Abstract
Hemodialysis vascular access recommendations promote arteriovenous (AV) fistulas first; however, it may not be the best approach for all hemodialysis patients, because likelihood of successful fistula placement, procedure-related and subsequent costs, and patient survival modify the optimal access choice. We performed a decision analysis evaluating AV fistula, AV graft, and central venous catheter (CVC) strategies for patients initiating hemodialysis with a CVC, a scenario occurring in over 70% of United States dialysis patients. A decision tree model was constructed to reflect progression from hemodialysis initiation. Patients were classified into one of three vascular access choices: maintain CVC, attempt fistula, or attempt graft. We explicitly modeled probabilities of primary and secondary patency for each access type, with success modified by age, sex, and diabetes. Access-specific mortality was incorporated using preexisting cohort data, including terms for age, sex, and diabetes. Costs were ascertained from the 2010 USRDS report and Medicare for procedure costs. An AV fistula attempt strategy was found to be superior to AV grafts and CVCs in regard to mortality and cost for the majority of patient characteristic combinations, especially younger men without diabetes. Women with diabetes and elderly men with diabetes had similar outcomes, regardless of access type. Overall, the advantages of an AV fistula attempt strategy lessened considerably among older patients, particularly women with diabetes, reflecting the effect of lower AV fistula success rates and lower life expectancy. These results suggest that vascular access-related outcomes may be optimized by considering individual patient characteristics.
Keywords: dialysis access, survival, hemodialysis
Arteriovenous (AV) fistula use in patients on hemodialysis is associated with better clinical outcomes, including lower infection, hospitalization, and mortality rates.1–3 These potential benefits led to the Fistula First Breakthrough Initiative, which targets AV fistulas in at least 50% of patients on incident hemodialysis and 66% of patients on prevalent hemodialysis on the basis of the reasonable conclusion that a mature, well functioning AV fistula is the preferred form of vascular access for most patients on hemodialysis.
Each form of hemodialysis vascular access has distinct advantages and disadvantages, with specific access types potentially better for certain individuals. Currently, central venous catheters (CVCs) are the dominant form of vascular access at hemodialysis initiation (used in 72% of incident patients),4 likely reflecting the relative ease of initial placement as well as systemic barriers to adequate preparation for kidney failure.5–7 In contrast, current guidelines suggest early AV fistula placement followed by prompt evaluation for maturation and additional interventions and/or new fistula creation if needed. AV grafts, a third type of vascular access, are neither recommended nor discouraged.8 Despite higher initial successful function rates compared with AV fistulas and lower infection rates compared with catheters, AV grafts tend to require more interventions and are more likely to fail after successful use compared with AV fistulas.2,9 Multiple studies show that older patients, women, and patients with vascular disease or vascular disease risk factors are at greatest risk of having an AV fistula fail to mature, potentially leaving them dependent on CVCs for vascular access.10–12 Accordingly, it is possible that these patients may benefit from initial AV graft placement because of higher rates of primary maturation.13,14
Many prior studies exploring vascular access choice have not assessed the effect of vascular access failure and more importantly, have not fully considered how patient characteristics affect both access failure and mortality. We, therefore, addressed the most common scenario facing North American patients on hemodialysis and hemodialysis facilities—a patient initiating hemodialysis with a CVC—and modeled subsequent vascular access choice, incorporating age, sex, and diabetes status into a decision analysis to address the rate of access failure and mortality associated with each vascular access option.
Results
Overview of the Simulation
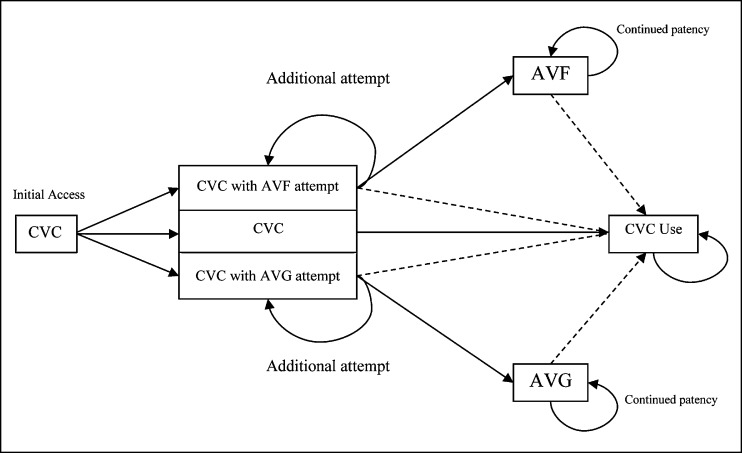
Model assumptions were obtained from the published literature or determined directly from observational cohorts when available (Tables 1 and 2). A simplified conceptual model showing decision points and patient flow is shown in Figure 1.
