Abstract
The rate of renal disease progression varies widely among patients with autosomal dominant polycystic kidney disease (ADPKD), necessitating optimal patient selection for enrollment into clinical trials. Patients from the Mayo Clinic Translational PKD Center with ADPKD (n=590) with computed tomography/magnetic resonance images and three or more eGFR measurements over ≥6 months were classified radiologically as typical (n=538) or atypical (n=52). Total kidney volume (TKV) was measured using stereology (TKVs) and ellipsoid equation (TKVe). Typical patients were randomly partitioned into development and internal validation sets and subclassified according to height-adjusted TKV (HtTKV) ranges for age (1A–1E, in increasing order). Consortium for Radiologic Imaging Study of PKD (CRISP) participants (n=173) were used for external validation. TKVe correlated strongly with TKVs, without systematic underestimation or overestimation. A longitudinal mixed regression model to predict eGFR decline showed that log2HtTKV and age significantly interacted with time in typical patients, but not in atypical patients. When 1A–1E classifications were used instead of log2HtTKV, eGFR slopes were significantly different among subclasses and, except for 1A, different from those in healthy kidney donors. The equation derived from the development set predicted eGFR in both validation sets. The frequency of ESRD at 10 years increased from subclass 1A (2.4%) to 1E (66.9%) in the Mayo cohort and from 1C (2.2%) to 1E (22.3%) in the younger CRISP cohort. Class and subclass designations were stable. An easily applied classification of ADPKD based on HtTKV and age should optimize patient selection for enrollment into clinical trials and for treatment when one becomes available.
Keywords: ADPKD, kidney volume, polycystic kidney disease, cystic kidney, progression of chronic renal failure, survival
Autosomal dominant polycystic kidney disease (ADPKD) is the most common monogenic kidney disease and the fourth leading cause of ESRD in adults worldwide.1,2 The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) has shown that total kidney volume (TKV) predicts the risk of developing renal insufficiency in patients with ADPKD, thus qualifying it as a prognostic biomarker.3,4 Kidney volume is now used as the primary or secondary endpoint in clinical trials of renin-angiotensin blockade,5 vasopressin V2 receptor antagonists,6,7 somatostatin analogues,8–11 mammalian target of rapamycin inhibitors,12–14 bosutinib (NCT01233869), and KD019 (NCT01559363). Volume measurements for this purpose require high precision and are laborious.15 Furthermore, kidney volume does not always predict change in renal function, as, for example, in patients with few large cysts or in patients with renal atrophy secondary to ischemia or urinary tract obstruction.
With the likely development of effective therapies for ADPKD, readily available, rapid, and reliable tools are needed to select patients who are appropriate for clinical trials or likely to benefit from an effective therapy. Here we describe and validate a practical classification of ADPKD to achieve this goal.
Results
TKV by Ellipsoid from Magnetic Resonance Imaging or Computed Tomography Correlates Strongly with TKV by Stereology
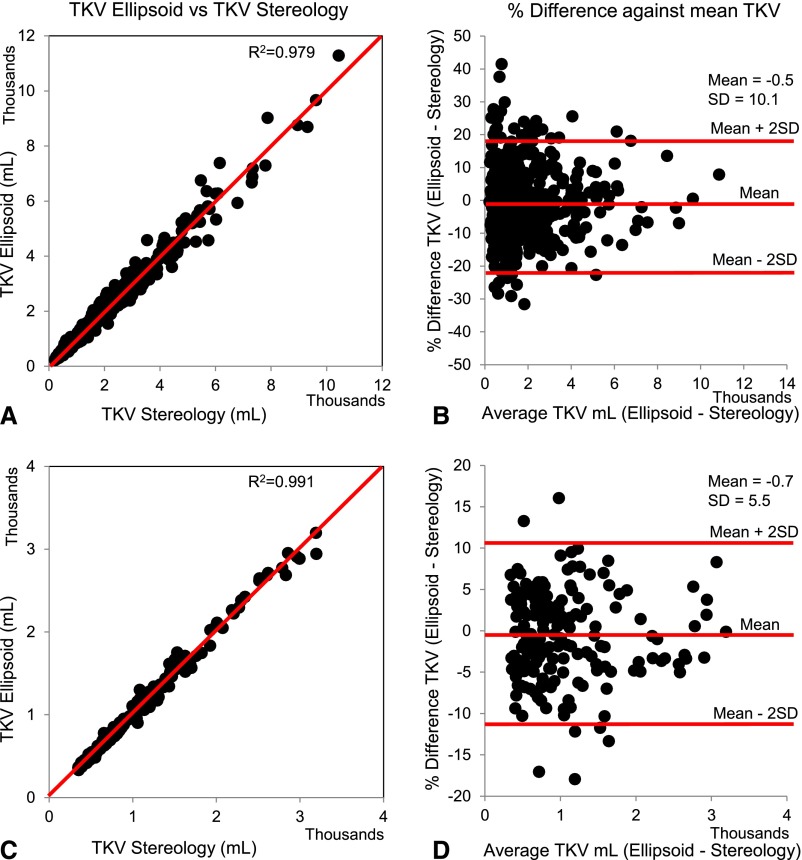
A total of 590 patients with ADPKD from the Mayo Clinic Translational Polycystic Kidney Disease Center (MTPC), age 15–80 years, had an electronic magnetic resonance imaging (MRI) or computed tomography (CT) scan with complete coverage of both kidneys, a serum creatinine level <5 mg/dl within 6 months from the imaging study, and at least two follow-up measurements of serum creatinine before ESRD. One hundred seventy-seven patients from the CRISP study were used for external validation (Figure 1). TKV by stereology (TKVs) and TKV by ellipsoid (TKVe) were measured in the 590 Mayo patients. The prediction of TKVs from TKVe was very high (R2=0.9998), with a predicted residual sum of squares statistic of 13.1 (Supplemental Table 1). In addition, TKVs and TKVe were strongly correlated (R2=0.979) (Figure 2A). TKVe did not systematically under- or overestimate TKVs, with a mean (±SD) percentage difference of −0.5%±10.1% (95% confidence interval [95% CI], −20.7% to 19.6%) (Figure 2B). TKVe was also measured in the 177 CRISP patients. TKVs (previously measured in the CRISP study) and TKVe were strongly correlated (R2=0.991, Figure 2C). TKVe did not systematically under- or overestimate TKVs, with a mean percentage difference of −0.7%±5.5% (95% CI, −11.7% to 10.3%) (Figure 2D). Overall, the prediction of TKVs from TKVe was very high and both TKV measurements were strongly correlated for the MTPC and CRISP patients. In 175 of the 590 MTPC patients (29.7%) and 10 of the 177 (5.6%) CRISP patients, the percentage difference between TKVe and TKVs exceeded 10%. The difference exceeded 20% in only 5.9% of the MTPC and in none of the CRISP patients. The average time needed to measure TKVs was 45 minutes, compared with 7 minutes for TKVe. Because results using TKVs and TKVe were similar, only those using TKVe are presented here.
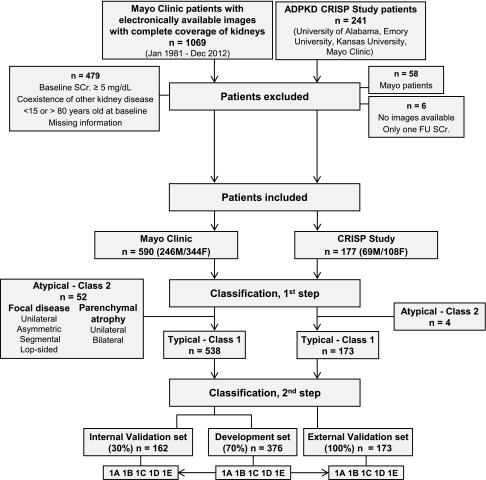
Figure 1.
