tag-free ILYd4 (5μg) did not form the aggregates under 25°C or 40°C over times. (B). Aggregate formation for the His-tagged ILYd4 (5μg).
Fig. (6). No significant change in the solubility of His-tagged and tag-free ILYd4 under the different temperatures.
After storing the proteins in PBS buffer in 25°C or 40°C at different time periods, we used 15% SDS-PAGE to determine the relative amount of protein in solution (S) and pellet (P) (total 5μg) obtained following centrifugation of the dialyzed proteins. (A). The solubility of tag-free ILYd4 by assessing their relative distribution after storing in PBS buffer in either 25°C or 40°C over different time periods. (B). The solubility of His-tagged ILYd4 by assessing their relative distribution after storing in PBS buffer in either 25°C or 40°C over different time periods.
DISCUSSION
Our previously published results show that His-tagged ILYd4 significantly enhances RTX or OFA-mediated CDC on primary B-lymphoma cells in vitro, RTX-resistant B-lymphoma cells in vitro and in vivo and primary CLL ex vivo through specific inhibition of hCD59’ anti-MAC activity [1, 16-18]. Here, we report that tag-free ILYd4 enhances a three-fold higher RTX-mediated CDC on primary and RTX-resistant B-cell malignancy cells in vitro than His-tagged ILYd4. This enhancement is attributed primarily to the improvement in the physical properties of the tag-free ILYd4, which include better solubility and greater stability of the monomer, but also to the higher inherent efficacy of the tag-free ILY4. Therefore, tag-free ILYd4 is an improved and “working” candidate for the development of a clinical adjuvant for Ab-based cancer therapy such as RTX or OFA-based B-cell malignancy therapy and provides the much needed means to overcome the devastating RTX-resistant B-cell malignancies, such as patients relapsed from RTX treatment. Additionally, based on our previous results [1, 16-18], we speculate that a combination of the tag-free ILYd4 with RTX or OFA may serve as the first line strategy to treat NHL and CLL patients who express high level of CD59 on their cancer cells. The selectivity of the tag-free ILYd4 toward cancer B-cells will originate from the higher expression of CD59 in these cancer cells especially those that became resistant to Ab-based therapy via over-expression of CD59. We aim to take advantage of the attractive therapeutic window offered by the double assault on B-cells by RTX or OFA targeting the uniquely expressed CD20 and by tag-free ILYd4 targeting malignant B-cells that over-express CD59. Consequently, under this combination therapy malignant B-cells will be specifically subjected to the CDC effect and undergo preferentially lysis. Importantly, ILYd4 by itself does not trigger lysis or ADCC effect in cells that are not targeted by these Abs e.g. erythrocytes (ex vivo and in vivo) [1, 16-18]. The latter express other complement regulators such as CD46 and CD55 that are able to compensate for the transient inhibition of CD59 by tag-free ILYd4 and protect them from CDC. Nevertheless, we plan to further investigate the potential side effect of hemolysis by tag-free ILYd4 in humanized hCD59 mice and clinical studies.
There has been a long standing debate concerning the enhancement of therapeutic Ab-mediated CDC on clinical outcome for the immune cancer therapy [30-37]. Recently, clinical studies with OFA and Lenalidomide on 34 CLL-relapsed patients previously treated with chemoimmunotherapy such as fludarabine, cyclophosphamide and RTX (FCR), directly highlights the role of CDC in the immune therapy. The results reported at the 53rd American Society of Hematology Annual Meeting, December 10-11, 2011 demonstrated that this particular combination therapy induced responses in 65% of patients with relapsed CLL [38]. Another clinical trials reported in the same meeting documented that as compared to traditional methods with RTX-based chemoimmunotherapy, OFA-based chemoimmunotheraoy appeared to have less hematologic toxicity and improved efficacy [39]. Supportively, it has been previously demonstrated in in vitro experiments that OFA induces much stronger CDC than RTX [23, 40], while it mediates ADCC and direct cell death at levels comparable to RTX [23, 40]. Taken together, the clinical trial results strongly suggest that the enhancement of CDC effect with OFA through the complement activation at early stage contribute significantly to the improved outcome in the treatment of B-cell malignancies in vivo. Recently, we reported that His-tagged ILYd4 also enhances CDC effects mediated by OFA on RTX-resistant B-cell malignancy cell lines and primary CLL cells [1, 17, 18]. The sensitivity to CDC effects mediated by OFA with or without ILYd4 negatively correlated with the level of CD59 [1, 17, 18]. Taken together, these results further support the notion that tag-free ILYd4, an agent with higher anti-hCD59 activity, better physical properties and more potent anti-hCD59 activity than His-tagged ILYd4, may be an important addition to the arsenal of anti-cancer therapeutics or an important adjuvant for cancer immune therapy. The latter is derived from its increased CDC activity at the terminal complement pathway, namely MAC formation.