Table 1.
| Transition | Probability | Data Source |
|---|---|---|
| CVC to functioning AVF on first attempt | Logistic regression: varying by age, sex, and diabetes status with 90-d transition time | REDUCE FTM (Lok et al.11) |
| CVC to functioning AVG on first attempt | 0.95 with 30-d transition time | DAC Study Group (Dixon et al.24) |
| Continue CVC | 1.0 | Assumed |
| CVC to functioning AVF on second attempt | Logistic regression: varying by age, sex, and diabetes status with 90-d transition time | REDUCE FTM (Lok et al.11) |
| CVC to functioning AVG on second attempt | 0.95 with 30-d transition time | DAC Study Group (Dixon et al.24) |
| Continued AVF function | Subsequent function after initial successful creation: 1 yr: 90%a; 3 yr: 80%a; subsequent function after successful second attempt: 1 yr: 75%a; 3 yr: 50%a | Xue et al.15 |
| Continued AVG function | 1 yr: 71.8%; 3 yr: 51.5%a | Gibson et al.21 |
| AVF to death | Survival curve on the basis of age, sex, and diabetes status | DOPPS 2 |
| AVG to death | Survival curve on the basis of age, sex, and diabetes status | DOPPS 2 |
| CVC to death | Survival curve on the basis of age, sex, and diabetes status | DOPPS 2 |
AVF, AV fistula; REDUCE FTM, risk equation determining unsuccessful cannulation events and failure to maturation in AV fistulas; AVG, AV graft; DAC, Dialysis Access Consortium.
Value extrapolated from published data.
Table 2.
| Event | Cost |
|---|---|
| AV fistula attempt | $792.74 (CMS) |
| AV graft attempt | $573.82 (CMS) |
| Total CVC patient cost per year | First-year cost: $128,900a; second-year cost: $90,110b |
| Total AV graft patient cost per year | First-year cost: $113,073a; second-year cost: $79,337b |
| Total AV fistula patient cost per year | First-year cost: $92,720a; second-year cost: $64,701b |
Figure 1.
Concept model of simulated progression across vascular access options beginning at hemodialysis initiation. Death can be reached from all states; in all failure states, dialysis persists with a CVC. Dashed lines represent failure to achieve or loss of access patency. AVF, AV fistula; AVG, AV graft.
Base Case Analyses
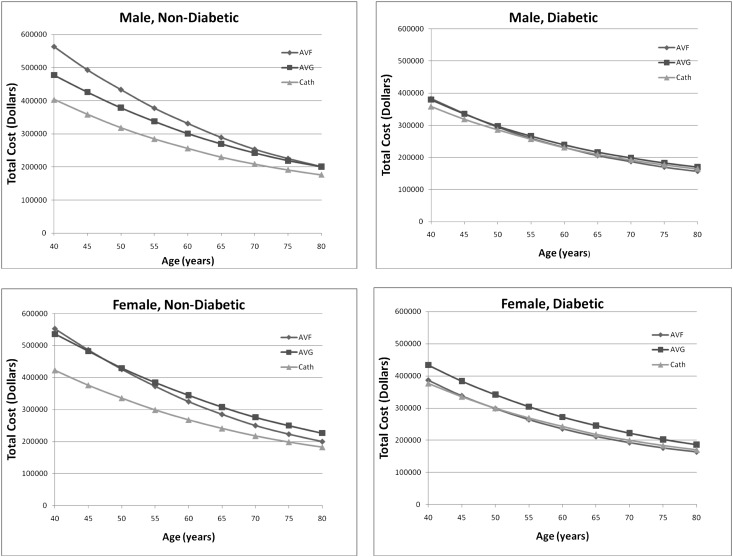
For the youngest modeled cohort (40 years old), placement of AV fistulas in men without diabetes maximized mean survival (7.94 years) (Table 3); the corresponding mean overall cost for this group was $563,000 (approximately $71,000 per year) (Figures 2 and 3). In contrast, the CVC strategy for a 40-year-old man with diabetes minimized mean survival (4.15 years) and because of the shorter lifespan, minimized overall costs ($358,000), albeit with higher annualized costs. Accordingly, for this age group when considering annual costs, the AV fistula attempt strategy was superior in regards to survival for each group combination, although the advantage was considerably reduced among women with diabetes. The AV graft attempt strategy, although inferior to an AV fistula strategy for survival, was associated with greater lifespan and lower annualized costs than a catheter-only strategy across all categories.
Table 3.