Flowchart of the study design and classification. We reviewed electronically available abdominal CT or MRI of 1069 patients with ADPKD from the Mayo Clinic, of whom 590 were classified as having typical ADPKD (class 1) or atypical ADPKD (class 2). The 538 class 1 patients were randomly partitioned into a development set (70%) or an internal validation set (30%). Assuming a theoretical starting HtTKV of 150 ml/m and a yearly increase of 1.5%, 3%, 4.5%, or 6%, HtTKV limits were calculated for each age in the development set. Patients were then subclassified as 1A–1E on the basis of the HtTKV for their specific age. The same classification criteria were applied to the internal validation set. In addition, the images from 177 non-Mayo CRISP participants were reviewed and 173 were classified as class 1 to form the external validation set. Atypical patients (class 2) were subclassified as 2A or 2B according to imaging characteristics (Supplemental Figure 1, Table 1). FU, follow-up; SCr, serum creatinine.
Figure 2.
TKV by ellipsoid correlates strongly with TKV by stereology. Comparison of TKVe from CT or MRI versus TKVs in the MTPC (A and B) and CRISP (C and D) cohorts. TKVs and TKVe were strongly correlated (A and C) without systematic under- or overestimation of TKVe (B and D) in both cohorts. In 175 of the 590 MTPC patients (29.7%; B) and in 10 of the 177 CRISP patients (5.6%; D), the percentage difference between TKVe and TKVs exceeded 10%. The difference exceeded 20% in only 5.9% of the MTPC and in none of the CRISP patients.
Image Classification and Baseline Clinical, Laboratory, and Genetic Characteristics
Most patients in the MTPC (91.2%) and CRISP (97.7%) datasets presented imaging characteristics typical for ADPKD and were classified as class 1; the remaining patients were classified as atypical or class 2 (Figure 1) on the basis of prespecified imaging findings (Supplemental Figure 1, Table 1). The main clinical, laboratory, and genetic characteristics at the time of TKV0 for all study participants are shown in Table 2. CRISP class 1 patients were younger (P<0.001), had higher eGFR (P<0.001), and had lower height-adjusted TKV (HtTKV) (P<0.001) compared with MTPC class 1 patients, reflecting the inclusion criteria for enrollment into CRISP and an earlier stage of the disease. A positive family history of ADPKD was present in 79.9% and 80.3% of class 1 MTPC and CRISP patients, respectively. Mutation detection rates and genotypes were also similar.
Table 1.
Classification of ADPKD patients by prespecified imaging findings
| Class, Subclass, and Term | Description |
|---|---|
| 1: Typical ADPKD | Bilateral and diffuse distribution, with mild, moderate, or severe replacement of kidney tissue by cysts, where all cysts contribute similarly to TKV. (Supplemental Figure 1, A and B) |
| 2: Atypical ADPKD | |
| A | |
| Unilateral | Diffuse cystic involvement of one kidney causing marked renal enlargement with a normal contralateral kidney defined by a normal kidney volume (<275 ml in men; <244 ml in women) and having no or only 1–2 cysts (Supplemental Figure 1C) |
| Segmental | Cystic disease involving only one pole of one or both kidneys and sparing the remaining renal tissue (Supplemental Figure 1D) |
| Asymmetric | Diffuse cystic involvement of one kidney causing marked renal enlargement with mild segmental or minimal diffuse involvement of the contralateral kidney defined by a small number of cysts (>2 but <10) and volume accounting for <30% of TKV (Supplemental Figure 1E) |
| Lopsided | Bilateral distribution of renal cysts with mild replacement of kidney tissue with atypical cysts where ≤5 cysts account for ≥50% TKV (the largest cyst diameter is used to estimate individual cyst volume) (Supplemental Figure 1F) |
| B | |
| Bilateral presentation with acquired unilateral atrophy | Diffuse cystic involvement of one kidney causing moderate to severe renal enlargement with contralateral acquired atrophy (Supplemental Figure 1G) |
| Bilateral presentation with bilateral kidney atrophy | Impaired renal function (serum creatinine≥1.5 mg/dl) without significant enlargement of the kidneys, defined by an average length <14.5 cm, and replacement of kidney tissue by cysts with atrophy of the parenchyma (Supplemental Figure 1H) |
Table 2.
Baseline characteristics of study patients
| Cohort/Class | Men/Women (n/n) | Median Age (yr) | Median eGFR (ml/min per 1.73 m2)a | Median HtTKV (ml/m)b | Family History of ADPKD—Yes/No/Unknown (n/n/n) | Genetic Analysis, n (%) | NMD, n (%) | PKD1, n (%) | PKD2, n (%) | Median Follow-Up SCrc (mg/dl) | Median Follow-Up (yr) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MTPC | |||||||||||
| 1 | 538 (218/320) | 44 (35–51) | 75 (45–97) | 668 (430–1234) | 430/71/37 | 308 (57)d | 20 (6)e | 239 (78)e | 49 (16)e | 6 (4–12) | 6 (4–10) |
| 2 | |||||||||||
| A | 36 (21/15) | 60 (50–71) | 67 (57–80) | 591 (476–1020) | 16/14/6 | 11 (31)d | 5 (45)d | 6 (55)e | 0 (0)e | 4 (2–8) | 6 (3–9) |
| B | 16 (7/9) | 62 (50–70) | 33 (21–50) | 351 (248–481) | 9/6/1 | 2 (13)d | 0 (0)d | 2 (100)e | 0 (0)e | 9 (2–14) | 8 (4–11) |
| CRISP | |||||||||||
| 1 | 173 (67/106) | 34 (25–40) | 94 (80–112) | 492 (356–744) | 139/11/23 | 171 (99)d | 14 (8)e | 133 (78)e | 24 (14)e | 5 (4–6) | 9 (8–10) |
| 2 | |||||||||||
| A | 3 (1/2) | 43 (38–46) | 52 (51–55) | 886 (754–1574) | 2/0/1 | 3 (100)d | 0 (0)d | 1 (33.3)e | 2 (66.7)e | 5 | 9 (8–9) |
| B | 1 (1/0) | 16 | 74 | 260 | 1/0/0 | 1 (100)d | 0 | 1 (100)e | 0 | 5 | 10 |
| Total | 767 (315/452) | 42 (15–80) | 78 (9–173) | 608 (141–6167) | 597/102/68 | 496 (65)d | 39 (8)e | 382 (77)e | 75 (15)e | 6 (2–49) | 7 (0.5–18) |
Medians are expressed with interquartile ranges in parentheses. NMD, no mutation detected; SCr, serum creatinine.
Estimated using Chronic Kidney Disease Epidemiology equation.
Calculated by ellipsoid formula.
Does not include baseline SCr.
Percentage is based on total number of patients for each class.
Percentage is based on patients with genetic analysis within each class.
HtTKV and Change in eGFR over Time Were Strongly Associated in Class 1 Patients
A longitudinal mixed-effects regression model for future eGFR as a function of sex, age at HtTKV0, eGFR at HtTKV0, log2HtTKV0, and years from HtTKV0 showed a statistically significant effect of all predictors without interactions, except for sex, in the class 1 patients (Table 3). However, the only significant interactions with time were log2HtTKV0 and age (Table 3). Contrary to class 1 patients, the only significant predictor at baseline in class 2 patients was eGFR at HtTKV0; no significant effect of log2HtTKV0 was detected. When interactions with time were analyzed, eGFR at HtTKV0 remained significant and log2HtTKV0 remained nonsignificant (Table 3).
Table 3.