The extension at the N-terminus of ILYd4 by an extraneous sequence that includes a His tag and an entrokinase cleavage site was introduced in our previous studies to facilitate the isolation and purification of ILYd4. However, there is not sufficient data to support the safety/non-immunogenicity of this extension in future clinical application. Furthermore, chelating effect 6 x His sequence in the His-tag of ILYd4 on transition metal could lead to unexpected effects when administered to the patients. For many years, it had been assumed that due to its small size and simple amino-acid composition the presence of His-tag in many recombinant proteins should not affect the function of target protein. However, extensively emerging data raise the concerns about His-tag’s roles in misfolding, aggregation [41], lost activity [19], or decreased solubility of His-tagged target proteins [42]. These results are consistent with the findings reported here that extraneous tag in the N-terminus of His-tagged ILYd4 changes the physical properties of ILYd4 to increase aggregation, reduce solubility, and negatively influence the anti-hCD59 activity of ILYd4. Previous results reported by Hughes et al, indicate that His tag in C-terminus of ILYd4 may not compromise the activity of ILYd4 because His-tagged ILYd4 has a potent anti-hCD59 activity with IC50 in the single digit nM range [27]. Our results suggest that the tag in the N-terminus of His-tagged ILYd4 has a distinctly negative effect on its anti-hCD59 activity, which may be different from that it has when introduced at the C-terminus of ILYd4. This further indicates that the N- but not C-terminal regions of ILYd4 may significantly contribute to the interaction with hCD59, a subject that requires further investigation.
Another key issue during protein therapeutic development is the physical stability of the drug candidate. Previously, we documented that His-tagged ILYd4 dramatically enhanced anti-CD20 Ab therapeutics with a good potency in vitro and in vivo [1, 17, 18]. However, an aqueous solution of His-tagged ILYd4 is very unstable and starts to precipitate, even at low concentration. The thermostability test of His-tagged and tag-free ILYd4 demonstrates that tag-free protein does not form significant aggregates and displays better thermostability that results with an undiminished anti-hCD59 activity even after 14 days at 40°C. While, His-tagged ILYd4 progressively loses its anti-hCD59 activity at 40°C and correlates with increased degree of aggregate formation. Taken together, tag-free ILYd4 displays more favorable physical properties (better thermal stability and lesser tendency to aggregate) under different temperatures and pH conditions than His-tagged ILYd4. Therefore, these results suggest that tag-free ILYd4 presents a promising pre-clinical lead and provides an excellent starting point for future drug optimization.
Nevertheless, the underlying mechanism by which the extraneous His-tag containing N-terminus extension in His-tagged ILYd4 compromises the solubility and thermal stability is not clear. Previously, several groups demonstrate that His-tag alters the binding characteristics or even the structure of recombinant protein [43, 44]. Also, Amor-Mahjoub et al. reported that the 6 x His-tag placed at the N-terminus of the interdomain linker deleted version of HSC70 stabilized its dimeric form, whereas the tag-free protein remains preferentially monomeric [21]. Consistently, analysis of both the His-tagged and tag-free ILYd4 on native gel electrophoresis indicates that His-tag at N-terminus of ILYd4 triggers aggregation. Interestingly, the prevalence of acidic amino acid at the N-terminal region of ILYd4 is pronouncedly high and their proximity to the 6 x His-tag is very notable. One can speculate that this situation may lead to extensive complementary charge interactions and lead to misfolding and oligomerization of His-tagged ILYd4, which could be accelerated by higher temperature (e.g. 40°C) and higher pHs (7.4 and 9.0).