Mean survival (discounted life years) for modeled patient characteristics
| Modeled Patient | AV Fistula Strategy | AV Graft Strategy | CVC-Only Strategy |
|---|---|---|---|
| 40-yr-old nondiabetic man | 7.95 | 5.36 | 4.44 |
| 40-yr-old nondiabetic woman | 7.71 | 6.05 | 4.69 |
| 40-yr-old diabetic man | 5.26 | 4.20 | 3.95 |
| 40-yr-old diabetic woman | 5.17 | 4.82 | 4.16 |
| 60-yr-old nondiabetic man | 4.55 | 3.30 | 2.81 |
| 60-yr-old nondiabetic woman | 4.34 | 3.81 | 2.93 |
| 60-yr-old diabetic man | 2.99 | 2.56 | 2.53 |
| 60-yr-old diabetic woman | 2.94 | 2.94 | 2.66 |
| 80-yr-old nondiabetic man | 2.60 | 2.12 | 1.93 |
| 80-yr-old nondiabetic woman | 2.49 | 2.41 | 2.01 |
| 80-yr-old diabetic man | 1.90 | 1.73 | 1.80 |
| 80-yr-old diabetic woman | 1.91 | 1.94 | 1.85 |
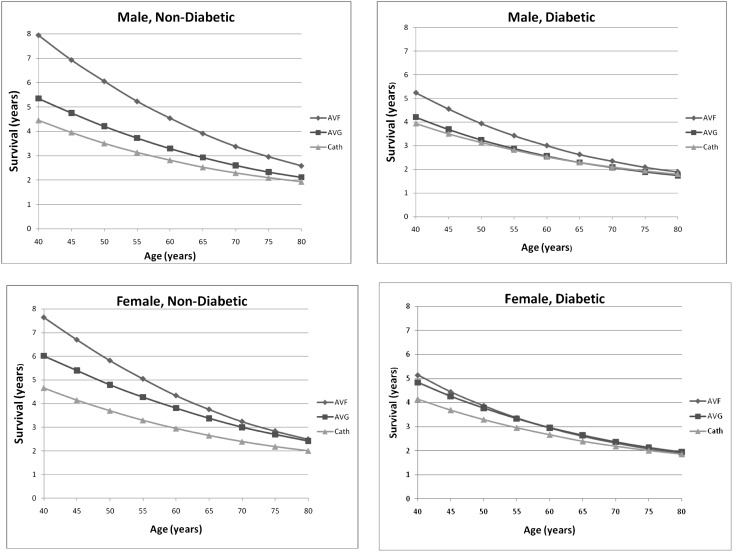
Figure 2.
Patient survival by access attempt strategy. Plots are stratified by sex and diabetes status. The x axis represents the age in years of modeled patients. The y axis represents the survival in years for modeled patients. Patient survival in years by age stratified by sex and diabetes status. AVF, AV fistula; AVG, AV graft; cath, CVC.
Figure 3.
Total life time in US dollars by access attempt strategy. Plots are stratified by sex and diabetes status. The x axis represents the age in years of modeled patients. The y axis represents the total lifetime cost in US dollars for modeled patients. Total costs in United States dollars for vascular access strategies by age stratified by sex and diabetes status. AVF, AV fistula; AVG, AV graft; cath, CVC.
For middle-aged (60-year-old) patients, placement of AV fistulas in men without diabetes also maximized mean survival (4.54 years), yielding overall costs of $331,000 per person. The catheter-only strategy in men with diabetes yielded the lowest mean survival (2.53 years) along with a mean overall cost of $231,000. The AV fistula attempt strategy yielded a higher survival compared with both an AV graft attempt strategy and catheter-only strategy across most categories. However, for women with diabetes, the AV fistula and AV graft strategies yielded the same survival (2.95 years), although the overall cost for the AV graft attempt strategy was $36,000 higher.
For the oldest (80-year-old) patients, there was a similar trend: placement of AV fistulas in men without diabetes yielded the longest mean survival (2.59 years) at an overall cost of $201,000 per person. For this age group, men with diabetes with an AV graft attempt strategy had the lowest mean survival (1.74 years), although there was little difference across access strategies, with an AV fistula strategy yielding a mean survival of 1.89 years and the catheter-only strategy yielding a mean survival of 1.79 years. For women with diabetes, an AV graft attempt strategy maximized mean survival (1.94 years) and was slightly higher than mean survival for the AV fistula strategy (1.91 years) or the catheter-only strategy (1.86 years). Overall costs associated with an AV graft attempt strategy were highest at $186,000 compared with $164,000 and $170,000 for AV fistula and catheter only, respectively.
Projecting outcomes at a finer set of age gradations for men and women with and without diabetes highlighted several trends, which are illustrated in Figure 2. For men without diabetes, the AV fistula strategy produced the greatest survival benefit across all ages, although this advantage decreased as age increased (Supplementary Figure 1). A similar but less pronounced pattern was evident in women. For men with diabetes, the AV fistula strategy yielded a modest survival benefit at younger ages, but the benefit diminished as age approached 80 years. Finally, for women with diabetes, there was little survival difference at all ages between AV fistula and AV graft strategies. For this particular category, both strategies were found to have better survival and lower cost compared with a catheter-only strategy at younger ages, with smaller differences seen at older ages.
For the majority of scenarios, the incremental cost-effectiveness ratio for the AV fistula strategy compared with the AV graft strategy was <$50,000 across all age categories, indicating that a fistula strategy likely is cost-effective. In older women with diabetes, survival among patients (Table 4) using an AV graft strategy was equal to or marginally better than among patients using an AV fistula strategy, with approximately 60 years of age being the threshold at which an AV graft attempt strategy first was associated with greater mean survival. A CVC-only strategy was inferior to both AV fistula and AV graft attempt strategies for all categories and across all ages; however, survival differences narrowed considerably at the oldest ages.