Longitudinal mixed-effects model for future eGFR using HtTKV as baseline predictor (class 1 and class 2 patients)
| Variable | Class 1 | Class 2 (A and B) | ||
|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| Intercept | 35.87 (20.31 to 51.42) | <0.001 | 22.78 (−13.64 to 59.19) | 0.21 |
| Sex (reference is male) | −0.95 (−2.89 to 0.99) | 0.34 | −2.65 (−8.09 to 2.79) | 0.33 |
| Age at HtTKV0 (yr) | −0.18 (−0.28 to −0.08) | <0.001 | −0.07 (−0.35 to 0.20) | 0.59 |
| eGFR at HtTKV0 (ml/min per 1.73 m2) | 0.90 (0.85 to 0.94) | <0.001 | 0.86 (0.71 to 1.00) | <0.001 |
| Log2(HtTKV0) (ml/m) | −1.89 (−3.00 to −0.79) | <0.001 | −0.90 (−3.99 to 2.19) | 0.56 |
| Years from HtTKV0 | 7.65 (3.39 to 11.92) | <0.001 | −0.81 (−9.30 to 7.68) | 0.85 |
| Sex, years from HtTKV0 (interaction) | −0.35 (−0.85 to 0.16) | 0.18 | 0.21 (−0.99 to 1.41) | 0.73 |
| Age at TKV, years from HtTKV0 (interaction) | 0.03 (0.002 to 0.055) | 0.03 | 0.03 (−0.02 to 0.09) | 0.21 |
| eGFR at HtTKV, years from HtTKV0 (interaction) | −0.004 (−0.017 to 0.009) | 0.55 | 0.05 (0.02 to 0.08) | 0.004 |
| Log2(HtTKV), years from HtTKV0 (interaction) | −1.15 (−1.46 to −0.85) | <0.001 | −0.65 (−1.36 to 0.06) | 0.07 |
Classification by HtTKV0 and Age at HtTKV0 Predicts the Change in eGFR over Time in Class 1 Patients
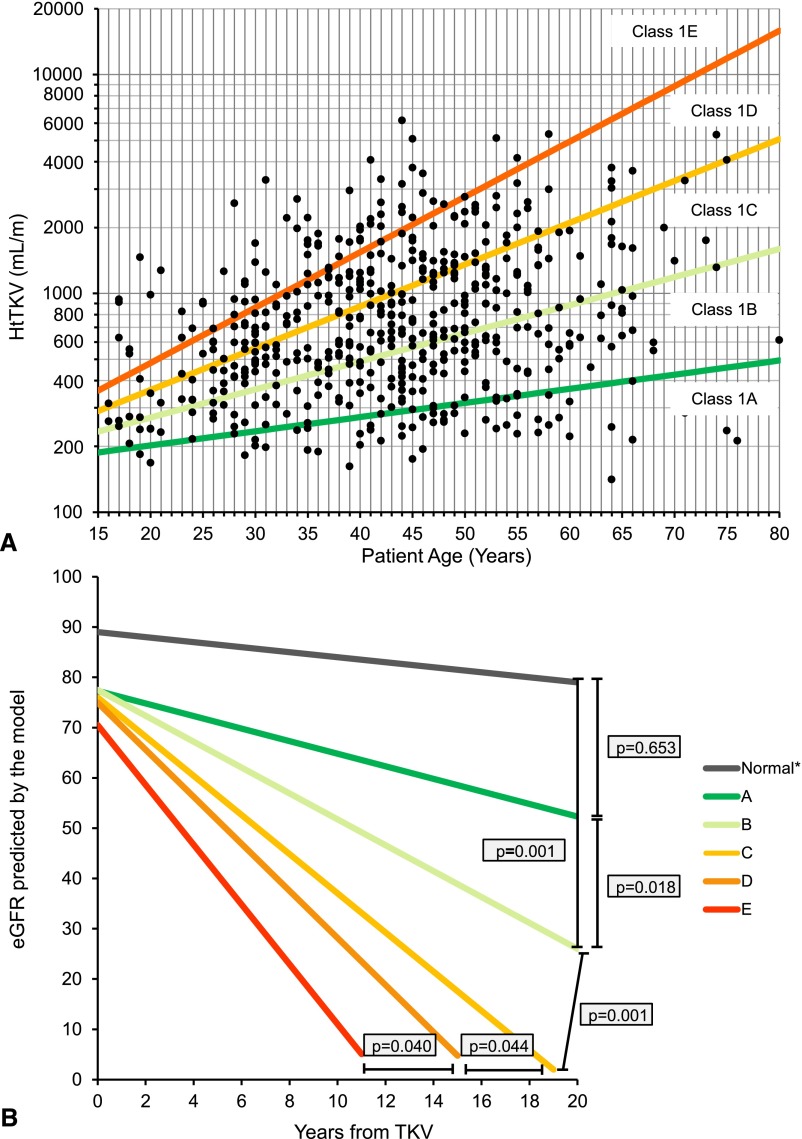
Class 1 patients from MTPC and CRISP were stratified into five subclasses (1A–1E) based on estimated kidney growth rates: yearly percentage increase of <1.5% (subclass 1A), 1.5%–3% (1B), 3%–4.5% (1C), 4.5%–6% (1D), or >6% (1E). (Figure 3A, Supplemental Table 2). The MTPC class 1 patients were randomly partitioned into a development (70% of the patients, n=376) and an internal validation (30% of the patients, n=162) set (Figure 1). All CRISP class 1 patients (100%, n=173) were used as an external validation set. The main clinical, laboratory, and genetic characteristics for the development, internal, and external validation sets are shown in Table 4. In the three sets of patients, the frequency of PKD1 mutations increased, whereas that of PKD2 mutations decreased with increasing disease severity.
Figure 3.
Classification by HtTKV0 and age at HtTKV0 predicts the change in eGFR over time in class 1 patients. (A) Subclassification of patients with class 1 ADPKD at baseline based on HtTKV limits for their age. Limits are defined based on estimated kidney growth rates of 1.5%, 3.0%, 4.5%, and 6.0%. (B) Slopes for men based on the model presented in Table 5. As a reference, the average eGFR at baseline (75 ml/min per 1.73 m2) and the average age at baseline (44 years) for all class 1 patients were used for the model. Estimated slopes (ml/min per 1.73 m2 per year) by subclass (A–E) are −0.23,−1.33, −2.63, −3.48, and −4.78, respectively, for men and 0.03, −1.13, −2.43, −3.29 and −4.58, respectively, for women (not plotted). Values for normal slope (*) were obtained from a population of healthy kidney donors.16
Table 4.