CONCLUSION
We report here that the removal of the extraneous His-tag containing sequence from the His-tagged ILYd4 markedly improves the physical properties and the anti-hCD59 activity of the tag-free ILYd4. Therefore, tag-free ILYd4 is a better preclinical candidate for further development into a potential therapeutic adjuvant for RTX and OFA treatment of RTX-resistant NHL and CLL. The anti-cancer efficacy and potential side effects such as hemolysis require further investigation. Moreover, potential immunogenicity of the tag-free ILYd4 is another critical aspect to be addressed in the course of developing a protein drug. Although, previous findings indicate that rILYd4 has low immunogenicity [45, 46] development of low- or even non-immunogenic form of ILYd4 or ILYd4-derived peptides will be essential for clinical application. Therefore, we plan to delineate the minimal bioactive sequence, the critical amino acids in ILYd4 essential for the interaction with CD59, and integrate this structural information in the design of more potent, non-immunogenic, and less costly forms of ILYd4 (ILYd4-mimetic) in the future.
Supplementary Material
ACKNOWLEDGEMENTS
The completion of this paper is in part supported by NIH RO1 AI061174 (X.B.) and NIH R21CA141324 (X.B.), Harvard Technology Development Accelerator Fund (X.B), and China Scholarship Council (2010623100) (L.W.) and (2011622129) (F. L).
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST
The authors declare that they have no conflict of interests.
REFERENCES
- [1].Ge X, Wu L, Hu W, et al. rILYd4, a human CD59 inhibitor, enhances complement-dependent cytotoxicity of ofatumumab against rituximab-resistant B-cell lymphoma cells and chronic lymphocytic leukemia. Clin Cancer Res. 2011;17(21):6702–11. doi: 10.1158/1078-0432.CCR-11-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist. 2008;13(9):954–66. doi: 10.1634/theoncologist.2008-0089. [DOI] [PubMed] [Google Scholar]
- [3].Yu Q, Yu R, Qin X. The good and evil of complement activation in HIV-1 infection. Cell Mol Immunol. 2010;7(5):334–40. doi: 10.1038/cmi.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mayer MM. Complement. Historical perspectives and some current issues. Complement. 1984;1(1):2–26. [PubMed] [Google Scholar]
- [5].Fisicaro N, Aminian A, Hinchliffe SJ, et al. The pig analogue of CD59 protects transgenic mouse hearts from injury by human complement. Transplantation. 2000;70(6):963–8. doi: 10.1097/00007890-200009270-00014. [DOI] [PubMed] [Google Scholar]
- [6].Qin X, Hu W, Song W, et al. Generation and phenotyping of mCd59a and mCd59b double-knockout mice. Am J Hematol. 2009 Feb;84(2):65–70. doi: 10.1002/ajh.21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sugita Y, Tobe T, Oda E, et al. Molecular cloning and characterization of MACIF, an inhibitor of membrane channel formation of complement. J Biochem. 1989;106(4):555–7. doi: 10.1093/oxfordjournals.jbchem.a122893. [DOI] [PubMed] [Google Scholar]
- [8].Golay J, Lazzari M, Facchinetti V, et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood. 2001;98(12):3383–9. doi: 10.1182/blood.v98.12.3383. [DOI] [PubMed] [Google Scholar]
- [9].Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol. 2003;40(2-4):109–23. doi: 10.1016/s0161-5890(03)00112-3. [DOI] [PubMed] [Google Scholar]
- [10].Dalle S, Dupire S, Brunet-Manquat S, Reslan L, Plesa A, Dumontet C. In vivo model of follicular lymphoma resistant to rituximab. Clin Cancer Res. 2009;15(3):851–7. doi: 10.1158/1078-0432.CCR-08-1685. [DOI] [PubMed] [Google Scholar]
- [11].Bannerji R, Kitada S, Flinn IW, et al. Apoptotic-regulatory and complement-protecting protein expression in chronic lymphocytic leukemia: relationship to in vivo rituximab resistance. J Clin Oncol. 2003;21(8):1466–71. doi: 10.1200/JCO.2003.06.012. [DOI] [PubMed] [Google Scholar]
- [12].Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95(12):3900–8. [PubMed] [Google Scholar]