Table 4.
Comparison of attempt strategies by mortality difference (discounted life years) and incremental cost-effectiveness (dollars per discounted life year)
| Modeled Patient | Survival Difference (AVF − AVG) | ICER (AVF Versus AVG) | Survival Difference (AVF − Catheter) | ICER (AVF Versus Catheter) |
|---|---|---|---|---|
| 40-yr-old nondiabetic man | 2.59 | $34,464 | 3.49 | $45,603 |
| 40-yr-old nondiabetic woman | 1.67 | $14,410 | 2.98 | $43,338 |
| 40-yr-old diabetic man | 1.06 | $10,311 | 1.30 | $18,863 |
| 40-yr-old diabetic woman | 0.35 | Dominant | 1.00 | $10,284 |
| 60-yr-old nondiabetic man | 1.25 | $29,208 | 1.73 | $43,330 |
| 60-yr-old nondiabetic woman | 0.52 | Dominant | 1.39 | $40,547 |
| 60-yr-old diabetic man | 0.44 | $350 | 0.48 | $1496 |
| 60-yr-old diabetic woman | −0.001 | >$100,000 | 0.28 | Dominant |
| 80-yr-old nondiabetic man | 0.48 | $18,102 | 0.66 | $36,600 |
| 80-yr-old nondiabetic woman | 0.08 | Dominant | 0.49 | $34,563 |
| 80-yr-old diabetic man | 0.17 | $172 | 0.10 | Dominant |
| 80-yr-old diabetic woman | −0.03 | >$100,000 | 0.05 | Dominant |
Survival differences are reported in years. AVF, AV fistula; AVG, AV graft; ICER, incremental cost-effectiveness ratio; dominant, AVF has lower cost and superior survival.
Sensitivity Analyses
First, we varied the probability of initial AV fistula success (Table 5). When initial success was decreased by 20%, we saw a shift toward a much smaller survival benefit for the AV fistula attempt strategy compared with the AV graft strategy for most scenarios. For women with diabetes, the AV graft strategy had a superior survival across the range of modeled ages. When the AV fistula probability was increased by 20%, the AV fistula survival benefit increased modestly for most scenarios, and for women with diabetes, parity was reached near 80 years of age. Second, we varied long-term patency rates for both AV fistulas and AV grafts. Increasing the AV graft long-term patency rate by 50% resulted in relatively little change from our base case. Decreasing the AV fistula long-term patency rate by 50% yielded results very similar to the base case as well. Finally, increasing the time period to achieve a functioning graft to 90 days (equal to that of AV fistula) resulted in nearly identical results to our base case. We also varied cost inputs in two ways. First, we assumed that the annual cost of an AV graft was equal to the corresponding cost for an AV fistula. This change made the incremental cost-effectiveness of the AV fistula attempt strategy less favorable, increasing it to >$50,000 dollars per life year for the majority of scenarios. For patients in which AV grafts had superior survival (60- and 80-year-old women with diabetes), this change made the cost-effectiveness ratio more favorable (smaller) but did not reduce it to below the common benchmark of $50,000 per life year. Second, the yearly cost of a catheter was increased by $25,000. This change had little effect on the incremental cost-effectiveness of the AV fistula attempt strategy, yielding results similar to our base case.
Table 5.
Sensitivity analyses showing the difference in life years in models that vary AV fistula and AV graft success and patency probability
| Model Patient | Base Case AVF Initial Success Rate (%) | Survival Difference between AVF and AVG in Life Years | |||||
|---|---|---|---|---|---|---|---|
| Base Case (AVF − AVG) | AVF Initial Success 20% Less Likely | AVF Initial Success 20% More Likely | AVG Long-Term Patency Improved by 50% | AVF Long-Term Patency Worsened by 50% | AVF and AVG Maturation Time Both 90 d | ||
| 40-yr-old nondiabetic man | 86 | 2.59 | 2.04 | 2.82 | 2.46 | 2.22 | 2.60 |
| 40-yr-old nondiabetic woman | 75 | 1.67 | 0.88 | 2.03 | 1.48 | 1.39 | 1.65 |
| 40-yr-old diabetic man | 78 | 1.06 | 0.83 | 1.20 | 1.04 | 0.99 | 1.09 |
| 40-yr-old diabetic woman | 63 | 0.35 | −0.004 | 0.52 | 0.27 | 0.24 | 0.30 |
| 60-yr-old nondiabetic man | 77 | 1.25 | 0.88 | 1.37 | 1.20 | 1.16 | 1.23 |
| 60-yr-old nondiabetic woman | 62 | 0.52 | 0.08 | 0.85 | 0.49 | 0.45 | 0.55 |
| 60-yr-old diabetic man | 66 | 0.44 | 0.31 | 0.49 | 0.43 | 0.41 | 0.44 |
| 60-yr-old diabetic woman | 49 | −0.001 | −0.13 | 0.09 | −0.01 | −0.03 | −0.021 |
| 80-yr-old nondiabetic man | 66 | 0.48 | 0.29 | 0.58 | 0.47 | 0.46 | 0.46 |
| 80-yr-old nondiabetic woman | 48 | 0.08 | −0.12 | 0.24 | 0.07 | 0.07 | 0.10 |
| 80-yr-old diabetic man | 52 | 0.17 | 0.11 | 0.19 | 0.15 | 0.16 | 0.16 |
| 80-yr-old diabetic woman | 35 | −0.03 | −0.06 | −0.01 | −0.05 | −0.05 | −0.03 |
AVF, AV fistula; AVG, AV graft.