Baseline characteristics of patients with class 1 ADPKD: development and validation sets
| Class | Men/Women (n/n) | Median Age (yr) | Median eGFR (ml/min per 1/.73 m2)a | Median HtTKV (ml/m)b | Family History of ADPKD—Yes/No/Unknown (n/n/n) | Genetic Analysis, n (%) | NMD, n (%) | PKD1, n (%) | PKD2, n (%) | Median Follow-Up SCr (mg/dl)c | Median Follow-Up (yr) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Development set | |||||||||||
| 1A | 40 (7/33) | 50d (44–58) | 84 (64–97) | 249 (214–280) | 23/12/5 | 16 (40) | 5 (31) | 7 (44) | 4 (25) | 6 (3–11) | 5 (3–10) |
| 1B | 88 (23/65) | 46e (36–53) | 78 (62–97) | 433 (322–565) | 67/14/7 | 58 (66) | 6 (10) | 37 (64) | 15 (26) | 8 (3–10) | 6 (3–9) |
| 1C | 122 (46/76) | 44f (36–50) | 71 (47–98) | 701 (514–1037) | 109/8/5 | 73 (60) | 3 (4) | 58 (80) | 12 (16) | 7 (4–14) | 6 (4–10) |
| 1D | 77 (40/37) | 41g (34–49) | 60 (36–96) | 1195 (843–1544) | 62/8/7 | 43 (56) | 0 (0) | 39 (91) | 4 (9) | 5 (3–12) | 6 (4–11) |
| 1E | 49 (28/21) | 36 (29–43) | 46 (26–94) | 1874 (1118–2609) | 37/9/3 | 20 (41) | 0 (0) | 20 (100) | 0 (0) | 6 (4–11) | 5 (3–8) |
| Subtotal | 376 (144/232) | 44 (35–51) | 71 (44–97) | 651 (431–1195) | 298/51/27 | 210 (56)h | 14 (7)i | 161 (77)i | 35 (16)i | 6 (4–11) | 6 (4–10) |
| Internal validation set | |||||||||||
| 1A | 16 (6/10) | 43d (34–54) | 97 (77–114) | 226 (193–247) | 10/3/3 | 10 (63) | 2 (20) | 5 (50) | 3 (27) | 5 (3–10) | 5 (4–11) |
| 1B | 37 (10/27) | 47e (40–55) | 77 (49–100) | 453 (342–527) | 32/4/1 | 23 (62) | 1 (4.3) | 18 (78.3) | 4 (17.4) | 7 (4–11) | 5 (4–9) |
| 1C | 48 (20/28) | 45f (35–55) | 69 (31–94) | 778 (536–1278) | 41/5/2 | 24 (50) | 2 (8) | 18 (75) | 4 (17) | 8 (5–16) | 7 (4–11) |
| 1D | 41 (25/16) | 42g (32–49) | 67 (30–91) | 1212 (822–1836) | 32/6/3 | 25 (61) | 1 (4) | 22 (88) | 2 (8) | 6 (3–13) | 5 (4–9) |
| 1E | 20 (13/7) | 34 (27–40) | 82 (60–91) | 1406 (920–2214) | 17/2/1 | 16 (80) | 0 (0) | 15 (94) | 1 (6) | 7 (5–14) | 8 (5–11) |
| Subtotal | 162 (74/88) | 43 (33–51) | 77 (46–98) | 725 (416–1306) | 132/20/10 | 98 (60)h | 6 (6)i | 78 (80)i | 14 (14)i | 7 (4–13) | 6 (4–11) |
| External validation set | |||||||||||
| 1A | 10 (2/8) | 40d (34–42) | 105 (92–106) | 231 (220–248) | 8/1/1 | 9 (90) | 2 (22) | 5 (56) | 2 (22) | 6 (5–6) | 9 (7–9) |
| 1B | 41 (17/24) | 36e (31–42) | 98 (83–112) | 354 (274–397) | 33/3/5 | 41 (100) | 5 (12) | 20 (49) | 16 (39) | 5 (4–6) | 9 (7–10) |
| 1C | 55 (21/34) | 35f (27–42) | 93 (77–114) | 537 (403–633) | 44/3/8 | 54 (98) | 4 (7.4) | 45 (83.3) | 5 (9.3) | 5 (4–6) | 9 (8–9) |
| 1D | 39 (15/24) | 32g (22–40) | 90 (77–111) | 759 (467–1016) | 34/1/4 | 39 (100) | 2 (5) | 37 (95) | 0 (0) | 5 (3–6) | 9 (8–9) |
| 1E | 28 (12/16) | 25 (18–31) | 93 (79–123) | 906 (643–1334) | 20/3/5 | 28 (100) | 1 (3.5) | 26 (94) | 1 (3.5) | 6 (5–6) | 9 (8–10) |
| Subtotal | 173 (67/106) | 34 (25–40) | 94 (80–112) | 492 (356–744) | 139/11/23 | 171 (99)h | 14 (8)i | 133 (78)i | 24 (14)i | 5 (4–6) | 9 (8–10) |
Median values are expressed with interquartile ranges in parentheses. NMD, no mutation detected; SCr, Serum creatinine.
Estimated using Chronic Kidney Disease Epidemiology equation. Class 1 patients classified based on percentage increase rate based on HtTKV ellipsoid.
Calculated by ellipsoid formula.
Does not include baseline SCr.
Subclass 1A versus 1B, P=0.01 (development set); P=NS (internal validation set); P=NS (external validation set).
Subclass 1B versus 1C, P=NS (development set); P=NS (internal validation set); P=NS (external validation set).
Subclass 1C versus 1D, P=NS (development set); P=NS (internal validation set); P=NS (external validation set).
Subclass 1D versus 1E, P=0.001 (development set); P=0.04 (internal validation set); P=0.01 (external validation set).
Percentage is based on total number of patients for each class.
Percentage is based on patients with genetic analysis within each class.
The previous model, applied to patients in the development set and using this classification (1A–1E) instead of HtTKV0 to model eGFR decline, showed that the only noninteraction terms with a statistically significant effect were age and baseline eGFR. However, the only significant interactions with time were the ones that considered patient’s subclass (Table 5). Estimated eGFR slopes derived from the model were significantly different among subclasses and were all significantly different from a control population of healthy kidney donors,16 except 1A (Figure 3B).
Table 5.
Longitudinal mixed-effects model for future eGFR using classification as baseline predictor (class 1 patients)
| Variable | Estimate (95% CI) | P Value |
|---|---|---|
| Intercept | 21.18 (9.75 to 32.61) | 0.003 |
| Sex (reference is male) | −1.26 (−3.65 to 1.12) | 0.30 |
| Age at HtTKV0 (yr) | −0.26 (−0.40 to −0.11) | <0.001 |
| eGFR at HtTKV0 (ml/min per 1.73 m2) | 0.90 (0.85 to 0.95) | <0.001 |
| Subclass 1Ba | 0.58 (−3.60 to 4.76) | 0.79 |
| Subclass 1Ca | −1.14 (−5.37 to 3.08) | 0.6 |
| Subclass 1Da | −1.93 (−6.63 to 2.76) | 0.42 |
| Subclass 1Ea | −6.26 (−11.91 to −0.62) | 0.03 |
| Years from HtTKV0 | −0.23 (−3.42 to 2.96) | 0.9 |
| Sex, years from HtTKV0 (interaction) | 0.19 (−0.42 to 0.81) | 0.54 |
| Age at HtTKV, years from HtTKV0 (interaction) | −0.02 (−0.06 to 0.02) | 0.32 |
| eGFR at HtTKV, years from HtTKV0 (interaction) | 0.00 (−0.02 to 0.01) | 0.9 |
| Subclass 1B,a years from HtTKV0 (interaction) | −1.33 (−2.44 to −0.23) | 0.02 |
| Subclass 1C,a years from HtTKV0 (interaction) | −2.63 (−3.74 to −1.51) | <0.001 |
| Subclass 1D,a years from HtTKV0 (interaction) | −3.48 (−4.73 to −2.23) | <0.001 |
| Subclass 1E,a years from HtTKV0 (interaction) | −4.78 (−6.35 to −3.20) | <0.001 |
Reference is subclass 1A. Patients were subclassified based on HtTKV ellipsoid.
eGFR was predicted in both validation sets separately using the equation derived from the model applied to the development set (Figure 4, A–D), and the performance of the model was evaluated by the expected predicted error (EPE). The EPE was 13.5 and 17.1 ml/min per 1.73m2 in the internal and external validation sets, respectively, and was lower for patients with an observed eGFR<60 ml/min per 1.73 m2 compared with those with an observed eGFR≥60 ml/min per 1.73 m2 (10.1 and 14.9 versus 16.2 and 17.7) (Supplemental Table 3).
Figure 4.