- [13].Gancz D, Fishelson Z. Cancer resistance to complement-dependent cytotoxicity (CDC): Problem-oriented research and development. Mol Immunol. 2009;46(14):2794–800. doi: 10.1016/j.molimm.2009.05.009. [DOI] [PubMed] [Google Scholar]
- [14].Takei K, Yamazaki T, Sawada U, Ishizuka H, Aizawa S. Analysis of changes in CD20, CD55, and CD59 expression on established rituximab-resistant B-lymphoma cell lines. Leuk Res. 2006;30(5):625–31. doi: 10.1016/j.leukres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- [15].Huang Y, Fedarovich A, Tomlinson S, Davies C. Crystal structure of CD59: implications for molecular recognition of the complement proteins C8 and C9 in the membrane-attack complex. Acta Crystallogr D Biol Crystallogr. 2007;63(Pt 6):714–21. doi: 10.1107/S0907444907015557. [DOI] [PubMed] [Google Scholar]
- [16].Hu W, Yu Q, Hu N, et al. A high-affinity inhibitor of human CD59 enhances complement-mediated virolysis of HIV-1: implications for treatment of HIV-1/AIDS. J Immunol. 2010;184(1):359–68. doi: 10.4049/jimmunol.0902278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hu W, Ge X, You T, et al. Human CD59 inhibitor sensitizes rituximab-resistant lymphoma cells to complement-mediated cytolysis. Cancer Res. 2011;71(6):2298–307. doi: 10.1158/0008-5472.CAN-10-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].You T, Hu W, Ge X, Shen J, Qin X. Application of a novel inhibitor of human CD59 for the enhancement of complement-dependent cytolysis on cancer cells. Cell Mol Immunol. 2011;8(2):157–63. doi: 10.1038/cmi.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fonda I, Kenig M, Gaberc-Porekar V, Pristovaek P, Menart V. Attachment of histidine tags to recombinant tumor necrosis factor-alpha drastically changes its properties. Scientific World Journal. 2002;2:1312–25. doi: 10.1100/tsw.2002.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cadel S, Gouzy-Darmon C, Petres S, et al. Expression and purification of rat recombinant aminopeptidase B secreted from baculovirus-infected insect cells. Protein Expr Purif. 2004;36(1):19–30. doi: 10.1016/j.pep.2004.03.013. [DOI] [PubMed] [Google Scholar]
- [21].Amor-Mahjoub M, Suppini JP, Gomez-Vrielyunck N, Ladjimi M. The effect of the hexahistidine-tag in the oligomerization of HSC70 constructs. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;844(2):328–34. doi: 10.1016/j.jchromb.2006.07.031. [DOI] [PubMed] [Google Scholar]
- [22].Hu W, Ferris SP, Tweten RK, et al. Rapid conditional targeted ablation of cells expressing human CD59 in transgenic mice by intermedilysin. Nat Med. 2008;14(1):98–103. doi: 10.1038/nm1674. [DOI] [PubMed] [Google Scholar]
- [23].Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104(6):1793–800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- [24].Kennedy AD, Solga MD, Schuman TA, et al. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood. 2003;101(3):1071–9. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- [25].Giddings KS, Zhao J, Sims PJ, Tweten RK. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat Struct Mol Biol. 2004;11(12):1173–8. doi: 10.1038/nsmb862. [DOI] [PubMed] [Google Scholar]
- [26].Tweten RK. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect Immun. 2005;73(10):6199–209. doi: 10.1128/IAI.73.10.6199-6209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hughes TR, Ross KS, Cowan GJ, et al. Identification of the high affinity binding site in the Streptococcus intermedius toxin intermedilysin for its membrane receptor, the human complement regulator CD59. Mol Immunol. 2009;46(7):1561–7. doi: 10.1016/j.molimm.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Haskard DO, Boyle JJ, Mason JC. The role of complement in atherosclerosis. Curr Opin Lipidol. 2008;19(5):478–82. doi: 10.1097/MOL.0b013e32830f4a06. [DOI] [PubMed] [Google Scholar]