Discussion
In patients initiating hemodialysis with a CVC, an AV fistula attempt strategy seems associated with better survival and lower annual per patient costs compared with either an AV graft or CVC-only strategy. However, for patients over the age of 60 years, particularly women or patients with diabetes (scenarios where AV fistula success may be less likely), any difference in survival across strategies is minimal, and differences in cost are modest. These findings suggest that heterogeneity within the hemodialysis population significantly influences vascular access-related outcomes and that, although attempting AV fistulas may be the optimal strategy for most patients, an individualized care plan is critical.
Previous studies have attempted to address similar questions about vascular access.15,16 A 2012 decision analysis compared AV fistula use with AV graft use, finding that an AV fistula attempt strategy was more effective when the probability of AV fistula maturation was 36% or greater.16 However, this study did not consider patient mortality, take into account patient-level characteristics, or include transition to CVCs as a potential state. A 2010 decision analysis modeled the choice between AV fistula and AV graft, included prematuration scenarios, and considered model patient mortality,15 concluding that AV fistulas were superior to AV grafts, although the reported difference was less than the differences reported in observational studies. This study also did not consider catheters as a potential vascular access option. Neither study accounted for variation in AV fistula failure rates because of patient-level characteristics, a key strength of our study.
Our results confirm that, for many patients initiating hemodialysis with a CVC, attempting to place an AV fistula for hemodialysis access is a reasonable transitional strategy, although it may not be the ideal strategy for all patients initiating with a catheter. Notably, recent guidelines and quality metrics focus on the presence or absence of AV fistulas rather than the individualized care plan,17 reflecting the reasonable conclusion that a well functioning AV fistula is the preferred type of vascular access. Our study supports this overall premise but does so convincingly only in younger patients initiating dialysis with a catheter. For older patients, particularly for women or individuals with diabetes, we found little mortality difference among the three vascular access types, whereas cost differences were modest. Accordingly, practice and reimbursement guidelines should potentially allow for more flexibility, possibly including an indicator of vascular access suitability and likelihood of success.18 In our model, age, sex, and diabetes status serve as predictors of both the probability of AV fistula failure and overall mortality. Because each of these factors has a strong effect on survival and cost differences among different access types, it is likely that better predictive models for both successful AV fistula creation and mortality could further assist in determining the optimum access choice for patients on hemodialysis. Finally, we also note that our findings apply to patients initiating hemodialysis with a catheter, a situation that may reflect patient education as well as systemic barriers to the optimum delivery of health care, including ability to pay.6,7 As such, changes to the way that we care for patients before ESRD have the potential to lead to different outcomes than the outcomes presented.
The Fistula First Breakthrough Initiative states that their mission is “to improve the survival and quality of life of patients on hemodialysis by optimizing vascular access selection—which for most patients will be an AV fistula.” Our findings may modify this statement. First, working AV fistulas may not be attainable in all patients, and therefore, the referral and evaluation process could be emphasized or rewarded instead. Second, the mission statement could suggest potential characteristics of patients for whom an AV fistula attempt may not be beneficial, including old age, women, and diabetic comorbidity. In our study, such patients had the lowest probability of fistula maturation and the highest mortality rate.