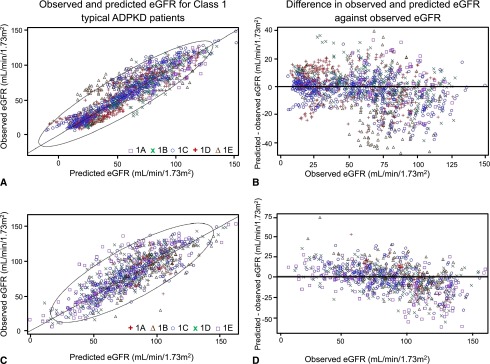
Equation derived from the development set predicted eGFR in both validation sets. Prediction of eGFR in patients with class 1 ADPKD: internal (A and B) and external (C and D) validation sets. (A and C) Scatterplot of the observed eGFR (estimated by Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation) versus the predicted eGFR derived from the model obtained from the development set. Ellipse represents the 95% CI. (B and D) Differences between predicted eGFR by the model and eGFR values by the CKD-EPI equation plotted against the eGFR values by CKD-EPI equation. The average predicted errors were 13.5 and 17.1 ml/min per 1.73 m2 in the internal and external validation sets, respectively, and were lower for patients with an observed eGFR<60 ml/min per 1.73 m2 than in those with an observed eGFR≥60 ml/min per 1.73 m2 (10.1 and 14.9 versus 16.2 and 17.7).
In addition, we explored the incremental value of polycystic kidney disease (PKD) class, using the combined validation sets, and found that upon applying the linear model (in Table 5) without and with PKD classes to predict the last eGFR, the R2 value increases from 69% to 72% when PKD class was included. The increase in R2 was greater in patients with higher levels of baseline eGFR. Similarly, we examined the gain in sensitivity/specificity when adding PKD class to a model to predict last eGFR≤45 ml/min per 1.73 m2 (binary endpoint, binary prediction; Supplemental Table 4). For those with baseline eGFR≥60 ml/min per 1.73 m2, specificity increased from 91% to 92% when PKD class was added and sensitivity increased from 40% to 60%. The net reclassification index for those with a baseline eGFR≥60 ml/min per 1.73 m2 was 19% with use of PKD class (20% of those with eGFR≤45 ml/min per 1.73 m2 were reclassified correctly −1% of those with eGFR>45 ml/min per 1.73 m2 reclassified incorrectly). With use of the model as a continuous predictor of last eGFR<45 ml/min per 1.73 m2, the receiver-operating characteristic curve area increased from 86% to 90% when PKD class was added for those with baseline eGFR≥60 ml/min per 1.73 m2. For predicting time to ESRD, using PKD classes increased the concordance statistic (beyond baseline eGFR) in all categories, particularly in patients with a baseline eGFR≥60 ml/min per 1.73 m2 (Supplemental Table 5).
Classification by HtTKV0 and Age Predicts Renal Survival in Patients with Class 1 ADPKD
MTPC Patients
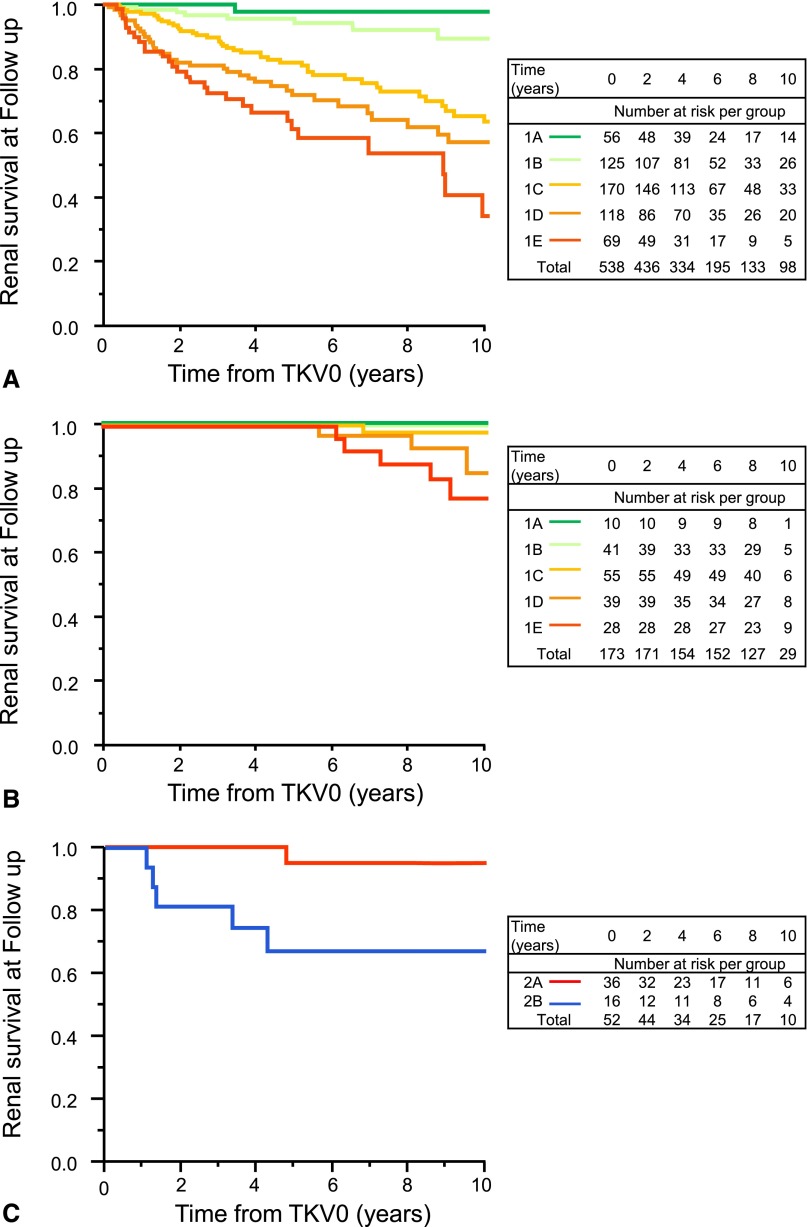
At the last clinical follow-up, a median of 6 (range, 0–18) years, 125 class 1 patients had reached ESRD (Figure 5A). Renal survival differed (P<0.001) between the subclasses. The estimated frequency of ESRD at 10 years increased from A to E (2.4%, 11.0%, 37.8%, 47.1%, and 66.9%, respectively). Median age at ESRD for patients who reached ESRD significantly decreased (P<0.001) from B to E (65%, 58%, 51%, and 43%, respectively). Only one patient in subclass 1A reached ESRD, because of complications from an abdominal malignancy. A multivariable Cox model controlling for eGFR at TKV0 showed an increased risk for ESRD for each step in the progression from 1A to 1E (hazard ratio, 1.84; 95% CI, 1.49 to 2.26; P<0.001) (Supplemental Table 6).
Figure 5.
Classification by HtTKV0 and age at HtTKV0 predicts renal survival in class 1 patients. Kaplan-Meier survival plots of time to ESRD by subclasses in the class 1 MTPC (A) and CRISP (B) patients and for the class 2 MTPC (C) patients.
CRISP Patients
At the last clinical follow-up, a median of 9 (range, 1–12) years, 18 class 1 patients had reached ESRD. Renal survival significantly differed (P<0.003) between the subclasses. No patient in subclass A reached ESRD and only 1 patient in B reached ESRD after 12 years of follow-up. The estimated frequency of ESRD at 10 years in the CRISP cohort, a cohort 12 years younger than the MTPC cohort, increased from C to E (2.2%, 14.6%, and 22.3%, respectively) (Figure 5B). Median age at ESRD for patients in each subgroup who reached ESRD decreased from C to E (54, 52, and 40 years, respectively). A multivariable Cox model controlling for eGFR at TKV0 showed an increased risk for ESRD for each step in the progression from 1A to 1E (hazard ratio, 4.67; 95% CI, 1.03 to 21.20; P=0.04) (Supplemental Table 6).