- [29].Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20(9):1325–36. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- [30].Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103(7):2738–43. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- [31].Cittera E, Leidi M, Buracchi C, et al. The CCL3 family of chemokines and innate immunity cooperate in vivo in the eradication of an established lymphoma xenograft by rituximab. J Immunol. 2007;178(10):6616–23. doi: 10.4049/jimmunol.178.10.6616. [DOI] [PubMed] [Google Scholar]
- [32].Golay J, Cittera E, Di GN, et al. The role of complement in the therapeutic activity of rituximab in a murine B lymphoma model homing in lymph nodes. Haematologica. 2006;91(2):176–83. [PubMed] [Google Scholar]
- [33].Di GN, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171(3):1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- [34].Klepfish A, Gilles L, Ioannis K, Rachmilewitz EA, Schattner A. Enhancing the action of rituximab in chronic lymphocytic leukemia by adding fresh frozen plasma: complement/rituximab interactions & clinical results in refractory CLL. Ann N Y Acad Sci. 2009;1173:865–73. doi: 10.1111/j.1749-6632.2009.04803.x. [DOI] [PubMed] [Google Scholar]
- [35].Xu W, Miao KR, Zhu DX, et al. Enhancing the action of rituximab by adding fresh frozen plasma for the treatment of fludarabine refractory chronic lymphocytic leukemia. Int J Cancer. 2011;128(9):2192–201. doi: 10.1002/ijc.25560. [DOI] [PubMed] [Google Scholar]
- [36].Weng WK, Levy R. Expression of complement inhibitors CD46, CD55, and CD59 on tumor cells does not predict clinical outcome after rituximab treatment in follicular non-Hodgkin lymphoma. Blood. 2001;98(5):1352–7. doi: 10.1182/blood.v98.5.1352. [DOI] [PubMed] [Google Scholar]
- [37].Wang H, Liu Y, Li ZY, Fan X, Hemminki A, Lieber A. A recombinant adenovirus type 35 fiber knob protein sensitizes lymphoma cells to rituximab therapy. Blood. 2010;115(3):592–600. doi: 10.1182/blood-2009-05-222463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ferrajoli A, O’Brien S, Wierda WG, et al. Combination Therapy with Ofatumumab and Lenalidomide in Patients with Relapsed Chronic Lymphocytic Leukemia (CLL): Results of a Phase II Trial. Blood (ASH Annual Meeting Abstracts) 2011;118:1788. [Google Scholar]
- [39].Shanafelt TD, Lanasa MC, Zent CS, et al. Ofatumumab Based Chemoimmunotherapy (CIT) for Patients with Previously Un-treated CLL. Blood (ASH Annual Meeting Abstracts) 2011;118:3898. [Google Scholar]
- [40].Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177(1):362–71. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- [41].Renzi F, Panetta G, Vallone B, et al. Large-scale purification and crystallization of the endoribonuclease XendoU: troubleshooting with His-tagged proteins. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62(Pt 3):298–301. doi: 10.1107/S1744309106006373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Woestenenk EA, Hammarstrom M, van den BS, Hard T, Berglund H. His tag effect on solubility of human proteins produced in Escherichia coli: a comparison between four expression vectors. J Struct Funct Genomics. 2004;5(3):217–29. doi: 10.1023/b:jsfg.0000031965.37625.0e. [DOI] [PubMed] [Google Scholar]
- [43].Gaberc-Porekar V, Menart V, Jevsevar S, Vidensek A, Stalc A. Histidines in affinity tags and surface clusters for immobilized metal-ion affinity chromatography of trimeric tumor necrosis factor alpha. J Chromatogr A. 1999;852(1):117–28. doi: 10.1016/s0021-9673(99)00374-x. [DOI] [PubMed] [Google Scholar]
- [44].Hang Q, Woods L, Feiss M, Catalano CE. Cloning, expression, and biochemical characterization of hexahistidine-tagged terminase proteins. J Biol Chem. 1999;274(22):15305–14. doi: 10.1074/jbc.274.22.15305. [DOI] [PubMed] [Google Scholar]
- [45].Ohkura K, Nagamune H, Kourai H. Structural analysis of human specific cytolysin intermedilysin aiming application to cancer immunotherapy. Anticancer Res. 2004;24(5C):3343–53. [PubMed] [Google Scholar]
- [46].Nagamune H, Ohkura K, Umezu K, Shouji H, Kourai H. A cell membrane modification technique using domain 4 of intermedilysin for immunotherapy against cancer. Anticancer Res. 2004;24(5C):3367–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.