There are several limitations to our study. First, model assumptions are largely on the basis of observational data with all its potential biases. Importantly, both the patency rates for fistulas and grafts and the overall survival estimates by the above access types are likely confounded by indication. This type of bias may be particularly important for studies of vascular access, because older, sicker patients may be more likely to receive catheters and to a lesser degree, AV grafts as their permanent access. In a related fashion, this bias may partially explain the greater survival time seen for women with an AV graft or catheter. Women, even those with few comorbid conditions, may be more likely to receive alternative forms of access because of their lower rate of AV fistula patency. Despite this potential for bias in observational data, we note that there are currently no completed randomized trials comparing vascular access strategies for patients on hemodialysis. Our study, which attempts to model vascular access scenarios as close to reality as possible while maintaining model simplicity, tries to address this bias in part through our sensitivity analyses, which incorporate additional variation in mortality and patency rates between access types. Our results remain largely consistent, even in these sensitivity analyses. However, we did not account for possible differences in outcomes that might be related to the reasons why patients initiated hemodialysis with a catheter. For example, the catheter-related mortality risks in patients whose fistula or graft created in the predialysis phase failed, necessitating initiation with a catheter, might differ from catheter-related mortality risks in patients who initiated dialysis with a catheter because of modality indecision, lack of dialysis preparation, or urgent start caused by intercurrent medical events. The effect of subsequent fistula creation (AV fistula–CVC–AV fistula) could be different from that modeled in our study (CVC–AV fistula). Indeed, our study results, which were produced by a simulation model and on the basis of population averages (not individual outcomes), cannot replace results from a prospective trial. We also acknowledge that, for the sake of model simplicity, several common scenarios were not modeled, including attempts at both AV fistula and AV graft creation in the same patient as well as incorporation of peritoneal dialysis as an access option. In addition, we did not vary the survival curves after a switch between access types; we note that a previous observational study showed a higher risk for mortality associated with switching from an AV fistula or AV graft to a catheter.19 We chose to limit these potential transitions to allow for easier interpretation of both the model and results. Furthermore, we assumed that, during the first 90 days after hemodialysis initiation, mortality risk does not depend on the access strategy selected. Although an oversimplification, our model aimed to explore longer-term differences in access-related mortality and cost. Additionally, we did not include other important factors that may influence the successful creation of a useable vascular access. Other risk factors, such as cardiovascular and peripheral vascular disease, heart failure, or use of additional diagnostic tests such as vessel mapping, may also be useful in predicting the likelihood of benefit for each access type but were not specifically modeled in this study. Finally, we note that costs associated with vascular access types are not static. For example, fistulas placed in patients with more marginal vessels may require costly interventional procedures to facilitate maturation (e.g., balloon-assisted maturation). As such, inclusion of all patients, even poor candidates, in an AV fistula attempt strategy would likely increase costs. These factors are partially accounted for in sensitivity analyses that equalize AV fistula and AV graft costs.
Our study also has several strengths. First, model assumptions were ascertained from large datasets that include a representative population of patients on hemodialysis. Use of such data makes it more likely that our results are generalizable to the wider dialysis population. Second, most access transitions that occur from hemodialysis initiation onward have been considered. Finally, by addressing the effect of age, sex, and diabetes status, the model accounts for heterogeneity in the hemodialysis population, allowing for assessment of how these factors influence differences between vascular access attempt strategies.
In conclusion, we show substantial heterogeneity in the cost and survival difference among access types when age, sex, and diabetes status are considered. Although being younger, a man, and without diabetes are characteristics indicating benefit of attempting AV fistulas, both AV graft and CVC-only strategies may be reasonable for patients with low likelihood of fistula success, such as older patients (including older women) and patients with diabetes. These results indicate that consideration of these subgroups may be crucial for the proper interpretation of future studies concerning vascular access.
Concise Methods
Decision Model
We developed a simulation model to reflect the progression from initial vascular access placement at hemodialysis initiation to death. Using a discrete event framework, at any time period in the model, patients were characterized as having one of three vascular access types: CVC, AV fistula, or AV graft. Beginning from hemodialysis initiation, we modeled the probability of vascular access failure and assessed the survival and cost associated with each vascular access modality.
Because most patients on hemodialysis in the United States initiate with a catheter,4 our base case assumed initiating hemodialysis through a tunneled CVC. We then compared three strategies: (1) continue CVC, (2) attempt AV fistula, and (3) attempt AV graft (Figure 1). After the initial 90-day period, each patient’s probability of death depended on age, sex, and diabetes status.3 Patients who survived retained CVC as their access until a functioning AV fistula or AV graft was achieved. A successful AV fistula was assumed to require 90 days for primary patency, whereas a successful AV graft was assumed to require 30 days for primary patency. Probabilities of primary and secondary patency depended on patient age, sex, and diabetes status as well as access type and whether the placement attempt was the first or second (the model assumed that no more than two placement attempts would be made; first attempts had a higher probability of success). AV graft and AV fistula failures were followed by return to a CVC, with a subsequent attempt to place a new AV fistula or graft. To simplify the model, AV fistula placement was not attempted in patients who first received an AV graft, and AV graft placement was not attempted in patients who first received an AV fistula. CVCs were assumed to carry no modeled risk of failure but did incur the cost of catheter removal, replacement, and hospitalizations as part of the overall total costs associated with having a CVC. Time to death (after the initial 90-day period) depended on age, sex, diabetes status, and access type (Figure 1). Comorbidity adjusters were deliberately limited to enhance model simplicity and applicability.
Our primary outcomes were mean survival in life years, mean medical costs in United States dollars, and the incremental cost-effectiveness ratio defined as the difference in costs between two competing strategies divided by the difference in survival in life years between strategies. Life years and costs were discounted at a rate of 3% per year.