Renal Survival in Patients with Class 2 ADPKD
Renal survival differed between the A and B subclasses of the MTPC class 2 patients (95.0% versus 67.0% at 10 years, respectively; P=0.002) (Figure 5C). Only one patient in subclass 2A, characterized by focal or markedly asymmetric disease, reached ESRD, at 72 years, presumably from hypertensive nephroangiosclerosis. In contrast, seven patients in subclass 2B reached ESRD at a median age of 70 (range, 55–77) years. There were too few class 2 patients in the CRISP cohort with which to ascertain renal survival.
Stability of Class and Subclass Designations during Imaging Follow-Up
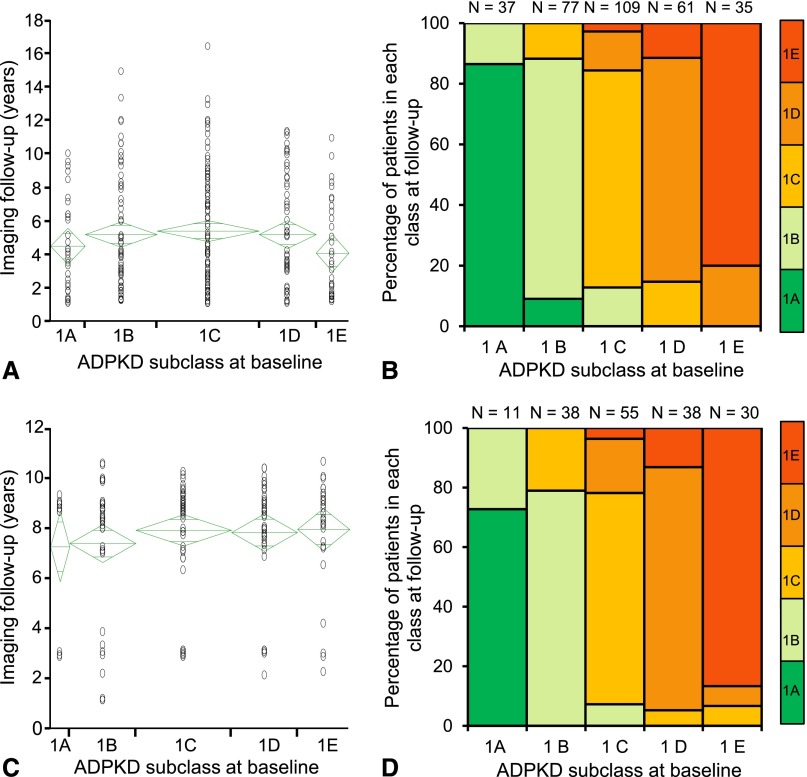
Change from one class to another was assessed in 319 patients from the 538 MTPC class 1 patients, 32 of the 52 MTPC class 2 patients, and 172 patients from the 173 CRISP class 1 patients who had at least one TKV (class 1) or one CT or MRI scan (class 2) at least one year after the first study. After a median follow-up of 4 years (Figure 6, A and B), a few patients in the MTPC cohort moved to the immediate lower subclass (seven from 1B to 1A, 14 from 1C to 1B, nine from 1D to 1C, and seven from 1E to 1D). Most patients in 1A (86.5%) remained within the pre-established limits for the subclass, while 13.5% of the patients moved to the range of subclass 1B. In subclasses 1B, 1C, and 1D, 11.7%, 15.6%, and 11.5% of patients, respectively, progressed to a higher subclass, in most cases the immediate higher subclass. Mean duration of imaging follow-up was longer in patients who changed subclass than in those who did not (7 years versus 4 years; P<0.001). After a median follow-up of 9 years (Figure 6, C and D), a few patients in the CRISP cohort moved to the immediate lower subclass (four from 1C to 1B, two from 1D to 1C, and two from 1E to 1D and 1C). Most patients in 1A (73%) remained within the pre-established limits for the subclass, although 27% of the patients moved to the range of subclass 1B. In subclasses 1B, 1C, and 1D, 21.0%, 21.8%, and 13.2% of patients, respectively, progressed to a higher subclass, in most cases the immediate higher subclass. Mean imaging follow-up was not significantly different between patients who changed subclass and those who did not. None of the patients switched from class 1 to class 2.
Figure 6.
Classification remains stable in most patients over time. Years of imaging follow-up and stability of patients with class 1 ADPKD. Years of imaging follow-up (A and C) and stability of patients with class 1 (typical) ADPKD (B and D) during imaging follow-up period in MTPC (A and B) and CRISP (C and D) patients. Most MTPC and CRISP patients remained in their baseline classification. A few patients moved to the immediate lower subclass, whereas some progressed to a higher class, in most cases the immediate higher class, at the end of imaging follow-up.
Discussion
ADPKD phenotypes associated with PKD2 mutations,17,18 nontruncating PKD1 mutations,19–21 mosaicism,22,23 and possibly other modifying genetic factors24 may be mild and pose a low risk for ESRD. With the increased use and resolution of imaging studies, mild ADPKD phenotypes are now diagnosed with increased frequency. Inclusion of patients with low risk of progression in clinical trials decreases the power to detect a treatment effect. When a treatment is proven to halt or slow the progression of ADPKD, its use in patients with a good prognosis would expose them to adverse events without a meaningful benefit. Therefore, identifying the most appropriate patients to enroll into clinical trials or decide when to initiate treatment in clinical practice is now crucial.
The CRISP study has shown that HtTKV measured by stereology predicts the risk of GFR decline, qualifying it as a prognostic biomarker.3,4 However, the predictive value of HtTKV is limited in particular conditions, such as in patients with a few large cysts or in patients with renal atrophy secondary to ischemia or urinary tract obstruction. Furthermore, measurement of kidney volume by stereology in ADPKD is time consuming and not readily available.
Our study presents an imaging classification of ADPKD that can guide in the selection of patients for clinical trials. In contrast to using TKV or HtTKV alone, the proposed classification recognizes that imaging characteristics of different patients have prognostic implications. It does not require stereology, which is time-consuming and depends on specialized software that is not available to all clinicians. The classification includes the estimation of HtTKV by the ellipsoid equation from a Digital Imaging and Communications in Medicine (DICOM) abdominal CT or magnetic resonance image. This requires only a few minutes and has sufficient precision to substitute stereology in the classification assignment. Because errors in TKV measurements using the ellipsoid equation could be higher compared with stereology at an individual patient level, TKV measurements using the ellipsoid equation should be used cautiously. In this context, and with the purpose of selecting patients into clinical trials, the misclassification error is modest. Therefore, the classification can be readily applied to any patient with ADPKD having a DICOM abdominal CT or MRI using the web application provided in the Supplemental Material.
TKVe estimates can also be obtained from ultrasonography-derived orthogonal dimensions. However, ultrasonographic measurements are operator dependent and more affected by body habitus. Previous studies have shown that ultrasonographic measurements of renal length and TKVe are less reproducible than those obtained from magnetic resonance images, even in normal kidneys.25 Furthermore, TKVe by ultrasonography in the CRISP study was 11% greater than TKVs by MRI, with an SD of 34%.26 Hence, we do not recommend the use of ultrasonography in the classification.
The proposed classification defines groups of patients with different risks for eGFR decline. Patients in subclasses 1A and 2A, who have a low risk for GFR decline, should not be included in clinical trials or subjected to treatments to slow down cyst growth with potential side effects. Because some patients in subclass 1A may progress to a higher subclass over time, re-evaluation after 3–5 years should be considered. Patients in subclasses 1C–1E, who have rapidly progressive disease, are optimal candidates for clinical trials and are most likely to benefit from a therapy. Patients in subclass 1B, who have an intermediate risk, could be re-evaluated at yearly intervals to more precisely determine their risk for progression. Patients in subclass 2B, with atrophy of the renal parenchyma out of proportion to TKV, are not likely to benefit from therapies directed to slowing kidney growth.
A responsibility of nephrologists counseling patients with ADPKD is to assess their prognosis. This is more challenging in patients whose renal function is still normal. At the present time, most nephrologists rely on the appearance of the kidneys on imaging studies. We believe that the proposed classification represents an improvement and is helpful in this regard.
The proposed classification predicts the decline of GFR and renal survival in patients with typical ADPKD over a broad range of CKD stages, even in patients at early stages of the disease with preserved renal function. Although the expected predicted error is slightly higher in patients with an eGFR≥60 ml/min per 1.73 m2, the predictive value of the classification was confirmed by an external validation set of patients who had been selected for participation in the observational CRISP study on the basis of preserved renal function and presence or absence of risk factors for progressive disease.27 The lower incidence of ESRD in the CRISP compared with the MTCP cohort is consistent with the younger age and earlier stage of the disease at baseline in the CRISP cohort.
Although the retrospective design is a potential limitation of the study, the inclusion criteria and the blinding of the evaluation of the imaging studies to clinical data were chosen to avoid selection bias. Therefore, the study patients are probably representative of the diagnosed patients with ADPKD in the United States. An exception is the under-representation of the African American population, which accounted for only 1.2% of study patients. Future studies will need to validate the proposed classification in this population.
Another limitation of this classification is that it is based on CT or MRI. For future studies and clinical practice, researchers and clinicians should know that MRI is superior to CT in measuring HtTKV and implementing the classification because it avoids radiation exposure and better defines the cysts without requiring the administration of contrast agents. A drawback of MRI is its cost. However, image acquisitions for measurements of TKV can be obtained in a few minutes, and the cost of MRI to measure TKV could be greatly reduced if in the United States, for example, the American Medical Association approved a specific Current Procedural Terminology code for this purpose. The availability of reasonably priced measurements of TKV would greatly help clinicians in the management of patients with ADPKD.
In summary, image-based criteria and HtTKV determined by CT or MRI using the ellipsoid equation allow a practical classification of ADPKD that can be used to select the most appropriate patients for clinical trials and identify patients with progressive disease likely to benefit from an effective therapy.
Concise Methods
Study Design
This multicenter, retrospective analysis was designed to develop and ascertain the value of a classification of ADPKD based on calculated HtTKV and age to predict decline of renal function. The classification was first derived and validated using the clinical imaging database of the MTPC and was subsequently validated using the clinical imaging database from the CRISP study. The Mayo Clinic Institutional Review Board approved the study and all patients informed consent.
Study Participants
Development and Internal Validation Sets
The MTPC clinical imaging database (see Supplemental Material ) was used to identify patients meeting the following criteria (Figure 1): Diagnosis of ADPKD,28,29 availability of one abdominal CT or MRI scan with complete coverage of both kidneys before ESRD (defined by initiation of dialysis or kidney transplant), baseline measurement of serum creatinine of <5 mg/dl within 6 months from the imaging study, at least two follow-up measurements of serum creatinine before ESRD, ages between 15 and 80 years at the time of imaging, and patient height availability. This dataset was used to derive and internally validate the imaging-based classification proposed in this article.
External Validation Set
To assess the predictive accuracy of the classification, participants in the CRISP study,3,4 who met the inclusion criteria were used as an external validation set. CRISP participants from the Mayo Clinic were excluded from this set (Figure 1). Detailed descriptions of the CRISP study have been published.3,4,27
Evaluation of CT and MRI and TKV Measurements
All DICOM files from CT and MRI were retrieved to a work station and inspected to confirm complete coverage of both kidneys and image quality. Two methods were used for TKV measurements. First, TKV was determined from 5- to 10-mm axial/coronal CT or MRI by TKVs using Analyze software (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). The same images were reanalyzed at a later time, and TKV was calculated using the ellipsoid equation as described in the Supplemental Material (Supplemental Figure 2). TKVe measurements were performed blinded to TKVs. The equivalence of TKVs measurements between MRI and CT images (Supplemental Figure 3) and reproducibility of TKVs and TKVe measurements by CT or MRI are shown in the Supplemental Material.
Classification of ADPKD into Typical (Class 1) and Atypical (Class 2) Patients
The patients were classified into typical (class 1) and atypical (class 2) cases on the basis of prespecified imaging findings (Supplemental Figure 1, Table 1). Images were classified by researchers blinded to clinical data. The classification by two observers of 50 randomly selected patients was highly reproducible (98%).
Classification of Patients with Class 1 ADPKD
Patients with class 1 ADPKD were stratified into five subclasses based on estimated kidney growth rates. TKV increase in patients at high risk for progression averages 5%–6% per year.3,7,30 Because our goal was to identify patients with slow, intermediate, and rapid progression, we calculated HtTKV ranges for each specific age assuming a theoretical starting HtTKV of 150 ml/m and yearly percentage increases of <1.5% (subclass 1A), 1.5%–3% (1B), 3%–4.5% (1C), 4.5%–6% (1D), or >6% (1E). Patients were subclassified into 1A–1E subclasses according to the HtTKV0 measurements and the HtTKV limits for their specific age (Supplemental Table 2). The web application in the Supplemental Material facilitates this classification.
Determination of Kidney Function and Disease Progression
The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.31 A conversion factor was used for serum creatinine values of MTPC patients obtained before October 18, 2006, when the method to measure serum creatinine was changed to isotope dilution mass spectrophotometry traceable.32 Serum creatinine values for CRISP patients were validated by the Cleveland Clinic.4 Baseline was the date of the first abdominal image with a serum creatinine within 6 months available. Clinical follow-up was defined as the period from TKV0 to the date at which time patients were last seen at the clinic. Disease progression was assessed by eGFR slopes and renal survival. Dates of ESRD were obtained from our MTPC database. In addition, to ensure that episodes of acute RRT for AKI were not coded as ESRD, the medical records of all patients having an ESRD date were reviewed.
Statistical Analyses
A multivariable longitudinal linear mixed-effects analysis with baseline predictors of sex, age at TKV0, HtTKV0 or image classification group, and eGFR at TKV0, along with follow-up years from TKV0 to subsequent visits (at time t), was used to predict eGFR follow-up readings at t years of follow-up.33 Inclusion of a linear year-of-follow-up term allows estimation of average and within-subject eGFR slopes. Interactions of follow-up year with image classification group were used to estimate slopes for each image class. Serum creatinine readings taken after ESRD were not considered. To minimize variability in the number of follow-up serum creatinine values, only the first serum creatinine was used when a patient had more than one reading during the same month. To reduce skewed results and the effect of TKV outliers, TKV was analyzed on a log2 scale. HtTKV was used for analysis.
This modeling approach was used to identify specific trends of renal function decline in class 1 and class 2 patients and among class 1 ADPKD subclasses. To assess the predictive accuracy of the model used for the subclassification of the typical ADPKD group, the MTPC class 1 patients were randomly assigned to a development (70% of the patients) or internal validation (30% of the patients) set. CRISP patients were used as an external validation set. The performance of the model for the change in eGFR created using the development set was evaluated by the EPE of the eGFR in both internal and external validation sets separately, using scatterplots comparing the actual eGFR value against the predicted by the model for each eGFR value. All models were controlled by sex, age at TKV0, TKV0 (ADPKD subclass for the typical group), and eGFR at TKV0. Time to ESRD after TKV0 was analyzed using Kaplan-Meier curves and Cox proportional hazards models.