Model Input Parameters
Event probabilities for each access type were extracted when possible from published literature regarding primary access placement success and subsequent access patency (Table 1). To estimate the probability of initial AV fistula placement success, we used data from the risk equation determining unsuccessful cannulation events and failure to maturation in AV fistulas, a longitudinal cohort study that examined risk factors for fistula failure.11 Predicted probability of success was calculated using terms for age, sex, and diabetes. The probability of initial AV graft success was extracted from the placebo group rate in the Dialysis Access Consortium AV graft trial.20 Rates for continued AV fistula and AV graft function were extrapolated from previously published reports15,21 and similar to cumulative patency rates reported in a recent work by Lok et al.9
Predicted survival associated with each access type (AV fistula, AV graft, and CVC) was derived from patient-level data in the Dialysis Outcomes and Practice Patterns Study 2 (DOPPS 2) cohort.3 Because death within the first 90 days of hemodialysis initiation was assumed to be less attributable to access choice for individuals initiating hemodialysis with CVCs, the same initial 90-day mortality rate was used for all patients followed by an access-specific post–90-day mortality model. The initial predicted 90-day mortality rate was determined using logistic regression from North American and European DOPPS 2 participants and included terms for age, sex, and diabetes status. For post–90-day mortality, a Cox proportional hazards model with terms for age, sex, and diabetes status was constructed for each access subgroup using DOPPS 2 data (n=2855).
Costs of access placement were estimated using reimbursement rates available through the Centers for Medicare and Medicaid Services (Table 2). Total yearly medical costs associated with each access type (which include access- and nonaccess-related costs as well as hospitalizations) were estimated using the 2010 US Renal Database System (USRDS) report.22 Because first-year hemodialysis costs are greater than subsequent yearly costs, an estimate of first-year access-specific costs was made using the USRDS data reported by Mau et al.23 Because first-year costs were not specified by access type in the study by Mau et al.,23 the costs associated with AV grafts were assigned the baseline yearly cost, with AV fistula (lower) and CVC (higher) costs adjusted downward and upward, respectively, to match the price ratios seen in the 2010 USRDS report.
Model Coding
Our model was programmed in Microsoft C-Sharp (version 2010). Cycle length varied, because the simulation was event-driven. The simulation was run 100,000 times for each model patient.
Sensitivity Analyses
AV fistula initial success rate varied between 35% and 86% depending on age range (40–80 years old), sex, and diabetes status (Table 5). To investigate alternative plausible model inputs, we varied the following parameters.
AV Fistula Initial Success Rate
Because patient, center, and surgeon factors can affect the probability of initial AV fistula success, the intercept of the base case logistic regression was modified to produce either an absolute 20% lower or higher success rate.
Long-Term Patency of AV Fistulas and AV Grafts
For AV fistulas, we investigated the effect of a relative decrease in the patency rate by 50%. For AV grafts, the long-term patency was increased by a relative 50%. This analysis accounted for upward bias in the patency rate for each access type caused by the choice of better candidates for AV fistula placement.
Time Needed before Use of Access Type
We investigated whether varying the time needed to achieve a functioning AV graft affected survival by increasing this time period from 30 to 90 days (the same time period as the AV fistula strategy).
Costs
Costs were varied in two different ways. First, the yearly cost of AV graft use was reduced to match that of AV fistula use. This change was made to account for the possibility that, because of selection bias, AV fistula candidates may typically be less likely to require interventions (and thus, have lower costs) or alternatively, with AV fistulas being placed in less healthy patients, that more frequent interventions could narrow the cost difference between AV fistulas and grafts. Second, the yearly cost of catheter use was increased to explicitly account for infection-related costs. Although these costs may already be incorporated in the yearly costs, the USRDS report did delineate how infection costs contributed to overall costs. We estimated infection-related costs to be an additional $25,000 per year on the basis of an assumed cost of $25,000 per episode and an assumed average of one infection episode per year.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Bruce Robinson, Dr. Ron Pisoni, and Brian Bieber of the Dialysis Outcomes and Practice Patterns Study (DOPPS) Coordinating Center at Arbor Research Collaborative for Health for use and technological descriptions regarding the DOPPS data used in this study that were integral to performing the decision analysis.
The study was funded by an American Society of Nephrology Research Fellowship Grant (to D.A.D.), the Kidney Research Scientist Core Education and National Training Program New Investigator Award (to N.T.; a joint initiative of the Kidney Foundation of Canada, the Canadian Society of Nephrology, and the Canadian Institute of Health Research), and a Carl Gottschalk Career Development Grant (to D.E.W.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Catheter Last, Fistula Not-So-First,” on pages 5–7.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2013111236/-/DCSupplemental.