All statistical analyses were conducted using SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Ms. T. L. Becker for the secretarial assistance and Mr. B. L. Dallman for the help with the web application. We thank the patients involved in the study for their participation and contribution.
This work has been supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK090728, DK058816), the Polycystic Kidney Disease Foundation, and Otsuka Pharmaceuticals. The CRISP study is supported by cooperative agreements from the NIDDK of the NIH (DK056943, DK056956, DK056957, DK056961) and by the National Center for Research Resources General Clinical Research Centers at each institution (RR000039, Emory University; RR00585, Mayo College of Medicine; RR23940, Kansas University Medical Center; RR000032, University of Alabama at Birmingham) and the National Center for Research Resources Clinical and Translational Science Awards at each institution (RR025008, Emory University; RR024150, Mayo College of Medicine; RR033179, Kansas University Medical Center; RR025777 and UL1 TR000165, University of Alabama at Birmingham; RR024153, University of Pittsburgh School of Medicine).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013101138/-/DCSupplemental.
References
- 1.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Grantham JJ: Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med 359: 1477–1485, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP, CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapman AB, Torres VE, Perrone RD, Steinman TI, Bae KT, Miller JP, Miskulin DC, Rahbari Oskoui F, Masoumi A, Hogan MC, Winklhofer FT, Braun W, Thompson PA, Meyers CM, Kelleher C, Schrier RW: The HALT polycystic kidney disease trials: Design and implementation. Clin J Am Soc Nephrol 5: 102–109, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higashihara E, Torres VE, Chapman AB, Grantham JJ, Bae K, Watnick TJ, Horie S, Nutahara K, Ouyang J, Krasa HB, Czerwiec FS, TEMPOFormula and 156-05-002 Study Investigators : Tolvaptan in autosomal dominant polycystic kidney disease: Three years’ experience. Clin J Am Soc Nephrol 6: 2499–2507, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 8.Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene-Iordache B, Remuzzi G, Epstein FH: Safety and efficacy of long-acting somatostatin treatment in autosomal-dominant polycystic kidney disease. Kidney Int 68: 206–216, 2005 [DOI] [PubMed] [Google Scholar]
- 9.van Keimpema L, Nevens F, Vanslembrouck R, van Oijen MG, Hoffmann AL, Dekker HM, de Man RA, Drenth JP: Lanreotide reduces the volume of polycystic liver: a randomized, double-blind, placebo-controlled trial. Gastroenterology 137: 1661–, e1–e2., 2009 [DOI] [PubMed] [Google Scholar]
- 10.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, Kim B, King BF, Glockner J, Holmes DR, 3rd, Rossetti S, Harris PC, LaRusso NF, Torres VE: Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol 21: 1052–1061, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caroli A, Perico N, Perna A, Antiga L, Brambilla P, Pisani A, Visciano B, Imbriaco M, Messa P, Cerutti R, Dugo M, Cancian L, Buongiorno E, De Pascalis A, Gaspari F, Carrara F, Rubis N, Prandini S, Remuzzi A, Remuzzi G, Ruggenenti P, ALADIN study group : Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): A randomised, placebo-controlled, multicentre trial. Lancet 382: 1485–1495, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt KU: Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Perico N, Antiga L, Caroli A, Ruggenenti P, Fasolini G, Cafaro M, Ondei P, Rubis N, Diadei O, Gherardi G, Prandini S, Panozo A, Bravo RF, Carminati S, De Leon FR, Gaspari F, Cortinovis M, Motterlini N, Ene-Iordache B, Remuzzi A, Remuzzi G: Sirolimus therapy to halt the progression of ADPKD. J Am Soc Nephrol 21: 1031–1040, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bae KT, Grantham JJ: Imaging for the prognosis of autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 96–106, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Poggio ED, Rule AD, Tanchanco R, Arrigain S, Butler RS, Srinivas T, Stephany BR, Meyer KH, Nurko S, Fatica RA, Shoskes DA, Krishnamurthi V, Goldfarb DA, Gill I, Schreiber MJ, Jr: Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int 75: 1079–1087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hateboer N, v Dijk MA, Bogdanova N, Coto E, Saggar-Malik AK, San Millan JL, Torra R, Breuning M, Ravine D: Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet 353: 103–107, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Harris PC, Bae KT, Rossetti S, Torres VE, Grantham JJ, Chapman AB, Guay-Woodford LM, King BF, Wetzel LH, Baumgarten DA, Kenney PJ, Consugar M, Klahr S, Bennett WM, Meyers CM, Zhang QJ, Thompson PA, Zhu F, Miller JP: Cyst number but not the rate of cystic growth is associated with the mutated gene in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 17: 3013–3019, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Rossetti S, Kubly V, Consugar M, Hopp K, Roy S, Horsley S, Chauveau D, Rees L, Barratt TM, van't Hoff W, Niaudet WP, Torres VE, Harris PC: Incompletely penetrant PKD1 alleles associated with mild, homozygous and in utero onset polycystic kidney disease. Kidney Int 75: 848–855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornec-Le Gall E, Audrézet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo MP, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang Y-H, Conklin J, Campbell K, He N, Wang K, Sundsbak JL, Heyer CM, Haider M, Harris PC, Pei Y: Hypomorphic PKD1 mutations are a common cause of mild ADPKD [Abstract]. J Am Soc Nephrol 23: 700A, 2012 [Google Scholar]
- 22.Connor A, Lunt PW, Dolling C, Patel Y, Meredith AL, Gardner A, Hamilton NK, Dudley CR: Mosaicism in autosomal dominant polycystic kidney disease revealed by genetic testing to enable living related renal transplantation. Am J Transplant 8: 232–237, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Reiterová J, Štekrová J, Merta M, Kotlas J, Elišáková V, Lněnička P, Korabečná M, Kohoutová M, Tesař V: Autosomal dominant polycystic kidney disease in a family with mosaicism and hypomorphic allele. BMC Nephrol 14: 59, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PC, Rossetti S: Determinants of renal disease variability in ADPKD. Adv Chronic Kidney Dis 17: 131–139, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakker J, Olree M, Kaatee R, de Lange EE, Moons KG, Beutler JJ, Beek FJ: Renal volume measurements: Accuracy and repeatability of US compared with that of MR imaging. Radiology 211: 623–628, 1999 [DOI] [PubMed] [Google Scholar]
- 26.O’Neill WC, Robbin ML, Bae KT, Grantham JJ, Chapman AB, Guay-Woodford LM, Torres VE, King BF, Wetzel LH, Thompson PA, Miller JP: Sonographic assessment of the severity and progression of autosomal dominant polycystic kidney disease: The Consortium of Renal Imaging Studies in Polycystic Kidney Disease (CRISP). Am J Kidney Dis 46: 1058–1064, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF, Jr, Glockner JF, Wetzel LH, Brummer ME, O’Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort : Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int 64: 1035–1045, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossetti S, Chauveau D, Walker D, Saggar-Malik A, Winearls CG, Torres VE, Harris PC: A complete mutation screen of the ADPKD genes by DHPLC. Kidney Int 61: 1588–1599, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Grantham JJ, Cook LT, Torres VE, Bost JE, Chapman AB, Harris PC, Guay-Woodford LM, Bae KT: Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int 73: 108–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rule AD, Larson TS: Do we need another equation to estimate GFR from serum creatinine in renal allograft recipients? Nephrol Dial Transplant 23: 2427–2428, author reply 2428, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rutter CM, Elashoff RM: Analysis of longitudinal data: Random coefficient regression modelling. Stat Med 13: 1211–1231, 1994 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.