References
- 1.Lacson E, Jr., Wang W, Hakim RM, Teng M, Lazarus JM: Associates of mortality and hospitalization in hemodialysis: Potentially actionable laboratory variables and vascular access. Am J Kidney Dis 53: 79–90, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Xue H, Ix JH, Wang W, Brunelli SM, Lazarus M, Hakim R, Lacson E, Jr.: Hemodialysis access usage patterns in the incident dialysis year and associated catheter-related complications. Am J Kidney Dis 61: 123–130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, Rayner HC, Saito A, Sands JJ, Saran R, Gillespie B, Wolfe RA, Port FK: Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: An instrumental variable analysis. Am J Kidney Dis 53: 475–491, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Fistual First Breakthrough Initiative. Available at: http://www.fistulafirst.org/AboutFistulaFirst/FFBIData.aspx. Accessed July 7, 2013
- 5.Foley RN, Chen SC, Collins AJ: Hemodialysis access at initiation in the United States, 2005 to 2007: Still “catheter first.” Hemodial Int 13: 533–542, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Allon M, Dinwiddie L, Lacson E, Jr., Latos DL, Lok CE, Steinman T, Weiner DE: Medicare reimbursement policies and hemodialysis vascular access outcomes: A need for change. J Am Soc Nephrol 22: 426–430, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Donca IZ, Wish JB: Systemic barriers to optimal hemodialysis access. Semin Nephrol 32: 519–529, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Vascular Access 2006 Work Group : Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S176–S247, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Lok CE, Sontrop JM, Tomlinson G, Rajan D, Cattral M, Oreopoulos G, Harris J, Moist L: Cumulative patency of contemporary fistulas versus grafts (2000-2010). Clin J Am Soc Nephrol 8: 810–818, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monroy-Cuadros M, Yilmaz S, Salazar-Bañuelos A, Doig C: Risk factors associated with patency loss of hemodialysis vascular access within 6 months. Clin J Am Soc Nephrol 5: 1787–1792, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lok CE, Allon M, Moist L, Oliver MJ, Shah H, Zimmerman D: Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 17: 3204–3212, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Miller CD, Robbin ML, Allon M: Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 63: 346–352, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Allon M, Lok CE: Dialysis fistula or graft: The role for randomized clinical trials. Clin J Am Soc Nephrol 5: 2348–2354, 2010 [DOI] [PubMed] [Google Scholar]
- 14.O’Hare AM: Vascular access for hemodialysis in older adults: A “patient first” approach. J Am Soc Nephrol 24: 1187–1190, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue H, Lacson E, Jr., Wang W, Curhan GC, Brunelli SM: Choice of vascular access among incident hemodialysis patients: A decision and cost-utility analysis. Clin J Am Soc Nephrol 5: 2289–2296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosas SE, Feldman HI: Synthetic vascular hemodialysis access versus native arteriovenous fistula: A cost-utility analysis. Ann Surg 255: 181–186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowling CB, O’Hare AM: Managing older adults with CKD: Individualized versus disease-based approaches. Am J Kidney Dis 59: 293–302, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes A, Schmidt R, Wish J: Re-envisioning Fistula First in a patient-centered culture. Clin J Am Soc Nephrol 8: 1791–1797, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lacson E, Jr., Wang W, Lazarus JM, Hakim RM: Change in vascular access and mortality in maintenance hemodialysis patients. Am J Kidney Dis 54: 912–921, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Dixon BS, Beck GJ, Vazquez MA, Greenberg A, Delmez JA, Allon M, Dember LM, Himmelfarb J, Gassman JJ, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Fenves AZ, Kaufman JS, Cotton JR, Jr., Martin KJ, McNeil JW, Rahman A, Lawson JH, Whiting JF, Hu B, Meyers CM, Kusek JW, Feldman HI, DAC Study Group : Effect of dipyridamole plus aspirin on hemodialysis graft patency. N Engl J Med 360: 2191–2201, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibson KD, Gillen DL, Caps MT, Kohler TR, Sherrard DJ, Stehman-Breen CO: Vascular access survival and incidence of revisions: A comparison of prosthetic grafts, simple autogenous fistulas, and venous transposition fistulas from the United States Renal Data System Dialysis Morbidity and Mortality Study. J Vasc Surg 34: 694–700, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L: Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis 55[1 Suppl 1]: S1–S420, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mau L-W, Liu J, Qiu Y, Guo H, Ishani A, Arneson TJ, Gilbertson DT, Dunning SC, Collins AJ: Trends in patient characteristics and first-year medical costs of older incident hemodialysis patients, 1995-2005. Am J Kidney Dis 55: 549–557, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Dixon BS, Beck GJ, Dember LM, Vazquez MA, Greenberg A, Delmez JA, Allon M, Himmelfarb J, Hu B, Greene T, Radeva MK, Davidson IJ, Ikizler TA, Braden GL, Lawson JH, Cotton JR, Jr., Kusek JW, Feldman HI, Dialysis Access Consortium (DAC) Study Group : Use of aspirin associates with longer primary patency of hemodialysis grafts. J Am Soc Nephrol 22: 773–781, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2010 Annual Data Report. Am J Kidney Dis 57[1 Suppl 1]: A8, e1–A8, e526, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.